Introduction
Liver cancer is the fourth leading cause of
cancer-related death worldwide. The main risk factors for its
development are liver cirrhosis, chronic hepatitis B or C virus
infection, and alcoholic and non-alcoholic fatty liver diseases
(1-3).
According to the latest global cancer burden statistics released by
the International Agency for Research on Cancer of the World Health
Organization, China reported the highest incidence of liver cancer
cases in 2020, with ~4,568,754 new cases that year. Moreover, liver
cancer is the second leading cause of all cancer deaths in China,
posing a serious threat to overall health and quality of life
(4). Exhaustive research on liver
cancer has yielded a considerable improvement in the monitoring
methods and treatment strategies available for this disease
(1). However, most patients with
liver cancer are diagnosed in the middle and advanced stages of the
disease, and therefore cannot undergo surgical resection.
Additionally, most available drugs for liver cancer are
non-specific, which causes liver cancer cells to easily develop
resistance to single-target drugs (2). Consequently, the mortality rate of
liver cancer remains high, with a 5-year survival rate of <5%
(3). Therefore, there is an urgent
need to develop new and effective drugs to improve the overall
survival of patients with liver cancer and their quality of
life.
Traditional Chinese medicine is widely used for
cancer treatment in China, as the numerous natural compounds
employed target various cellular processes and cause relatively few
adverse reactions (5,6). Additionally, certain compounds have
demonstrated high efficiency and reduced toxicity as curative
agents for liver cancer (7).
Curcuma zedoaria (referred to as Ezhu in traditional Chinese
medicine) is a herb commonly used for alleviating blood stasis and
stagnation (8) or for treating
heart and abdominal pain, swelling or food stagnation (9,10);
it is also widely prescribed in traditional Chinese medicine for
anti-tumor therapy and displays anti-carcinogenic properties
believed to promote the flow of Qi, a concept in traditional
Chinese medicine that represents the vital life force dredging the
meridians, reducing lumps and relieving pain (11,12).
Several studies have confirmed that Curcuma zedoaria yields
a beneficial effect in the treatment of various tumors; it also
exerts a substantial inhibitory effect on the proliferation of
cervical and breast cancer, and other tumors (13-15).
The chemical composition of Curcuma zedoaria is complex and
may be roughly categorized into volatile oils and curcumin
(9). Germacrone (GM) is a volatile
oil present in all varieties of Curcuma zedoaria at
relatively stable concentrations. GM has been demonstrated to
possess a wide range of pharmacological properties, thereby
rendering it an efficacious therapeutic candidate against several
types of cancer (16,17). Although several studies have
reported that GM inhibits HepG2 cell growth (18,19),
the mechanism underlying this inhibition remains unclear.
Pyroptosis is a newly discovered mechanism of
pro-inflammatory programmed cell death distinct from apoptosis,
necrosis and autophagy (20-22).
The main manifestation is cell swelling and lysis, accompanied by
the release of pro-inflammatory factors (22). Classic pyroptosis is mediated by
caspases-1/4/5/11, which cleave the effector gasdermin D (GSDMD),
canceling the auto-inhibition that the C-terminal domain of GSDMD
exerts over the pro-active N-terminal GSDMD domain (23-25).
Thereafter, the activated N-terminal GSDMD fragment oligomerizes
and perforates the cellular membrane, causing pyroptosis (25,26).
Wang et al (26) found that
there is a very conservative caspase-3 tetrapeptide cleavage site
in the GSDME. In specific cells expressing GSDME, activation of
caspase-3, under certain conditions, can specifically cleave GSDME
and release the N-terminus, thus forming membrane pores in the
plasma membrane and triggering the lytic death of the cells.
Therefore, GSDME may act as an effector of secondary necrosis in
response to certain physiological stimuli. This strategy may
provide an effective host response when in contact with certain
pathogens that block the apoptotic pathway (26,27).
Curcumin has been reported to promote the lysis of GSDME protein in
HepG2 cells, while also inducing pyrolysis and exerting anticancer
effects (28). Additionally, Zhang
et al (29) found that
miltirone induces HepG2 cell death through GSDME-dependent
pyroptosis, which indicates that HepG2 cells have high levels of
GSDME protein expression. Another study demonstrated that the
concentration of GM induces a dose-dependent increase in activated
caspase-3 expression in HepG2 cells (18). Despite these indications, it
remains unclear whether GM activates caspase-3, inducing the
cleavage of GSDME and cell pyroptosis, and whether it exerts
anticancer effects.
The present study investigated the effects of GM on
HepG2 cells using in vitro and in vivo models, and
elucidated the possible molecular mechanisms, with a special focus
on the ability of GM to induce pyroptosis in HepG2 cells.
Materials and methods
Reagents
Antibodies against caspase-3 (cat. no. ab184787),
GSDMD-N (cat. no. ab215203), GSDMD (cat. no. ab210070),
pro-caspase-1 + p10 + p12 (cat. no. ab179515) and
GSDME/GSDME-N-terminal (cat. no. ab215191) were purchased from
Abcam. Antibodies against cleaved caspase-3 (cat. no. 19677-1-AP)
and GAPDH (cat. no. 60004-1-1g), and horseradish
peroxidase-conjugated secondary antibodies (cat. no. SA00001-1)
were purchased from ProteinTech Group, Inc. The Cell Counting Kit-8
(CCK-8) (cat. no. C0037), BeyoClick™, EdU Cell
Proliferation Kit with Alexa Fluor 488 (cat. no. C0071S), propidium
iodide (PI; cat. no. ST511), 7-AAD Cell Viability Assay Kit (cat.
no. C1053S) were purchased from Beyotime Institute of
Biotechnology. GM (cat. no. S9311; purity: 98.2%) and
N-acetylcysteine (NAC; cat. no. S1623) were purchased from Selleck
Chemicals.
Cell culture and treatment
HepG2 cells were purchased from the Cell Bank of the
Chinese Academy of Sciences and authenticated by STR profiling.
HepG2 cells were primarily cultured in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; Cytiva) supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin-streptomycin in an incubator at 37˚C,
under 5% CO2 (18).
Cells were treated with 0, 50, 100, 150 and 200 µM GM at 37˚C for
12, 24 or 48 h, following which different assays were performed.
HepG2 cells were pretreated with or without 5 mM NAC for 2 h before
treatment with 200 µM of GM for 24 h, following which different
assays were performed.
PI staining
HepG2 cells were pretreated with with 0, 50, 100,
150 and 200 µM GM at 37˚C for 24 h, a PI solution (Beyotime
Institute of Biotechnology) was added to the medium and further
incubated for 30 min at 37˚C in the dark. Observed under a
fluorescence microscope (Leica Microsystems, Inc.), in three random
sections of each sample, three different areas (or more) were
randomly selected for the capture of images. Analyses were
performed using ImageJ 1.8.0 software (National Institutes of
Health). Results are shown as the mean ± SD.
Cell viability assay
Cell viability was evaluated using CCK-8 and colony
formation assays. For the colony formation assay, the cells were
plated at a density of 500 cells/well in a 6-well plate (Corning,
Inc.) and then cultured for 7 days in the aforementioned culture
medium. The following day, the cells were treated with the desired
doses (0, 50, 100, 150 and 200 µM) of GM for 24 h, and then the
culture medium was refreshed. The culture was continued for 7 days
and the medium was changed every 3 days. The cells were then washed
twice with PBS, fixed in methanol for 10 min at 37˚C and stained
with 1% crystal violet for 10 min at 37˚C and manually counted.
Cell groups consisting of >50 cells were considered colonies.
For the CCK-8 assay, a total of 10,000 cells per well were seeded
into 96-well plates, incubated for 12 h and subsequently exposed to
the desired doses of GM (0, 50, 100, 150, and 200 µM) for 12, 24 or
48 h. Cell viability was measured with a Cell Counting Kit-8 after
GM treatment at a wavelength of 450 nm. Cell proliferation was
measured with a BeyoClick™EdU Cell Proliferation Kit
with Alexa Fluor 488 after GM (0, 50, 100, 150 and 200 µM)
treatment for 24 h. For microscopic analysis, images of the same
area were obtained from three different experiments. Analyses were
performed using ImageJ 1.8.0 software (National Institutes of
Health). Results are expressed as the mean ± SD.
Flow cytometry
Flow cytometry was performed using an Annexin
V-FITC/PI apoptosis detection kit (Nanjing KeyGen Biotech Co.,
Ltd.), according to the manufacturer's instructions. Briefly, HepG2
cells treated with/without GM for 24 h were digested with trypsin
in the absence of EDTA. After digestion, the harvested cells were
washed with PBS and resuspended in 500 µl binding buffer. Cells
were then incubated with a binding buffer containing 5 µl Annexin
V-FITC and 5 µl PI for 15 min at 25˚C in the dark. The cells were
analyzed using CytExpert 2.0 software (Beckman Coulter, Inc.).
LDH release assay
HepG2 cells in the logarithmic growth phase were
seeded in a 96-well plate at a density of 5x103 cells
per well. Cells were incubated overnight in a cell incubator at
37˚C under 5% CO2, and subsequently exposed to different
GM concentrations (0, 50, 100, 150 and 200 µM) for 12, 24 or 48 h.
The LDH release assay was performed according to instructions in
the LDH Cytotoxicity Assay Kit (Beyotime Institute of
Biotechnology).
Cell death assay
For the cell death assay, HepG2 cells were
pretreated with GM at different concentrations (0, 50, 100, 150 and
200 µM) for 24 h. Cells were collected and initially stained with
7-AAD (2 µg/ml in PBS; Nanjing KeyGen Biotech Co., Ltd.) for 30
min, and washed three times with PBS (for 3 min each), and
resuspended in 500 µl 1X Assays Buffer. The cells were then
analyzed using CytExpert 2.0 software (Beckman Coulter, Inc.), and
data were collected for analysis.
Western blotting
Cells or tumor tissues were washed with cold PBS
twice and prepared in radioimmunoprecipitation assay (RIPA) lysis
buffer (Beyotime Institute of Biotechnology) containing
protease/phosphatase inhibitor cocktail. The BCA Protein Assay Kit
(Beyotime Institute of Biotechnology) was used to measure the
protein concentration. Total proteins (30 µg) were separated on 12%
gels using SDS-PAGE and then transferred onto nitrocellulose
membranes. Next, the membranes were blocked in Tris-buffered saline
containing 0.1% Tween-20 and 5% fat-free milk for 1 h at 25˚C,
followed by incubation with primary antibody solutions (1:1,000)
for 18 h at 4˚C. Subsequently, the membranes were incubated with
secondary antibody solutions (1:5,000) at 25˚C for 1 h. Enhanced
chemiluminescence (ECL) reagent (MilliporeSigma) or ECL Plus
Amersham; Cytiva) were used to detect the immunoreactive bands and
visualized with the ChemiDoc XRS system (Bio-Rad Laboratories,
Inc.). Densitometry analysis for western blotting was performed
using Gelpro32 imaging software (Media Cybernetics, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from the cells or tissues
using an RNAprep FastPure kit (cat. no. TSP413; TsingKe Biological
Technology), according to the manufacturer's instructions. Total
RNA was reverse transcribed into complementary DNA using an RT6
cDNA Synthesis Kit (cat. no. TSK302M; TsingKe Biological
Technology), according to the manufacturer's instructions. qPCR was
performed using a CFX96 Real-time System (Bio-Rad Laboratories,
Inc.) with SYBR Green I (cat. no. TSE202; TsingKe Biological
Technology) and thermocycling conditions as follows: 95˚C for 10
sec, 61˚C for 30 sec and 72˚C for 30 sec, for 40 cycles. Relative
gene expression levels were calculated using the 2-ΔΔCq
method (30). The primer sense and
antisense sequences were as follows: β-actin forward,
5'-CCTGGCACCCAGCACAAT-3' and reverse, 5'-GGGCCGGACTCGTCATAC-3'; and
caspase-3 forward, 5'-TGGAACAAATGGACCTGTTGACC-3' and reverse,
5'-AGGACTCAAATTCTGTTGCCACC-3'. β-actin was used as an internal
control for quantification.
Transmission electron microscopy
(TEM)
HepG2 cells were pretreated with GM at different
concentrations (0, 50, 100, 150 and 200 µM) for 24 h. The cells
were fixed with 4% paraformaldehyde at 4˚C for 48 h, post-fixed in
1% osmium tetroxide at 4˚C for 1.5 h, dehydrated in a graded
ethanol series, infiltrated with propylene oxide, embedded in epoxy
resins at 37˚C for 12 h and sectioned to a thickness of 70 nm.
After double staining with uranyl acetate (25˚C for 30 min) and
lead citrate (25˚C for 5 min), ultrathin sections were examined
using a model HT-7700 transmission electron microscope (Hitachi
Ltd.).
Cell transfection
HepG2 cells were transfected with caspase-3 small
interfering (si)RNA (5'-GCAGCAAACCTCAGGGAAATT-3') and control siRNA
(5'-TTCTCCGAACGUGUCACGUTT-3'), purchased from Guangzhou RiboBio
Co., Ltd., following the manufacturer's instructions. siRNA (100
nM) transfections were performed using Lipofectamine®
2000 transfection reagent (Gibco; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions at 37˚C for 48 h.
Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) transfection
medium was replaced with complete culture medium with 10% fetal
bovine serum 5 h after transfection. All experiments were performed
48 h after the transfection. The expression of caspase-3 was
measured by RT-qPCR and western blotting.
Nude mouse xenograft model
Female nude mice (BALB/c; age, 4 weeks; weight,
20-22 g), were purchased from the Laboratory Animal Center of
Guangzhou University of Chinese Medicine (Shenzhen, China). All
mice were housed under the following conditions: A 12 h light/dark
cycle (lights on, 07:00; lights off, 19:00), a temperature of
22±2˚C, a humidity of 50±10%, and free access to standard diet and
water. All animal experiments were approved by the Animal Ethics
Committee of Guangzhou University of Chinese Medicine (approval no.
20210303042). As described in a previous study (29), mice (n=30) were randomly assigned
to five treatment groups (n=6), and then treated with GM solution
(5, 10, 15 or 20 mg/kg body weight) or the same dose of vehicle
(PBS). Briefly, 1x106 HepG2 cells were resuspended in
100 µl PBS and injected subcutaneously into the right side of the
mouse. Starting 6 days after cell injection, the nude mice were
treated intragastrically with GM solution (5, 10, 15 or 20 mg/kg
body weight), or vehicle (PBS) for 21 consecutive days. A caliper
was used to monitor the length and width of the tumor every 5 days.
The tumor volume (V) was calculated using the following formula:
V=(a x b2)/2, where a and b are the maximum and minimum
diameters in millimeters, respectively. On the 30th day after cell
injection, the tumor burden was <10% of the body weight and the
longest diameter of a single tumor was 12 mm, in line with animal
ethics requirements. There were no ulcerated, necrotic or infected
tumors. The mice were euthanized with an intraperitoneal injection
of pentobarbital sodium (200 mg/kg), and the xenograft tumors were
excised and measured.
Quantitative determination of
oxidative stress and hematoxylin-eosin staining
Xenograft tumors and livers were collected after the
mice were sacrificed under anesthesia. Samples were subsequently
fixed with 4% paraformaldehyde in PBS for 24 h at room temperature,
dehydrated with an ethanol gradient, cleared with xylene, embedded
in paraffin and then cut into 4-µm sections. Dihydroethidium (DHE;
Thermo Fisher Scientific, Inc.) staining was used to determine ROS
levels in the xenograft tumors. HepG2 cells and tissue sections
were stained with 5 mmol/l DHE (in PBS) for 20 min at room
temperature. Nuclei were stained with DAPI at 25˚C for 5 min. For
H&E staining, the sections were dewaxed and dehydrated,
subsequently washed with PBS, and then stained with H&E at 25˚C
for 2 min each. Observed under a light microscope (XI71; Olympus
Corporation), in three random sections of each sample, three
different areas (or more) were randomly selected for the capture of
images at x40 magnification. Analyses were performed using ImageJ
1.8.0 software (National Institutes of Health). Results are shown
as the mean ± SD (n=6 mice per group).
Germacrone-interaction proteins
comprehensive analysis
The protein network with germacrone was built using
the STITCH tool (https://stitch.embl.de/), which includes direct
physical interactions between germacrone and the interacting
proteins, as well as the inner functional correlation between these
proteins. After importing germacrone into the Search Tool STITCH,
the germacrone-interaction protein network information was
obtained.
Statistical analysis
Each experiment was repeated independently at least
three times. The animals were randomly divided into five
experimental groups. Survival analysis was performed using the
log-rank test. Body weight analysis was performed using repeated
measures ANOVA. Unpaired Student's t-test was used to compare two
groups. Statistical comparisons between three or more groups were
performed using one-way ANOVA followed by Tukey's multiple
comparison test. P<0.05 was used to indicate a statistically
significant difference. All analyses were performed using GraphPad
Prism 7 (GraphPad Software, Inc.).
Results
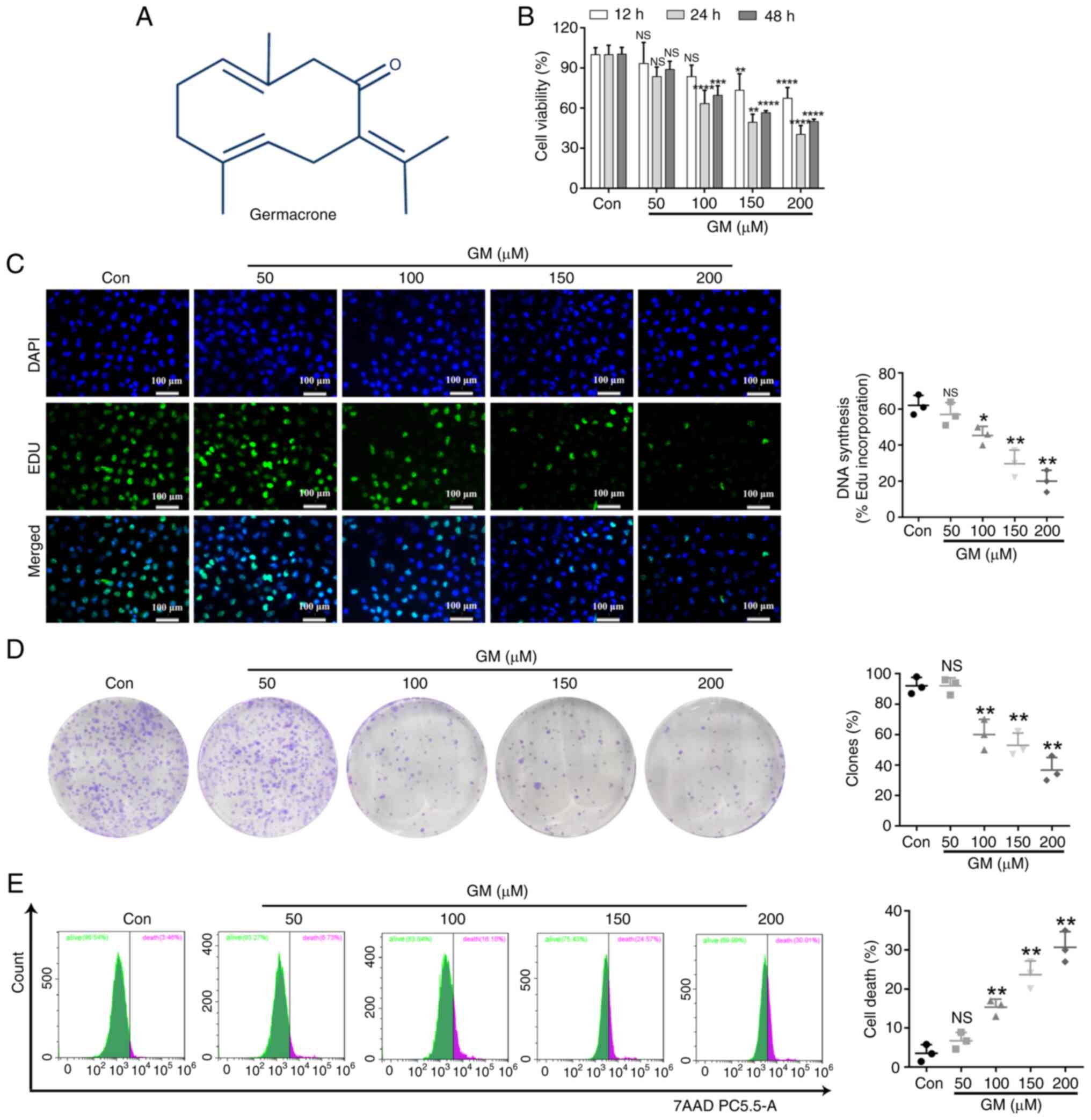
Effects of GM on HepG2 cell
proliferation
The chemical structure of GM is shown in Fig. 1A. To determine whether GM inhibited
HepG2 cell viability, cells were treated with different
concentrations of GM (0, 50, 100, 150 and 200 µM) for 12, 24 or 48
h and cell viability was detected using the CCK-8 assay. GM
markedly hampered the viability of HepG2 cells in a dose-dependent
manner (Fig. 1B), and treatment
with 200 µM of GM for 24 h had the most obvious effect (Fig. 1B). Furthermore, the effect of GM on
proliferation was investigated by treating HepG2 cells with
different GM concentrations for 24 h, and then performing the
colony-formation assay and EdU staining. These two methods
demonstrated that cell proliferation was substantially inhibited by
GM treatment, compared with the cell proliferation rate in the
control group (Fig. 1C and
D). Thereafter, cellular damage or
death was evaluated using a 7-AAD cell viability assay kit and flow
cytometry. The results revealed that GM increased the damage or
death in HepG2 cells in a dose-dependent manner (Fig. 1E).
GM induces plasma membrane
permeabilization and pyroptosis in HepG2 cells
Microscopic study of the cells revealed
membranolysis and the presence of large bubbles emerging from the
plasma membrane in GM-treated HepG2 cells, which were distinct from
the morphological features of apoptotic cells, but similar to the
features of pyroptotic cell morphology (Fig. 2A). To further understand whether
these cells underwent pyroptosis in response to GM, the
concentration of cytosolic compounds that are released as a result
of membrane disruption during pyroptosis, such as LDH, was
measured. GM treatment markedly increased the release of LDH into
the supernatant in a dose-dependent manner (Fig. 2B). Staining with PI showed that
treatment with GM increased PI fluorescence in a dose-dependent
manner (Fig. 2C), further
indicating the breakdown of plasma membrane integrity. Furthermore,
GM treatment substantially increased the cell population positive
for Annexin V and PI in a dose-dependent manner, as detected using
flow cytometry (Fig. 2D).
Altogether, these data indicated that GM induced pyroptotic cell
death by plasma membrane permeabilization in HepG2 cells.
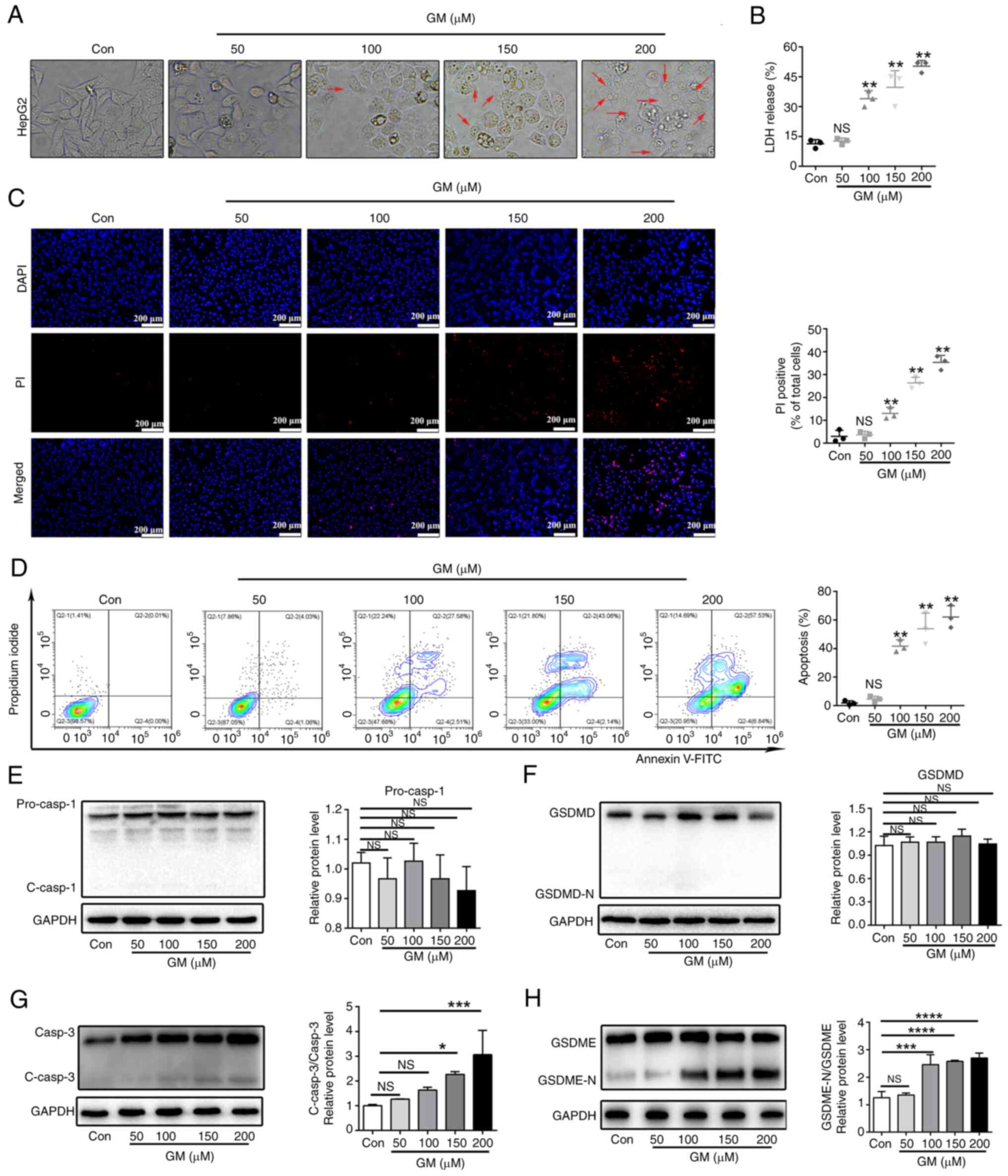 | Figure 2GM induces plasma membrane
permeabilization and pyroptosis in HepG2 cells. (A) Representative
microscopic images of HepG2 cells treated with GM at different
concentrations for 24 h (magnification, x400). (B) Release of LDH
from HepG2 cells treated with GM at different concentrations for 24
h. (C) Fluorescence microscopy images showing PI staining in HepG2
cells. Scale bar, 200 µm. (D) Representative flow cytometry scatter
plots. HepG2 cells were treated with GM at different concentrations
for 24 h and then analyzed using flow cytometry. Representative
immunoblot analysis of (E) pro-caspase-1 and cleaved caspase-1, (F)
GSDMD and GSDMD-N, (G) caspase-3 and cleaved caspase-3 (H) GSDME
and GSDME-N, and GADPH protein expression levels were detected
using western blotting analysis in HepG2 cells treated with GM at
different concentrations for 24 h. Results are presented as the
mean ± SD of three independent experiments. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001 vs. control. Pro-casp, pro-caspase;
GSDMD, gasdermin D; GSDMD-N, gasdermin D-N-terminal; C-casp,
cleaved caspase; LDH, lactate dehydrogenase; PI, propidium iodide;
Con, control; NS, not significant. |
According to previous studies, caspases-1/4/5/11 can
cleave GSDMD, releasing its N-terminal domain from the membrane and
permitting pore formation by GSDMD, thereby inducing pyroptosis
(22). Although caspase-1 and
GSDMD are expressed in HepG2 cells (31), in the present study, GM treatment
did not induce the cleavage of GSDMD and caspase-1, as detected by
western blot analysis (Fig. 2E and
F). This implied that GSDMD is not
involved in GM-induced HepG2 cell death. The discovery and
characterization of GSDME are relatively recent and it has been
proposed to act as a molecular switch between apoptotic and
pyroptotic cell death. When caspase-3 cleaves the N-terminal
fragment of GSDME (GSDME-N), apoptotic cell death is converted into
pyroptotic cell death (26).
Therefore, whether the effector of cell pyrolysis, GSDME (32), is involved in GM-induced cell death
was evaluated in the present study. Treatment with GM (150/200 µM)
increased the cleavage of caspase-3, with concomitant increase in
the expression levels of GSDME-N (Fig.
2G and H). These data
indicated that GM induced the caspase-3-mediated cleavage of GSDME,
which is involved in pyroptosis in HepG2 cells.
Caspase-3-mediated cleavage of GSDME
involves GM-induced pyroptosis in HepG2 cells
We hypothesized that the activation of caspase-3 is
essential for GM-induced pyroptosis. To validate this hypothesis,
stable caspase-3-knockdown HepG2 cells were generated and validated
by analyzing the expression levels of caspase-3 using RT-qPCR and
western blotting (Fig. 3A and
B). Knockdown of caspase-3 rescued
cell viability in response to GM treatment but resulted in a
prominent reduction in GM-induced LDH release and plasma membrane
ballooning (Fig. 3C-E). PI
staining showed that caspase-3 knockdown decreased the rate of cell
death, as indicated by PI fluorescence (Fig. 3F). Meanwhile, HepG2 cells treated
with GM proceeded rapidly to the Annexin V and PI double-positive
stage, while caspase-3 knockdown delayed the process by decreasing
the percentage of double-positive cells (Fig. 3G). Finally, western blot analyses
showed that the expression level of GSDME-N was notably reduced in
GM-treated HepG2 cells in which caspase-3 knockdown was performed
compared with that in GM-treated cells without caspase-3 knockdown
(Fig. 3H and I). These results showed that GM activated
caspase-3, which in turn cleaved GSDME to induce pyroptosis.
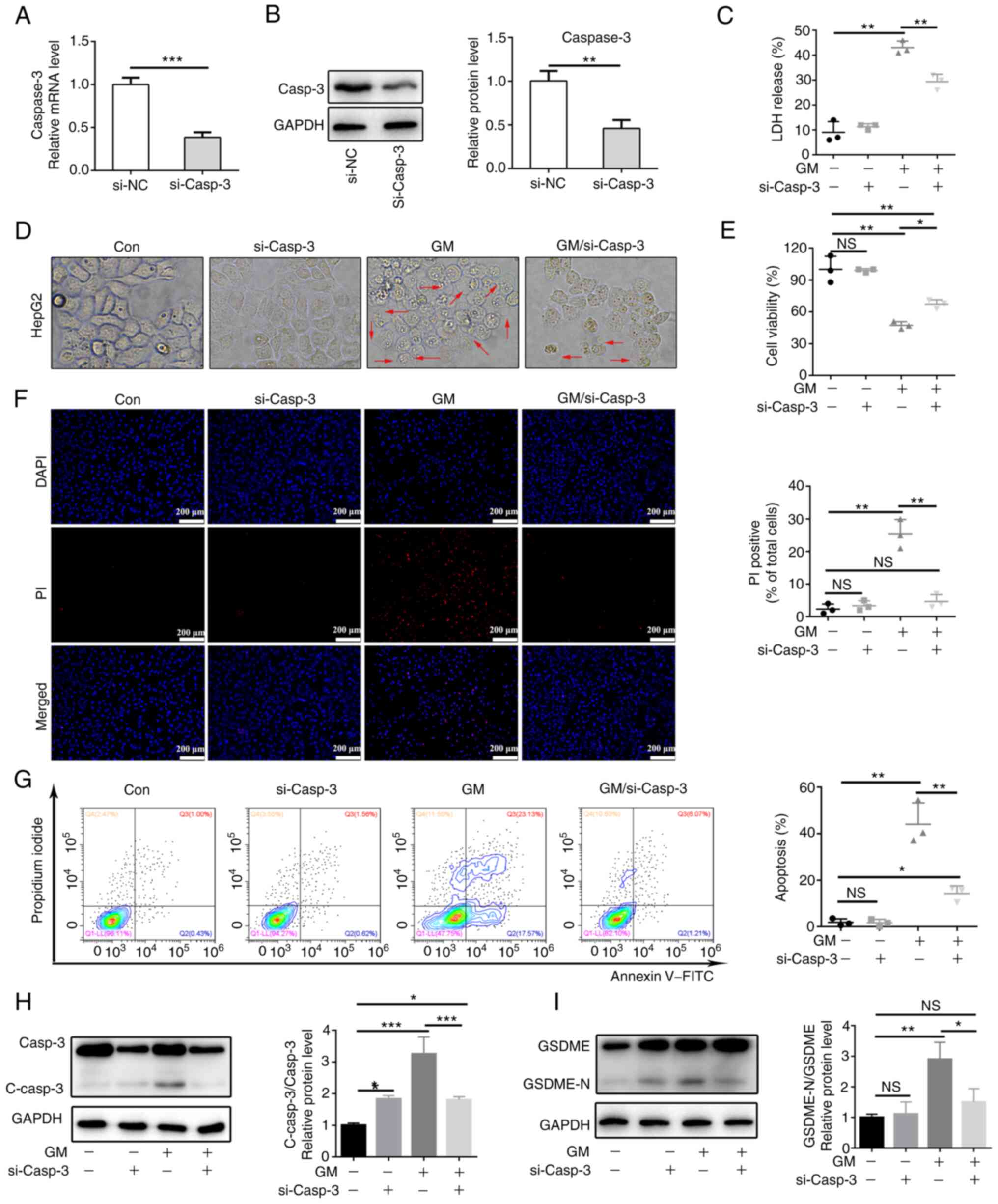 | Figure 3Caspase-3-mediated cleavage of GSDME
is involved in GM-induced pyroptosis in HepG2 cells. Expression
levels of caspase-3 in si-NC and si-Casp3 cells were detected using
(A) reverse transcription-quantitative PCR and (B) western
blotting. HepG2 cells and caspase-3-knockdown HepG2 cells were
treated with 200 µM GM for 24 h and (C) LDH release was measured,
as well as (D) cell morphology (magnification, x400) and (E) cell
viability using a Cell Counting kit-8 assay. (F) Fluorescent
microscopy images showing PI staining. Scale bar, 200 µm. (G)
Representative flow cytometry scatter plots. Representative
immunoblot analysis for (H) Casp3 and C-casp3 and (I) GSDME and
GSDME-N. Results are represented as the mean ± SD of three
independent experiments. *P<0.05,
**P<0.01 and ***P<0.001. si-NC, small
interfering RNA negative control; Casp3, caspase-3; C-casp3,
cleaved caspase-3; GSDME, gasdermin E; GSDME-N, gasdermin
E-N-terminal; LDH, lactate dehydrogenase; PI, propidium iodide; GM,
germacrone; NS, not significant; Con, control. |
GM induces mitochondrial damage and
enhances ROS production in HepG2 cells
In order to elucidate the underlying mechanism by
which GM induces caspase-3/GSDME-mediated pyroptosis, the targets
of GM were predicted using the STITCH database. The results showed
that GM mainly acts on the cytochrome P450 (CYP) system of the
mitochondria (Fig. 4A). Since
previous studies have shown that mitochondrial damage is closely
linked to GSDME-mediated pyroptosis (33-36),
the present study investigated whether GM-induced pyroptosis was
associated with mitochondrial damage. TEM images showed that the
cells treated with GM exhibited markedly increased mitochondrial
swelling compared with the control cells (Fig. 4B). Considering that mitochondrial
damage is closely associated with the generation of ROS, we
hypothesized that GM increases the levels of cellular ROS. DHE
staining revealed that cellular ROS was markedly increased upon
treatment with GM in a dose-dependent manner (Fig. 4C).
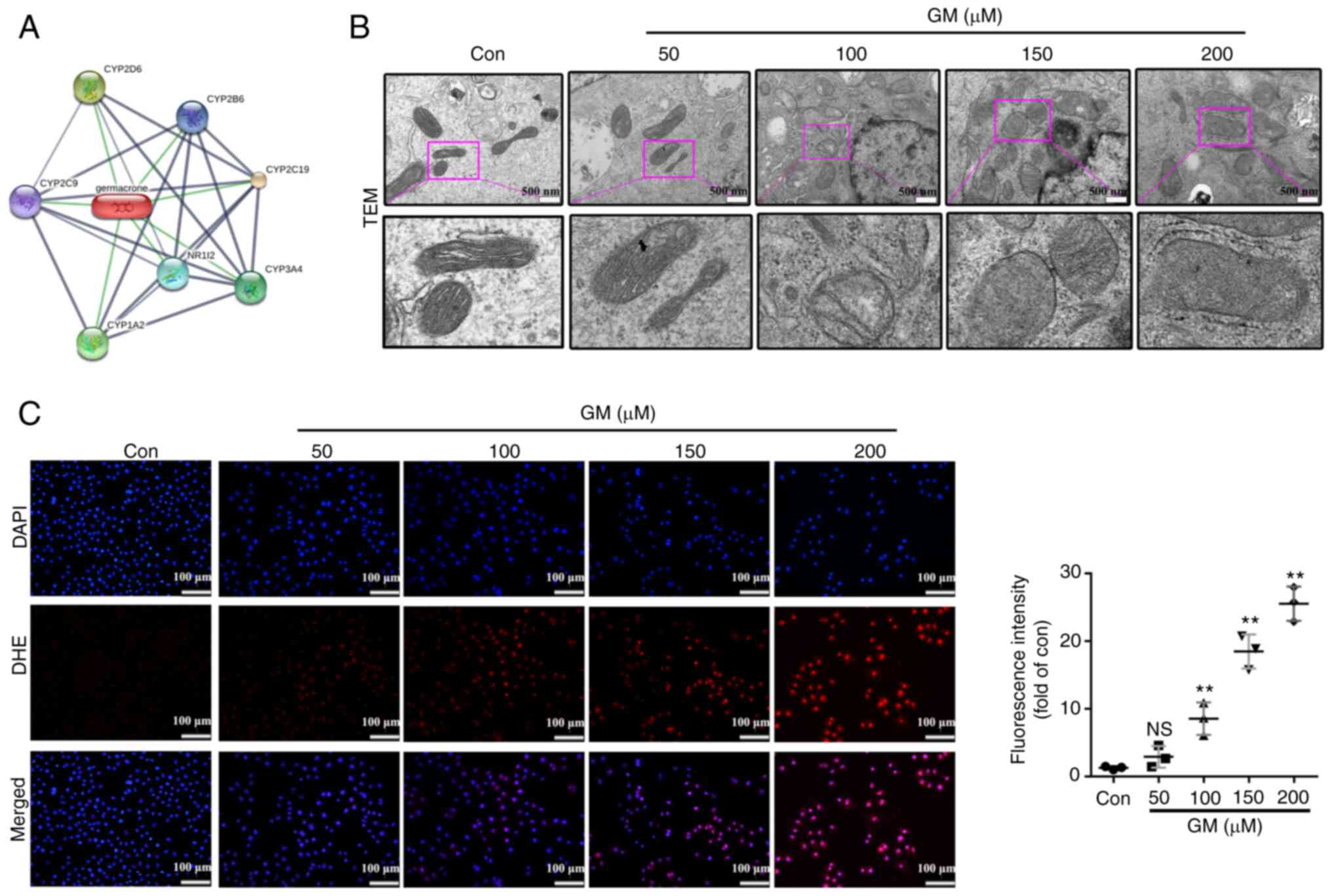 | Figure 4GM induces mitochondrial damage and
increases the production of ROS in HepG2 cells. (A) Representative
transmission electron microscopy images of HepG2 cells treated with
the indicated concentrations of GM for 24 h. Scale bar, 500 nm. (B)
Protein-protein interactions between GM-interactors are shown in
gray, whereas interactions between GM and its targets are shown in
green. Stronger associations are represented by thicker lines. (C)
HepG2 cells were treated with the indicated concentrations of GM
for 24 h, and cellular ROS levels were detected using DHE (red) and
DAPI (blue) staining. Scale bar, 100 µm. Results are represented as
the mean ± SD of three independent experiments.
**P<0.01 vs. control. ROS, reactive oxygen species;
NS, not significant; Con, control; DHE, dihydroethidium; GM,
germacrone; TEM, transmission electron microscopy. |
GM promotes pyroptosis through the
induction of ROS in HepG2 cells
It has been reported that caspase-3/GSDME-mediated
pyroptosis is closely associated with an increase in ROS production
(32). The present study suggested
that GM treatment results in an elevation of cellular ROS levels,
thereby regulating HepG2 cell pyroptosis. To verify this
hypothesis, HepG2 cells were pretreated with the ROS scavenger NAC
(5 mM) for 2 h and then treated with GM for 24 h. Notably, NAC
substantially attenuated the pyroptotic characteristics, including
balloon-like bubbling, the release of LDH, PI staining, pyroptosis,
and double positivity for Annexin V and PI (Fig. 5A-E). Additionally, NAC
substantially attenuated the cleavage of caspase-3 and GSDME in
GM-treated HepG2 cells (Fig. 5F
and G). These results implied that
GM, by damaging the mitochondria, increased the generation of
cellular ROS, and induced pyroptosis by activating caspase-3 and
GSDME in HepG2 cells.
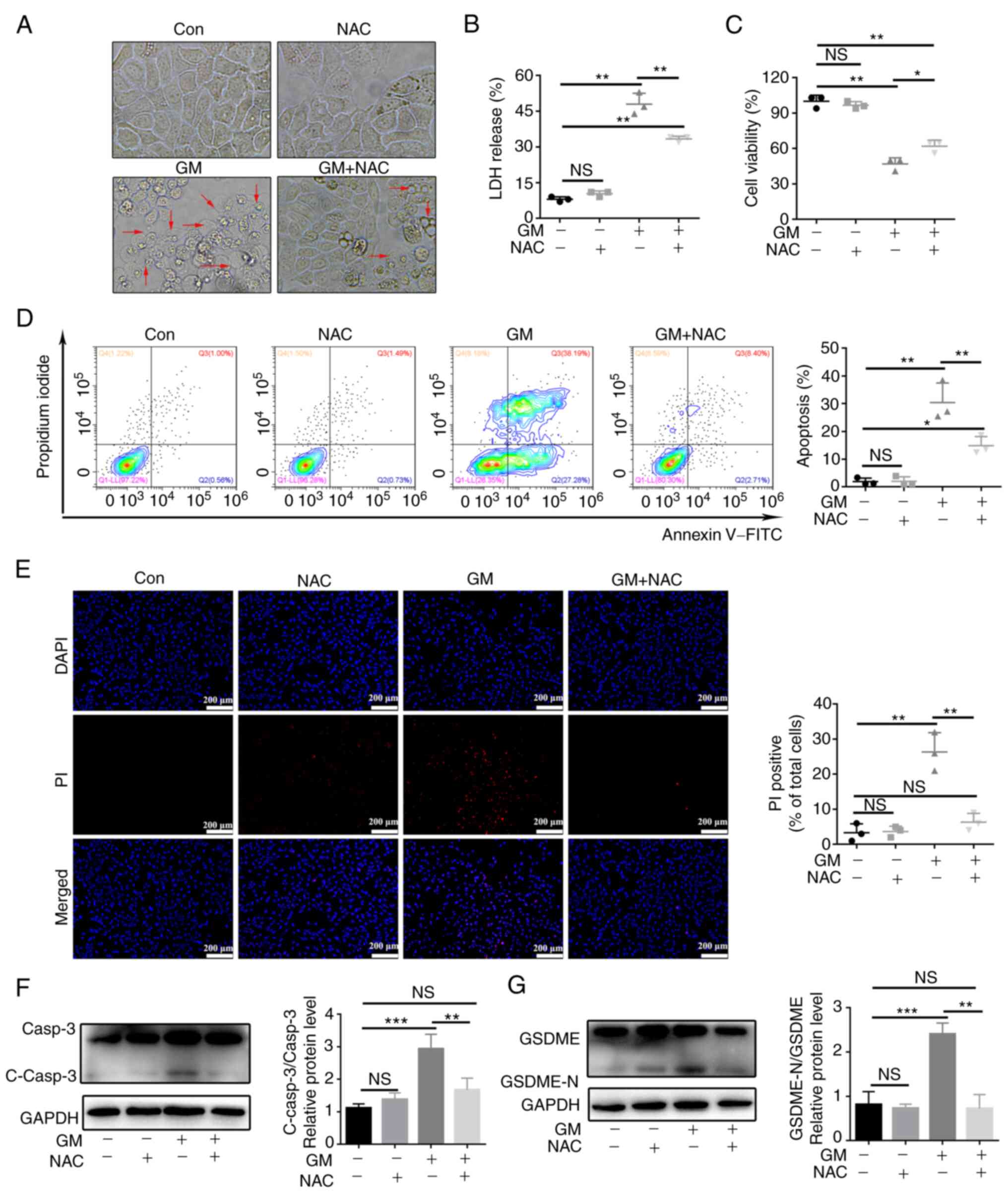 | Figure 5GM promotes pyroptosis through
induction of reactive oxygen species in HepG2 cells. HepG2 cells
were pretreated with or without 5 mM NAC for 2 h before treatment
with 200 µM of GM for 24 h, following which different assays were
performed. (A) HepG2 cell morphology (magnification, x400). (B)
Results of the LDH assay. (C) Analysis of cell viability using a
Cell Counting kit-8 assay. (D) Representative flow cytometry
scatter plots. (E) Fluorescence microscopy images showing PI
staining. Scale bar, 200 µm. (F and G) Representative immunoblot
analysis for (F) Casp3 and C-casp3, and (G) GSDME and GSDME-N.
Results are presented as mean ± SD of three independent
experiments. *P<0.05, **P<0.01 and
***P<0.001. Casp3, caspase-3; C-casp3,
cleaved-caspase-3; GSDME, gasdermin E; GSDME-N, gasdermin
E-N-terminal; NAC, N-acetylcysteine; LDH, lactate dehydrogenase;
PI, propidium iodide; NS, not significant; Con, control; GM,
germacrone. |
GM inhibits tumor growth and induces
pyroptosis in a xenograft model
The aforementioned in vitro experiments
demonstrated that GM-induced pyroptosis via cleaved caspase-3 to
exert antitumor effects. To investigate the antitumor effects of GM
in vivo, HepG2 cells were injected subcutaneously into the
right side of BALB/C nude mice and the xenograft tumor volume was
measured every 5 days. At 6 days post-cell injection, the nude mice
were treated intragastrically with GM solution (5 10, 15 or 20
mg/kg body weight) or vehicle for 21 consecutive days. The body
weight of the mice did not differ between the GM- and the
vehicle-treated groups (Fig. 6A).
However, administration of GM markedly attenuated the volume and
the weight of the tumors in a dose-dependent manner compared with
the corresponding parameters in the vehicle group (Fig. 6B-D). Moreover, higher levels of
ROS, determined by stronger fluorescence staining with DHE, were
also observed in the GM treatment groups (Fig. 6E). In addition, GM administration
substantially promoted the formation of cleaved caspase-3 and
GSDME-N in a dose-dependent manner (Fig. 6F and G), which was consistent with the results
of the in vitro studies on HepG2 cells. Therefore, these
results indicated that GM treatment could reduce tumor volume by
inducing pyroptosis in a mouse xenograft model. In addition, no
significant morphological changes were observed in the liver
tissues of the GM-treated mice, suggesting that GM had no
significant toxicity in the mice (Fig.
6H).
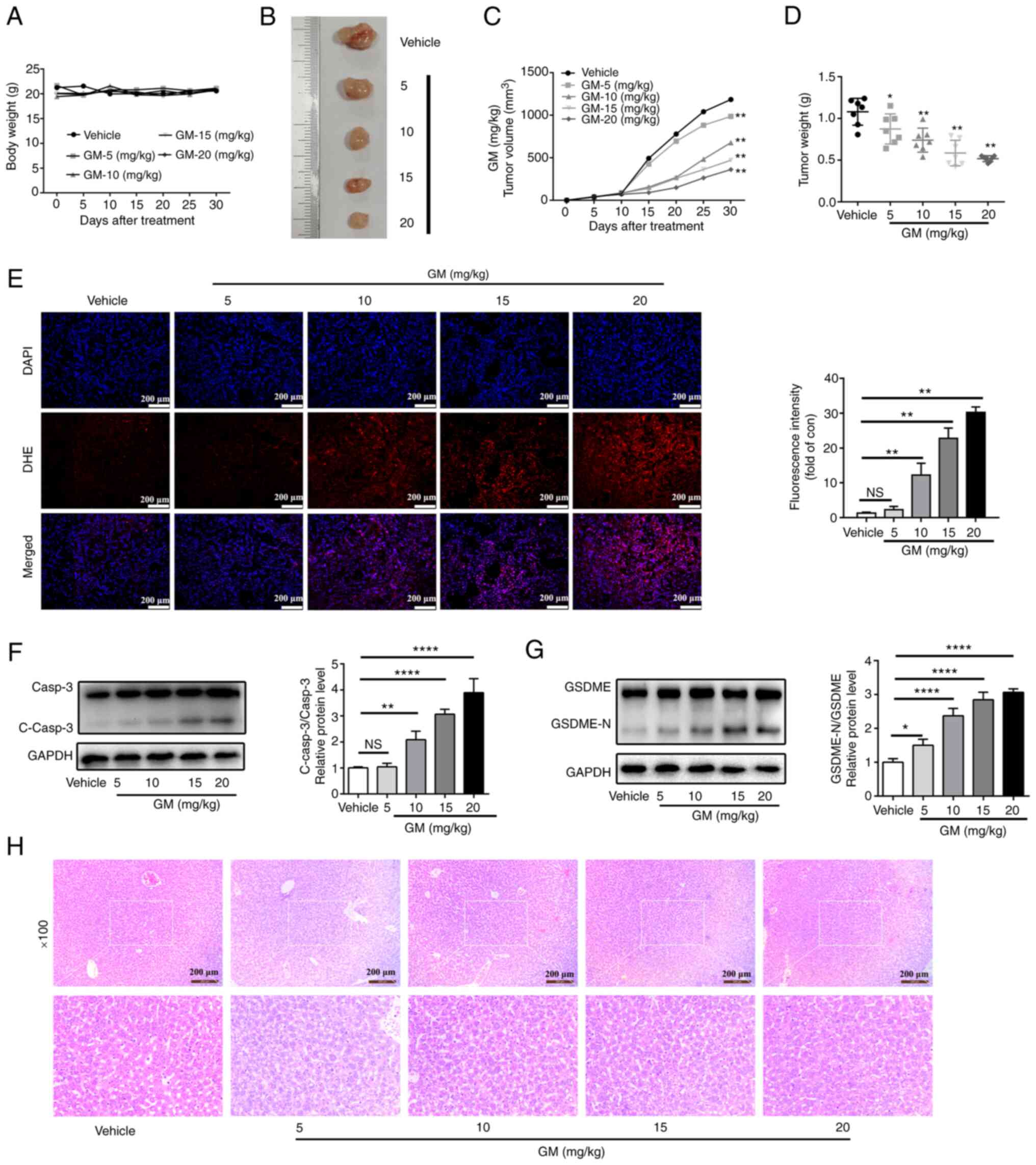 | Figure 6GM inhibits tumor growth and induces
pyroptosis in a xenograft model. A total of 1x106 HepG2
cells were inoculated into BALB/c nude mice to establish a tumor
model. Starting six days after cell injection, the nude mice were
treated intragastrically with germacrone solution (0, 5, 10, 15 or
20 mg/kg body weight) for 21 consecutive days. (A) Body weight of
mice at 30 days (n=6). (B) Representative images of HepG2
cell-derived xenograft tumors on day 30. (C) Tumor volume was
measured every 5 days. (D) Tumor weight on day 30. (E) Fluorescence
images of DHE (red) and DAPI (blue) staining showing reactive
oxygen species levels in tumor sections (n=3). Scale bar, 100 µm.
(F and G) Western blot images of protein expression of (F) Casp3
and C-casp3, and (G) GSDME and GSDME-N in tumors derived from
treatment with GM or vehicle. (H) Representative liver HE staining
images of all experimental groups at day 30. Results are presented
as mean ± SD of three independent experiments.
*P<0.05, **P<0.01 and
****P<0.0001, vs. vehicle. Casp3, caspase-3; C-casp3,
cleaved-caspase-3; GSDME, gasdermin E; GSDME-N, gasdermin
E-N-terminal; NS, not significant; DHE, dihydroethidium; GM,
germacrone. |
Discussion
Pyroptosis, a type of programmed cell death, is not
cell-type specific and possesses a possible beneficial role in
tumor cell therapy. Previous studies have confirmed that bacteria
or lipopolysaccharides induce pyroptosis through GSDMD cleavage
(37,38). Recent studies have demonstrated
that treatment with several chemotherapeutic drugs activates
caspase-3/GSDME and triggers secondary necrosis after apoptosis or
pyroptosis (26,39), which challenges a long-standing
view that chemotherapy acts most potently by stimulating apoptosis.
Previous studies have shown that GM exerts anticancer effects
through multiple molecular mechanisms. For example, GM treatment
effectively induces apoptosis by inducing cell cycle arrest in
human esophageal squamous cell carcinoma cells, breast cancer cell
lines and A549 cells (40,41). Furthermore, other studies have
shown that GM induces apoptosis of gastric cancer cells via the
regulation of the hepatitis B virus X-interacting protein-mediated
cell cycle and promoting the formation of autophagosomes, (42) and induces apoptosis in HepG2 cells
by downregulating the activation of the JAK2/STAT3 pathway and
upregulating the expression of p53 and Bax (43). However, whether pyroptosis is
involved in the chemotherapeutic use of GM in HepG2 cells still
remains unclear. The present study demonstrated that GM induced
pyroptosis of HepG2 cells via a ROS-caspase3-GSDME pathway. In
HepG2 cells, GM caused mitochondrial damage and elevated ROS,
consequently activating caspase-3, and initiated the cleavage of
GSDME, ultimately inducing pyroptosis of HepG2 cells. These
findings shed light on the interassociation between GM and
pyroptosis, and suggested a functional role for GM-mediated
pyroptosis in antitumor treatment.
Consistent with previous results, cell viability and
proliferation assays demonstrated that GM effectively inhibited the
proliferation of HepG2 cells in a concentration-dependent manner.
Moreover, a flow cytometry assay revealed that GM treatment induced
an increase in the HepG2 cell mortality rate. In addition, it was
observed that GM induced LDH release and cell swelling, and these
results suggested that other types of cell death, such as
pyroptosis and necroptosis, may also be involved in GM-induced
HepG2 cell death. Further experiments showed that GSDMD and
caspase-1 were not activated by GM in HepG2 cells; however, GSDME
activation was observed both in vivo and in vitro.
Furthermore, the in vivo and in vitro results showed
that the level of cleaved-caspase-3 protein was positively
associated with the sensitivity of cells to pyroptosis induced by
GM exposure. These results indicated that caspase-3 may be crucial
for GM-induced pyroptosis. Notably, in caspase-3-depleted HepG2
cells, the LDH release and pyroptosis induced by GM were decreased,
indicating that the pyroptosis induced by GM was
caspase-3-dependent. Consistently, flow cytometry data showed that
caspase-3 knockdown rescued cell death caused by GM and that it
reduced the proportion of Annexin V-PI single-positive cells, while
it decreased the proportion of double-positive cells. Altogether,
these data indicated that GM can induce pyroptosis via the
caspase-3/GSDME pathway in HepG2 cells.
Next, the mechanism by which GM regulates
caspase-3/GSDME-mediated pyroptosis in HepG2 cells was explored by
predicting the protein targets of GM using STITCH. It was found
that GM mainly acts on the CYPs of the mitochondria. CYPs are
primarily located in the inner membrane of the mitochondria and are
the pivotal enzymes involved in the oxidative metabolism of drugs
and exogenous substances (44-46).
Dysfunctional CYPs can affect the cellular redox balance, resulting
in increased cellular ROS production (47-49).
Previous studies have shown that ROS play an important role in
pyroptosis (26,50). For example, lobaplatin induces
caspase-3/GSDME-mediated pyrolysis by increasing cellular ROS
levels in colon cancer cells (32). Iron-activated ROS promotes the
oxidation of the outer mitochondrial membrane protein Tom20 in
melanoma cells and induces pyrolysis by activating the
Bax/caspase-3/GSDME pathway (50).
Another critical study showed that GM increases ROS production and
induces apoptosis in HepG2 cells (43). These reports collectively suggest
that besides apoptosis, ROS can induce cell death towards the
pyroptosis pathway. Hence, in the present study, we hypothesized
that GM might enhance the generation of ROS to promote apoptosis
and caspase-3/GSDME-mediated pyroptosis by targeting the
mitochondria in HepG2 cells. Notably, TEM showed that GM markedly
increased mitochondrial swelling compared with that observed in the
control cells, and that it increased ROS levels. However, the
addition of the antioxidant NAC markedly blocked pyroptotic
characteristics after GM treatment. Moreover, NAC blocked the
release of LDH and cleaved caspase-3/GSDME induced by GM. These
results indicated that GM markedly elevated the generation of
cellular ROS by damaging mitochondria to induce
caspase-3/GSDME-mediated pyroptosis in HepG2 cells.
However, the present study has some limitations.
First, further investigations are waranted to confirm the effect of
GM on liver cancer cells. Therefore, future studies will include
the verification of the inhibitory effect and mechanism of GM in
different liver cancer cell lines. Furthermore, the expression of
GSDME should have been suppressed to demonstrate that the cell
death observed in HepG2 cells was due to GSDME-mediated pyroptosis
and not the other types of cell death. Although GM increases the
production of ROS by inducing mitochondrial damage, the mechanism
of GM-induced mitochondrial damage is not fully elucidated. Future
studies should investigate the effect of GM on the regulatory
mechanism related to mitochondria in liver cancer cells.
The findings of the present study demonstrated that
GM might be a potential candidate for the treatment of liver
cancer, and that GM triggered pyroptosis by activating
caspase-3/GSDME. These findings provide a novel insight that
ROS/caspase-3/GSDME-dependent pyroptosis is a possible mechanism
through which GM exerts its therapeutic action.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by grants from the
Shenzhen Science and Technology Project (nos. JCYJ20170817094901026
and JCYJ20180302173542393).
Availability of data and materials
The raw data and original images from the study are
included in the Figshare database (https://doi.org/10.6084/m9.figshare.19233756.v1).
Further inquiries can be directed to the corresponding authors.
Authors' contributions
XZZ and XFS conceived the study concept and revised
the manuscript. XFS, XZ and WFM performed the experiments and
drafted the original manuscript. WXF, QH, MQM, MLL and RH
participated in conducting the study and the data analysis. ZYH and
JL analyzed the data, contributed to writing the paper, and revised
the paper. XZZ was responsible for the funding acquisition. XFS and
XZ confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by and performed in
accordance with the relevant guidelines and regulations drafted by
the Animal Ethics Committee of Guangzhou University of Chinese
Medicine (approval no. 20210303042).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clark T, Maximin S, Meier J, Pokharel S
and Bhargava P: Hepatocellular carcinoma: Review of epidemiology,
screening, imaging diagnosis, response assessment, and treatment.
Curr Probl Diagn Radiol. 44:479–486. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hartke J, Johnson M and Ghabril M: The
diagnosis and treatment of hepatocellular carcinoma. Semin Diagn
Pathol. 34:153–159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oravecz M and Mészáros J: Traditional
Chinese medicine: Theoretical background and its use in China. Orv
Hetil. 153:723–731. 2012.PubMed/NCBI View Article : Google Scholar : (In Hungarian).
|
|
6
|
Chan HHL and Ng T: Traditional Chinese
medicine (TCM) and allergic diseases. Curr Allergy Asthma Rep.
20(67)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou P: Traditional Chinese medicine. Comb
Chem High Throughput Screen. 13(836)2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Momenkiaei F and Raofie F: Preparation of
Curcuma longa L. extract nanoparticles using supercritical solution
expansion. J Pharm Sci. 108:1581–1589. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dosoky NS and Setzer WN: Chemical
composition and biological activities of essential oils of
Curcuma species. Nutrients. 10(1196)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun J, Chen F, Braun C, Zhou YQ, Rittner
H, Tian YK, Cai XY and Ye DW: Role of curcumin in the management of
pathological pain. Phytomedicine. 48:129–140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ayati Z, Ramezani M, Amiri MS, Moghadam
AT, Rahimi H, Abdollahzade A, Sahebkar A and Emami SA: Ethnobotany,
phytochemistry and traditional uses of Curcuma spp. and
pharmacological profile of two important species (C. longa and C.
zedoaria): A review. Curr Pharm Des. 25:871–935. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, Wu Y, Li Y and Guo F: Review of the
traditional uses, phytochemistry, and pharmacology of Curcuma
wenyujin Y. H. Chen et C. Ling. J Ethnopharmacol.
269(113689)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dosoky NS, Satyal P and Setzer WN:
Variations in the volatile compositions of Curcuma species.
Foods. 8(53)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nutr Cancer. 62:919–930. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Giordano A and Tommonaro G: Curcumin and
cancer. Nutrients. 11(2376)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Burapan S, Kim M, Paisooksantivatana Y,
Eser BE and Han J: Thai Curcuma species: Antioxidant and
bioactive compounds. Foods. 9(1219)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Srivilai J, Waranuch N, Tangsumranjit A,
Khorana N and Ingkaninan K: Germacrone and sesquiterpene-enriched
extracts from Curcuma aeruginosa Roxb. Increase skin penetration of
minoxidil, a hair growth promoter. Drug Deliv Transl Res.
8:140–149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Y, Wang W, Fang B, Ma F, Zheng Q, Deng
P, Zhao S, Chen M, Yang G and He G: Anti-tumor effect of germacrone
on human hepatoma cell lines through inducing G2/M cell cycle
arrest and promoting apoptosis. Eur J Pharmacol. 698:95–102.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Riaz A, Rasul A, Kanwal N, Hussain G, Shah
MA, Sarfraz I, Ishfaq R, Batool R, Rukhsar F and Adem Ş:
Germacrone: A potent secondary metabolite with therapeutic
potential in metabolic diseases, cancer and viral infections. Curr
Drug Metab. 21:1079–1090. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang R, Hao J, Guo K, Liu W, Yao F, Wu Q,
Liu C, Wang Q and Yang X: Germacrone inhibits cell proliferation
and induces apoptosis in human esophageal squamous cell carcinoma
cells. Biomed Res Int. 2020(7643248)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of pyroptosis. Trends Cell Biol. 27:673–684.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jia C, Chen H, Zhang J, Zhou K, Zhuge Y,
Niu C, Qiu J, Rong X, Shi Z, Xiao J, et al: Role of pyroptosis in
cardiovascular diseases. Int Immunopharmacol. 67:311–318.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z,
Zhang Z and Dai Z: Propofol directly induces caspase-1-dependent
macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell
Death Dis. 10(542)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jiang M, Qi L, Li L and Li Y: The
caspase-3/GSDME signal pathway as a switch between apoptosis and
pyroptosis in cancer. Cell Death Discov. 6(112)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liang WF, Gong YX, Li HF, Sun FL, Li WL,
Chen DQ, Xie DP, Ren CX, Guo XY, Wang ZY, et al: Curcumin activates
ROS signaling to promote pyroptosis in hepatocellular carcinoma
HepG2 cells. In Vivo. 35:249–257. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang X, Zhang P, An L, Sun N, Peng L,
Tang W, Ma D and Chen J: Miltirone induces cell death in
hepatocellular carcinoma cell through GSDME-dependent pyroptosis.
Acta Pharm Sin B. 10:1397–1413. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhan ZY, Wu M, Shang Y, Jiang M, Liu J,
Qiao CY, Ye H, Lin YC, Piao MH, Sun RH, et al: Taxifolin ameliorate
high-fat-diet feeding plus acute ethanol binge-induced
steatohepatitis through inhibiting inflammatory caspase-1-dependent
pyroptosis. Food Funct. 12:362–372. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J,
Wang K, Sun X and Zheng J: Cleavage of GSDME by caspase-3
determines lobaplatin-induced pyroptosis in colon cancer cells.
Cell Death Dis. 10(193)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kleih M, Böpple K, Dong M, Gaißler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis.
10(851)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li Q, Liang X, Yang Y, Zeng X, Zhong X and
Huang C: Panax notoginseng saponins ameliorate cisplatin-induced
mitochondrial injury via the HIF-1α/mitochondria/ROS pathway. FEBS
Open Bio. 10:118–126. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mazat JP, Devin A and Ransac S: Modelling
mitochondrial ROS production by the respiratory chain. Cell Mol
Life Sci. 77:455–465. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Q, Shi N, Cai C, Zhang M, He J, Tan Y
and Fu W: The role of mitochondria in pyroptosis. Front Cell Dev
Biol. 8(630771)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang K, Sun Q, Zhong X, Zeng M, Zeng H,
Shi X, Li Z, Wang Y, Zhao Q, Shao F and Ding J: Structural
mechanism for GSDMD targeting by autoprocessed caspases in
pyroptosis. Cell. 180:941–955.e20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu LS, Liu Y, Wang XW, Xu B, Lin YL, Song
Y, Dong Y, Liu JL, Wang XJ, Liu S, et al: LPS enhances the
chemosensitivity of oxaliplatin in HT29 cells via GSDMD-mediated
pyroptosis. Cancer Manag Res. 12:10397–10409. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
An H, Heo JS, Kim P, Lian Z, Lee S, Park
J, Hong E, Pang K, Park Y, Ooshima A, et al: Tetraarsenic hexoxide
enhances generation of mitochondrial ROS to promote pyroptosis by
inducing the activation of caspase-3/GSDME in triple-negative
breast cancer cells. Cell Death Dis. 12(159)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao Y, Cai J, Shi K, Li H, Du J, Hu D,
Liu Z and Wang W: Germacrone induces lung cancer cell apoptosis and
cell cycle arrest via the Akt/MDM2/p53 signaling pathway. Mol Med
Rep. 23(452)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhong Z, Chen X, Tan W, Xu Z, Zhou K, Wu
T, Cui L and Wang Y: Germacrone inhibits the proliferation of
breast cancer cell lines by inducing cell cycle arrest and
promoting apoptosis. Eur J Pharmacol. 667:50–55. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fang X, Tan T, Gao B, Zhao Y, Liu T and
Xia Q: Germacrone regulates HBXIP-mediated cell cycle, apoptosis
and promotes the formation of autophagosomes to inhibit the
proliferation of gastric cancer cells. Front Oncol.
10(537322)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu YY, Zheng Q, Fang B, Wang W, Ma FY,
Roshan S, Banafa A, Chen MJ, Chang JL, Deng XM, et al: Germacrone
induces apoptosis in human hepatoma HepG2 cells through inhibition
of the JAK2/STAT3 signalling pathway. J Huazhong Univ Sci Technolog
Med Sci. 33:339–345. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cui Y, Peng Y, Zhang Q, Xia S, Ruan B, Xu
Q, Yu X, Zhou T, Liu H, Zeng D, et al: Disruption of EARLY LESION
LEAF 1, encoding a cytochrome P450 monooxygenase, induces ROS
accumulation and cell death in rice. Plant J. 105:942–956.
2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lu Y and Cederbaum AI: Cytochrome P450s
and alcoholic liver disease. Curr Pharm Des. 24:1502–1517.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chien Y, Rosal K and Chung BC: Function of
CYP11A1 in the mitochondria. Mol Cell Endocrinol. 441:55–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Peter Guengerich F and Avadhani NG: Roles
of cytochrome P450 in metabolism of ethanol and carcinogens. Adv
Exp Med Biol. 1032:15–35. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guengerich FP: Cytochrome P450 2E1 and its
roles in disease. Chem Biol Interact. 322(109056)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL,
Cheng K, Sun RY, Zhou D, Han J and Wu Q: Tom20 senses
iron-activated ROS signaling to promote melanoma cell pyroptosis.
Cell Res. 28:1171–1185. 2018.PubMed/NCBI View Article : Google Scholar
|