Introduction
The intervertebral disc (IVD) is a key connective
tissue between vertebrae. Its degeneration can lead to
morphological and physiological changes in the disc, lower back
pain, and subsequently to impaired load-bearing capacity (1). Intervertebral disc degeneration (IDD)
is a common musculoskeletal disorder with multifactorial pathology.
It is caused by several factors, such as genetic susceptibility,
inflammation, cellular senescence, non-physiological mechanical
stresses, and apoptosis (2).
Currently, treatments for IDD are mainly targeted to alleviate the
symptoms, and no effective measures can reverse the pathological
changes in the IVD (3). Therefore,
it is necessary to explore the exact cause and pathogenesis of IDD
and develop more effective conservative methods for IVD
therapy.
Extracellular matrix (ECM) degradation and nucleus
pulposus (NP) cell loss are two major pathological features of IDD
(4). NP cells play an essential
role in maintaining the homeostasis and structural integrity of the
ECM; their degradation can further destruct the normal function of
IVDs (5). Apart from ECM
degradation, inflammation is also considered to be a critical event
during IDD, which distinguishes asymptomatic from symptomatic disc
degeneration (6). During IDD
progression, NP cells increasingly secrete pro-inflammatory
molecules, such as tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, IL-6, and IL-17, among which TNF-α is the most prominent
and has been reported to be highly upregulated in disc tissue
(7). These proinflammatory
molecules trigger a range of inflammatory responses, and further
influence disc cell senescence, death, autophagy, and apoptosis
(8). Therefore, targeting
TNF-α-induced inflammation and NP cell senescence may be promising
for the treatment of IDD.
Luteolin, or 3',4',5,7-tetrahydroxy flavone, is a
flavonoid, which is widely distributed in various plants including
flowers, vegetables, and medicinal herbs and spices (9). It has been demonstrated that luteolin
possesses various biological activities, such as anti-inflammatory
(10), anticancer (11), antioxidant (12), neuroprotective (13), and antidiabetic (14) activity. Accumulating evidence
indicates that the anti-inflammatory effects of luteolin are
associated with the downregulation of the expression levels of
proinflammatory molecules, including TNF-α, nitric oxide,
cyclooxygenase-2 (COX-2), and IL-1β (15). A recent study reported that
luteolin significantly inhibited IL-1β-induced degradation of
collagen II and attenuated the progression of osteoarthritis
(16). In addition, Yang et
al (17) demonstrated that
luteolin prevented the production of proinflammatory factors of
neutrophils and diminished inflammatory tissue injury. However,
whether luteolin can mediate the inflammatory processes in IDD and
improve disc impairment has not been previously investigated.
The sirtuin (Sirt) family comprises nicotinamide
adenine dinucleotide (NAD+)-dependent protein
deacetylases, which participate in multiple biological processes
including inflammation, aging, cellular redox homeostasis, and
metabolic homeostasis (18-20).
Sirt6 is a member of the Sirt family, which is mainly enriched in
the nucleus (21). A recent study
demonstrated that the expression levels of Sirt6 were significantly
decreased during the progression of IDD, while upregulation of
Sirt6 expression prevented IL-1β-induced NP ECM degradation and
ameliorated IDD development (22).
Moreover, Chen et al (23)
demonstrated that Sirt6 prevented NP cell senescence and death by
triggering autophagy. These findings indicate that Sirt6 plays a
crucial role in the progression of ID. However, its detailed
functions in the pathogenesis of IDD remain poorly understood.
Notably, luteolin has been reported to act as a Sirt6 activator and
trigger its expression by binding to the Sirt6-specific acyl
binding channel (24).
Based on previous findings, the purpose of the
present study was to investigate whether luteolin exerts protective
effects on NP cells and inhibits IDD progression. Moreover, the
mechanistic pathway of Sirt6 involvement in IDD was explored.
Materials and methods
Cell culture
Immortalized human nucleus pulposus cells (HNPCs)
were obtained from AcceGen Biotechnology (cat. no. ABI-TC102D). The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
with F12 nutrient mixture (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Hyclone; Cytiva), and 1%
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA) at 37˚C in the
presence of 5% CO2. The cells in the logarithmic growth
phase were used for subsequent experiments.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) cell viability assay
MTT was first prepared as a stock solution of 5
mg/ml in phosphate-buffered saline (PBS, pH 7.2) and filtered. The
cell suspension was transferred to a 96-well plate at a density of
5x103 cells/well. The cells were treated with different
concentrations of luteolin (1, 2, and 4 µM; Sigma-Aldrich; Merck
KGaA) or co-treated with luteolin (1, 2, and 4 µM) and 50 ng/ml
TNF-α for 24 h. To investigate the effect of Sirt6 knockdown on
TNF-α-induced HNPC viability in the presence of luteolin (4 µM),
the Sirt6 knockdown HNPCs were co-treated with 5 ng/ml TNF-α and
luteolin (4 µM) for 24 h. Subsequently, 10 µl MTT solution was
added to each well. Following incubation for 4 h at 37˚C, 100 µl
dimethyl sulfoxide (Adamas-Beta, Ltd.) was added to each well. The
96-well plate was shaken gently, and the absorbance (abs) of each
sample was read by a microplate reader at 570 nm to determine cell
viability. The viable cells produced a dark blue formazan product,
whereas this type of staining was not formed in the non-viable
cells. The percentage of the viable cells was calculated using the
following formula: (%)=[100x (sample abs)/(control abs)].
TUNEL assay
The induction of apoptosis was detected by TUNEL
using In Situ Cell Death Detection kit (cat. no.
11684817910; Roche Diagnostics GmbH) according to the
manufacturer's protocol. Briefly, the cells were washed with PBS
three times and subsequently fixed at room temperature (20-25˚C)
with 4% paraformaldehyde (Beyotime Institute of Biotechnology) for
15 min. Subsequently, 0.15% Triton X-100 was added to the cells at
room temperature for an additional 5 min. FITC-deoxyuridine
triphosphate solution (Roche Diagnostics GmbH) was added to the
cells and incubated at 37˚C for 60 min in the dark. The detection
solution was discarded and the cells were washed three times with
PBS. An inverted fluorescence microscope (Olympus Corp.) was used
to measure the excitation and emission wavelengths in the range of
450-500 nm and 515-565 nm (green fluorescence), respectively.
Detection kit
ELISA kits (Beyotime Institute of Biotechnology)
were applied to detect the expression levels of inflammatory
cytokines, including IL-6 (cat. no. P1326) and IL-1β (cat. no.
PI305). Senescence β-galactosidase staining kit (Beyotime Institute
of Biotechnology, cat. no. C0602) was used to detect the activity
levels of β-galactosidase in the cells. ELISA kit (SenBeiJia
Biological Technology Co., Ltd.; cat. no. SBJ-H1920) was used to
detect the activity of telomerase. The effect of luteolin on
TNF-α-induced Sirt6 activity was detected by the SIRT6 activity
assay kit (Abcam, cat. no. ab156068).
Bioinformatics website
The Protein Data Bank (PDB) database (https://www.rcsb.org/) was used to obtain the
three-dimensional structure of Sirt6 protein (PDB ID:3k35), and
molecular docking was performed using Autodock (version 4.2; The
Scripps Research Institute). The pose with the lowest free energy
(-8.3 kcal/mol) was selected for visualization.
Cell transfection
The cells (3x105 cells/well) were
incubated in 6-well plates and cultured for 24 h at 37˚C with 5%
CO2. Following incubation, the cells were transfected
with Sirt6-targeting short interfering RNA (si-Sirt6#1,
5'-TTCTTCCACAAACATGTTC-3'; si-Sirt6#2, 5'-TCTTCCACAAACATGTTCC-3')
and short interfering negative control (si-NC,
5'-ACGTGACACGTTCGGAGAATT-3') at a concentration of 25 nM. All
plasmids were synthesized by Shanghai GenePharma Co., Ltd. and
transfected using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Blank control group cells were not treated. Following
transfection, the cells were cultured for 48 h and the transfection
efficiency was assessed by western blot analysis.
Western blot analysis
The total proteins from the cells were extracted
with RIPA lysis buffer (Beyotime Institute of Biotechnology) and
determined using a BCA protein assay kit (Thermo Fisher Scientific
Inc.). The proteins were transferred on 12% SDS gels and analyzed
with PAGE. Subsequently, they were transferred to PVDF membranes.
The membranes were blocked with 5% non-fat milk for 4 h at room
temperature. Subsequently, the membranes (Abcam) were incubated
overnight at 4˚C with the following primary antibodies: anti-Bcl-2
(1:1,000; cat. no. ab182858), anti-Bax (1:1,000; cat. no. ab32503),
anti-cleaved caspase 3 (1:1,000; cat. no. ab32042), anti-p16
(1:1,000; cat. no. ab51243), anti-p21 (1:1,000; cat. no. ab109520),
anti-Sirt6 (1:1,000; cat. no. ab289970), anti-p-NF-κB p65 (1:1,000;
cat. no. ab239882), anti-NF-κB p65 (1:1,000; cat. no. ab207297),
anti-p53 (1:1,000; cat. no. Ab32389), anti-H3K9ac (1:500; cat. no.
Ab32129), anti-histone H3 (H3; 1:1,000; cat. no. Ab1791) and
anti-GAPDH (1:1,000; cat. no. ab8245; all from Abcam). Following
primary antibody incubation, the membranes were incubated with a
goat anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibody (1:5,000; cat. no. ab150077, Abcam) at room temperature
for 4 h. Finally, the images of the protein bands were visualized
using ECL reagents (MilliporeSigma). The protein expression levels
were semi-quantified using ImageJ software (version 1.46; National
Institutes of Health) with GAPDH or histone H3 as the loading
control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA extraction was performed from HNPCs using
RNAzol RT (Sigma-Aldrich; Merck KGaA), followed by reverse
transcription of the RNA into cDNA with a cDNA reverse
transcription kit (Qiagen GmbH), according to the manufacturer's
protocol. Subsequently, qPCR was performed using a QuantiTect SYBR
Green PCR kit (Qiagen GmbH), according to the manufacturer's
protocol. The following thermocycling conditions were used for
qPCR: 95˚C for 10 min, followed by 40 cycles of 95˚C for 10 sec and
60˚C for 60 sec. The following primers (GenScript) were used for
qPCR analysis: Sirt6 forward, 5'-TCCCCGACTTCAGGGGTC-3' and reverse,
5'-GTTCTGGCTGACCAGGAAGC-3'; and GAPDH forward,
5'-AGCCACATCGCTCAGACAC-3' and reverse, 5'-GCCCAATACGACCAAATCC-3'.
The mRNA expression levels were quantified using the
2-ΔΔCq method (25) and
normalized to the expression levels of the internal reference gene
GAPDH.
Statistical analysis
The measured data were expressed as mean ± standard
deviation from ≥3 independent experiments and GraphPad Prism 8.0
software (GraphPad Software, Inc.) was used to plot the figures.
One-way ANOVA followed by Tukey's post hoc test was used for
comparison between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
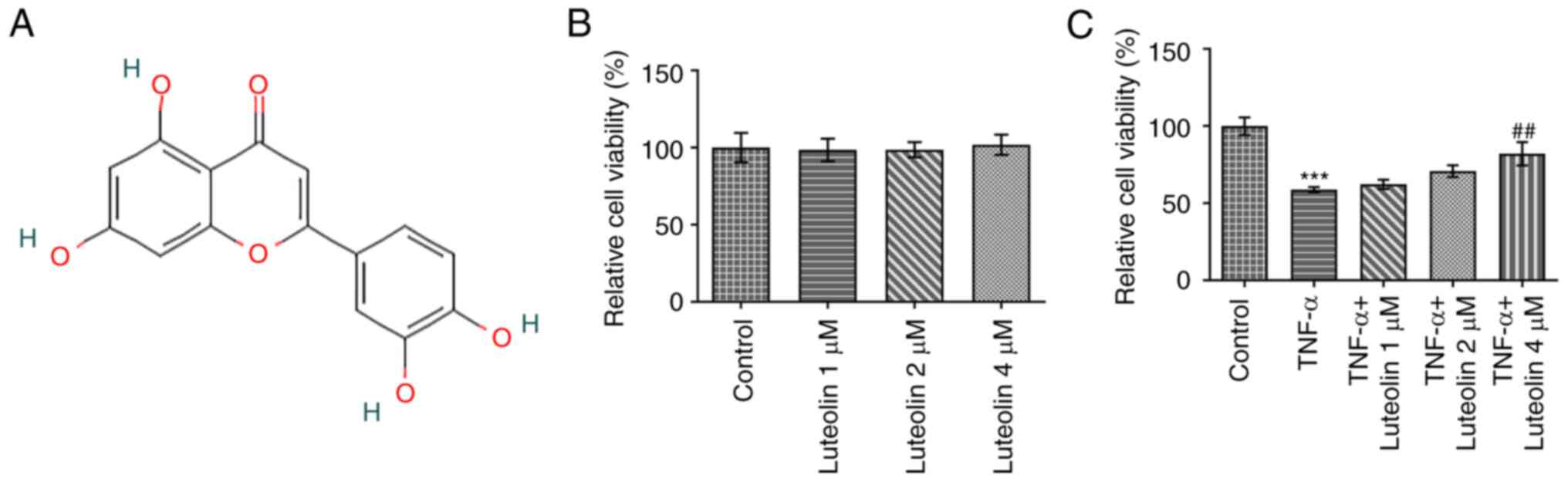
Luteolin enhances the viability of
TNF-α-induced HNPCs
The viability of HNPCs was detected by the MTT
assay. The results indicated that luteolin (Fig. 1A) exerted no apparent reduction in
the viability of HNPCs at the concentration range of 1-4 µM,
demonstrating optimal biocompatibility (Fig. 1B). Subsequently, the effects of
luteolin were examined on TNF-α-induced HNPC viability. Treatment
of the cells with luteolin indicated a concentration-dependent
increase in HNPC viability (Fig.
1C).
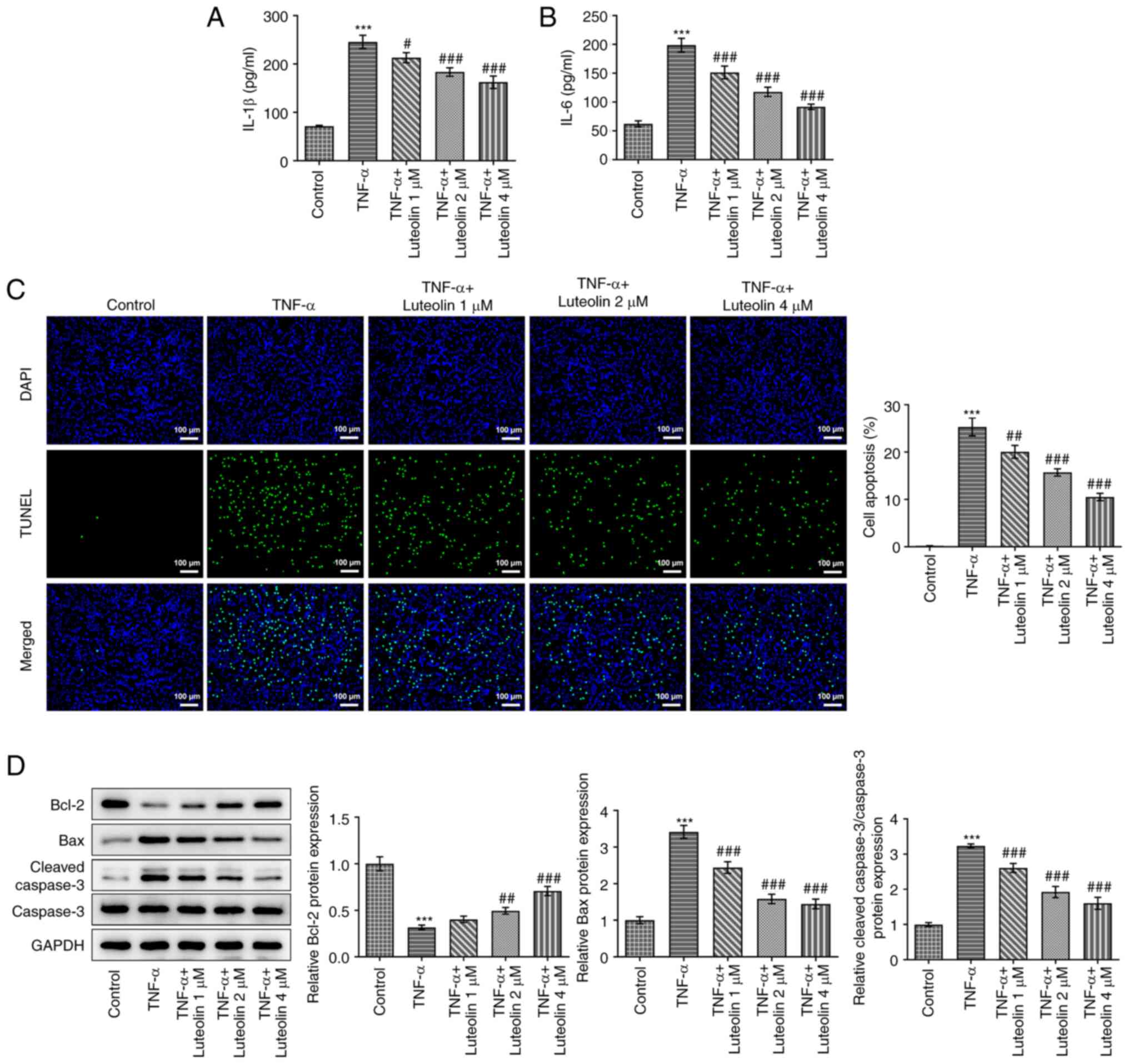
Luteolin inhibits TNF-α-induced HNPC
inflammatory injury
ELISA was performed to detect the levels of specific
inflammatory factors produced by TNF-α-induced HNPCs. The results
demonstrated that TNF-α induced a significant increase in IL-1β
(Fig. 2A) and IL-6 (Fig. 2B) levels, while luteolin treatment
decreased the expression levels of these inflammatory factors in a
concentration-dependent manner. Subsequently, TUNEL staining was
used to detect the induction of apoptosis of HNPCs, as shown in
Fig. 2C. Compared with the TNF-α
group, luteolin decreased TNF-α-induced apoptosis in a
concentration-dependent manner. To further verify this result, the
expression levels of specific apoptosis-related proteins were
examined by western blot analysis (Fig. 2D). The results demonstrated that
TNF-α induction increased the expression levels of the
pro-apoptotic proteins Bax and cleaved caspase 3, whereas it
reduced the expression level of the anti-apoptotic protein Bcl-2.
However, treatment of the cells with luteolin reversed these
effects. The effects noted were positively correlated with the
treatment concentration of luteolin.
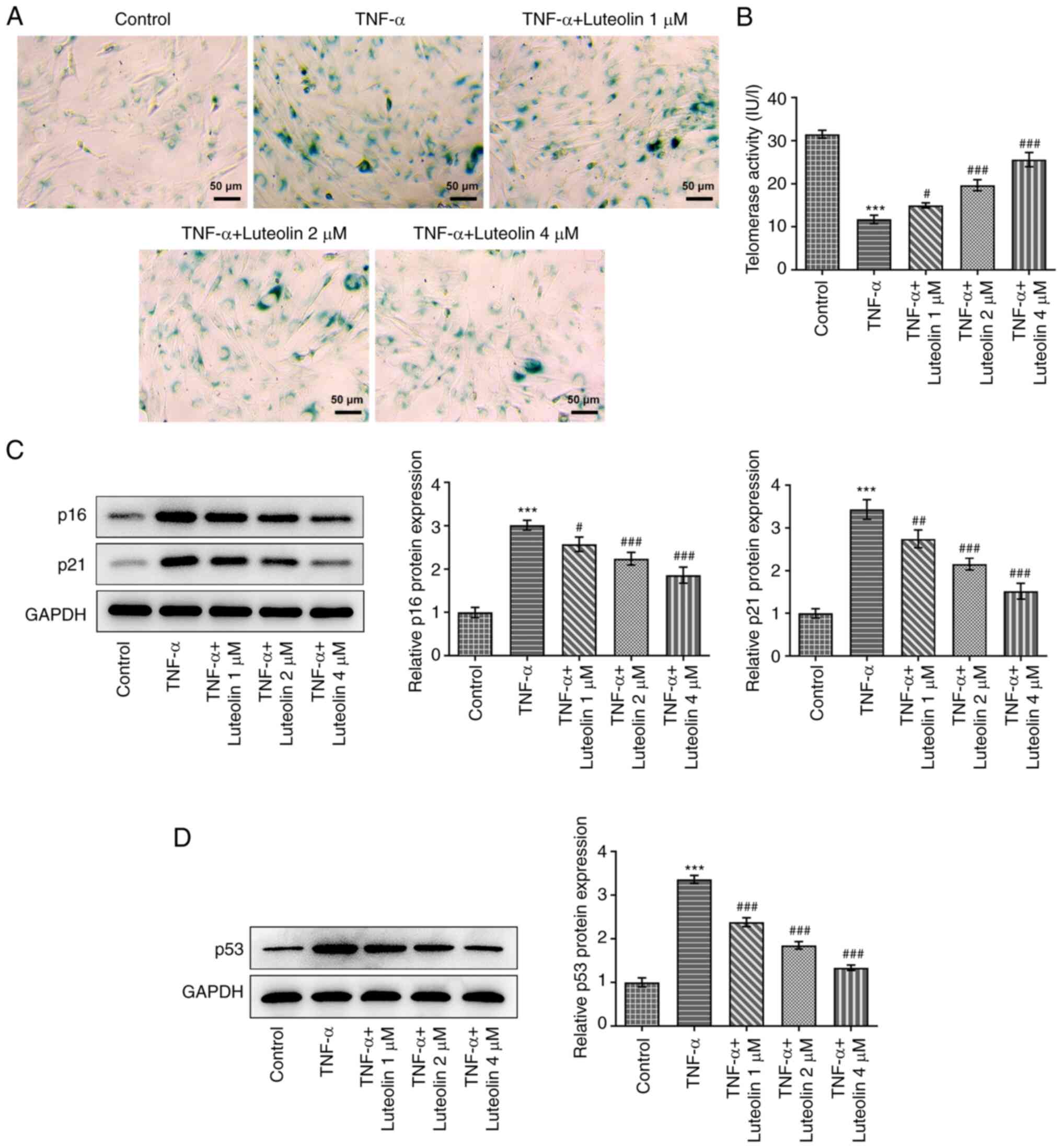
Luteolin suppresses TNF-α-induced
senescence of HNPCs
HNPC senescence was detected by the
senescence-associated β-galactosidase (SA-β-Gal) staining kit. In
senescent cells, a dark blue product was generated following the
catalysis of senescence-specific β-galactosidase (26). TNF-α-induced HNPC senescence
indicated a marked dark-blue color production, which was alleviated
by treatment of the cells with different concentrations of luteolin
(Fig. 3A). Telomerase activity is
reduced in senescent cells (27);
therefore, the effect of luteolin was examined on intracellular
telomerase activity stimulated by TNF-α. Similar results to those
of the senescent assay were obtained (Fig. 3B). Subsequently, the expression
levels of aging-related proteins were examined, and the results
indicated that TNF-α promoted the expression levels of p16 and p21
proteins, while luteolin treatment reversed these effects in a
concentration-dependent manner (Fig.
3C). in addition, we also examined the effect of TNF-α
induction on the expression of p53, an aging-related protein. The
results showed that TNF-α induction increased the expression of
p53, while luteolin treatment annulled this effect in a
concentration-dependent manner (Fig.
3D).
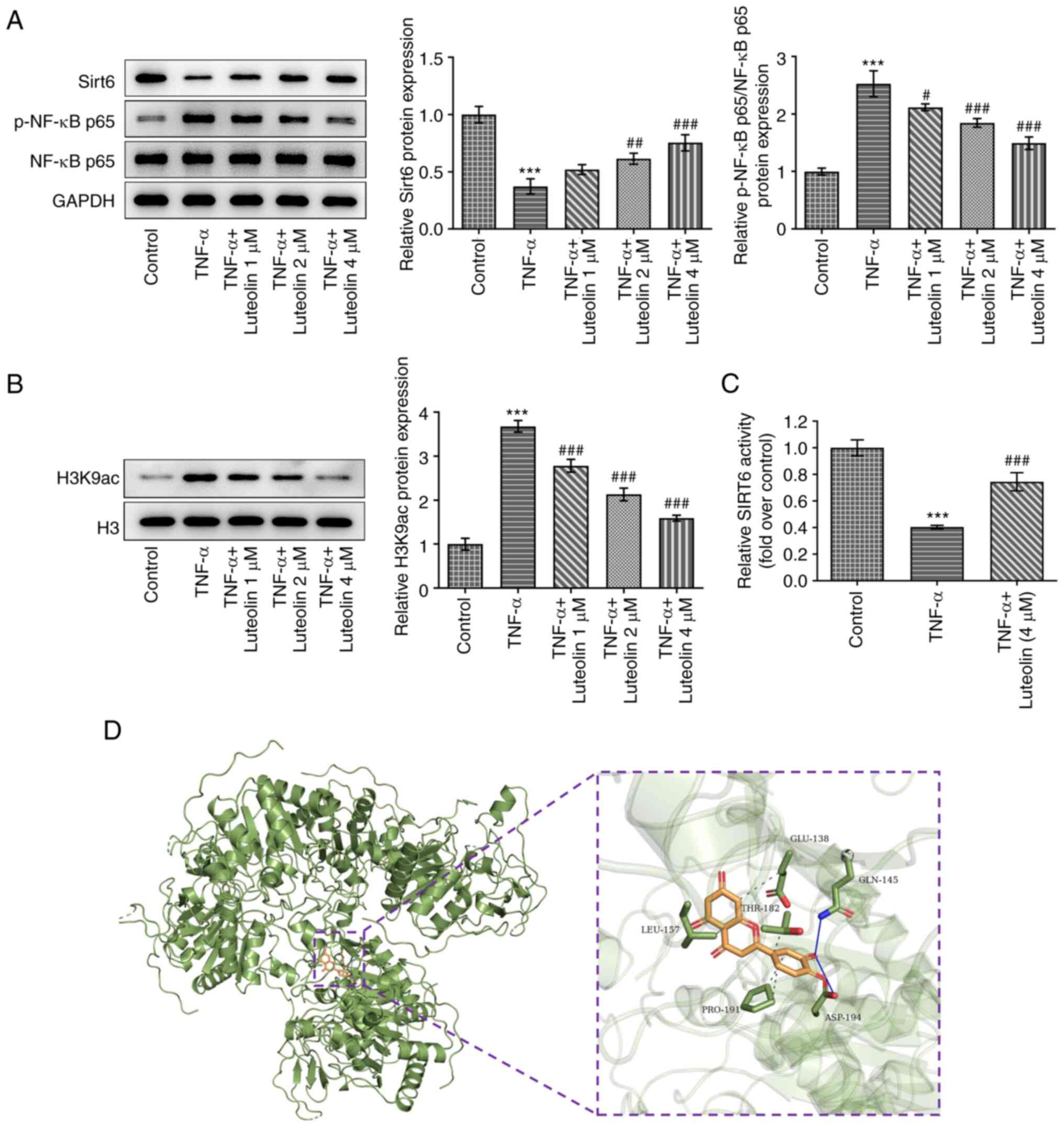
Luteolin regulates the Sirt6/NF-κB
pathway
To further investigate the mechanism of action of
luteolin, western blot analysis was used to detect the expression
levels of Sirt6/NF-κB pathway proteins. The results indicated that
TNF-α induced downregulation of Sirt6 protein expression and
upregulation of phosphorylated (p-)NF-κB p65 protein expression,
while luteolin treatment reversed these effects in a
concentration-dependent manner (Fig.
4A). Furthermore, the effect of TNF-α on histone acetylation
was examined. TNF-α increased the histone acetylation-related
H3K9ac expression level, while luteolin reversed this effect in a
concentration-dependent manner (Fig.
4B). The effect of luteolin on TNF-α-induced Sirt6 activity was
detected by SIRT6 activity assay kit, as shown in Fig. 4C. Luteolin at 4 µM significantly
abrogated the TNF-α-induced decrease in Sirt6 activity. The
three-dimensional structure of Sirt6 protein (PDB id: 3k35) was
also obtained using the PDB database (https://www.rcsb.org/), and molecular docking was
carried out using Autodock. The data indicate that Sirt6 interacts
with luteolin (Fig. 4D).
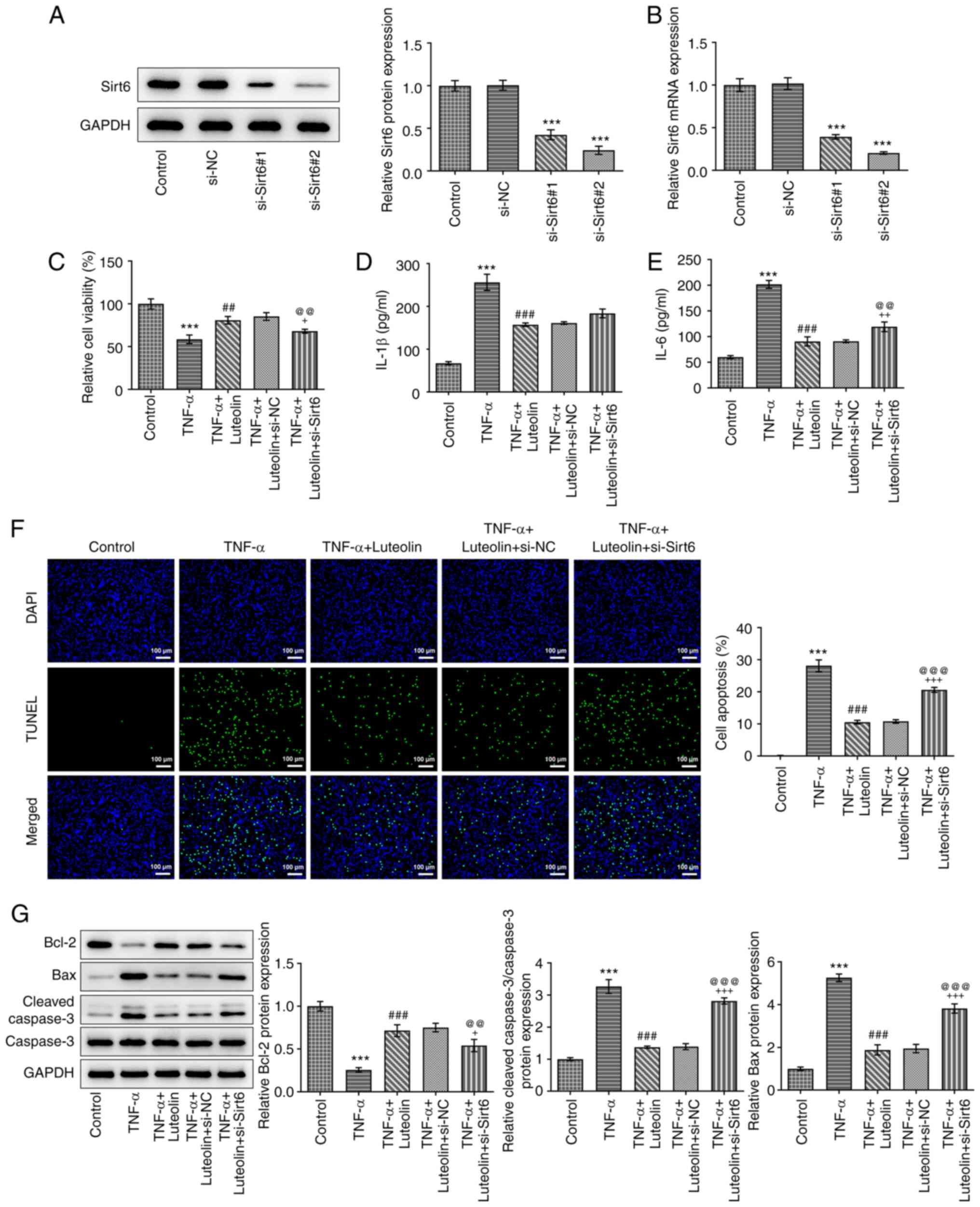
Knockdown of Sirt6 expression
partially reverses the protective effect of luteolin on
TNF-α-induced HNPCs
Western blot and RT-qPCR analyses were used to
assess the knockout efficiency of Sirt6. The Sirt6 protein
(Fig. 5A) and mRNA (Fig. 5B) expression levels were lower in
the si-Sirt6#2 group compared with those noted in the si-Sirt6#1
group, and therefore si-Sirt6#2 was selected for subsequent
experiments. Subsequently, MTT (Fig.
5C) and ELISA assays (Fig. 5D
and E) were employed to detect the
effects of Sirt6 knockdown on cell viability and expression of
inflammatory factors, respectively. Compared with the TNF-α +
luteolin + si-NC group, the TNF-α + luteolin + si-Sirt6 group
demonstrated significantly decreased cell viability and
upregulation of IL-1β and IL-6 expression levels. Subsequently,
TUNEL staining (Fig. 5F) and
western blot (Fig. 5G) analyses
were used to detect cell apoptosis. Knockdown of Sirt6 expression
reversed the inhibitory effects of luteolin on TNF-α-induced
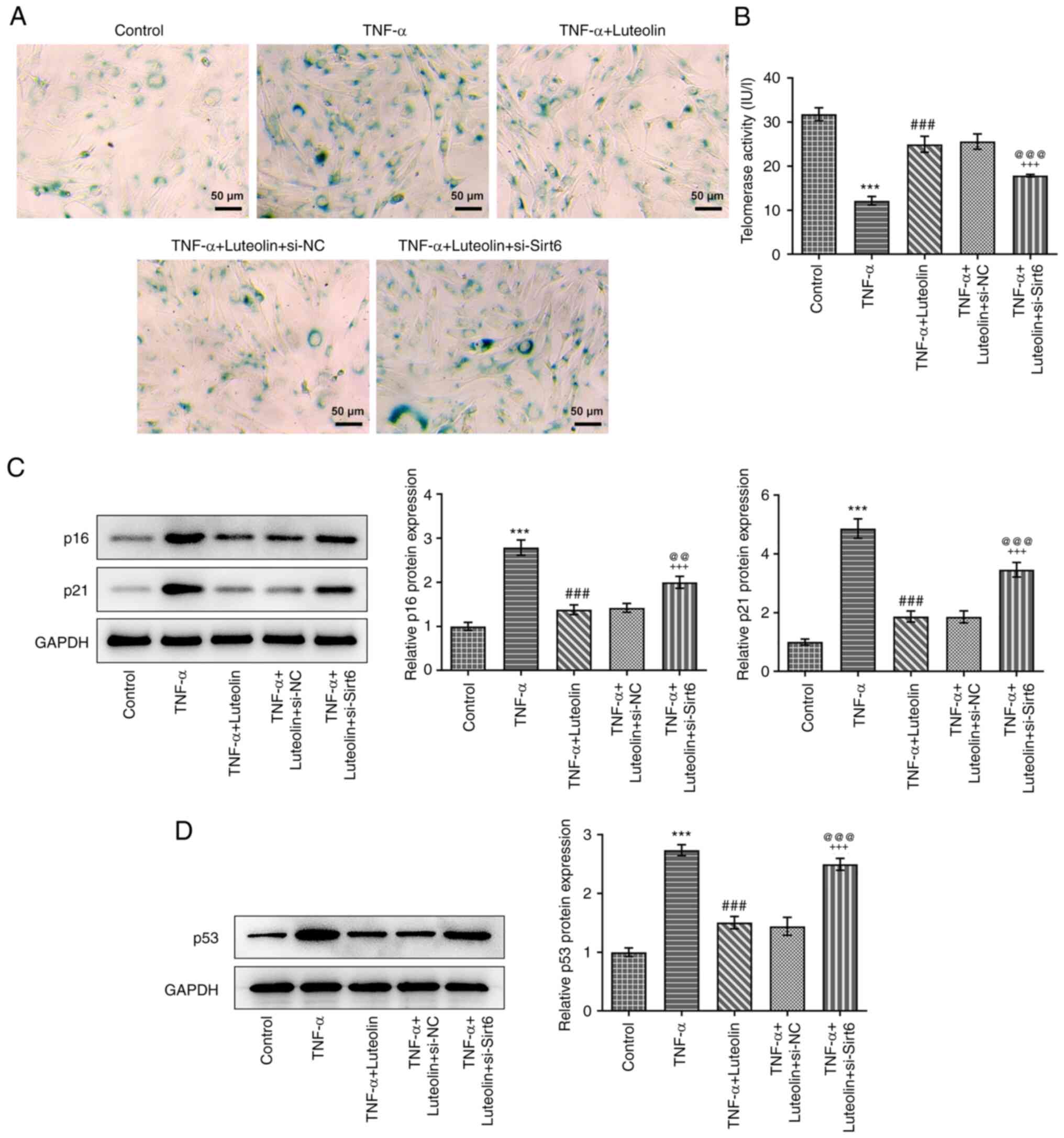
apoptosis of HNPCs. Additional investigation of the knockdown
effects of Sirt6 on cell senescence yielded similar results and
this effect manifested as an increase in the blue product of
β-galactosidase (SA-β-Gal) staining (Fig. 6A), a decrease in telomerase
activity (Fig. 6B), and an
upregulation in the expression levels of senescence-related
proteins (p16, p21 and p53) (Fig.
6C and D).
Discussion
Intervertebral disc degeneration (IDD) is the main
cause of low back pain, which leads to the loss of labor capacity
and causes a serious burden to human society (28). However, until recently no effective
drugs and treatment methods have been reported for IDD progression.
Recently, traditional Chinese medicine has attracted considerable
attention due to its high efficiency, safety, and low toxicity
(29). Luteolin is a flavonoid
with significant anti-inflammatory activity in various disease
types (30). However, it is still
unknown whether luteolin possesses protective effects in IDD.
Therefore, the effects of luteolin on intervertebral disc (IVD)
degeneration and the underlying mechanism of action were
investigated. The present study demonstrated that luteolin could
ameliorate TNF-α-induced nucleus pulposus (NP) cell senescence and
apoptosis by targeting the Sirt6/NF-κB pathway.
NP cellular senescence and apoptosis are the major
biochemical changes that contribute to the development of IVD
degeneration (31). Notably,
inflammation and oxidative stress may accelerate this chronic
process. In addition, a relevant study has reported that ECM
stiffness aggravated oxidative stress-induced senescence and
apoptosis in human NP cells (32).
Tumor necrosis factor (TNF)-α is one of the TNF superfamily
ligands, which possesses proinflammatory activity and is considered
to be linked to the development of multiple diseases (33). Recent research has indicated that
TNF-α can lead to inflammatory disc degeneration (34). In a TNF-α-injected porcine model,
Kang et al (35)
demonstrated that TNF-α resulted in early-stage disc degeneration
as shown by NP cell cluster, ECM degeneration, vascularization, and
IL-1β secretion. These results indicate that TNF-α serves as a
crucial early pathogenetic driver in IDD (35). In the present study, 50 ng/ml TNF-α
was used to establish the inflammatory cell damage model and to
mimic the pathophysiology of IDD in vitro. TNF-α induced the
senescence of human nucleus pulposus cells (HNPCs), while luteolin
treatment reduced the number of SA-β-Gal-positive cells and the
expression of senescence-related proteins, such as p16 and p21.
Moreover, it is worth mentioning that cellular senescence is
closely related to the inflammatory response, interrupted
proliferation, as well as damaged NP tissue self-repair (36). A previous study has reported that
IL-1β and IL-6 can upregulate the levels of matrix-degrading
proteases, such as MMP-13 and thus directly promote matrix
degradation (37). In the present
study, after TNF-α induction, it was shown that the expression
levels of IL-1β, IL-6, and Bax cleaved caspase-3 in HNPCs were
markedly increased. This finding was accompanied by decreased Bcl-2
level. However, luteolin administration significantly increased
cell viability, inhibited cell apoptosis, and decreased the
expression levels of specific cytokines. Similarly, Xia et
al (38) highlighted that
luteolin could target TNF-α-induced cell inflammation injury,
whereas the anti-inflammatory effect of luteolin was linked to the
selective inhibition of the NADPH oxidase 4/reactive oxygen species
(ROS)/NF-κB and MAPK pathways. In terms of the toxicity of
luteolin, Ali and Siddique reported about the bio-availability of
luteolin in the plasma, and human clinical trials indicated no dose
limiting toxicity when administered at a dose of 100 mg/day
(39). Taken together, these
findings support the hypothesis that luteolin at a certain
concentration range exerts protective effects against TNF-α-induced
NP cell senescence and inflammation injury.
Sirt6, a member of the Sirt proteins, has been
implicated in various cellular processes including inflammation,
aging, energy metabolism, stress resistance, and cancer (40). Sirt6 plays a negative regulatory
role in the regulation of cellular senescence. Loss of Sirt6 is
known to be associated with cellular senescence and apoptosis
(41). For example, Sirt6 can
suppress the senescence of smooth muscle cells and thus reduce
atherogenesis (42). Sirt6 has
also been shown to protect nerve cells from amyloid-β1-42
oligomer-induced cellular senescence in Alzheimer's disease
(43). Kang et al (22) demonstrated that overexpression of
Sirt6 could prevent ECM degradation in human NP cells and thus
inhibit the progression of IDD, indicating that Sirt6 exerts
protective effects on NP cells. The results of the present study
indicated that Sirt6 expression was significantly downregulated in
TNF-α-treated HNPCs. However, luteolin promoted the expression of
Sirt6 in HNPCs. Luteolin has also been shown to upregulate Sirt6
levels under in vitro diabetic conditions (44). These results indicate that the
protective effects of luteolin on IDD may be associated with the
regulation of Sirt6.
It has been reported that the NF-κB signaling
pathway can promote ECM degradation by increasing the activity of
matrix-degrading proteases in NP cells (45). Hyperactive NF-κB signaling
contributes to premature and normal aging (46). The interference with NF-κB
signaling is regarded as a targeted therapeutic strategy for IDD
(47). Moreover, Li et al
(48) indicated that Sirt6 could
directly target NF-κB p65 to reduce the activation of NF-κB and
therefore prevent the NF-κB-mediated expression of specific
inflammatory cytokines. Similarly, Kang et al (22) reported that Sirt6 overexpression
inhibited NF-κB signaling and therefore prevented matrix
degradation in IDD. These results suggest that Sirt6 plays a
negative regulatory role in NF-κB activation in IDD. P65 was found
to be expressed simultaneously in the nucleus and cytoplasm of NP
cells after induction of TNF-α for 24 h, which increased the
nuclear translocation and phosphorylation of P65(49). Consistent with this report, the
present study demonstrated that downregulation of Sirt6 expression
in TNF-α-treated HNPCs was accompanied by the phosphorylation of
NF-κB (p65 subunit), while luteolin inhibited NF-κB p65 subunit
phosphorylation. These results suggest that the protective role of
luteolin is linked to the regulation of the Sirt6/NF-κB pathway.
Luteolin has also been shown to exhibit anti-inflammatory activity
in various diseases, such as intracerebral hemorrhage (50), osteoarthritis (16), and diabetic cardiomyopathy
(14). This type of activity is
mediated by inhibiting the activation of the NF-κB pathway. In
addition, the present study demonstrated that downregulation of
Sirt6 led to decreased cell viability, cell senescence, and
increased secretion of IL-1β and IL-6, indicating that inhibition
of Sirt6 could partially abrogate the protective effects of
luteolin in IDD. However, the present study still has the following
deficiencies. First of all, the present study was based solely on
in vitro data. Therefore, the mechanisms of luteolin in IDD
require further assessment in future in vivo studies.
Second, since luteolin has been shown to affect cellular
senescence, its effect on longevity needs to be further validated
in animal studies. In addition, the mechanism of luteolin effect on
the NF-κB/Sirt6 pathway remains to be further explored.
In summary, the present study indicated that
luteolin attenuates TNF-α-mediated NP cell inflammation injury and
senescence by targeting Sirt6 and inhibiting the activation of the
downstream NF-κB signaling pathway. These findings provide novel
molecular insight into the anti-inflammatory effects of luteolin,
which can be considered a potential candidate for IDD therapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant from Wuhan
Municipal Health Commission (grant no. WZ15B09).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TX and JY conceived and designed the study. LM, PL
and RP performed the experiments. JY and LM wrote the paper. TX, PL
and RP reviewed and edited the manuscript. TX, LM and RP confirm
the authenticity of the data. All authors read and approved the
final manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luoma K, Riihimäki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cazzanelli P and Wuertz-Kozak K: MicroRNAs
in intervertebral disc degeneration, apoptosis, inflammation, and
mechanobiology. Int J Mol Sci. 21(3601)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu PH, Kim HS and Jang IT: Intervertebral
disc diseases PART 2: A review of the current diagnostic and
treatment strategies for intervertebral disc disease. Int J Mol
Sci. 21(2135)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kos N, Gradisnik L and Velnar T: A brief
review of the degenerative intervertebral disc disease. Med Arch.
73:421–424. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang Y, He F, Chen Z, Su Q, Yan M, Zhang
Q, Tan J, Qian L and Han Y: Melatonin modulates IL-1β-induced
extracellular matrix remodeling in human nucleus pulposus cells and
attenuates rat intervertebral disc degeneration and inflammation.
Aging (Albany NY). 11:10499–10512. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guo Z, Gao WS, Wang YF, Gao F, Wang W and
Ding WY: MiR-502 suppresses TNF-α-induced nucleus pulposus cell
apoptosis by targeting TARF2. Biomed Res Int.
2021(5558369)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Purmessur D, Walter BA, Roughley PJ,
Laudier DM, Hecht AC and Iatridis J: A role for TNFα in
intervertebral disc degeneration: A non-recoverable catabolic
shift. Biochem Biophys Res Commun. 433:151–156. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aziz N, Kim MY and Cho JY:
Anti-inflammatory effects of luteolin: A review of in vitro, in
vivo, and in silico studies. J Ethnopharmacol. 225:342–358.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Imran M, Rauf A, Abu-Izneid T, Nadeem M,
Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, et al:
Luteolin, a flavonoid, as an anticancer agent: A review. Biomed
Pharmacother. 112(108612)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu RZ, Li BS, Gao SS, Seo JH and Choi BM:
Luteolin inhibits H2O2-induced cellular
senescence via modulation of SIRT1 and p53. Korean J Physiol
Pharmacol. 25:297–305. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kempuraj D, Thangavel R, Kempuraj DD,
Ahmed ME, Selvakumar GP, Raikwar SP, Zaheer SA, Iyer SS,
Govindarajan R, Chandrasekaran PN and Zaheer A: Neuroprotective
effects of flavone luteolin in neuroinflammation and neurotrauma.
Biofactors. 47:190–197. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li L, Luo W, Qian Y, Zhu W, Qian J, Li J,
Jin Y, Xu X and Liang G: Luteolin protects against diabetic
cardiomyopathy by inhibiting NF-κB-mediated inflammation and
activating the Nrf2-mediated antioxidant responses. Phytomedicine.
59(152774)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang L, Wang X, Zhang L, Virgous C and Si
H: Combination of curcumin and luteolin synergistically inhibits
TNF-α-induced vascular inflammation in human vascular cells and
mice. J Nutr Biochem. 73(108222)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fei J, Liang B, Jiang C, Ni H and Wang L:
Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and
attenuates osteoarthritis progression in a rat model. Biomed
Pharmacother. 109:1586–1592. 2019.
|
|
17
|
Yang SC, Chen PJ, Chang SH, Weng YT, Chang
FR, Chang KY, Chen CY, Kao TI and Hwang TL: Luteolin attenuates
neutrophilic oxidative stress and inflammatory arthritis by
inhibiting Raf1 activity. Biochem Pharmacol. 154:384–396.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shahgaldi S and Kahmini FR: A
comprehensive review of Sirtuins: With a major focus on redox
homeostasis and metabolism. Life Sci. 282(119803)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
O'Callaghan C and Vassilopoulos A:
Sirtuins at the crossroads of stemness, aging, and cancer. Aging
Cell. 16:1208–1218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mendes KL, Lelis DF and Santos SHS:
Nuclear sirtuins and inflammatory signaling pathways. Cytokine
Growth Factor Rev. 38:98–105. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang HC and Guarente L: SIRT1 and other
sirtuins in metabolism. Trends Endocrinol Metab. 25:138–145.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kang L, Hu J, Weng Y, Jia J and Zhang Y:
Sirtuin 6 prevents matrix degradation through inhibition of the
NF-κB pathway in intervertebral disc degeneration. Exp Cell Res.
352:322–332. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen J, Xie JJ, Jin MY, Gu YT, Wu CC, Guo
WJ, Yan YZ, Zhang ZJ, Wang JL, Zhang XL, et al: Sirt6
overexpression suppresses senescence and apoptosis of nucleus
pulposus cells by inducing autophagy in a model of intervertebral
disc degeneration. Cell Death Dis. 9(56)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Akter R, Afrose A, Rahman MR, Chowdhury R,
Nirzhor SSR, Khan RI and Kabir MT: A comprehensive analysis into
the therapeutic application of natural products as SIRT6 modulators
in Alzheimer's disease, aging, cancer, inflammation, and diabetes.
Int J Mol Sci. 22(4180)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Su L, Dong Y, Wang Y, Wang Y, Guan B, Lu
Y, Wu J, Wang X, Li D, Meng A and Fan F: Potential role of
senescent macrophages in radiation-induced pulmonary fibrosis. Cell
Death Dis. 12(527)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bernardes de Jesus B and Blasco MA:
Telomerase at the intersection of cancer and aging. Trends Genet.
29:513–520. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao CQ, Jiang LS and Dai LY: Programmed
cell death in intervertebral disc degeneration. Apoptosis.
11:2079–2088. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu L, Yu C, Zhang X, Yu Z, Zhan F, Yu X,
Wang S, He F, Han Y and Zhao H: The treatment of intervertebral
disc degeneration using traditional Chinese medicine. J
Ethnopharmacol. 263(113117)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gendrisch F, Esser PR, Schempp CM and
Wölfle U: Luteolin as a modulator of skin aging and inflammation.
Biofactors. 47:170–180. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang B, Ke W, Wang K, Li G, Ma L, Lu S,
Xiang Q, Liao Z, Luo R, Song Y, et al: Mechanosensitive ion channel
piezo1 activated by matrix stiffness regulates oxidative
stress-induced senescence and apoptosis in human intervertebral
disc degeneration. Oxid Med Cell Longev.
2021(8884922)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Horiuchi T, Mitoma H, Harashima S,
Tsukamoto H and Shimoda T: Transmembrane TNF-alpha: Structure,
function and interaction with anti-TNF agents. Rheumatology
(Oxford). 49:1215–1228. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hong J, Yan J, Chen J, Li S, Huang Y,
Huang Z, Chen W, Liang A and Ye W: Identification of key potential
targets for TNF-α/TNFR1-related intervertebral disc degeneration by
bioinformatics analysis. Connect Tissue Res. 62:531–541.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kang R, Li H, Rickers K, Ringgaard S, Xie
L and Bünger C: Intervertebral disc degenerative changes after
intradiscal injection of TNF-α in a porcine model. Eur Spine J.
24:2010–2016. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sprung CN, Ivashkevich A, Forrester HB,
Redon CE, Georgakilas A and Martin OA: Oxidative DNA damage caused
by inflammation may link to stress-induced non-targeted effects.
Cancer Lett. 356:72–81. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen F, Jiang G, Liu H, Li Z, Pei Y, Wang
H, Pan H, Cui H, Long J, Wang J and Zheng Z: Melatonin alleviates
intervertebral disc degeneration by disrupting the
IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res.
8(10)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K
and Sun H: Luteolin protects HUVECs from TNF-α-induced oxidative
stress and inflammation via its effects on the Nox4/ROS-NF-κB and
MAPK pathways. J Atheroscler Thromb. 21:768–783. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ali F and Siddique YH: Bioavailability and
pharmaco-therapeutic potential of luteolin in overcoming
Alzheimer's disease. CNS Neurol Disord Drug Targets. 18:352–365.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ng F and Tang BL: Sirtuins' modulation of
autophagy. J Cell Physiol. 228:2262–2270. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lee OH, Woo YM, Moon S, Lee J, Park H,
Jang H, Park YY, Bae SK, Park KH, Heo JH and Choi Y: Sirtuin 6
deficiency induces endothelial cell senescence via downregulation
of forkhead box M1 expression. Aging (Albany NY). 12:20946–20967.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Grootaert MOJ, Finigan A, Figg NL, Uryga
AK and Bennett MR: SIRT6 protects smooth muscle cells from
senescence and reduces atherosclerosis. Circ Res. 128:474–491.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang J, Zheng B, Yang S, Wang F, Wang Z
and Wang J: The protective effects of Agomelatine against Aβ1-42
oligomers-induced cellular senescence mediated by SIRT6 and
Agomelatine's potential in AD treatment. Hum Cell. 34:1734–1743.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim A, Lee W and Yun JM: Luteolin and
fisetin suppress oxidative stress by modulating sirtuins and
forkhead box O3a expression under in vitro diabetic conditions.
Nutr Res Pract. 11:430–434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22.
2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kawahara TL, Michishita E, Adler AS,
Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang
HY and Chua KF: SIRT6 links histone H3 lysine 9 deacetylation to
NF-kappaB-dependent gene expression and organismal life span. Cell.
136:62–74. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang GZ, Liu MQ, Chen HW, Wu ZL, Gao YC,
Ma ZJ, He XG and Kang XW: NF-κB signalling pathways in nucleus
pulposus cell function and intervertebral disc degeneration. Cell
Prolif. 54(e13057)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li P, Jin Y, Qi F, Wu F, Luo S, Cheng Y,
Montgomery RR and Qian F: SIRT6 acts as a negative regulator in
dengue virus-induced inflammatory response by targeting the DNA
binding domain of NF-κB p65. Front Cell Infect Microbiol.
8(113)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen J, Yan J, Li S, Zhu J, Zhou J, Li J,
Zhang Y, Huang Z, Yuan L, Xu K, et al: Atorvastatin inhibited TNF-α
induced matrix degradation in rat nucleus pulposus cells by
suppressing NLRP3 inflammasome activity and inducing autophagy
through NF-κB signaling. Cell Cycle. 20:2160–2173. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang Y, Tan X, Xu J, Wang T, Liang T, Xu
X, Ma C, Xu Z, Wang W, Li H, et al: Luteolin alleviates
neuroinflammation via downregulating the TLR4/TRAF6/NF-κB pathway
after intracerebral hemorrhage. Biomed Pharmacother.
126(110044)2020.PubMed/NCBI View Article : Google Scholar
|