Introduction
In 2020, the term metabolic dysfunction-associated
fatty liver disease (MAFLD) was suggested as a replacement for the
term non-alcoholic fatty liver (NAFL) disease for describing a
diseased condition of the liver, which is characterized by hepatic
steatosis and fat accumulation, and induced by metabolic disorders
in addition to alcohol and other factors (1). Currently, the global prevalence of
MAFLD is 25%. MAFLD includes pathological features, such as NAFL
with or without mild inflammation, non-alcoholic steatohepatitis
(NASH) and different degrees of hepatic fibrosis (HF); moreover,
severe MAFLD cases may develop into liver cirrhosis and
hepatocellular carcinoma (HCC) (2). NASH is a critical subtype of MAFLD,
with ~20% of patients with NAFL developing NASH, 10-29% of patients
with NASH developing HF or cirrhosis and 4-27% of the latter
progressing to developing HCC with cirrhosis (3). NASH-related fibrosis (NASH-F) is the
last reversible step before progressing to HCC (4). Therefore, the treatment of NASH-F is
an important target in MAFLD therapy.
HF is a multipurpose wound-healing process that is
presented as the key early stage of liver cirrhosis. In the absence
of liver transplantation, HF can eventually develop into HCC
(5). HF is a complex pathological
process, and its exact pathogenesis remains unknown. It is
characterized by mass production of extracellular matrix (ECM) by
activated hepatic stellate cells (HSCs) that undergo myofibroblast
transformation; these activated HSCs can be identified by the
expression of α-smooth muscle actin (α-SMA) (6,7).
Increasing evidence indicates that the transforming growth factor-β
(TGF-β) pathway plays a major role in the development of HF
(8,9). The Smad protein family comprises
TGF-β downstream signaling molecules, through which TGF-β signals
from the cell surface to the nucleus. TGF-β1 mediates the
activation and proliferation of HSCs through the classical
TGF-β1/Smad2/3 signaling pathway (10). Previous research has demonstrated
that TGF-β signaling participates in all stages of liver disease
progression, with high TGF-β signaling activity leading to the
activation of HSCs, their transdifferentiation to myofibroblasts
and the cell death of a large number of hepatocytes, all of which
contribute to the development of HF (11). Therefore, targeting the TGF-β
pathway appears to be a promising strategy for the treatment of
various pathological mechanisms associated with HF (12).
TGF-β is a major pro-fibrotic cytokine that plays a
critical role in the development of HF by regulating the activation
of HSCs and the mass production of ECM (13,14).
Mechanistically, TGF-β1 binds to the TGF-β type I (TGF-βRI) and
type II receptors and activates Smad2/3. Subsequently, activated
Smad2/3 associates with Smad4, and the complex translocates to the
nucleus where it acts as a transcription factor regulating the
expression of specific genes (TGF-β canonical pathway) (11). Smad signaling also induces the
transcription of fibrosis-related Smad7 target genes; whereas Smad3
is known to promote TGF-β signaling, Smad7 acts as an inhibitor of
the pathway (15). Specifically,
the Smad7 protein is an inhibitory protein that inhibits TGF-β1
receptor and phosphorylation of its downstream mediators Smad2/3,
thereby blocking the TGF-β1 signaling cascade (16). Thus, in the context of HF, Smad7
can attenuate the TGF-β1-mediated activation of HSCs, reduce the
expression of pro-fibrotic genes, such as collagen type I and
α-SMA, and inhibit the production of ECM, thereby reversing
fibrosis (16). The TGF-β1/Smad
signaling pathway plays an important role in the occurrence and
development of HF, given that TGF-β1 can stimulate the
proliferation and activation of HSCs. TGF-β1 signaling is
transduced from the plasma membrane to the nucleus through the Smad
proteins, where they control laminin (LN) and hyaluronic acid (HA)
expression, eventually leading to the occurrence and progression of
HF (17,18). Taken together, the TGF-β1/Smad
pathway plays an important regulatory role both in development and
reversion of HF. Therefore, regulation of the TGF-β1/Smad signaling
pathway can be used as an important research target both for
preventing and treating NASH-F.
Traditional Chinese Medicine (TCM) and its effective
components present great potential in the treatment of fibrosis.
For example, Peng et al (19) demonstrated that Salvia
miltiorrhiza treatment could significantly alleviate elevated
levels of serum aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) and could reduce liver inflammation and
fibrosis in a CCl4-induced animal model. Tanshinone IIA (TIIA) is
the most abundant lipid-soluble constituent of Salvia
miltiorrhiza Bunge, and has been widely used in the treatment
of cardiovascular diseases (20),
atherosclerosis (21) and
Alzheimer's disease (22). In
recent years, TIIA has also been used for the treatment of visceral
fibrosis, including lung, heart, kidney and uterus fibrosis, and it
has been demonstrated that TIIA mediates its anti-fibrotic effects
through TGF-β/Smad, NF-κB and nuclear factor erythroid 2-related
factor 2(23). Furthermore, Shi
et al (24) found that TIIA
attenuated liver injury, alleviated ECM accumulation, and decreased
HSC proliferation and activation in a CCl4-induced rat model of HF,
thus exerting potent anti-fibrotic effects. In addition, TIIA has
been shown to significantly inhibit the activation of LX-2 induced
by TGF-β1(25). However, the
precise underlying molecular mechanism of action of TIIA in hepatic
fibrosis remains unknown. Therefore, the present study investigated
the potential beneficial effects of TIIA and its underlying
mechanism in alleviating liver fibrosis.
Materials and methods
Animals and treatments
Specific pathogen-free 7-week-old 15 male and 15
females Wistar rats (160-200 g) were purchased from the Institute
of Medical Biology, Chinese Academy of Medical Sciences [SCXK
(Dian) K2019-0002]. Before the start of the experiment, rats were
acclimated for 7 days in the facility under standard environmental
conditions (23±2˚C; humidity, 60±5%; 12/12 h light/dark cycle with
lights on at 8:00 a.m.) and had access to food and water ad
libitum. All animals received humane care according to
institutional animal care guidelines approved by the Experimental
Animal Ethics Committee of Yunnan University of Traditional Chinese
Medicine (Kunming, China; approval no. R-062021021). The animals
were then randomly divided into three groups (n=10 per group): i)
Control group, continuously fed standard chow; ii) NASH-F group,
fed methionine choline deficiency (MCD) diet; and iii) TIIA group,
fed MCD diet and being administered TIIA. The groups were treated
with the standard or MCD diet for 8 weeks. Rats in the drug
treatment group received TIIA (20 mg/kg/day) via intraperitoneal
injection during MCD diet feeding and were weighed daily, while the
rats in the other groups were given an equal volume of normal
saline via intraperitoneal injection. The doses of TIIA were
calculated according to previous research (Liu et al,
unpublished data) on mice induced by the MCD diet, adjusted to
reflect the differences in the body surface area between animals.
At the end of the treatment period the rats were anesthetized with
30 mg/kg intraperitoneal sodium pentobarbital injection and
sacrificed via cervical dislocation after collection of blood from
the abdominal veins of each animal in each group. Serum was
collected at 377.325 x g for 10 min at 4˚C by centrifugation and
preserved at -80˚C until further analysis. Liver tissues were
immediately obtained and fixed in 4% paraformaldehyde for ≥48 h at
room temperature.
Reagents
TIIA was purchased from Shanghai Aladdin Biochemical
Technology Co., Ltd. (cat. no. H31022558). MCD and standard diet
were obtained from Nantong Chem-Base Co., Ltd. (cat. nos. TP3622657
and TP3622647C). AST (cat. no. C0010-2-1), ALT (cat. no. C009-2-1),
triglycerides (TG) (cat. no. A110-1-1), total cholesterol (TC)
(cat. no. A111-1-1), total bilirubin (TBIL) (cat. no. C019-1) and
total bile acid (TBA) (cat. no. E003-2-1) test kits were purchased
from Nanjing Jiancheng Bioengineering Institute. HA, LN, type III
collagen (PC-III) and type Ⅳ collagen (IV-C) in the rat serum were
measured using ELISA assay kits (Meimian Industrial Co., Ltd.).
RIPA buffer, PMSF and bicinchoninic acid (BCA) protein
concentration kit were purchased from Beyotime Institute of
Biotechnology. Antibodies against TGF-β1 (cat. no. Ab215715),
Smad2/3 (cat. no. Ab202445), Smad7 (cat. no. Ab272928), α-SMA (cat.
no. Ab124964), phosphorylated (p)-Smad2/3 (cat. no. Ab254407) and
β-actin (cat. no. Ab8226) were purchased from Abcam, Inc.
TRIzol® was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. (cat. no. 15596026). ReverTra Ace™ qPCR
RT Master Mix with gDNA Remover and SYBR-Green Realtime PCR Master
Mix were purchased from Toyobo Life Science. The primer sequences
of the target genes were synthesized by Sangon Biotech Co., Ltd.
Recombinant human TGF-β1 (cat. no. 100-21-10 µg ) was purchased
from PeproTech, Inc.
Biochemical parameters
Liver function was evaluated based on the levels of
AST, ALT, TBIL and TBA in the rat serum. The kits were used
according to the manufacturer's instructions, and the optical
density (OD) values for ALT, AST, TC, TG, TBIL and TBA were
obtained using a Spark 10M multimode reader (Tecan Group, Ltd.),
using the appropriate wavelengths. The concentrations of the
aforementioned indicators were calculated using the OD value.
ELISA
HA, IV-C, LN and PC-III in the rat serum were
detected using ELISA assay kits according to the manufacturer's
instructions. The absorbance was measured using a microplate reader
at 450 nm, and the concentrations of the aforementioned indicators
were calculated using the OD value.
Histological analysis
Liver tissues were fixed in 4% paraformaldehyde for
at least 48 h, dehydrated, embedded in paraffin, and cut into 5-µm
thick sections. Liver sections were deparaffinized in xylene for 45
min, rehydrated in a graded alcohol series, then stained with
hematoxylin for 3 min and eosin for 30 sec. All steps were carried
out at room temperature. For Masson's trichrome staining, the
sections were deparaffinized and rehydrated as previously
described. Then, sections were stained with hematoxylin,
differentiated with acid ethanol, stained in Masson's blue
solution, followed by staining with Fuchsin for 8 min. The tissues
were then washed with phosphomolybdic acid for 2 min and stained
with aniline blue for 5 min. All these steps were performed at room
temperature. The sections were sealed with neutral resin and images
were captured using a slide scanning image system
(SQS-1000SQS-1000, Shenzhen Shengqiang Technology Co., Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the liver tissues using
TRIzol®, and reverse-transcribed into cDNA using the
ReverTra Ace qPCR RT kit according to the manufacturer's protocol.
qPCR was conducted on a Roche LightCycler 96 real-time PCR system
using SYBR-Green as the detection fluorophore (95˚C, 5 sec; 60˚C,
34 sec). The expression of TGF-β1, Smad2, Smad3, Smad7 and α-SMA
was normalized to that of the housekeeping gene β-actin. The
relative mRNA expression was quantified using the 2-ΔΔCq
method (26). The primer sequences
used are listed in Table I.
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| TGF-β1 | |
|
Forward |
CTCCCGTGGCTTCTAGTGC |
|
Reverse |
GCCTTAGTTTGGACAGGATCTG |
| Smad2 | |
|
Forward |
ATGTCGTCCATCTTGCCATTC |
|
Reverse |
AACCGTCCTGTTTTCTTTAGCTT |
| Smad3 | |
|
Forward |
CACGCAGAACGTGAACACC |
|
Reverse |
GGCAGTAGATAACGTGAGGGA |
| Smad7 | |
|
Forward |
GGCCGGATCTCAGGCATTC |
|
Reverse |
TTGGGTATCTGGAGTAAGGAGG |
| α-SMA | |
|
Forward |
GCGTGGCTATTCCTTCGTGACTAC |
|
Reverse |
CATCAGGCAGTTCGTAGCTCTTCTC |
| β-actin | |
|
Forward |
CACGATGGAGGGGCCGGACTCATC |
|
Reverse |
TAAAGACCTCTATGCCAACAC |
Western blotting
A total of 2 ml LX-2 cells were incubated in 6-well
plates at a density of 5x105 cells/ml and divided into
control, a model (TGF-β1) group and TIIA 20 or 40 µmol/l groups.
The control group was treated with complete culture medium, model
group with 10 ng/ml TGF-β1 and the TIIA group with either 20 or 40
µmol/l TIIA and 10 ng/ml TGF-β1 by sequential for 24 h. Liver
tissues and LX-2 cells were lysed in RIPA lysis buffer and PMSF.
The protein concentration was quantified using a BCA protein
concentration kit. The proteins (30 µg/lane) were separated by 10%
SDS-PAGE Gel Fast Preparation Kit (Epizyme, Inc.). The separated
proteins were subsequently transferred to a PVDF membranes, blocked
with 5% skimmed milk for 2 h at room temperature, and blotted
overnight at 4˚C with primary antibodies. Primary antibodies
included TGF-β1 (1:1,000), Smad2/3 (1:1,500), p-Smad2/3 (1:1,500),
Smad7 (1:2,500) and α-SMA (1:1,500). β-actin (1:2,500) was used as
loading control. Blots were rinsed by TBST buffer (Tween-20, 1%)
and the membranes were incubated with a goat anti-rabbit
horseradish peroxidase-conjugated IgG secondary antibody (1:500,
cat. no. Ab6721; Abcam) at room temperature for 4 h. Following
another triple wash, proteins were detected using an ECL reagent
(ZenBioScience). The intensities of protein bands were quantified
using the ImageJ software (version 1.52; National Institutes of
Health).
Cell viability assay
A total of 105/ml human LX-2 cells (cat.
no. HTX2168) [Otwo Biotech (Shenzhen) Inc.] were cultured in
96-well plates, grown to 80-90% confluence, and pre-treated with
100 µl TIIA solution at 10, 20, 40, 80, 160 or 320 µmol/l for 24 h
at 37˚C with 5% CO2. Subsequently, 20 µl MTS (Promega
Corporation) was added to each well, and the plates were further
incubated for 1-4 h at 37˚C with 5% CO2. Absorbance was
measured at 490 nm using a Spark 10M multimode reader.
Statistical analysis
Results were analyzed with SPSS version 25.0 (IBM
Corp.) and GraphPad Prism version 8.0 software (GraphPad Software,
Inc.). Data are presented as the mean ± SD from at least three
independent experiments. Differences between multiple groups were
determined using one-way ANOVA followed by Bonferroni's post hoc
test as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
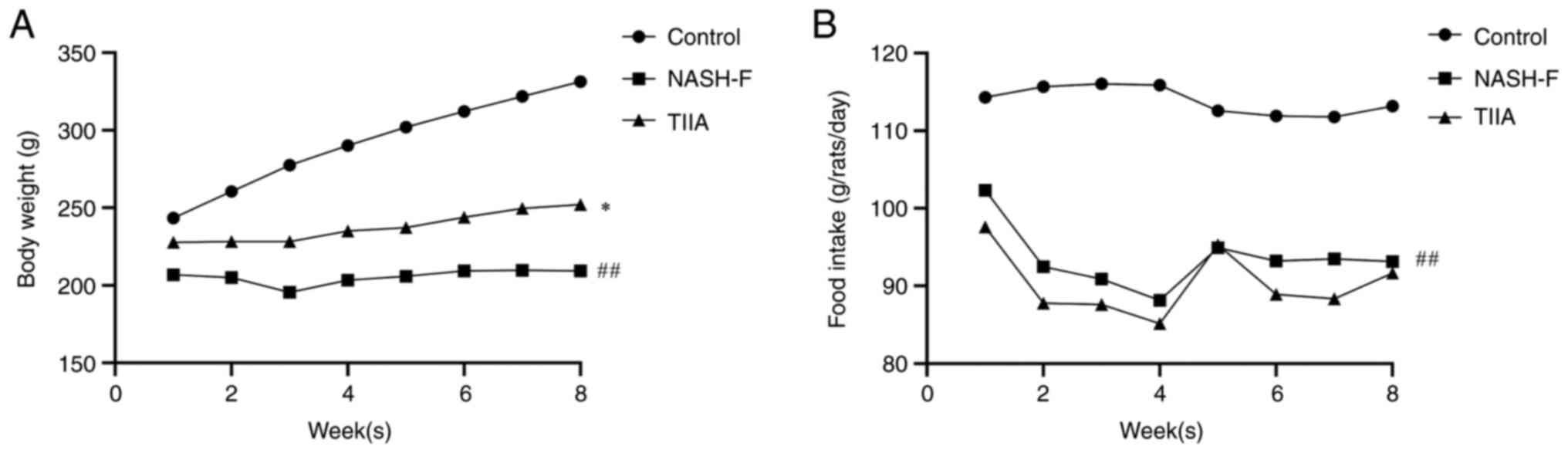
Effect of TIIA on body weight and food
intake in rats induced by MCD diet
To explore the effect of TIIA on the treatment of
NASH-F, Wistar rats were fed a normal or MCD diet for up to 8 weeks
and changes in body weight and food intake were observed. The body
weight of rats in the NASH-F group was decreased at 2-8 weeks
compared with the control group. The body weight of rats in the
TIIA group was increased at 2-8 weeks as compared with the NASH-F
group (Fig. 1A). The food intake
of rats in the control group remained stable during the modeling
period, and the food intake of rats in the NASH-F and TIIA groups
decreased at 2-8 weeks (Fig. 1B).
TIIA treatment increased body weight of MCD-induced NASH-F rats,
but there is no difference in its food intake.
TIIA changes liver function indexes in
rats with MCD-induced NASH-F
MCD-induced rats developed steatohepatitis similar
to NASH-F. NASH-F group had a notable increase in serum ALT and AST
(Fig. 2A), which are markers used
to indicate liver dysfunction. Interestingly, TIIA treatment
suppressed this increase in liver transaminase (ALT and AST) levels
observed in the NASH-F group (Fig.
2A). Similarly, NASH-F group had markedly reduced serum TC and
TG levels compared with the control group, and both serum lipid
markers showed a tendency for increase in the TIIA group compared
with the NASH-F group, but there was no statistical significance.
(Fig. 2B). Finally, NASH-F group
had higher serum TBIL and TBA levels compared with the control
group, and the increase in both bile metabolism markers was
suppressed by TIIA treatment (Fig.
2C). Collectively, these serum biochemical data suggested that
TIIA can improve liver function, blood cholesterol and bile
metabolism in MCD-fed rats.
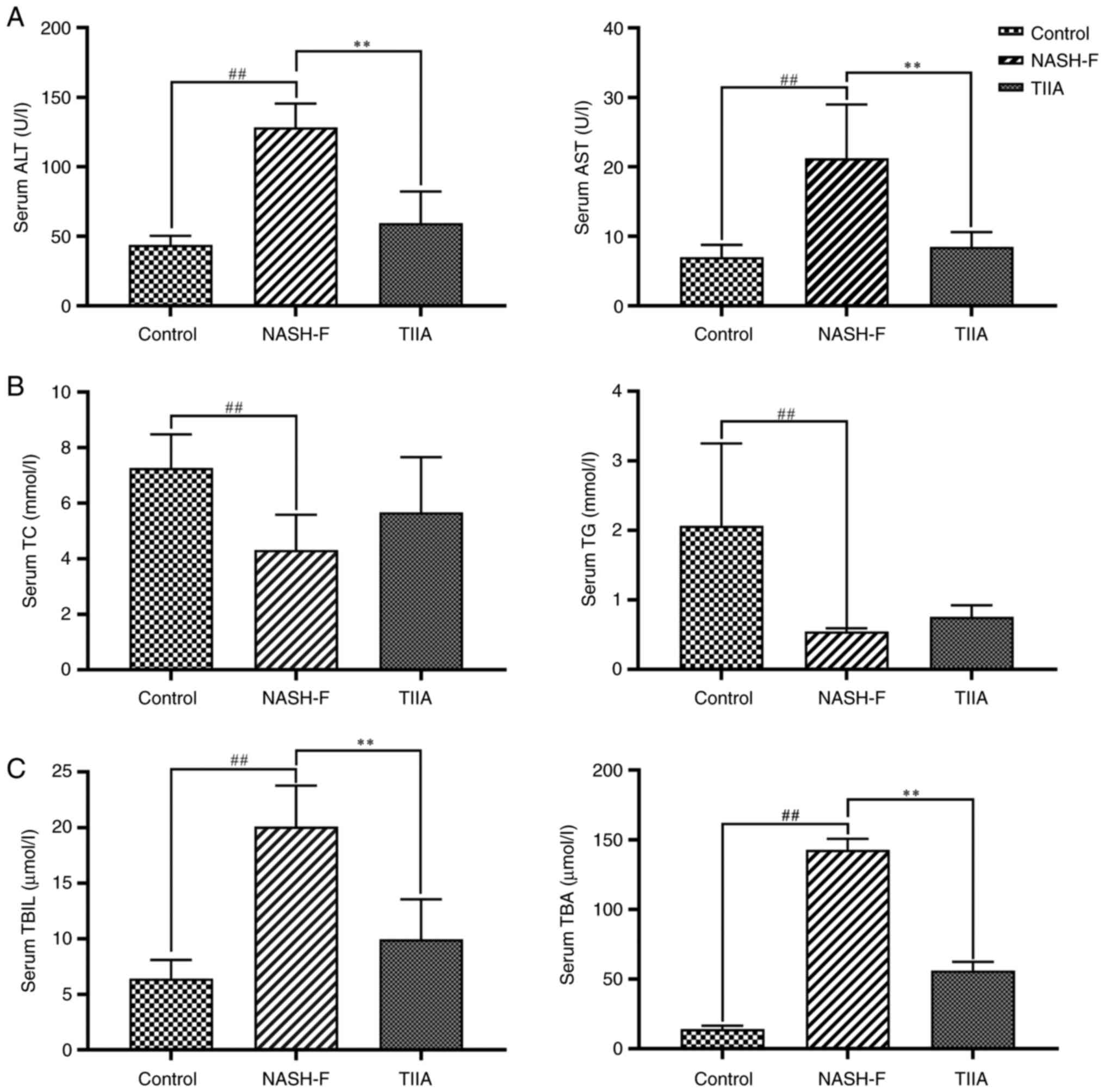 | Figure 2Effects of TIIA on serum
transaminase, lipid, TBIL and TBA levels in methionine choline
deficiency diet-fed rats. (A) Serum transaminase levels in rats
(ALT, NASH-F vs. control, P=0.001; and TIIA vs. NASH-F, P=0.008;
AST, NASH-F vs. control, P=0.002; and TIIA vs. NASH-F, P=0.006).
(B) Serum lipid levels in rats (TC, NASH-F vs. control, P=0.005;
and TIIA vs. NASH-F, P=0.147; TG, NASH-F vs. control, P<0.001;
and TIIA vs. NASH-F, P=0.074). (C) Serum TBIL and TBA levels in
rats (TBIL, NASH-F vs. control, P<0.001; and TIIA vs. NASH-F,
P<0.001; TBA, NASH-F vs. control, P<0.001; and TIIA vs.
NASH-F, P=0.004). ##P<0.01; **P<0.01
(n=6). NASH-F, non-alcoholic steatohepatitis-related fibrosis;
TIIA, tanshinone IIA; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; TC, total cholesterol; TG, triglycerides; TBIL,
total bilirubin; TBA, total bile acid. |
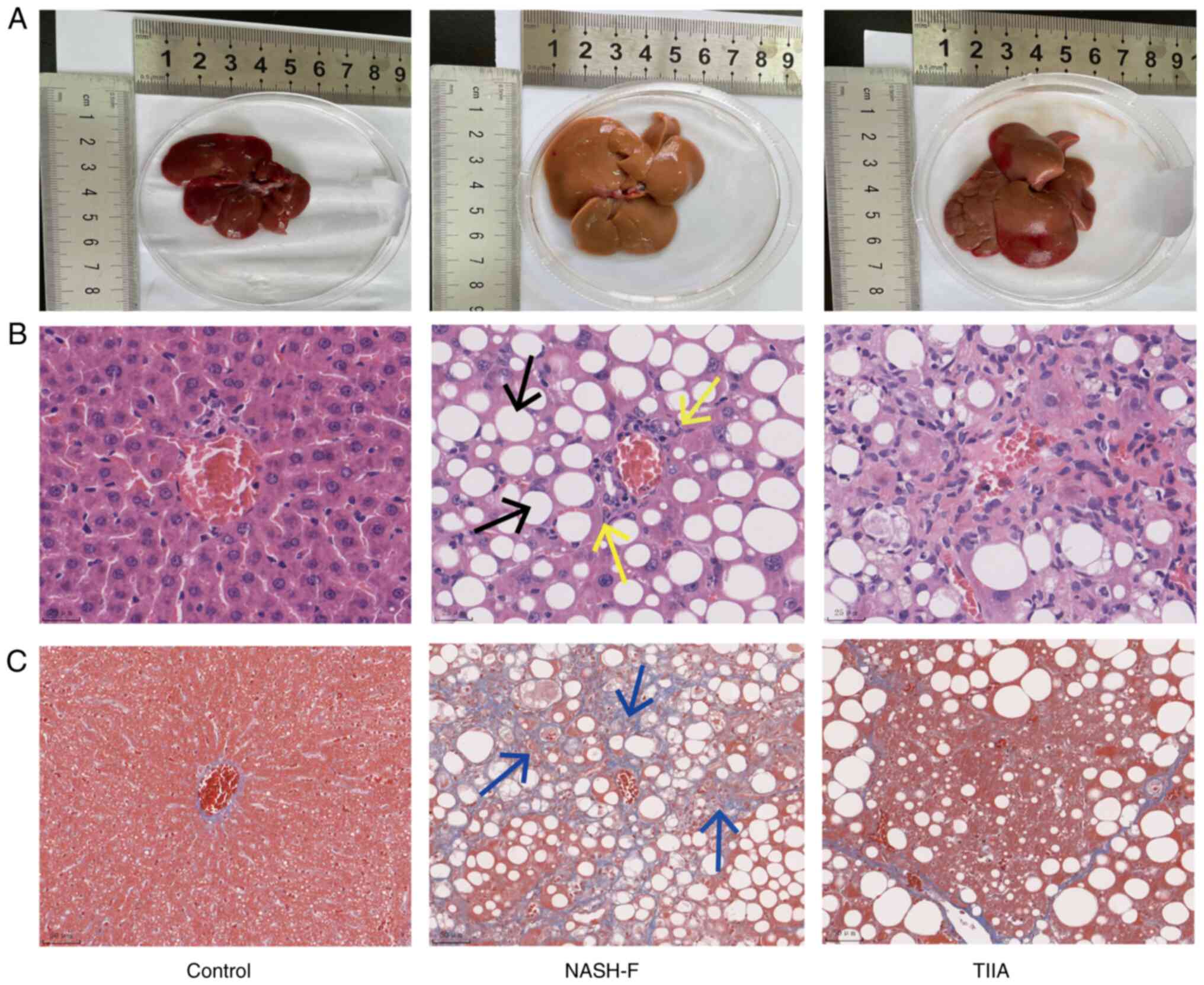
TIIA ameliorates steatosis and liver
fibrosis in rats with MCD-induced NASH-F
The livers of the MCD-induced rats had a pale yellow
color and increased volume 8 weeks after the start of the MCD diet,
compared with the control group (dark red). However, in the TIIA
treatment group, the livers were of a mixed yellow and red color
(Fig. 3A). H&E staining
revealed fat vacuoles and inflammatory cell infiltration in the
liver tissue of MCD-induced NASH-F rats compared with control
animals. Interestingly, TIIA treatment markedly reduced both the
number of fat vacuoles and the level of inflammatory cell
infiltration (Fig. 3B). In order
to further evaluate the liver fibrosis, Masson's trichrome staining
was performed. The results showed hepatic lobule structure
disorder, central vein area and collagen fibres accumulated in the
NASH-F group. TIIA treatment was associated with improved liver
structure, with only a small amount of collagen deposition in the
hepatic hilar region (Fig. 3C). In
summary, histological analysis showed that TIIA notably reduced the
number of fat vacuoles, inflammatory infiltration and fibrosis in
NASH-F rats.
TIIA decreases the serum levels of
liver fibrosis indicators in rats with MCD-induced NASH-F
To determine whether TIIA can improve liver
fibrosis, ELISA was performed to determine whether TIIA can reduce
the serum levels of four liver fibrosis indicators in NASH-F rats.
Compared with the control group, the serum levels of HA, IV-C, LN
and PC-III were markedly elevated in NASH-F group, indicating
increased HF. TIIA treatment notably suppressed the serum levels of
all four indicators of fibrosis, thereby preventing the increase of
fibrosis observed in MCD-fed rats (Fig. 4A-D). Collectively, these data
confirmed the beneficial effects of TIIA on liver fibrosis
developed in MCD-fed rats.
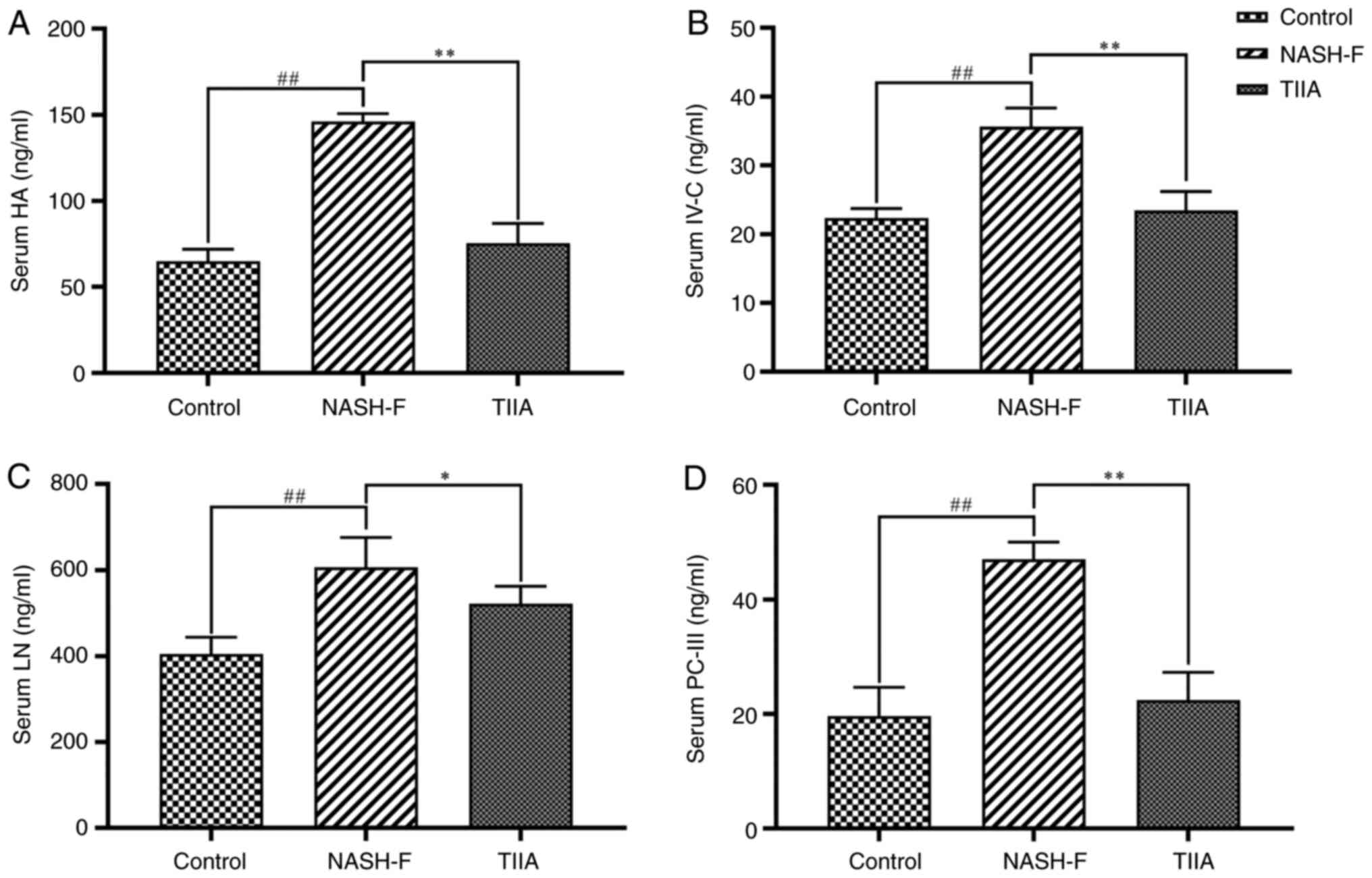 | Figure 4Effects of TIIA on serum levels of
four liver fibrosis indicators in methionine choline deficiency
diet-fed rats. ELISA analyses of (A) HA (NASH-F vs. control,
P<0.001; and TIIA vs. NASH-F, P<0.001), (B) IV-C (NASH-F vs.
control, P<0.001; and TIIA vs. NASH-F, P<0.001), (C) LN
(NASH-F vs. control, P<0.001; and TIIA vs. NASH-F, P=0.013) and
(D) PC-III (NASH-F vs. control, P<0.001; and TIIA vs. NASH-F,
P<0.001). ##P<0.01; *P<0.05;
**P<0.01 (n=6). NASH-F, non-alcoholic
steatohepatitis-related fibrosis; TIIA, tanshinone IIA; HA,
hyaluronic acid; IV-C, type Ⅳ collagen; LN, laminin; PC-III, type
III collagen. |
Effect of TIIA on mRNA expression
levels of the TGF-β1/Smad signaling pathway components in rats with
MCD-induced NASH-F
To explore whether TIIA can inhibit the progression
of fibrosis, qPCR analysis was performed to determine whether TIIA
can reduce the expression of TGF-β1, α-SMA, Smad2 and Smad3, and
increase the expression of Smad7 in NASH-F rats. The mRNA
expression levels of TGF-β1, α-SMA, Smad2 and Smad3 were markedly
increased in the livers of NASH-F group compared with the control
group and this increase was notably suppressed by TIIA treatment
(Fig. 5A-D). The opposite pattern
was observed for Smad7, with its mRNA levels being markedly
suppressed in the livers of MCD-fed rats compared with control
group, which was reversed by TIIA treatment (Fig. 5E).
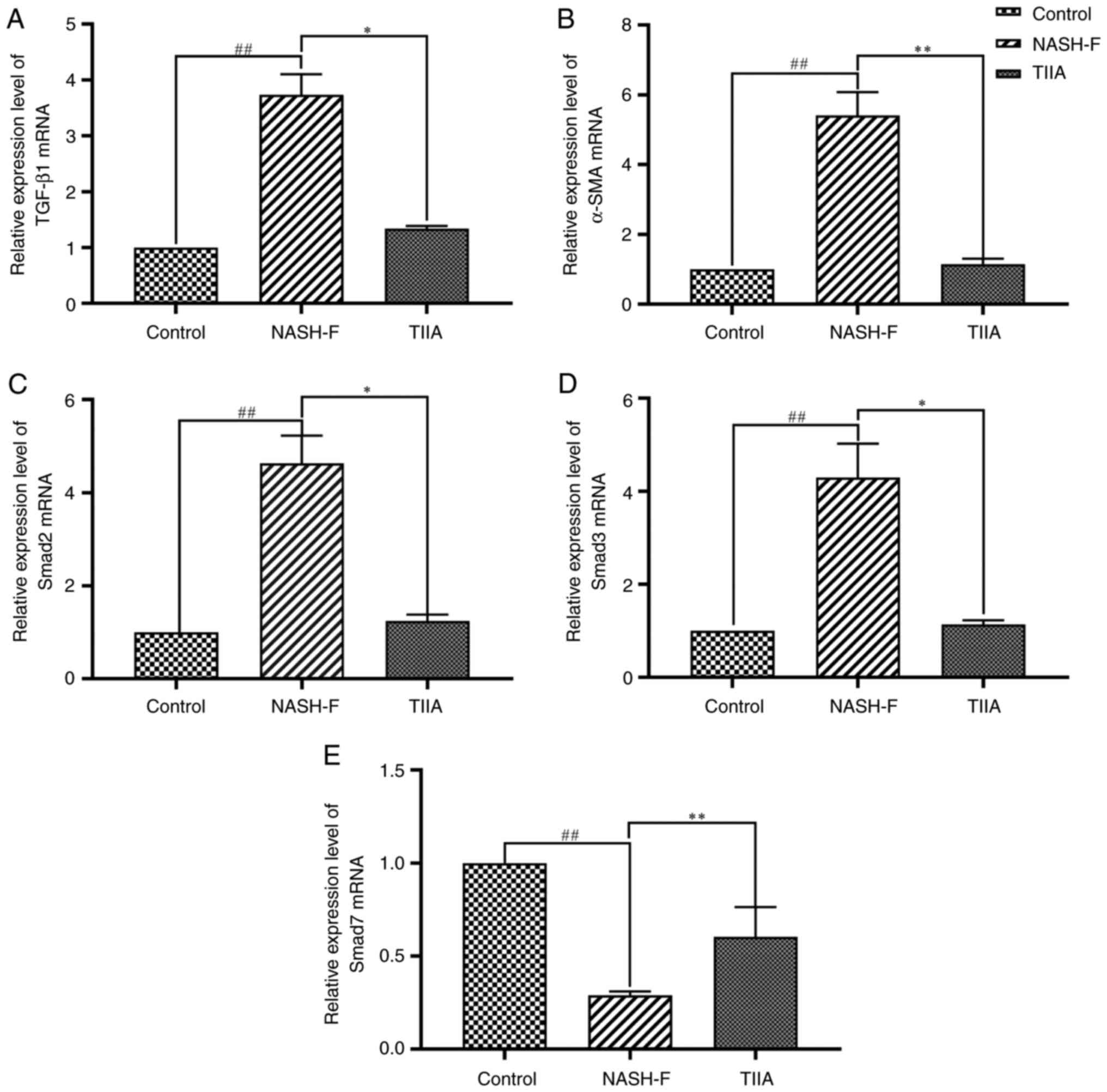 | Figure 5Effects of TIIA on the mRNA levels of
the TGF-β1/Smad pathway molecules in methionine choline deficiency
diet-fed rats. Reverse transcription-quantitative PCR analyses of
(A) TGF-β1 (NASH-F vs. control, P=0.006; and TIIA vs. NASH-F,
P=0.024), (B) α-SMA (NASH-F vs. control, P<0.001; and TIIA vs.
NASH-F, P<0.001), (C) Smad2 (NASH-F vs. control, P=0.006; and
TIIA vs. NASH-F, P=0.024), (D) Smad3 (NASH-F vs. control, P=0.006;
and TIIA vs. NASH-F, P=0.024) and (E) Smad7 (NASH-F vs. control,
P<0.001; and TIIA vs. NASH-F, P=0.006). ##P<0.01;
*P<0.05; **P<0.01 (n=6). NASH-F,
non-alcoholic steatohepatitis-related fibrosis; TIIA, tanshinone
IIA; α-SMA, α-smooth muscle actin. |
TIIA modulates protein expression of
the TGF-β1/Smad signaling pathway components in rats with
MCD-induced NASH-F
To further investigate the effect of TIIA on the
TGF-β/Smad signaling pathway, the protein levels of TGF-β1,
p-Smad2/3, Smad2/3, Smad7 and α-SMA were examined by western
blotting. The protein levels of TGF-β1, p-Smad2/3 and α-SMA were
markedly increased in the NASH-F group compared with the control
group. TIIA treatment reduced TGF-β1, α-SMA and p-Smad2/3 protein
levelscompared with the NASH-F group, similarly to the observations
in the mRNA level. The opposite pattern was observed for Smad7,
with its protein levels being reduced in the livers of NASH-F rats
and this decrease being reversed by TIIA treatment, but there was
no statistical significance. (Fig.
6A-E). As the Smad2/3 protein levels remained unchanged, TIIA
was associated with both a reduction in p-Smad2/3 and the ratio of
p-Smad2/3 to total Smad2/3 compared with the NASH-F group (Fig. 6C-E).
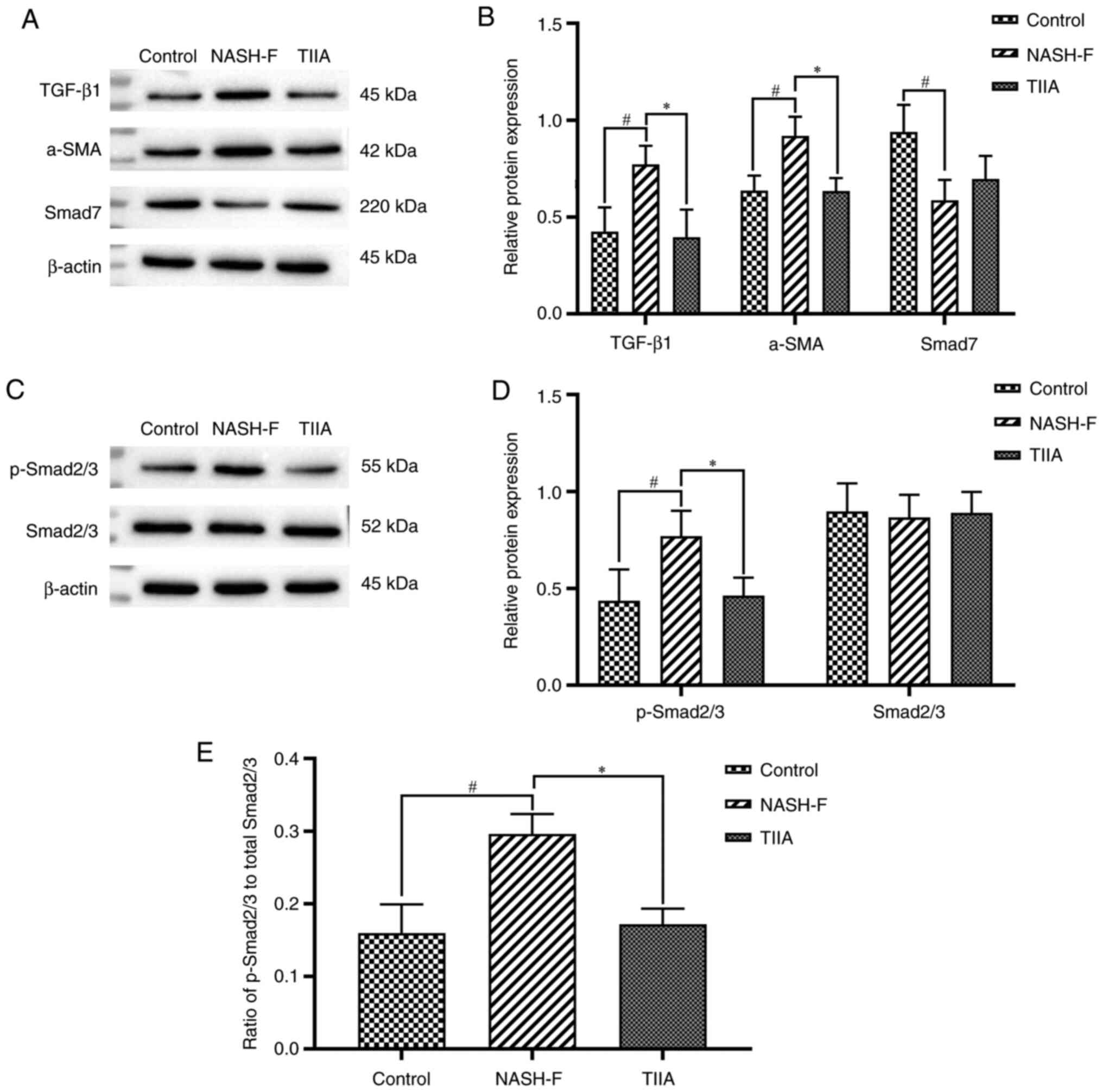 | Figure 6TIIA modulates protein expression of
the TGF-β1/Smad pathway molecules. (A) Western blot analysis of
TGF-β1, α-SMA and Smad7 protein levels in rat livers. Results were
normalized relative to β-actin. (B) Quantification of TGF-β1
(NASH-F vs. control, P=0.013; and TIIA vs. NASH-F, P=0.024), α-SMA
(NASH-F vs. control, P=0.019; and TIIA vs. NASH-F, P=0.018) and
Smad7 (NASH-F vs. control, P=0.012; and TIIA vs. NASH-F, P=0.300)
shown in A. (C) Western blot analysis of p-Smad2/3 and Smad2/3
protein levels in rat livers. Results were normalized relative to
β-actin. (D) Quantification of p-Smad2/3 (NASH-F vs. control
P=0.049; and TIIA vs. NASH-F, P=0.031) and Smad2/3 (NASH-F vs.
control, P=0.776; and TIIA vs. NASH-F, P=0.778) shown in C. (E)
Ratio of p-Smad2/3 over total Smad2/3 expression (NASH-F vs.
control, P=0.022; and TIIA vs. NASH-F, P=0.022).
#P<0.05; *P<0.05 (n=3). NASH-F,
non-alcoholic steatohepatitis-related fibrosis; TIIA, tanshinone
IIA; p, phosphorylated; α-SMA, α-smooth muscle actin. |
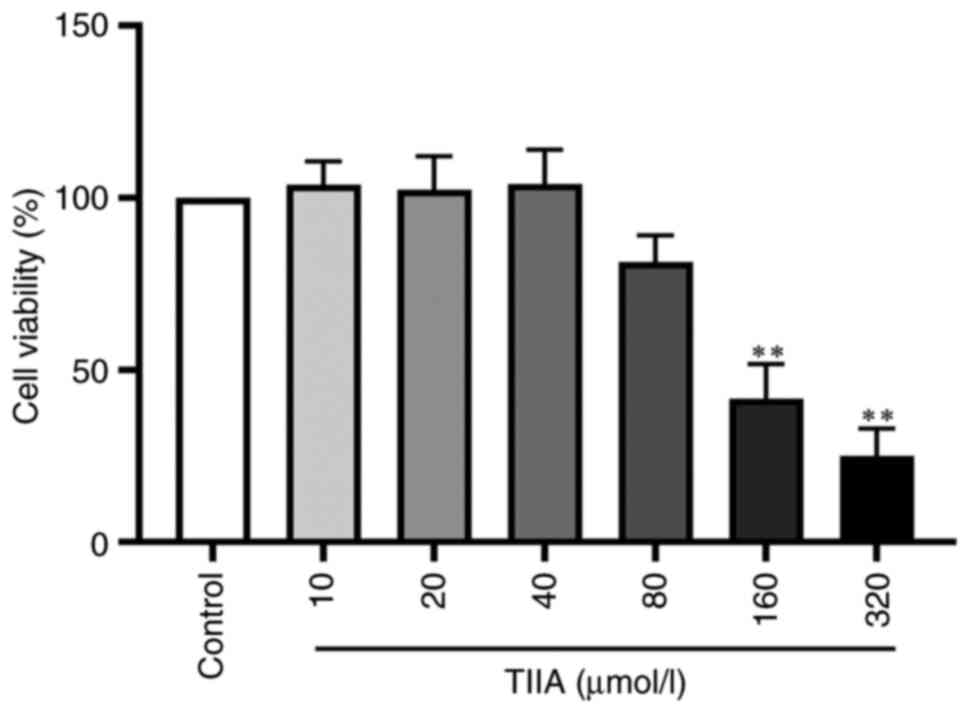
Effect of TIIA on LX-2 cell
viability
To study whether TIIA was toxic in LX-2 cells, the
effect of different concentrations of TIIA was examined on LX-2
cell viability using MTS. Compared with the control group, 10, 20,
40 and 80 µmol/l TIIA had no effect on cell viability, whereas 160
and 320 µmol/l TIIA significantly reduced the viability of LX-2
cells (Fig. 7). Therefore,
concentrations of 20 and 40 µM were selected for subsequent
experiments.
TIIA modulates protein expression of
the TGF-β1/Smad pathway molecules in the LX-2 cell line
To further investigate the effect of TIIA on the
TGF-β1/Smad signaling pathway, the expression levels of α-SMA,
Smad2/3, p-Smad2/3 and Smad7 were examined in each group using
western blotting. The expression of α-SMA in the model group was
significantly increased compared with the control group.
Concomitant treatment with TIIA reversed the increase in α-SMA
expression induced by TGF-β1. Moreover, as the expression of
Smad2/3 remained unchanged, TIIA treatment was associated with a
reduction in p-Smad2/3 and the ratio of p-Smad2/3 to total Smad2/3
(Fig. 8A-C). Finally, the reverse
pattern was observed for Smad7, with its protein levels being
reduced by TGF-β1 and this reduction being reversed by TIIA
treatment (Fig. 8D and E). However, there was no difference
between the two doses of TIIA.
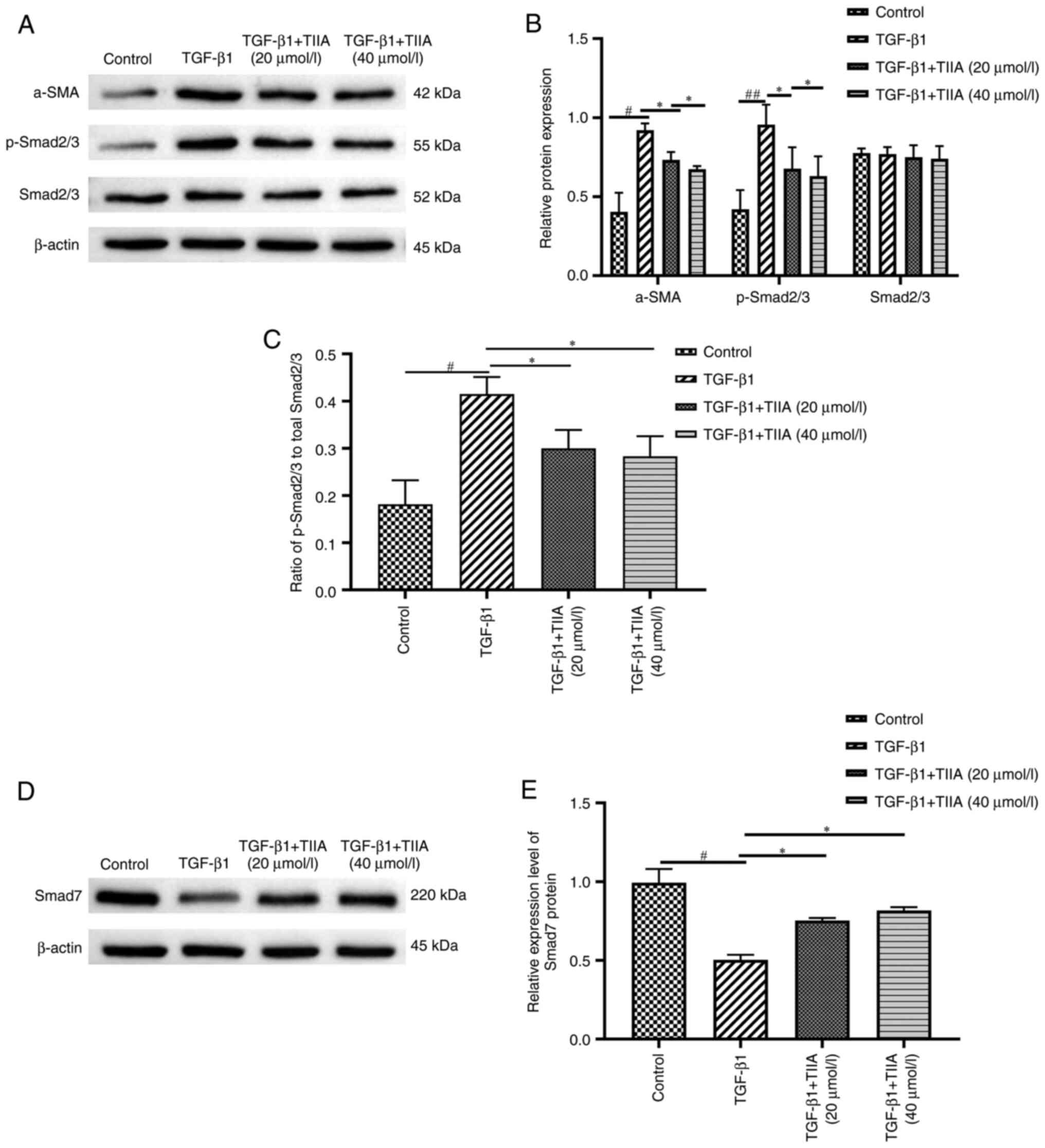 | Figure 8TIIA modulates protein expression of
the TGF-β1/Smad pathway molecules in vitro. (A) Western blot
analysis of α-SMA, p-Smad2/3 and Smad2/3 protein levels in LX-2
cells. Results were normalized relative to β-actin. (B)
Quantification of α-SMA [TGF-β1 vs. control, P=0.036; and TIIA vs.
TGF-β1, P=0.035 (20 µmol/l) or 0.013 (40 µmol/l), p-Smad2/3 (TGF-β1
vs. control, P=0.001; and TIIA vs. TGF-β1, P=0.027 (20 µmol/l) or
0.014 (40 µmol/l)] and Smad2/3 [TGF-β1 vs. control, P=0.781; and
TIIA vs. TGF-β1, P=0.783 (20 µmol/l) or 0.782 (40 µmol/l)] shown in
A. (C) Ratio of p-Smad2/3 over total Smad2/3 expression [TGF-β1 vs.
control, P=0.035; and TIIA vs. TGF-β1, P=0.025 (20 µmol/l) or 0.015
(40 µmol/l)]. (D) Western blot analysis of Smad7 protein levels in
LX-2 cells. Results were normalized relative to expression of
β-actin. (E) Quantification of Smad7 expression shown in C [TGF-β1
vs. control, P=0.018; and TIIA vs. TGF-β1, P=0.026 (20 µmol/l) or
0.021 (40 µmol/l)]. #P<0.05; ##P<0.01;
*P<0.05 (n=3). p, phosphorylated; TIIA, tanshinone
IIA; α-SMA, α-smooth muscle actin. |
Discussion
NASH is an important subtype of MAFLD. Its main
characteristic is liver inflammation and progressive fibrosis,
which eventually damages liver function and the patient's health
(27). Liver fibrosis is observed
in 37-84% of patients with NASH. thus, HF is the main predictor of
progression and death in these patients (28,29).
MCD-induced HF in rats is a well-established model of NASH-F,
characterized by elevated serum aminotransferase levels and liver
histological changes similar to the human NASH-F (30). To explore the effect of TIIA on
NASH-F, an MCD diet rat model of HF was used. According to previous
research, MCD diet can lead to abnormal liver metabolism,
significant fat accumulation and weight loss (31). In the present study, TIIA treatment
could partially prevent the MCD diet-induced weight loss observed
in NASH-F rats. Moreover, compared with the NASH-F group, the serum
of animals from the TIIA group had lower levels of the liver
function (AST, ALT, TBIL, TBA) and liver fibrosis (HA, LN, IV-C,
PC-III) indicators and higher levels of TC and TG. These
observations, combined with the respective pathology results,
indicated that TIIA could markedly improve the degree of
MCD-induced NASH-F in rats. The results further indicated that TIIA
may reduce liver injury and improve liver function by exerting an
anti-fibrotic effect.
HSCs and a small number of portal myofibroblasts are
activated in NASH-F, resulting in the production and accumulation
of ECM (32). Activation of HSCs
can cause an imbalance in ECM synthesis and degradation, and induce
collagen accumulation, thereby leading to extensive hyperplasia of
the fibrous tissue as well as high expression of α-SMA protein
(33,34). Therefore, HSCs are activated in the
resting state and become myofibroblasts, which is a key factor in
promoting the development of HF (35). In response to liver injury, HSCs
are stimulated to proliferate and are characterized by high
expression of α-SMA, which results from TGF-β1-stimulation that is
mediated by activated Kupffer cells (36). High α-SMA expression is a typical
feature of myofibroblasts, and its induction is the most reliable
marker of HSC activation, given that it is absent from other
resident liver cells in either normal or damaged liver, except for
smooth muscle cells surrounding large vessels (37).
TGF-β/Smad signaling is the classical pathway
leading to HSC activation. Upon stimulation with the major TGF
subtype (TGF-β1), the TGF-β1 ligand binds to surface receptors on
the HSCs, mediating their activation. The Smad2 and Smad3
downstream mediators are then recruited to TGF-β receptors and are
activated by phosphorylation (38). Smad2 and Smad3 mainly promote HF,
while Smad7 inhibits TGF-β1 signaling, thereby inhibiting HF
(39). Specifically, Smad7 is a
negative regulatory protein participating in the TGF-β/Smad
signaling pathway, which acts in a competitive fashion against
TGF-βRI to prevent phosphorylation of Smad2/3, thus inhibiting the
activation of the TGF-β/Smad cascade (40). The present study demonstrated that,
compared with the NASH-F group, TIIA markedly reduced α-SMA mRNA
and protein expression in the rat liver tissue, indicating that
TIIA could inhibit the activation of HSCs and reduce the synthesis
of ECM, thereby preventing the development of NASH-F. Furthermore,
as TGF-β1/Smad signaling is a key pathway leading to HF, the
mechanisms via which TIIA attenuates MCD-induced NASH-F was
investigated by examining the TGF-β1/Smad signaling pathway. The
results revealed that TIIA notably inhibited the expression of
TGF-β1 mRNA and protein levels, decreased the expression of Smad2
and Smad3 mRNA and p-Smad2/3 protein levels, and increased Smad7
mRNA and protein levels in the liver tissue, compared with the
NASH-F group. In vitro experiments further confirmed the
in vivo observations. Firstly, the cytotoxicity of different
concentrations of TIIA was examined in LX-2 cells and it was
revealed that 10, 20, 40 and 80 µmol/l TIIA did not affect the
viability of these cells. Subsequently, a cell-based experiment to
investigate the effect of TIIA on the TGF-β1/Smad signaling pathway
was performed by stimulating LX-2 cells with recombinant human
TGF-β1, while at the same time treating the cells with TIIA. The
results showed that the effects of TIIA on expression of the
TGF-β1/Smad signaling protein were the same both in vivo and
in vitro. Overall, these observations suggested that the
TGF-β1/Smad signaling pathway may be involved in the development of
NASH-F. The present study investigated the effect of TIIA on
TGF-β1/Smad signaling pathway in NASH-F rats without TGF-β
antibodies to neutralize bioactivity; this should be included in
future research.
In conclusion, the present study investigated the
effects of an anti-HF TCM. It also provided convincing evidence
demonstrating that TIIA can effectively prevent MCD-induced NASH-F,
improve the liver function and prevent the destruction of hepatic
tissue and fibrosis progression in a rat model of HF. Finally, the
present study demonstrated that TIIA exerted its effects by
downregulating the expression of TGF-β1 and p-Smad2/3 and by
increasing the expression of Smad7. These observations suggested
that TIIA may be useful in inhibiting or reversing progression of
NASH-F, and may offer the possibility of developing a novel
therapeutic drug for the treatment of chronic liver diseases.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant nos. 81960814, 81760818 and 82160898),
Science and Technology Program of Yunnan Science and Technology
Department [grant nos. 2018FF001(-006), 2019FF002(-079) and
202101AZ070001-042] and Yunnan Provincial Key Laboratory of
Molecular Biology for Sinomedicine (Yunnan University of
Traditional Chinese Medicine; grant no. 2019DG016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX conceived the study and wrote the manuscript. LX,
YZ, NJ and YD carried out the animal model construction and all the
animal experiments. LX, TJ, SW and WW acquired, analyzed and
interpreted the data and revised the final manuscript. SZ and WC
made substantial contributions to the conception and design of the
study. LX and SZ confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments of the current study were
approved by the Experimental Animal Ethics Committee of Yunnan
University of Traditional Chinese Medicine (Kunming, China;
approval no. R-062021021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eslam M, Newsome PN, Sarin SK, Anstee QM,
Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour
JF, Schattenberg JM, et al: A new definition for metabolic
dysfunction-associated fatty liver disease: An international expert
consensus statement. J Hepatol. 73:202–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arab JP, Arrese M and Trauner M: Recent
insights into the pathogenesis of nonalcoholic fatty liver disease.
Annu Rev Pathol. 13:321–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Powell EE, Wong VW and Rinella M:
Non-alcoholic fatty liver disease. Lancet. 397:2212–2224.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thibaut R, Gage MC, Pineda-Torra I,
Chabrier G, Venteclef N and Alzaid F: Liver macrophages and
inflammation in physiology and physiopathology of non-alcoholic
fatty liver disease. FEBS J: Apr 15, 2021 (Epub ahead of
print).
|
|
5
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang Y, Miao H, Yan H, Sheng Y and Ji L:
Hepatoprotective effect of forsythiae fructus water extract against
carbon tetrachloride-induced liver fibrosis in mice. J
Ethnopharmacol. 218:27–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu J, Kong D, Qiu J, Xie Y, Lu Z, Zhou C,
Liu X, Zhang R and Wang Y: Praziquantel ameliorates
CCl4-induced liver fibrosis in mice by inhibiting
TGF-β/Smad signalling via up-regulating Smad7 in hepatic stellate
cells. Br J Pharmacol. 176:4666–4680. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Walton KL, Johnson KE and Harrison CA:
Targeting TGF-β mediated SMAD signaling for the prevention of
fibrosis. Front Pharmacol. 8(461)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Karin D, Koyama Y, Brenner D and Kisseleva
T: The characteristics of activated portal
fibroblasts/myofibroblasts in liver fibrosis. Differentiation.
92:84–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Massagué J: TGF-β signalling in context.
Nat Rev Mol Cell Biol. 13:616–130. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Fabregat I, Moreno-Càceres J, Sánchez A,
Dooley S, Dewidar B, Giannelli G and Ten Dijke P: IT-LIVER
Consortium. TGF-β signalling and liver disease. FEBS J.
283:2219–2232. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Giannelli G, Mikulits W, Dooley S,
Fabregat I, Moustakas A, Ten Dijke P, Portincasa P, Winter P,
Janssen R, Leporatti S, et al: The rationale for targeting TGF-β in
chronic liver diseases. Eur J Clin Invest. 46:349–361.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yan C, Wang L, Li B, Zhang BB, Zhang B,
Wang YH, Li XY, Chen JX, Tang RX and Zheng KY: The expression
dynamics of transforming growth factor-β/Smad signaling in the
liver fibrosis experimentally caused by clonorchis sinensis.
Parasit Vectors. 8(70)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li B, Yan C, Wu J, Stephane K, Dong X,
Zhang YZ, Zhang Y, Yu Q and Zheng KY: Clonorchis sinensis ESPs
enhance the activation of hepatic stellate cells by a cross-talk of
TLR4 and TGF-β/Smads signaling pathway. Acta Trop.
205(105307)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
El Nashar EM, Alghamdi MA, Alasmari WA,
Hussein MMA, Hamza E, Taha RI, Ahmed MM, Al-Khater KM and
Abdelfattah-Hassan A: Autophagy promotes the survival of adipose
mesenchymal stem/stromal cells and enhances their therapeutic
effects in cisplatin-induced liver injury via modulating
TGF-β1/Smad and PI3K/AKT signaling pathways. Cells.
10(2475)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dooley S, Hamzavi J, Breitkopf K,
Wiercinska E, Said H, Lorenzen J, Ten Dijke P and Gressner AM:
Smad7 prevents activation of hepatic stellate cells and liver
fibrosis in rats. Gastroenterology. 125:178–191. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Choi JH, Jin SW, Choi CY, Kim HG, Lee GH,
Kim YA, Chung YC and Jeong HG: Capsaicin inhibits
dimethylnitrosamine-induced hepatic fibrosis by inhibiting the
TGF-β1/Smad pathway via peroxisome proliferator-activated receptor
gamma activation. J Agric Food Chem. 65:317–326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bai G, Yan G, Wang G, Wan P and Zhang R:
Anti-hepatic fibrosis effects of a novel turtle shell decoction by
inhibiting hepatic stellate cell proliferation and blocking
TGF-β1/Smad signaling pathway in rats. Oncol Rep. 36:2902–2910.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng Y, Yang T, Huang K, Shen L, Tao Y and
Liu C: Salvia miltiorrhiza ameliorates liver fibrosis by
activating hepatic natural killer cells in vivo and in vitro. Front
Pharmaco. 9(762)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yan Q, Mao Z, Hong J, Gao K, Niimi M,
Mitsui T and Yao J: Tanshinone IIA stimulates cystathionine γ-lyase
expression and protects endothelial cells from oxidative injury.
Antioxidants (Basel). 10(1007)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Z, Gao X, Jiao Y, Qiu Y, Wang A, Yu
M, Che F, Li S, Liu J, Li J, et al: Tanshinone IIA exerts
anti-inflammatory and immune-regulating effects on vulnerable
atherosclerotic plaque partially via the TLR4/MyD88/NF-κB signal
pathway. Front Pharmacol. 10(850)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Subedi L and Gaire BP: Tanshinone IIA: A
phytochemical as a promising drug candidate for neurodegenerative
diseases. Pharmacol Res. 169(105661)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bi Z, Wang Y and Zhang W: A comprehensive
review of tanshinone IIA and its derivatives in fibrosis treatment.
Biomed Pharmacother. 137(111404)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi MJ, Yan XL, Dong BS, Yang WN, Su SB
and Zhang H: A network pharmacology approach to investigating the
mechanism of Tanshinone IIA for the treatment of liver fibrosis. J
Ethnopharmacol. 253(112689)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun X, Tan Y, Lyu J, Liu HL, Zhao ZM and
Liu CH: Active components formulation developed from fuzheng huayu
recipe for anti-liver fibrosis. Chin J Integr Med: Sep 28, 2021
(Epub ahead of print).
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American association for the study of
liver diseases. Hepatology. 67:328–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wong VW, Wong GL, Choi PC, Chan AW, Li MK,
Chan HY, Chim AM, Yu J, Sung JJ and Chan HL: Disease progression of
non-alcoholic fatty liver disease: A prospective study with paired
liver biopsies at 3 years. Gut. 59:969–974. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Taylor RS, Taylor RJ, Bayliss S, Hagström
H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V,
Peleg N, et al: Association between fibrosis stage and outcomes of
patients with nonalcoholic fatty liver disease: A systematic review
and meta-analysis. Gastroenterology. 158:1611–1625.e12.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li R, Li J, Huang Y, Li J, Yan S, Lin J,
Chen Y, Wu L, Liu B, Wang G and Lan T: Polydatin attenuates
diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int
J Biol Sci. 14:1411–1425. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li X, Wang TX, Huang X, Li Y, Sun T, Zang
S, Guan KL, Xiong Y, Liu J and Yuan HX: Targeting ferroptosis
alleviates methionine-choline deficient (MCD)-diet induced NASH by
suppressing liver lipotoxicity. Liver Int. 40:1378–1394.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zisser A, Ipsen DH and Tveden-Nyborg P:
Hepatic stellate cell activation and inactivation in
NASH-fibrosis-roles as putative treatment targets? Biomedicines.
9(365)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Czaja AJ: Hepatic inflammation and
progressive liver fibrosis in chronic liver disease. World J
Gastroenterol. 20:2515–2532. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Eom YW, Shim K and Baik SK: Mesenchymal
stem cell therapy for liver fibrosis. Korean J Intern Med.
30:580–589. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Seki E and Brenner DA: Recent advancement
of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat
Sci. 22:512–518. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu S, Mao Y, Wu J, Feng J, Li J, Wu L, Yu
Q, Zhou Y, Zhang J and Chen J: TGF-β/Smad and JAK/STAT pathways are
involved in the anti-fibrotic effects of propylene glycol alginate
sodium sulphate on hepatic fibrosis. J Cell Mol Med. 24:5224–5237.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Friedman SL: Hepatic stellate cells:
Protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Z, Wang Z, Dong F, Shi W, Dai W, Zhao
J, Li Q, Fang ZE, Ren L, Liu T, et al: Germacrone attenuates
hepatic stellate cells activation and liver fibrosis via regulating
multiple signaling pathways. Front Pharmacol.
12(745561)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yu K, Li Q, Shi G and Li N: Involvement of
epithelial-mesenchymal transition in liver fibrosis. Saudi J
Gastroenterol. 24:5–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhou QY, Yang HM, Liu JX, Xu N, Li J, Shen
LP, Zhang YZ, Koda S, Zhang BB, Yu Q, et al: MicroRNA-497 induced
by clonorchis sinensis enhances the TGF-β/Smad signaling pathway to
promote hepatic fibrosis by targeting Smad7. Parasit Vectors.
14(472)2021.PubMed/NCBI View Article : Google Scholar
|