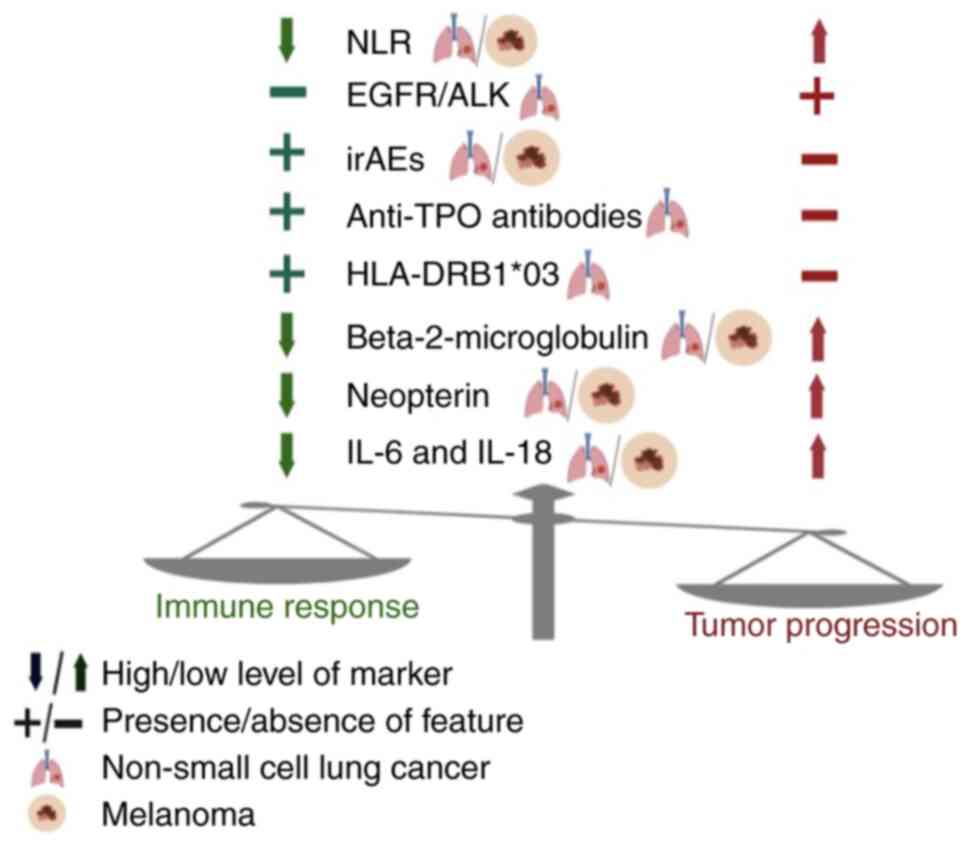
Introduction
Immune checkpoint inhibitors (ICI) demonstrate
unprecedented results in the treatment of variety advanced solid
tumors, such as non-small cell lung cancer (NSCLC) and melanoma
(1,2). Immunotherapy has led to an advantage
in progression-free survival (PFS) and overall survival (OS) over
conventional chemotherapy; unfortunately, 60-70% of patients do not
respond to ICI (3). Given the high
cost of ICI and the high possibility of severe immune-related
adverse events (irAEs), there is a growing need of immunotherapy
predictive biomarkers, as well as biomarkers for monitoring
response to the therapy.
PD-L1 expression on tumor and immune cells is a
potential predictor of sensitivity to ICI and was initially
approved by the FDA in 2015(4). In
the KEYNOTE-024 trial it was shown that patients with metastatic
NSCLC with high PD-L1 expression (≥50%) had longer PFS and OS, when
using anti-PD-1 antibodies compared with chemotherapy (4). However, PD-L1 expression is useful
for only a few tumors: NSCLC, bladder cancer, gastric cancer,
cervical cancer, head and neck squamous cell carcinoma, esophageal
cancer and triple negative breast cancer (5). In addition, patients with low and
negative PD-L1 expression also can response to the therapy
(5). Thus, the main challenge is
to find additional universal predictive markers to determine the
indications for ICI in various malignant tumors.
Tumors with microsatellite instability (MSI)
demonstrate high sensitivity to anti-PD-1 therapy regardless of a
histological type (6). MSI is the
first approved indication for tissue-agnostic treatment (6). Another FDA-approved marker, which
allows to select patients for ICI, is high tumor mutational burden
(TMB-h) (7). However, low
prevalence of MSI or TMB-h leads to limitations for ICI appointment
for most of patients.
Clinical evaluation of predictive markers in tumors
is limited by intratumoral heterogeneity and subsequent biopsies.
Blood-based biomarkers are more accessible and could be used for
non-invasive therapeutic monitoring of the treatment efficacy. In
addition, blood could provide a holistic view on the patient's
immune response, which is one of the key factors of the
effectiveness of cancer immunotherapy (8).
Systemic inflammation causes tumor growth and
progression and, as a result, is associated with poor survival in
various types of cancer (3).
Changes in ratios of peripheral blood biomarkers, for example,
based on changes in the number of lymphocytes
[neutrophil-lymphocyte (NLR) and platelet-lymphocyte ratio (PLR)]
and level of cytokines can serve as a reflection of this process
among patients with malignant tumors (8). In particular, a high level of NLR has
been described as a predictor of poor survival regardless of
treatment in various tumors (3). A
number of studies have shown that immunological markers, such as
NLR, PLR and IL-6, are also predictors of the effectiveness of ICI
(8-10).
The present study also suggested that peripheral markers of T-cell
immune response activation [in particular, β-2 microglobulin
(B2-MG) and IL-18], and markers of macrophages activation, the best
known of which is neopterin (NPT), could be used for monitoring the
response to ICI. As irAEs are associated with the response to ICI,
the present study performed the determination of various
autoantibodies, which could serve as early sign of autoimmune
reactions during ICI, as well as the study of the most well-known
gene in the mosaic of autoimmunity, human leukocyte antigen
(HLA)-DRB1. The aim of the present study was to
define novel immunological markers for monitoring the response to
ICI in advanced NSCLC and melanoma (Fig. 1).
Materials and methods
Study population, treatment and
response evaluation
The present retrospective study included 74 patients
with advanced malignant tumors who received ICI (groups 1 and 2).
The present study also included 30 patients with advanced NSCLC
(aNSCLC), who received initial platinum chemotherapy (group 3).
The present study was conducted from September 2018
to July 2021 at Pavlov First Saint Petersburg State Medical
University. Patients eligible for the study had to be >18 years
old, histologically confirmed NSCLC or cutaneous melanoma, stage
IIIC-IV for cutaneous melanoma and stage IIIB-IVB for NSCLC and
Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0,
1 or 2(4) All patients with NSCLC
and cutaneous melanoma were classified based on Tumor Node
Metastasis staging and the American Joint Committee on Cancer (8th
edition, 2017) (11). Patients who
had a concomitant infection including human immunodeficiency virus
or hepatitis, received systemic steroids, had previous
immunotherapy, concomitant or previous radiotherapy and previous or
ongoing autoimmune disease were excluded. Patient characteristics
are summarized in Table I.
 | Table IClinical and epidemiological data of
patients included in three groups. |
Table I
Clinical and epidemiological data of
patients included in three groups.
|
Characteristics | Group 1 (n=45) | Group 2 (n=29) | Group 3 (n=30) |
|---|
| Sex, n (%) | | | |
|
Male | 30 (66.7) | 16 (55.2) | 21 (70.0) |
|
Female | 15 (33.3) | 13 (44.8) | 9 (30.0) |
| Age, median (IQR),
n (%) | 62 (59-69) | 57 (53-62) | 64 (59-70) |
|
<60 | 25 (55.6) | 14 (48.3) | 12 (40.0) |
|
>60 | 30 (44.4) | 15 (51.7) | 18 (60.0) |
| Histology, n
(%) | | | |
|
Squamous
cell lung cancer | 27(60) | 0 (0.0) | 18 (60.0) |
|
Adenocarcinoma
of the lung | 18(40) | 0 (0.0) | 12 (40.0) |
|
Cutaneous
melanoma | - | 29(100) | - |
| Stage, n (%) | | | |
|
Locally
advanced | 9(20) | 2 (6.9) | 6 (20.0) |
|
Metastatic | 36(80) | 27 (93.1) | 24 (80.0) |
| Disease progression
within the first six months, n (%) | | | |
|
Yes | 19 (42.2) | 7 (24.1) | 17 (56.7) |
|
No | 26 (57.8) | 22 (75.9) | 13 (43.3) |
| Immunotherapy, n
(%) | | | |
|
Nivolumab | 12 (26,6) | 29 (100.0) | - |
|
Pembrolizumab | 30 (66,7) | 0 (0.0) | - |
|
Atezolizumab | 3 (6,7) | 0 (0.0) | - |
| Systemic therapy, n
(%) | | | |
|
First-line | 0 (0,0) | 29 (100.0) | - |
|
Second-line | 36(80) | 0 (0.0) | - |
|
Third-line | 9(20) | 0 (0.0) | - |
| First-line therapy,
n (%) | | | |
|
Chemotherapy | 0 (0,0) | | 30 (100.0) |
|
ALK
inhibitors | 36(80) | | 0 (0.0) |
|
EGFR
inhibitors | 9(20) | | 0 (0.0) |
| Mutational status,
n (%) | | | |
|
EGFR+ | 2 (4,4) | 0 (0.0) | 0 (0.0) |
|
ALK+ | 3 (6,7) | 0 (0.0) | 0 (0.0) |
|
EGFR/ALK- | 21 (46,7) | 0 (0.0) | 5 (16.7) |
|
No data | 19 (42,2 | 29 (100.0) | 25 (83.3) |
| PD-L1 expression, n
(%) | | | |
|
<1% | 16 (35,6) | 9 (31.1) | 7 (23.3) |
|
1-49% | 20 (44,4) | 13 (44.8) | 3 (10.0) |
|
>50% | 9(20) | 0 (0.0) | 0 (0.0) |
|
No data | 0 (0,0) | 7 (24.1) | 20 (66.7) |
Radiological assessment was performed according to
the criteria RECIST 1.1(12). The
primary endpoint for all groups was response rate over a six-month
follow-up. Response was defined as the patient's disease control
rate (DCR) in the course of treatment. This way, the responder
subgroup comprised patients, who showed signs of clinical benefit
within the first six months of treatment, which included a complete
response (CR), a partial response (PR) and stable disease (SD).
Objective response was defined as the cases with CR and PR. The
non-responder group included every patient who discontinued
treatment due to disease progression within the first six months of
the treatment. Progression was defined as a measurable increase in
tumor size according to the criteria RECIST 1.1 or a presence of
new metastatic sites. The secondary endpoint for all groups was the
assessment of PFS. OS has not been assessed due to the immaturity
of the data. For group 1, the median follow-up was 7.8 months
[interquartile range (IQR): 6.5-11.8 months]. For group 2, the
median follow-up was 8.4 months (IQR: 6.1-9.7 months), for group 3,
7.6 months (IQR: 6.3-9.1 months).
For all of the patients a measurement of
immunological markers in peripheral blood was performed. All serum
samples were collected by venipuncture of arm and stored as minimum
of 2 ml aliquots at -80°C until analysis at Pavlov First Saint
Petersburg State Medical University as previously described
(10). The present study protocol
conformed to the ethical guidelines of the Declaration of Helsinki
and was approved by the ethics committee of the Pavlov First Saint
Petersburg State Medical University (approval no. 246-2021). All
participants signed informed consent forms.
Group 1 included 45 patients with locally advanced
and metastatic NSCLC which were treated with ICI in subsequent-line
setting: anti-PD-1 (nivolumab, pembrolizumab) or anti-PD-L1 therapy
(atezolizumab; Table I). The
patients had previously progressed on a platinum-based chemotherapy
(n=40) or EGFR/ALK inhibitors (n=5). Group 1 was divided into
subgroups based on the duration of response to ICI: responders
(n=26), and non-responders (n=19).
Group 2 included 29 patients with unresectable
locally advanced (stage IIIC and IIID) or metastatic melanoma who
received first-line nivolumab as monotherapy. According to the
results of radiological evaluation, the group was also divided into
2 subgroups: responders (n=22), and non-responders (n=7).
Measurement of tissue and serum
markers
PD-L1 expression was assessed in paraffin tissue
sections (4-µm thick) by the Dako PD-L1 IHC clone 22C3 pharmDx
(Agilent Technologies, Inc.) and the Ventana PD-L1 IHC clone SP142
(Ventana Medical Systems, Inc.) assay according to manufacturer's
instructions. NLR and PLR were also calculated before and after two
months the start of the treatment in groups 1 and 2. All patients
participated the study had no history of autoimmune diseases before
starting ICI. Among patients from groups 1 and 2 level of
thyroid-stimulating hormone was within the range 0.4-4.0 mU/l
before starting anti-PD-1/PD-L1 therapy.
In groups 1 and 2 peripheral blood samples were
taken after two months from the start of ICI. Blood samples were
taken both before the start of the next cycle of ICI, and
immediately after the completion of the ICI infusion. The last
sampling point was chosen in order to assess the direct effect of
ICI on cytokine levels. Due to retrospective nature of the study
some patients from group 1 and all patients from group 2 did not
have preserved in advance baseline blood samples that were taken
before the initiation of ICI. In group 1, 16 patients also had
additional points of taking serum samples: before the start of ICI
and six months after the start of therapy. These points were used
to study the possible dynamic changes in the level of markers. In
group 3, peripheral blood was taken before the start of the
infusion of three or subsequent cycles of the chemotherapy.
B2-microglobulin (B2-MG) was determined by an
immunoturbidimetric method, using a test system manufactured by
Biosystems S.A. Due to the fact that the level of B2-MG in the
blood depends on the renal function, the value of serum creatinine
was studied among patients from the three groups before the start
and two months after treatment. The study of neopterin (NPT) was
performed using enzyme-linked immunosorbent assay (ELISA) kit of
IBL International GmbH (cat. no. RE59321) according to the
manufacturer's instructions. The level of cytokines, IL-6 (cat. no.
A8768) and IL-18 (cat. no. A8770) were determined by ELISA with
test systems of the Vector-Best also according to the kit
instructions.
The majority of autoantibodies were determined by
ELISA using commercial test systems of Orgentec Diagnostika GmbH:
antibodies to extractable nuclear antigens (cat. no. 416-5140),
anticardiolipin antibodies (IgG and IgM; cat. no. 416-5150),
anti-MCV antibodies (cat. no. 416-5480). Anti-thyroid peroxidase
autoantibodies (anti-TPO) (cat. no. EA 1012-9601 G), antibodies to
thyroid stimulating hormone receptor (cat. no. EA 1015-9601 G),
antibodies to β-2-glycoprotein (cat. no. EA 1632-9601 G) were
evaluated using commercial ELISA kits manufactured by Euroimmun
Medizinische Labordiagnostika AG. Anti-neutrophil cytoplasmic
antibodies IgG (cat. no. FA 1200-1005), antinuclear antibodies
(diagnostic titer >1: 160) (cat. no. FA 1510-0010-1),
anti-mitochondrial antibody, anti-liver kidney microsomal antibody
and anti-smooth muscle antibody (cat. no. FA 1300-1005-8) were
determined by indirect immunofluorescence using a commercial kit of
Euroimmun Medizinische Labordiagnostika AG. The detection of the
listed autoantibodies was carried out according to manufacturer's
instructions.
Determination of allelic variant of the
HLA-DRB1 gene was performed by reverse transcription PCR
using a commercial test system HLA-DNA-TECH (DNA-Technology LLC;
cat. no. R1-H001-S3/5EU) also according to kit instructions.
Statistical analysis
Statistical data processing was performed using
GraphPad Prism (version 9.3.1; GraphPad Software Inc.). Fisher's
exact test was applied for the comparative analysis of qualitative
characteristics. Evaluation of differences in quantitative
parameters between two compared groups was performed using the
Mann-Whitney U-test. Optimal cut-off values for immunological
markers and the level of PD-L1 expression were determined using
receiver operating characteristic curve (ROC) analysis for the
subsequent study of PFS. Differences in PFS between two compared
groups were analyzed using a log-rank test with hazard ratio
analysis, as well as a graphical presentation using the
Kaplan-Meier method. In group 1 and 2 univariate and multivariate
Cox regression analysis were used to assess the effect of clinical,
morphological data and immunological markers on PFS. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical and morphological parameters
of ICI efficiency NSCLC
There were no significant differences between
responders and non-responders in sex (P=0.891), age (P=1.0), ECOG
PS (P=1.0), smoking status (P=0.068), body mass index (BMI)
(P=0.719), histological type (P=0.283), PD-L1 expression levels on
tumor cells (P=0.152) and the presence of EGFR/ALK mutations (P=
0.146) in group 1. No differences were observed between responders
and non-responders in NLR before (P=0.546) and two months after
initiation of therapy (P=0.132), as well as PLR before (P=0.244)
and two months after initiation (P=0.428).
Using ROC analysis optimal cut-off level of PD-L1
expression to determine efficacy of ICI was ≥50%. However, PD-L1
≥50% was not significantly associated with longer PFS in univariate
Cox regression analysis [hazard ratio (HR): 0.28, 95% confidence
interval (95% CI) 0.04-0.99; P=0.091]. In univariate regression
analysis, the presence of EGFR/ALK mutations (HR: 5.18, 95% CI
0.75-22.68; P=0.045) and NLR ≥5 (HR: 8.02, 95% CI 1.21-32.24,
P=0.009) were associated with shorter PFS (Table II). In multivariate analysis, only
the presence of EGFR/ALK mutations (HR: 8.13, 95% CI 1.13-64.97;
P=0.018) was a predictor of short PFS (Table II).
 | Table IIUnivariate and multivariate
regression analysis of clinical, morphological and immunological
parameters associated with PFS in NSCLC. |
Table II
Univariate and multivariate
regression analysis of clinical, morphological and immunological
parameters associated with PFS in NSCLC.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥75 vs. <75
years) | 1.41
(0.49-3.20) | 0.332 | - | - |
| Sex (male vs.
female) | 1.03
(0.36-2.65) | 0.914 | - | - |
| ECOG PS (0/1 vs.
2) | 1.11
(0.56-2.49) | 0.834 | - | - |
| Smoking status
(former/current vs. never) | 1.53
(0.43-4.25) | 0.452 | - | - |
| BMI (≥25 vs. <25
kg/m2) | 0.85
(0.60-1.45) | 0.623 | - | - |
| Histology
(non-squamous vs. squamous) | 0.45
(0.16-1.17) | 0.110 | - | - |
| None vs. presence
EGFR/ALK mutation | 5.18
(0.75-22.68) | 0.045 | 8.13
(1.13-64.97) | 0.018 |
| Level of PD-L1
expression (<50 vs. ≥50) | 0.28
(0.04-0.99) | 0.091 | - | - |
| irAEs (presence vs.
none) | 2.88
(1.10-8.45) | 0.038 | 3.46
(1.01-14.78) | 0.064 |
| NLR before
initiation of therapy (<5 vs. ≥5) | 8.02
(1.21-32.24) | 0.009 | 8.36
(0.78-91.11) | 0.068 |
| B2-MG (≥2.5 vs.
<2.5) | 0.27
(0.09-0.69) | 0.009 | 0.13
(0.03-0.40) | 0.006 |
| Neopterin (≥12 vs.
<12) | 0.23
(0.07-0.64) | 0.007 | 0.35
(0.13-0.87) | 0.027 |
| IL-6 (≥10 vs.
<10) | 0.46
(0.18-1.16) | 0.091 | - | - |
| IL-18 (≥273 vs.
<273) | 0.23
(0.05-1.06) | 0.056 | - | - |
| Anti-TPO (none vs.
presence) | 0.31
(0.05-1.09) | 0.118 | - | - |
Melanoma
No differences were observed between the responders
and non-responders in age (P=0.811), sex (P=0.665), ECOG PS
(P=1.0), disease stage (P=1.0), category M (P=0.690), level of
serum LDH and PD-L1 expression (P=0.792) in group 2. NLR ≥5 before
initiation of ICI was significantly associated with progression of
disease <six months (P=0.007). There were no significant
differences between responders and non-responders in NLR after two
months of starting ICI (P=0.068), as well as PLR before (P=0.922)
and two months after initiation (P=0.546).
In melanoma optimal cut-off level of PD-L1
expression to determine efficacy of therapy was ≥5%. At the same
time, the level of PD-L1 ≥5% was also not correlated with longer
PFS in univariate Cox regression analysis (HR: 0.28, 95% CI
0.01-1.59; P=0.237) (Table III).
Only NLR ≥5 before initiation of ICI was associated with short PFS
when using univariate (HR: 8.95, 95% CI 2.45-32.67; P=0.0006), as
well as multivariate regression analysis (HR: 7.93, 95% CI
1.80-40.91; P=0.007; Table
III).
 | Table IIIUnivariate and multivariate
regression analysis of clinical, morphological and immunological
parameters associated with PFS in melanoma. |
Table III
Univariate and multivariate
regression analysis of clinical, morphological and immunological
parameters associated with PFS in melanoma.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Melanoma (group
2) | | | | |
| Age (≥65 vs.
<65) | 0.78
(0.11-4.78) | 0.792 | - | - |
| Sex (male vs.
female) | 0.82
(0.04-9.58) | 0.873 | - | - |
| ECOG PS (0/1 vs.
2) | 2.60
(0.51-11.36) | 0.209 | - | - |
| Disease stage (III
vs. IV) | 0.87
(0.11-5.68) | 0.882 | - | - |
| Category M (M1a-b
vs. M1c) | 0.43
(0.02-2.72) | 0.443 | - | - |
| Serum LDH (elevated
vs. normal) | 1.95
(0.55-9.43) | 0.343 | - | - |
| Level of PD-L1
expression (≤5 vs. >5) | 0.28
(0.01-1.59) | 0.237 | - | - |
| irAEs (presence vs.
none) | 4.72
(1.42-21.36) | 0.020 | 5.21
(1.07-38.67) | 0.058 |
| NLR before
initiation of therapy (<5 vs. ≥5) | 8.95
(2.45-32.67) | 0.0006 | 7.93
(1.80-40.91) | 0.007 |
| B2-MG (≥2.5 vs.
<2.5) | 0.10
(0.02-0.39) | 0.003 | 0.09
(0.01-0.44) | 0.008 |
| NPT (≥12 vs.
<12) | 0.62
(0.14-1.21) | 0.184 | - | - |
| IL-6 (≥10 vs.
<10) | 0.25
(0.07-0.84) | 0.015 | 0.57
(0.31-1.67) | 0.516 |
| IL-18 (≥273 vs.
<273) | 1.69
(0.47-8.31) | 0.459 | - | - |
| Anti-TPO (none vs.
presence) | 0.21
(0.01-1,07) | 0.133 | - | - |
Autoimmune markers and ICI
efficiency
In group 1 irAEs developed in 37.8% of cases. All
cases were represented by 1-2 grade and included the following
diseases: Autoimmune thyroiditis (n=7), rash (n=4), hepatitis
(n=3), pneumonitis (n=2), colitis (n=1). The presence of irAEs was
associated with the duration of the response >6 months: 53.9% of
cases (14/26) in responders compared with 15.8% (3/19) in
non-responders (P=0.013). Univariate regression analysis showed
that irAEs was associated with longer PFS (HR: 2.88, 95% CI
1.10-8.45; P=0.038). However, this relationship was not found in
multivariate analysis (P=0.064).
In group 2 irAEs developed in 44.8% of cases and
represented the following diseases: rash (n=6), autoimmune
thyroiditis (n=5), hepatitis (n=1), pneumonitis (n=1). Only the
case of hepatitis was represented by grade 3 of toxicity, the rest
were grade 1-2. The appearance of irAEs during ICI was associated
with prolonged PFS in univariate (HR: 4.72, 95% CI 1.42-21.36;
P=0.020), but not in multivariate regression analysis (HR: 5.21,
95% CI 1.07-38.67; P=0.058).
Autoantibodies
Anti-TPO antibodies (according to the manufacturer's
instructions the positive threshold value was >50 IU/ml) were
detected in all cases of autoimmune thyroiditis (n=7) in group 1.
The appearance of antibodies after two months from the start of ICI
was significantly associated with a response ≥six months: in
responders a presence of the marker was observed in 26.9% (7/26) of
cases and in non-responders-0% (0/19) (P=0.016). Using univariate
regression analysis, no association between anti-TPO antibodies and
PFS was demonstrated in group 1 (P=0.118). In group 2, anti-TPO
antibodies were also detected in all cases of autoimmune
thyroiditis (n=5). No association was demonstrated between the
appearance of anti-TPO antibodies and duration of response ≥6
months and also PFS (P>0.05).
Diagnostic titer of antinuclear antibodies and
antibodies to extractable nuclear antigens was detected in all
cases of immune-related hepatitis in group 1 (n=3) and in group 2
(n=1). In group 1 and 2 none of studied autoantibodies was detected
in other immune-related adverse events, such as rash, pneumonitis
and colitis.
In group 1 and 2 there was no statistically
significant association between the duration of response to
anti-PD-1/PD-L1 therapy, PFS and the presence of one of the
following autoantibodies: Antinuclear antibodies, antibodies to
extractable nuclear antigens, anticardiolipin antibodies, anti-MCV
antibodies, antibodies to thyroid stimulating hormone receptor,
antibodies to β-2-glycoprotein, anti-neutrophil cytoplasmic
antibodies, anti-mitochondrial antibody, anti-liver kidney
microsomal antibody and anti-smooth muscle antibody
(P>0,05).
HLA-DRB1
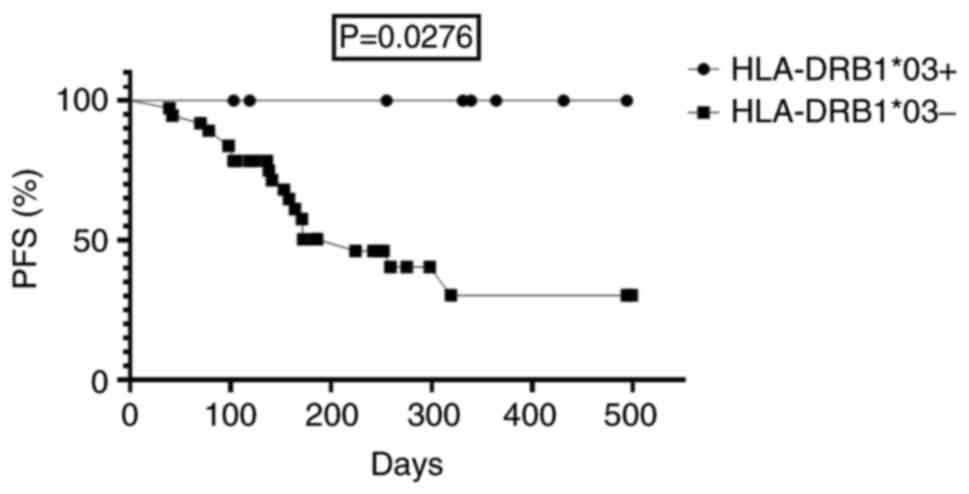
The HLA-DRB1*03 genotype was associated with
response to therapy among patients in group 1: 26.9% of cases
(7/26) were responders, 0% (0/19) non-responders (P=0.016). At the
same time, six patients with the HLA-DRB1*03 in group 1 had
a partial response after six months from the start of therapy, and
one patient had a complete response. Among patients with the
HLA-DRB1*03 PFS was statistically significantly longer
compared with patients with other variants of alleles of the
HLA-DRB1 gene in group 1: median was not reached compared
with 224 days, respectively (HR=3.6; 95% CI 1.2-11.2; P=0.0276;
Fig. 2). However, no association
was observed between this genotype and PFS when using univariate
Cox regression analysis (P>0.05).
Immunological markers and ICI
efficiency B2-MG
Among patients with aNSCLC receiving ICI, a level of
B2-MG after two months was significantly lower in responders
compared with non-responders in group 1: Median was 1.7 mg/l (95%
CI 1.6-2.3 mg/l) compared with 2.9 mg/l (95% CI 2.5-3.3 mg/l),
respectively (P<0,0001; Fig.
3A). There were no significant differences between cases with
objective response and SD (P=0.284). In group 1, in patients with
B2-MG ≥2.5 mg/l, PFS was lower than among patients with a level of
less than 2.5 mg/l: Median was 168 days and not reached,
respectively (HR 2.8; 95% CI 1.2-6.9; P=0.017; Fig. 3C). Also, B2-MG ≥2.5 mg/ml was
associated with shorter PFS in univariate (HR: 0.27, 95% CI
0.09-0.69; P=0.009) and multivariate regression analysis (HR: 0.13,
95% CI 0.03-0.40; P=0.006; Table
II).
In 16 patients with NSCLC receiving ICI no
association was observed between pretreatment level of B2-MG and
PFS (P=0.805). The change in B2-MG from baseline to measurements
after two months was associated with PFS. In 16 patients increased
level of marker during two months of treatment >2.7 times was
associated with short PFS: Median was 319 days compared with not
reached, respectively (P=0.021). Also in group 1, 16 patients with
NSCLC median level of the marker after two and six months were
comparable (P=0.912).
B2-MG was also statistically significantly lower in
responders than in non-responders in group 2: median was 1.8 mg/l
(95% CI 1.6-2.3 mg/l) compared with 3.6 mg/l (95% CI 3.1-4.0 mg/l),
respectively (P=0.0001) (Fig. 3B).
There were no significant differences between the cases with
objective response and SD (P=0.094). Among patients with melanoma
with B2-MG ≥2.5 mg/l PFS was shorter than among patients with the
level of the marker <2.5 mg/l: median was 178 days and not
reached, respectively (HR 10.0; 95% CI 2.9-34.4; P=0.0002)
(Fig. 3D). B2-MG ≥2.5 mg/l was
associated with shorter PFS in both univariate (HR: 0.10, 95% CI
0.02-0.39; P=0.003) and multivariate analysis (HR: 0.09, 95% CI
0.01-0.44; P=0.008).
Among patients from groups 1 and 2 creatinine
corresponded to the reference value both before the start and after
two months of ICI. This indicates the absence of effect of renal
function on the level of the marker in two groups.
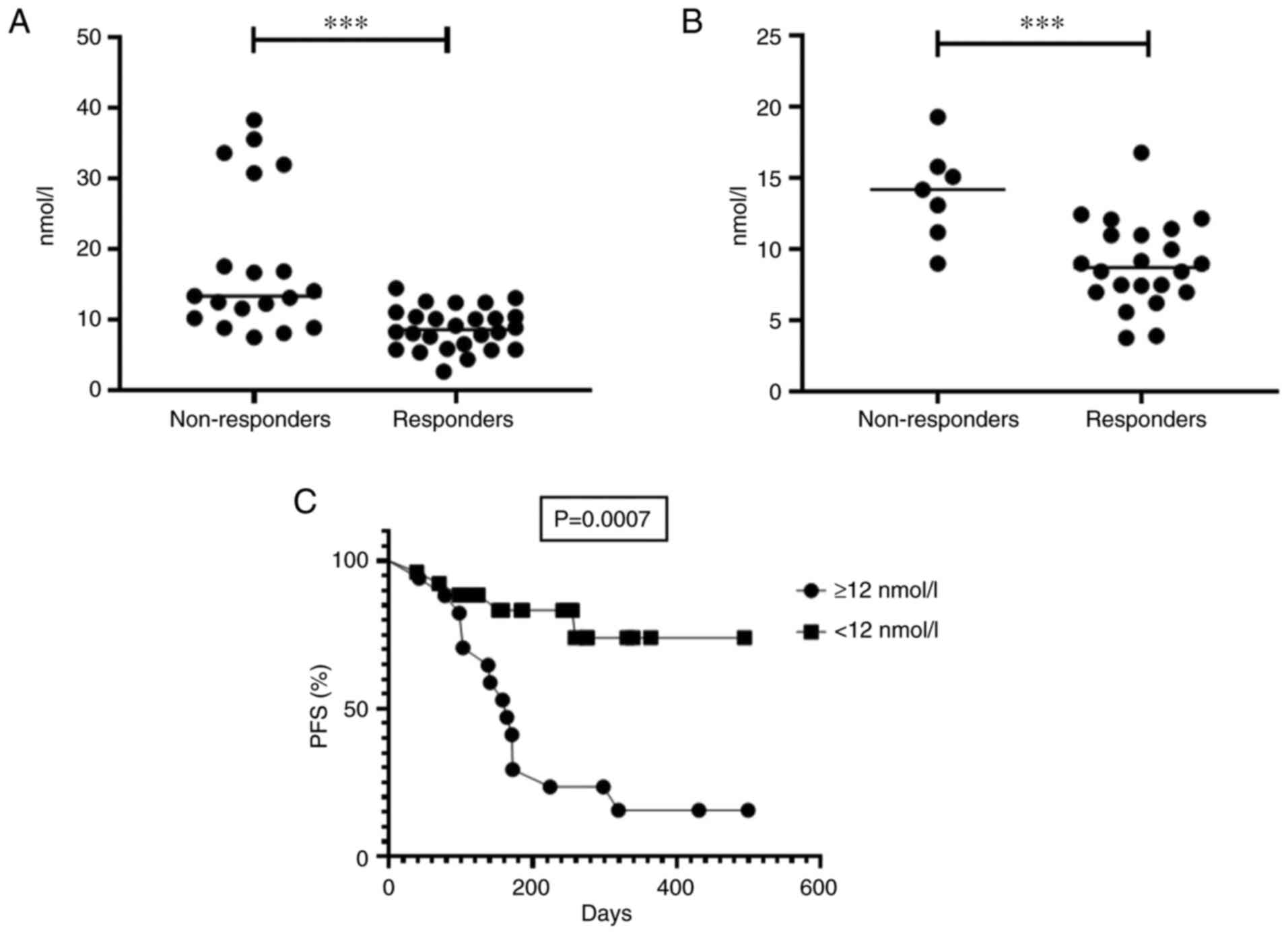
NPT
Median of NPT was lower in responders compared with
non-responders in group 1: 8.6 nmol/l (95 % CI 7.6-10.0 nmol/l)
compared with 13.4 nmol/l (95% CI 13.0-23.0 nmol/l), respectively
(P<0.0001; Fig. 4A). There was
also no significant difference in the level of the marker between
patients with objective response and SD (P=0.151). Among patients
with aNSCLC with NPT ≥12 nmol/l PFS was significantly lower than
among patients with NPT <12 nmol/l: median was 164 days compared
with not reached, respectively (HR=4.8; 95% CI 1.9-12.3; P=0.0007)
(Fig. 4C). NPT ≥12 nmol/l was
associated with shorter PFS in both univariate (HR: 0.23, 95% CI
0.07-0.64; P=0.007) and multivariate analysis (HR: 0.35, 95% CI
0.13-0.87; P=0.027).
In group 1 in 16 patients with NSCLC high baseline
level of NPT (defined as >6.8 nmol/l) was associated with short
PFS: Median was 224 days compared with not reached (P=0.0018). No
association was observed between the change in NPT from baseline to
measurements after two months of therapy initiation and PFS
(P=0.067). Also, 16 patients demonstrated no differences in the
level of the marker at two control points: after two and six months
of the start of ICI (P=0.736).
There was a statistically significant difference of
the level of NPT in responders and non-responders in group 2:
Median was 8.7 nmol/l (95% CI 7.6-10.3 nmol/l) compared with 14.2
nmol/l (95% CI 10.9-17.0 nmol/l), respectively (P=0.0016) (Fig. 4B). There was also no significant
difference in the level of the marker between patients with
objective response and SD (P=0.151). However, there was no
statistically significant relationship between NPT ≥12 nmol/l and
shorter PFS while using log-rank test (HR: 2.88, 95% CI 0.86-9.64;
P=0.052) and univariate Cox regression analysis (HR: 0.62, 95% CI
0.14-1.21; P=0.184).
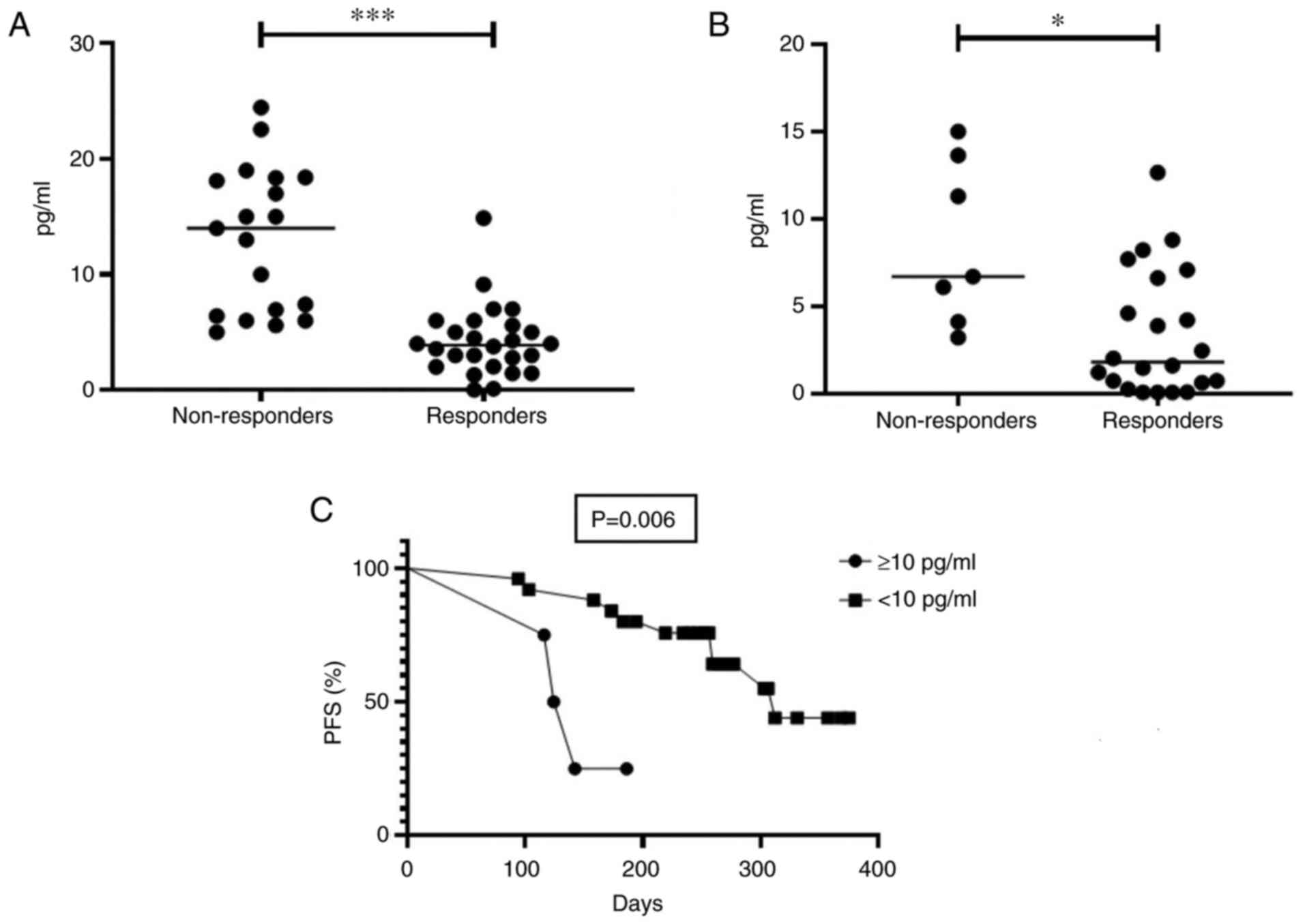
IL-6
A level of IL-6 after two months of the start of ICI
was lower in responders compared with non-responders in group 1:
Median was 3.9 pg/ml (95% CI 2.8-5.0 pg/ml) compared with 14 pg/ml
(95% CI 6.0-18.4 pg/ml), respectively (P<0.0001) (Fig. 5A). There were no statistically
significant differences between patients with objective response
and SD (P=0.217). Using univariate regression analysis there was no
significant association between a level of IL-6 and PFS
(P=0.091).
In group 1 in 16 patients with NSCLC no significant
association was observed between lower IL-6 at baseline (P=0.209),
decreasing the level of the marker during first two months of ICI
(P=0.091) and PFS. Also, levels of the marker were comparable after
two and six months in 16 patients in group 1 (P=0.334).
A level of IL-6 after two months was lower in
responders compared with non-responders in group 2: Median was 1.8
pg/ml (95% CI 1.8-5.0 pg/ml) and 6.7 pg/ml (95% CI 4.2-12.9 pg/ml),
respectively (P=0.013; Fig. 5B).
There were no differences in the level of the marker among patients
with objective response and SD (P=0.891). Among patients with
melanoma with a level of IL-6 ≥10 pg/ml PFS was lower than among
patients with IL-6 <10 pg/ml: median was 133 and 312 days,
respectively (HR=4.9; 95% CI 0.5-49.7; P=0.006; Fig. 5C). A level of IL-6 ≥10 pg/ml after
two months of initiating ICI was associated with low PFS in
univariate Cox regression analysis (HR: 0.25, 95% CI 0.07-0.84;
P=0.015), but not in multivariate analysis (HR: 0.57, 95% CI
0.31-1.67; P=0.516).
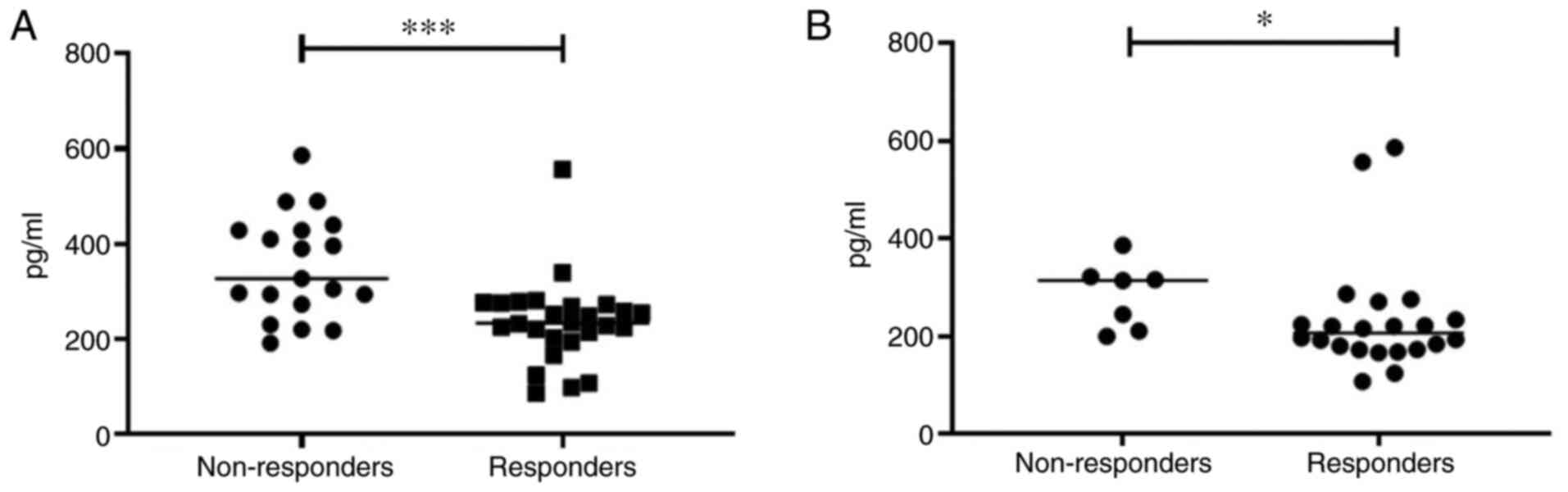
IL-18
The level of IL-18 in responders was significantly
lower compared with non-responders in group 1: Median was 233.3
pg/ml (95% CI 198.9-271.8 pg/ml) compared with 327.4 pg/ml (95% CI
300.5-405.5 pg/ml), respectively (P=0.0003; Fig. 6A). There was no significant
difference in the level of this marker among patients with
objective response and SD in group 1 (P=0.432). Using univariate
analysis, the relationship between the level of IL-18 and PFS has
not been demonstrated (P=0.056).
In 16 patients with NSCLC receiving ICI no
association was observed between the baseline level of IL-18
(P=0.641), change in the level of the marker during two months of
treatment (P=0.067) and PFS. As for IL-6, 16 patients had similar
levels of IL-18 at two and six months after therapy initiation
(P=1.0).
The level of IL-18 was significantly lower in
responders compared with non-responders in group 2: Median was
206.3 pg/ml (95% CI 183.0-287.1 pg/ml) and 314.0 pg/ml (95% CI
222.0-347.7 pg/ml), respectively (P=0.032) (Fig. 6B). Using univariate analysis, no
association between a level of IL-18 and PFS was demonstrated
(P=0.459).
Immunological markers in chemotherapy
and ICI
In group 3 there were no differences between
responders and non-responders in sex (P=1.0), age (P=0.411), ECOG
PS (P=0.892), smoking status (P=0.384), body mass index (P=1.0),
histological type (P=0.460) and stage of disease (P=1.0), NLR
before (P=0.196) and two months after initiation of therapy
(P=0.104), as well as PLR before (P=0.627) and two months after
initiation (P=0.359). Also, there were no differences between two
subgroups in B2-MG (P=1.0), NPT (P=0.233), IL-6 (P=0.893) and IL-18
(P=1.0). None of the studied autoantibodies was found during the
treatment. For the group 3, no statistical significance was
observed between any of allelic variants of the HLA-DRB1 and
the duration of the response to the chemotherapy (P>0,05). In
univariate analysis no association between clinical and laboratory
parameters and PFS was shown (Table
IV).
 | Table IVUnivariate regression analysis of
clinical, morphological and immunological markers associated with
PFS. |
Table IV
Univariate regression analysis of
clinical, morphological and immunological markers associated with
PFS.
| | Univariate
analysis |
|---|
|
Characteristics | HR (95% CI) | P-value |
|---|
| Age (≥75 vs.
<75) | 0.83
(0.48-1.66) | 0.485 |
| Sex (male vs.
female) | 0.97
(0.92-1.18) | 0.672 |
| ECOG PS (0/1 vs.
2) | 1.32
(0.83-1.92) | 0.213 |
| Smoking status
(former/current vs. never) | 1.24
(0.91-1.71) | 0.153 |
| Histology
(non-squamous vs. squamous) | 0.91
(0.85-1.83) | 0.532 |
| NLR before
initiation of therapy (<3 vs. ≥3) | 1.26
(0.85-1.93) | 0.196 |
| NPT (≥10 vs.
<10) | 0.61
(0.42-1.19) | 0.145 |
Group 3 allowed the assessment of the specificity of
changes in immunological parameters in relation to ICI in groups 1
and 2. Measurement of these markers in group 3 could help to
indirectly assess the level of markers before the start of ICI
among patients in group 1 who previously had received chemotherapy.
Group 1 and group 3 did not statistically differ in sex (P=0.806),
age (P=0.655), histology (P=1.0) and TNM stage (P=1.0).
Among patients from group 1, previously treated with
platinum-containing chemotherapy, a level of B2-MG after the
initiation of ICI was significantly higher compared with patients
from group 3 who received chemotherapy: Median was 2.1 mg/l (95% CI
2.0-2.5 mg/l) compared with 1.1 mg/l (95% CI 1.0-1.2 mg/l),
respectively (P<0.0001). During the study of another
inflammatory marker NPT, a significant difference was also
demonstrated among patients from group 1 and group 3: median was
9.8 nmol/l (95% CI 10.4-14.3 nmol/l) and 6.2 nmol/l (95% CI 5.7-7.5
nmol/l), respectively (P<0.0001). A statistically significant
difference in the level of IL-6 and IL-18 was observed among
patients with aNSCLC, receiving ICI and chemotherapy
(P<0.0001).
For 16 patients from group 1, median level of B2-MG
and NPT evaluated before the start of ICI was 1.2 mg/l (95% CI
1.0-1.4 mg/l) and 5.9 nmol/l (95% CI 5.2-6.9 nmol/l), respectively.
These values were comparable to the same in group 3. In 16 patients
median of IL-6 before the start of ICI was similar to level of the
marker in the comparison group receiving platinum-based
chemotherapy in group 1: 1.9 pg/ml (95% CI 1.4-2.6) and 2.0 pg/ml
(95% CI 1.7-3.0), respectively. Similar values of IL-18 were shown
in two groups: ICI (group 1)-158.4 pg/ml (95% CI 141.3-169.8
pg/ml), chemotherapy (group 3)-165.5 pg/ml (95% CI 142.9-181.3
pg/ml).
Discussion
The present study demonstrated a predictive role of
some markers of chronic inflammation in NSCLC and cutaneous
melanoma for the first time, to the best of the authors' knowledge.
It has shown that NLR, B2-MG, NPT, IL-6 and IL-18 were associated
with response to ICI. An association between autoimmune reactions
and the effectiveness of ICI was also demonstrated.
According to the study of potential predictive tumor
markers, only the presence of EGFR/ALK mutations was an independent
predictor of shorter PFS among patients with NSCLC. In the present
study, no relationship was found between PD-L1 expression and
response to therapy in NSCLC and melanoma.
ICI suppresses negative co-stimulatory signals of T
cells, thereby enhancing the antitumor response (13). ICI can also activate autoreactive T
cells through the immunostimulating mechanism. This, in turn, can
lead to impairment of tolerance of T cells to autoantigens and to
the activation of autoreactive B cells. This ultimately leads to
the formation of autoantibodies that are early predictors of irAEs
(13). In the present study, the
appearance of irAEs among patients with NSCLC and melanoma was
associated with longer PFS only in univariate analysis. Hussaini
et al (14) demonstrated in
meta-analysis that irAEs were independent predictors of increased
survival regardless of tumor type and the type of ICI. The present
study also found that the presence of a diagnostic titer of
anti-TPO antibodies was associated with the response to the therapy
only among patients with NSCLC during ICI, while the generation of
the autoantibodies was not observed in non-responders. However, no
relationship was shown between anti-TPO antibodies and PFS among
patients with NSCLC and melanoma. Music et al (15) also showed that an increase in the
level of anti-TPO antibodies before starting the 3rd cycle of
therapy was associated with an increase OS among patients receiving
pembrolizumab.
Another autoimmune marker of ICI efficacy is poorly
studied allelic variants of gene HLA class II HLA-DRB1. HLA
class II molecules play a role in cross-priming, T and B cell
modulation, and the development of autoimmunity (16). The present study, for the first
time to the best of the authors' knowledge, showed the relationship
between the HLA-DRB1*03 allele and the response to ICI. The
frequency of HLA-DRB1*03 allele in patients with NSCLC
receiving ICI was 26.9%. The similar prevalence of the allele in
patients with NSCLC receiving chemotherapy was 23,3% (7/30).
Kapustin et al (17)
analyzed 200 healthy individuals from the same region and showed
that the frequency of the HLA-DRB1*03 allele in general
population was 16.0%.
Among patients with aNSCLC receiving ICI,
HLA-DRB1*03 was associated with response duration ≥6 months
compared with other allelic variants of the gene. All patients with
HLA-DRB1*03 achieved objective response within six months.
Moreover, HLA-DRB1*03 was associated with longer PFS in the
log-rank test, but not in Cox regression analysis.
NLR is a convenient blood marker of systemic
inflammation that reflects the balance of the antitumor immune
response (18). Traditionally,
neutrophils demonstrate tumor-promoting activity in the tumor
microenvironment, while lymphocytes are effective suppressors of
tumor growth (18). An increase of
level of NLR indicates a high level of absolute neutrophil count
and/or a low level of absolute lymphocyte count and, as a result, a
decrease in the antitumor response (19). The present study found that a high
level of NLR, measured before starting ICI, was associated with
shorter PFS among patients with NSCLC in univariate analysis and
among patients with melanoma in multivariate analysis. Similar
results are demonstrated in a number of other studies (18-20).
B2-MG is a non-glycosylated protein that is a
component of HLA class I (21).
After release from the cell surface, B2-MG is separated from the
complex and circulates as a monomer (21). Serum levels of B2-MG are markers of
cellular activation of the immune system, as well as markers of
poor prognosis in certain lymphoproliferative disorders such as
multiple myeloma (22). The
present study showed that B2-MG could be used as a marker for
monitoring response to anti-PD-(L)1 therapy. Responders with NSCLC
and melanoma, who received first-line and subsequent ICI, had
significantly lower B2-MG levels than non-responders. At the same
time, no differences were observed in the level of B2-MG among
patients with objective response and SD. It was shown that a high
level of B2-MG (≥2.5 mg/l) among patients with aNSCLC and cutaneous
melanoma that received ICI was associated with shorter PFS in
multivariate analysis. Also, in 16 patients with NSCLC increased
level of the marker during ICI was associated with short PFS. A
possible explanation of the relationship between B2-MG and the
resistance to ICI is that high concentrations of serum B2-MG in
vitro lead to a decrease in the antigen-presenting ability, as
well as to inhibition of the T-cell immune response (23). Also, high protein levels lead to
retardation in the differentiation of monocytes into functional
dendritic cells (23). Moreover,
it has been shown that high concentrations of B2-MG can also
promote tumor growth and survival by increasing the production of
cytokines such as IL-6 and IL-10 by monocytes (23).
NPT is a marker of macrophage activation (24). In malignant tumors, NPT production
is a result of chronic stimulation of macrophages and reflects an
inability of immune surveillance to inhibit tumor growth (25). In the present study the possibility
of using NPT was investigated for predicting response to ICI. NPT
was significantly lower in responders with NSCLC and melanoma, who
received ICI, than in non-responders. At the same time, the level
of the marker did not differ in objective response and stable
disease. In 16 patients with NSCLC the high baseline level of the
marker during treatment was associated with short PFS. Also, a high
level of NPT (≥12 nmol/l) after two months was independent
predictor of short PFS only among patients with aNSCLC, receiving
subsequent-line ICI monotherapy.
Cytokines are potential markers of response to ICI.
IL-6 is one of the most promising predictive cytokine markers. A
high level of IL-6 production is associated with the formation of
an immunosuppressive tumor microenvironment due to myeloid-derived
suppressor cells, M2 macrophage cells and Treg cells (26). Also, IL-6 is responsible for the
immune evasion by tumor cells via enhancing of PD-L1 expression
(26). The present study found
that level of IL-6 after two months of initiating ICI was
statistically significantly higher in responders with NSCLC and
melanoma that received ICI, than in non-responders. No differences
were observed in the level of IL-6 between cases of objective
response and stable disease. Also, a high level of IL-6 among
patients with melanoma was associated with short PFS only in
univariate analysis. Laino et al (27) analyzed CheckMate-064, 066 and 067
studies and found that high IL-6 levels were associated with poor
response and shorter survival among patients with melanoma treated
with nivolumab.
A similar association was shown between a high level
of IL-18 during ICI and early disease progression among patients
with NSCLC and melanoma who received ICI. However, no association
was shown between a high level of the marker and PFS in both groups
of patients. Although preclinical and some clinical studies suggest
that IL-18 has antitumor activity, other studies shown that IL-18
plays a dual role in tumors, as it can exhibit pro-invasive and
pro-angiogenic activity in various tumors (28-30).
In a recent retrospective study of patients with aNSCLC, Wang et
al (29) demonstrated that a
high level of IL-18 before starting ICI was associated with
response to the therapy. However, the same study noted a decreased
level of IL-18 among patients who achieved a partial response
(29). A possible explanation for
the data obtained in the present study, as well as in the work of
Wang et al (29) is that
IL-18 produced by tumor cells reduces the antimetastatic activity
of NK cells in a PD-1-dependent manner (30).
A limitation of the present study was the absence of
an index that included some of the studied markers, which allows
the most accurate determination of patients who will respond to
immunotherapy. Different combination of predictive markers before
initiation of treatment and also during immunotherapy did not show
greater statistically significance by univariate Cox regression
analysis than using single markers.
Despite the fact that the dynamic changes in B2-MG,
NPT, IL-6 and IL-18 were not assessed among all patients who
received ICI before the start and two months after initiation of
the treatment, these markers significantly change only during
immunotherapy. It was shown that levels of B2-MG, NPT, IL-6 and
IL-18 were higher among patients with aNSCLC who received ICI
(regardless of response or progression) than among patients with
the same disease who received chemotherapy. Moreover, the levels of
these markers in 16 patients with aNSCLC before initiating ICI were
similar to those among patients receiving chemotherapy. Thus, a
platinum-based chemotherapy does not affect the level of the
inflammation markers. Also, 16 patients showed no significant
changes in differences of B2-MG, NPT, IL-6, IL-18 after two and six
months of starting ICI.
The use of immunological markers, such as NLR,
B2-MG, NPT, IL-6, IL-18, HLA-BRB1, anti-TPO antibodies,
could determine the efficacy of ICI among patients with NSCLC and
melanoma. However, further prospective study is warranted.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AAM was responsible for development of research
design, review of publications on the topic of the article,
analysis of the data obtained, design of illustrative material,
statistical analysis and article writing. SVL was responsible for
development of research design, methodology and analysis of the
data obtained. MAU, SVOd, IVC and AMU were involved in curation of
patients, performed experiments and curated data. ALA and SVOr were
responsible for idea and design development, scientific editing,
and research management. AAM and SVOr confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Pavlov First Saint Petersburg State Medical
University (approval no. 246-2021). All participants signed
informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li S, Zhang C, Pang G and Wang P: Emerging
blood-based biomarkers for predicting response to checkpoint
immunotherapy in non-small-cell lung cancer. Front Immunol.
11(603157)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Möller M, Turzer S, Schütte W, Seliger B
and Riemann D: Blood immune cell biomarkers in patient with lung
cancer undergoing treatment with checkpoint blockade. J Immunother.
43:57–66. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Davis AA and Patel VG: The role of PD-L1
expression as a predictive biomarker: An analysis of all US food
and drug administration (FDA) approvals of immune checkpoint
inhibi. J Immunother Cancer. 7(278)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Marcus L, Lemery SJ, Keegan P and Pazdur
R: FDA approval summary: Pembrolizumab for the treatment of
microsatellite instability-high solid tumors. Clin Cancer Res.
25:3753–3758. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prasad V and Addeo A: The FDA approval of
pembrolizumab for patients with TMB >10 mut/Mb: Was it a wise
decision? No. Ann Oncol. 31:1112–1114. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bai R, Lv Z, Xu D and Cui J: Predictive
biomarkers for cancer immunotherapy with immune checkpoint
inhibitors. Biomark Res. 8(34)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim
TM, Kim DW, Heo DS and Lee JS: Post-treatment
neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients
with advanced non-small cell lung cancers treated with anti-PD-1
antibody. Cancer Immunol Immunother. 67:459–470. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Keegan A, Ricciuti B, Garden P, Cohen L,
Nishihara R, Adeni A, Paweletz C, Supplee J, Jänne PA, Severgnini
M, et al: Plasma IL-6 changes correlate to PD-1 inhibitor responses
in NSCLC. J Immunother Cancer. 8(e000678)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun JY and Lu XJ: Cancer immunotherapy:
Current applications and challenges. Cancer Lett. 480:1–3.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hussaini S, Chehade R, Boldt RG, Raphael
J, Blanchette P, Maleki Vareki S and Fernandes R: Association
between immune-related side effects and efficacy and benefit of
immune checkpoint inhibitors-a systematic review and meta-analysis.
Cancer Treat Rev. 92(102134)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Music M, Iafolla M, Soosaipillai A,
Batruch I, Prassas I, Pintilie M, Hansen AR, Bedard PL, Lheureux S,
Spreafico A, et al: Predicting response and toxicity to PD-1
inhibition using serum autoantibodies identified from immuno-mass
spectrometry. F1000Res. 9(337)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Scally SW, Petersen J, Law SC, Dudek NL,
Nel HJ, Loh KL, Wijeyewickrema LC, Eckle SB, van Heemst J, Pike RN,
et al: A molecular basis for the association of the HLA-DRB1 locus,
citrullination, and rheumatoid arthritis. J Exp Med. 210:2569–2582.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kapustin S, Lyshchov A, Alexandrova J,
Imyanitov E and Blinov M: HLA class II molecular polymorphisms in
healthy Slavic individuals from North-Western Russia. Tissue
Antigens. 54:517–520. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lalani AA, Xie W, Martini DJ, Steinharter
JA, Norton CK, Krajewski KM, Duquette A, Bossé D, Bellmunt J, Van
Allen EM, et al: Change in neutrophil-to-lymphocyte ratio (NLR) in
response to immune checkpoint blockade for metastatic renal cell
carcinoma. J Immunother Cancer. 6(5)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang
G, Chen R, Jiao S and Wang J: Pretreatment neutrophil-to-lymphocyte
ratio (NLR) may predict the outcomes of advanced non-small-cell
lung cancer (NSCLC) patients treated with immune checkpoint
inhibitors (ICIs). Front Oncol. 10(654)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jin J, Yang L, Liu D and Li W: Association
of the neutrophil to lymphocyte ratio and clinical outcomes in
patients with lung cancer receiving immunotherapy: A meta-analysis.
BMJ Open. 10(e035031)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li L, Dong M and Wang XG: The implication
and significance of beta 2 microglobulin: A conservative
multifunctional regulator. Chin Med J (Engl). 129:448–455.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rossi D, Fangazio M, De Paoli L, Puma A,
Riccomagno P, Pinto V, Zigrossi P, Ramponi A, Monga G and Gaidano
G: Beta-2-microglobulin is an independent predictor of progression
in asymptomatic multiple myeloma. Cancer. 116:2188–2200.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xie J, Wang Y, Freeman ME III, Barlogie B
and Yi Q: Beta 2-microglobulin as a negative regulator of the
immune system: High concentrations of the protein inhibit in vitro
generation of functional dendritic cells. Blood. 101:4005–4012.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Volgger BM, Windbichler GH, Zeimet AG,
Graf AH, Bogner G, Angleitner-Boubenizek L, Rohde M, Denison U,
Sliutz G, Fuith LC, et al: Long-term significance of urinary
neopterin in ovarian cancer: A study by the Austrian association
for gynecologic oncology (AGO). Ann Oncol. 27:1740–1746.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Melichar B, Spisarová M, Bartoušková M,
Krčmová LK, Javorská L and Študentová H: Neopterin as a biomarker
of immune response in cancer patients. Ann Transl Med.
5(280)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu C, Yang L, Xu H, Zheng S, Wang Z, Wang
S, Yang Y, Zhang S, Feng X, Sun N and Wang Y: Systematic analysis
of IL-6 as a predictive biomarker and desensitizer of immunotherapy
responses in patients with non-small cell lung cancer. BMC Med.
20(187)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Laino AS, Woods D, Vassallo M, Qian X,
Tang H, Wind-Rotolo M and Weber J: Serum interleukin-6 and
C-reactive protein are associated with survival in melanoma
patients receiving immune checkpoint inhibition. J Immunother
Cancer. 8(e000842)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fabbi M, Carbotti G and Ferrini S:
Context-dependent role of IL-18 in cancer biology and
counter-regulation by IL-18BP. J Leukoc Biol. 97:665–675.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y, Chen H, Zhang T, Yang X, Zhong J,
Wang Y, Chi Y, Wu M, An T, Li J, et al: Plasma cytokines
interleukin-18 and C-X-C motif chemokine ligand 10 are indicative
of the anti-programmed cell death protein-1 treatment response in
lung cancer patients. Ann Transl Med. 9(33)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Terme M, Ullrich E, Aymeric L, Meinhardt
K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G,
et al: IL-18 induces PD-1-dependent immunosuppression in cancer.
Cancer Res. 71:5393–5399. 2011.PubMed/NCBI View Article : Google Scholar
|