Introduction
Homocysteine (HCY) is an important intermediate
product of methionine and cysteine metabolism. HCY is normally
metabolized in vivo and the HCY concentration is usually low
(1). However, MTHFR gene mutation
or decreased enzyme activity, as well as lack of folic acid,
vitamin B6, vitamin B12 and other factors may affect HCY
metabolism, which can lead to hyperhomocysteinaemia (HHCY)
(2). Hypertension can damage renal
function and when combined with HHCY renal damage is worse and
occurs earlier (3). Zhao et
al (4) reported that the
synergistic effect of hypertension and HHCY increases the risk of
all-cause mortality in middle-aged and older adults in the United
States. In China, ~75% of patients with hypertension have HHCY
(5) and the renal damage in these
patients is worse compared with hypertensive patients without HHCY
(6). Therefore, it is necessary to
study the mechanism by which HHCY aggravates hypertensive renal
damage to identify effective treatments.
The pathological underlying mechanism of
HHCY-induced renal disease is complex and numerous factors have
been implicated, such as oxidative stress, endoplasmic reticulum
stress, inflammation and hypomethylation (7). A previous study demonstrated that
oxidative stress is more severe in patients with hypertension with
HHCY than in patients without HHCY (8). Moreover, our previous study
demonstrated that HHCY aggravates hypertensive renal damage due to
the abnormal expression of NADPH oxidase (NOX)2/NOX4 caused by
oxidative stress (9). Furthermore,
folic acid (FA) was reported to improve renal damage in
spontaneously hypertensive rats (SHRs), which was caused by HHCY
(10). However, the effective dose
of FA remains controversial and one study has suggested that FA
increases the risk of cancer (11). Therefore, it is important to find a
safe, effective drug to improve renal function damage worsened by
HHCY.
A diet rich in fruits and vegetables is beneficial
to health, and an epidemiological study has reported that it has a
positive role in preventing kidney disease and renal cancer
(12). Resveratrol (RSV) is a
polyphenolic antioxidant found in the extracts of peanuts, grapes
and plant roots. RSV has various biological activities, including
antioxidant, anti-inflammatory, anticancer, antiproliferative and
angioregulatory effects (13).
Numerous studies have examined the effects of RSV on antioxidant
stress, which demonstrated that it has vascular protective and
anti-aging effects (14,15). RSV also significantly decreases the
serum HCY levels in rats (16) and
reverses HCY-induced oxidative stress (17). Using this information, it was
hypothesized that RSV, as a natural antioxidant, may improve
hypertensive renal damage aggravated by HHCY.
Therefore, the aim of the present study was to use a
HHCY-associated hypertension animal model to investigate whether
RSV could reduce renal injury via the inhibition of oxidative
stress, and to compare the efficacy of RSV and FA.
Materials and methods
Animal experiments
The animal care and experimental procedures in the
present study complied with the regulations of and was approved by
the Medical Ethics Committee of Shandong Provincial Qianfoshan
Hospital (Jinan, China). Male SHRs (age, 8-10 weeks; weight,
180-200 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. and were housed in the animal room of
Shandong Provincial Qianfoshan Hospital Medical Research Centre.
The SHRs were kept in eight cages under a 12-h light/dark cycle at
23-24˚C with a humidity of 45-55%. All animals were allowed free
access to standard rodent food and water. The SHRs were acclimated
to their environment for 1 week. Subsequently, the animals were
randomly divided into four groups of eight SHRs as follows: i)
Control; ii) HHCY; iii) HHCY + FA; and iv) HHCY + RSV. HHCY was
induced by administering 2% DL-HCY (5 ml/kg; cat. no. H4628;
Sigma-Aldrich; Merck KGaA) twice a day intraperitoneally for 12
weeks. From the fifth week, the SHRs in the HHCY group were treated
with 0.9% saline (0.5 ml/day) by gavage, those in the HHCY + FA
group were treated with FA (0.4 mg/kg/day; cat. no. F7876;
Sigma-Aldrich; Merck KGaA) and the HHCY + RSV group was treated
with RSV (30 mg/kg/day; cat. no. SRT501; MedChemExpress) for 8
weeks. Throughout the experiment, the control SHRs were given only
an equal volume of 0.9% saline (0.5 ml/day). After 12 weeks of
treatment, the SHRs were anaesthetised with pentobarbital sodium
(40 mg/kg; Sigma-Aldrich; Merck KGaA) and 1 ml blood was then
collected via cardiac puncture and the kidney tissues were
dissected. Finally, all SHRs were sacrificed using pentobarbital
sodium (150 mg/kg). Death was verified by the cessation of
breathing and heartbeat.
Blood pressure (BP) measurement
The BP of conscious SHRs was assessed using the tail
cuff method and a non-invasive BP system (cat. no. BP-2010A;
Softron Beijing Biotechnology Co., Ltd.) as previously described
(18). The BP of the SHRs in each
group was assessed before the start of the experiment and after 4,
8 and 12 weeks of the experiment. Each time, the BP was determined
three times and then averaged.
Plasma HCY and serum oxidative stress
biomarker analysis
At week 4, ~1 ml of rat tail blood was collected and
used to detect HCY levels and assess if the SHR HHCY model had
worked. The plasma HCY and creatinine levels were analysed using a
Cobas8000 automatic biochemistry analyser (Roche Diagnostics) with
Cobas 8000 data manager (Version 1.06.05.0516; Roche Diagnostics).
Superoxide dismutase (SOD) is an important antioxidant enzyme
(19). Malondialdehyde (MDA) is
the end-product of lipid oxidation and reflects the degree of
oxidative stress (19). The serum
MDA and SOD levels were quantified using commercial kits (MDA Assay
Kit; cat. no. A003-1; SOD Assay Kit; cat. no. A001-3; Nanjing
Jiancheng Bioengineering Institute), according to the
manufacturer's instructions.
Detection of renal function
indexes
After 12 weeks of treatment, SHRs were placed
individually in metabolic cages for urine collection. Serum and
urine biochemistry were determined using an automatic biochemical
analyser (Roche Diagnostics) with Cobas 8000 data manager (Version
1.06.05.0516, Roche Diagnostics). The urinary albumin creatinine
ratio (UACR) and glomerular filtration rate (GFR) were used as the
two main indicators of renal function. UACR and GFR were determined
as follows: UACR=urinary microalbumin/urinary creatinine; and
GFR=(urine creatinine/plasma creatinine) x urine volume/body
weight.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the kidney tissue
samples using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Subsequently, complementary (c)DNA was synthesised using the
Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics)
according to the manufacturer's protocol. The mRNA expression
levels were analysed using an ViiA 7 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the SYBR Green
real-time PCR reagent (Roche Diagnostics). The thermocycling
conditions for qPCR were as follows: Initial denaturation at 95˚C
for 10 min; 40 cycles of denaturation at 95˚C for 15 sec, and
annealing and elongation at 60˚C for 60 sec; and a final extension
at 95˚C for 15 sec, 60˚C for 60 sec and 95˚C for 15 sec. The
relative change in mRNA expression levels was determined using the
2-ΔΔCq method (20)
with GAPDH as the internal reference gene. The qPCR primer
sequences (BioSune Biotechnology) of the target genes were as
follows: NOX2 forward (F), 5'-CTGCCAGTGTGTCGGAATCT-3' and reverse
(R) 5'-TGTGAATGGCCGTGTGAAGT-3'; NOX4 F, 5'-ATGTTGGGCCTAGGATTGTGT-3'
and R, 5'-TCCTGCTAGGGACCTTCTGT-3'; nephrin F,
5'-CCTGACCATCCTGGCCAACTC-3' and R, 5'-ATCTTCCAGCCTCTCTCCTTCCT-3';
and GAPDH F, 5'-CCCCCAATGTATCCGTTGTG-3' and R,
5'-TAGCCCAGGATGCCCTTTAGT-3'.
Western blotting
Total protein was extracted from renal tissue using
the RIPA protein extraction solution (Beyotime Institute of
Biotechnology; cat. no. P0013B). Protein concentrations were
determined by BCA Protein Assay Kit (Beyotime Institute of
Biotechnology; cat. no. P0012). The loading amount was calculated
according to the standard of 50 µg protein in each lane. Total
protein was separated using SDS-PAGE on a 10% gel and separated
proteins were transferred onto PVDF membranes. Subsequently, the
membranes were blocked with 5% skimmed milk at room temperature for
2 h. The membranes were then incubated with the primary antibodies
at 4˚C overnight. After three washes using TBS with 0.05% Tween-20
(TBST), the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit antibody (cat. no.
SA00001-2; 1:9,000; Proteintech Group, Inc.) at room temperature
for 1 h. After three more washes with TBST, the protein bands were
visualised using the Ultra High Sensitivity ECL Kit (cat. no.
E412-01; Vazyme Biotech Co., Ltd.) and the ChemiDoc™ Touch Gel
Imaging System (Bio-Rad Laboratories, Inc.). Finally, the protein
bands were semi-quantified using ImageJ software (V1.4.3.67;
National Institutes of Health) and were normalised to GAPDH. The
primary antibodies used were as follows: anti-GAPDH (cat. no.
ab181602, 1:10,000), anti-NOX2 (cat. no. ab31092, 1:1,000),
anti-NOX4 (cat. no. ab133303, 1:4,000) and anti-nephrin (cat. no.
ab216341,1:200), which were all purchased from Abcam.
Statistical analysis
All data are presented as the mean ± SE and were
analysed using SPSS version 24.0 (IBM Corp.). Groups were
statistical compared using one-way ANOVA followed by the Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference. All experiments were performed at least
three times.
Results
RSV reduces HCY levels in the SHR HHCY
model, but to a lesser extent compared with FA
Fig. 1A and B
present the systolic and diastolic BP data for the control, HHCY,
HHCY + FA and HHCY + RSV groups at weeks 4, 8 and 12. The results
demonstrated that there was no significant difference in BP among
the groups.
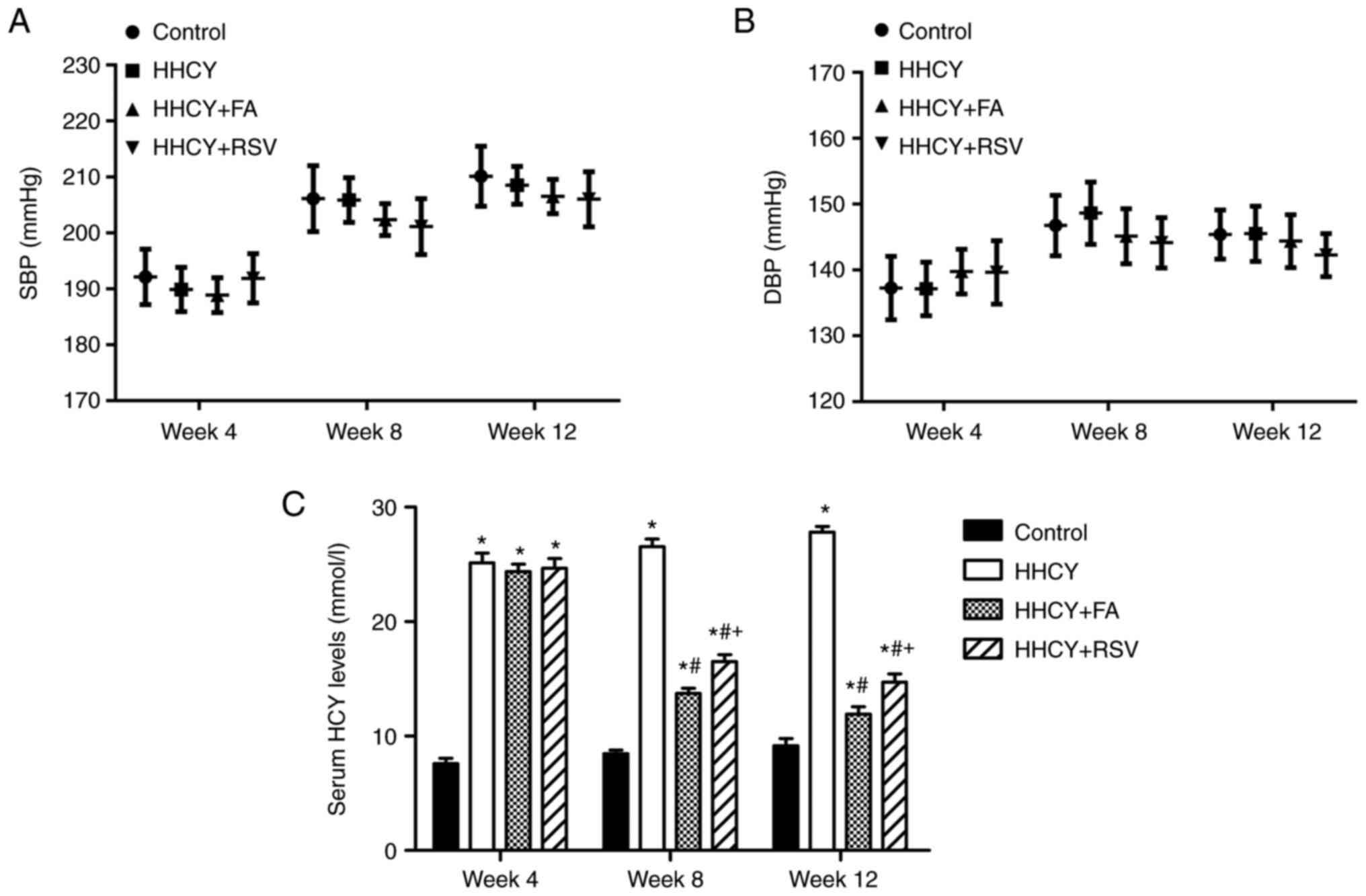 | Figure 1Levels of plasma HCY, SBP and DBP. (A
and B) BP levels and (C) serum HCY levels in the control, HHCY,
HHCY + FA and HHCY + RSV groups at weeks 4, 8 and 12. Data are
presented as the mean ± SE (n=8). *P<0.05 vs.
control; #P<0.05 vs. HHCY; and +P<0.05
vs. HHCY + FA. BP, blood pressure; HCY, homocysteine; HHCY,
hyperhomocysteine; FA, folic acid; RSV, resveratrol; SBP, systolic
BP; DBP, diastolic BP. |
After 4 weeks of intraperitoneal injection of DL-HCY
in the HHCY, HHCY + FA and HHCY + RSV groups, the HCY levels
(25.14±0.85, 24.35±0.66 and 24.66±0.84 µmol/l, respectively) were
significantly higher compared with the control (7.58±0.47 µmol/l).
There was no significant difference between the HHCY, HHCY + FA and
HHCY + RSV groups. Subsequently the HHCY, HHCY + FA and HHCY + RSV
groups were treated with DL-HCY intraperitoneally for a further 8
weeks. The serum HCY levels in the HHCY group markedly increased in
a time-dependent manner (week 8, 26.54±0.66 µmol/l; week 12,
27.81±0.5 µmol/l). Meanwhile, from week 5, FA was administered to
the HHCY + FA group and RSV was administered to the HHCY + RSV
group. The serum HCY levels were significantly lower in the HHCY +
FA and HHCY + RSV groups compared with the HHCY group at both week
8 and week 12 (week 8, 13.73±0.47 and 16.52±0.58 µmol/l; and week
12, 11.92±0.63 and 14.72±0.72 µmol/l in the HHCY + FA and HHCY +
RSV groups, respectively). Furthermore, compared with that in the
HHCY + RSV group, the serum HCY levels in the HHCY + FA group was
significant decreased at weeks 8 and 12 (Fig. 1C).
FA and RSV improve the HHCY-induced
UACR increase and GFR decrease
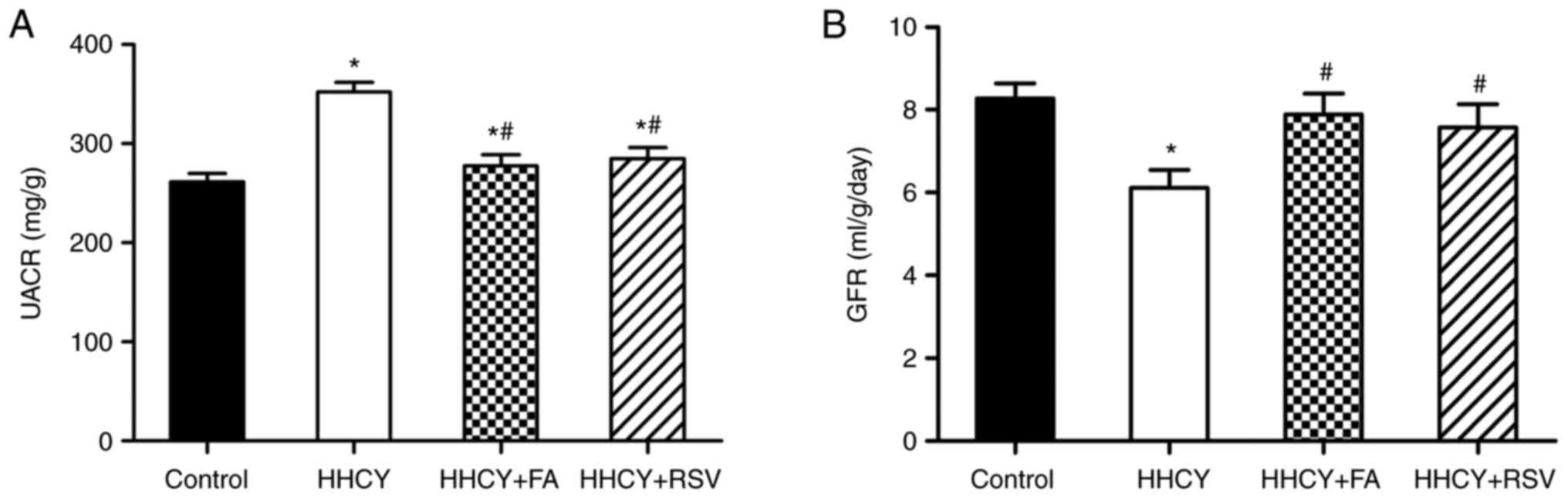
The results demonstrated the the UACR was
significantly higher in the HHCY group (351.97±9.69 mg/g) compared
with the control (261.35±8.65 mg/g) (Fig. 2A). FA and RSV treatment both
significantly reduced the UACR compared with the HHCY group.
However, there was no significant difference between the HHCY + FA
(277.34±11.52 mg/g) and HHCY + RSV (284.99±10.75 mg/g) groups.
Furthermore, compared with the control (8.27±0.37 ml/g/day), the
GFR of the HHCY group decreased significantly (6.11±0.44 ml/g/day)
(Fig. 2B). However, even though
the GFR significantly increased in the HHCY + FA (7.89±0.51
ml/g/day) and HHCY + RSV (7.57±0.57 ml/g/day) groups, there was no
significant difference between the two groups. Therefore, these
results demonstrated that both FA and RSV treatment may have
significantly decreased the HHCY-induced UACR increase and improved
the HHCY-induced GFR decrease.
FA and RSV inhibit HHCY-induced
oxidative stress
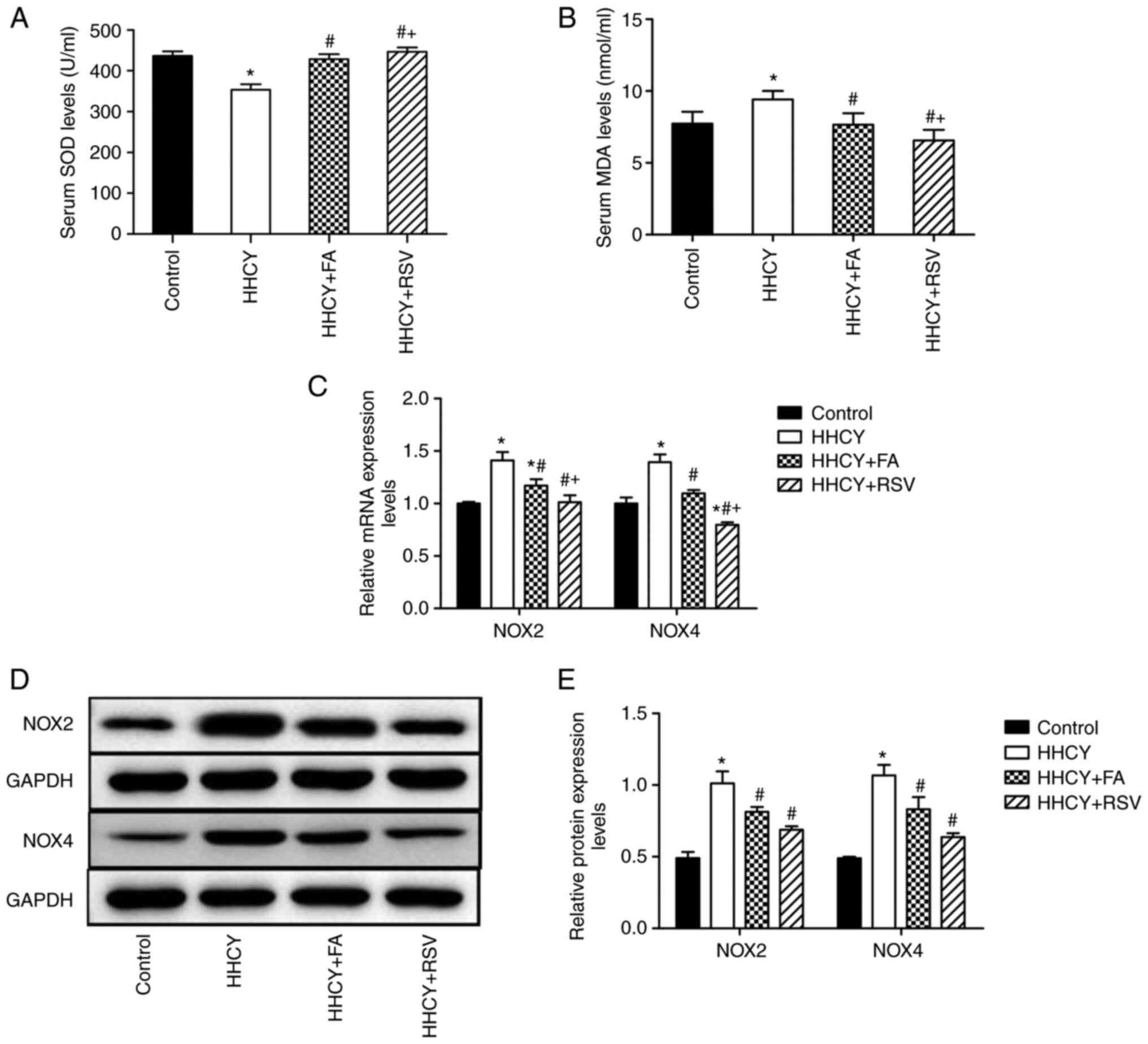
The serum SOD concentration in the HHCY group
(353.52±13.17 U/ml) was significantly decreased compared with the
control (436.33±11.23 U/ml). However, the SOD concentration in the
HHCY + FA (428.67±12.14 U/ml) and HHCY + RSV (446.63±10.69 U/ml)
groups demonstrated a significant reversal of the HHCY-induced
reduction, compared with the HHCY group (Fig. 3A). Moreover, the MDA concentration
was significantly higher in the HHCY group (9.41±0.59 nmol/ml)
compared with the control (7.72±0.83 nmol/ml). However, compared
with the HHCY group, FA and RSV treatment significantly reduced the
increase in MDA induced by HHCY (HHCY + FA, 7.65±0.81 nmol/ml; HHCY
+ RSV, 6.56±0.73 nmol/ml) (Fig.
3B). Furthermore, compared with the HHCY + FA group, the HHCY +
RSV group exhibited significantly different results for both SOD
and MDA.
FA and RSV inhibit HHCY-induced NOX2
and NOX4 mRNA and protein expression
Compared with the control, the NOX2 and NOX4 mRNA
and protein expression levels in the HHCY group were significantly
increased (Fig. 3C-E). Compared
with the HHCY group both FA and RSV treatment significantly
inhibited the HHCY-induced NOX2 and NOX4 mRNA and protein
expression. Furthermore, the NOX2 and NOX4 mRNA expression levels
in the HHCY + RSV group were significantly weaker compared with the
HHCY + FA group. However, there was no significant difference in
the protein expression levels of NOX2 and NOX4 between these two
groups.
FA and RSV treatment reverse the
HHCY-induced decrease in nephrin expression levels
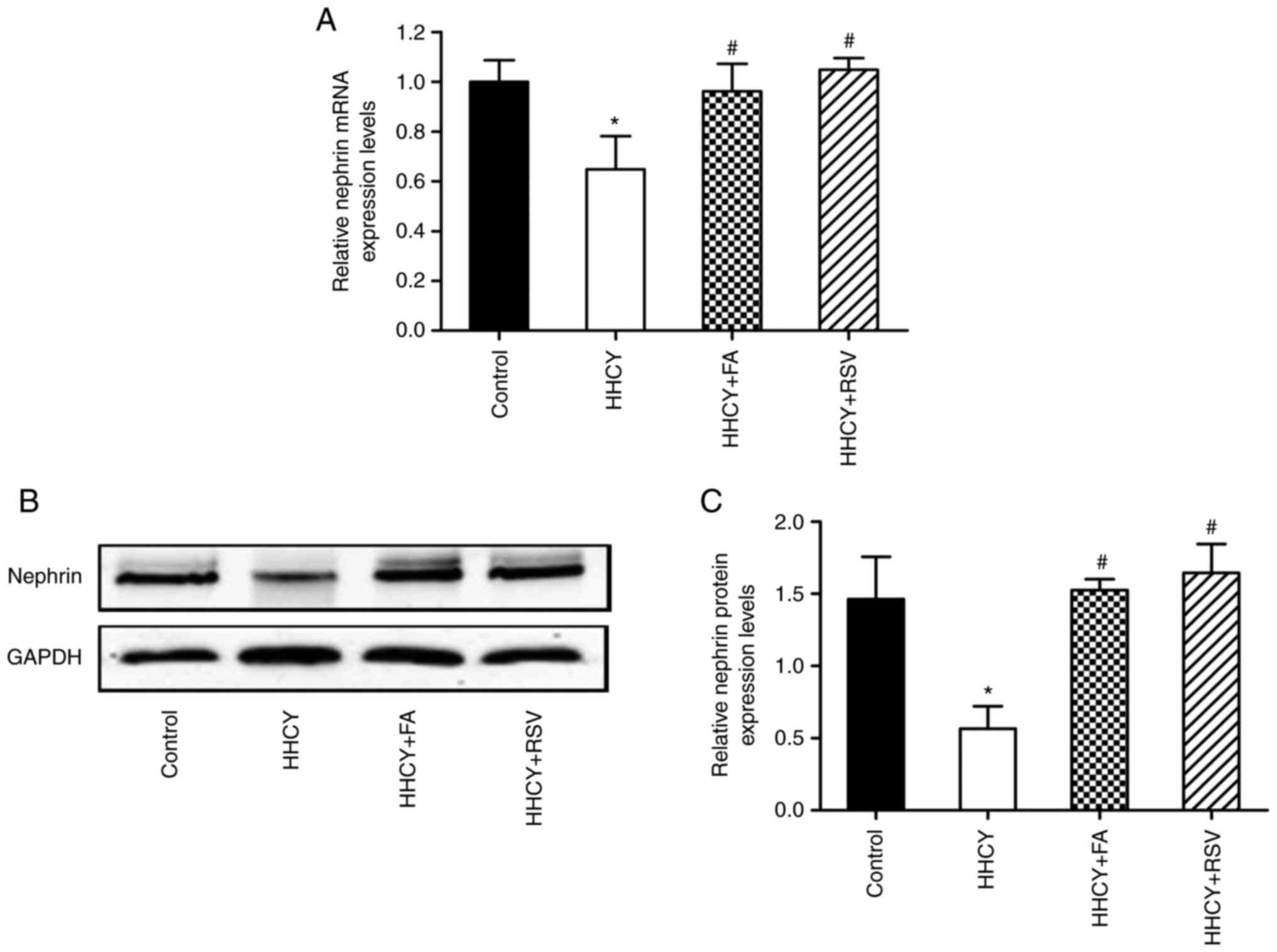
RT-qPCR demonstrated that nephrin mRNA expression
levels were significantly decreased in the HHCY group compared with
the control. Compared with the HHCY group both FA and RSV treatment
significantly reversed this change in nephrin mRNA expression
levels (Fig. 4A). Furthermore,
compared with the control, nephrin protein expression levels were
significantly reduced in the HHCY group, which was significantly
reversed via FA or RSV treatment (Fig.
4B and C). However, there was
no significant difference in nephrin mRNA and protein expression
levels between the HHCY + FA and HHCY + RSV groups.
Discussion
Hypertension is a common cause of renal damage and
HHCY aggravates renal function impairment in patients with
hypertension (21). In China, a
large proportion of patients with hypertension also have HHCY and
most of these individuals have renal damage, which may be related
to insufficient dietary FA (3). An
intervention study demonstrated that FA reduces HCY and improves
renal function (7). However, the
effective dose of FA has not been determined and certain studies
have suggested that FA increases the risk of cancer (11,22).
Therefore, it is necessary to identify an effective drug with
minimal side-effects that can improve the hypertensive renal damage
that is aggravated by HHCY.
In our previous study it was demonstrated that HHCY
aggravates hypertensive renal damage via the activation of
oxidative stress (9). The natural
antioxidant RSV improves diabetic kidney damage and delays kidney
aging (23,24). Moreover, it has previously been
confirmed that RSV can reduce HCY levels (16). Therefore, RSV may be a potential
treatment for HHCY-induced hypertensive renal damage. In the
present study, SHRs were treated with intraperitoneal DL-HCY for 12
weeks to establish a hypertensive rat model with HHCY. The serum
HCY levels in the HHCY group markedly increased in a time-dependent
manner, which indicated that the model had been established.
In the present study, RSV and FA were used as
treatments in the HHCY group and changes were observed in BP, serum
HCY levels, oxidative stress and renal function. The results
demonstrated that there was no significant change in BP in any of
the experimental groups throughout the experiment, which conflicts
with previous reports on the anti-hypertensive effects of RSV
(25,26). A study showed that feeding SHRs
with food containing RSV (4 g/kg) meant that 146 mg/kg/day RSV was
available, which decreased the blood pressure of the SHRs
significantly (27). The results
in the present study may be different because of the dosage, the
mode of administration and the effective concentration of RSV.
The results of the present study demonstrated that
compared with the control the UACR, an important index of early
renal damage, was significantly higher in the HHCY group, whereas
the GFR was significantly lower. Consistent with a Mendelian
randomisation study (28), a high
HCY level potentially lead to a decreased GFR in the present study.
The present study also demonstrated that RSV significantly
ameliorated the increase in UACR and decrease in GFR caused by
HHCY; however, there was no significant difference compared with
the FA treatment group. Furthermore, the significant change in GFR
in the HHCY group was potentially related to the decreased mRNA and
protein expression levels of nephrin, a podocyte gap molecule that
serves an important role in maintaining the glomerular filtration
barrier (29,30). HHCY reduces nephrin expression in
kidney tissue (31), which was
also confirmed in our previous study (9). In the present study, RSV
supplementation increased nephrin expression levels and
significantly improved the GFR in SHRs with HHCY; however there was
no significant difference compared with the FA treatment group.
It was also previously observed that HHCY aggravated
hypertensive renal damage and its underlying mechanism are related
to oxidative stress (9). RSV
reduces the expression and activity of NOXs in endothelial cells
(32). In the present study, HHCY
resulted in decreased serum SOD and increased MDA levels; however,
treatment with FA or RSV significantly counteracted these effects.
Compared with the control, NOX2 and NOX4 mRNA and protein
expression levels in the HHCY group were significantly increased,
which was offset by the FA and RSV treatments. In the present
study, the rats in the control group were SHRs, and a previous
study confirmed that SHR themselves are in a state of oxidative
stress (33). RSV, as an
antioxidant, can improve the oxidative stress of SHRs, which
explains the phenomenon of the expression of NOX4 mRNA being lower
in HHCY + RSV group than that of the control group. Furthermore, it
was demonstrated that compared with the HHCY + FA group, the
indexes related to oxidative stress (SOD, MDA and the mRNA
expression levels of NOX2 and NOX4) improved more significantly in
the HHCY + RSV group. However, there was no significant difference
for the protein expression levels of NOX2 and NOX4 between the HHCY
+ FA and HHCY + RSV groups. As previous stated, mRNA is the
template for protein synthesis, but due to differential
translation, protein degradation, contextual confounders among
other reasons, protein may not be present in proportional
quantities (34). This may explain
why there was no difference in the expression of NOX2 and NOX4
proteins between the HHCY + RSV and HHCY + FA groups.
Furthermore, the results of the present study
demonstrated that RSV reduced the serum HCY levels, which is
consistent with previous research (16). However, this change was not as
significant as for FA treatment, as there was no significant
difference between FA and RSV in terms of improving renal function.
These results suggested that RSV potentially improved the renal
damage induced by HHCY via a mechanism other than that of reducing
serum HCY levels.
In summary, RSV, like FA, improved the renal damage
aggravated by HHCY in SHRs. Furthermore, RSV potentially reduced
the renal damage, not only by decreasing HCY levels but also, and
mainly, by reducing oxidative stress. We consider that RSV alone or
in combination with FA may become a safe and effective new choice
to improve hypertensive renal damage aggravated by HHCY in the
clinic. However, certain limitations need to be explored in future
studies. For example, the effect of different doses of RSV is
unclear and future studies should examine the effect of combined
treatment with FA and RSV on improving hypertensive renal damage
aggravated by HHCY.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shandong
Provincial Key Research and Development Programme Foundation (grant
no. 2018GSF118009), the Technology Program Foundation of Jinan,
China (grant no. 201805060), the Shandong Provincial Medical
Science and Technology Development Programme Foundation (grant no.
2019WS039) and the National Science Foundation for Incubation Fund
of Shandong Provincial Qianfoshan Hospital (grant no.
QYPY2020NSFC1011).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RX, NG and QCH conceived and designed the
experiments. QCH and XZ performed the experiments. QCH, XZ and PC
analysed the data. RX, NG and PC provided reagents and advice. XZ
was a major contributor in writing the manuscript. XZ and QCH
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was established according
to the ethical guidelines and was approved by the Medical Ethics
Committee of Shandong Provincial Qianfoshan Hospital (approval no.
2020-S292).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finkelstein JD and Martin JJ:
Homocysteine. Int J Biochem Cell Biol. 32:385–389. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zaric BL, Obradovic M, Bajic V, Haidara
MA, Jovanovic M and Isenovic ER: Homocysteine and
Hyperhomocysteinaemia. Curr Med Chem. 26:2948–2961. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou YF and Guan YF: Hyperhomocysteinemia
and kidney diseases. Sheng Li Xue Bao. 70:607–611. 2018.PubMed/NCBI(In Chinese).
|
|
4
|
Zhao W, Gao F, Lv L and Chen X: The
interaction of hypertension and homocysteine increases the risk of
mortality among middle-aged and older population in the United
States. J Hypertens. 40:254–263. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ye Z, Wang C, Zhang Q, Li Y, Zhang J, Ma
X, Peng H and Lou T: Prevalence of homocysteine-related
hypertension in patients with chronic kidney disease. J Clin
Hypertens (Greenwich). 19:151–160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xie D, Yuan Y, Guo J, Yang S, Xu X, Wang
Q, Li Y, Qin X, Tang G, Huo Y, et al: Hyperhomocysteinemia predicts
renal function decline: A prospective study in hypertensive adults.
Sci Rep. 5(16268)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Long Y and Nie J: Homocysteine in renal
injury. Kidney Dis (Basel). 2:80–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guo G, Sun W, Liu G, Zheng H and Zhao J:
Comparison of oxidative stress biomarkers in hypertensive patients
with or without hyperhomocysteinemia. Clin Exp Hypertens.
40:262–266. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gao N, Zhang Y, Li L, Lei L, Cao P, Zhao
X, Lin L and Xu R: Hyperhomocysteinemia-induced oxidative stress
aggravates renal damage in hypertensive rats. Am J Hypertens.
33:1127–1135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gao N, Zhang Y, Lei L, Li L, Cao P, Zhao
X, Lin L and Xu R: Low doses of folic acid can reduce
hyperhomocysteinemia-induced glomerular injury in spontaneously
hypertensive rats. Hypertens Res. 43:1182–1191. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shulpekova Y, Nechaev V, Kardasheva S,
Sedova A, Kurbatova A, Bueverova E, Kopylov A, Malsagova K, Dlamini
JC and Ivashkin V: The concept of folic acid in health and disease.
Molecules. 26(3731)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Den*Hartogh DJ and Tsiani E: Health
benefits of resveratrol in kidney disease: Evidence from in vitro
and in vivo studies. Nutrients. 11(1624)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cheng CK, Luo JY, Lau CW, Chen ZY, Tian XY
and Huang Y: Pharmacological basis and new insights of resveratrol
action in the cardiovascular system. Br J Pharmacol. 177:1258–1277.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Breuss JM, Atanasov AG and Uhrin P:
Resveratrol and its effects on the vascular system. Int J Mol Sci.
20(1523)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pyo IS, Yun S, Yoon YE, Choi JW and Lee
SJ: Mechanisms of aging and the preventive effects of resveratrol
on age-related diseases. Molecules. 25(4649)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Atazadegan MA, Bagherniya M, Askari G,
Tasbandi A and Sahebkar A: The effects of medicinal plants and
bioactive natural compounds on homocysteine. Molecules.
26(3081)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koz ST, Etem EO, Baydas G, Yuce H, Ozercan
HI, Kuloğlu T, Koz S, Etem A and Demir N: Effects of resveratrol on
blood homocysteine level, on homocysteine induced oxidative stress,
apoptosis and cognitive dysfunctions in rats. Brain Res.
1484:29–38. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li D, Cui Z, Xu S, Xu T, Wu S, Bouakaz A,
Wan M and Zhang S: Low-intensity focused ultrasound stimulation
treatment decreases blood pressure in spontaneously hypertensive
rats. IEEE Trans Biomed Eng. 67:3048–3056. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Reastuty R and Haryuna TSH: Correlation of
SOD and MDA expression in the organ of corti and changes in the
function of outer hair cells measured by DPOAE examination in
noise-exposed rat cochlea. Rep Biochem Mol Biol. 10:41–49.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu C, Lin L and Xu R: Elevated
homocysteine and differential risks of the renal function decline
in hypertensive patients. Clin Exp Hypertens. 42:565–570.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pieroth R, Paver S, Day S and Lammersfeld
C: Folate and its impact on cancer risk. Curr Nutr Rep. 7:70–84.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Salami M, Salami R, Mafi A, Aarabi MH,
Vakili O and Asemi Z: Therapeutic potential of resveratrol in
diabetic nephropathy according to molecular signaling. Curr Mol
Pharmacol: Dec 17, 2021 (Epub ahead of print).
|
|
24
|
Li KX, Ji MJ and Sun HJ: An updated
pharmacological insight of resveratrol in the treatment of diabetic
nephropathy. Gene. 780(145532)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chudzińska M, Rogowicz D, Wołowiec Ł,
Banach J, Sielski S, Bujak R, Sinkiewicz A and Grześk G:
Resveratrol and cardiovascular system-the unfulfilled hopes. Ir J
Med Sci. 190:981–986. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Parsamanesh N, Asghari A, Sardari S,
Tasbandi A, Jamialahmadi T, Xu S and Sahebkar A: Resveratrol and
endothelial function: A literature review. Pharmacol Res.
170(105725)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dolinsky VW, Chakrabarti S, Pereira TJ,
Oka T, Levasseur J, Beker D, Zordoky BN, Morton JS, Nagendran J,
Lopaschuk GD, et al: Resveratrol prevents hypertension and cardiac
hypertrophy in hypertensive rats and mice. Biochim Biophys Acta.
1832:1723–1733. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park S, Lee S, Kim Y, Cho S, Kim K, Kim
YC, Han SS, Lee H, Lee JP, Joo KW, et al: Causal effects of
homocysteine, folate, and cobalamin on kidney function: A mendelian
randomization study. Nutrients. 13(906)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wartiovaara J, Ofverstedt LG, Khoshnoodi
J, Zhang J, Mäkelä E, Sandin S, Ruotsalainen V, Cheng RH, Jalanko
H, Skoglund U and Tryggvason K: Nephrin strands contribute to a
porous slit diaphragm scaffold as revealed by electron tomography.
J Clin Invest. 114:1475–1483. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Tryggvason K and Wartiovaara J: Molecular
basis of glomerular permselectivity. Curr Opin Nephrol Hypertens.
10:543–549. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xia M, Conley SM, Li G, Li PL and Boini
KM: Inhibition of hyperhomocysteinemia-induced inflammasome
activation and glomerular sclerosis by NLRP3 gene deletion. Cell
Physiol Biochem. 34:829–841. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li H, Xia N, Hasselwander S and Daiber A:
Resveratrol and vascular function. Int J Mol Sci.
20(2155)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luo M, Cao C, Niebauer J, Yan J, Ma X,
Chang Q, Zhang T, Huang X and Liu G: Effects of different
intensities of continuous training on vascular inflammation and
oxidative stress in spontaneously hypertensive rats. J Cell Mol
Med. 25:8522–8536. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Buccitelli C and Selbach M: mRNAs,
proteins and the emerging principles of gene expression control.
Nat Rev Genet. 21:630–644. 2020.PubMed/NCBI View Article : Google Scholar
|