Introduction
Interstitial pulmonary diseases are a group of
diseases caused mainly by pathological changes in the alveolar wall
(1). The basic pathological changes
of interstitial pulmonary diseases include diffuse lung parenchyma,
alveolitis and interstitial fibrosis, and their clinical
manifestations comprise active dyspnea, diffuse shadow in X-ray
chest film, restrictive ventilation disorder, decreased diffusion
function and hypoxemia (1,2). Interstitial pulmonary diseases have a
variety of subtypes, among which idiopathic pulmonary fibrosis
(IPF) is the most harmful one (3).
IPF is a chronic, progressive and fatal interstitial pulmonary
disease, and there is no effective treatment due to its unknown
pathogenesis (4,5). The incidence rate of IPF varies
according to different regions, different populations and different
occupations (6). In developed
countries, the incidence rate is as low as three in
100,000(6). In developing
countries, its incidence is slightly higher. Overall, the incidence
rate of males is higher than that of females (6). It is believed that repeated pulmonary
epithelial cell injury and myofibroblast activation are the direct
causes of pulmonary fibrosis (7,8). A
large number of fibrosis-promoting cytokines produced in the
process of injury repair can induce fibroblasts to differentiate,
proliferate, migrate and invade through the autocrine or paracrine
tissues (9). This results in
continuous deposition of extracellular matrix (ECM), hinders normal
injury repair and ultimately leads to the destruction of lung
structure and the occurrence of fibrosis (9,10).
Abnormal proliferation of fibroblasts and increase
of ECM are the basic pathological features of interstitial
pulmonary diseases, indicating that fibroblasts serve. Important
biological roles in the diseases (11,12).
Fibroblasts are the main cell components in lung connective
tissues, differentiated from mesenchymal cells with a spindle flat
star shape and protuberances (13,14).
Fibroblasts have large cell bodies and their cytoplasm has weak
basophilia (15). Under normal
conditions, the main functions of fibroblasts include sustaining
normal lung morphology, synthesizing and releasing ECM,
participating in gas exchange in lung tissues and maintaining
normal physiological functions of pulmonary epithelial and
endothelial cells such as secretion (16). However, fibroblasts demonstrate
abnormal proliferation and differentiation and secrete a large
amount of ECM in interstitial pneumonia (17).
Cytokines serve important roles in the occurrence
and development of interstitial pneumonia (18). For example, TGF-β is the strongest
fibrogenic cytokine that can stimulate the proliferation of
pulmonary fibroblasts and make them transform into myofibroblasts,
hence it is an important target for the inhibition of pulmonary
fibrosis (19). Studies on
cytokines that are involved in pulmonary fibrosis have important
clinical implications (20).
Interleukin (IL)-10 is a type of inflammatory cytokine that is
involved in tumor development and progression, interstitial
pulmonary diseases and autoimmune diseases (21,22).
IL-10 is secreted mainly by lymphocytes, macrophages and mast cells
and it can inhibit macrophages and Th1 cells and enhance the
biological function of B cells (23). In addition, IL-10 reduces the
production of inflammatory cytokines, such as IL-2 and IFN-γ, thus
inhibiting inflammatory responses (24). It has also been reported that IL-10
participates in the occurrence and development of pulmonary
fibrosis (25). However, the role
of IL-10 in pulmonary fibroblasts remains unclear. The present
study aimed to investigate the regulatory relationship between
IL-10 and lung fibroblasts at a cellular and molecular level and to
provide experimental basis for clinical targeted intervention in
interstitial pneumonia.
Materials and methods
Subjects
A total of 42 patients with IPF (31 males and 11
females; age range, 53-71 years; mean age, 62.4±7.3 years) were
diagnosed at Lishui People's Hospital (Lishui, China) between July
012 and July 2018 according to IPF non-traumatic diagnostic
criteria formulated by ATS/ERS/JRS/ALAT in 2011(26). The exclusion criteria for the
patients were: i) Not having chronic obstructive pulmonary disease;
ii) having collagen angiopathy complicated with interstitial
pulmonary diseases; iii) having unstable angina pectoris; iv)
having interstitial pulmonary diseases caused by occupational and
environmental exposures; and v) having interstitial pulmonary
diseases and other serious diseases caused by drugs or known
causes. The inclusion criterion was IPF patients diagnosed in
Lishui People's Hospital (Lishui, China) who had no history of
cancer, diabetes, hypertension, autoimmune diseases or chronic
medication. Among the 42 patients, 38 were smokers and 4 were
non-smokers. Twenty healthy subjects were included as control group
(age range, 33-45 years; 15 males and 5 females).
From all subjects, 10 ml blood was drawn from the
elbow vein. Of the 10 ml blood, 8 ml was used for separating serum
after centrifugation at 1,500 x g and 4˚C for 10 min, and 2 ml was
used to obtain peripheral mononuclear lymphocytes for flow
cytometry. The serum and peripheral mononuclear lymphocytes were
stored at -80˚C until use. All procedures performed in the current
study were approved by the Ethics Committee of Wenzhou Medical
University (Lishui, China). Written informed consent was obtained
from all patients or their families.
Cells and transfection
Lung tissues were collected during lobectomy and cut
into 1 mm3 size pieces. Then, the tissues were cultured
in DMEM supplemented with 20% fetal bovine serum (Thermo Fisher
Scientific, Inc.) at 37˚C and 5% CO2. Every 2 days, the
tissues were observed and the medium was replenished. Subsequently,
~3 weeks later, primary fibroblasts crawled out of tissue mass and
continued growing. On reaching 70-80% confluency, the fibroblasts
were passaged at a volume ratio of 1:3 (cell suspension vs. medium)
and cultured in DMEM medium supplemented with 10% fetal bovine
serum at 37˚C and 5% CO2.
MRC-5 cells (2x105; Cell Bank of the
Chinese Academy of Sciences, Shanghai, China) in logarithmic growth
phase were seeded into 24-well plates, and cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) at 37˚C and 5% CO2. When cells reached
70% confluency, they were transfected with the small-interfering
RNA of IL-10RA1 (siR-IL-10RA1; 5'-GGTCTACAGCATCGAGTAT-3'; Hanbio
Biotechnology Co., Ltd.) or siR-NC (non-targeting siRNA sequences;
Hanbio Biotechnology Co., Ltd.) using Lipofectamine
3000® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Briefly, 1.5 µl
siR-IL-10RA1 or siR-NC (50 pmol/µl; Hanbio Biotechnology Co., Ltd.)
was mixed with 50 µl Opti Mem medium (Thermo Fisher Scientific,
Inc.) in a vial. In another vial, 1 µl Lipofectamine
3000® (Invitrogen; Thermo Fisher Scientific, Inc.) was
mixed with 50 µl Opti Mem medium. After standing for 5 min, the two
vials were combined for further incubation at room temperature for
another 20 min. Then, the mixtures were added onto cells in
thesiR-IL-10RA1 and siR-NC groups. Next, 6 h later, the medium was
replaced with RPMI-1640 medium containing 10% fetal bovine serum.
After culturing at 37˚C for 48 h, the cells were collected for
subsequent experimentation. To examine the effect of IL-10 on
fibroblasts, MRC-5 cells were cultured with the serum of IPF
patients (serum group) or the serum of IPF patients containing
IL-10 antibody (serum+IL-10 antibody group) at 37˚C and 5%
CO2 for 24 h. Untreated MRC-5 cells were used as
negative control (NC) group.
The present study included eight cases of primary
cell culture and five cases were successful. Cellular experiments
were performed on the five successful cases.
Enzyme-linked immunosorbent assay
(ELISA)
HumanIL-10 ELISA kit (abs510005-96T; Absin
Bioscience Inc.) was used to determine the concentration of IL-10
in serum. In microplates, standards (100 µl) and samples (100 µl
serum) were added into predefined wells, while blank wells were
left empty. In the wells for standards and samples, horseradish
peroxidase-labelled conjugates (100 µl) were added before sealing
the plates for incubation at 37˚C for 1 h. After washing the plates
5 times, substrates A (50 µl) and B (50 µl) were added into each
well. After incubation at 37˚C for 15 min, stop solution (50 µl)
was added into each well, and absorbance of each well was measured
at 450 nm within 15 min.
Reverse transcription-quantitative
(RT-q) PCR
Transfected cells (1x106) were directly
lysed with 1 ml TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific Inc.) at room temperature for 30 min. Total RNA
was extracted using the phenol chloroform method. The concentration
and quality of RNA was measured using ultraviolet spectrophotometry
(NanoDrop ND2000; Thermo Fisher Scientific Inc.). The acceptable
concentration was 50-150 ng/µl and the acceptable ratio of
A260/A280 was between 1.8 and 2.0. Next, cDNA was obtained by
reverse transcription from 1 µg RNA and stored at -20˚C. Reverse
transcription of mRNA was performed at 50˚C for 45 min using
TIANScript II cDNA First Strand Synthesis kit (Tiangen Biotech Co.,
Ltd.) according to the manufacturer's manual. SuperReal PreMix
(SYBR-Green) RT-qPCR kit (Tiangen Biotech Co., Ltd.) was used to
detect mRNA expression of IL-10R1, using GAPDH as an internal
reference gene. The sequences of IL-10R1 were: forward,
5'-TGAAAACAAGAGCAAGGCCG-3' and reverse, 5'-ATCCCTCCGAGACACTGGAA-3'.
The sequences of GAPDH used were: forward,
5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'. The reaction system (20 µl) was
composed of 10 µl SYBR Premix EXTaq, 0.5 µl upstream primer, 0.5 µl
downstream primer, 2 µl cDNA and 7 µl ddH2O. The
thermocycling conditions used were as follows: Initial denaturation
at 95˚C for 10 min; denaturation at 95˚C for 1 min and annealing at
60˚C for 30 sec (40 cycles); elongation at 72˚C for 30 sec and were
performed using the iQ5 instrument (Bio-Rad Laboratories Inc.). The
2-ΔΔCq method (27) was
used to calculate the relative expression of IL-10R1 mRNA against
GAPDH. Each sample was tested in triplicate.
CCK-8 assay
MRC-5 cells were trypsinized and seeded into 96-well
plates at a density of 2x103/well. At 0, 24, 48 and 72
h, the medium was discarded, and the cells were washed with
phosphate-buffered saline (PBS) twice, followed by addition of DMEM
medium and 10 µl CCK-8 reaction reagent (5 g/l; Beyotime Institute
of Biotechnology). After incubation at 37˚C and 5% CO2
for 2 h, the absorbance of each well was measured at 490 nm for
plotting cell viability curves.
Flow cytometry
Flow cytometry was used for cell cycle analysis. At
24 h after transfection or treatments with serum, 1x106
MRC-5 cells of NC, serum and serum + IL-10 antibody groups or
siR-NC and siR-IL-10R1 groups were washed twice with precooled PBS.
The centrifugation was at 2,000 x g for 10 min and 4˚C. BD
Cycletest Plus DNA Reagent kit (catalogue no. 340242; BD
Biosciences) was used to perform the cell cycle analysis according
to the manufacturer's instructions. The cells were permeabilized
using the liquid provided in BD Cycletest Plus DNA Reagent kit
(catalogue no. 340242; BD Biosciences). The staining buffer
contained RNAse. The cells were incubated with 200 µl liquid A for
10 min and 150 µl liquid B for another 10 min. Then, the cells were
incubated with 120 µl liquid C in the dark for 10 min before flow
cytometry (FACSCalibur; BD Biosciences). The result was analyzed
using ModFit software version 3.2 (Verity Software House Inc.).
For separating peripheral mononuclear lymphocytes, 3
ml sterile mononuclear cell separation solution was added into 15
ml tube before gently adding 2 ml peripheral blood on top of the
solution. After centrifugation at 100 x g at room temperature for
20 min, the layer of peripheral mononuclear lymphocytes was gently
aspirated and mixed with 10 ml sterile PBS, before centrifugation
at 100 x g at room temperature for 5 min. Then, the supernatant was
discarded and 5 ml PBS was added to resuspend the cells. After
centrifugation at 100 x g at room temperature for 5 min, 250 µl BD
Cytofix/Cytoperm reagent (BD Biosciences) was used to resuspend the
cells, which were incubated at 4˚C for 20 min and permeabilized.
Then, 1 ml PBS was added to stop perforation. After centrifugation
at 100 x g at room temperature for 5 min, the cells were collected
and stained with IL-10 antibody (1:3,000; cat.no. PI528; Beyotime
Institute of Biotechnology) in the dark at room temperature for 30
min. Finally, the cells were examined by flow cytometry
(FACSCalibur; BD Biosciences). The result was analyzed using ModFit
software version 3.2 (Verity Software House Inc.).
Western blotting
After treatment for 48 h, the cells in NC, serum and
serum+IL-10 antibody groups or siR-NC and siR-IL-10R1 groups were
collected and washed with PBS twice. Then, the cells were lysed
with 600 µl precooled Radio-Immunoprecipitation Assay (RIPA) lysis
buffer (Beyotime Institute of Biotechnology) for 5 min on ice. The
mixture was centrifuged at 13,000 x g and 4˚C for 10 min. The
supernatant was used to determine protein concentration using the
bicinchoninic acid (BCA) protein concentration determination kit
(RTP7102; Real-Times Biotechnology Co., Ltd.). Subsequently, the
samples were mixed with 5X sodium dodecyl sulfate loading buffer
before denaturation in a boiling water bath for 10 min.
Subsequently, the protein samples (20 µg/lane) were subjected to
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at
100 V. The resolved proteins were transferred to polyvinylidene
difluoride membranes on ice (250 mA; 1 h) and blocked with 5%
skimmed milk at room temperature for 1 h. Then, the membranes were
incubated with rabbit anti-human IL-10R1 (1:1,000; abs136163),
IL-10R2 (1:1,000; abs138477), collagen type I α1 chain (COL1a1;
1:1,000; abs118788), collagen type I α2 chain (COL1a2; 1:1,000;
abs101119) or GAPDH (1:4,000; abs132004) polyclonal primary
antibodies (all from Absin Bioscience Inc.) at 4˚C overnight. After
extensive washing with PBS with 0.1% Tween-20 3 times (each wash
for 15 min), the membranes were incubated with goat anti-rabbit
IgG-horseradish peroxidase-conjugated secondary antibody (1:4,000;
abs20040; Absin, Shanghai, China) for 1 h at room temperature
before washing with PBS with Tween 20 3 times (each wash for 15
min). Subsequently, membrane development was performed using the
enhanced chemiluminescence detection kit (Abcam) for imaging. Image
lab v.3.0 software (Bio-Rad Laboratories Inc.) was used to acquire
and analyze imaging signals. GAPDH was used as the loading
control.
Immunohistochemistry
Sterilized cover glasses were placed in a 90 mm
culture dish, and then the cells were seeded in the culture dish at
a density of 2x104/ml for cell climbing. On the
following day, the cells attached on the cover slip were washed
with PBS for three times of 2 min. Then, the cells were fixed with
4% paraformaldehyde at room temperature for 15 min and dried under
room temperature for 5 min. After washing with PBS for 3 times of 2
min, the cells were incubated with 0.5% Triton X-100 (dissolved in
DPBS) at room temperature for 20 min before washing again with PBS
for 3 times of 2 min. After addition of 3%
H2O2, the cells were incubated for 15 min at
room temperature. Following washing with PBS for 3 times of 2 min,
the cells were incubated with blocking serum at room temperature
for 20 min. Then, primary vimentin antibody (abs131996; Absin,
Shanghai, China) was added before incubation at 40˚C overnight.
Subsequently, the cells were incubated with secondary antibody
pv6001 at 37˚C for 30 min. After washing with PBS for 5 times of 2
min, 1 drop of diaminobenzidine (DAB) liquid was added to the
section was dripped with. After 10 min, the section was washed with
water for 5 min. After staining with hematoxylin at room
temperature for 10 min, the section was washed with water for 5 min
before being sealed in gum.
Statistical analysis
Results were analyzed using SPSS 18.0 (IBM Corp.).
The data were expressed as means ± standard deviations. The number
of biological replicates was three. Comparison between two groups
was carried out using paired Student's t-tests. Multiple group
comparisons were performed by one-way analysis of variance followed
by a post hoc Student-Newman-Keuls test. P<0.05 was considered
to indicate a statistically significant difference.
Results
IL-10 promotes the viability and
collagen synthesis of MRC-5 cells and primary pulmonary
fibroblasts
To measure the levels of IL-10 in serum samples from
42 IPF patients and 20 healthy subjects, ELISA was performed. The
data demonstrated that the level of IL-10 in serum from patients
with (135.7±12.5 ng/µl) was significantly higher compared with that
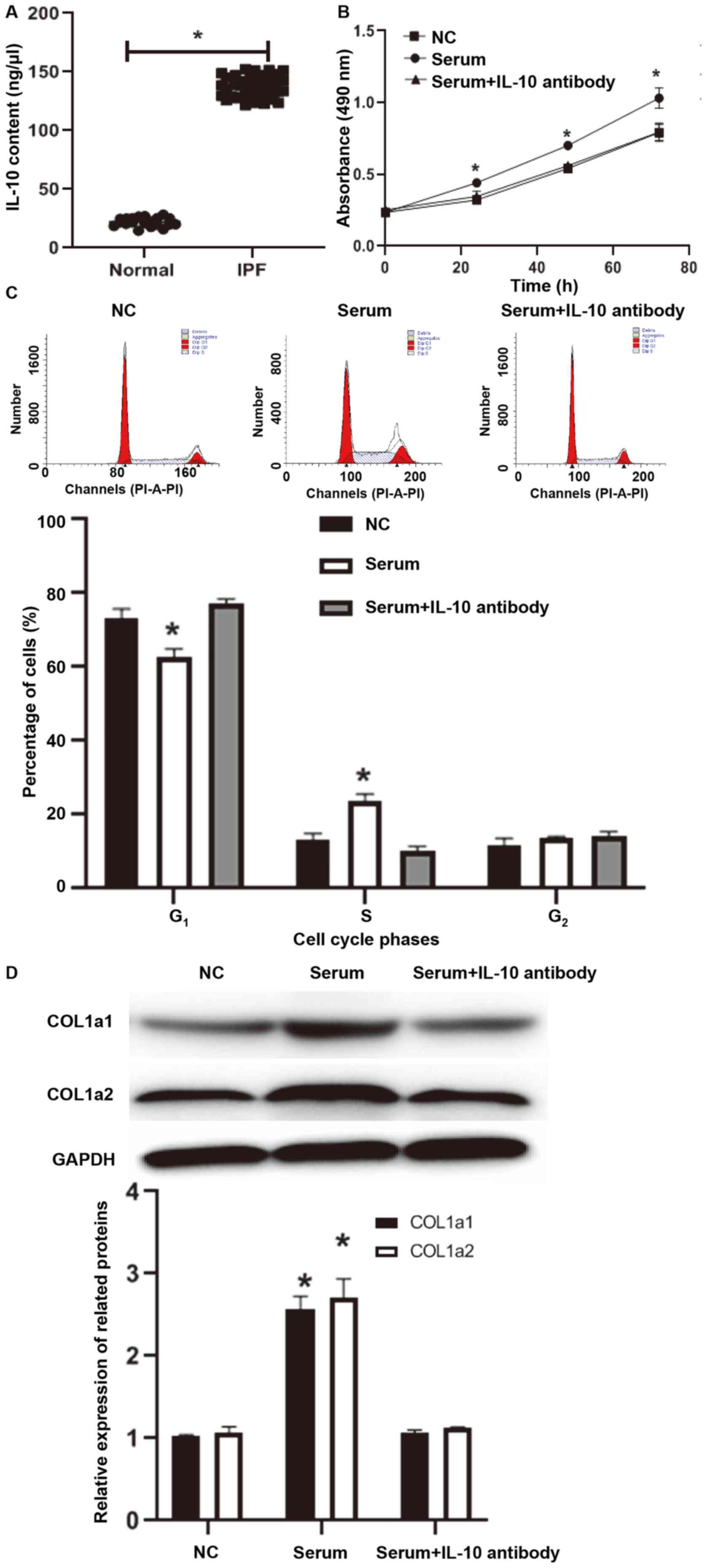
in normal subjects (21.6±5.8 ng/µl) (P<0.05; Fig. 1A). To examine the effect of IL-10 on
fibroblasts, MRC-5 cells were cultured with the serum of patients
with IPF for 24 h in the absence or presence of IL-10 antibody. The
CCK-8 assay demonstrated that the absorbance of MRC-5 cells
stimulated with serum from patients with IPF was significantly
higher compared with that of MRC-5 cells treated with serum from
healthy subjects at 24, 48 and 72 h (P<0.05 for all time points;
Fig. 1B), while co-incubation with
IL-10 antibody reduced the absorbance of MRC-5 cells to a level
similar to that of MRC-5 cells treated with serum from healthy
subjects (P>0.05; Fig. 1B). Flow
cytometry demonstrated that, compared with MRC-5 cells that were
not treated with serum (NC group), the transition from
G1 to S phase in MRC-5 cells treated with serum from IPF
patients was accelerated (P<0.05; Fig. 1C), while treatment with IL-10
antibody reversed this to a level similar to NC group (P>0.05;
Fig. 1C). Western blotting
demonstrated that COL1a1 and COL1a2 protein expression in MRC-5
cells treated with serum from IPF patients was significantly higher
compared with that in the MRC-5 cells of the negative control group
(P<0.05; Fig. 1D), while
treatment with IL-10 antibody reversed this to a level similar to
the negative control group (P>0.05; Fig. 1D). To further confirm the effect of
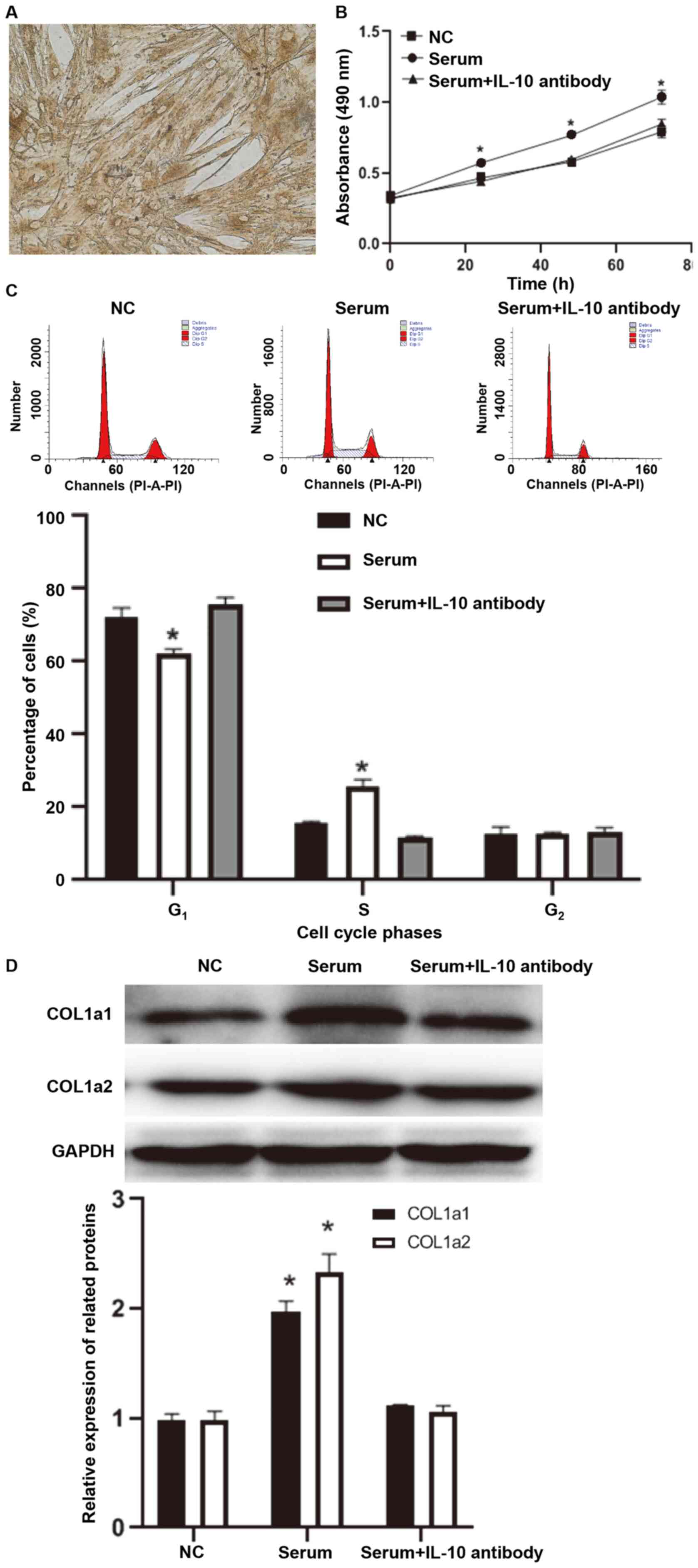
IL-10 on fibroblasts, primary fibroblasts were isolated and
identified. Microscopy demonstrated that primary pulmonary
fibroblasts were spindle-shaped (yellow-brown indicated positive
signals), and immunohistochemistry detected the marker protein
vimentin in the fibroblasts (Fig.
2A). The present study included 8 cases of primary cell culture
and 5 cases were successful. Cellular experiments were performed on
the 5 successful cases. Similar results were observed in primary
fibroblasts compared to those from MRC-5 cells (Fig. 2B-D). The aforementioned results
suggested that IL-10 promotes the viability and collagen synthesis
of MRC-5 cells and primary pulmonary fibroblasts.
IL-10 and IL-10R1 play regulatory
roles in the viability and collagen synthesis of MRC-5 cells
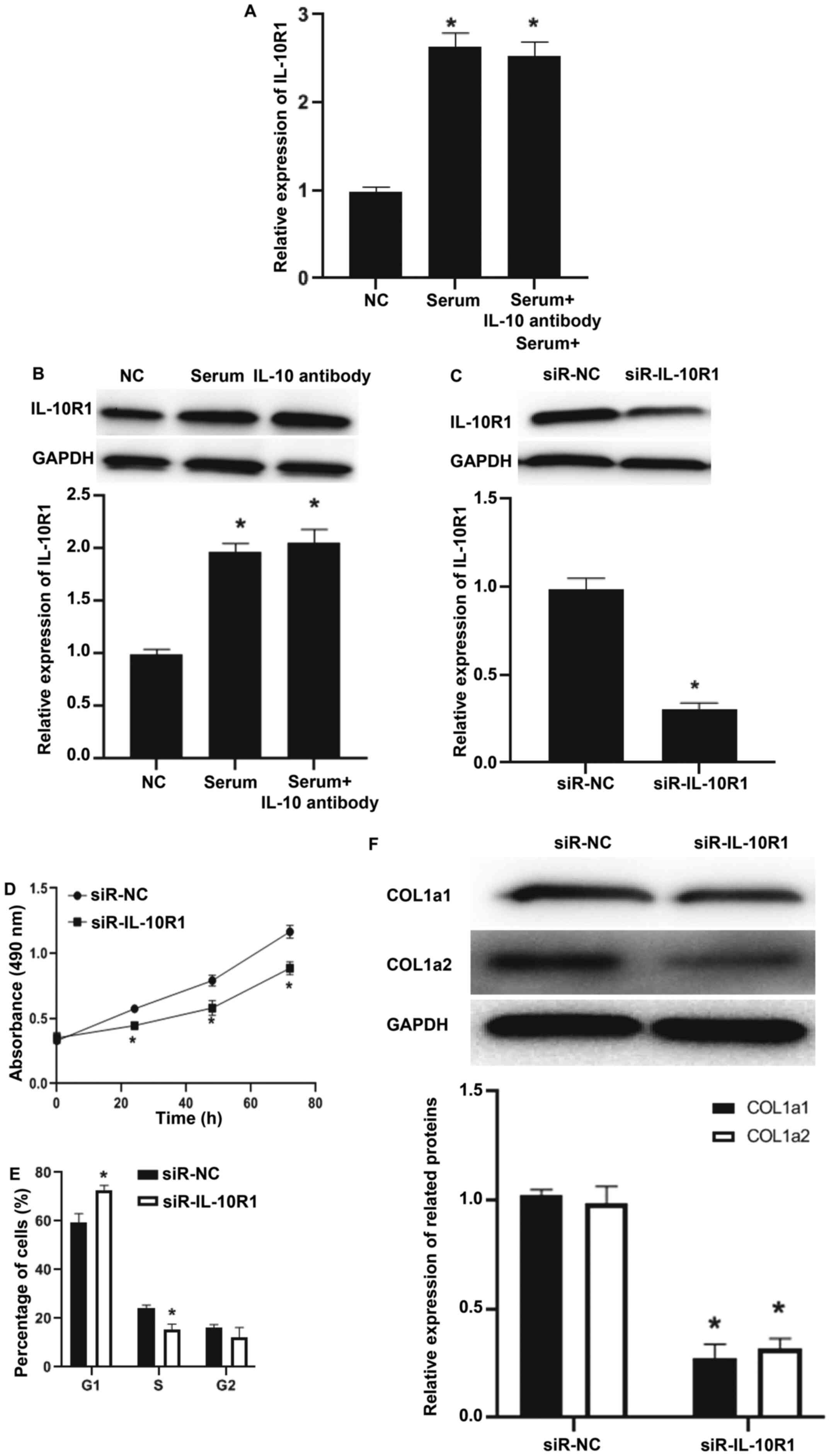
To investigate the mechanism by which IL-10
regulates MRC-5 cells, the mRNA and protein expression of IL-10
receptor IL-10R1. RT-qPCR and western blotting demonstrated that
IL-10R1 mRNA and protein expression in MRC-5 cells treated with
serum from patients with IPF was significantly higher compared with
that in the negative control group (P<0.05; Fig. 3A and B), while addition of IL-10 antibody did
not decrease the level of IL-10R1 mRNA and protein compared with
serum group (P>0.05; Fig. 3A and
B). After transfecting MRC-5 cells
with siR-IL-10R1, the expression of IL-10R1 protein was decreased
compared with the siR-NC group (P<0.05; Fig. 3C). The CCK-8 assay demonstrated that
the viability of MRC-5 cells in the siR-IL-10R1 group was decreased
compared with the siR-NC group (P<0.05 at all time points;
Fig. 3D). Flow cytometry
demonstrated that siR-IL-10R1 slowed down the transition from
G1 phase to S phase compared with that of siR-NC group
(P<0.05; Fig. 3E). In addition,
expression of COL1a1 and COL1a2 in siR-IL-10R1 group was lower
compared with that in the siR-NC group (Fig. 3F). The aforementioned results
suggested that IL-10 and IL-10R1 serve regulatory roles in the
viability and collagen synthesis of MRC-5 cells.
The ratio of peripheral mononuclear
lymphocytes with positive expression of IL-10 is elevated in
peripheral blood from patients with IPF compared with healthy
subjects
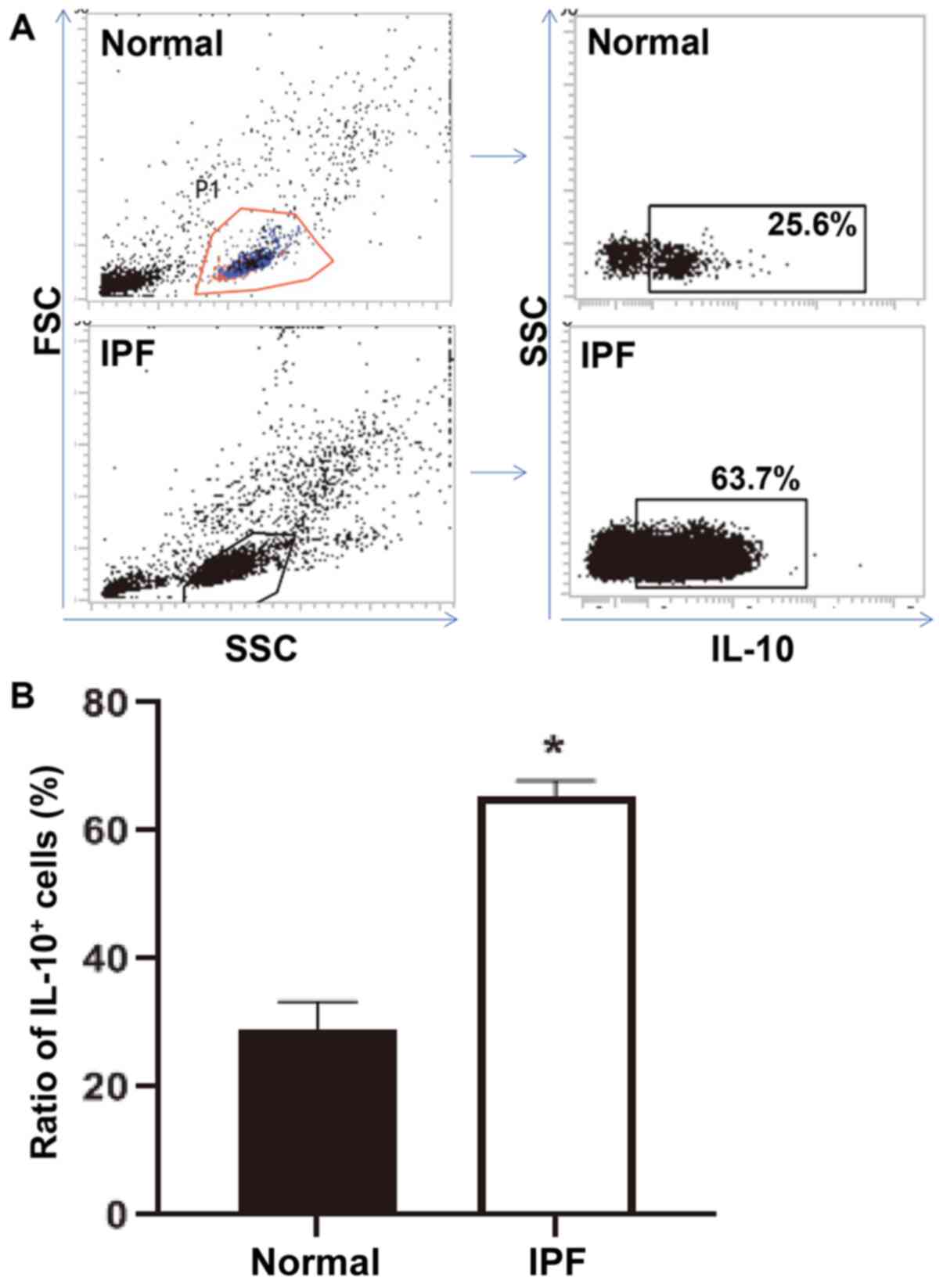
To detect the expression of IL-10 in peripheral
mononuclear lymphocytes, flow cytometry was performed. The data
demonstrated that the ratio of IL-10+ peripheral
mononuclear lymphocytes in patients with IPF was significantly
higher compared with that in normal subjects (P<0.05; Fig. 4A and B). Peripheral mononuclear lymphocyte
population itself looked much smaller in the normal subjects
compared with the patients with IPF, possibly because of the
proliferation of inflammatory cells caused by IPF inflammation. The
aforementioned result indicated that the ratio of peripheral
mononuclear lymphocytes with positive expression of IL-10 is
elevated in peripheral blood from patients with IPF.
Discussion
Chronic airway inflammation, myofibroblast
proliferation and ECM synthesis are the main mechanisms of
pulmonary subepithelial fibrosis in patients with interstitial
pneumonia (28,29). In recent years, pulmonary
fibroblasts have become important targets in improving airway
remodeling (30). Hence, the role
of pulmonary fibroblasts in airway remodeling in asthma has
attracted increasing attention. IL-10 is a type of cytokine that
has attracted a lot of attention in recent years (31). Numerous tissues and cells, such as T
cells, B cells, macrophages, bronchial epithelial cells and tumor
cells produce IL-10 (32,33). IL-10 is a multidirectional
immunoregulatory factor and its main activity is immunosuppression
(34). Studies have demonstrated
that IL-10 has different biological functions in a number of other
cells. For example, IL-10 induced the expression of E-selectin on
the surface of vascular endothelial cells and regulated the barrier
function of endothelial cells (35). Treatment targeting IL-10 inhibited
the apoptosis of CD8+ cells induced by dendritic cells
(36). In addition, IL-10 serves
important roles in lung injury and pneumonia. For example, the
expression of IL-10 and TGF-β in biopsy tissues of patients with
IPF was significantly increased compared with that from healthy
subjects and involved in pulmonary fibrosis, indicating that they
are important therapeutic targets (37). In addition, a study demonstrated
that IL-10 delivered by hydrogel improved treatment of
bleomycin-induced lung fibrosis in mice (25). Consistent with a previous report
(38), the present study revealed
that expression of IL-10 in peripheral blood of patients with IPF
was significantly increased compared with normal subjects,
suggesting that IL-10 may serve a role in pulmonary fibrosis.
Pulmonary fibroblasts serve important roles in pulmonary fibrosis
and secrete cytokines that are involved in the local inflammatory
response and collagen secretion inducing matrix microenvironment
remodeling (37). During the
occurrence and development of interstitial pneumonia, abnormal
proliferation of pulmonary fibroblasts occurs and the ability of
collagen synthesis increases significantly (39). In the present study, primary
pulmonary fibroblasts were cultured with the serum of patients with
IPF which stimulated the viability and collagen synthesis of MRC-5
cells and primary pulmonary fibroblasts. Following addition of
IL-10 antibody, the stimulation effect by IPF serum was reduced
which suggested that IL-10 promoted the viability and collagen
synthesis of lung fibroblasts.
IL-10 is a secretory cytokine that transmits
extracellular signals mainly by binding to its membrane surface
receptor IL-10R (40). IL-10R
mainly consists of IL-10R1 and IL-10R2 subunits, and IL-10 first
binds to IL-10R1 and then binds to IL-10R2 to activate downstream
signaling pathways (41).
IL-10/IL-10R pathway is involved in numerous pathological processes
(42). For example, detection of
IL-10 expression in serum may help predict the prognosis of
patients with rheumatoid arthritis (43). In addition, IL-10 promoted drug
resistance in non-small cell lung cancer (44). The present study demonstrated that
the expression of IL-10R1, but not IL-10R2, in primary fibroblasts
was increased significantly compared with untreated primary
fibroblasts after treatment with serum from IPF patients. Serum
from IPF patients could promote collagen secretion of fibroblasts,
and IL-10 antibody was able to block this effect. Following
interference with the expression of IL-10R1, the treatment effect
of IPF serum on primary fibroblasts was significantly weakened in
the present study. This suggested that IL-10/IL-10R1 promoted the
viability and collagen secretion of primary fibroblasts. IL-10 can
be secreted by a variety of cells, of which monocytes are the main
cells (45). Using flow cytometry,
the present study demonstrated that the number of IL-10-positive
mononuclear lymphocytes in peripheral blood of patients with IPF
was significantly higher compared with that in normal subjects,
which suggested that mononuclear lymphocytes secreted IL-10 in
patients with IPF and IL-10 promoted the viability and collagen
secretion of lung fibroblasts leading to pulmonary fibrosis.
The present study had several limitations. In the
current study, the heterogeneity of primary fibroblasts was very
large and even the generational differences between fibroblasts
from the same patient were very large. If cell sorting was used to
select monoclonal cells, the heterogeneity could have been avoided
to some extent, but diversity would have been lost at the same
time. In addition, the present study only suggested that monocytes
secrete IL-10 that acts on fibroblasts as there is infiltration by
a lot of monocytes in interstitial pneumonia. In order to prove
that IL-10 is derived from monocytes, more evidences required from
future studies.
In conclusion, the present study demonstrated that
the IL-10 level in peripheral blood of patients with IPF is
increased significantly compared with normal subjects. Activation
of the IL-10/IL-10R1 signaling pathway promotes the viability and
collagen synthesis of pulmonary fibroblasts leading to pulmonary
fibrosis. The present study explored the mechanism of IL-10 in the
occurrence and development of interstitial pneumonia, and provided
experimental basis for understanding the role of inflammatory
factors in this disease.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Subject Fund of Zhejiang
Provincial Science and Technology Hall (grant no. LGF19H010006) and
Lishui Science and Technology Bureau Project (grant no.
2016GYX29).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY, JP, XC, ZC, EC and JQ contributed to the design
of the study. HY, JP, XC, ZY, LL, EG, CX and HZ performed the
experiments. HY and JP confirm the authenticity of all the raw
data. HY, JP and XC analyzed the data. HY, JP, XC, ZC, EC and JQ
interpreted results and drafted the manuscript. All authors have
read and approved the manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Wenzhou Medical University
(Lishui, China) with approval no. IACUC-20180901-28. Written
informed consent was obtained from all subjects or their
families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hobbs BD, Putman RK, Araki T, Nishino M,
Gudmundsson G, Gudnason V, Eiriksdottir G, Zilhao Nogueira NR,
Dupuis J, Xu H, et al: Overlap of genetic risk between interstitial
lung abnormalities and idiopathic pulmonary fibrosis. Am J Respir
Crit Care Med. 200:1402–1413. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pereira CAC, Soares MR, Boaventura R,
Castro MDC, Gomes PS, Gimenez A, Fukuda C, Cerezoli M and Missrie
I: Squawks in interstitial lung disease prevalence and causes in a
cohort of one thousand patients. Medicine (Baltimore).
98(e16419)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Singh P, Thakur B, Mohapatra AK and Padhan
P: Clinical features and outcome of acute exacerbation in
connective tissue disease-associated interstitial lung disease: A
single-center study from India. Int J Rheum Dis. 22:1741–1745.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gasperini ML, Gigante A, Iacolare A,
Pellicano C, Lucci S and Rosato E: The predictive role of lung
ultrasound in progression of scleroderma interstitial lung disease.
Clin Rheumatol. 39:119–123. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Willemsen AECAB, Tol J, van Erp NP, Jonker
MA, de Boer M, Meek B, de Jong PC, van Moorsel C, Gerritsen WR,
Grutters JC and van*Herpen CML: Prospective study of drug-induced
interstitial lung disease in advanced breast cancer patients
receiving everolimus plus exemestane. Target Oncol. 14:441–451.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saito S, Alkhatib A, Kolls JK, Kondoh Y
and Lasky JA: Pharmacotherapy and adjunctive treatment for
idiopathic pulmonary fibrosis (IPF). J Thorac Dis. 11 (Suppl
14):S1740–S1754. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ussavarungsi K, Nugent K, Gerke AK,
Krasowski MD, Tuetken RS and Lenert PS: Interstitial lung disease
associated with anti-PM-Scl antibody: A single center experience.
Autoimmun Rev. 18(102355)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chai GT, Tan TC, Lee YS, Kaw GJ, Chuah KL,
Lim YJ, Abisheganaden JA and Thong BY: Impact of an interstitial
lung disease service in the diagnosis and management of
interstitial lung disease in Singapore. Singapore Med J.
61:302–307. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen Z, Wang X and Ye S: Tofacitinib in
Amyopathic Dermatomyositis-associated interstitial lung disease. N
Engl J Med. 381:291–293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hoffmann-Vold AM, Fretheim H, Halse AK,
Seip M, Bitter H, Wallenius M, Garen T, Salberg A, Brunborg C,
Midtvedt Ø, et al: Tracking impact of interstitial lung disease in
systemic sclerosis in a complete nationwide cohort. Am J Respir
Crit Care Med. 200:1258–1266. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ye Y, Fu Q, Wang R, Guo Q and Bao C: Serum
KL-6 level is a prognostic marker in patients with anti-MDA5
antibody-positive dermatomyositis associated with interstitial lung
disease. J Clin Lab Anal. 33(e22978)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee JU, Son JH, Shim EY, Cheong HS, Shin
SW, Shin HD, Baek AR, Ryu S, Park CS, Chang HS and Park JS: Global
DNA methylation pattern of fibroblasts in idiopathic pulmonary
fibrosis. DNA Cell Biol. 38:905–914. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kheirollahi V, Wasnick RM, Biasin V,
Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J,
Zhang JS, Kwapiszewska G, et al: Metformin induces lipogenic
differentiation in myofibroblasts to reverse lung fibrosis. Nat
Commun. 10(2987)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Caporarello N, Meridew JA, Jones DL, Tan
Q, Haak AJ, Choi KM, Manlove LJ, Prakash YS, Tschumperlin DJ and
Ligresti G: PGC1α repression in IPF fibroblasts drives a pathologic
metabolic, secretory and fibrogenic state. Thorax. 74:749–760.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Woodley DT: Distinct fibroblasts in the
papillary and reticular dermis: Implications for wound healing.
Dermatol Clin. 35:95–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schruf E, Schroeder V, Kuttruff CA, Weigle
S, Krell M, Benz M, Bretschneider T, Holweg A, Schuler M, Frick M,
et al: Human lung fibroblast-to-myofibroblast transformation is not
driven by an LDH5-dependent metabolic shift towards aerobic
glycolysis. Respir Res. 20(87)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Woodcock HV, Eley JD, Guillotin D, Platé
M, Nanthakumar CB, Martufi M, Peace S, Joberty G, Poeckel D, Good
RB, et al: The mTORC1/4E-BP1 axis represents a critical signaling
node during fibrogenesis. Nat Commun. 10(6)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ebrahimpour A, Shrestha S, Bonnen MD,
Eissa NT, Raghu G and Ghebre YT: Nicotine modulates growth factors
and MicroRNA to promote inflammatory and fibrotic processes. J
Pharmacol Exp Ther. 368:169–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nie Y, Zhang D, Qian F and Wu Y: Baccatin
III ameliorates bleomycin-induced pulmonary fibrosis via
suppression of TGF-β1 production and TGF-β1-induced fibroblast
differentiation. Int Immunopharmacol. 74(105696)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nenov MN, Konakov MV, Teplov IY and Levin
SG: Interleukin-10 facilitates glutamatergic synaptic transmission
and homeostatic plasticity in cultured hippocampal neurons. Int J
Mol Sci. 20(3375)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Krakow S, Crescimone ML, Bartels C,
Wiegering V, Eyrich M, Schlegel PG and Wölfl M: Re-expression of
CD14 in response to a combined IL-10/TLR stimulus defines
monocyte-derived cells with an immunoregulatory phenotype. Front
Immunol. 10(1484)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiao S, Huang G, Wei Z, Nie K, Liu Z, Deng
C and Wang D: IL-10 Gene-modified human amniotic mesenchymal stem
cells augment regenerative wound healing by multiple synergistic
effects. Stem Cells Int. 2019(9158016)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sheridan MP, Browne JA, Doyle MB,
Fitzsimons T, McGill K and Gormley E: IL-10 suppression of IFN-γ
responses in tuberculin-stimulated whole blood from Mycobacterium
bovis infected cattle. Vet Immunol Immunopathol. 189:36–42.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shamskhou EA, Kratochvil MJ, Orcholski ME,
Nagy N, Kaber G, Steen E, Balaji S, Yuan K, Keswani S, Danielson B,
et al: Hydrogel-based delivery of Il-10 improves treatment of
bleomycin-induced lung fibrosis in mice. Biomaterials. 203:52–62.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deterding RR, DeBoer EM, Cidon MJ,
Robinson TE, Warburton D, Deutsch GH and Young LR: Approaching
clinical trials in childhood interstitial lung disease and
pediatric pulmonary fibrosis. Am J Respir Crit Care Med.
200:1219–1227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Algamdi M, Sadatsafavi M, Fisher JH,
Morisset J, Johannson KA, Fell CD, Kolb M, Manganas H, Cox G,
Gershon AS, et al: Costs of workplace productivity loss in patients
with fibrotic interstitial lung disease. Chest. 156:887–895.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wen J, Lin X, Gao W, Qu B, Zuo Y, Liu R
and Yu M: Inhibition of LPA1 Signaling impedes
conversion of human Tenon's fibroblasts into myofibroblasts via
suppressing TGF-β/Smad2/3 signaling. J Ocul Pharmacol Ther.
35:331–340. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Saraiva M and O'Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Y, Sun SN, Liu Q, Yu YY, Guo J, Wang
K, Xing BC, Zheng QF, Campa MJ, Patz EF Jr, et al: Autocrine
complement inhibits IL10-dependent T-cell-mediated antitumor
immunity to promote tumor progression. Cancer Discov. 6:1022–1035.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang S, Tang X, Sheng X, Xing J and Zhan
W: Analysis of the role of IL-10 in the phagocytosis of
mIgM+ B lymphocytes in flounder (Paralichthys
olivaceus). Fish Shellfish Immunol. 92:813–820. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ramakrishna C, Kujawski M, Chu H, Li L,
Mazmanian SK and Cantin EM: Bacteroides fragilis polysaccharide A
induces IL-10 secreting B and T cells that prevent viral
encephalitis. Nat Commun. 10(2153)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vora M, Romero LI and Karasek MA:
Interleukin-10 induces E-selectin on small and large blood vessel
endothelial cells. J Exp Med. 184:821–829. 1996.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Qiao J, Liu Z, Dong C, Luan Y, Zhang A,
Moore C, Fu K, Peng J, Wang Y, Ren Z, et al: Targeting Tumors with
IL-10 prevents dendritic cell-mediated CD8+ T cell
apoptosis. Cancer Cell. 35:901–915.e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bergeron A, Soler P, Kambouchner M,
Loiseau P, Milleron B, Valeyre D, Hance AJ and Tazi A: Cytokine
profiles in idiopathic pulmonary fibrosis suggest an important role
for TGF-beta and IL-10. Eur Respir J. 22:69–76. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Papiris SA, Tomos IP, Karakatsani A,
Spathis A, Korbila I, Analitis A, Kolilekas L, Kagouridis K,
Loukides S, Karakitsos P and Manali ED: High levels of IL-6 and
IL-8 characterize early-on idiopathic pulmonary fibrosis acute
exacerbations. Cytokine. 102:168–172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wollin L, Distler JH, Redente EF, Riches
DW, Stowasser S, Schlenker-Herceg R, Maher TM and Kolb M: Potential
of nintedanib in treatment of progressive fibrosing interstitial
lung diseases. Eur Respir J. 54(1900161)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kotlarz D, Beier R, Murugan D,
Diestelhorst J, Jensen O, Boztug K, Pfeifer D, Kreipe H, Pfister
ED, Baumann U, et al: Loss of interleukin-10 signaling and
infantile inflammatory bowel disease: Implications for diagnosis
and therapy. Gastroenterology. 143:347–355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Glocker EO, Kotlarz D, Boztug K, Gertz EM,
Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan
D, et al: Inflammatory bowel disease and mutations affecting the
interleukin-10 receptor. N Engl J Med. 361:2033–2045.
2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Engelhardt KR, Shah N, Faizura-Yeop I,
Kocacik Uygun DF, Frede N, Muise AM, Shteyer E, Filiz S, Chee R,
Elawad M, et al: Clinical outcome in IL-10- and IL-10
receptor-deficient patients with or without hematopoietic stem cell
transplantation. J Allergy Clin Immunol. 131:825–830.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
McDonald S, Reed R and Baricevic-Jones I:
Biologics in Rheumatoid Arthritis Genetics and Genomics Study
Syndicate. Ling S, Plant D and Barton A: Can serum interleukin-17
and interleukin-10 levels predict response to biologic treatments
in patients with rheumatoid arthritis? Rheumatology (Oxford).
58:1872–1873. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vahl JM, Friedrich J, Mittler S, Trump S,
Heim L, Kachler K, Balabko L, Fuhrich N, Geppert CI, Trufa DI, et
al: Interleukin-10-regulated tumour tolerance in non-small cell
lung cancer. Br J Cancer. 117:1644–1655. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lopes RL, Borges TJ, Zanin RF and Bonorino
C: IL-10 is required for polarization of macrophages to M2-like
phenotype by mycobacterial DnaK (heat shock protein 70). Cytokine.
85:123–129. 2016.PubMed/NCBI View Article : Google Scholar
|