Introduction
Pre-eclampsia (PE) is a progressive multisystemic
disease that affects pregnancy and poses a serious threat to
maternal and fetal health (1,2). It
typically manifests as new-onset hypertension and proteinuria after
~week 20 of gestation (1,2). To the best of our knowledge, there is
no definitive therapeutic strategy for PE. Current treatment
methods, including delivery of the fetus, antihypertensive therapy
and excessive fluid administration, are mainly focused on
controlling the disease, prolonging the gestational age and
maintaining the safety of both the mother and fetus (3). Medication is mainly provided for
symptom relief, which is complemented with close monitoring of the
maternal and fetal condition (4).
The etiology and specific pathophysiological
mechanism of PE remain unclear. However, PE is generally considered
to be the result of the joint action of a number of factors,
mechanisms and pathways that need to be investigated, with a
particular focus on the pregnant woman, placenta and fetus
(3). Numerous studies have
reported that aberrant utero-placental vascular structural
modelling, inflammatory immune overactivation, vascular endothelial
cell injury, inadequate trophoblast invasion and increased
apoptosis are key processes of PE pathogenesis (5-7).
Among these factors, trophoblast dysfunction is considered to serve
a key role in the pathogenic process of PE (8,9). In
addition, obstructed placental blood flow caused by the reduced
angiogenic ability of the placenta is known to be another
contributing factor in PE, since uterine spiral arteries of
patients with PE tend to be more prone to deficient remodeling
(10-12).
Therefore, restoring trophoblast invasion and placental
angiogenesis may be an effective treatment strategy for PE.
It has been recently reported that pregnant women
with PE have significantly lower expression levels of proteasome
26S subunit, non-ATPase 14 (PSMD14) in the placenta, compared with
those with normal blood pressure (13). In view of this finding, PSMD14 was
proposed to be a key gene in PE with potential prognostic and
therapeutic value (13).
Furthermore, PSMD14 expression has been previously found to be
increased in liver cancer tissues, which promotes the
proliferation, migration and invasion of hepatocellular carcinoma
cells to facilitate tumor growth in vivo (14). However, it remains to be
investigated if PSMD14 can regulate the proliferation and invasion
of trophoblasts and placental angiogenesis. Therefore, the aim of
the present study was to investigate the role of PSMD14 in PE and
clarify the underlying mechanism.
Materials and methods
Cell culture and treatment
A HTR-8/SVneo trophoblast cell line (15,16)
and HUVECs were purchased from Procell Life Science &
Technology Co., Ltd. It was generated using freshly isolated
extravillous cytotrophoblasts from first trimester placenta and
transfected with a plasmid containing the simian virus 40 large T
antigen (15,16). A recent study demonstrated that
this cell line contains two populations, one of epithelial and one
of mesenchymal origin (15,16).
The complete culture medium used for the HTR-8/SVneo cell line was
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) and HUVECs
were incubated in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc.). Both RPMI-1640 medium and DMEM were
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), 100
µg/ml streptomycin and 100 U/ml penicillin (Sigma-Aldrich; Merck
KGaA). HTR-8/SVneo cells and HUVECs were incubated in a humidified
atmosphere at 37˚C with 5% CO2.
Cell transfection
PSMD14-specific pc-DNA3.1 overexpression vector
(oe-PSMD14; BC009524) and corresponding negative control (oe-NC),
short hairpin RNA (sh) plasmids targeting HEY1 (sh-HEY1-1/2) and
the control shRNA (sh-NC) and a pcDNA3.1 expression vector
containing full-length human HEY1 (pcDNA3.1-HEY1; BC001873) and
corresponding negative control (pcDNA3.1-NC; V790-20) were
constructed by Shanghai GenePharma Co., Ltd. Briefly, after
annealing, shRNA fragments were integrated into a lentiviral GV493
vector (hU6-MCS-CBh-GFP-IRES-puromycin). The sequence for sh-HEY1-1
was 5'-GCAAGGATCTGCTAAGCTA-3'. The sequence for sh-HEY1-2 was
5'-AGATTAAGGTGTTGTATAA-3'. The negative control shRNA sequence was
5'-CCGGCAACAAGATGAAGAGCACCAACTC-3'. A final concentration of 100 nM
plasmids was transfected into HTR-8/SVneo cells and HUVECs using
2.5 µl/ml Lipofectamine 2000® (Thermo Fisher Scientific,
Inc.) for 24 h at 37˚C according to the manufacturer's protocol.
After 48 h transfection, cells were collected for subsequent
experiments.
Total RNA extraction
After the culture medium was discarded, HTR-8/SVneo
cells were washed twice with PBS, incubated with 1 ml
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) for
5 min and centrifuged for 10 min at 16,000 x g at 4˚C. The
supernatant was collected, mixed with chloroform (Shanghai Aladdin
Biochemical Technology Co., Ltd.) for 10 min and centrifuged at
7,155 x g for 15 min at 4˚C. Following the addition of 75% ethanol
and centrifugation at 7,155 x g for 5 min at 4˚C, the RNA sample
was dissolved in 50 µl RNase-free H2O (Takara Bio,
Inc.). Finally, the total RNA concentration was quantified by using
NanoDrop® 2000 (Thermo Fisher Scientific, Inc.) at 260
and 280 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following total RNA extraction, 2 µg RNA was
converted to cDNA using PrimeScript™ RT Master Mix (Takara Bio,
Inc.) following the manufacturer's protocol as follows: 25˚C for 5
min, 42˚C for 60 min and 70˚C for 5 min. qPCR was performed in an
ABI PRISM™ 7000 Sequence Detection Systems Spectral Calibration Kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) under the
following conditions: Pre-denaturation for 30 sec at 95˚C, followed
by 40 cycles of denaturation for 5 sec at 95˚C and annealing for 30
sec at 60˚C. The primer sequences for PCR are presented as follows:
PSMD14 forward, 5'-GTCAGGAACAGGTGTCAGTGT-3' and reverse,
5'-AACCAACAACCATCTCCGGC-3'; HEY1 forward,
5'-CGGCTCTAGGTTCCATGTCC-3' and reverse, 5'-GCTTAGCAGATCCCTGCTTCT-3'
and GAPDH forward, 5'-GGGAAACTGTGGCGTGAT-3' and
5'-GAGTGGGTGTCGCTGTTGA-3'. The relative mRNA level was normalized
with GAPDH using the 2-ΔΔCq method (17).
Western blotting
Protein samples were isolated from HTR-8/SVneo cells
using RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.), quantified by an Enhanced Bicinchoninic Acid Protein
Assay kit (Beyotime Institute of Biotechnology) and separated using
10% SDS-PAGE (30 µg per lane) and transferred onto nitrocellulose
membranes. The membranes were blocked with 5% skim milk powder in
Tris-buffered saline containing 0.1% Tween-20 for 2 h at room
temperature, followed by incubation with the following primary
antibodies overnight at 4˚C: Anti-PSMD14 (1:1,000; cat. no.
ab182762; Abcam), anti-nuclear protein Ki67 (1:1,000; cat. no.
ab15580; Abcam), anti-proliferating cell nuclear antigen (PCNA;
1:1,000; cat. no. ab29; Abcam), anti-MMP2 (1:1,000; cat. no.
ab92536; Abcam), anti-MMP9 (1:1,000; cat. no. ab76003; Abcam) and
anti-HEY1 (1:1,000; cat. no. ab154077; Abcam). The secondary
antibodies, goat anti-rabbit horseradish peroxidase-conjugated IgG
secondary antibody (1:3,000; cat. no. ab6721; Abcam) and goat
anti-mouse horseradish peroxidase-conjugated IgG secondary antibody
(1:3,000; cat. no. ab6728; Abcam), were incubated at room
temperature for 2 h. Super ECL Detection Reagent (Shanghai Yeasen
Biotechnology Co., Ltd.) and ImageJ software v1.8.0 (National
Institutes of Health) were used to observe and analyze the
blots.
MTT assay
HTR-8/SVneo cells were inoculated into 96-well
plates (5x103/well), cultured for 24, 48 and 72 h at
37˚C, following which they were incubated with 10 µl MTT solution
(5 mg/ml; Beyotime Institute of Biotechnology) and the mixture was
incubated for 4 h at 37˚C. Next, 200 µl DMSO was added to each well
to dissolve the formazan (Shanghai Aladdin Biochemical Technology
Co., Ltd.) for 4 h. Absorbance was measured at 570 nm on a
microplate reader (Model 550; Bio-Rad Laboratories, Inc.). The cell
proliferation was calculated with the following formula: Cell
proliferation (%)=[OD570 nm of treated group)/(OD570 nm of control
group)] x100.
5-Ethynyl-2'-deoxyuridine (EdU)
staining
Following the indicated transfection of HTR-8/SVneo
cells and centrifugation at 72 x g/min for 3 min at 4˚C, the cells
(1x104 cells/well) were resuspended in EdU (50 µM per
well; Beyotime Institute of Biotechnology)-containing RPMI-1640
medium for 2 h at 37˚C. Subsequently, the cells were fixed in 4%
paraformaldehyde for 30 min at room temperature, permeabilized with
1% Triton X100 and incubated with click solution (catalog no.
C0075L-3; Beyotime Institute of Biotechnology) for 30 min at room
temperature for nuclear staining with 0.1 µg/ml DAPI (Beyotime
Institute of Biotechnology) for 20 min at room temperature.
Following centrifugation at 600 x g for 10 min at room temperature
and resuspension in PBS, cytospin slides were prepared for
observation under a fluorescence microscope (Leica DM3000k; Leica
Microsystems, Inc.; magnification, x200).
Wound healing assay
Following transfection, HTR-8/SVneo cells were
collected and seeded (1x106/well) in 6-well plates for
24 h. A wound on the cell monolayer was made using a sterile
toothpick. Unattached cells were removed by washing with PBS, and
the rest of the cells were incubated in RPMI-1640 medium containing
10% FBS with 5% CO2 at 37˚C. Images were captured at 0
and 24 h of incubation under a fluorescence microscope (Leica
DM3000k; magnification, x100) and migration distance calculated as
follows: Migration (%)=[(0 h average scratch distance-24 h average
scratch distance)/0 h average scratch distance] x100.
Transwell assay
An invasion assay was conducted to measure cell
invasion using Transwell chambers (6.5-mm in diameter; 8-µm
pore-size; Corning, Inc.). The Transwell chambers were first coated
with 200 µg/ml Matrigel (BD Biosciences) at 37˚C for 1 h. The
transfected cells were harvested and suspended to a final
concentration of 5x105 cells/chamber in serum-free
RPMI-1640 medium. These cell suspensions were loaded into the upper
chamber whilst media containing 10% FBS were added into the lower
chamber. Following 24 h of incubation at 37˚C, the cells were fixed
with 4% paraformaldehyde at room temperature for 15 min and stained
with 0.5% crystalline violet dye (Gibco; Thermo Fisher Scientific,
Inc.) at room temperature for 30 min. The number of invasive cells
was counted under a light microscope (magnification, x100). In
total, five randomly chosen fields were counted for each
chamber.
HUVEC tube formation assay
Transfected HTR-8/SVneo cells were seeded
(1x106/well) in 6-well plates at 37˚C for 24 h and then
the media of the HTR-8/SVneo cells (conditioned medium) was
collected, centrifuged at 500 x g for 5 min and stored in aliquots
at -80˚C. Matrigel (Corning, Inc.) diluted in DMEM was evenly added
into 12-well plates at 300 µl per well to incubate HUVECs
(1x104 cells/well) for 30 min at 37˚C. HUVECs were
selected for digestion, centrifugation and counting. Subsequently,
1x105 HUVECs were added to the culture dish from the EP
tube and the conditioned medium was added into the cells. Images of
tube formation were captured after 12 h of incubation at 37˚C using
an inverted light microscope (magnification, x40). Quantification
of tube formation was performed by using IncuCyte angiogenesis
version 2.0 image analysis (Essen Bioscience).
Dual-luciferase reporter assay
The wild-type (WT) and corresponding mutational
PSMD14 promoter fragments covering predicted HEY1 sites were cloned
into the firefly luciferase reporter plasmid, pGL3-basic vector
(Promega Corporation). Wild-type PSMD14 (PSMD14-WT) or mutant
PSMD14 (PSMD14-MUT) was co-transfected with 10 nM pcDNA3.1-NC or
pcDNA3.1-HEY1 into HTR-8/SVneo cells using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) at 37˚C for 48 h. Luciferase
activity was detected by using a dual luciferase reporter assay
system (Promega Corporation). Firefly luciferase activity was
normalized to Renilla luciferase activity.
Chromatin immunoprecipitation (ChIP)
assay
HTR-8/SVneo cells (1x107/well) were fixed
with 1% methanol at room temperature for 10 min, centrifuged at 300
x g for 3 min at 25˚C and lysed using 0.25% SDS buffer (Beyotime
Institute of Biotechnology). After rupturing the chromatin using
ultrasound (0˚C, 50% power with 4 cycles of 5 sec on, 5 sec off), 2
µg anti-HEY1 (catalog no. 19929-1-AP; Proteintech) and normal
rabbit IgG (catalog no. #3900; CST) antibodies were added to the
cell supernatant for overnight incubation at 4˚C. The precipitate
of the crosslinked protein-DNA complexes was collected for DNA
purification by phenol/chloroform extraction and ethanol
precipitation followed by verification using RT-qPCR as
aforementioned. The analyses of the relative promoter precipitation
levels were carried out by quantifying the intensity of the PCR
product in the immunoprecipitated DNA vs. the DNA input control
using the 2-ΔΔCq method as described previously
(17).
Cell Counting Kit 8 (CCK-8) assay
HTR-8/SVneo cells were seeded into a 96-well plate
(5x103/well) and washed with PBS following incubation
for 24, 48 and 72 h at 37˚C. A total of 10 µl CCK-8 solution
(Beyotime Institute of Biotechnology) was then added into each well
and the cells were incubated for 2 h at 37˚C. The plate was then
placed in a pre-heated microplate analyzer (Model 550; Bio-Rad
Laboratories, Inc.) to measure the absorbance of each well at 450
nm.
Statistical analysis
Data are shown as the mean ± standard deviation from
three independent experiments. Data were analyzed using SPSS 19.0
software (IBM Corp.). In total, two groups of data were compared
using an unpaired, two-tailed Student's t-test. The data were in
accordance with the normal distribution by Shapiro-Wilk test and
significant differences between multiple groups were analyzed by
one-way ANOVA followed by Bonferroni post hoc comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Bioinformatics tools
The Human Transcription Factor Database (https://bioinfo.life.hust.edu.cn/HumanTFDB/#!/)
predicted the binding site of HEY1 on the promotor region of
PSMD14.
Results
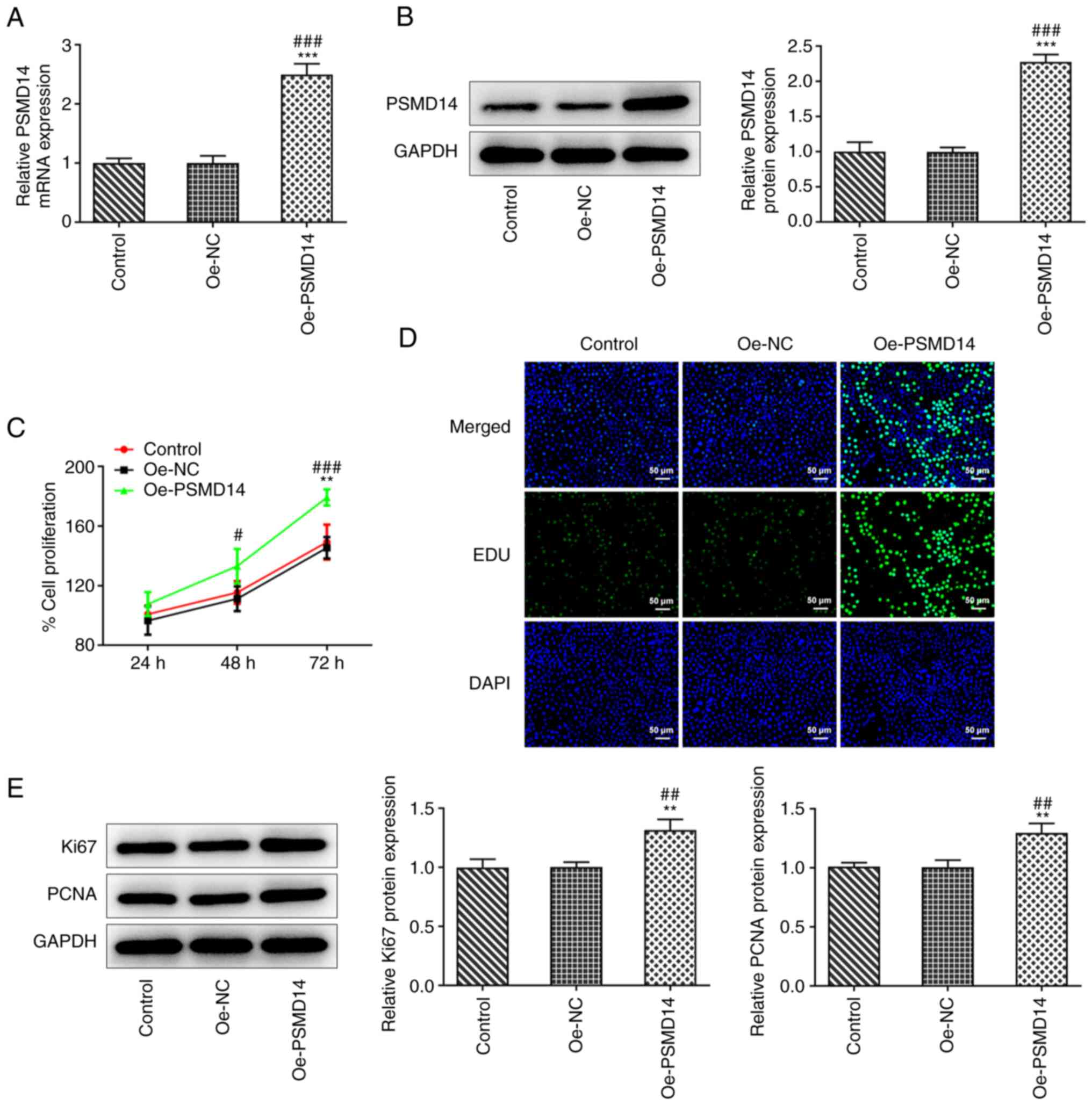
PSMD14 overexpression improves
trophoblast proliferation
To examine the role of PSMD14 in trophoblast
function, a PSMD14-expressing plasmid was transfected into
HTR-8/SVneo cells. RT-qPCR and western blotting revealed a
significant increase in the expression of PSMD14 following cell
transfection compared with that in cells transfected with Oe-NC
(Fig. 1A and B). MTT assay results showed a significant
increase in the proliferation of PSMD14-overexpressing HTR-8/SVneo
cells, compared with that in the Oe-NC group at 48 and 72 h
(Fig. 1C). Similarly, the EdU
staining results showed that the number of proliferating cells was
markedly increased following PSMD14 overexpression compared with
that in the Oe-NC group (Fig. 1D).
Furthermore, western blotting results revealed that PSMD14
overexpression significantly promoted the expression of
proliferation markers Ki67 and PCNA in HTR-8/SVneo cells (Fig. 1E). These results support the
findings from MTT assay and EdU staining in that PSMD14
overexpression promoted HTR-8/SVneo cell proliferation.
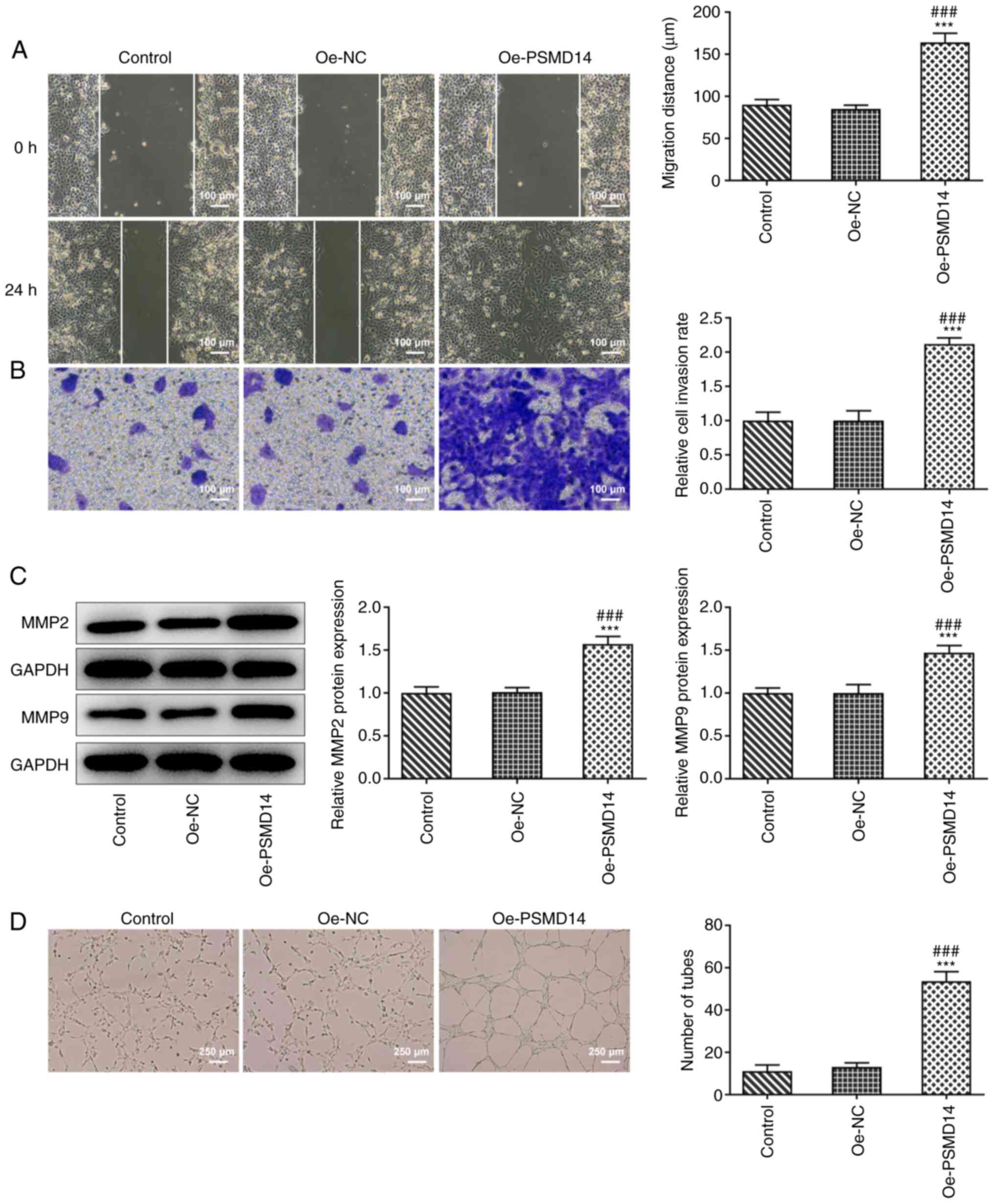
PSMD14 overexpression enhances
trophoblast migration and invasion in addition to promoting HUVEC
angiogenesis
As shown in Fig. 2A
and B, HTR-8/SVneo cells
transfected with oe-PSMD14 possessed significantly higher migratory
and invasive capacities compared with those in the oe-NC group. In
addition, significant increases in the expression levels of
migration-related proteins MMP2 and MMP9 were observed following
PSMD14 overexpression in HTR-8/SVneo cells compared with those in
cells transfected with Oe-NC (Fig.
2C). Tube formation assay results subsequently revealed a
stimulating effect of cell culture supernatant of the HTR-8/SVneo
trophoblasts overexpressing PSMD14 on the tube-like structure
formation by HUVECs (Fig. 2D).
These results suggest that PSMD14 overexpression enhances
trophoblast migration and invasion, in addition to HUVEC
angiogenesis.
Transcription factor HEY1 activates
PSMD14 expression
The Human Transcription Factor Database predicted
the binding site of HEY1 in the PSMD14 promoter region (Fig. 3A). The role of HEY1 in the
expression mechanism of PSMD14 was therefore examined following
HEY1 overexpression or knockdown in HTR-8/SVneo cells. The
transfection efficiency of pcDNA3.1-HEY1 or sh-HEY1-1/2 was
detected by RT-qPCR and western blotting. The results revealed that
HEY1 expression was prominently increased after transfection of
pcDNA3.1-HEY1. shRNA transfection decreased the expression levels
of HEY1, and sh-HEY1-1 exhibited superior transfection efficacy
(Fig. 3B and C). Therefore, sh-HEY1-1 was selected for
subsequent experiments. PSMD14 expression was also significantly
higher in HTR-8/SVneo cells transfected with pcDNA3.1-HEY1 compared
with that in the pcDNA3.1-NC group, whereas HTR-8/SVneo cells
transfected with the sh-HEY1 exhibited significantly lower PSMD14
expression levels compared with those in the sh-NC group (Fig. 3D and E).
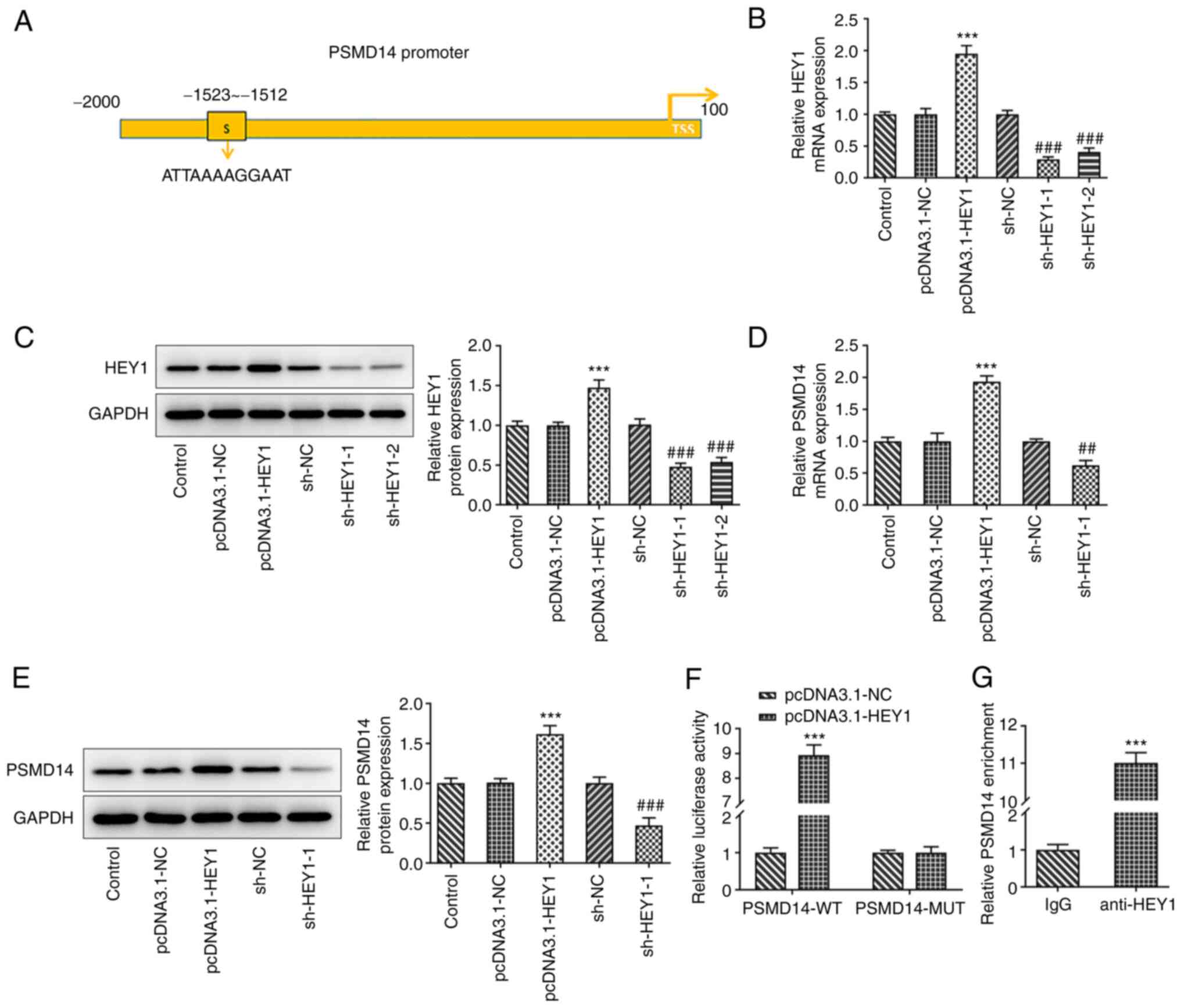 | Figure 3Transcription factor HEY1 activates
PSMD14 expression. (A) The binding site of HEY1 on the PSMD14
promoter was predicted using HumanTFDB. (B) mRNA and (C) protein
expression of HEY1 in HTR-8/SVneo cells transfected with
pcDNA3.1-NC, pcDNA3.1-HEY1, sh-NC, sh-HEY-1 or sh-HEY1-2 were
detected by RT-qPCR and western blotting, respectively.
***P<0.001 vs. pcDNA3.1-NC and
###P<0.001 vs. sh-NC. (D) mRNA and (E) protein
expression of PSMD14 in HTR-8/SVneo cells transfected with
pcDNA3.1-NC, pcDNA3.1-HEY1, sh-NC, sh-HEY1-1 or sh-HEY1-2 were
detected by RT-qPCR and western blotting, respectively.
***P<0.001 vs. pcDNA3.1-NC; ##P<0.01
and ###P<0.001 vs. sh-NC. (F) Relative luciferase
activity of HTR-8/SVneo cells co-transfected with pcDNA3.1-NC or
pcDNA3.1-HEY1 and PSMD14-WT or PSMD14-MUT were detected by
dual-luciferase reporter assay. ***P<0.001 vs. NC.
(G) Relative PSMD14 enrichment in HTR-8/SVneo cell lysates
incubated with IgG or anti-HEY1 antibody were detected by chromatin
immunoprecipitation assay. ***P<0.001 vs. IgG. HEY1,
Hes-related family BHLH transcription factor with YRPW motif 1;
TSS, transcription start sites; PSMD14, proteasome 26S subunit,
non-ATPase 14; sh, short interfering; RT-qPCR, reverse
transcription-quantitative PCR; WT, wild-type; MUT, mutant. |
Dual-luciferase reporter assay results revealed
significantly higher luciferase activities in HTR-8/SVneo cells
co-transfected with pcDNA3.1-HEY1 and PSMD14-WT compared with those
in cells co-transfected with pcDNA3.1-NC and PSMD14-WT, but
luciferase activity remain unchanged between the pcDNA3.1-HEY1 and
PSMD14-MUT or pcDNA3.1-NC and PSMD14-MUT groups (Fig. 3F). ChIP assay results also revealed
significantly higher PSMD14 enrichment in the anti-HEY1 group
compared with that in the IgG group (Fig. 3G). Collectively, these results
suggest that the transcription factor HEY1 can activate PSMD14
expression in HTR-8/SVneo trophoblast cells.
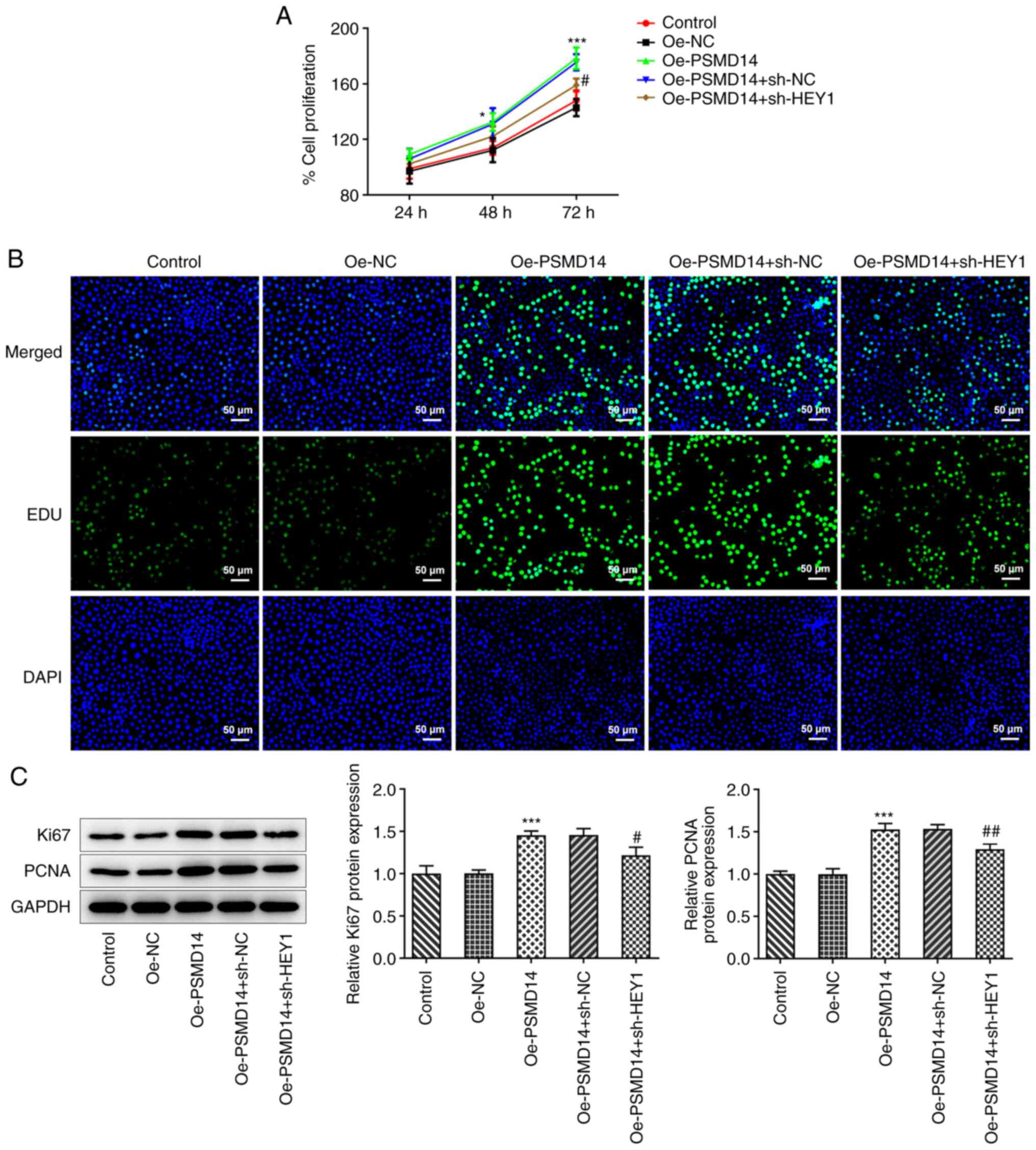
HEY1 silencing reverses the positive
effects of PSMD14 overexpression on trophoblast proliferation
PSMD14 overexpression was found in the present study
to promote the proliferation of HTR-8/SVneo trophoblast cells.
Following HEY1 knockdown, PSMD14-overexpressing HTR-8/SVneo cells
exhibited significantly lower cell proliferation levels (Fig. 4A). Similarly, HEY1 knockdown also
decreased the number of proliferating cells following PSMD
overexpression, as evidenced by the EdU staining results (Fig. 4B). The expression of Ki67 and PCNA
in PSMD14-overexpressing HTR-8/SVneo cells was also significantly
downregulated following HEY1 knockdown compared with that in
PSMD14-overexpressing cells transfected with sh-NC (Fig. 4C). These results suggest that
knocking down HEY1 expression weakens the positive effects of
PSMD14 overexpression on trophoblast proliferation.
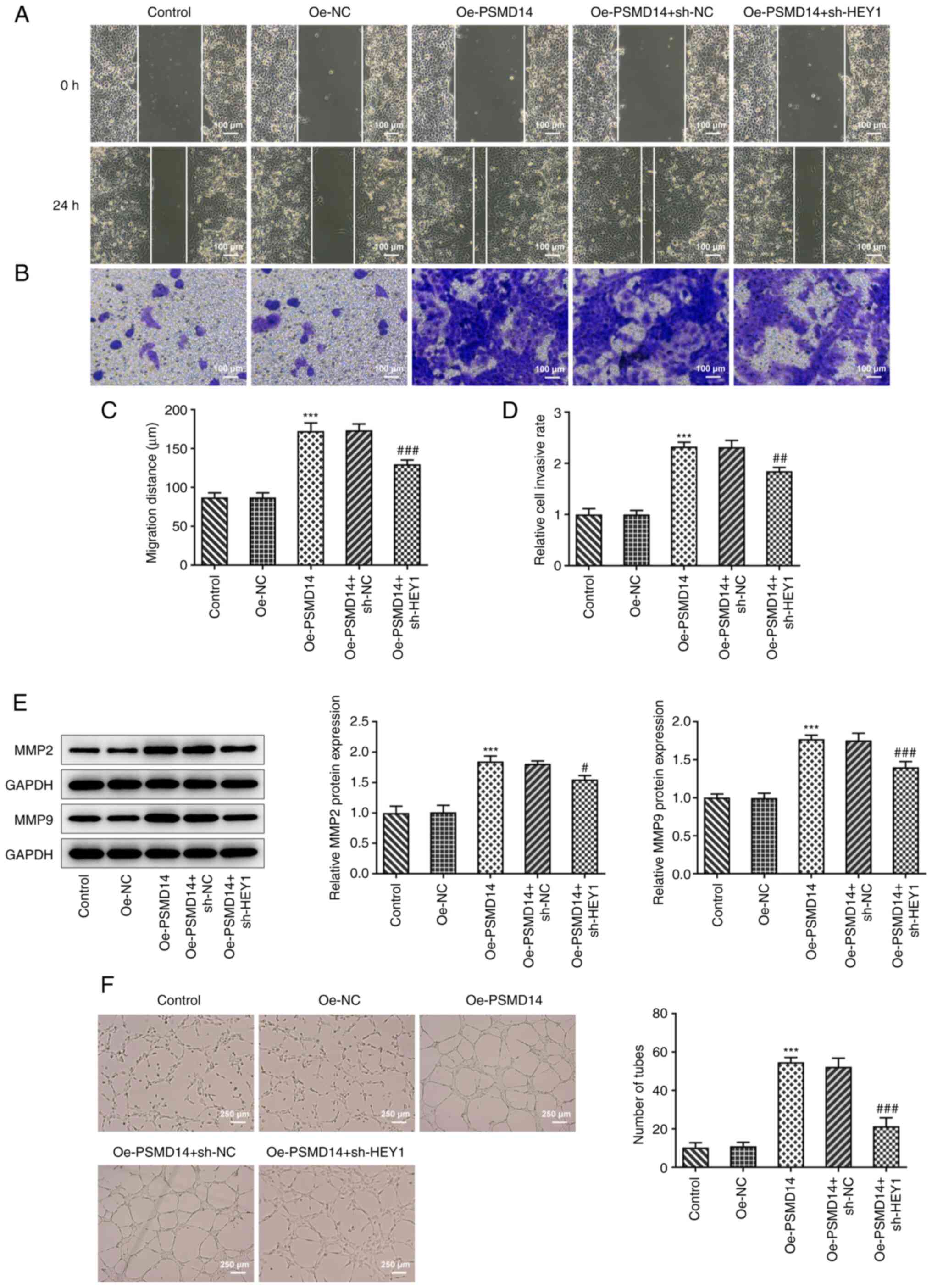
HEY1 knockdown inhibits the promoting
effects of PSMD14 overexpression on trophoblast migration, invasion
and angiogenesis
It was observed from wound healing and Transwell
assays that HEY1 knockdown significantly decreased the migratory
and invasive capabilities of PSMD14-overexpressing trophoblasts
compared with those in PSMD14-overexpressing cells transfected with
sh-NC (Fig. 5A-D). In addition,
MMP2 and MMP9 expression in PSMD14-overexpressing HTR-8/SVneo cells
was also significantly reduced by HEY1 knockdown compared with
those in PSMD14-overexpressing cells transfected with sh-NC
(Fig. 5E). Furthermore, the PSMD14
overexpression-activated stimulation of cell culture supernatant of
the HTR-8/SVneo trophoblasts on the tube-like structure formation
ability by HUVECs was significantly reversed by HEY1 knockdown
(Fig. 5F). Therefore, although
PSMD14 overexpression enhanced trophoblast migration, invasion and
HUVEC angiogenesis, HEY1 knockdown was able to at least partially
reverse these changes.
Discussion
PE is a hypertensive disorder that typically occurs
during pregnancy and can become a serious obstetric complication in
addition to being a major cause of maternal and fetal mortality
(3). Globally, 4-5% pregnant women
are affected by PE, who face higher risks of seizures or falling
into coma if the condition deteriorates into eclampsia, which
threatens the life of both the mother and the baby (18,19).
The past two decades have witnessed promising advances in terms of
the accuracy and efficiency of PE diagnosis, in addition to
improvements in the clinical management of this disease (20,21).
However, the mechanism underlying the pathogenesis of PE remains to
be fully elucidated (20,21). Trophoblasts can differentiate into
highly proliferative and invasive extravillous trophoblasts with
physiology similar to that of cancer cells, which can invade the
endometrium and anchor the placenta to the uterus (22). There they promote angiogenesis,
which provide nutrients and oxygen supply that is essential for
fetal development (22). Defective
trophoblast function has been previously shown to be a major cause
of PE development (23-25).
In addition, insufficient angiogenesis of vascular endothelial
cells, which contributes to dysfunctional vascular recast, has also
been proposed to be a an important cause of PE, which ultimately
leads to placental ischemia and hypoxia (23-25).
To the best of our knowledge, the molecular mechanism underlying
these pathological processes remain unclear.
Recently, a gene expression profiling study revealed
that PSMD14 expression was aberrantly reduced in pregnant women
with PE in comparison with healthy pregnant women (13), suggesting a role for PSMD14 in PE
pathogenesis. PSMD14 has been previously reported to be a potential
prognostic biomarker and treatment target in several types of
cancer, including breast cancer (26), lung adenocarcinoma (27) and melanoma (28). In hepatocellular carcinoma, PSMD14
was found to serve an oncogenic role, where its upregulation
promoted cell proliferation, migration and invasion in vitro
and tumor growth in vivo (14). Furthermore, elevated PSMD14
expression has been observed in breast cancer tissues compared with
that in adjacent non-tumor tissues, such that PSMD14 knockdown was
found to inhibit proliferation and migration whilst facilitating
apoptosis and G0/G1 arrest (29). It was therefore hypothesized in the
present study that PSMD14 expression may also affect the cellular
processes of trophoblasts. In the present study, increased
proliferation was observed alongside enhanced expression levels of
Ki67 and PCNA following PSMD14 overexpression in HTR-8/SVneo cells.
Previous studies have shown that cell viability and invasion of
trophoblast cells are closely associated with PE progression
(30,31). Extravillous trophoblasts (EVTs) are
progenies trophoblasts following epithelial-mesenchymal transition
and are highly invasive, which enables them to migrate away from
the attached embryo and invade the uterine epithelium and spiral
arteries to establish maternal-fetal linkage (32). Dysfunctional EVT migration and
invasion frequently result in the failure of establishing
maternal-fetal connection, which has been previously associated
with PE pathogenesis (33). In the
present study, HTR/SVneo cell invasion and migration after PSMD14
upregulation were both promoted according to results from wound
healing and Transwell assays, which were coupled with significantly
increased levels of MMP2 and MMP9 expression. In addition, the
supernatant of PSMD14-overexpressing trophoblasts also improved the
tube formation capabilities of HUVECs. Therefore, PSMD14
overexpression was suggested to promote trophoblast function and
HUVEC angiogenesis.
The potential relationship between the transcription
factor HEY1 and the PSMD14 promoter was predicted using
bioinformatics analysis. This prediction was meaningful as HEY1 has
been previously shown to be expressed at significantly lower levels
in patients with PE compared with those in healthy pregnant women
(34). In hepatocellular
carcinoma, HEY1 upregulation has been identified to be a
tumorigenic factor, which correlated negatively with prognosis,
overall survival and recurrence-free survival (35). In addition, high HEY1 expression
levels have been detected in clinical glioblastoma samples, the
silencing of which has been shown to be able to reduce the
invasion, migration and proliferation of 4910 and 5310 cells
(36). The present study supported
the possible interaction between HEY1 and PSMD14, which showed that
HEY1 knockdown can at least partially reverse the positive effects
of PSMD14 overexpression on trophoblast physiology and HUVEC
angiogenesis. However, the present study only investigated the role
of PSMD14 and its transcriptional regulation by HEY1 in
vitro. Further in vivo and clinical studies are required
to verify the findings in the present study.
In conclusion, the present study demonstrated that
HEY1 can activate PSMD14 expression, thereby promoting trophoblast
proliferation, invasion and migration, in addition to downstream
endothelial angiogenesis. PSMD14 and HEY1 are therefore promising
therapeutic targets for PE. However, further research on PSMD14 and
HEY1 is warranted to obtain an in-depth understanding of their
roles in PE pathogenesis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Provincial Natural
Science Foundation of China Hainan (grant no. 821MS128) and
Provincial General scientific research project in the health and
family planning industry of China Hainan (grant no. 20A200001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and FC designed the study. LZ, SZ and FC
performed the experiments and analyzed the data. All authors read
and approved the final manuscript. LZ and SZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mol BWJ, Roberts CT, Thangaratinam S,
Magee LA, de Groot CJM and Hofmeyr GJ: Pre-eclampsia. Lancet.
387:999–1011. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Burton GJ, Redman CW, Roberts JM and
Moffett A: Pre-eclampsia: Pathophysiology and clinical
implications. BMJ. 366(l2381)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Phipps EA, Thadhani R, Benzing T and
Karumanchi SA: Pre-eclampsia: Pathogenesis, novel diagnostics and
therapies. Nat Rev Nephrol. 15:275–289. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Duley L, Gulmezoglu AM, Henderson-Smart DJ
and Chou D: Magnesium sulphate and other anticonvulsants for women
with pre-eclampsia. Cochrane Database Syst Rev.
2010(CD000025)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Warrington JP, George EM, Palei AC,
Spradley FT and Granger JP: Recent advances in the understanding of
the pathophysiology of preeclampsia. Hypertension. 62:666–673.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fisher SJ: Why is placentation abnormal in
preeclampsia? Am J Obstet Gynecol. 213 (Suppl 4):S115–S22.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chaiworapongsa T, Chaemsaithong P, Yeo L
and Romero R: Pre-eclampsia part 1: Current understanding of its
pathophysiology. Nat Rev Nephrol. 10:466–480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zou Y, Li S, Wu D, Xu Y, Wang S, Jiang Y,
Liu F, Jiang Z, Qu H, Yu X, et al: Resveratrol promotes trophoblast
invasion in pre-eclampsia by inducing epithelial-mesenchymal
transition. J Cell Mol Med. 23:2702–2710. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Escudero C, Puebla C, Westermeier F and
Sobrevia L: Potential cell signalling mechanisms involved in
differential placental angiogenesis in mild and severe
pre-eclampsia. Curr Vasc Pharmacol. 7:475–85. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Staff AC, Fjeldstad HE, Fosheim IK, Moe K,
Turowski G, Johnsen GM, Alnaes-Katjavivi P and Sugulle M: Failure
of physiological transformation and spiral artery atherosis: Their
roles in preeclampsia. Am J Obstet Gynecol. 226 (Suppl):S895–S906.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Virtanen A, Huttala O, Tihtonen K, Toimela
T, Heinonen T and Uotila J: Angiogenic capacity in pre-eclampsia
and uncomplicated pregnancy estimated by assay of angiogenic
proteins and an in vitro vasculogenesis/angiogenesis test.
Angiogenesis. 22:67–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu Z, Wu C, Liu Y, Wang N, Gao S, Qiu S,
Wang Z, Ding J, Zhang L, Wang H, et al: Identifying key genes and
drug screening for preeclampsia based on gene expression profiles.
Oncol Lett. 20:1585–1596. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv J, Zhang S, Wu H, Lu J, Lu Y, Wang F,
Zhao W, Zhan P, Lu J, Fang Q, et al: Deubiquitinase PSMD14 enhances
hepatocellular carcinoma growth and metastasis by stabilizing GRB2.
Cancer Lett. 469:22–34. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abou-Kheir W, Barrak J, Hadadeh O and
Daoud G: HTR-8/SVneo cell line contains a mixed population of
cells. Placenta. 50:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abalos E, Cuesta C, Grosso AL, Chou D and
Say L: Global and regional estimates of preeclampsia and eclampsia:
A systematic review. Eur J Obstet Gynecol Reprod Biol. 170:1–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wallis AB, Saftlas AF, Hsia J and Atrash
HK: Secular trends in the rates of preeclampsia, eclampsia, and
gestational hypertension, United States, 1987-2004. Am J Hypertens.
21:521–526. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Abraham C and Kusheleva N: Management of
Pre-eclampsia and eclampsia: A simulation. MedEdPORTAL.
15(10832)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin Y, Chen Y, Huang J, Chen H, Shen W,
Guo W, Chen Q, Ling H and Gan X: Efficacy of premedication with
intranasal dexmedetomidine on inhalational induction and
postoperative emergence agitation in pediatric undergoing cataract
surgery with sevoflurane. J Clin Anesth. 33:289–295.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu Z, Wang J, Zhang P and Ding W:
Ulinastatin attenuates vascular endothelial cell damage in pregnant
women with severe pre-eclampsia. An Acad Bras Cienc.
91(e20180746)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abbas Y, Turco MY, Burton GJ and Moffett
A: Investigation of human trophoblast invasion in vitro. Hum Reprod
Update. 26:501–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ridder A, Giorgione V, Khalil A and
Thilaganathan B: Preeclampsia: The relationship between uterine
artery blood flow and trophoblast function. Int J Mol Sci.
20(3263)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qi L, Zhou B, Chen J, Hu W, Bai R, Ye C,
Weng X and Zheng S: Significant prognostic values of differentially
expressed-aberrantly methylated hub genes in breast cancer. J
Cancer. 10:6618–6634. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang L, Xu H, Ma C, Zhang J, Zhao Y, Yang
X, Wang S and Li D: Upregulation of deubiquitinase PSMD14 in lung
adenocarcinoma (LUAD) and its prognostic significance. J Cancer.
11:2962–2971. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yokoyama S, Iwakami Y, Hang Z, Kin R, Zhou
Y, Yasuta Y, Takahashi A, Hayakawa Y and Sakurai H: Targeting
PSMD14 inhibits melanoma growth through SMAD3 stabilization. Sci
Rep. 10(19214)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Luo G, Hu N, Xia X, Zhou J and Ye C: RPN11
deubiquitinase promotes proliferation and migration of breast
cancer cells. Mol Med Rep. 16:331–338. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zuo Q, Huang S, Zou Y, Xu Y, Jiang Z, Zou
S, Xu H and Sun L: The Lnc RNA SPRY4-IT1 modulates trophoblast cell
invasion and migration by affecting the epithelial-mesenchymal
transition. Sci Rep. 6(37183)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu J, Xia Y, Zhang H, Guo H, Feng K and
Zhang C: Overexpression of long non-coding RNA H19 promotes
invasion and autophagy via the PI3K/AKT/mTOR pathways in
trophoblast cells. Biomed Pharmacother. 101:691–697.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ge H, Yin N, Han TL, Huang D, Chen X, Xu
P, He C, Tong C and Qi H: Interleukin-27 inhibits trophoblast cell
invasion and migration by affecting the epithelial-mesenchymal
transition in preeclampsia. Reprod Sci. 26:928–938. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bai R, Kusama K, Nakamura K, Sakurai T,
Kimura K, Ideta A, Aoyagi Y and Imakawa K: Down-regulation of
transcription factor OVOL2 contributes to epithelial-mesenchymal
transition in a noninvasive type of trophoblast implantation to the
maternal endometrium. FASEB J. 32:3371–3384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fragkiadaki P, Soulitzis N, Sifakis S,
Koutroulakis D, Gourvas V, Vrachnis N and Spandidos DA:
Downregulation of notch signaling pathway in late preterm and term
placentas from pregnancies complicated by preeclampsia. PLoS One.
10(e0126163)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yu H, Jia R, Zhao L, Song S, Gu J and
Zhang H: LDB2 inhibits proliferation and migration in liver cancer
cells by abrogating HEY1 expression. Oncotarget. 8:94440–94449.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsung AJ, Guda MR, Asuthkar S, Labak CM,
Purvis IJ, Lu Y, Jain N, Bach SE, Prasad DVR and Velpula KK:
Methylation regulates HEY1 expression in glioblastoma. Oncotarget.
8:44398–44409. 2017.PubMed/NCBI View Article : Google Scholar
|