Introduction
Heart failure is the serious and terminal stage of
all kinds of cardiovascular diseases. There are one million cases
of chronic heart failure per year, with five-year mortality rates
as high as 50% (1). Delaying the
progression of heart failure and improving the quality of life are
the main treatment goals for patients with heart failure.
Sacubitril/valsartan is the first angiotensin II
receptor blocker neprilysin inhibitor (ARNI), which inhibits both
angiotensin II receptor and neprilysin (2). A series of clinical studies have
shown that sacubitril/valsartan has a good therapeutic effect on
cardiac function (3-5).
Sacubitril/valsartan was approved by the US Food and Drug
Administration in 2015 and was recommended for patients with heart
failure by American College of Cardiology/American Heart
Association/Heart Failure Society of America (6). The European Society of Cardiology
(ESC) also recommends ARNI as an alternative to
angiotensin-converting enzyme inhibitor (ACEI) for patients with
persistent symptoms despite treatment with an ACEI, β-blocker and
an angiotensin receptor blocker (ARB) or aldosterone receptor
antagonist. It can reduce hospitalization and mortality (7,8).
There are few studies about the effect of
sacubitril/valsartan on patients during the perioperative period of
cardiac surgery. The present study focused on patients during the
perioperative period of cardiac surgery with consideration of the
comorbidities and concomitant medication of the patient. To explore
the effect of sacubitril/valsartan on cardiac function for patients
during cardiac perioperative period patients were divided into two
groups: heart failure with reduced ejection fraction (HFrEF) group
and heart failure with preserved ejection fraction (HFpEF) group.
The present study was conducted to investigate the therapeutic
effect of taking sacubitril/valsartan in patients with HFREF and
HFPEF during cardiac perioperative period.
Materials and methods
The present study was an observational self-control
study. The patients with heart failure who underwent cardiac
surgery were recruited from Beijing Anzhen Hospital (Beijing,
China). All patients were treated with sacubitril/valsartan during
the perioperative period (within 7 days prior to and following the
surgery). The variations of left ventricular ejection fraction
(LVEF) and left ventricular end-diastolic diameter (LVED) were
evaluated at least 28 days after treatment. A total of 59 patients
with heart failure between May 2019 and November 2020, were
included in the present study (Table
I). Patients who met the following inclusion criteria were
eligible: i) ≥18 years old; ii) initiation of sacubitril/valsartan
therapy during the cardiac perioperative period (within 7 days
prior to and following the surgery); iii) LVEF <50 or LVEF ≥50
and B-type natriuretic peptide (BNP) >35 pg/ml and/or N-terminal
B-type natriuretic peptide (NT-proBNP) >125 pg/ml and any of the
following: Related structural heart disease and/or diastolic
dysfunction; current symptomatic heart failure. The major exclusion
criteria were: i) patients succumbed during hospitalization; ii)
duration of sacubitril/valsartan treatment <28 days; iii) lack
of echocardiography prior to and following treatment of
sacubitril/valsartan. According to the value of LVEF, 31 patients
with LVEF<50% were divided into HFrEF group and 28 patients with
LVEF ≥50% into HFrEF group.
 | Table IBaseline characteristics of the study
population. |
Table I
Baseline characteristics of the study
population.
| Characteristic | Total | HFrEF | HFpEF |
|---|
| Cases, n | 59 | 31 | 28 |
| Mean age ± SD,
years | 56.3±12.8 | 55.3±12.9 | 57.1±12.8 |
| Sex, n (%) | | | |
|
Male | 44 (74.6) | 25 (80.6) | 19 (67.9) |
|
Female | 15 (25.4) | 6 (19.4) | 9 (32.1) |
| Mean body-mass index
± SD, kg/m2 | 24.7±3.1 | 24.7±2.9 | 24.7±3.4 |
| Medical history, n
(%) | | | |
|
Valvular
disease | 45 (76.3) | 19 (61.3) | 26 (92.9) |
|
Hypertension | 28 (47.4) | 20 (64.5) | 8 (28.5) |
|
Coronary
heart disease, n (%) | 27 (45.7) | 20 (64.5) | 7 (25.0) |
|
Hyperlipidemia | 15 (25.4) | 11 (35.4) | 4 (14.2) |
|
Diabetes
mellitus | 10 (16.9) | 7 (22.5) | 3 (10.7) |
|
Atrial
fibrillation | 9 (15.2) | 4 (12.9) | 5 (17.8) |
|
Renal
dysfunction | 4 (6.7) | 3 (9.6) | 1 (3.5) |
| NYHA functional
class, n (%) | | | |
|
II | 16 (27.1) | 4 (12.9) | 12 (42.8) |
|
III | 34 (57.6) | 21 (67.7) | 13 (46.4) |
|
IV | 9 (15.2) | 6 (19.3) | 3 (10.7) |
| Smoker, n (%) | 26 (44.1) | 14 (45.2) | 12 (42.9) |
| Drinker, n (%) | 18 (30.6) | 10 (32.3) | 8 (28.6) |
| History of PCI, n
(%) | 5 (8.4) | 5 (16.1) | 0 (0.0) |
| Concomitant
medication, n (%) | | | |
|
Loop
diuretics | 56 (94.9) | 29 (93.5) | 27 (96.4) |
|
β-blockers | 35 (59.3) | 22 (70.9) | 13 (46.4) |
|
Digoxin | 21 (35.5) | 11 (35.4) | 10 (35.7) |
|
Spironolactone | 12 (20.3) | 10 (32.2) | 2 (7.1) |
|
ARB | 3 (5.0) | 0 (0.0) | 3 (10.7) |
|
ACEI | 1 (1.6) | 0 (0.0) | 1 (3.5) |
| Laboratory
findings | | | |
|
Cr,
µmol/la | 76.8 (63.9,92.4) | 77.0 (66.5,95.8) | 75.1 (61.8,89.0) |
|
BUN,
mmol/la | 6.8 (5.3,9.0) | 6.5 (5.32,11.1) | 7.0 (5.4,9.0) |
|
UA,
µmol/l | 172.0±97.7 | 161.0±105.0 | 184.2±88.9 |
|
BNP,
pg/mla | 358.0
(137.8,759.5) | 470.0
(152.0,782.0) | 189.0
(65.0,688.0) |
|
AST,
U/la | 22.0
(17.0,38.0) | 23.0
(17.0,33.0) | 21.5
(18.0,38.8) |
|
ALT,
U/la | 19.0
(14.0,35.0) | 20.0
(14.0,51.0) | 18.5
(12.0,32.8) |
| Follow-up
duration/daya | 107 (90.5,157) | 109.5
(90.3,158) | 106 (90.3,158) |
The medicine was sacubitril/valsartan tablets
(Novartis International AG) with a range of dosage of 25-200 mg.
The present study had been approved by the Medical Clinical
Research Ethics Committee of Beijing Anzhen Hospital (approval no.
201808) and patient privacy was protected. All patients signed
informed consent before they were recruited. The length of
follow-up was 28 days or longer by phone, email, WeChat and return
visit. Baseline characteristics, liver and kidney function, BNP and
echocardiogram results of the study population were recorded. The
therapeutic effect on patients with sacubitril/valsartan was
assessed by the values of LVEF and LVED, which were the primary
endpoints. The renal safety of patients was assessed by serum
creatinine (Cr) and blood urea nitrogen (BUN), which were the
secondary endpoints.
Statistical analysis
Statistical analysis was performed with SPSS17.0
software (SPSS, Inc.). Normal and continuous variables were
expressed as mean ± standard deviation. Data were compared by using
paired t-test across the groups. Non-normal variables were
expressed as quartiles. Count data were expressed as percentages.
In the subgroup analysis stratified by concomitant medication, the
values of variations in LVEF and LVED prior to and following
treatment were compared. The values were sampled 1,000 times and
the results were shown as average and 95% confidence interval (CI).
The differences between subgroups were compared using unpaired
t-test and expressed by Pinteraction. P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline characteristics of the study
population
According to the inclusion and exclusion criteria, a
total of 59 patients were included, 31 in HFrEF group and 28 in
HFpEF group. The participants were between 20 and 80 years old with
a mean age of 56.3±12.8 years. A total of 44 patients (74.5%) were
male and the average BMI was 24.7±3.1 kg/m2. As shown in
Table I, concomitant medication
included diuretics, digoxigenin and antihypertensive drugs. 56
(94.9%) patients were given a combination of loop diuretics; 35
(59.3%) patients were given a combination of β-blocker. A total of
45 (76.3%) patients had valvular disease including valvular
insufficiency, valve prolapse and valvular stenosis, 28 (47.4%)
patients had hypertension; 27 (45.7%) patients had coronary heart
disease. Other complications including hypertension, coronary heart
disease, type 2 diabetes mellitus, hyperlipidemia, atrial
fibrillation and renal dysfunction were shown in Table I.
Baseline characteristics of laboratory examination
of patients were collected. Renal function of patients was
evaluated by Cr, BUN and uric acid (UA); liver function was
evaluated by alanine aminotransferase (ALT) and aspartate
aminotransferase (AST). Cr, BUN, UA values and follow-up duration
were non-normally distributed, which were expressed by median and
quartile.
As shown in Table
II, the types of cardiac surgery patients underwent included
heart valve replacement, coronary-artery-bypass-grafting, heart
valvuloplasty, Bentall and transcatheter aortic valve
implantation.
 | Table IITypes of cardiac surgery. |
Table II
Types of cardiac surgery.
| Type of cardiac
surgery | Number of
patients | Percentage |
|---|
| HVR | 15 | 25.42 |
| CABG | 13 | 22.03 |
| HVP | 9 | 15.26 |
| Bentall | 8 | 13.56 |
| HVR + CABG | 4 | 6.78 |
| TAVI | 4 | 6.78 |
| Others | 4 | 6.78 |
| Bentall + CABG | 2 | 3.39 |
| Total | 59 | 100.00 |
As shown in Table
III, in the present study, the maintenance dose of
sacubitril/valsartan was 100 mg bid in 37 (62.7%) patients and 50
mg bid in 11 (18.6%) patients.
 | Table IIIDosage of sacubitril/valsartan. |
Table III
Dosage of sacubitril/valsartan.
| Medication
dose | Total (n=59) | HFrEF (n=31) | HFpEF (n=28) |
|---|
| Initial dose in
mg/day, n (%) | | | |
|
200 | 9 (15.2) | 4 (12.9) | 5 (17.8) |
|
100 | 39 (66.1) | 21 (67.7) | 18 (64.2) |
|
50 | 10 (16.9) | 5 (16.1) | 5 (17.8) |
|
25 | 1 (1.6) | 1 (3.2) | 0 (0.0) |
| Maintenance dose in
mg/day, n (%) | | | |
|
200 | 10 (16.9) | 5 (16.1) | 5 (17.8) |
|
150 | 1 (1.6) | 1 (3.2) | 0 (0.0) |
|
100 | 37 (62.7) | 19 (61.2) | 18 (64.2) |
|
50 | 11 (18.6) | 6 (19.3) | 5 (17.8) |
Sacubitril/valsartan and LVEF and
LVED
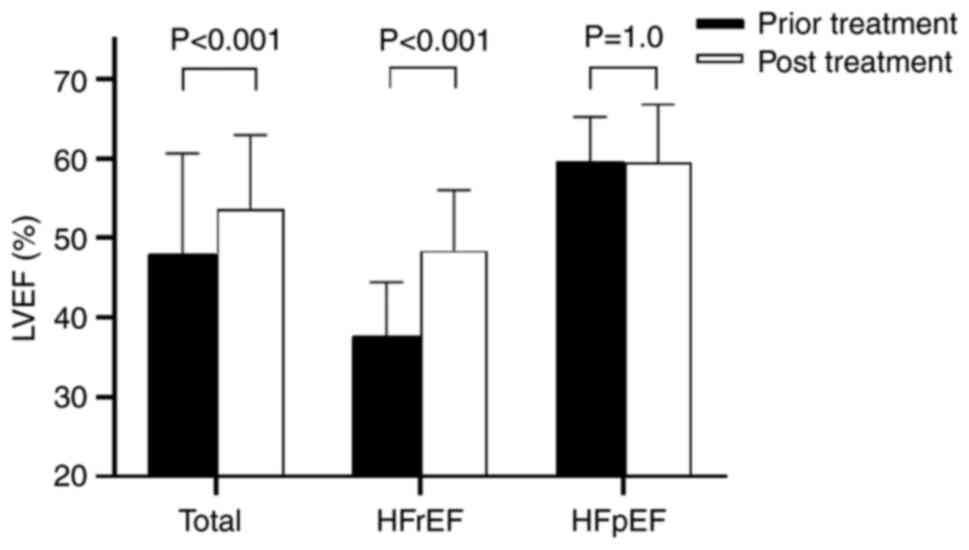
As shown in Fig. 1,
the LVEF showed an increase of 5.5% (P<0.001) in overall
patients; the LVEF increased by 10.7% (P<0.001) in the HFrEF
group; there was no significant difference of LVEF in HFpEF group.
The results suggested that sacubitril/valsartan could increase
significantly values of LVEF and improve cardiac function of
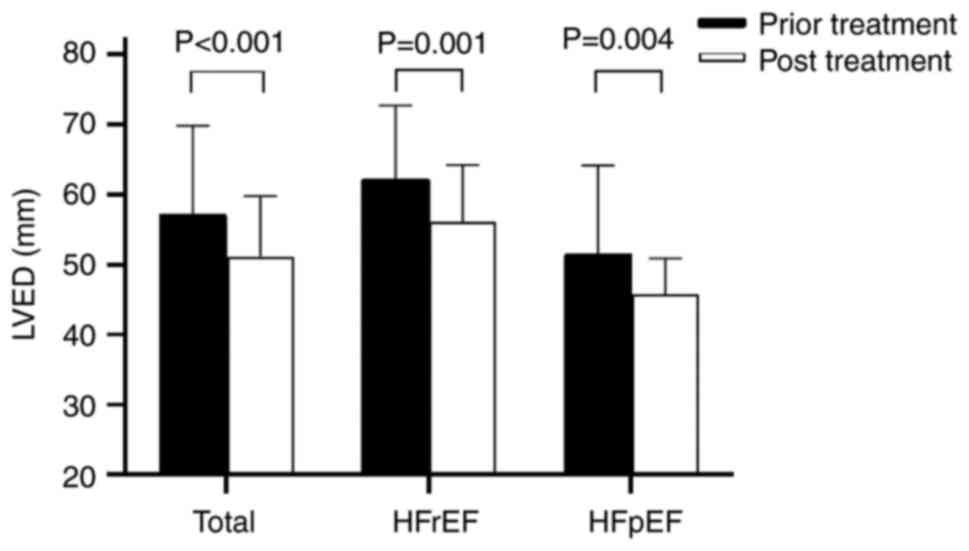
patients in HFrEF group. As shown in Fig. 2, the changes in LVEF and LVED were
analyzed. The LVED decreased by 10.8 mm (P=0.001) in HFrEF group;
the LVED results decreased by 10.4 mm (P=0.004) in HFpEF group. The
sizes of left ventricular could be significantly decreased by
sacubitril/valsartan in both groups, which indicated that
sacubitril/valsartan could reverse ventricular remodeling and
improve cardiac function.
Sacubitril/valsartan and concomitant
medication
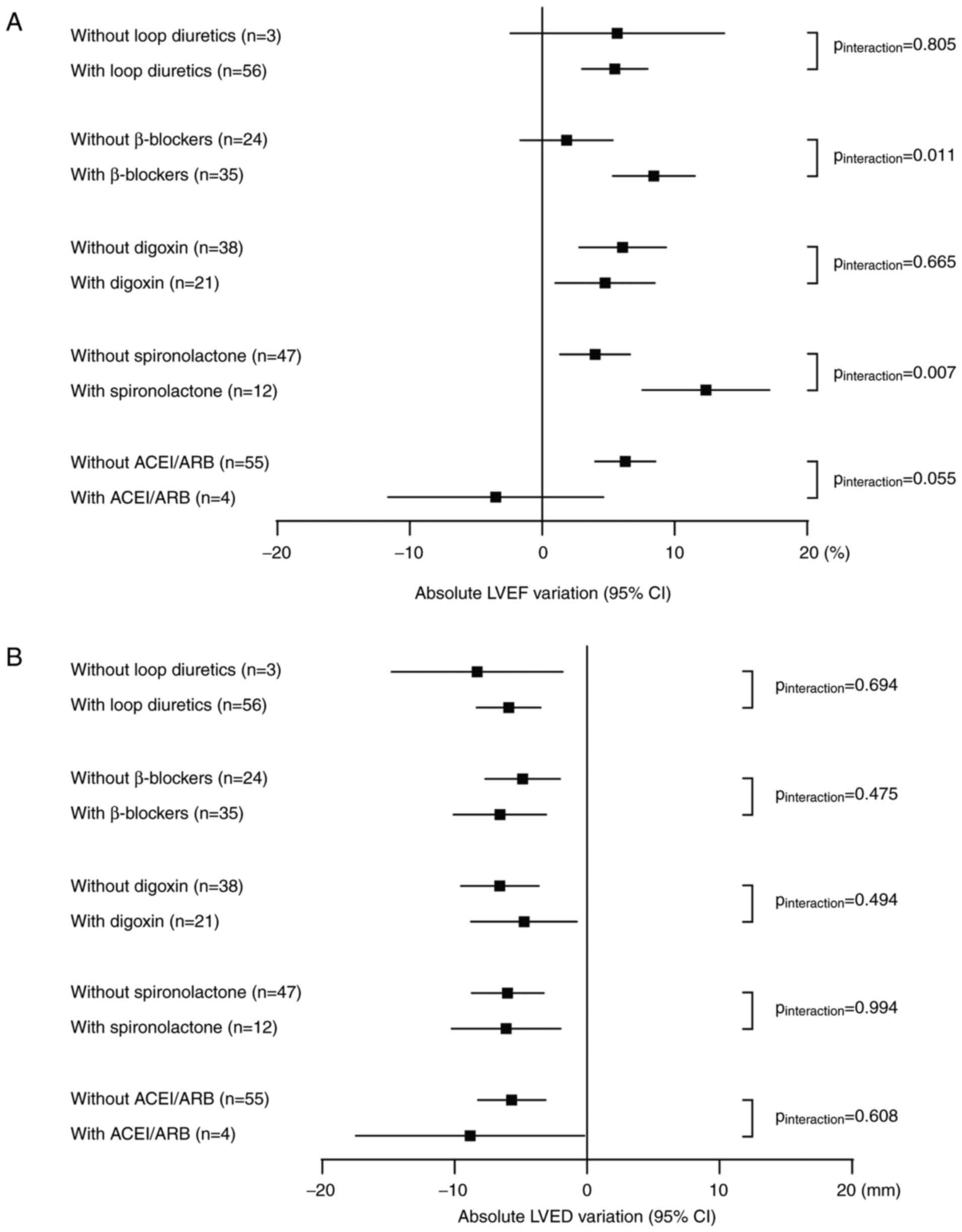
As shown in Fig.
3A, according to the subgroup analysis stratified by the
concomitant medication, the results showed that the combination of
loop diuretics, digoxin and ACEI/ARB had no significant effect on
the variation of LVEF. The combination of β-blocker and
spironolactone could further improve LVEF of patients on the basis
of sacubitril/valsartan (Pinteraction=0.011 and
Pinteraction=0.007). In addition, whether patients were
treated with loop diuretics, β-blockers, digoxin, spironolactone
and ACEI/ARB or not, the LVED of patients decreased significantly.
And there was no significant difference in variation of LVED with
or without concomitant medication, as shown in Fig. 3B.
Sacubitril/valsartan and renal
function
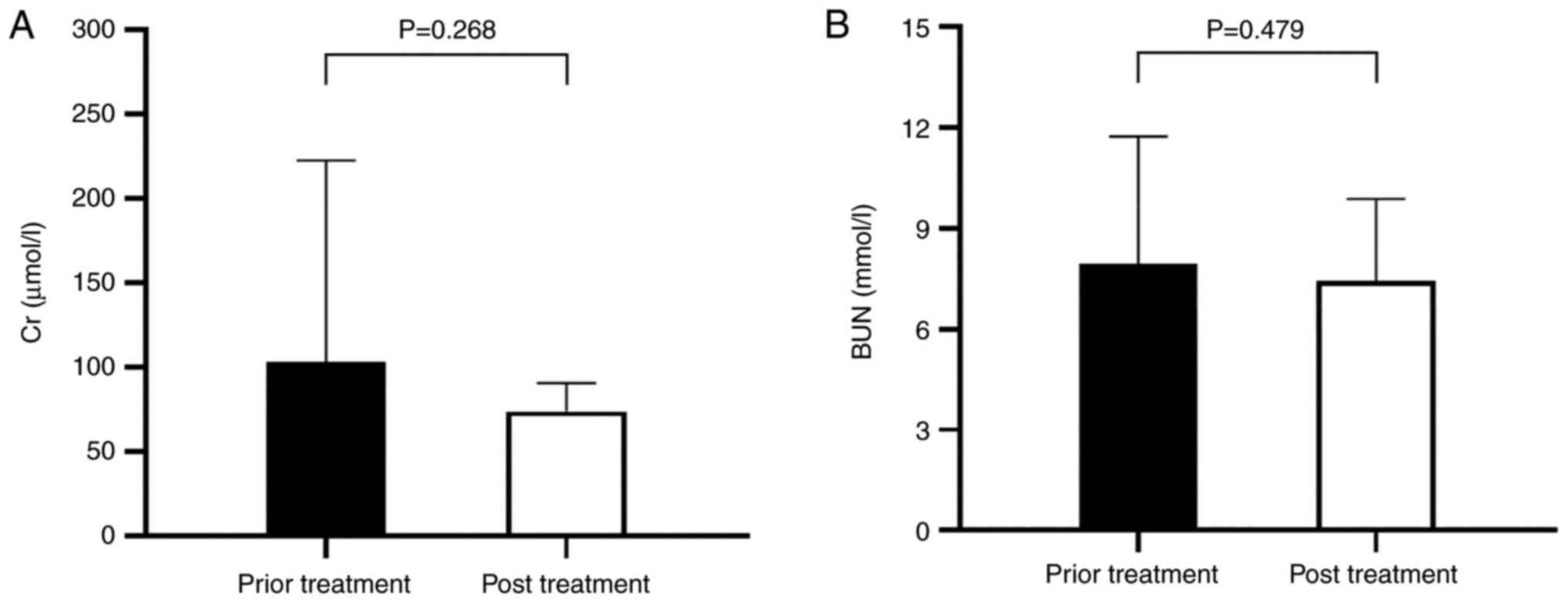
As shown in Fig. 4,
compared with prior treatment, the changes in Cr and BUN were no
statistical significance (PCr=0.95,
PBUN=0.55). There were no significant changes in renal
function, which indicated that sacubitril/valsartan had little
effect on renal function.
Discussion
The present study included patients with cardiac
dysfunction during the cardiac perioperative period, including
HFrEF group (31 cases) and HFpEF group (28 cases). HFrEF was
generally caused by impaired ventricular contractile function, with
eccentric cardiac hypertrophy and ventricle volume overload. HFpEF
was generally caused by impaired ventricular diastolic function
with increased ventricular stiffness, elevated left ventricular
filling pressure, concentric cardiac hypertrophy and ventricular
hypertrophy (9).
The primary indicators were the variations of LVEF
and LVED in echocardiography following sacubitril/valsartan
therapy. LVEF is the ratio of heart stroke volume (SV) to left
ventricular end-diastolic volume (EDV) and reflects the systolic
function of the left ventricle. LVEF is the commonest parameter to
evaluate cardiac function in patients with heart failure. Patients
with heart failure were divided into three groups by LVEF including
heart failure with preserved ejection fraction (≥50%), heart
failure with mid-range ejection fraction (40-49%) and heart failure
with reduced ejection fraction (<40%). The HFrEF group in the
present study included patients with heart failure mid-range
ejection fraction and heart failure with reduced ejection fraction.
The HFpEF group in the present study were the patients with
preserved ejection fraction defined by ESC. A Meta-analysis Global
Group in Chronic Heart Failure (10) collated the data from 39,372
patients data from 31 studies which showed LVEF was associated with
the prognosis of heart failure and the lower the LVEF, the higher
the risk of all-cause death. However, when LVEF >50%, there was
no significant association between LVEF and mortality (10,11).
LVED can reflect the size of the left ventricle and the degree of
myocardial remodeling. A study by Tognon et al (12) found that the degree of left
ventricular enlargement was significantly correlated with the
decline of heart function and the increase of NT-pro BNP.
Therefore, in the present study, the variations of LVEF and LVED
were regarded as the primary indicators for evaluating the
therapeutic effect of sacubitril/valsartan on cardiac function.
The present study showed that the LVEF results of
patients in HFrEF group increased significantly following
sacubitril/valsartan treatment (P<0.001) and the LVED decreased
significantly (P=0.001). The results suggested that
sacubitril/valsartan could significantly increase the cardiac
ejection function in patients with heart failure during
perioperative period of cardiac surgery and reverse ventricular
remodeling. Following sacubitril/valsartan treatment, there was no
significant change of the LVEF value in patients of HFpEF group,
but the LVED decreased significantly (P=0.001). These results
suggested that sacubitril/valsartan could significantly decrease
the size of the ventricle in patients with heart failure during
perioperative period of cardiac surgery, reverse ventricular
remodeling and improve cardiac function.
A large, randomized, double-blind, multicenter
PARADIGM-HF study compared the efficacy of sacubitril/valsartan and
enalapril in patients with HFrEF (13). The study included patients with New
York Heart Association (NYHA) Class ~II-IV heart failure, with an
LVEF ≤40% and BNP ≥150 pg/ml or NT-pro BNP ≥600 pg/ml. The primary
outcome was a composite of mortality from cardiovascular causes or
hospitalization for heart failure. As compared with enalapril,
sacubitril/valsartan reduced the risk of hospitalization for heart
failure by 21% (incidence of the composite endpoint 21.8% compared
with 26.5%; hazard ratio in the sacubitril/valsartan group, 0.80;
95% CI, 0.73-0.87; P<0.001) and the results showed that
sacubitril/valsartan did not increase the incidence of renal
dysfunction, hyperkalemia and cough in patients, but the incidence
of symptomatic hypotension increased (13-16).
There was still a high mortality rate for patients
with HFpEF. However, there was a lack of effective treatments for
patients with HFpEF. A randomized, double-blind, multicenter phase
II clinical trial PARAGON-HF study compared the efficacy of
sacubitril/valsartan and valsartan in patients with HFpEF (17). The study included patients with
NYHA Class II~IV heart failure, ejection fraction of ≥45%, elevated
level of natriuretic peptides and structural heart disease to
receive sacubitril/valsartan or valsartan. Compared with valsartan,
the difference was not statistically significant (rate ratio, 0.87;
95% CI, 0.75-1.01; P=0.06). NYHA class improved in the
sacubitril/valsartan group which was higher compared with the
valsartan group (15% compared with 12.6%, odds ratio, 1.45; 95% CI,
1.13-1.86) and deterioration of renal function in
sacubitril/valsartan group was lower (1.4% compared with 2.7%,
hazard ratio, 0.50; 95% CI, 0.33-0.77). There was no significant
difference in heart failure hospitalization and cardiovascular
mortality between the two groups (17,18).
The existing studies of sacubitril/valsartan were
mainly on ischemic cardiomyopathy. In the sacubitril/valsartan
group in PARADIGM-HF study, 59.9% of patients had ischemic
cardiomyopathy and 43.4% had a history of myocardial infarction,
without a history of valvular disease. In the sacubitril/valsartan
group in PARAGON-HF study, 37.4% of patients had ischemic
cardiomyopathy and 23.3% were hospitalized for myocardial
infarction, without a history of valvular disease. In the present
study, 45.7% of patients had coronary heart disease and 76.3% had a
history of valvular disease. The present study mainly focused on
the therapeutic effect of sacubitril/valsartan in patients with
heart failure during perioperative period.
There are no reports on the treatment of
sacubitril/valsartan in patients during cardiac perioperative
period, to the best of the authors' knowledge. The population in
the present study were patients within 7 days before and after the
cardiac surgery. The results suggested that sacubitril/valsartan
might serve a role in patients with cardiac insufficiency. Of 27.1%
of patients with class (NYHA) II heart failure, 67.7% of patients
with class (NYHA) III heart failure and 15.2% of patients with
class (NYHA) IV heart failure were included in the present study.
However, 71.6% of patients with class (NYHA) II heart failure in
sacubitril/valsartan group, 23.1% of patients with class (NYHA) III
heart failure and 0.8% of patients with class (NYHA) IV heart
failure were included in PARADIGM-HF study. In total, 77.5% of
patients with class (NYHA) II heart failure in sacubitril/valsartan
group, 19.0% of patients with class (NYHA) III heart failure and
0.3% of patients with class (NYHA) IV heart failure were included
in PARAGON-HF study. Most patients in the present study were class
(NYHA) III heart failure and had the worse cardiac function. The
patients usually had low blood pressure following cardiac surgery,
so the initial dose of the sacubitril/valsartan was restricted. The
low maintenance dose of sacubitril/valsartan might be associated
with the lack of poor compliance and regular follow-up. Therefore,
was difficult to reach the target dose of sacubitril/valsartan of
200 mg bid. Only 11 (18.6%) patients finally reached the target
dose in the present study. The LVEF and LVED improved
significantly, even if the lower dose might not fully reflect the
efficacy of sacubitril/valsartan. The lower dose of
sacubitril/valsartan might have a certain therapeutic effect on
heart failure.
Sacubitril/valsartan decomposes to sacubitril and
valsartan in body. Valsartan is a selective AT 1 blocker that
inhibits angiotensin-II-dependent aldosterone release (19). Therefore, other medications that
inhibit the renin aniotension aldosterone system should avoid
combining with sacubitril/valsartan, which increases the risk of
angioedema without improving outcomes (20,21).
There were three (5.0%) patients in the present study combined with
ARB and one (1.6%) patient combined with ACEI. This combination
should be avoided as much as possible to reduce the risk of
angioedema in patients. No significant drug interactions were
observed between sacubitril/valsartan and other commonly used
medicines for heart failure and there was no induction or
inhibition of Cytochrome P450 (CYP450) (22,23).
In the present study, subgroup analysis was conducted considering
the effect of concomitant medication on the results. Diuretics and
β-blockers are first-line drugs for the treatment of heart failure
(24,25). The results showed that there were
only significant differences in variation of LVEF when combined
with β-blocker and spironolactone, while the combination of other
drugs had no significant effect on the variation of LVEF and LVED.
These results did not reverse the conclusions of the whole study,
which suggested that β-blocker and spironolactone could further
improve LVEF of patients on the basis of sacubitril/valsartan. As
for the weight of each medication for the treatment of patients
with heart failure, further large sample studies are still
needed.
Due to the blood pressure-lowering effect of
sacubitril/valsartan, it might cause symptomatic hypotension for
patients with low baseline blood pressure. In the present study,
all patients started taking sacubitril/valsartan during their
hospitalization and there were no drug disruptions due to cough,
angioedema and hyperkalemia. On the other hand,
sacubitril/valsartan had a certain effect on renal function. In the
PARADIGM-HF study, 3.3% of Cr values increased by 2.5 mg/dl and
1.5% by 3.0 mg/dl in patients. There were fewer patients in the
LCZ696 group compared with the enalapril group who interrupted
treatment because of renal impairment (0.7% vs. 1.4%; P=0.002).
Overall, there was no significant effect on Cr and BUN in the
present study.
There were several limitations in the present study:
i) The study was a single-center study with a small sample and
lacked a randomized control group; ii) there might be confounding
factors in the effect of the medicine, because the cardiac surgery
itself might also affect the cardiac function of patients; iii) the
bias from other concomitant medications for heart failure might
affect the results of the study, thus a stratified analysis was
conducted based on each combination to minimize these biases; iv)
the maintenance dose of sacubitril/valsartan was low due to
postoperative hypotension, poor compliance, therefore it was
difficult to reach the target dose of sacubitril/valsartan of 200
mg bid and the low dose might not fully reflect the efficacy of
sacubitril/valsartan; and v) due to the small sample size, the
weight of each medication for the treatment of patients with heart
failure and the effect of different cardiac surgeries on the
results were not accessed. Therefore, large sample studies are
needed in the future.
The present study explored the therapeutic effect of
sacubitril/valsartan on patients with cardiac insufficiency during
the perioperative period. The results showed that
sacubitril/valsartan could increase the LVEF of patients with
HFrEF, reduce the LVED of all patients and improve the heart
function of patients. The results also indicated that there were
few side effects on renal function. Furthermore, therapeutic
effects were observed even with the lower dose of
sacubitril/valsartan. Finally, the present study indicated that
sacubitril/valsartan had a therapeutic effect on patients with
cardiac insufficiency during the perioperative period, with
relatively good tolerance.
Acknowledgements
The authors wish to acknowledge Dr Bo Yang and Dr
Huanyu Qiao (Department of Cardiac Surgery, Beijing Anzhen
Hospital, Capital Medical University) for their assistance in
patient enrollment.
Funding
Funding: The present study was supported in part by Beijing
Municipal Administration of Hospitals Clinical Medicine Development
of Special Funding Support (grant no. ZYLX201805), National Major
Scientific and Technological Special Project for Significant New
Drugs Development during the Thirteenth five-year Plan Period
(grant no. 2017ZX09304017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WP designed the study and participated in the data
analysis and manuscript writing. XL participated in analysis and
interpretation of data, drafting the manuscript and manuscript
writing. YL participated in writing and revising the article, and
contributed to the conception of the study. WP and YL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study had been approved by the Medical Clinical
Research Ethics Committee of Beijing Anzhen Hospital and patient
privacy was protected (approval no. 201808). All patients provided
written informed consent before they were recruited.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Braunwald E: The path to an angiotensin
receptor antagonist-neprilysin inhibitor in the treatment of heart
failure. J Am Coll Cardiol. 65:1029–1041. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khder Y, Shi V, McMurray JJV and Lefkowitz
MP: Sacubitril/valsartan (LCZ696) in heart failure. Handb Exp
Pharmacol. 243:133–165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Solomon SD, Rizkala AR, Gong J, Wang W,
Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, et al:
Angiotensin receptor neprilysin inhibition in heart failure with
preserved ejection fraction: rationale and design of the PARAGON-HF
trial. JACC Heart Fail. 5:471–482. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Barghash MH and Desai AS: First-in-class
composite angiotensin receptor-neprilysin inhibitors (ARNI) in
practice. Clin Pharmacol Ther. 102:265–268. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Mann DL, Greene SJ, Givertz MM, Vader JM,
Starling RC, Ambrosy AP, Shah P, McNulty SE, Mahr C, Gupta D, et
al: Sacubitril/valsartan in advanced heart failure with reduced
ejection fraction: Rationale and design of the LIFE trial. JACC
Heart Fail. 8:789–799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey Jr DE, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, et
al: 2016 ACC/AHA/HFSA focused update on new pharmacological therapy
for heart failure: An update of the 2013 ACCF/AHA guideline for the
management of heart failure: A report of the american college of
cardiology/american heart association task force on clinical
practice guidelines and the heart failure society of america. J Am
Coll Cardiol. 68:1476–1488. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure: The task force for
the diagnosis and treatment of acute and chronic heart failure of
the european society of cardiology (ESC)developed with the special
contribution of the heart failure association (HFA) of the ESC. Eur
Heart J. 37:2129–2200. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Russo-Vorms L, Meyer P and Reny JL: [«
ARNI » (Angiotensin Receptor-Neprilysin Inhibitor): When, for whom
and how?]. Rev Med Suisse. 15:1882–1886. 2019.PubMed/NCBI(In French).
|
|
9
|
Tanai E and Frantz S: Pathophysiology of
heart failure. Compr Physiol. 6:187–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pocock SJ, Ariti CA, McMurray JJ, Maggioni
A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA,
et al: Predicting survival in heart failure: A risk score based on
39 372 patients from 30 studies. Eur Heart J. 34:1404–1413.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mele D, Nardozza M and Ferrari R: Left
ventricular ejection fraction and heart failure: An indissoluble
marriage? Eur J Heart Fail. 20:427–430. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tognon AP, Foppa M, Luft VC, Chambless LE,
Lotufo P, El Aouar LM, Fernandes LP and Duncan BB: Reproducibility
of left ventricular mass by echocardiogram in the ELSA-Brasil. Arq
Bras Cardiol. 104:104–111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
McMurray JJ, Packer M, Desai AS, Gong J,
Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg
K, et al: Angiotensin-neprilysin inhibition versus enalapril in
heart failure. N Engl J Med. 371:993–1004. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lewis EF, Claggett BL, McMurray JJV,
Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai
AS, et al: Health-related quality of life outcomes in PARADIGM-HF.
Circ Heart Fail. 10(e003430)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Böhm M, Young R, Jhund PS, Solomon SD,
Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K,
et al: Systolic blood pressure, cardiovascular outcomes and
efficacy and safety of sacubitril/valsartan (LCZ696) in patients
with chronic heart failure and reduced ejection fraction: Results
from PARADIGM-HF. Eur Heart J. 38:1132–1143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Velazquez EJ, Morrow DA, DeVore AD,
Ambrosy AP, Duffy CI, McCague K, Hernandez AF, Rocha RA and
Braunwald E: Rationale and design of the comParIson Of
sacubitril/valsartaN versus enalapril on effect on nt-pRo-bnp in
patients stabilized from an acute heart failure episode
(PIONEER-HF) trial. Am Heart J. 198:145–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Solomon SD, McMurray JJV, Anand IS, Ge J,
Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B,
et al: PARAGON-HF investigators and committees.
Angiotensin-neprilysin inhibition in heart failure with preserved
ejection fraction. N Engl J Med. 381:1609–1620. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Solomon SD, Zile M, Pieske B, Voors A,
Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J,
et al: The angiotensin receptor neprilysin inhibitor LCZ696 in
heart failure with preserved ejection fraction: A phase 2
double-blind randomised controlled trial. Lancet. 380:1387–1395.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Książczyk M and Lelonek M: Angiotensin
receptor/neprilysin inhibitor-a breakthrough in chronic heart
failure therapy: Summary of subanalysis on PARADIGM-HF trial
findings. Heart Fail Rev. 25:393–402. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chng BLK, Hon JS, Chan H, Zheng Y, Gao F,
Teo LYL and Sim KLD: Safety and tolerability of
sacubitril/valsartan initiation in inpatient versus outpatient
setting: A retrospective real world study. Heart Lung Circ.
30:674–682. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dani SS, Ganatra S and Vaduganathan M:
Angioedema with sacubitril/valsartan: Trial-level meta-analysis of
over 14,000 patients and real-world evidence to date. Int J
Cardiol. 323:188–191. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ayalasomayajula S, Langenickel T, Pal P,
Boggarapu S and Sunkara G: Clinical pharmacokinetics of
sacubitril/valsartan (LCZ696):A novel angiotensin
receptor-neprilysin inhibitor. Clin Pharmacokinet. 56:1461–1478.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Flarakos J, Du Y, Bedman T, Al-Share Q,
Jordaan P, Chandra P, Albrecht D, Wang L, Gu H, Einolf HJ, et al:
Disposition and metabolism of ((14)C) Sacubitril/Valsartan
(formerly LCZ696) an angiotensin receptor neprilysin inhibitor, in
healthy subjects. Xenobiotica. 46:986–1000. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Writing Committee Members and ACC/AHA
Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the
management of heart failure. J Card Fail. 28:e1–e167.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Authors/Task Force Members. McDonagh TA,
Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler
J, Čelutkienė J, et al: 2021 ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure: Developed by the task
force for the diagnosis and treatment of acute and chronic heart
failure of the european society of cardiology (ESC). With the
special contribution of the heart failure association (HFA) of the
ESC. Eur J Heart Fail. 24:4–131. 2022.PubMed/NCBI View Article : Google Scholar
|