Introduction
X-linked adrenoleukodystrophy (X-ALD), caused by
mutations in the ATP-binding cassette subfamily D member 1 (ABCD1)
gene, is the most common peroxisomal disorder. ABCD1 is located on
Xq28 and encodes the peroxisomal transporter of very-long-chain
fatty acids (VLCFAs) (1). Defects
in ABCD1 protein prevent β-oxidation of VLCFAs, leading to its
accumulation in tissue and plasma (1). According to age of onset, affected
site and progression rate, multiple phenotypes of X-ALD are
recognized, including cerebral ALD, adrenomyeloneuropathy,
Addison's and asymptomatic (2).
As in the case of numerous types of X-linked
recessive disease, female carriers are assumed to remain
asymptomatic; however, ~20% of X-ALD female carriers develop
symptoms, usually in their thirties, and the incidence rate is up
to 88% in those aged >60 years (3,4). In
general, female X-ALD carriers display adrenomyeloneuropathy-like
phenotype. The primary clinical manifestations of this phenotype
are progressive spastic gait, sensory deficit and bladder
dysfunction, commonly without adrenal insufficiency (4,5).
The present report describes the case of a
7-year-old girl with cerebral ALD. We aimed to discover the
possible pathogenesis of this patient in the present study.
Materials and methods
Sample collection
Peripheral blood (3 ml) was collected from a female
patient with X-ALD (age, 8 years in 2021), as well as from members
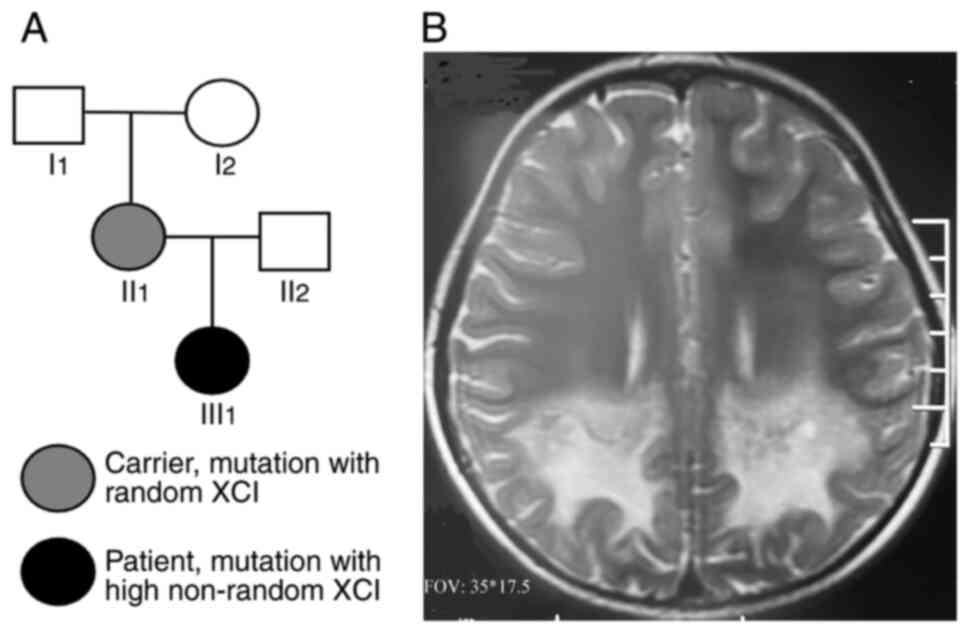
of their family (Fig. 1A). The
ages of family members I1, I2, II1
and II2 were 62, 60, 36 and 35 years, respectively. The
present study was approved by the Ethics Committee of the Shengjing
Hospital of China Medical University (approval no. 2021PS209K).
Written informed consent was obtained from the patient's parents
and the other adults in the family.
Biochemical measurements
Serum or plasma (0.5 ml) was collected after
centrifugation of blood samples at 1,200 x g for 5 min at room
temperature. Plasma adrenocorticotrophic hormone (ACTH) and serum
cortisol levels were measured according to a chemiluminescence
method (6), and serum VLCFAwas
measured by gas chromatography and mass spectrometry (7) at Jinyu Medical Laboratory Center.
Whole-exome and Sanger sequencing
Genomic DNA was extracted using QIAamp DNA Mini kit
(Qiagen China Co., Ltd.). The concentration of extracted DNA was
quantified with Nanodrop 2000 (NanoDrop; Thermo Fisher Scientific,
Inc.). Genomic DNA was fragmented to an average size of 150 bp
using a S220 Focusedultrasonicator (Covaris, LLC). The DNA library
was prepared using the GenCap NGS Fast DNA Library Prep Set for
Illumina (cat. no. MG16001M; MyGenostics Technologies Inc.)
according to the manufacturer's instructions. The DNA library was
captured using a whole-exome capture kit (GenCap Human Gene
Enrichment Kit; cat. no. MG16101M, MyGenostics Technologies Inc.).
The captured DNA was eluted with buffer, and amplified for 13
cycles using the following program: 95˚C for 4 min (1 cycle); 98˚C
for 30 sec, 65˚C for 30 sec, 72˚C for 30 sec (13 cycles); 72˚C for
5 min (1 cycle). The PCR product was purified using SPRI beads
(Beckman Coulter, Inc.) according to the manufacturer's protocol.
The enriched libraries were sequenced using 150-bp paired-end reads
on the Illumina HiSeq X Ten platform (Illumina, Inc.). Raw data
were stored in the FASTQ format. Illumina sequencing adapters and
low-quality reads (<80 bp) were filtered. Clean reads were
aligned to the UCSC hg19 human reference genome using the
Burrows-Wheeler Alignment tool 0.7.17 (http://maq.sourceforge.net). Duplicated reads were
removed using Picard (broadinstitute.github.io/picard). Insertion, deletion
and single nucleotide polymorphism (SNP) variants were detected and
filtered using the Genome Analysis Toolkit 4.0.8.1 (https://www.broadinstitute.org/gatk). The
identified variants were annotated using ANNOVAR (http://annovar.openbioinformatics.org/en/latest) and
assessed via the following databases: 1000 Genomes(https://www.internationalgenome.org), Exome
Aggregation Consortium (http://gnomad.broadinstitute.org), Human Gene Mutation
Database(http://www.hgmd.cf.ac.uk/ac/index.php), Mutation
Taster (https://www.mutationtaster.org), Sorting Intolerant
From Tolerant (http://provean.jcvi.org/index.php), PolyPhen-2
(http://genetics.bwh.harvard.edu/pph2)
and Genomic Evolutionary Rate Profiling (http://mendel.stanford.edu/SidowLab/downloads/ger).
Variation sites were identified by comparing DNA sequences with the
corresponding GenBank reference sequences using Mutation Surveyor
software (SoftGenetics, Inc.). The pathogenicity of mutations was
assessed in accordance with the American College of Medical
Genetics and Genomics Guidelines (ACMG) (8). The mutation was also confirmed via
Sanger sequencing on ABI Prism 3730 Genetic Analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the following
primers: Forward, 5'-TGTGTGAGTGGCACTGGGAGA-3' and reverse,
5'-GCTCCAGCATAACATACCACA-3'. SNP haplotype analysis was performed
using Infinium Asian Screening Array-24 v1.0 kit (Illumina, Inc.).
In brief, DNA samples were incubated with corresponding reagents in
the kit at 37˚C overnight for PCR amplification. The amplified DNA
was then fragmented in the heat block, precipitated with isopropyl
alcohol, resuspended after centrifugation and hybridized with the
chip at 48˚C overnight, followed bywashing, extension and chip
dying. The prepared chipwasscanned in Iscan chip scanner (Illumina,
Inc.). Data were uploaded to ChromGo (Yikon Genomics) for
analysis.
X chromosome inactivation (XCI)
analysis in peripheral blood
Peripheral blood (1 ml) was used for genomic DNA
extraction (Qiagen China Co., Ltd.). The XCI pattern was determined
by quantifying the CAG repeat in exon 1 of the androgen receptor
gene using a commercial kit according to the manufacturer's
instructions (XCI analysis kit, MyGenosticsGenCap Enrichment
Technologies). In brief, when the X chromosome is inactivated, the
binding site of the methylation-sensitive restriction endonuclease
HpaII, which is located near the CAG repeat, is methylated
and cannot be cleaved, whereas the non-methylated site of the
active X chromosome can be cleaved by HpaII. Therefore, if
the genomic DNA is digested with HpaII and then amplified
via PCR, only the inactivated X chromosome produces the expected
bands (9).
Results
Patient clinical information
description
At the age of 7 years, from April 2020 the patient
(III1; Fig. 1A) began
to experience occasional confusion and needed to be called several
times before answering. She was found to have a poor sense of
direction and failed to find her way home when she went out. Her
eyesight declined and she had 30˚ strabismus. She was afraid of
moving to higher positions and her ability to balance worsened. Her
electroencephalogram revealed slow waves. Brain magnetic resonance
imaging (MRI) revealed asymmetric demyelination of bilateral white
matter, suggesting the acute phase of ALD (Fig. 1B). The patient did not receive any
treatment. Eight months later (December 2020), she was unable to
hear, see or speak and became paralyzed. In May 2021, the patient's
mother (II1) visited the obstetric clinic (Shengjing
Hospital of China Medical University, Shenyang, China) and wished
to have a healthy child via assisted reproductive technology. The
clinical information, particularly the MRI, demonstrated that she
was affected with ALD.
Patient exhibits high plasma VLCFA
levels
Biochemical measurement analysis demonstrated that
the C26:0 and C26:0/C22:0 levels in III1 were notably
increased (Table I). Moreover,
C24:0/C22:0 and ACTH and cortisol levels in III1 were
slightly elevated. By contrast, VLCFAs, ACTH and cortisol levels
were close to normal in II1 and I2, with
C26:0 level and C26:0/C22:0 slightly increased in II1.
The results showed the patient exhibited high plasma VLCFA
levels.
 | Table IResults of biochemical
measurement. |
Table I
Results of biochemical
measurement.
| Type | Sample ID | III1 | II1 | I2 | Reference value |
|---|
| ACTH | | 73.560↑ | 18.240 | 10.760 | 7.200-63.400
pg/ml |
| Cortisol | | 26.180↑ | 8.390 | 9.790 | 4.260-24.850
µg/dl |
| VLCFA | C22:0 | 50.800 | 53.000 | 62.000 | ≤96.300 nmol/ml |
| | C24:0 | 91.000 | 63.400 | 57.100 | ≤91.400 nmol/ml |
| | C26:0 | 3.850 ↑↑ | 1.320 ↑ | 0.780 | ≤1.300 nmol/ml |
| | C24:0/C22:0 | 1.790 ↑ | 1.200 | 0.920 | ≤1.390 |
| | C26:0/C22:0 | 0.076 ↑↑ | 0.025 ↑ | 0.013 | ≤0.023 |
Patient harbors a heterozygous ABCD1
mutation
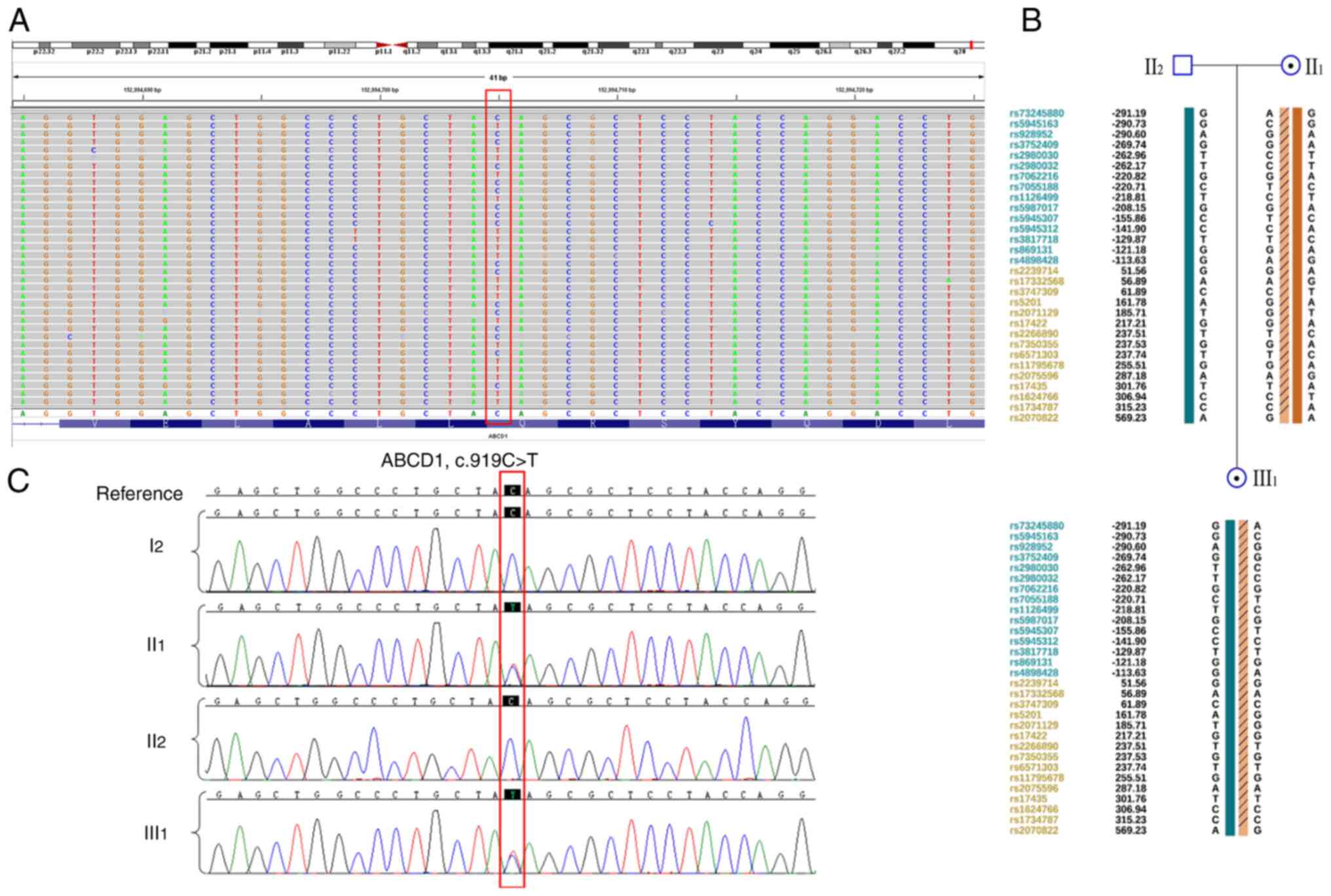
Whole-exome sequencing performed on II1,
II2 and III1 (Fig. 2A). II1 and
III1 harbored the ABCD1 c.919C>T (p.Q307X)
heterozygous mutation, which was confirmed using Sanger sequencing
(Fig. 2B). Based on ACMG
guidelines, this mutation was identified as pathogenic. The
pathogenic mutation in the patient was inherited from her mother
who did not demonstrate any symptoms. In addition, SNP haplotype
analysis was constructed for the linkage analysis (Fig. 2C); a total of15 available SNP sites
upstream of the gene and 15 sites downstream were selected for the
haplotype construction. These findings indicated that the patient
harbors a heterozygous ABCD1 mutation from her asymptomatic
mother.
Paternal X chromosome is inactivated
in the patient
Although both II1 and III1
harbored the same ABCD1 heterozygous mutation, III1
showed obvious clinical manifestations, whereas II1 was
an asymptomatic carrier. As an X-linked recessive disease, female
ABCD1 heterozygous carriers are typically asymptomatic, especially
in childhood (4). To identify the
pathogenic cause in III1, other pathogenic ABCD1
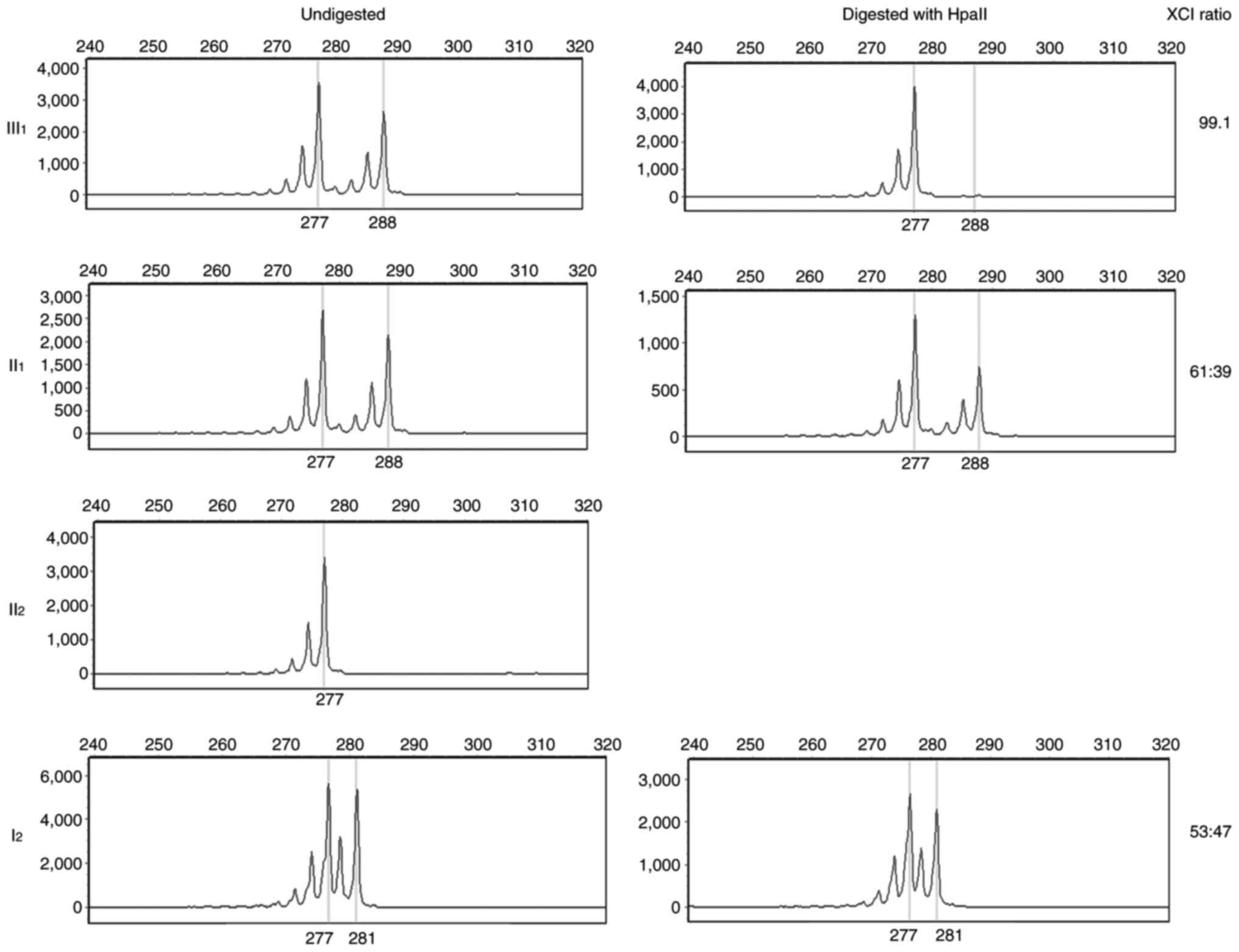
mutations were excluded. As XCI is involved in ALD, the
polymorphism of the CAG repeat in exon 1 of the androgen receptor
gene was investigated. X chromosome in III1 displayed
highly non-random inactivation, implying that her paternal X
chromosome was almost completely inactivated (Fig. 3). This indicated that the severe
clinical symptoms in III1 were caused by maternal ABCD1
mutation and paternal XCI.
Discussion
The present study describes the case of a patient
with X-ALD caused by maternal ABCD1 mutation and paternal XCI. The
incidence of X-ALD is 1 in 20,000-50,000 individuals (10). According to the age of onset,
affected site and progression rate, X-ALD can be classified into
four major groups (2): i) Cerebral
ALD, which is characterized by demyelination of white matter of the
brain; ii) adrenomyeloneuropathy, with spinal cord demyelination
and axonal degeneration; iii) Addison-like phenotype due to
adrenocortical insufficiency and iv) asymptomatic type. Childhood
cerebral ALD and adrenomyeloneuropathy are the most common types
observed during childhood and adulthood, with incidences of 31-35
and 20-60%, respectively (11,12).
Onset of childhood cerebral ALD usually occurs at
the age of 3-10 years, with a peak age onset of 7 years, and is
characterized by progressive deterioration of cognitive,
behavioral, and motor function. The patient initially presents with
learning and behavioral problems, as well as attention deficit,
followed by cognitive decline, ataxia, cortical blindness, deafness
and seizures within 6 months to 2 years. This can progress to a
vegetative state and death within years of diagnosis (1,8). The
lower the onset age, the faster the disease progression (13). Brain MRI shows demyelination in the
white matter of the brain and this finding can precede symptoms
(13). In the present study, the
patient's clinical manifestations was typical, including hearing,
vision and behavioral abnormality, which progressed to the
vegetative state rapidly. The subsequent genetic analysis
identified the ABCD1 heterozygous pathogenic mutation.
To date, >900 variants of ABCD1 have been
reported (14). Although
Campopiano et al (15)
addressed the genotype-phenotype correlation in a large family,
most studies have showed weak correlation between genotype and
phenotype (1,2). Thus, ongoing clinical symptoms and
prognosis cannot be determined by the patient genotype. It is
accepted that other factors, including genetic, epigenetic or
environmental, may affect rate of progression (2).
As in the case of many X-linked diseases, X-ALD
female carriers typically remain asymptomatic; however, 18-88% of
female carriers develop symptoms (4). The incidence rate of X-ALD in ABCD1
heterozygous female carriers is associated with age; 18% of female
carriers aged <40 years develop symptoms and this increases to
88% of those aged >60 years (4). Horn et al (16) also found that nearly all female
carriers aged >50 years develop neurological symptoms. One of
the earliest descriptions of an adrenomyeloneuropathy-like X-ALD
phenotype was a female patient (17).
In X-ALD, XCI is an important factor. XCI refers to
silencing of one of the two X chromosomes in mammalian female cells
that ensures equal expression of X-linked genes between female and
male. This process occurs during early development; one X
chromosome is transcriptionally silenced while the other remains
active (18,19). Which X chromosome is inactivated is
usually random; therefore, ~50% of all cells express genes from the
paternal-derived X chromosome and the remaining 50% from the
maternal chromosome (5). When 80%
of cells demonstrate a preferential inactivation of the same X
chromosome, it is defined as skewed XCI (5). Extremely skewed XCI implies
inactivation of paternal or maternal-derived X chromosome in
>95% of all cells (20). To
date, whether skewed XCI is associated with symptomatic X-ALD
female carriers is unclear. Maier et al (21) reported that the manifestation of
symptoms in 22 X-ALD carriers was associated with skewed XCI and
that there was a significant correlation between the extent of
skewing and the severity of neurological abnormality. By contrast,
Watkiss et al (22) and
Salsano et al (5) did not
find any association between neurological manifestation and XCI
pattern. The present study suggested that extremely skewed XCI
served an important role in the patient's severe clinical
presentation. In general, XCI skewing appears to favor the inactive
mutant allele, which may explain why female carriers may be
asymptomatic in severe X-linked disorders with a highly skewed XCI
(18,19). However, ABCD1 mutations in X-ALD
demonstrate a proliferative advantage rather than a disadvantage,
caused by XCI skewing in favor of the normal X chromosome (5). The present findings were consistent
with this hypothesis as the normal paternal X chromosome was almost
completely inactivated. However, in the present study, the
mechanism by which the paternal X chromosome was inactivated was
not clarified. Further experiments and samples (from this and other
families) will be needed to study the mechanism in-depth.
In addition to X-ALD, XCI serves an important role
in other common X-linked recessive diseases. Yang et al
(23) found skewed XCI in a female
patient who had a heterozygous missense mutation in the FIX gene
and a complete clinical manifestation of moderate hemophilia B was
noted. A total of 28% of heterozygous females with FVIII or FIX
variants have clinical manifestations and exhibit skewed XCI
(24). In Becker muscular
dystrophy, Viggiano et al (25) reported that skewed XCI serves a
crucial role in the onset of symptoms in female carriers. Skewed
XCI also appears to have a correlation with a severe phenotype in
Duchenne muscular dystrophy (26).
He et al (9) found that XCI
was implicated in symptomatic female carriers of dystrophinopathies
and XCI analysis of amniocytes may be useful in predicting the risk
of dystrophinopathy in fetal carriers. Thus, the incidence of
skewed XCI may be common and contribute to the manifestation of
symptoms in carriers of recessive X-linked disorders (18,19).
In conclusion, X-AD in the present patient was
caused by both maternal ABCD1 mutation and paternal XCI. This
suggests that XCI may be a key factor in X-ALD.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the 345 Talent
Project of Shengjing Hospital (grant no. M0298).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the Sequence Read Archive database
(submission number: SUB11494821, Bio Sample accession number:
SAMN28555541; https://www.ncbi.nlm.nih.gov/sra/PRJNA841819).
Authors' contributions
ZL provided obstetrical service, collected the
samples and clinical information, performed the biochemical
measurements, and wrote the paper. GL performed the sequencing and
analyzed the data, provided genetic counseling, and revised the
manuscript. ZL and GL confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Shengjing Hospital of China Medical University
(approval no. 2021PS209K). Written informed consent was obtained
from the patient's parents.
Patient consent for publication
Consent for publication was obtained from the
patient's parents. Patient personal information was removed from
the picture.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu J, Eichler F, Biffi A, Duncan CN,
Williams DA and Majzoub JA: The changing face of
adrenoleukodystrophy. Endocr Rev. 41:577–593. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Palakuzhiyil SV, Christopher R and Chandra
SR: Deciphering the modifiers for phenotypic variability of
X-linked adrenoleukodystrophy. World J Biol Chem. 11:99–111.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schmidt S, Träber F, Block W, Keller E,
Pohl C, von Oertzen J, Schild H, Schlegel U and Klockgether T:
Phenotype assignment in symptomatic female carriers of X-linked
adrenoleukodystrophy. J Neurol. 248:36–44. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Engelen M, Barbier M, Dijkstra IM, Schür
R, de Bie RM, Verhamme C, Dijkgraaf MG, Aubourg PA, Wanders RJ, van
Geel BM, et al: X-linked adrenoleukodystrophy in women: A
cross-sectional cohort study. Brain. 137:693–706. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Salsano E, Tabano S, Sirchia SM,
Colapietro P, Castellotti B, Gellera C, Rimoldi M, Pensato V,
Mariotti C, Pareyson D, et al: Preferential expression of mutant
ABCD1 allele is common in adrenoleukodystrophy female carriers but
unrelated to clinical symptoms. Orphanet J Rare Dis.
7(10)2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ye YJ, Liu B and Qin BZ: Clinical analysis
of patients of cirrhosis complicated with adrenal insufficiency.
Eur Rev Med Pharmacol Sci. 20:2667–2672. 2016.PubMed/NCBI
|
|
7
|
Hadj Ahmed S, Koubaa N, Kharroubi W,
Zarrouk A, Mnari A, Batbout F, Gamra H, Hammami S, Lizard G and
Hammami M: Identification of long and very long chain fatty acids,
plasmalogen-C16:0 and phytanic acid as new lipid biomarkers in
Tunisian coronary artery disease patients. Prostaglandins Other
Lipid Mediat. 131:49–58. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Richards S, Aziz N, Bale S, Bick D, Das S,
Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al:
Standards and guidelines for the interpretation of sequence
variants: A joint consensus recommendation of the American College
of Medical Genetics and Genomics and the Association for molecular
pathology. Genet Med. 17:405–424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He WB, Du J, Xie PY, Zhou S, Zhang YX, Lu
GX, Lin G, Li W and Tan YQ: X-chromosome inactivation pattern of
amniocytes predicts the risk of dystrophinopathy in fetal carriers
of DMD mutations. PrenatDiagn. 39:603–608. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Olgac A, Kasapkara ÇS, Derinkuyu B, Yüksel
D, Çetinkaya S, Aksoy A, Ceylaner S, Güleray N, Yeşilipek A, Aydın
Hİ, et al: Retrospective evaluation of patients with X-linked
adrenoleukodystrophy with a wide range of clinical presentations: A
single center experience. J Pediatr Endocrinol Metab. 34:1169–1179.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Engelen M, Kemp S, de Visser M, van Geel
BM, Wanders RJ, Aubourg P and Poll-The BT: X-linked
adrenoleukodystrophy (X-ALD): Clinical presentation and guidelines
for diagnosis, follow-up and management. Orphanet J Rare Dis.
7(51)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mohn A, Polidori N, Aiello C, Rizzo C,
Giannini C, Chiarelli F and Cappa M: ABCD1 gene mutation in an
Italian family with X-linkedadrenoleukodystrophy: Case series.
Endocrinol Diabetes Metab Case Rep. 2021:20–0125. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moser HW, Loes DJ, Melhem ER, Raymond GV,
Bezman L, Cox CS and Lu SE: X-Linked adrenoleukodystrophy: Overview
and prognosis as a function of age and brain magnetic resonance
imaging abnormality. A study involving 372 patients.
Neuropediatrics. 31:227–239. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
ALD info: The information platform to all
aspects of adrenoleukodystrophy and the worldwide registry for
ABCD1 variants. https://adrenoleukodystrophy.info/. Accessed March 1,
2022.
|
|
15
|
Campopiano R, Femiano C, Chiaravalloti MA,
Ferese R, Centonze D, Buttari F, Zampatti S, Fanelli M, Amatori S,
D'Alessio C, et al: A large family with p.Arg554His mutation in
ABCD1: Clinical features and genotype/phenotype correlation in
female carriers. Genes (Basel). 12(775)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Horn MA, Retterstøl L, Abdelnoor M,
Skjeldal OH and Tallaksen CM: Adrenoleukodystrophy in Norway: High
rate of de novo mutations and age-dependent penetrance. Pediatr
Neurol. 48:212–219. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Penman RW: Addison's disease in
association with spastic paraplegia. Br Med J.
1(402)1960.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shvetsova E, Sofronova A, Monajemi R,
Gagalova K, Draisma HHM, White SJ, Santen GWE, Chuva de Sousa Lopes
SM, Heijmans BT, van Meurs J, et al: Skewed X-inactivation is
common in the general female population. Eur J Hum Genet.
27:455–465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pereira G and Dória S: X-chromosome
inactivation: Implications in human disease. J Genet.
100(63)2021.PubMed/NCBI
|
|
20
|
Wang Z, Yan A, Lin Y, Xie H, Zhou C and
Lan F: Familial skewed x chromosome inactivation in
adrenoleukodystrophy manifesting heterozygotes from a Chinese
pedigree. PLoS One. 8(e57977)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Maier EM, Kammerer S, Muntau AC, Wichers
M, Braun A and Roscher AA: Symptoms in carriers of
adrenoleukodystrophy relate to skewed X inactivation. Ann Neurol.
52:683–688. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Watkiss E, Webb T and Bundey S: Is skewed
X inactivation responsible for symptoms in female carriers for
adrenoleucodystrophy? J Med Genet. 30:651–654. 1993.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang C, Yu Z, Zhang W, Cao L, Ouyang W, Hu
F, Zhang P, Bai X and Ruan C: A novel missense mutation, p.
Phe360Cys, in FIX gene results in haemophilia B in a female patient
with skewed X-inactivation. Haemophilia. 24:e68–e70.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miller CH and Bean CJ: Genetic causes of
haemophilia in women and girls. Haemophilia. 27:e164–e179.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Viggiano E, Picillo E, Ergoli M, Cirillo
A, Del Gaudio S and Politano L: Skewed X-chromosome inactivation
plays a crucial role in the onset of symptoms in carriers of Becker
muscular dystrophy. J Gene Med. 19:2017.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Viggiano E, Ergoli M, Picillo E and
Politano L: Determining the role of skewed X-chromosome
inactivation in developing muscle symptoms in carriers of Duchenne
muscular dystrophy. Hum Genet. 135:685–698. 2016.PubMed/NCBI View Article : Google Scholar
|