Introduction
Osteoarthritis (OA) is a joint disease that is
caused by a variety of pathogenic factors. One of the main
pathological features of OA is the gradual degradation of hyaline
cartilage (1,2). Other manifestations include increased
risk of fracture or the loss of articular cartilage, exposure of
the subchondral bone and the formation of osteophytes (3). The articular cartilage, which is the
hyaline cartilage, mainly consists of type II collagen and
proteoglycans (4). During the
onset of OA, type II collagen is degraded and proteoglycans are
lost in large quantities, resulting in reductions in compressive
strength and elasticity of the cartilage whilst increasing the
pressure load, which aggravates mechanical damage (5). Patients with OA predominantly
experience arthralgia, swelling, deformity, limited range of motion
and loss of function, which seriously affects their quality of
life, particularly among the middle-aged and elderly population
(6,7).
Achyranthes bidentata has been extensively
used for the treatment of OA due to its reported anti-inflammatory
and antioxidant properties (8,9).
Achyranthes bidentata polysaccharides (ABPS) is a
water-soluble form of fructan that can be extracted from the
Achyranthes bidentate plant (10). The chemical structure of ABPS is
dominated by furan rings, where its molecular weight is in the
range of 1.4-3.4 kDa (11). ABPS
has been widely applied for the treatment of bone-related diseases,
such as osteoporosis and OA, due to its observed protective effects
on the bone (12,13). As an anti-inflammatory agent, ABPS
has been previously shown to interfere with the inflammatory
activation that occurs during OA and the metabolic pathway of
arachidonic acid (13). In a
castrated rat model of osteoporosis, ABPS has been documented to
significantly increase the bone-mineral content and biomechanical
properties of the femur, which protected these rats from
osteoporosis by stimulating bone formation (14). ABPS has also been shown to
effectively promote the proliferation of chondrocytes by promoting
G1/S cell cycle progression, suggesting that ABPS may be
a potential drug for the treatment of OA (15). However, the precise role and
mechanism of action mediated by ABPS on OA remains unclear.
Therefore, the present study investigated the specific mechanism of
action ABPS on OA from the perspective of cartilage matrix
homeostasis.
Emerging studies have shown that long non-coding
RNAs (lncRNA) serve an important role in OA (16). In particular, the expression of the
lncRNA growth arrest-specific transcript 5 (GAS5) has been found to
be upregulated in the OA cartilage, which may exacerbate cartilage
degeneration by promoting chondrocyte apoptosis (17). Therefore, the aim of the present
study is to verify if the mechanism of ABPS on OA acts through
lncRNA GAS5 and associated signaling molecules. This hypothesis was
further tested using viral transfection for in vivo and
in vitro experiments.
Materials and methods
Main reagents
ABPS (cat. no. B25872; Lot no. C22M7Y11580;
Shanghai Yuanye Bio-Technology Co., Ltd). HE Staining Kit (cat. no.
G1120; Lot no. 20180111; Beijing Solarbio Science & Technology
Co., Ltd.). RIPA buffer (Beyotime Institute of Technology). PMSF
(Beijing Solarbio Science & Technology Co., Ltd.). HiScript II
1st Strand cDNA Synthesis Kit (cat. no. R211-02; lot no.
L/N:7E460H0; Vazyme Biotech Co., Ltd.). ChamQ Universal
SYBR® qPCR Master Mix (cat. no. Q711-02; lot no.
7E410L0; Vazyme Biotech Co., Ltd.). MMP-9 (1:1,000; cat. no.
ab76003; lot. no. GR324586-17; Abcam) primary antibody. MMP-13
(1:1,000; cat. no. 18165-1-AP, lot no. 00089106; Proteintech Group,
Inc.) primary antibody. Tissue inhibitor of metalloproteinases
(TIMP) 1 (1:1,000; cat. no. 16644-1-AP, lot. no. 00023865;
Proteintech Group; Inc.) primary antibody. TIMP-3 (1:1,000; cat.
no. 10858-1-AP, lot. no. 00091772; Proteintech Group, Inc.) primary
antibody. Type II collagen (1:1,000; cat. no. ab188570, lot. no.
GR3233600-8; Abcam) primary antibody. GAPDH rabbit polyclonal
(1:1,000; cat. no. 10494-1-AP, cat. no. 00098110; Proteintech
Group, Inc.) primary antibody. Goat Anti-Rabbit IgG (H+L) HRP
conjugate (1:5,000; cat. no. SA00001-2; lot no. 20000258;
Proteintech Group, Inc.) secondary antibody.
In vivo experiments
Animals were purchased from Shanghai Slack
Laboratory Animal Co., Ltd. [Qualification No. SCXK (Shanghai)
2017-0005]. The temperature of the rearing environment was
controlled at 23-25˚C, the humidity was 55-60%, the air was changed
16 times per h and the cyclic light conditions were automatically
controlled at 12-h light/dark cycle. Experimental animals were
provided food and water ad libitum. The breeding environment
and dietary conditions of experimental animals are in line with the
standards of the Experimental Animal Center of Fujian University of
Traditional Chinese Medicine [license no. SYXK (Fujian;
2019-0007)]. In total, 45 2-month-old male Sprague-Dawley rats were
fed adaptively during the first week, weighed, numbered and
randomly divided into three groups. Randomization was performed
using the ‘RAND’ function on Microsoft Excel (Microsoft
Corporation) 2016(18).
Ultimately, 45 animals were assigned to each of the three following
groups: Normal group (n=15), model group (n=15) and the ABPS group
(n=15). An OA model was constructed according to a modified version
of the Hulth method (19). The
model group and the ABPS group were modeled by the modified Hulth
method. In the normal group, only the skin was incised (the joint
capsule and ligament were not incised) and finally sutured. The
rats were first anaesthetized with 5% isoflurane at 2 l/min
O2 using a mask and maintained at 2%. They were then
fixed in a supine position, depilated at the right knee and locally
disinfected with 75% ethanol. A 1-cm longitudinal incision was then
made on the medial side of the right knee. The medial collateral
ligament and the anterior cruciate ligament were cut through the
incision, and the medial meniscus was removed. Finally, the joint
capsule and the skin were sutured and disinfected again with 75%
ethanol. After modeling, penicillin 20x104 U was
injected intraperitoneally for 3 days to prevent site infection.
From week 6 after modeling, the normal and model groups were given
normal saline at a dose of 10 ml/(kg·day), whereas the ABPS group
was given a clinically equivalent dose of ABPS at 400 mg/(kg·day)
(20). Almost all previous studies
opted for the oral intake method for ABPS for treating bone
diseases, such as osteoporosis (12,20,21)
and OA (13). Since the present
study was focused on exploring the protective mechanism of ABPS
against OA, the most common delivery method, which is the oral
gavage, was adopted for the rats. The intragastric dose was
adjusted on a weekly basis according to body weight to 10
ml/(kg·day). The gavage time was between 9 AM and 1 PM. The daily
gavage sequence was random, where each animal was treated at a
different time on each day. Rats were dosed six times a week for a
total of 8 weeks of treatment.
The experiment lasted for a total of 14 weeks. After
ABPS or vehicle treatment, each group was deeply anesthetized by 5%
isoflurane followed by euthanasia using cervical dislocation. All
the experiments were performed under the ethical principles of
animal experiments to reduce the suffering of experimental animals.
All experimental procedures were approved by the Animal Use and
Care Committee of Fujian University of Chinese Traditional Medicine
(approval no. 2017026).
Histological evaluation
Knee joint cartilage tissue and subchondral bone
tissue were first fixed in 4% paraformaldehyde at room temperature
for 2 days and placed in ethylenediaminetetraacetic acid disodium
salt (EDTA-2Na) for 8 weeks at room temperature. Tissues were
sequentially placed in 50, 70, 80, 95, 100% ethanol to complete
dehydration for 45 min at room temperature for each gradient. The
embedding frame containing the tissue was then placed in xylene I
and xylene II in sequence, and placed in a 65˚C oven for 1 h each.
Finally, the dissolved paraffin is introduced into the embedding
frame, and after the wax block is completely solidified, the
embedding frame was disassembled and the wax block taken out.
Paraffin blocks were cut into 8-µm serial sections for standard HE
staining and Safranin O-fast green staining (Beijing Solarbio
Science & Technology Co., Ltd.). In HE staining, after the
paraffin sections were baked in a 65˚C oven for 30 min, they were
placed at room temperature with xylene I and xylene II for 5 min
each. Then rehydrate with graded ethanol of 100, 95, 85 and 75% for
3 min. Then, a series of operations such as hematoxylin staining (5
min), differentiation solution (2 min) and eosin staining (1 min)
were used, and finally dehydration and mounting were performed. In
Safranin O-fast green staining, the paraffin sections were first
baked in a 65˚C oven for 30 min and then the paraffin sections were
deparaffinized and rehydrated. Subsequent steps were carried out at
room temperature. Weigert staining was performed for 3 min, acid
differentiation solution for 10 sec, and fast green staining
solution for 5 min. Following washing with weak acid solution for
10 sec, staining in safranine staining solution for 5 min and
finally dehydrating and sealing. Histopathological changes in the
cartilage and subchondral bone were observed under a light
microscope (DM4000 B; Leica Microsystems GmbH). Original
magnification, x200. Rat tissue sections were evaluated using the
modified Wakitani scoring system (22,23),
which consists of the following five items: Cell morphology (0-4),
matrix-staining or metachromatic staining (0-3), surface regularity
(0-3), thickness of the cartilage (0-2) and integration of repaired
tissue to the surrounding articular cartilage (0-2). The total
score is 0 to 14 points (n=3 rats in each group), divided into
three groups of three repetitions, each repetition of three fields
of view, for a total of nine fields of view.
LncRNA GAS5 in OA cartilage as
detected using reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using an RNA isolator Total
RNA Extraction Reagent (cat. no. R401-01; Lot no. 7E490L0; Vazyme
Biotech Co., Ltd.). The total RNA was then reverse transcribed into
cDNA according to the protocols of the reverse transcription kit.
Next, the cDNA was configured and loaded according to the steps of
the qPCR reaction kit. Gene expression was detected on the Bio-Rad
CFX96 real-time fluorescent quantitative PCR instrument. The
following primers pairs were used for the qPCR: LncRNAGAS5 (Rat)
forward, 5'-GGGATGGTGGAGTTTGAATCAG-3' and reverse,
5'-GCTTGCCATGCCTTCAGTTA-3' and GAPDH forward,
5'-ACGGCAAGTTCAACGGCACAG-3' and reverse,
5'-GAAGACGCCAGTAGACTCCACGAC-3'.
Western blotting for the in vivo
experiment
The rats were deeply anesthetized by the inhalation
of 5% isoflurane, before the cartilage tissue from the right
femoral condyle and tibial plateau was obtained. Total protein was
then extracted from the cartilage tissue using the RIPA buffer
(Beyotime Technology, Shanghai, China). The protein concentration
was determined using the BCA method, before 20 µg protein sample
was loaded into each well of a 10% sodium lauryl
sulfate-polyacrylamide gel, after which electrophoresis separation
was performed. The semi-dry proteins were then transferred onto a
PVDF membrane, followed by washing with TBST containing 0.05% Tween
20. The membrane was then incubated in NcmBlot Blocking Buffer
(Alexan Biotech Co., Ltd.) at room temperature for 2 h. Next, the
membrane was incubated in either of the following primary
antibodies overnight at 4˚C: (Rabbit polyclonal) MMP-9, MMP-13,
TIMP-1, TIMP-3, type II collagen and GAPDH. The following day, the
membranes were washed three times with TBST to remove any residual
antibodies. The membranes were then incubated in the Goat
Anti-Rabbit IgG (H+L), HRP conjugate for 1 h at room temperature,
followed by another round of washing with TBST. An appropriate
amount of BeyoECL Plus (Beyotime Institute of Biotechnology) was
then added to the membrane and the reaction was stopped after 1
min. The membrane was then exposed and developed. Using Image Lab
software version 3.0 (Bio-Rad Laboratories, Inc.), GAPDH was used
as the internal reference to analyze the gray value ratio of the
target protein.
Chondrocyte culture
The rats were anesthetized under 5.0% isoflurane and
sacrificed by cervical dislocation after they lost consciousness.
The articular cartilage was then separated from both knee joints,
transferred to PBS containing penicillin and streptomycin and
washed three times. The cartilage was cut into ~1 mm3,
then 5 ml of 0.2% type II collagenase was added and put into a
37˚C, 5% CO2 incubator for digestion for 4 h and then
transferred to a sterile centrifuge tube for centrifugation (137.4
x g for 3 min in a 4˚C centrifuge.). The supernatant was removed, 5
ml of DMEM containing 10% FBS was added to the lower sedimentary
tissue to terminate the digestion, and it was transferred to a
culture flask for culture at 37˚C in an incubator containing 5%
CO2. The culture medium was changed every 2 days and
primary chondrocytes were finally obtained. Type II collagenase,
FBS and DMEM were obtained from HyClone (Cytiva). In culture, the
large number of dedifferentiated chondrocytes were subsequently
passaged three to four times, after which the chondrocyte phenotype
was lost. Therefore, only second-generation chondrocyte cultures
were used for the present study.
Construction of lncRNA GAS5-knockdown
chondrocytes
A third-generation lentiviral packaging system was
used in the present study to infect chondrocytes with Lenti-lncRNA
GAS5. The lentivirus was packaged by Zolgene Biotechnology Co.,
Ltd. lncRNA GAS5-shRNA (cat. no. ZL-21P1048). 293FT cells
(Invitrogen, USA) were plated into 10 cm-dish (5 million/dish) and
incubated at 37˚C and 5% CO2 in DMEM (Invitrogen; Thermo
Fisher Scientific, Inc.). On the next day, the 293FT cells were
~80% confluent and the lentiviral transfection was performed by
adding pMDLg/pRRE (6 µg), pRSV-Rev (3 µg), pMD2.g (2 µg), shuttle
plasmid (9 µg) and Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.; 40 µl). The full name of the shRNA
plasmid was plvx-shRNA-puro. Following 72 h incubation at 37˚C, the
293FT lentiviral supernatant was harvested and used at 1, 4, 8, 12
and 16 multiplicities of infections (MOI) to transfect the
chondrocyte cells in the log phase of the growth plated in six-well
plates, using 5 µg/ml of polybrene per well at 37˚C overnight.
After 6 h of infection, 1 ml DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) containing 10% FBS was added into each well and
incubated at 37˚C overnight. After 24 h of transfection, the
virus-containing medium was aspirated before 2 ml fresh medium was
added and the culturing was continued. GFP was transiently
expressed 50 h after transfection, and the green fluorescent signal
observed under a fluorescence microscope (Carl Zeiss AG;
magnification, x100). At least three fields of view were checked
for each MOI value and the MOI value with the fluorescence area
>80% and the best cell viability was generally chosen.
Fluorescence in situ hybridization
(FISH)
FISH assay was performed to measure the local
expression of the lncRNA GAS5 in chondrocytes. In the present
study, GAS5 RNA probe labeled by digoxigenin (DIG) were selected.
All probes were custom synthesized by General Biosystems (Anhui)
Corporation Limited. Gas5-rat probe:
5'-CCACCATCCCATTTTCTGGCTTCCCATTCT(TTTCATCATCATACATCATCAT)30-3'; and
DIG-labeled probe: 5'-DIG-TTATGATGATGTATGATGATGT-3'.
(TTTCATCATCATACATCATCAT) 30-3' is a repeated nucleic acid sequence
with 30 repeats. Gene ID for GAS5: 81714. The second-labeled probe
had a digoxigenin label, and the second-labeled probe was
complementary to the repeating sequence of the first-labeled probe
and can be completely combined. The GAS5 probe sequence was
untagged. The slides were immersed in chondrocyte culture medium to
transfer chondrocytes to the slides for growth. Slides were fixed
with 4% paraformaldehyde (DEPC) for 20 min at 37˚C and washed with
PBS (pH 7.4) in a decolorizing shaker. Proteinase K (20 µg/ml; cat.
no. G1205; Wuhan Service bio Technology Co., Ltd.) was added to
cover the tissue and incubated at 37˚C for 2 h. The hybridization
buffer without probe (cat. no. G3016-3; Wuhan Service bio
Technology Co., Ltd.) was added onto the specimen and incubated at
37˚C for 1 h. The solution covering the samples was then replaced
with hybridization buffer containing GAS5-rat probe at a
concentration of 500 nM and hybridized overnight in a humidified
chamber at 42˚C. After removing the hybridization solution,
gradient washes were performed under saline-sodium citrate (SSC)
buffer (2X SSC at 37˚C for 10 min, 1X SSC at 37˚C for 10 min, and
0.5X SSC at 37˚C for 10 min). The DIG-labeled (1:400) probe was
added dropwise and incubated at 42˚C for 3 h. The blocking solution
(Wuhan Service bio Technology Co., Ltd.) was added to the specimen
and incubated at room temperature for 30 min. The blocking solution
was replaced by the anti-DIG-HRP antibody (1:10,000; cat. no.
200-032-156; Jackson ImmunoResearch Laboratories INC.) and
incubated at 37˚C for 40 min, before the specimen was washed four
times for 5 min in PBS. The sample was dried and CY3-TSA reagent
added to the sample and the reaction was performed at room
temperature for 5 min in the dark. After washing with PBS, the
nuclei were counterstained with DAPI and incubated in the dark for
8 min. After rinsing, anti-fluorescence quenching mounting medium
was added dropwise to mount the slides. (CY3-TSA reagent, DAPI
staining solution and anti-fluorescence quenching mounting medium
were purchased from Wuhan Service bio Technology Co., Ltd). Imaging
was performed using a fluorescence microscope (magnification,
x100).
Relative expression of lncRNA GAS5 in
lncRNA GAS5-silenced chondrocytes
RT-qPCR was performed to measure the relative
expression of the lncRNA GAS5 in the cultured chondrocytes
transfected with or without the sh-lncRNA GAS5. For this
experiment, cultured chondrocytes were divided into the following
groups: blank + Lenti-control, blank + Lenti-lncRNA GAS5, IL-1β +
Lenti-NC, and IL-1β + Lenti-lncRNA GAS5 group. Chondrocytes were
treated with 10 ng/ml IL-1β (cat. no. MKCL5731; MilliporeSigma) at
37˚C for 24 h. The RT-qPCR protocol for in vitro cell
experiments was the same as that used for the in vivo
samples described above.
Protein expression of MMP-9, MMP-13,
TIMP-1, TIMP-3 and type II collagen in lncRNA GAS5-silenced
chondrocytes
In vitro cell experiments used the same
western blotting protocol as described above for in vivo
samples.
Relative expression of lncRNA GAS5 in
IL-1β-treated chondrocytes following ABPS treatment
Cultured chondrocytes were divided into the
following treatment groups: Control group, IL-1β group (24) and ABPS groups (15). The IL-1β group was treated with 10
ng/ml IL-1β at 37˚C for 24 h, and the ABPS group was treated with
50, 100 and 150 µg/ml ABPS at 37˚C for 48 h, respectively. The
RT-qPCR protocol for in vitro cell experiments is the same
as that used for the in vivo samples described above. Total
RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was reverse transcribed into cDNA. The subsequent RNA detection
was performed according to the real-time quantitative PCR kit.
Briefly, 5 µl RNA sample was mixed with DEPC water solution at a
ratio of 1:10, before the optical density (OD) value was measured
on a DNA quantitative photometer. The OD260/OD280 of the RNA sample
was qualified to be between 1.8 and 2.0. A value <1.8 mean a
higher ratio of proteins than the extracted mRNA, which was deemed
to be of insufficient quality. After the reaction, RT-qPCR
amplification and dissolution curves were confirmed before the
results were quantified.
Protein expression of MMP-9, MMP-13,
TIMP-1, TIMP-3 and type II collagen in IL-1β-treated chondrocytes
after ABPS treatment
In vitro cell experiments used the same
western blotting protocol as described above for in vivo
samples. Protein was extracted from cultured chondrocytes by RIPA
buffer to generate a cell lysate, before the protein concentration
was subsequently determined using the BCA protein concentration
assay. Electrophoresis, film transfer, sealing, primary antibody
incubation, secondary antibody incubation, substrate color
development, image acquisition by gel imaging analysis system and
quantitative analysis of the absorbance value of each strip were
used to calculate the ratio of each sample to an internal reference
protein absorbance value.
Statistical analysis
Data were analyzed using the SPSS 26 software (IBM
Corp.) and were presented as the mean ± standard deviation. The
measurement data were tested using one-way analysis of variance
followed by Tukey's post hoc test. Wakitani scores were analyzed by
Kruskal-Wallis test followed by Dunn's post hoc test. P<0.05 was
considered to indicate a statistically significant difference. Each
experiment was replicated three times in the in vitro
experiments. In the in vivo experiments, 15 animals were
assigned to each of the three following groups: Normal group, model
group and the ABPS group.
Results
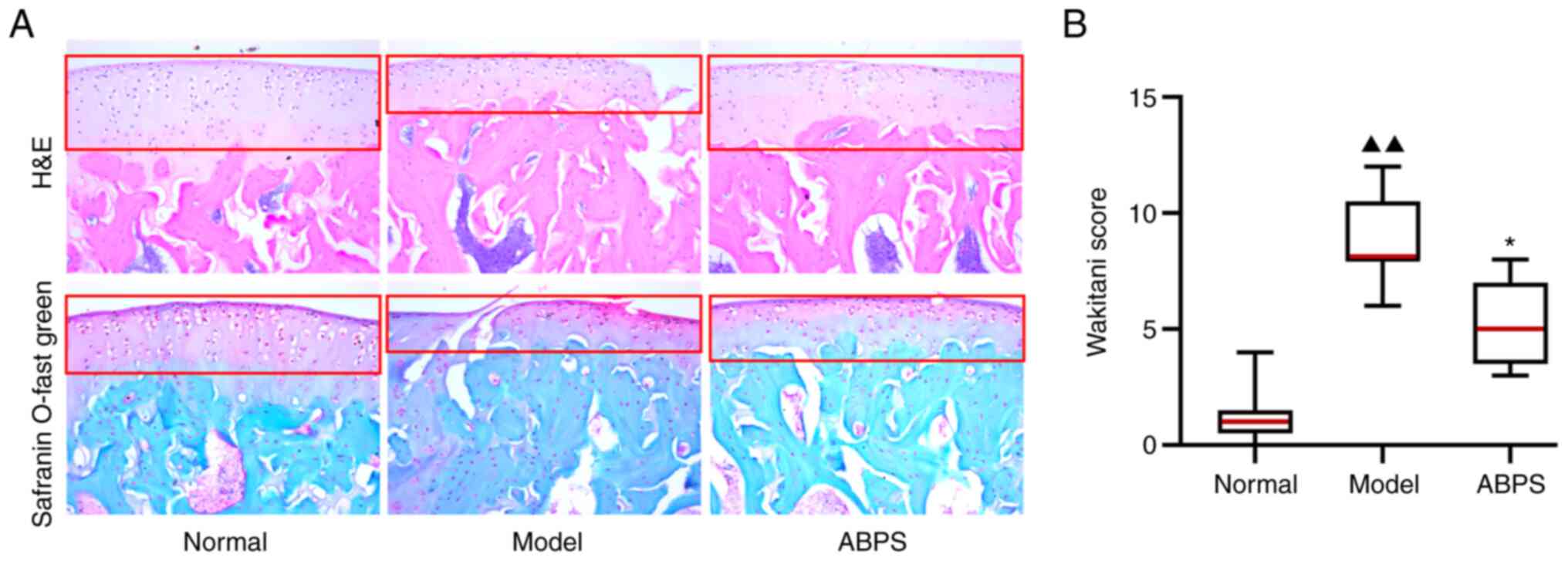
Histological evaluation results
The morphology of cartilage collected from control,
OA model and ABPS-treatment groups was analyzed using HE-staining
and Safranin O-fast green staining of tissue sections of the
femoral condyle. The results demonstrated that the transparent
cartilage layer in the OA model group displayed a rough surface and
a discontinuous structure compared with that in the control and
ABPS group, which received ABPS for eight weeks after OA modeling
(Fig. 1A). Subsequently, the
average modified Wakitani score in the model group was found to be
significantly higher compared with that in the normal group
(P<0.01), which was significantly reversed after ABPS
administration (P<0.01; Fig.
1B). suggesting that ABPS can promote the repair of articular
cartilage in rats with OA.
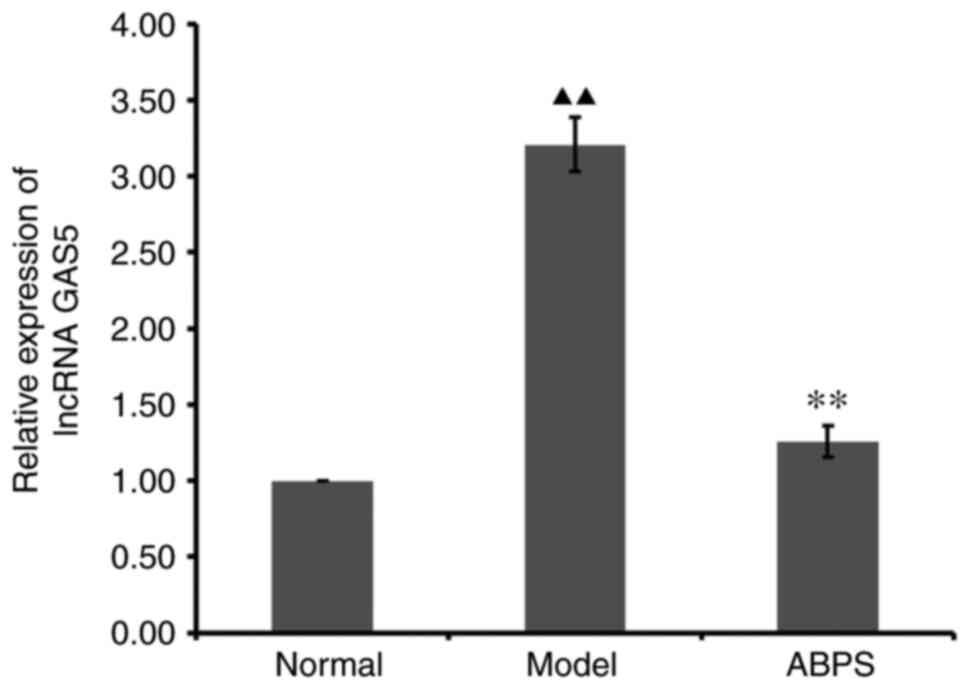
RT-qPCR results of in vivo
chondrocytes treated with ABPS
ABPS demonstrated regulatory ability in the
expression of lncRNA GAS5 (Fig.
2). Compared with that in the normal group, the expression of
lncRNA GAS5 in the OA cell model group was significantly increased
(P<0.01; Fig. 2). Compared with
that in the OA cell model group, the ABPS-treated group exhibited
significantly reduced lncRNA GAS5 expression levels (P<0.01;
Fig. 2).
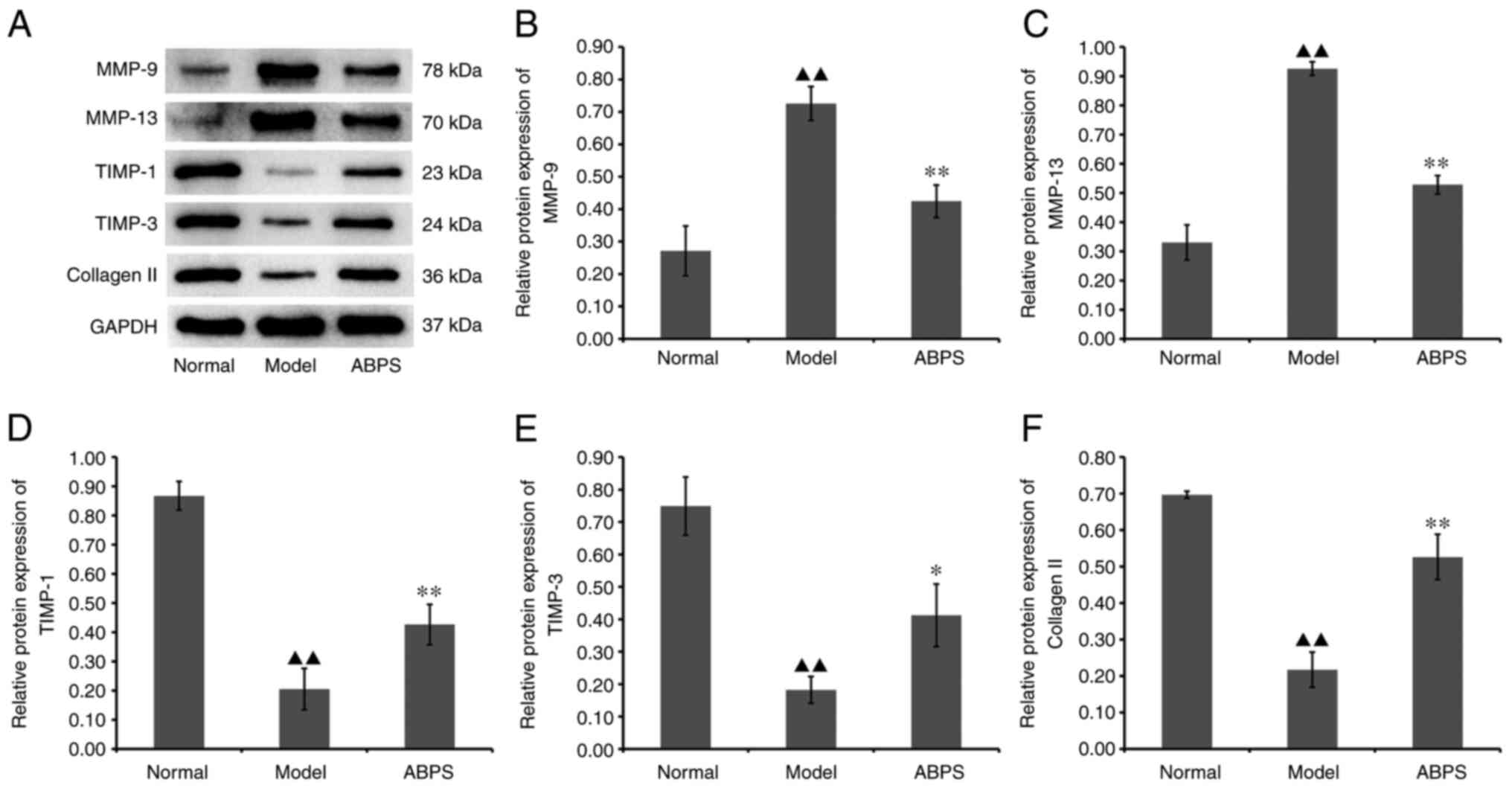
Western blotting results after in vivo
chondrocyte ABPS treatment
ABPS was demonstrated to exert a regulatory effect
on the expression of MMP-9, MMP-13, TIMP-1, TIMP-3 and type II
collagen in the cartilage of rats with OA (Fig. 3). Compared with that in the normal
group, the expression of MMP-9 and MMP-13 in the model group was
significantly increased, whereas that of TIMP-1, TIMP-3 and type II
collagen was significantly downregulated (P<0.01; Fig. 3). Compared with that in the model
group, the ABPS group showed significantly reduced MMP-9 and MMP-13
protein expression, whilst TIMP-1, TIMP-3 and type II collagen
protein expression was significantly increased (P<0.05 or
P<0.01; Fig. 3). These results
suggest that ABPS can inhibit the expression of lncRNA GAS5 to
delay the degradation of extracellular matrix (ECM) proteins by
chondrocytes in OA.
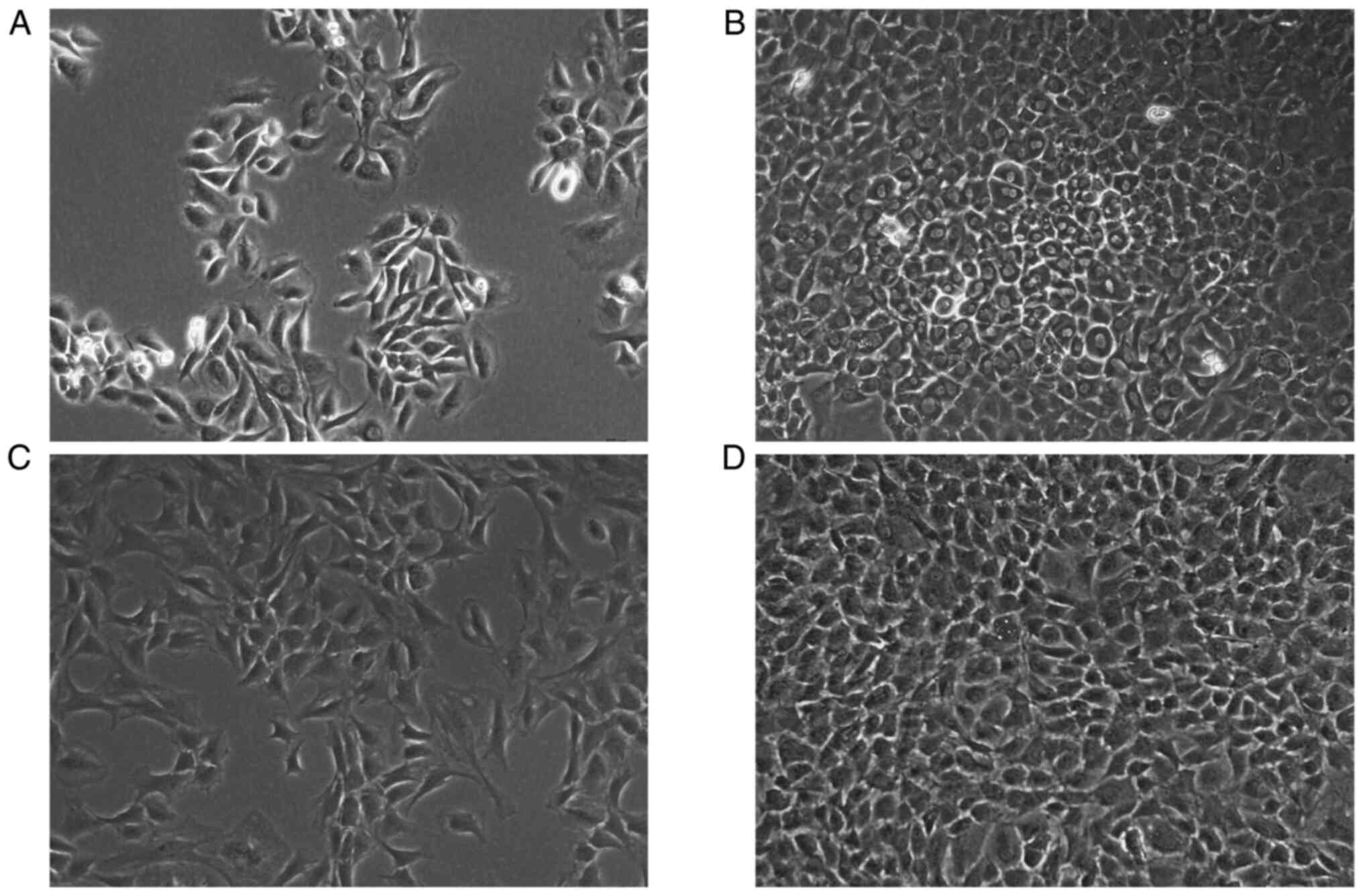
Observation of primary
chondrocytes
Chondrocytes were observed at different times of
culture to monitor their proliferation and distribution
characteristics in primary culture. The primary chondrocytes were
isolated from normal rats and seeded into culture flasks, which
displayed irregular, polygonal shapes after 6 days of culture
(Fig. 4A). Primary chondrocytes
began to grow in clusters on the 7th day, and the number of
chondrocytes accounted for more than 90% of the space under the
microscope (Fig. 4B). At first
passage, the chondrocytes assembled into uniformly distributed
cells by day 3 (Fig. 4C). After
second passage, the chondrocytes occupied >80% of the culture
flask (Fig. 4D). Therefore,
cultured second-generation primary chondrocytes were chosen to
conduct subsequent experiments.
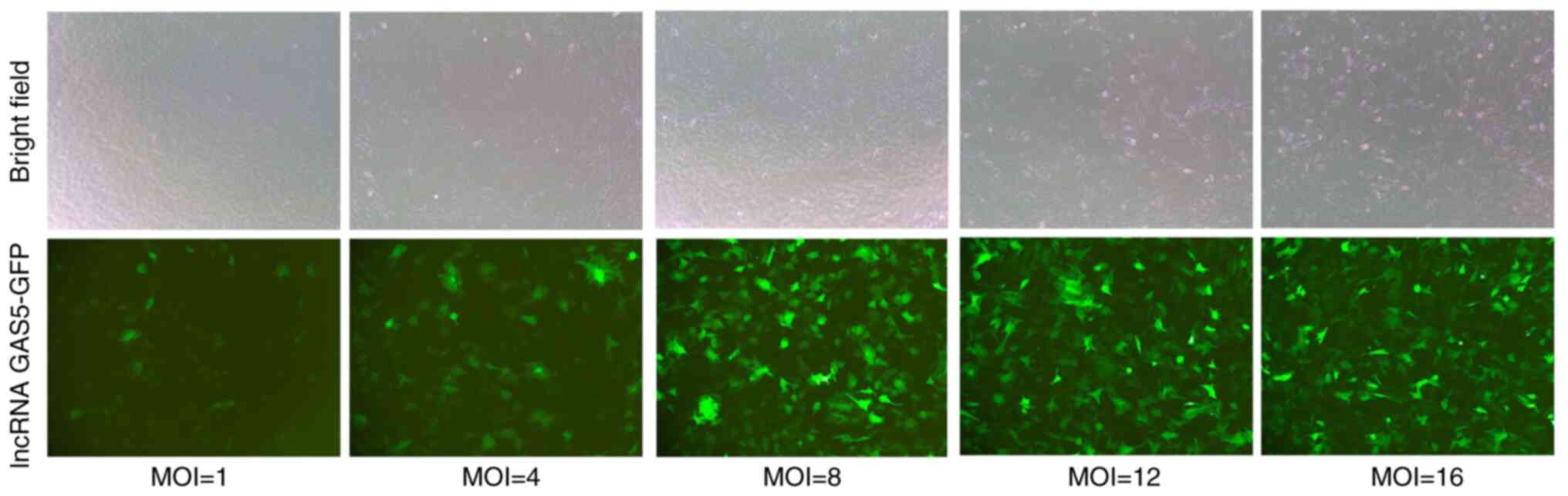
MOI of lncRNA GAS5 transfection
The logarithmic growth of chondrocytes whose density
was 50% on the second culture day were seeded into six-well plates.
Polybrene (5 µg/ml) and lncRNA GAS5 in 1, 4, 8, 12 and 16 MOIs were
added into each of the six-well plates. Fluorescent images were
taken 50 h after transfection. At MOI=8, the transfection
efficiency of lncRNA GAS5 was considered to be high, at which point
the survival rate of chondrocytes remained optimal (Fig. 5).
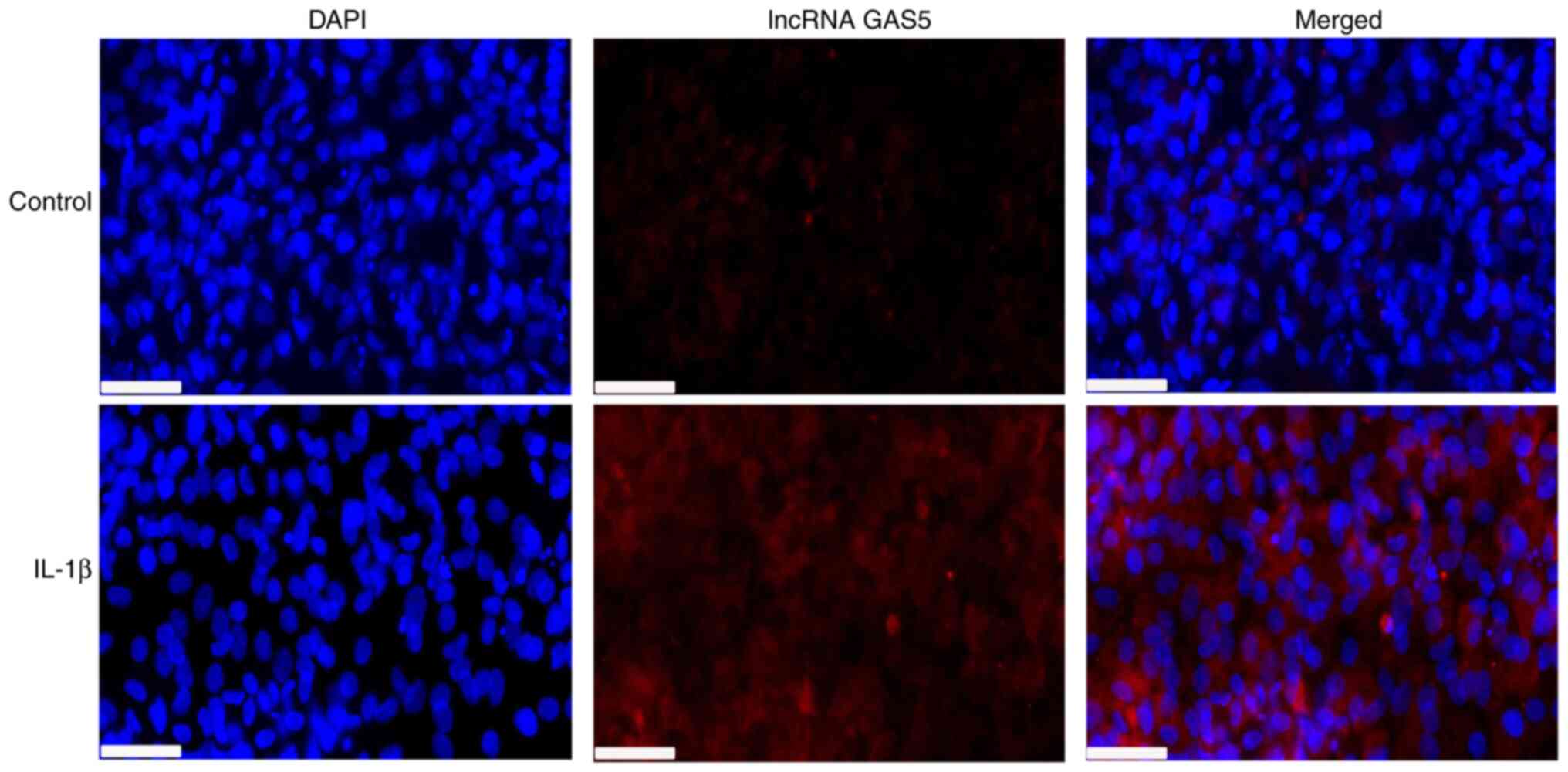
FISH results
LncRNA GAS5 was found to be mainly expressed in the
chondrocyte cytoplasm, suggesting that lncRNA GAS5 could interact
with other RNAs or proteins in the cytoplasm of chondrocytes. IL-1β
treatment was found to increase the expression of lncRNA GAS5 in
chondrocytes (Fig. 6).
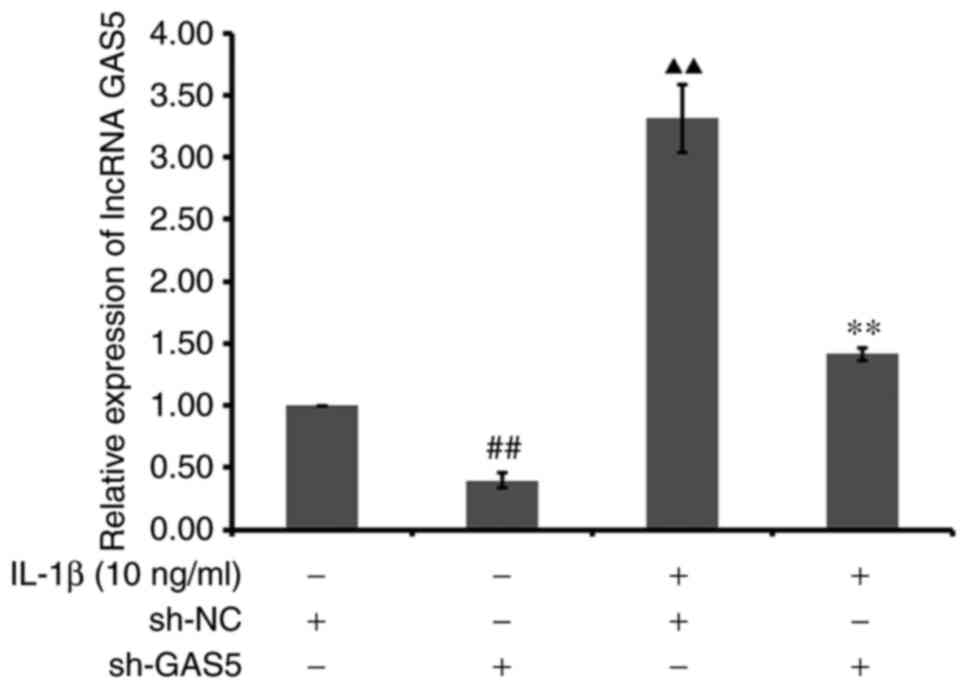
Sh-lncRNA GAS5 transfection can
effectively regulate the homeostasis of ECM proteins by
chondrocytes treated with IL-1β
The transfection efficiency of sh-lncRNA GAS5 was
verified using RT-qPCR, which showed that the relative expression
of lncRNA GAS5 in chondrocytes in the IL-1β + Lenti-lncRNA GAS5
group was significantly reduced compared with that in the IL-1β +
Lenti-NC group (P<0.01). Compared with blank + Lenti-NC group,
the relative expression of lncRNA GAS5 in chondrocytes of blank +
Lenti-lncRNA GAS5 group was significantly decreased (P<0.01;
Fig. 7).
After lncRNA GAS5 expression was knocked down, the
expression of MMP-9, MMP-13, TIMP-1, TIMP-3 and type II collagen
were normalized in chondrocytes treated with IL-1β. Compared with
that in the blank+Lenti-NC group, the expression of MMP-9 and
MMP-13 in the IL-1β + Lenti-NC group was significantly increased
(P<0.01), whereas that of TIMP-1, TIMP-3 and type II collagen
was significantly downregulated (P<0.01; Fig. 8). Furthermore, the expression of
MMP-9 and MMP-13 in the IL-1β + Lenti-lncRNA GAS5 group was
significantly decreased (P<0.01), whereas that of TIMP-1
(P<0.05), TIMP-3 (P<0.05) and type II collagen was
upregulated (P<0.01), compared with that in the IL-1β + Lenti-NC
group (Fig. 8). These results
suggest that lncRNA GAS5 serves an important role in regulating
chondrocyte physiology in terms of ECM homeostasis.
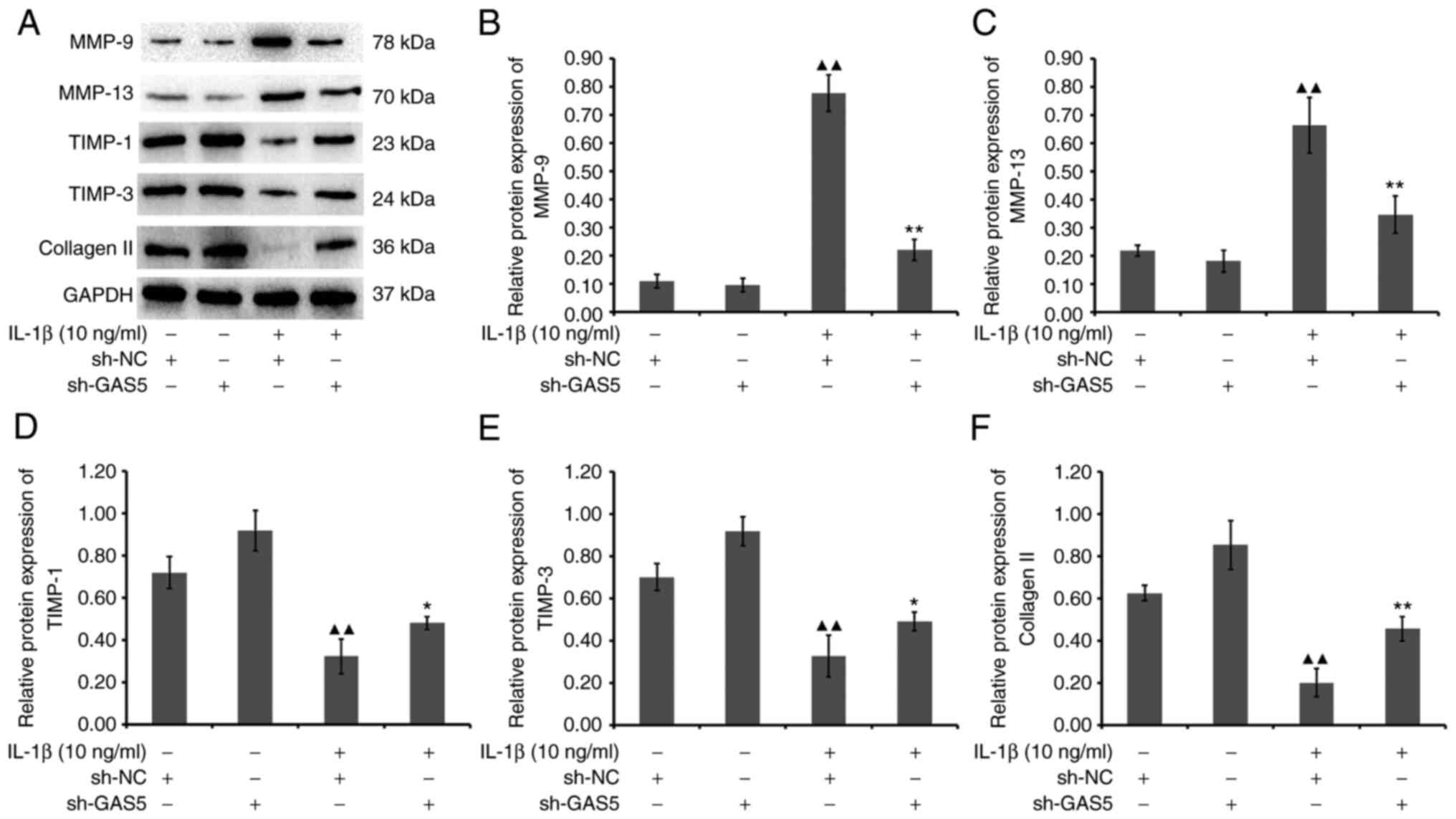 | Figure 8Effect of lncRNA GAS5 silencing on
the protein expression levels of MMP-9, MMP-13, TIMP-1, TIMP-3 and
type Ⅱ collagen in chondrocytes. (A) Western blot analysis of the
protein expression levels of MMP-9, MMP-13, TIMP-1, TIMP-3 and type
II collagen. The protein expression levels of (B) MMP-9, (C)
MMP-13, (D) TIMP-1, (E) TIMP-3 and (F) type II collagen were
quantified. ▲▲P<0.01 vs. blank + Lenti-NC;
**P<0.01 and *P<0.05 vs. IL-1β +
Lenti-NC. lncRNA, long non-coding RNA; GAS5, growth arrest specific
5; TIMP, tissue inhibitor of metalloproteinase. |
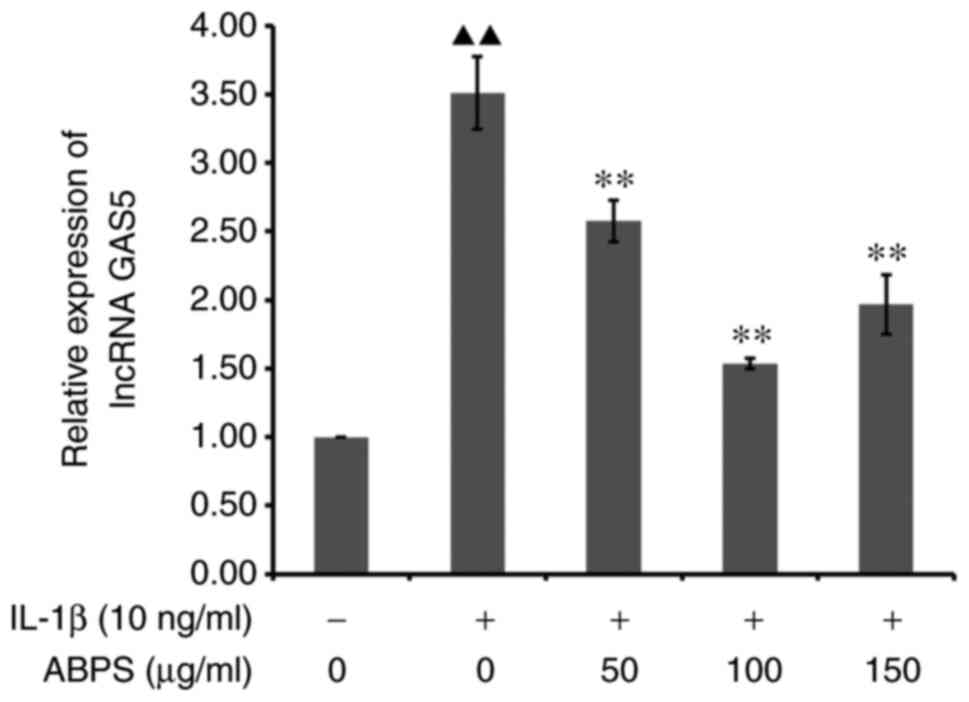
ABPS may regulate chondrocyte ECM
homeostasis via lncRNA GAS5 in IL-1ß-treated chondrocytes
RT-qPCR results showed that the relative expression
of lncRNA GAS5 in the ABPS group was significantly reduced in the
presence of IL-1β compared with that in the IL-1β alone group
(P<0.01; Fig. 9). However,
there was no statistical difference among the three groups of 50,
100 and 150 µg/ml ABPS, although it can be seen in Fig. 9 that 100 µg/ml of ABPS exerted the
optimal response. Subsequent western blot analysis revealed that
the expression of MMP-9 and MMP-13 in the ABPS group was
significantly decreased (P<0.01), whereas that TIMP-1, TIMP-3
and type II collagen was significantly upregulated, compared with
that in the IL-1β-alone group (P<0.01 or P<0.05; Fig. 10). These results suggest that ABPS
can exert protective effects by regulating the expression of
ECM-associated proteins in an in vitro model of OA
chondrocytes.
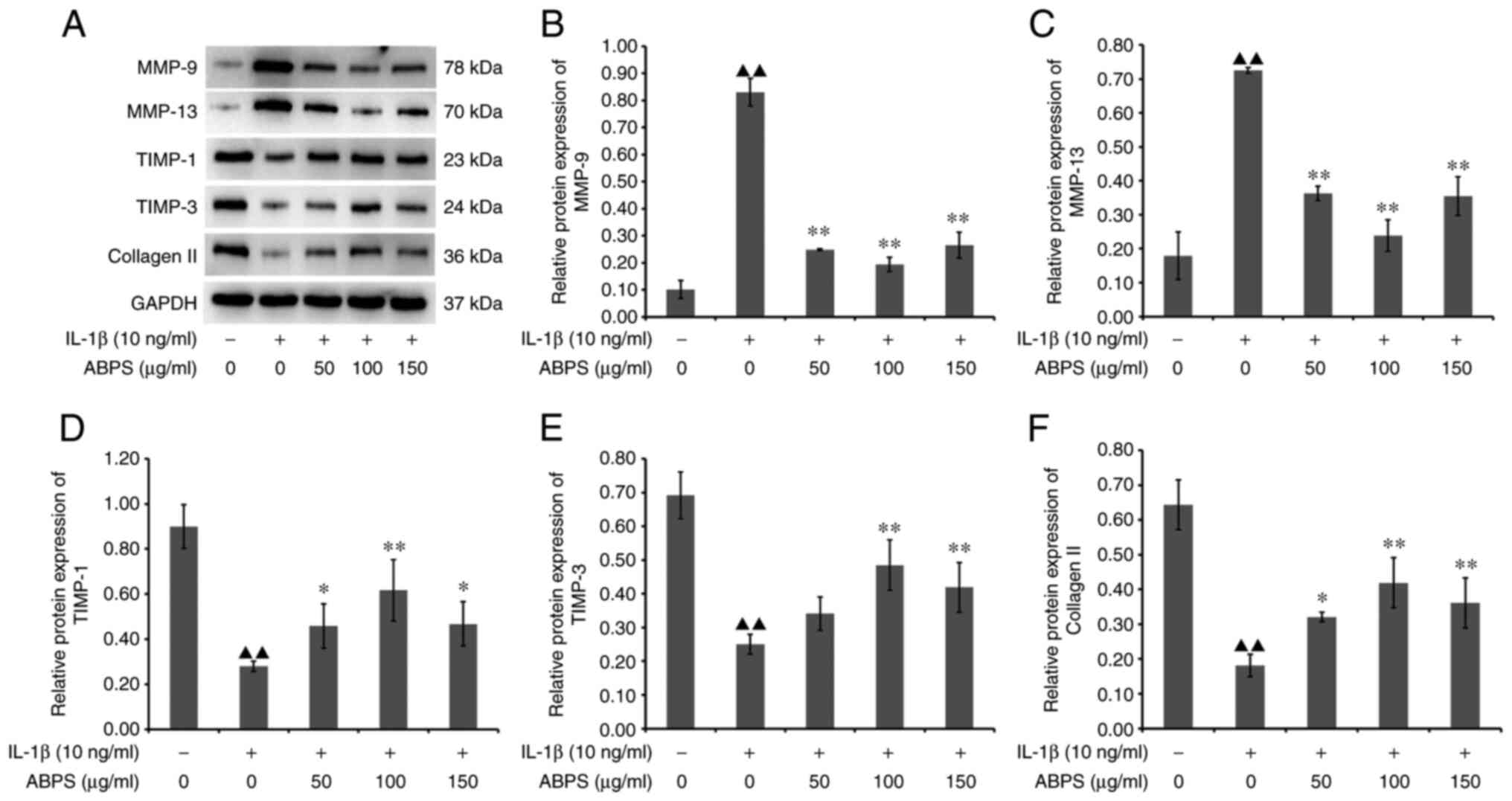 | Figure 10Effect of ABPS on the protein
expression levels of MMP-9, MMP-13, TIMP-1, TIMP-3 and type II
collagen chondrocytes treated with IL-1β. (A) Western blot analysis
of the protein expression levels of MMP-9, MMP-13, TIMP-1, TIMP-3
and type II collagen. The protein expression levels of (B) MMP-9,
(C) MMP-13, (D) TIMP-1, (E) TIMP-3 and (F) type II collagen were
quantified. ▲▲P<0.01 vs. Control;
**P<0.01 and *P<0.05 vs. IL-1β alone.
ABPS, achyranthes bidentata polysaccharides; TIMP, tissue
inhibitor of metalloproteinase. |
Discussion
OA is a common chronic degenerative joint disease
that seriously affects the quality of life of patients (25). Achyranthes bidentata is a
commonly used traditional Chinese medicine that is widely used for
the clinical treatment of OA. The China Food and Drug
Administration has already approved numerous proprietary
traditional Chinese medicines containing achyranthes
bidentata, such as Mailuoning granules/injection/oral
liquid and Danxi granules (9). An epidemiological survey on the use
of traditional Chinese herbal medicine by patients with OA revealed
that achyranthes bidentata is one of the most commonly
prescribed Chinese herbs included in the Chinese herbal formula
(23). As one of the bioactive
components contained within achyranthes bidentata, ABPS has
been reported to serve a key role in the regulation of bone
metabolism (20). ABPS alleviates
OA by reducing the inflammatory response through the arachidonic
acid Pathway (11). In addition,
it confers bone protective effects by promoting cell proliferation
and bone formation (10). In the
present study, it was found that ABPS can improve cartilage ECM
homeostasis, thereby alleviating articular cartilage degeneration.
This therefore provides a theoretical basis for the clinical
application of achyranthes bidentata for OA treatment. A
previous study has shown that ABPS can promote G1/S cell
cycle progression, which effectively promotes chondrocyte
proliferation (15). In addition,
ABPS can inhibit ECM degradation by increasing the expression of
type II collagen (26). However,
the mechanism of action remains unclear. The present study
therefore investigated if ABPS can regulate chondrocyte physiology
and ECM homeostasis through lncRNA GAS5 in an OA setting. The
results of HE staining showed that the tangent line of the
superficial layer in the ABPS group is parallel and continuous with
the articular surface, where the transitional, radiation, calcified
and other layers are relatively clear and identifiable, especially
on the cartilage surface. Improvements in the cartilage matrix
status was also observed; compared with the normal group, the
thickness of the cartilage matrix was thinner in the model group
and this trend was significantly improved after ABPS intervention.
According to results from the morphological analysis, ABPS was
found to delay cartilage degeneration by maintaining the
homeostasis of ECM by chondrocytes. In vivo RT-qPCR and
western blotting results showed that ABPS could inhibit the
expression of lncRNA GAS5, while increasing the protein expression
levels of TIMP-1, TIMP-3 and type II collagen and inhibiting the
protein expression of MMP-9 and MMP-13 in cartilage tissue of OA
rats.
Previous studies have shown that lncRNAs are
involved in the pathological process of OA by regulating apoptosis
and the homeostasis of cartilage ECM. The lncRNAs may accelerate
the degeneration of articular cartilage by causing ECM degradation
leading to abnormal cartilage remodeling (27,28).
A previous study has found that the expression of the lncRNA GAS5
is upregulated in cartilage tissues with OA (29). RT-qPCR and FISH analysis revealed
that the relative expression of lncRNA GAS5 was markedly increased
in IL-1β-treated chondrocytes, which was mainly localized to the
cytoplasm of chondrocytes. Tan et al (30) previously revealed that GAS5 is
mainly located in the cytoplasm of nucleus pulposus cells in a
model of intervertebral disc degeneration, such that GAS5 knockout
can ameliorate nucleus pulposus cell degeneration by increasing ECM
synthesis. Therefore, it could be speculated that ABPS can increase
ECM synthesis by inhibiting the expression of GAS5 to relieve
articular cartilage degeneration during OA.
In cartilages with OA, the homeostasis of cartilage
ECM is disrupted and cells produce large quantities of MMPs
(31). Type II collagen is one of
the most important components of the ECM, accounting for ~90% of
all types of collagen in the ECM and serves to provide tensile
strength to the cartilage (32,33).
MMPs accelerate the breakdown of collagen in the articular
cartilage, which thins collagen fibers and promote cartilage
degeneration (34). MMP-9 and
MMP-13 are important regulators of OA, since their expression was
previously demonstrated to be elevated in patients with OA, which
in turn reduced the expression of collagen and proteoglycans by
activating other collagenases (35,36).
According to the western blot analysis in the present study, the
expression of MMP-9 and MMP-13 was found to be increased whereas
the expression of type II collagen was decreased in IL-1β-treated
chondrocytes, a trend that was reversed by lncRNA GAS5 knockdown.
These results suggest that lncRNA GAS5 can promote the expression
of MMP-9 and MMP-13 and be involved in cartilage degradation by
cleaving type II collagen.
TIMPs are secretory proteins that function to
inhibit MMPs and are present in the majority of organs and bodily
fluids (37). The balance between
ECM synthesis and degradation in the articular cartilage is
typically disrupted in OA (38).
One of the main reasons for this is the imbalance in the expression
and activity of MMPs/TIMPs (39).
Maintaining a healthy MMP/TIMP balance can prevent cartilage damage
(40). In the present study, it
was shown that IL-1β could increase the expression levels of MMPs
whilst decreasing the expression of TIMPs in chondrocytes,
resulting in the imbalance of the MMPs/TIMPs ratio to aggravate ECM
degradation. Treatment of these chondrocytes with ABPS was able to
reduce the expression of lncRNA GAS5, which in turn could decrease
the ratio of MMPs/TIMPs. These results suggest that ABPS can
promote ECM synthesis by modulating the balance between MMP and
TIMP expression.
The present study showed that for the treatment of
OA, ABPS could function at least in part by regulating the
expression of lncRNA GAS5. Therefore, further studies will need to
be performed to explore the regulatory mechanism of ABPS on lncRNA
GAS5 in OA. GAS5 has a number of different variants produced by
alternate splicing during transcription (41). In addition, serum starvation and
rapamycin administration can increase the expression of lncRNA GAS5
through the mTOR pathway in T cells (42). However, the effects of ABPS on the
transcription or alternate splicing of lncRNA GAS5 during OA remain
unclear. Therefore, the mechanism of ABPS-induced downregulation of
lncRNA GAS5 expression requires further exploration in future
studies of OA.
To conclude, results from the present study suggest
that lncRNA GAS5 can serve an important role in regulating
chondrocyte cell physiology and in turn ECM homeostasis. In
addition, it was shown that ABPS can preserve the articular
cartilage structure in OA by downregulating the expression of
lncRNA GAS5 and regulating the expression of ECM-associated
proteins in chondrocytes. This suggests that ABPS can inhibit the
expression of lncRNA GAS5 and maintain the homeostasis of ECM,
ultimately delaying the process of cartilage degeneration in
OA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82104888); Scientific
Research Foundation for the High-level Talents Fujian University of
Traditional Chinese Medicine (grant nos. X2019011-talents and
X2021007-talents).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CLF, ZWQ and YFH performed the experiments and
analyzed the data. YYM, QL, JWZ and WHZ performed the experiments.
DZM designed the study and analyzed the data. CLF, ZWQ and YFH
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All the experimental procedures were approved by the
Animal Use and Care Committee of Fujian University of Chinese
Traditional Medicine (approval no. 2019122; Fuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma L: Osteoarthritis of the Knee. N
Engl J Med. 384:51–59. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Berenbaum F and Walker C: Osteoarthritis
and inflammation: A serious disease with overlapping phenotypic
patterns. Postgrad Med. 132:377–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Quicke JG, Conaghan PG, Corp N and Peat G:
Osteoarthritis year in review 2021: Epidemiology & therapy.
Osteoarthr Cartilage. 30:196–206. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun X, Zhang J, Li Y, Ren W and Wang L:
Etomidate ameliorated advanced glycation end-products
(AGEs)-induced reduction of extracellular matrix genes expression
in chondrocytes. Bioengineered. 12:4191–4200. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kumavat R, Kumar V, Malhotra R, Pandit H,
Jones E, Ponchel F and Biswas S: Biomarkers of joint damage in
osteoarthritis: Current status and future directions. Mediat
Inflamm. 2021(5574582)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
D Adamo S, Cetrullo S, Panichi V, Mariani
E, Flamigni F and Borzì RM: Nutraceutical activity in
osteoarthritis biology: A focus on the nutrigenomic role. Cells.
9(1232)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stack J and McCarthy GM: Cartilage
calcification and osteoarthritis: A pathological association?
Osteoarthritis Cartilage. 28:1301–1302. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu XX, Zhang XH, Diao Y and Huang YX:
Achyranthes bidentate saponins protect rat articular chondrocytes
against interleukin-1β-induced inflammation and apoptosis in vitro.
Kaohsiung J Med Sci. 33:62–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang J, Liu J, Jiao D, Zhang G, Qu C, Chen
H, Chen C, Yu S and Xiangyanga L: Prediction of the molecular
mechanism of eucommiae Cortex-Achyranthis bidentatae radix in the
treatment of osteoarthritis: Network pharmacology and molecular
docking. Drug Dev Ind Pharm. 30:1235–1247. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fan S, Wang Y, Zhang Y, Wu Y and Chen X:
Achyranthes bidentata Polysaccharide activates nuclear factor-Kappa
B and promotes cytokine production in J774A.1 cells through
TLR4/MyD88 signaling pathway. Front Pharmacol.
12(753599)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He X, Wang X, Fang J, Chang Y, Ning N, Guo
H, Huang L and Huang X: The genus Achyranthes: A review on
traditional uses, phytochemistry, and pharmacological activities. J
Ethnopharmacol. 203:260–278. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang D, Wang C, Hou X and Yan C:
Structural characterization and osteoprotective effects of a
polysaccharide purified from Achyranthes bidentata. Int J Biol
Macromol. 139:1063–1073. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li Z, Ma D, Peng L, Li Y, Liao Z and Yu T:
Compatibility of Achyranthes bidentata components in reducing
inflammatory response through Arachidonic acid pathway for
treatment of osteoarthritis. Bioengineered. 13:1746–1757.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yi J, Li X, Wang S, Wu T and Liu P: Steam
explosion pretreatment of Achyranthis bidentatae radix: Modified
polysaccharide and its antioxidant activities. Food Chem.
375(131746)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu F, Li X, Cai L, Li H, Chen J, Wong X,
Xu H, Zheng C, Liu X and Ye H: Achyranthes bidentata
polysaccharides induce chondrocyte proliferation via the promotion
of the G1/S cell cycle transition. Mol Med Rep. 7:935–940.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tu J, Huang W, Zhang W, Mei J and Zhu C:
The emerging role of lncRNAs in chondrocytes from osteoarthritis
patients. Biomed Pharmacother. 131(110642)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao ST, Yu YM, Wan LP, Liu ZM and Lin JX:
LncRNA GAS5 induces chondrocyte apoptosis by down-regulating
miR-137. Eur Rev Med Pharmacol Sci. 24:10984–10991. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu W, La AT, Evans A, Gao S, Yu Z, Bu D
and Ma L: Supplementation with sodium butyrate improves growth and
antioxidant function in dairy calves before weaning. J Anim Sci
Biotechnol. 12(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li X, Zhang Z, Liang W, Zeng J, Shao X, Xu
L, Jia L, He X, Li H, Zheng C, et al: Tougu Xiaotong capsules may
inhibit p38 MAPK pathway-mediated inflammation: In vivo and in
vitro verification. J Ethnopharmacol. 249(112390)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang M, Wang Y, Zhang Q, Wang C, Zhang D,
Wan JB and Yan C: UPLC/Q-TOF-MS-based metabolomics study of the
anti-osteoporosis effects of Achyranthes bidentata polysaccharides
in ovariectomized rats. Int J Biol Macromol. 112:433–441.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang S, Zhang Q, Zhang D, Wang C and Yan
C: Anti-osteoporosis activity of a novel Achyranthes bidentata
polysaccharide via stimulating bone formation. Carbohyd Polym.
184:288–298. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Unno H, Hasegawa M, Suzuki Y, Iino T,
Imanaka-Yoshida K, Yoshida T and Sudo A: Tenascin-C promotes the
repair of cartilage defects in mice. J Orthop Sci. 25:324–330.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wakitani S, Goto T, Pineda SJ, Young RG,
Mansour JM, Caplan AI and Goldberg VM: Mesenchymal cell-based
repair of large, full-thickness defects of articular cartilage. J
Bone Joint Surg Am. 76:579–592. 1994.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang P, Ye Y, Yuan W, Tan Y, Zhang S and
Meng Q: Curcumin exerts a protective effect on murine knee
chondrocytes treated with IL-1β through blocking the NF-κB/HIF-2α
signaling pathway. Ann Transl Med. 9(940)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weng X, Lin P, Liu F, Chen J, Li H, Huang
L, Zhen C, Xu H, Liu X, Ye H and Li X: Achyranthes bidentata
polysaccharides activate the Wnt/β-catenin signaling pathway to
promote chondrocyte proliferation. Int J Mol Med. 34:1045–1050.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang J, Sun Y, Liu J, Yang B, Wang T,
Zhang Z, Jiang X, Guo Y and Zhang Y: Roles of long noncoding RNA in
osteoarthritis (Review). Int J Mol Med. 48(133)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiang S, Liu Y, Xu B, Zhang Y and Yang M:
Noncoding RNAs: New regulatory code in chondrocyte apoptosis and
autophagy. Wires RNA. 11(e1584)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xing D, Liang JQ, Li Y, Lu J, Jia HB, Xu
LY and Ma XL: Identification of long noncoding RNA associated with
osteoarthritis in humans. Orthop Surg. 6:288–293. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tan L, Xie Y, Yuan Y and Hu K: LncRNA GAS5
as miR-26a-5p sponge regulates the PTEN/PI3K/Akt axis and affects
extracellular matrix synthesis in degenerative nucleus pulposus
cells in vitro. Front Neurol. 12:2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lucafò M, Pugnetti L, Bramuzzo M, Curci D,
Di Silvestre A, Marcuzzi A, Bergamo A, Martelossi S, Villanacci V,
Bozzola A, et al: Long non-coding RNA GAS5 and intestinal MMP2 and
MMP9 expression: A translational study in pediatric patients with
IBD. Int J Mol Sci. 20(5280)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang X, Dong Y, Dong H, Cui Y, Du Q, Wang
X, Li L and Zhang H: Telmisartan mitigates TNF-α-induced Type II
collagen reduction by upregulating SOX-9. ACS Omega. 6:11756–11761.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Majumdar MK, Askew R, Schelling S, Stedman
N, Blanchet T, Hopkins B, Morris EA and Glasson SS: Double-knockout
of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal
animals and prevents the progression of osteoarthritis. Arthritis
Rheum. 56:3670–3674. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma X, Zhang Z, Shen M, Ma Y, Li R, Jin X,
Gao L and Wang Z: Changes of type II collagenase biomarkers on
IL-1β-induced rat articular chondrocytes. Exp Ther Med.
21(582)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Slovacek H, Khanna R, Poredos P, Poredos
P, Jezovnik M, Hoppensteadt D, Fareed J and Hopkinson W:
Interrelationship of MMP-9, Proteoglycan-4, and inflammation in
osteoarthritis patients undergoing total hip arthroplasty. Clin
Appl Thromb Hemost. 27(1076029621995569)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jackson HW, Defamie V, Waterhouse P and
Khokha R: TIMPs: Versatile extracellular regulators in cancer. Nat
Rev Cancer. 17:38–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rahmati M, Nalesso G, Mobasheri A and
Mozafari M: Aging and osteoarthritis: Central role of the
extracellular matrix. Ageing Res Rev. 40:20–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sang W, Xue S, Jiang Y, Lu H, Zhu L, Wang
C and Ma J: METTL3 involves the progression of osteoarthritis
probably by affecting ECM degradation and regulating the
inflammatory response. Life Sci. 278(119528)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Iannone F and Lapadula G: The
pathophysiology of osteoarthritis. Aging Clin Exp Res. 15:364–372.
2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou Z, Chen J, Huang Y, Liu D, Chen S and
Qin S: Long noncoding RNA GAS5: A new factor involved in bone
diseases. Front Cell Dev Biol. 9(807419)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mourtada-Maarabouni M, Hasan AM, Farzaneh
F and Williams GT: Inhibition of human T-Cell proliferation by
mammalian target of rapamycin (mTOR) antagonists requires noncoding
RNA growth-arrest-specific transcript 5 (GAS5). Mol Pharmacol.
78:19–28. 2010.PubMed/NCBI View Article : Google Scholar
|