Introduction
Coronary heart disease (CHD) mainly refers to
coronary atherosclerotic heart disease. The basic pathological
process of CHD is the lack of blood supply of the coronary artery
caused by atherosclerosis (AS), which leads to ischemia and hypoxia
of myocardial cells (1). There are
numerous reasons for its occurrence. Lipid infiltration, oxidative
stress and inflammation may all lead to the occurrence of AS
(2). However, the specific
mechanisms remain to be fully elucidated; thus, the treatment and
prevention of CHD remain challenging.
A large number of clinical studies have demonstrated
that vascular endothelial injury causes AS, which in turn leads to
CHD, and CHD aggravates vascular endothelial injury (3-5).
Vascular endothelial dysfunction and integrity destruction are the
initial stages of AS, which may lead to a variety of cardiovascular
diseases (6). Endothelial cells
have high metabolic activity and endocrine regulation function to
ensure normal blood flow in the vascular wall (7). In addition, endothelial cells are
constantly affected by blood flow, inflammatory factors and their
own metabolic disorders, including permeability and diffuse
calcification that lead to structural and functional impairment,
resulting in morphological and functional changes of endothelial
cells (7). A previous study has
demonstrated that microRNA-26a-5p promotes the proliferation and
inhibits apoptosis of endothelial cells in mice with CHD by
activating the PI3K/AKT signaling pathway targeting PTEN, thereby
affecting the pathogenesis of CHD (8). In addition, several studies have
investigated the severity of the disease by examining apoptosis,
inflammation and senescence of vascular endothelial cells in CHD
(9,10).
Ginsenosides are the main active substances of the
Chinese herbal medicine ginseng, which have antitumor,
immunity-enhancing and protective effects on the vascular system
(11-13).
Ginsenoside Rg1 (Rg1) has been widely used in cardiovascular and
cerebrovascular diseases due to its unique therapeutic effects. For
example, Rg1 is able to enhance myocardial contractility and
maintain the integrity of the myocardial cell membrane, which has a
marked effect on the protection of the cardiovascular system and
the prevention and treatment of cardiovascular diseases, including
AS and hypertension (14-16).
Rg1 has been reported to alleviate high glucose-induced endothelial
barrier dysfunction in human umbilical vein endothelial cells
(HUVECs) by protecting the endothelial glycocalyx (17). However, to the best of our
knowledge, the effect of Rg1 on vascular endothelial cells in CHD
has remained elusive.
Therefore, in the present study, oxidized
low-density lipoprotein (ox-LDL) was used to induce apoptosis,
senescence and oxidative stress in vascular endothelial cells, and
the effects of Rg1 on the ox-LDL-induced apoptosis, senescence and
oxidative stress of vascular endothelial cells were explored and
the associated mechanism was investigated. Therefore, the present
study lays a foundation for the potential future treatment of CHD
using Rg1.
Materials and methods
Reagents and cell culture
HUVECs (cat. no. BNCC337616), obtained from BeNa
Culture Collection, were cultured in DMEM supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin in a humidified incubator with 5%
CO2 at 37˚C. HUVECs were treated with different
concentrations of Rg1 (1, 5 or 10 µM; Shanghai YaJi Biotechnology
Co., Ltd.) for 24 h at 37˚C (18,19).
The AMP-activated protein kinase (AMPK) inhibitor compound C (CC; 5
mM; MilliporeSigma) was used to pretreat HUVECs for 2 h at 37˚C. In
addition, HUVECs were stimulated with 100 mg/l ox-LDL (Beijing
Solarbio Science & Technology Co., Ltd.) for 48 h at 37˚C to
construct an in vitro AS model. To evaluate the effects of
Rg1 on ox-LDL-treated HUVECs, the cells were pretreated with Rg1
for 30 min prior to ox-LDL treatment. Untreated cells were regarded
as the control group.
Cell counting kit-8 (CCK-8) assay
Transfected HUVECs were seeded into 96-well plates
with complete medium at a density of 2x104. Following
treatment with Rg1 for 24 h, cell viability was detected using a
CCK-8 assay (Beyotime Institute of Biotechnology). A total of 10 µl
CCK-8 solution was added to each well of HUVECs. After incubation
for 2 h, the absorbance value of each well was measured at 450 nm
using a microplate reader (Bio-Rad Laboratories, Inc.).
TUNEL staining
Cells (1x104) were cultured on cover
slips in a 24-well plate used for fluorescent detection. Following
treatment with Rg1 for 24 h, cells were fixed in 4%
paraformaldehyde for 30 min at room temperature and then incubated
with TUNEL BrightGreen Apoptosis Detection kit (cat. no. A112-02;
Vazyme Biotech Co., Ltd.) for 1 h. Cells were incubated with DAPI
for 15 min at 37˚C in the dark, followed by fluorescence microscopy
(magnification, x200). Images from 5 random fields were analyzed
using ImageJ software (version 1.46; National Institutes of
Health).
Western blot analysis
HUVECs were lysed in RIPA buffer (MilliporeSigma)
and protein concentrations were measured using a BCA Protein Assay
Kit (Beyotime Institute of Biotechnology). A total of 30 µg protein
per lane was separated by SDS-PAGE using a 10% polyacrylamide gel
and electroblotted onto a PVDF membrane. After blocking with 5%
skimmed milk powder for 1.5 h at room temperature, the membrane was
incubated at 4˚C with primary antibodies overnight. After three
washes with TBS with 0.1% Tween-20, the membrane was incubated with
a secondary goat antibody (dilution, 1:5,000; cat. no. ab6721;
Abcam) for 1 h at room temperature. Signals were detected with
Pierce™ Enhanced Chemiluminescence Western Blotting
Substrate (Thermo Fisher Scientific, Inc.). The images were
analyzed using ImageJ 1.8.0 software. The following antibodies were
used (dilution, 1:5,000; all from Abcam): Anti-Bcl-2 (cat. no.
ab32124, Abcam), anti-Bax (cat. no. ab32503), anti-cleaved
caspase-3 (cat. no. ab32042), anti-caspase-3 (cat. no. ab32351),
anti-p16 (cat. no. ab51243), anti-p21 (cat. no. ab109520),
anti-AMPKα (cat. no. ab32047), anti-phosphorylated (p)-AMPKα (cat.
no. ab133448), anti-sirtuin (SIRT)3 (cat. no. ab217319),
anti-acetyl-p53 (cat. no. ab183544), anti-p53 (cat. no. ab179477)
and anti-GAPDH (cat. no. ab9485). GAPDH was used as a loading
control.
Senescence-associated β-galactosidase
(SA-β-gal) staining
Cells were cultured in 24-well plates
(1x104 cells/well). After treatment as aforementioned,
the cells were washed with PBS and fixed in 3% formaldehyde at room
temperature for 5 min. SA-β-gal staining was performed using a
senescence β-galactosidase staining kit (cat. no. 9860; Cell
Signaling Technology, Inc.) according to the manufacturer's
instructions. A total of three fields of view were randomly
selected and the number of senescent cells with blue staining was
determined using a light microscope (magnification, x200).
Measurement of reactive oxygen species
(ROS), malondialdehyde (MDA) and superoxide dismutase (SOD)
levels
The levels of ROS (cat. no. ab139476; Abcam), MDA
(cat. no. A003-1-2; Nanjing Jiancheng Bioengineering Institute) and
SOD (cat. no. A001-3-2; Nanjing Jiancheng Bioengineering Institute)
were detected using commercial kits according to the manufacturers'
protocols.
ELISA
In brief, 100 µl cell culture supernatant was added
into duplicate wells and incubated at 37˚C for 90 min. IL-1β (cat.
no. H002), IL-6 (cat. no. H007-1-1) and TNF-α (cat. no. H052-1)
levels were determined using ELISA (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's protocol.
Photometric measurements were performed at 450 nm using a
microplate reader (Varioskan Flash; Thermo Fisher Scientific,
Inc.).
Statistical analysis
Values are expressed as the mean ± standard
deviation. All experiments were repeated three times. All data were
analyzed using SPSS version 16 (SPSS, Inc.). Significant
differences among more groups were evaluated using one-way ANOVA
followed by the Tukey-Kramer honest significant difference post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Rg1 increases the viability of
ox-LDL-induced HUVECs
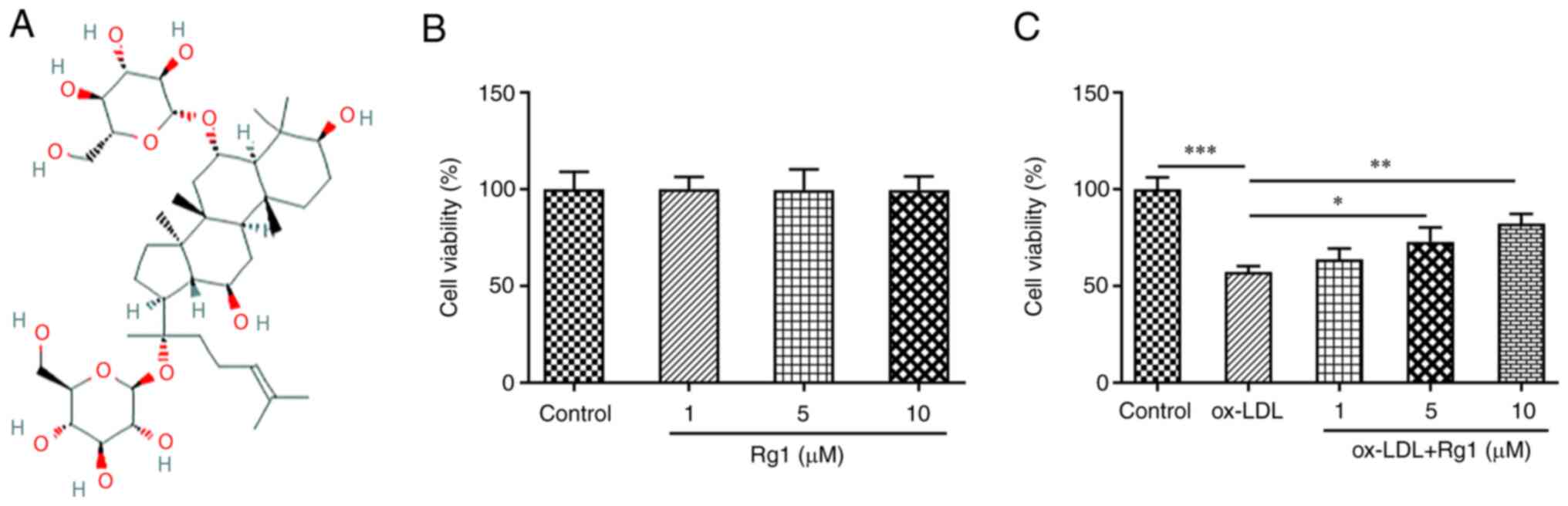
Fig. 1A presents
the chemical structural formula of Rg1. CCK-8 assay revealed no
significant change in cell viability after Rg1 administration
(Fig. 1B). Subsequently, the
effect of Rg1 on the viability of ox-LDL-induced cells was
detected. The results demonstrated that the viability of
ox-LDL-induced cells was markedly decreased compared with that of
the control group. Compared with the ox-LDL group, the cell
viability was enhanced with the increase of the Rg1 concentration
(Fig. 1C).
Rg1 inhibits the apoptosis of
ox-LDL-induced HUVECs
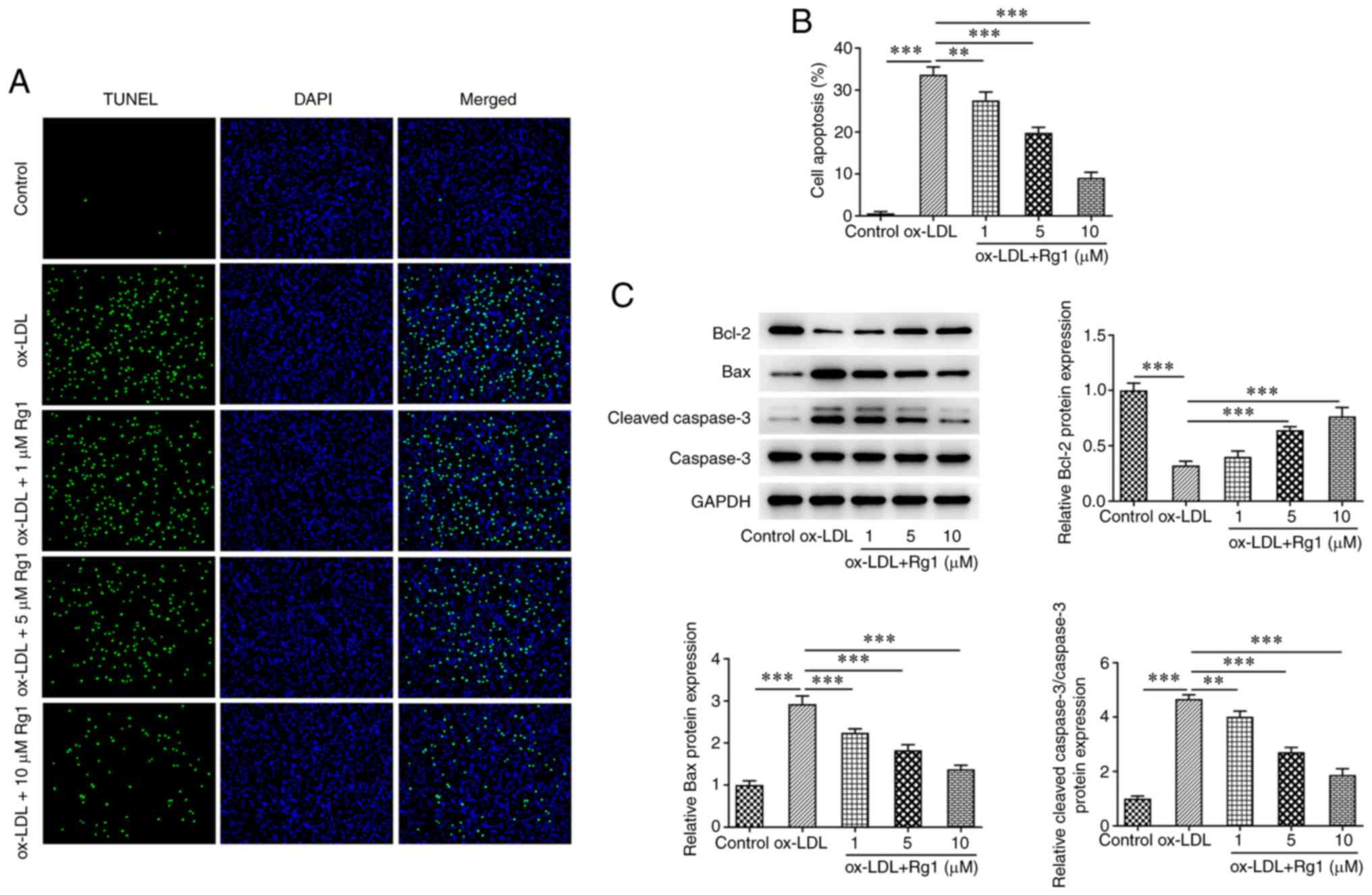
TUNEL assay results demonstrated that apoptosis was
markedly increased following treatment with ox-LDL compared with
the control group. Compared with that in the ox-LDL group,
apoptosis decreased in cells treated with Rg1 in a dose-dependent
manner (Fig. 2A and B). Subsequently, western blot analysis
was used to detect the expression levels of apoptosis-related
proteins. The results revealed that, compared with those in the
control group, the expression levels of Bcl-2 were decreased and
the expression levels of Bax and cleaved caspase-3 were increased
after ox-LDL induction. Compared with the ox-LDL group, Rg1 was
able to markedly inhibit the expression of Bax and cleaved
caspase-3, while it increased the expression levels of Bcl-2 and
caspase-3 expression remained unchanged (Fig. 2C).
Rg1 inhibits the senescence of
ox-LDL-induced HUVECs
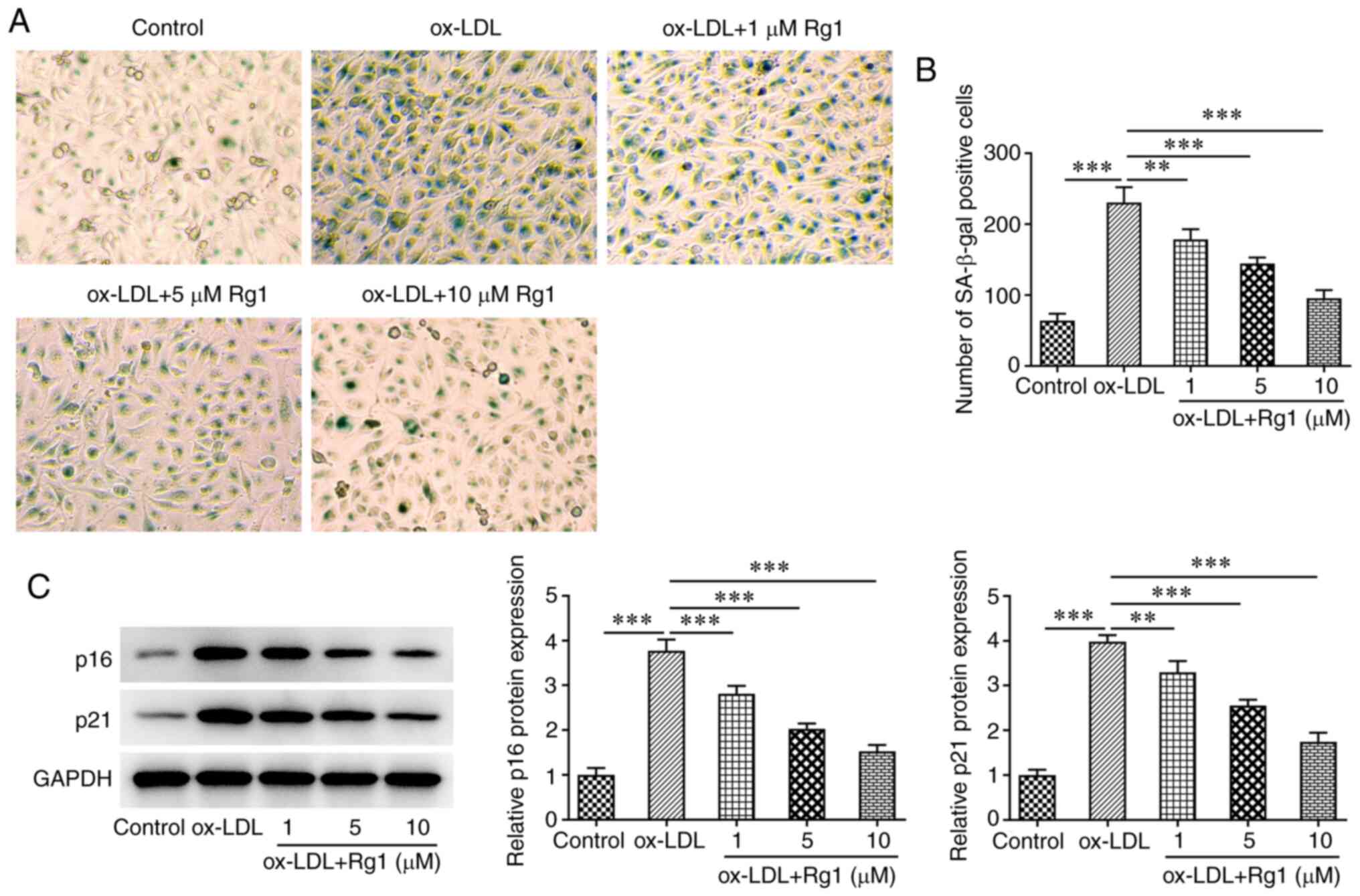
SA-β-gal staining was used to detect cell
senescence. The results revealed that ox-LDL markedly promoted cell
senescence compared with the control group. Rg1 markedly reduced
the senescence of ox-LDL-induced cells (Fig. 3A and B). Subsequently, western blot analysis
was used to detect the expression levels of the senescence-related
proteins p16 and p21. The results demonstrated that, compared with
those in the control group, the expression levels of p16 and p21
were markedly increased following challenge with ox-LDL. Of note,
compared with the ox-LDL group, the expression levels of p16 and
p21 were decreased in a dose-dependent manner after Rg1
administration (Fig. 3C).
Rg1 inhibits oxidative stress and
inflammation in ox-LDL-induced HUVECs
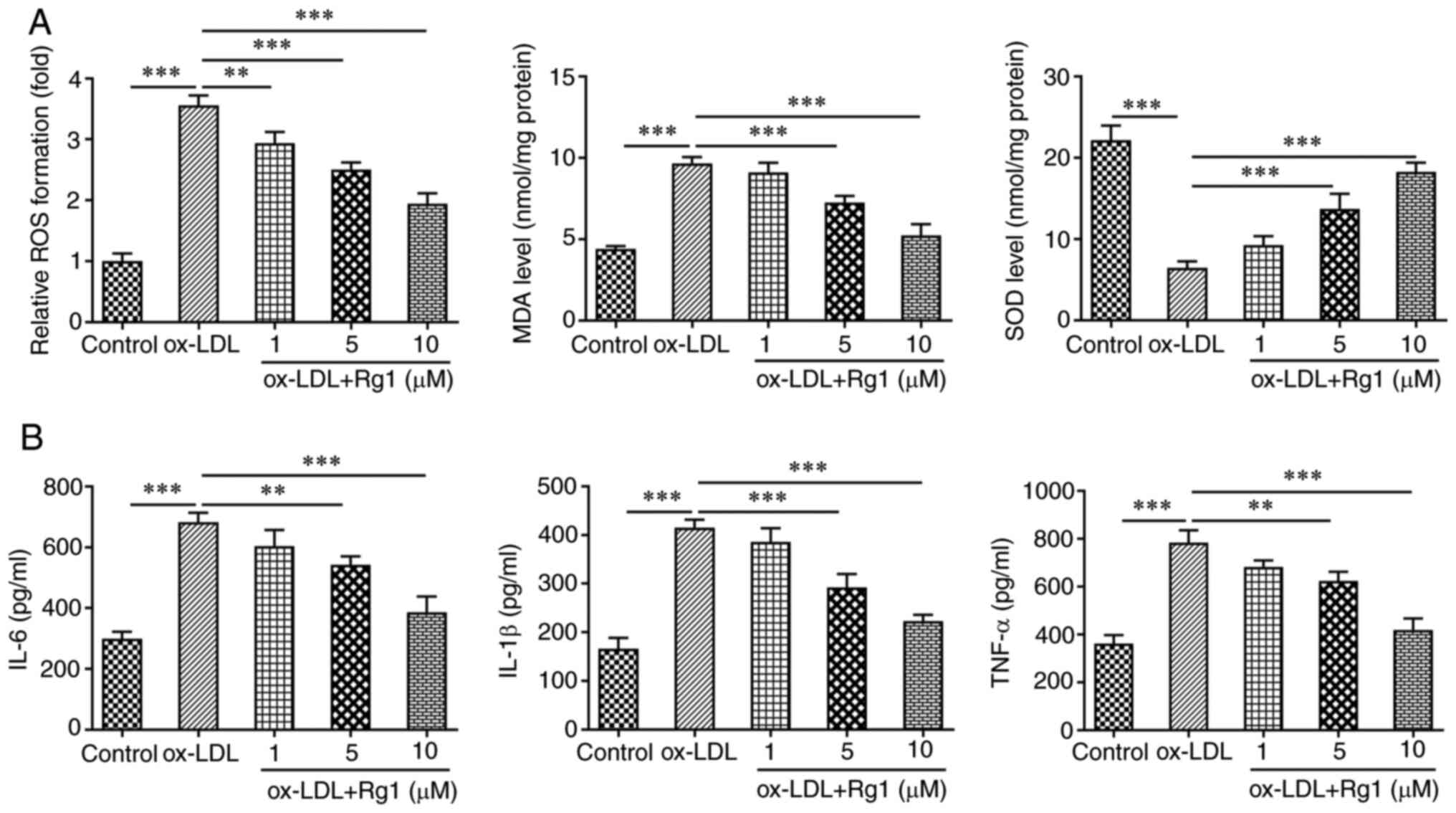
The levels of oxidative stress-related indicators,
including ROS, MDA and SOD, were subsequently detected. The results
revealed that compared with the control group, the levels of ROS
and MDA in ox-LDL-induced cells were markedly increased, while the
levels of SOD were markedly decreased. The effects of ox-LDL
induction on ROS, MDA and SOD levels were reversed after Rg1
administration (Fig. 4A). ELISA
kits were used to detect the expression levels of inflammatory
cytokines, including IL-6, IL-1β and TNF-α. It was revealed that
IL-6, IL-1β and TNF-α levels were markedly increased in the ox-LDL
group compared with the control group. Compared with those in the
ox-LDL group, the expression levels of IL-6, IL-1β and TNF-α in the
ox-LDL + Rg1 group were markedly decreased (Fig. 4B).
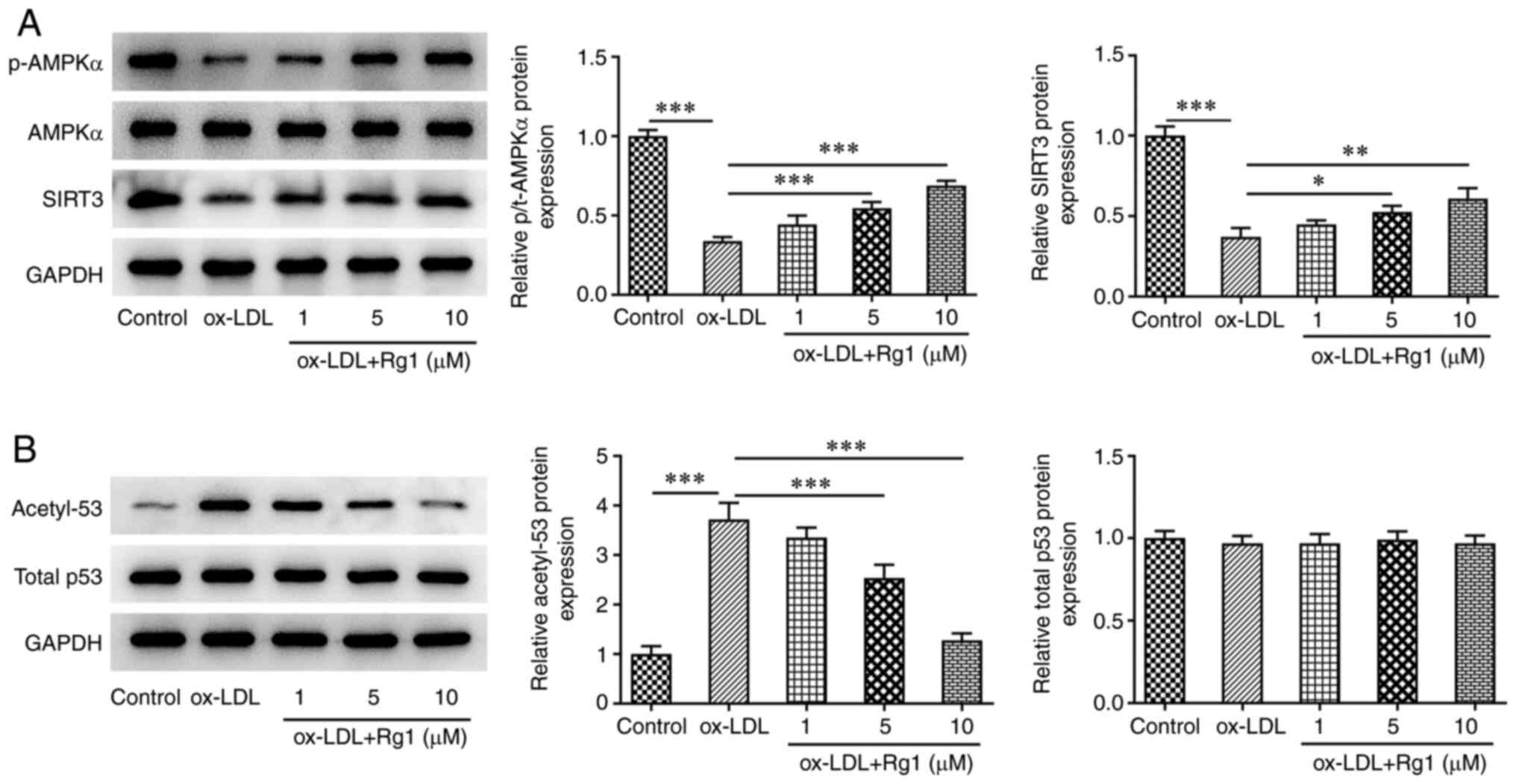
Rg1 regulates the AMPK/SIRT3/p53
signaling pathway
After ox-LDL induction, the levels of p-AMPK and
SIRT3 were markedly decreased, the expression of acetyl-p53 was
markedly increased and no apparent changes were observed in total
AMPK and total p53 expression, compared with the control group.
After Rg1 administration, compared with those in the ox-LDL group,
the levels of p-AMPK and SIRT3 increased, while acetyl-p53 levels
decreased in a dose-dependent manner (Fig. 5A and B). It was thus indicated that the
AMPK/SIRT3/p53 signaling pathway was abnormally regulated after
ox-LDL induction. The results suggested that 10 µM Rg1 exhibited
the most marked effect on this pathway, and therefore this
concentration was selected for subsequent experiments.
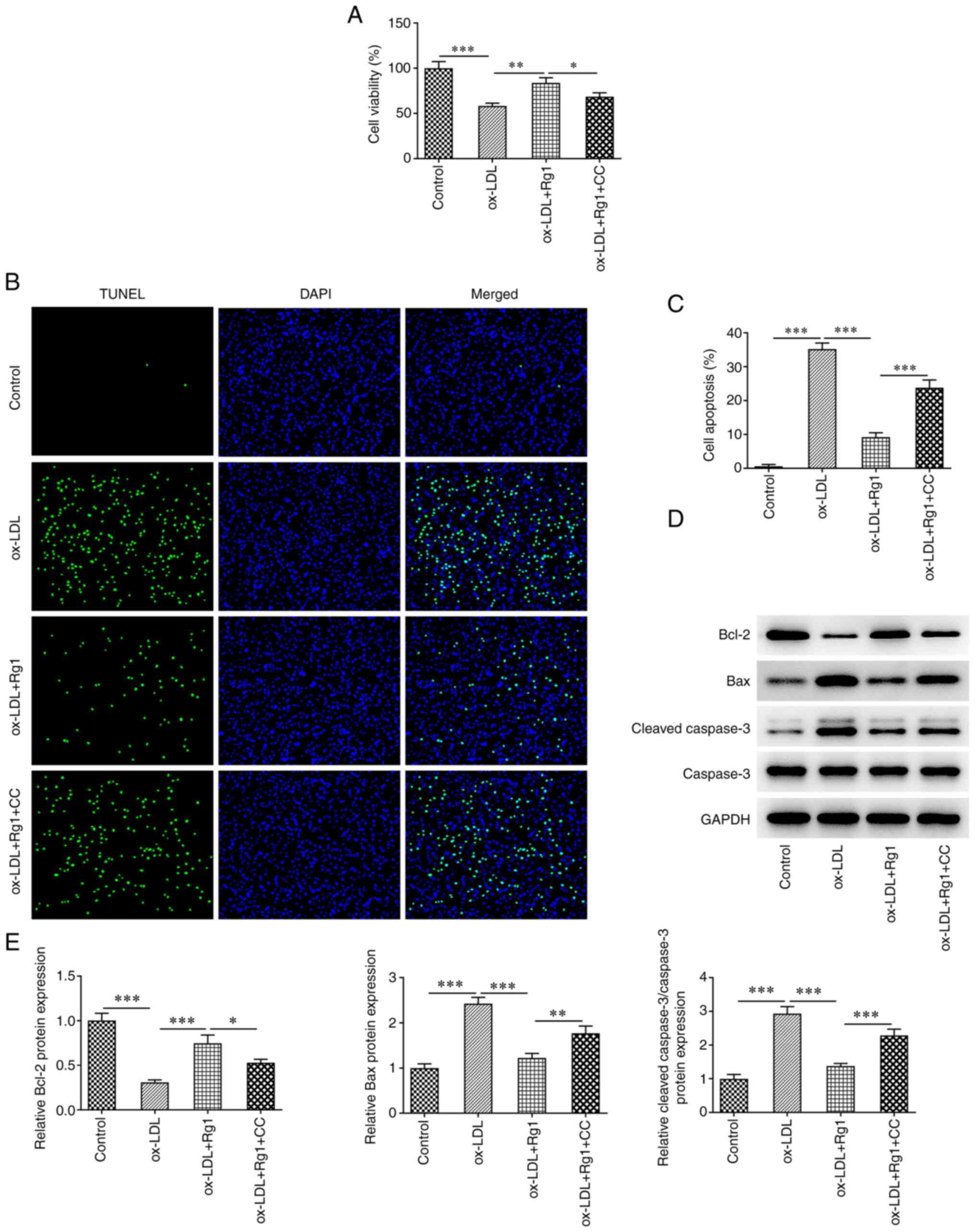
Pretreatment with CC partially
reverses the protective effect of Rg1 on ox-LDL-induced HUVECs
The mechanism of Rg1 acting on ox-LDL-induced HUVECs
was further explored by pretreating the cells with the AMPK
inhibitor CC. The results of the CCK-8 assay demonstrated that cell
viability in the ox-LDL +Rg1 + CC group was markedly decreased
compared with that in the ox-LDL + Rg1 group (Fig. 6A). TUNEL staining revealed that
apoptosis was markedly increased in the ox-LDL + Rg1 + CC group
compared with the ox-LDL + Rg1 group (Fig. 6B and C). Western blot analysis demonstrated
that compared with those in the ox-LDL + Rg1 group, Bax and cleaved
caspase-3 levels were increased, and Bcl-2 expression was decreased
in the ox-LDL + Rg1 + CC group (Fig.
6D and E). These results
indicated that CC was able to reverse the promoting effect of Rg1
on the viability of ox-LDL-induced HUVECs. Subsequently, cell
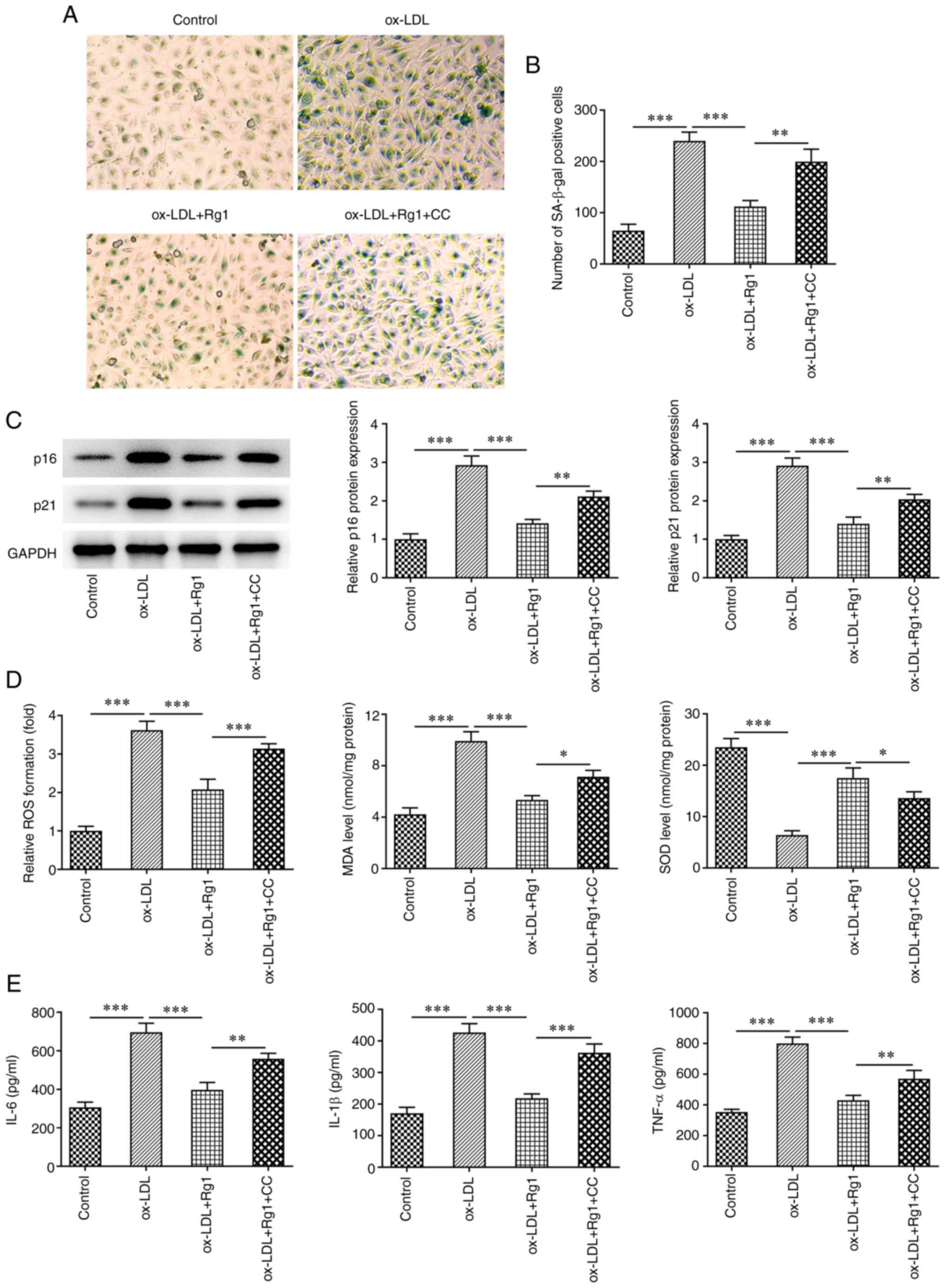
senescence was assessed, and it was revealed that SA-β-gal-positive
cells were markedly increased in the ox-LDL + Rg1 + CC group
compared with the ox-LDL + Rg1 group (Fig. 7A and B). Furthermore, the expression levels of
p16 and p21 in cells were markedly increased after CC treatment
compared with the ox-LDL + Rg1 group (Fig. 7C). Next, oxidative stress and
inflammation were detected, and the results demonstrated that
compared with those in the ox-LDL + Rg1 group, the levels of ROS
and MDA in the ox-LDL + Rg1 + CC group were increased, while the
levels of SOD were decreased (Fig.
7D). Furthermore, the expression levels of the inflammatory
cytokines IL-6, IL-1β and TNF-α were also increased after CC
treatment compared with those in the ox-LDL + Rg1 group (Fig. 7E). These results suggested that
pretreatment with CC partially reversed the protective effect of
Rg1 on ox-LDL-induced HUVECs.
Discussion
Injury to the coronary artery endothelium is a key
initiating factor in the formation of coronary AS (20). Oxidative modification of LDL may
promote the formation of atherosclerotic plaque, which is the most
important factor causing AS (21).
It is also the main factor leading to injury of endothelial cells
and smooth muscle cells (22). In
in vitro studies, ox-LDL was used to induce injury to HUVECs
to simulate an AS model (23-25).
In the present study, ox-LDL was used to treat HUVECs and induce
injury, simulating the damage of ox-LDL to blood vessels in the
process of coronary AS. The present results demonstrated that after
ox-LDL induction, the viability of HUVECs decreased, apoptosis and
senescence occurred, and oxidative stress and inflammation in cells
increased, indicating successful model establishment in
vitro.
Rg1 has a wide range of pharmacological effects
including neurotrophic, neuroprotective and effects (26,27).
In the present study, it was indicated that Rg1 was able to enhance
ox-LDL-induced cell viability and inhibit apoptosis. A previous
study has reported that Rg1 inhibits myocardial apoptosis and
inflammation through the Toll-like receptor 4/NF-κB/NLR family
pyrin domain containing 3 signaling pathway (28). In addition, the present study
revealed that Rg1 inhibited the senescence of ox-LDL-induced
HUVECs. Rg1 has been indicated to inhibit senescence and promotes
differentiation of human bone marrow mesenchymal stem cells through
GSK-3β and β-catenin (29). The
present study demonstrated that Rg1 inhibited ox-LDL-induced
oxidative stress and the inflammatory response in HUVECs. This is
consistent with the study of Qin et al (30), which demonstrated that Rg1 improved
cardiac oxidative stress and inflammation in streptozotocin-induced
diabetic rats. At present, only one study has investigated the
utility Rg1 in AS. For example, Rg1-notoginsenoside
R1-protocatechuic aldehyde was observed to reduce AS and attenuate
low-shear stress-induced vascular endothelial cell dysfunction in
apolipoprotein E-knockout mice (31). The novelty of the present study
lied to the demonstration that Rg1 ameliorates ox-LDL-induced
apoptosis, senescence and oxidative stress in HUVECs.
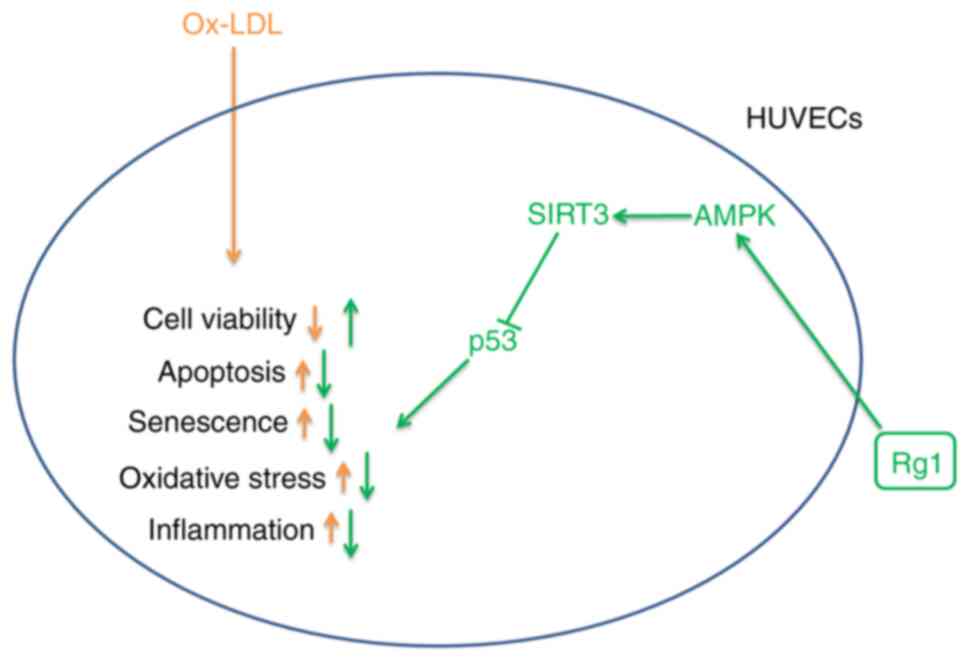
The present study revealed that the AMPK/SIRT3/p53
signaling pathway was inhibited in ox-LDL-induced HUVECs, while Rg1
was able to activate the AMPK/SIRT3/p53 signaling pathway after
ox-LDL induction. A previous study has demonstrated that Rg1 exerts
anti-senescence effects by inhibiting mitochondrial
pathway-mediated apoptosis of hematopoietic stem/progenitor cells
and activating the SIRT3/SOD2 signaling pathway (32). SIRT3 protects endothelial cells
from high glucose-induced senescence and dysfunction through the
p53 signaling pathway (33). In
addition, activation of the AMPK/SIRT3 signaling pathway may reduce
the levels of vascular endothelial mitochondrial ROS, and thus
increase the mitochondrial antioxidant capacity of vascular
endothelial cells (34).
Therefore, it was hypothesized that Rg1 exerts its role through the
AMPK/SIRT3/p53 signaling pathway. In the present study, CC was
added to further explore the associated mechanism. It was revealed
that CC inhibited cell viability, increased apoptosis, accelerated
cell senescence and increased oxidative stress and inflammatory
response in cells treated with ox-LDL + Rg1. It was able to reverse
the protective effect of Rg1 on ox-LDL-induced HUVECs. Therefore,
it was preliminarily concluded that Rg1 ameliorated ox-LDL-induced
apoptosis, senescence and oxidative stress of HUVECs at least
partially through the AMPK/SIRT3/p53 signaling pathway. The
specific mechanisms are illustrated in Fig. 8.
Of note, the present study presents certain
limitations. The effect of Rg1 on coronary AS and its mechanism
were only investigated at the cellular level, but not further
verified in animals, which will be performed in future studies. An
adequate dose of Rg1 for AS would also be required to be determined
in animal models in future studies. In addition, the mechanism was
only investigated using the AMPK pathway inhibitor CC and the
mechanism was not further verified with pathway agonists, which is
also one of the limitations of the present study. The conclusions
of the present study will be further verified with the use of
pathway agonists in future experiments. In addition, in the present
study, the effect of Rg1 was only assessed on vascular endothelial
cells. The effects on other cell types, such as the ventricular
cardiomyocyte cell line H9c2, remain to be examined.
In conclusion, the present study revealed that Rg1
ameliorates apoptosis, senescence and oxidative stress in
ox-LDL-induced HUVECs via the AMPK/SIRT3/p53 signaling pathway. The
present study provided an important theoretical basis for the
potential use of Rg1 in the clinical treatment of CHD.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shanghai Putuo
District Xinglin Excellence Program (grant no. ptxlyq2101), the
Clinical Advantage Discipline of Health System of Putuo District in
Shanghai (grant no. 2019ysxk01), the Shanghai Key Medical
Specialties Construction Project (grant no. ZK2019A11) and the
National Health Commission of Yangpu District ‘good doctor’
construction project.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TJL and ZXZ conceived and designed the study. JC and
ZJL performed the experiments. ZXZ and JC analyzed the experimental
data. TJL and ZJL wrote and revised the manuscript. ZXZ and JC
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang F and Wang Y: Coronary heart disease
and atrial fibrillation: A vicious cycle. Am J Physiol Heart Circ
Physiol. 320:H1–H12. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wirtz PH and von Känel R: Psychological
stress, inflammation, and coronary heart disease. Curr Cardiol Rep.
19(111)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gimbrone MA Jr and Garcia-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Deanfield JE, Halcox JP and Rabelink TJ:
Endothelial function and dysfunction: Testing and clinical
relevance. Circulation. 115:1285–1295. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Palombo C and Kozakova M: Arterial
stiffness, atherosclerosis and cardiovascular risk:
Pathophysiologic mechanisms and emerging clinical indications.
Vascul Pharmacol. 77:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Balta S: Endothelial dysfunction and
inflammatory markers of vascular disease. Curr Vasc Pharmacol.
19:243–249. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tesauro M, Mauriello A, Rovella V,
Annicchiarico-Petruzzelli M, Cardillo C, Melino G and Di Daniele N:
Arterial ageing: From endothelial dysfunction to vascular
calcification. J Intern Med. 281:471–482. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jing R, Zhong QQ, Long TY, Pan W and Qian
ZX: Downregulated miRNA-26a-5p induces the apoptosis of endothelial
cells in coronary heart disease by inhibiting PI3K/AKT pathway. Eur
Rev Med Pharmacol Sci. 23:4940–4947. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gao ZF, Ji XL, Gu J, Wang XY, Ding L and
Zhang H: microRNA-107 protects against inflammation and endoplasmic
reticulum stress of vascular endothelial cells via KRT1-dependent
Notch signaling pathway in a mouse model of coronary
atherosclerosis. J Cell Physiol. 234:12029–12041. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu X, Zheng W, Jin P, Hu J and Zhou Q:
Role of IGFBP1 in the senescence of vascular endothelial cells and
severity of aging-related coronary atherosclerosis. Int J Mol Med.
44:1921–1931. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li H, Huang N, Zhu W, Wu J, Yang X, Teng
W, Tian J, Fang Z, Luo Y, Chen M and Li Y: Modulation the crosstalk
between tumor-associated macrophages and non-small cell lung cancer
to inhibit tumor migration and invasion by ginsenoside Rh2. BMC
Cancer. 18(579)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun M, Ye Y, Xiao L, Duan X, Zhang Y and
Zhang H: Anticancer effects of ginsenoside Rg3 (review). Int J Mol
Med. 39:507–518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou P, Xie W, He S, Sun Y, Meng X, Sun G
and Sun X: Ginsenoside Rb1 as an anti-diabetic agent and its
underlying mechanism analysis. Cells. 8(204)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang R, Yin D, Yang D, Liu X, Zhou Q, Pan
Y, Li J and Li S: Xinnaokang improves cecal microbiota and lipid
metabolism to target atherosclerosis. Lett Appl Microbiol.
73:779–792. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang P, Ling L, Sun W, Yang J, Zhang L,
Chang G, Guo J, Sun J, Sun L and Lu D: Ginsenoside Rg1 inhibits
apoptosis by increasing autophagy via the AMPK/mTOR signaling in
serum deprivation macrophages. Acta Biochim Biophys Sin (Shanghai).
50:144–155. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pan C, Huo Y, An X, Singh G, Chen M, Yang
Z, Pu J and Li J: Panax notoginseng and its components decreased
hypertension via stimulation of endothelial-dependent vessel
dilatation. Vascul Pharmacol. 56:150–158. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu T, Wang H, Wang L, Zhong X, Huang W,
Deng X, Guo H, Xiong J, Xu Y and Fan J: Ginsenoside Rg1 attenuates
high glucose-induced endothelial barrier dysfunction in human
umbilical vein endothelial cells by protecting the endothelial
glycocalyx. Exp Ther Med. 17:3727–3733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen J, Zhang X, Liu X, Zhang C, Shang W,
Xue J, Chen R, Xing Y, Song D and Xu R: Ginsenoside Rg1 promotes
cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in
ischemic mice. Eur J Pharmacol. 856(172418)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Ding S, Chen Y, Sun Z, Zhang J,
Han Y, Dong X, Fang Z and Li W: Ginsenoside Rg1 alleviates
lipopolysaccharide-induced neuronal damage by inhibiting NLRP1
inflammasomes in HT22 cells. Exp Ther Med. 22(782)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Krankel N, Luscher TF and Landmesser U:
Novel insights into vascular repair mechanisms. Curr Pharm Des.
20:2430–2438. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q,
Li YF, Zhang Y, Guo YL, Wu NQ, Zhu CG, et al: Long-term prognostic
utility of low-density lipoprotein (LDL) triglyceride in real-world
patients with coronary artery disease and diabetes or prediabetes.
Cardiovasc Diabetol. 19(152)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kattoor AJ, Kanuri SH and Mehta JL: Role
of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 26:1693–1700.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen L, Yang W, Guo Y, Chen W, Zheng P,
Zeng J and Tong W: Exosomal lncRNA GAS5 regulates the apoptosis of
macrophages and vascular endothelial cells in atherosclerosis. PLoS
One. 12(e0185406)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Che J, Zhao H, Tang J and Shi G:
Paeoniflorin attenuates oxidized low-density lipoprotein-induced
apoptosis and adhesion molecule expression by autophagy enhancement
in human umbilical vein endothelial cells. J Cell Biochem.
120:9291–9299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang D, Bi Z, Li Y, Zheng H, Li L, Ouyang
J, Wang B and Bi Y: Sodium ferulate modified gene expression
profile of oxidized low-density lipoprotein-stimulated human
umbilical vein endothelial cells. J Cardiovasc Pharmacol Ther.
14:302–313. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G
and Sun X: Protective effects and target network analysis of
ginsenoside Rg1 in cerebral ischemia and reperfusion injury: A
comprehensive overview of experimental studies. Cells.
7(270)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gao Y, Chu S, Zhang Z and Chen N:
Hepataprotective effects of ginsenoside Rg1-a review. J
Ethnopharmacol. 206:178–183. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Luo M, Yan D, Sun Q, Tao J, Xu L, Sun H
and Zhao H: Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and
inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem.
121:2994–3004. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Z, Jiang R, Wang L, Chen X, Xiang Y,
Chen L, Xiao M, Ling L and Wang Y: Ginsenoside Rg1 improves
differentiation by inhibiting senescence of human bone marrow
mesenchymal stem cell via GSK-3β and β-catenin. Stem Cells Int.
2020(2365814)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Qin Q, Lin N, Huang H, Zhang X, Cao X,
Wang Y and Li P: Ginsenoside Rg1 ameliorates cardiac oxidative
stress and inflammation in streptozotocin-induced diabetic rats.
Diabetes Metab Syndr Obes. 12:1091–1103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang L, Li Y, Ma X, Liu J, Wang X, Zhang
L, Li C, Li Y and Yang W: Ginsenoside Rg1-notoginsenoside
R1-protocatechuic aldehyde reduces atherosclerosis and attenuates
low-shear stress-induced vascular endothelial cell dysfunction.
Front Pharmacol. 11(588259)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou Y, Wang YP, He YH and Ding JC:
Ginsenoside Rg1 performs anti-aging functions by suppressing
mitochondrial pathway-mediated apoptosis and activating sirtuin 3
(SIRT3)/superoxide dismutase 2 (SOD2) pathway in Sca-1(+) HSC/HPC
cells of an aging rat model. Med Sci Monit.
26(e920666)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen T, Ma C, Fan G, Liu H, Lin X, Li J,
Li N, Wang S, Zeng M, Zhang Y and Bu P: SIRT3 protects endothelial
cells from high glucose-induced senescence and dysfunction via the
p53 pathway. Life Sci. 264(118724)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Han L, Li J, Li J, Pan C, Xiao Y, Lan X
and Wang M: Activation of AMPK/Sirt3 pathway by phloretin reduces
mitochondrial ROS in vascular endothelium by increasing the
activity of MnSOD via deacetylation. Food Funct. 11:3073–3083.
2020.PubMed/NCBI View Article : Google Scholar
|