Introduction
The lung is a complex and primary organ for gas
exchange in mammals that has the largest epithelial surface in
close contact with the external environment (1). Acute microbial (bacterial or viral)
infections may trigger severe damage to the lungs, incurring the
occurrence of lung-related diseases such as acute lung injury (ALI)
(2). ALI is defined as increased
alveolar-capillary permeability triggered by severe noncardiogenic
factors, leading to severe tissue damage and even irreversible
pulmonary damage in severe conditions (1,3).
Recently, the number of patients with ALI due to long-time exposure
to particulate matter has increased, which has gained worldwide
attention (4).
Ropivacaine is a commonly used local anesthetic in
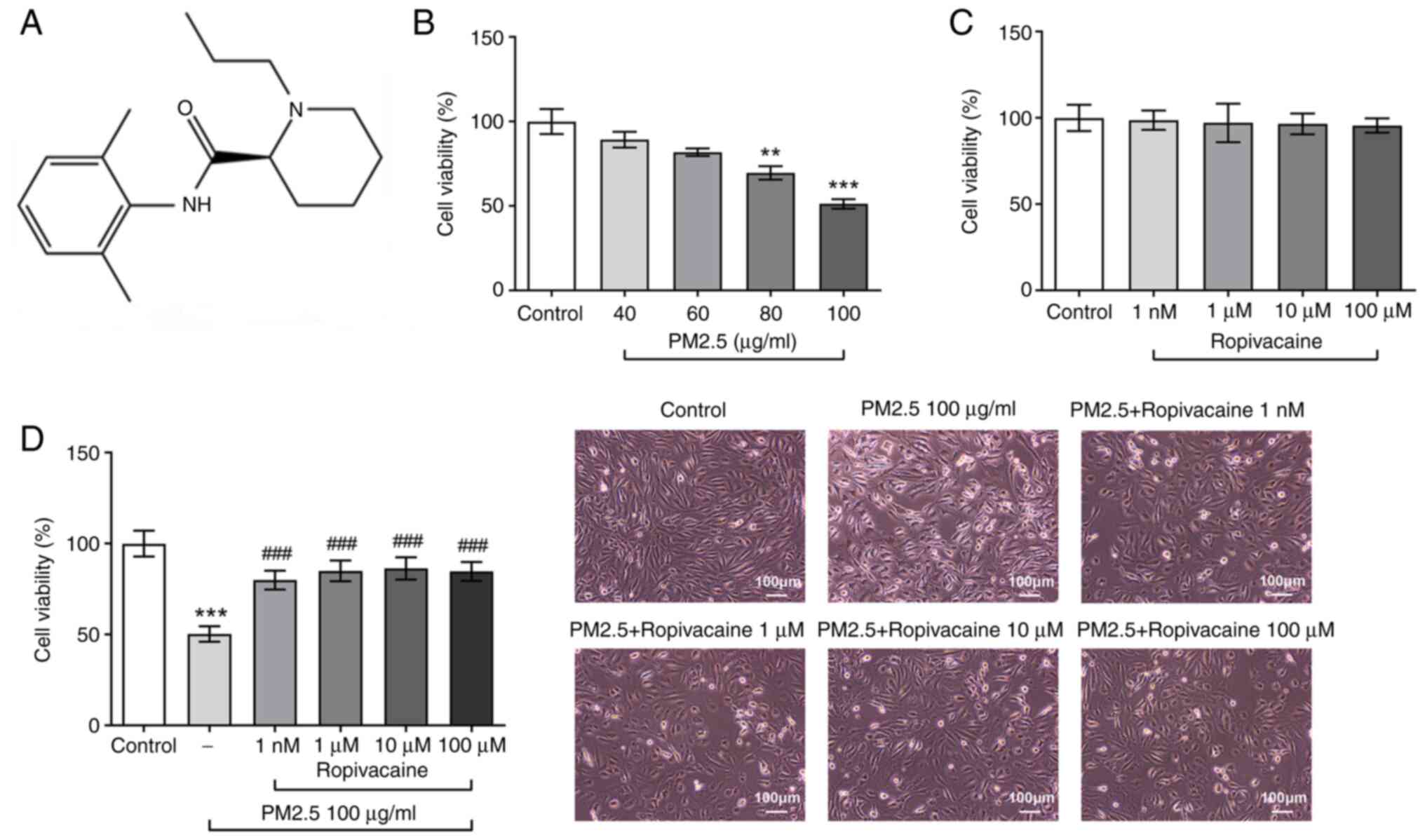
the clinic, whose structural formula is presented in Fig. 1. It is currently regarded as one of
the most potent anesthetics due to its low toxicity to the
cardiovascular system and central nervous system, good tolerance
and high clearance rate (5). It
has been reported that ropivacaine is able to significantly improve
the inflammatory response induced by tumor necrosis factor (TNF)-α,
and it may reduce the activities of matrix metalloproteinases
(MMPs), nuclear factor erythroid 2-related factor 2 and oxidative
stress in brain microvascular endothelial injury induced by high
glucose (6,7). In the model of acute hypertension,
inhibition of endothelial nitric oxide synthase activity reduces
the pressure-induced permeability of pulmonary vascular endothelial
cells (8). Ropivacaine exerts
anti-inflammatory effects by inhibiting the MAPK and NF-κB
signaling pathway in macrophages (9). Accumulating studies have focused on
the effect of ropivacaine on lung-related diseases. It may reduce
endotoxin-induced ALI by reducing neutrophil infiltration, block
inflammatory SRC proto-oncogene, non-receptor tyrosine kinase (SRC)
signal transduction and ICAM-1 expression, alleviate endotoxin
combined with hyperinflation-induced ALI and improve pulmonary
edema in N-formylmethionyl-leucyl-phenylalanine (fMLP)-induced ALI
mice (10-12).
The above results have demonstrated that ropivacaine
has obvious lung-protective effects, but the effect of ropivacaine
on lung injury caused by particulate matter with a diameter of ≤2.5
µm (PM2.5) has remained elusive. Therefore, the present study aimed
to explore whether ropivacaine is able to alleviate PM2.5-induced
inflammation and oxidative stress in lung epithelial cells.
Materials and methods
Cell culture and treatment
The human bronchial epithelial cell line BEAS-2B was
procured from the Type Culture Collection of the Chinese Academy of
Sciences. Cells were cultured in DMEM (MilliporeSigma) with 10%
fetal bovine serum (MilliporeSigma), 100 mg/ml penicillin and 100
mg/ml streptomycin in an incubator with 5% CO2 at
37˚C.
The collection of PM2.5 was performed as previously
described (13). A total of 50 mg
PM2.5 particles were collected by high-volume impactors assembled
with a glass fiber filter (Cytiva) from the mouth of the Yangtze
River at China's central eastern coast in Shanghai at a flow rate
of 1.13 m3/min for every 24 h between October 2019 and
April 2020. PM2.5 samples were dissolved in DMSO at the
concentrations of 100 µg/ml and stored at -80˚C for subsequent use.
Ropivacaine was diluted in VascuLife Basal Medium (Kurabo) at the
concentrations of 1 nM and 1, 10 and 100 µM for further use. For
the induction of ALI, the cultured BEAS-2B cells were collected and
exposed to PM2.5. Furthermore, the cells were treated with
monosodium urate (MSU; MilliporeSigma), an NLR family pyrin domain
containing 3 (NLRP3) agonist, at 150 µg/ml 48 h to study the
mechanism.
CCK-8 assay
Cells were exposed to PM2.5 in medium for 24 h. A
CCK8 kit (Dojindo Laboratories, Inc.) was used to measure the cell
viability of BEAS-2B cells in accordance with the manufacturer's
protocol, and the absorbance at 490 nm was read by an EnSpire
microplate reader (PerkinElmer, Inc.).
Measurement of reactive oxygen species
(ROS), malondialdehyde (MDA) and major endogenous antioxidant
glutathione (GSH)
BEAS-2B cells were seeded in 96-well plates at a
density of 2x104 cells/well and cultured overnight with
5% CO2 at 37˚C. The cells were then treated with
ropivacaine for 24 h prior to the addition of the
dichloro-dihydro-fluorescein diacetate (DCFH-DA) probe for 45 min.
After washing with PBS, cells were fixed with 4% of
polyformaldehyde at room temperature for 30 min. Finally, a
confocal fluorescence microscope (EX/EM: 488/525; Leica
Microsystems GmbH) was used to observe the cells. The fluorescence
intensity was analyzed by using ImageJ v1.8 software (National
Institutes of Health). The intracellular contents of MDA and GSH
were measured by a commercial assay kit (Nanjing Jiancheng Bio Co.,
Ltd.) according to the manufacturer's protocol.
ELISA
The levels of interleukin (IL)-6 (cat. no. D6050),
IL-8 (cat. no. D8000C) and TNF-α (cat. no. DTA00D) in the BEAS-2B
cell supernatants were determined by a commercially available ELISA
kits from R&D Systems, following the recommendations provided
by the manufacturer.
Western blot analysis
Cells were washed in PBS and total protein was
extracted in RIPA lysis buffer (Beyotime Institute of
Biotechnology). Lysed cells were subjected to centrifugation at
16,100 x g for 10 min at room temperature. Quantification of the
protein samples was performed using a BCA assay kit (Tiangen
Biotech, Co., Ltd.). Protein extracts were electrophoresed on 10%
SDS-PAGE and transferred onto PVDF membranes (MilliporeSigma). The
membranes were then blocked with 5% skimmed milk (Absin Bioscience,
Inc.) in Tris-buffered saline for 2 h at room temperature, and
incubated overnight at 4˚C with primary antibodies against MMP9
(cat. no. ab76003; 1:1,000 dilution; Abcam), MMP12 (cat. no.
ab52897; 1:1,000 dilution; Abcam), NLRP3 (cat. no. IMG-6668A; 1:200
dilution; Novus Biologicals, LLC), apoptosis-associated speck-like
protein (ASC; cat. no. sc-514414; 1:200, Santa Cruz Biotechnology,
Inc.), caspase-1 p20 (cat. no. PA5-99390; 1:500 dilution;
Invitrogen; Thermo Fisher Scientific, Inc.) and GAPDH (cat. no.
#5174; 1:1,000 dilution; Cell Signaling Technology, Inc.).
HRP-conjugated anti-rabbit IgG (cat. no. 14708; 1:15,000 dilution;
Cell Signaling Technology, Inc.) or anti-mouse IgG (cat. no.
ab205719; 1:2,000 dilution; Abcam) was used as a secondary antibody
for incubation at room temperature for 2 h. Immunoreactive bands
were visualized using an Pierce enhanced chemiluminescence
detection kit (cat. no. 32209; Thermo Fisher Scientific, Inc.).
Gray values were analyzed by using ImageJ software (v1.8; National
Institutes of Health).
Reverse transcription-quantitative
(RT-q)PCR assay
Total RNA from BEAS-2B cells was extracted by
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA (2 µg)
was then reverse-transcribed into cDNA with the Transcriptor 1st
strand cDNA Synthesis Kit (Roche Diagnostics). Real-time qPCR was
performed using the OneStep TB Green RT-PCR Kit (Takara Bio, Inc.).
The primers used are listed in Table
I. The thermocycling program was 95˚C for 10 min followed by 40
cycles of 95˚C for 15 sec and 60˚C for 60 sec. The relative
expression of each gene was calculated by the 2-ΔΔCq
method (13) and GAPDH was used as
the internal control.
 | Table IPrimers used for quantitative PCR. |
Table I
Primers used for quantitative PCR.
| Name/direction | Primer sequence |
|---|
| MMP9 | |
|
Forward |
5'-GAACCAATCTCACCGACAGG-3' |
|
Reverse |
5'-GCCACCCGAGTGTAACCATA-3' |
| MMP12 | |
|
Forward |
5'-AGTTTTGATGCTGTCACTACCG-3' |
|
Reverse |
5'-CACTGGTCTTTGGTCTCTCAGAA-3' |
| NLRP3 | |
|
Forward |
5'-GGTCCTCTTTACCATGTGCTTC-3' |
|
Reverse |
5'-AAGTCATGTGGCTGAAGCTGTA-3' |
| Caspase-1 p20 | |
|
Forward |
5'-AGAAAAGCCATGGCCGACAA-3' |
|
Reverse |
5'-ACGTGCTGTCAGAGGTCTTG-3' |
| ASC | |
|
Forward |
5'-GATCCAGGCCCCTCCTCAG-3' |
|
Reverse |
5'-GCATCTTGCTTGGGTTGGTG-3' |
| GAPDH | |
|
Forward |
5'-AGCCACATCGCTCAGACAC-3' |
|
Reverse |
5'-GCCCAATACGACCAAATCC-3' |
Statistical analysis
Quantitative data were expressed as the mean ±
standard deviation. GraphPad Prism 6 (GraphPad Software Inc.) was
used for data analysis. Data among groups were analyzed by ANOVA,
followed by Tukey's post-hoc test. P<0.05 was considered to
indicate statistical significance.
Results
Impact of ropivacaine on the viability
of PM2.5-induced BEAS-2B cells
To determine the impact of ropivacaine on ALI, the
changes in the viability of BEAS-2B cells induced by PM2.5 were
first assessed. As clearly presented in Fig. 1B, the viability of BEAS-2B cells
was decreased by PM2.5 in a dose-dependent manner. However, no
significant change in viability was observed in BEAS-2B cells
exposed to different doses of ropivacaine, demonstrating the
relative nontoxicity of ropivacaine used on BEAS-2B cells (Fig. 1C). Subsequently, BEAS-2B cells were
induced with PM2.5 and treated with ropivacaine. In comparison with
the control, PM2.5 markedly reduced the viability and altered the
morphology of BEAS-2B cell, which was rescued by ropivacaine
(Fig. 1D).
Impact of ropivacaine on oxidative
stress and inflammation of PM2.5-induced BEAS-2B cells
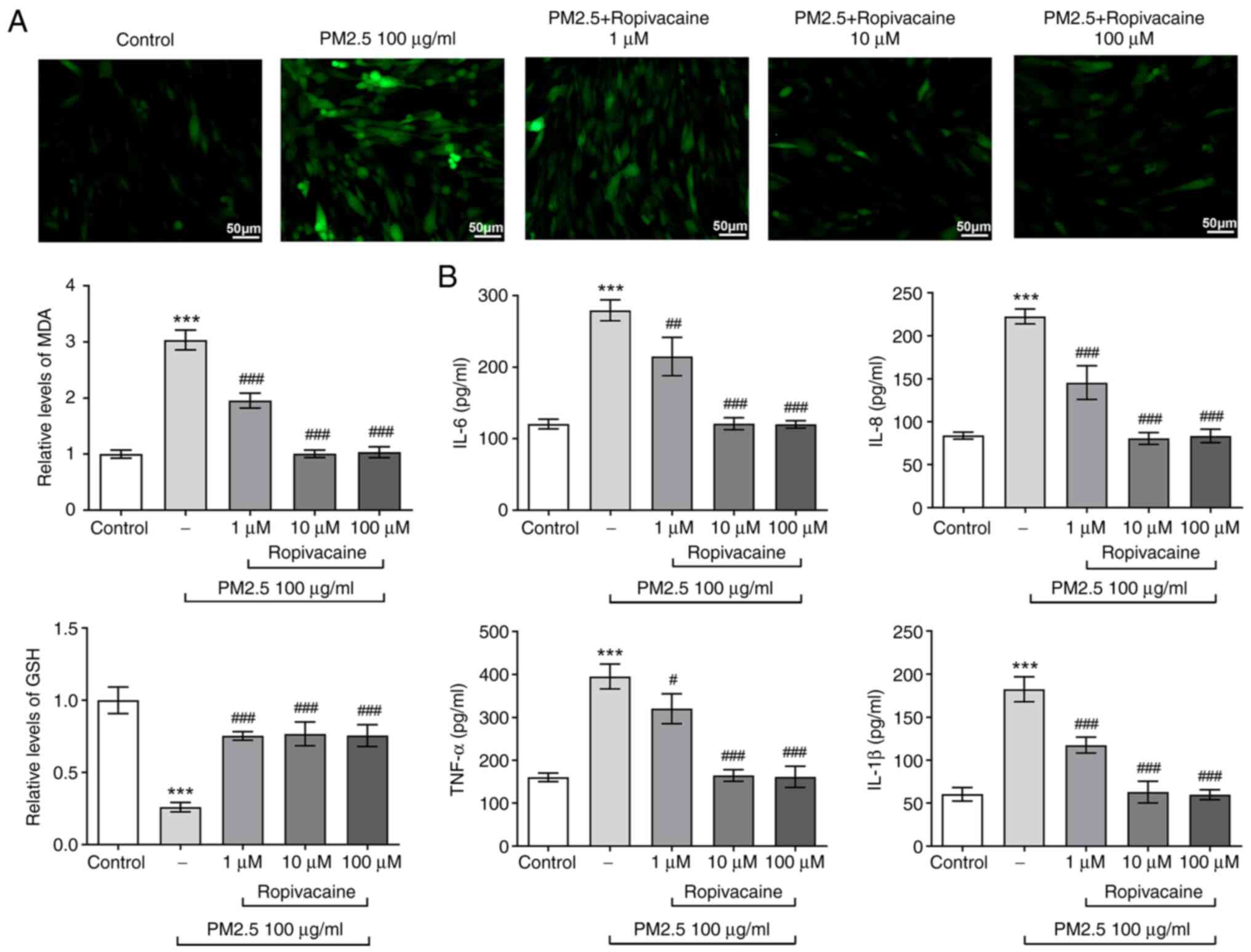
Since oxidative stress and inflammation are
well-recognized hallmarks of ALI, the changes of oxidative stress
and inflammation in PM2.5-induced BEAS-2B cells exposed to
ropivacaine were then determined. The DCFH-DA staining results
indicated that the ROS content in BEAS-2B cells was stimulated by
PM2.5, while it was reduced by different concentrations of
ropivacaine (Fig. 2A). A similar
effect was observed in terms of MDA levels, whereas GSH exhibited
the opposite trend. With regard to inflammatory response, the
inflammatory factors IL-6, IL-8, IL-1β and TNF-α shared similar
expression changes in PM2.5-induced BEAS-2B cells exposed to
ropivacaine, as demonstrated by the result that PM2.5 induced high
levels of inflammatory factors, while ropivacaine led to lower
levels of these factors (Fig. 2B).
Collectively, ropivacaine relieves oxidative stress and
inflammation of PM2.5-induced BEAS-2B cells.
Impact of ropivacaine on the
expression levels of MMPs in PM2.5-induced BEAS-2B cells
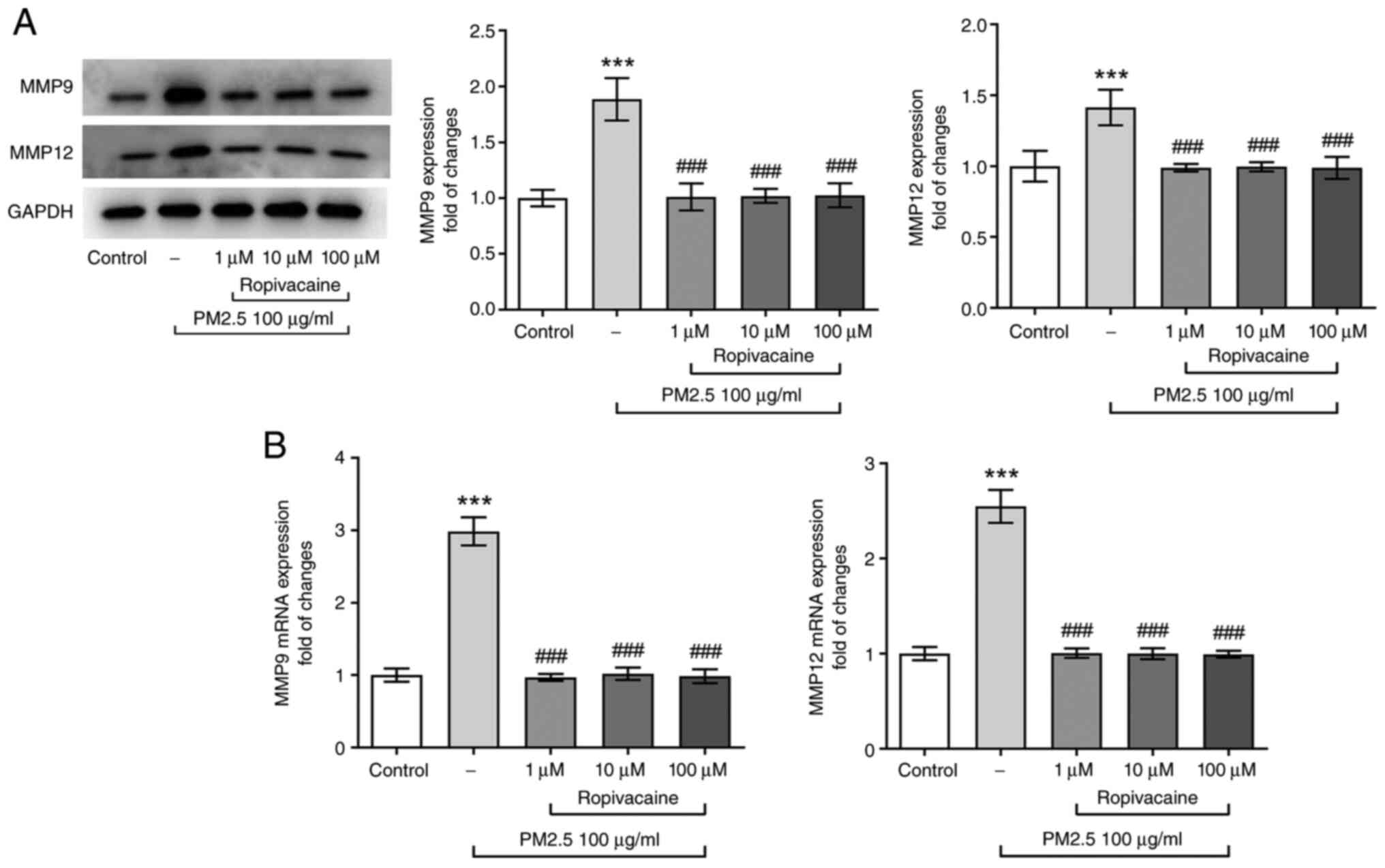
MMPs have an important role in regulating cellular
homeostasis by degrading extracellular matrix (ECM), particularly
MMP12 and MMP9, which are related to airway remodeling and tissue
damage. PM2.5 may significantly increase the expression of MMP9 and
MMP12 (14-16).
Thus, the expression of MMP-12 and MMP-9 was detected in the
present study. It was observed that PM2.5 induced abnormally high
levels of MMP12 and MMP9, whereas these effects were partially
abolished by ropivacaine (Fig. 3A
and B). These results implied the
potential effects of ropivacaine on the restoration of airway
remodeling and tissue damage.
Regulatory role of ropivacaine in
immune response and oxidative stress by NLRP3/Caspase-1
signaling
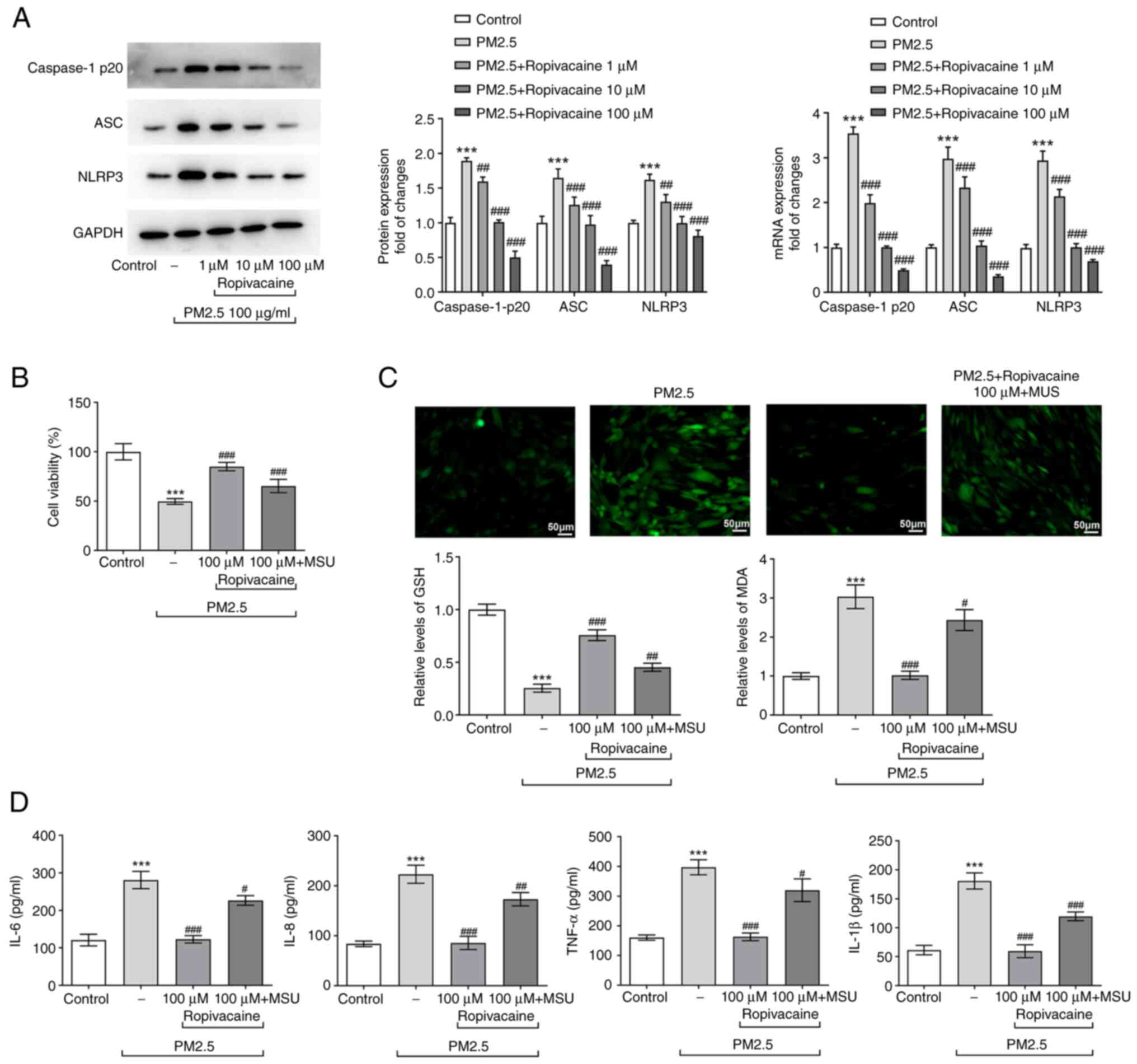
A previous study reported that PM2.5 triggered the
immune response in the lung by activating NLRP3/Caspase-1 signaling
(17), whereas ropivacaine
prevented the activation of NLRP3(18). Thus, in the present study, it was
hypothesized that ropivacaine exerted effects on ALI via
NLRP3/Caspase-1 signaling. As presented in Fig. 4A, PM2.5 activated the expression of
NLRP3, Caspase-1 p20 and ASC, which was reversed by increasing
doses of ropivacaine. Subsequently, BEAS-2B cells were treated with
the NLRP3 agonist MSU (150 µg/ml). The cell viability was
decreased, while oxidative stress and inflammatory response were
aggravated in BEAS-2B cells treated with MSU, which was abrogated
by ropivacaine exposure (Fig.
4B-D). Of note, the cellular changes in MSU-treated BEAS-2B
cells were consistent with those in PM2.5-treated BEAS-2B cells
upon ropivacaine exposure. Thus, it was concluded that ropivacaine
regulates the immune response and oxidative stress in PM2.5-induced
BEAS-2B cells by NLRP3/Caspase-1 signaling.
Discussion
The lung is a sensitive organ that may easily suffer
damage from inhaling substances such as PM2.5(19). Compelling evidence has suggested
the significance of PM2.5 in inducing various lung-related
diseases. Lung parenchymal injury and lung ischemia-reperfusion
injury were observed in rodents even after short-term exposure to
PM2.5, indicating the threat of PM2.5 to lung function (20). It was also previously indicated
that PM2.5 promotes the progression of allergic asthma in mice by
enhancing inflammation and suppressing autophagy (21). Consistently, PM2.5 induced
inflammation and oxidative stress in BEAS-2B cells, as demonstrated
by elevated contents of ROS and MDA, and suppressed activity of
GSH. These observations coincide with the fact that redundant ROS
release is one of the essential factors for PM2.5-induced cell
damage (19).
Accumulating evidence has demonstrated the
protective effects of ropivacaine against lung-associated diseases.
A study has indicated that ropivacaine attenuates inflammatory
response in experimental endotoxin-induced lung damage in
vivo (10). Concurrent with
this, the present study indicated that the release of inflammatory
factors in the supernatant of BEAS-2B cells was inhibited,
accompanied by suppressed oxidative stress. Thus, ropivacaine may
function as a potent anti-inflammatory substance for the treatment
of lung injury.
The MMP family, which is able to degrade ECM and
other substrates, participates in inflammation and tissue
remodeling, which are essential for the pathogenesis of various
lung-associated diseases (22).
Well-orchestrated expression of MMPs, particularly MMP9 and MMP12,
is required for the stabilized development of the lung. In the
present study, PM2.5 stimulation elevated the expression of MMP9
and MMP12, thereby promoting the development of ALI. However,
ropivacaine controlled their expression to relatively normal
levels, which may inhibit this condition.
Inflammation induced by a large number of immune
cells is critical for pulmonary inflammation upon challenge with
PM2.5 (23,24). Once activated, the NLRP3
inflammasome enhances the expression of caspase-1 and convert
pro-IL-1β and pro-IL-18 into their mature bioactive forms (24). Furthermore, inhibition of the
NLRP3/caspase-1 pathway was conducive to the reduction of
inflammatory response in a PM2.5-induced mouse model (17). Thus, the present study suggested
that ropivacaine exerted protective effects on the PM2.5-induced
in vitro ALI model by modulation of the NLRP3/caspase-1
pathway. To validate this notion, MSU was substituted for PM2.5 as
the inducer of the ALI model. An essential finding was that MSU led
to decreased cell viability and promoted oxidative stress and
inflammation, which was consistent with the effects of PM2.5.
Furthermore, ropivacaine partially abolished the effect of MSU on
oxidative stress and inflammation of BEAS-2B cells, demonstrating
that ropivacaine exerted protective effects on PM2.5-induced ALI
via NLRP3/Caspase-1 signaling.
In conclusion, the present study indicated that
ropivacaine exerts protective effects on PM2.5-induced ALI and this
effect may be relative to NLRP3/Caspase-1 signaling. The present
study provided evidence that targeting this signaling may be a
feasible strategy for ALI treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ wrote the manuscript and participated in the
planning and execution of the experiments. XYL and YGH participated
in the major experiments and analyzed the results. RZ and XYL
confirm the authenticity of the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar V: Pulmonary innate immune response
determines the outcome of inflammation during pneumonia and
sepsis-associated acute lung injury. Front Immunol.
11(1722)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rezoagli E, Fumagalli R and Bellani G:
Definition and epidemiology of acute respiratory distress syndrome.
Ann Transl Med. 5(282)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H and Kong X: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 271:26–37.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gu LZ, Sun H and Chen JH: Histone
deacetylases 3 deletion restrains PM2.5-induced mice lung injury by
regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed
Pharmacother. 85:756–762. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou Y, Yang TB, Wei J, Zeng C, Li H, Yang
T and Lei GH: Single-dose intra-articular ropivacaine after
arthroscopic knee surgery decreases post-operative pain without
increasing side effects: A systematic review and meta-analysis.
Knee Surg Sports Traumatol Arthrosc. 24:1651–1659. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Piegeler T, Schläpfer M, Dull RO, Schwartz
DE, Borgeat A, Minshall RD and Beck-Schimmer B: Clinically relevant
concentrations of lidocaine and ropivacaine inhibit TNFα-induced
invasion of lung adenocarcinoma cells in vitro by blocking the
activation of Akt and focal adhesion kinase. Br J Anaesth.
115:784–791. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lv H, Cheng Q, Li Y, Zhang Y, Chen J and
Chen W: The protective effects of ropivacaine against high
glucose-induced brain microvascular endothelial injury by reducing
MMPs and alleviating oxidative stress. Neurotox Res. 39:851–859.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Patel M, Chignalia AZ, Isbatan A,
Bommakanti N and Dull RO: Ropivacaine inhibits pressure-induced
lung endothelial hyperpermeability in models of acute hypertension.
Life Sci. 222:22–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu L, Li L, Wang F, Wu X, Zhao X and Xue
N: Anti-inflammatory effect of local anaesthetic ropivacaine in
lipopolysaccharide-stimulated RAW264.7 macrophages. Pharmacology.
103:228–235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Blumenthal S, Borgeat A, Pasch T, Reyes L,
Booy C, Lambert M, Schimmer RC and Beck-Schimmer B: Ropivacaine
decreases inflammation in experimental endotoxin-induced lung
injury. Anesthesiology. 104:961–969. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Piegeler T, Dull RO, Hu G, Castellon M,
Chignalia AZ, Koshy RG, Votta-Velis EG, Borgeat A, Schwartz DE,
Beck-Schimmer B and Minshall RD: Ropivacaine attenuates endotoxin
plus hyperinflation-mediated acute lung injury via inhibition of
early-onset Src-dependent signaling. BMC Anesthesiol.
14(57)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schley MT, Casutt M, Haberthür C, Dusch M,
Rukwied R, Schmelz M, Schmeck J, Schüpfer GK and Konrad CJ:
Long-acting local anesthetics attenuate FMLP-induced acute lung
injury in rats. Anesth Analg. 109:880–885. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zou W, Wang X, Hong W, He F, Hu J, Sheng
Q, Zhu T and Ran P: PM2.5 induces the expression of inflammatory
cytokines via the Wnt5a/Ror2 pathway in human bronchial epithelial
cells. Int J Chron Obstruct Pulmon Dis. 15:2653–2662.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao J, Li M, Wang Z, Chen J, Zhao J, Xu
Y, Wei X, Wang J and Xie J: Role of PM2.5 in the development and
progression of COPD and its mechanisms. Respir Res.
20(120)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin CI, Tsai CH, Sun YL, Hsieh WY, Lin YC,
Chen CY and Lin CS: Instillation of particulate matter 2.5 induced
acute lung injury and attenuated the injury recovery in ACE2
knockout mice. Int J Biol Sci. 14:253–265. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jia H, Liu Y, Guo D, He W, Zhao L and Xia
S: PM2.5-induced pulmonary inflammation via activating of the
NLRP3/caspase-1 signaling pathway. Environ Toxicol. 36:298–307.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang X, Jiang J, Huang L, Ren Q, Gao X
and Yu S: Ropivacaine prevents the activation of the NLRP3
inflammasome caused by high glucose in HUVECs. ACS Omega.
5:23413–23419. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang L, Liu G and Li X, Xia Z, Wang Y, Lin
W, Zhang W, Zhang W and Li X: Small GTPase RAB6 deficiency promotes
alveolar progenitor cell renewal and attenuates PM2.5-induced lung
injury and fibrosis. Cell Death Dis. 11(827)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee FY, Lee MS, Wallace CG, Huang CR, Chu
CH, Wen ZH, Huang JH, Chen XS, Wang CC and Yip HK: Short-interval
exposure to ambient fine particulate matter (PM2.5) exacerbates the
susceptibility of pulmonary damage in setting of lung
ischemia-reperfusion injury in rodent: Pharmacomodulation of
melatonin. Biomed Pharmacother. 113(108737)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang J, Chen Y, Yu Z, Ding H and Ma Z: The
influence of PM2.5 on lung injury and cytokines in mice. Exp Ther
Med. 18:2503–2511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Greenlee KJ, Werb Z and Kheradmand F:
Matrix metalloproteinases in lung: Multiple, multifarious, and
multifaceted. Physiol Rev. 87:69–98. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen GQ, Benthani FA, Wu J, Liang D, Bian
ZX and Jiang X: Artemisinin compounds sensitize cancer cells to
ferroptosis by regulating iron homeostasis. Cell Death Differ.
27:242–254. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou Y, Zhang CY, Duan JX, Li Q, Yang HH,
Sun CC, Zhang J, Luo XQ and Liu SK: Vasoactive intestinal peptide
suppresses the NLRP3 inflammasome activation in
lipopolysaccharide-induced acute lung injury mice and macrophages.
Biomed Pharmacother. 121(109596)2020.PubMed/NCBI View Article : Google Scholar
|