1. Introduction
Cognition includes intellectual abilities used for
perceiving, acquiring, understanding and responding to information
presented to a person. The term cognitive dysfunction (CD), known
also as ‘brain fog’, refers to deficits in attention, verbal and
non-verbal learning, short-term and working memory, visual and
auditory processing, mathematic problem solving, processing speed,
focusing on a specific topic, and motor functioning (1-3).
It has been documented that normal aging is
associated with reduction of cerebral blood flow by approximately
20% at age 60, compared to the age of 20 years (4). Normal cognitive function (CF) depends
on a continuous and optimally regulated blood supply (5), and any pathology that further reduces
cerebral blood perfusion in addition to that caused by aging could
damage or destroy vulnerable neurons of the brain (5). Glucose plays a crucial role as the
primary fuel source for the mammalian brain and any disturbance in
its circulating concentrations could directly affect brain function
(6).
Our aim is to review the existing literature about
the assessment of CD and the role(s) of various diseases in the
development of this disorder. We will emphasize the added value of
a combined brain-heart magnetic resonance imaging (MRI) evaluation
in the early diagnosis of CD.
2. Indications for CD screening
People should be screened for CD in the following
cases: i) if the person himself or herself, family members, or
others express concerns about changes in his/her memory or
thinking, ii) if problems/changes in the patient's memory or
thinking are observed by the physician, iii) if the patient is age
80 or older, because the risk of dementia increases rapidly after
this age (7). Other risk factors
that could further support the need for dementia screening include:
a) low education, b) history of type 2 diabetes mellitus, c)
stroke, d) depression, and e) difficulties in managing money or
medications (7).
Several standardized measures of CF have been used
and include the Montreal Cognitive Assessment (MoCA) (8), the Mini-Mental State Exam (MMSE)
(9) and the Mini-Cog (10). All three tests measure mental
functions through a series of questions and/or performance of
simple tasks. Although cognitive testing cannot show the specific
cause of impairment, it can assess whether the patient needs
further evaluation (8). The main
limitation in the evaluation of CF is the lack of robust evidence
to support all available screening tests (11). In addition, they have a relatively
high rate of intra-subject variability, which reduces their ability
to identify mild deficits or preclinical disease (12). There is no ideal test for any type
of CD, and this has resulted in the development of many specialized
tests for various types of the disorder (12). Finally, CF testing is unable to
provide specific information about the neural structures
responsible for any dysfunction identified. For example, although
it appears that white matter functions, such as processing speed,
attention, and visual-spatial processing, are particularly affected
by diabetes mellitus, localization of this dysfunction to white or
gray matter is not possible using the standard tests that assess
neurocognition (12).
3. Diseases implicated in the development of
cognitive dysfunction
Various diseases have been implicated in the
development of CD. The main groups responsible for CD development
encompass the following entities:
Cardiovascular diseases
Cardiovascular diseases (CVD) are classified as
cardiomyopathies, coronary artery, valvular, and congenital heart
diseases. All these entities may finally lead to rhythm
disturbances and heart failure (HF) (13). Any type of structural or functional
CVD decreasing cerebral blood flow will also increase the risk for
Alzheimer disease (AD) (14). The
association between CVD and CD, known as cardiogenic dementia, was
initially described in patients with cardiac arrhythmias (14). Atrioventricular block and
arrhythmias lower cardiac output and lead to persistent CD and
dementia (14,15). However, CD could be attenuated or
even reversed after cardiac pacing (14,15),
due to improvement of cerebral perfusion (14).
Brain hypoperfusion, as a result of low cardiac
output or hypotension may lead to CD (16) and finally to AD (17-19).
There is also an association between atrial fibrillation (AF) and
CD leading to AD in the absence of stroke, hypertension and
diabetes, because AF induces significant brain hypoperfusion
(20,21). Furthermore, HF, thrombotic events,
coronary artery disease, valvular disease and AF that are more
common in the elderly, can contribute to CD affecting mainly the
performance in executive functions, attention, learning,
psychomotor speed, verbal fluency, mental alertness and memory
(22,23). Additionally, a reduced left
ventricular ejection fraction is associated with impairment of
continuous vigilance and discriminability (24). Furthermore, low cardiac output is
associated with impairment of executive function, including
sequencing and planning (25). In
these cases, cardiac resynchronization may reverse CD by improving
cardiac hemodynamics and consequently brain perfusion (26,27).
Neural diseases
CD is a common expression of various neural
disorders including:
Dementia
Several types of dementia have been described: AD,
vascular dementia (VaD), dementia with Lewy bodies, frontotemporal
dementia and dementia associated with Parkinson´s disease. Dementia
may also be secondary to the human immuno-deficiency virus
(HIV-associated dementia) or other infectious agents and this may
be of particular importance in younger adults in specific areas.
Other rarer causes of dementia include Huntington's disease, prion
disease and head trauma (28).
AD is the most frequent subtype, corresponding to
about 55% of all diagnoses in humans aged >65 years (28). Next in frequency is VaD, a common
condition, especially in older patients (29,30),
estimated to represent 15% of all cases (28). Although AD can be identified with a
satisfactory degree of accuracy, at present, there is no consensus
on how to define ‘mixed’ dementia in clinical practice. Moreover,
overlapping symptoms and co-morbidities make the distinction more
difficult and a differential diagnosis is further complicated by
the fact that many patients have concomitant AD and cerebrovascular
disease (31).
Multiple sclerosis
CD occurs in 40-65% of multiple sclerosis (MS)
patients. It involves complex attention, information processing
speed, episodic memory and executive functions. It can be found in
both subclinical and clinically overt MS. In pediatric-onset MS,
cognition worsens relatively rapidly. CD usually affects personal
life and vocational status. In relapsing-remitting MS timely and
adequate disease-modifying treatment may stabilize or improve CD.
Cognitive behavioral therapy, including exercise and education
programs, is a promising intervention to improve CD (32).
Neuromuscular disorders
Neuromuscular disorders mainly affect the motor
functioning of the patient. However, the cognitive effects of these
diseases are also important. This is due to molecular defects that
significantly affect neuromotor functioning, but also participate
in the functioning of neural networks involved in cognitive
processes, leading to impairment of executive, behavioral and
psychosocial functions (33).
Although most Duchenne muscular dystrophy (DMD)
patients are not intellectually disabled, the risk for CD is
increased, with up to 30% of DMD patients presenting intellectual
disability. Apart from intellectual abilities, neurocognitive
dysfunction has been frequently reported. Deficits in short-term
memory, executive functions, visuospatial ability, as well as
deficits in attention, problems with narrative, linguistic and
reading skills have been described, irrespective of general
intelligence. Moreover, a higher incidence of neuro-psychiatric
disorders, such as autism, attention deficit, hyperactivity,
obsessive-compulsive disorders and social behavior problems, has
also been reported (34).
Psychiatric disorders
Schizophrenia
There is a broad range of CD in schizophrenia and
includes problems in perception, attention, memory and
problem-solving (35). Various
studies suggest that the working memory system is of limited
capacity in schizophrenia (36-39).
Working memory deficits are significantly correlated with formal
thought disorders (40), and
deficits in long-term memory (41). Schizophrenic patients may also show
deficits in executive function (42,43),
linked to disease severity and poor medication compliance (44,45).
Mood disorders
Neurocognitive deficits are common in mood
disorders. In major depression, they can mimic severe dementia
(46). In the acute phase of
bipolar disorder, they may progress to stupor. Cognitive deficits
in mood disorders include impaired performance in tests of
attention, executive function and memory. The deficits are
correlated with both the number of affective episodes and the
overall disease duration (47).
Performance on memory and executive tasks has been correlated with
illness episodes.
Obsessive-compulsive disorder
Patients with obsessive-compulsive disorder (OCD)
may show impairment on numerous tests of non-verbal memory
including visual reproduction and delayed recognition of figures,
maze learning and intermediate/delayed figure copying. Most studies
suggest that encoding and retrieval are impaired in OCD, while
storage of information remains intact. Patients with OCD often
function remarkably well in their daily lives, despite severe
symptomatology and cognitive difficulties, which are apparent only
on specific testing. In contrast to non-verbal memory deficits,
verbal memory is generally preserved in OCD (48,49).
Patients with OCD demonstrate normal general intelligence and
language abilities.
Somatic symptoms disorders
Somatic symptoms disorders include somatic,
psychopathological and neuropsychological symptoms. Cognitive
complaints include poor concentration, decreased memory of recent
events and poor word-finding abilities (50). Approximately 50-85% of patients
with somatoform/chronic fatigue syndrome report CD, which
contributes significantly to their social and occupational
dysfunction (51,52).
Attention deficit/hyperactivity
disorder
Poor performance on tests of executive function,
sustained attention and memory, are the most common
neuropsychological deficits reported in children and adults with
attention deficit/hyperactivity disorder (ADHD). There is little
evidence for deficits in basic motor, visual, spatial or sensory
functioning in ADHD, with the possible exception of olfactory
function (53,54).
Substance abuse
CD has been identified following substance abuse and
affects mental activities that involve acquiring, storing,
retrieving, and using information. This dysfunction plays an
important role in the development of the addictive process and
rehabilitation of substance abusers (55-58).
Metabolic diseases
The mechanism of CD in diabetes mellitus (DM) is
complex and includes a) factors related to DM per se (direct effect
of altered glucose metabolism on the brain) and b) DM-related CVD
and microvascular dysfunction. Chronic hyperglycemia triggers
neuronal damage and endothelial dysfunction, leading to CD over
time (59-62).
In the Diabetes Control and Complications Trial (DCCT)/Epidemiology
of Diabetes Interventions and Complications (EDIC) cognitive
follow-up study, 1,144 participants were followed up for 18 years.
High long-term hemoglobin A1c (HbA1c) levels, advanced age, lower
level of education and two clinical microvascular complications,
such as proliferative diabetic retinopathy and nephropathy, were
associated with CD (63,64).
DM is also a major risk factor for stroke,
particularly ischemic stroke, with type 2 DM alone known to
increase stroke risk 1.5 to 4-fold (63). Furthermore, chronic hyperglycemia
dramatically increases the risk of diabetic microvascular disease
including retinopathy, nephropathy and neuropathy. A positive
correlation between nephropathy and/or retinopathy and CD has been
found (64,65). Microvascular disease may also
directly lead to CD (66).
The effect of DM on cerebrovascular impairment can
be explained by the increased burden of inflammatory cytokines
e.g., interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α),
and subsequent chronic inflammation, which contributes to CD
(67). Finally, the apolipoprotein
E (APOE) ‘4 allele’, a documented risk factor for CVD and AD in the
general population, is also implicated in the development of CD in
DM. Additionally, studies involving mainly patients with type 2 DM,
have revealed APOE ‘4-negative’ diabetic participants to be more
likely to develop AD, compared with APOE ‘4-positive’ diabetic
patients (68,69).
In contrast to hyperglycemia, the role of
hypoglycemia is controversial but the relationship between
hypoglycemia and CD may become clear in elderly patients. Although
severe hypoglycemia has not been associated with long-term CD in
young patients with type 1 DM (DCCT follow-up study) (68), recent studies in adults >60
years with type 1 DM showed a greater prevalence of CD (60).
Autoimmune rheumatic diseases
For people with autoimmune rheumatic diseases (ARDs)
including sarcoidosis, avoiding CD is crucial to successfully
perform everyday tasks, including medical treatment adherence or
planning activities. Most ARDs have been associated with various
degrees of CD, which has been mainly documented in rheumatoid
arthritis, systemic lupus erythematosus, systemic sclerosis, and
sarcoidosis.
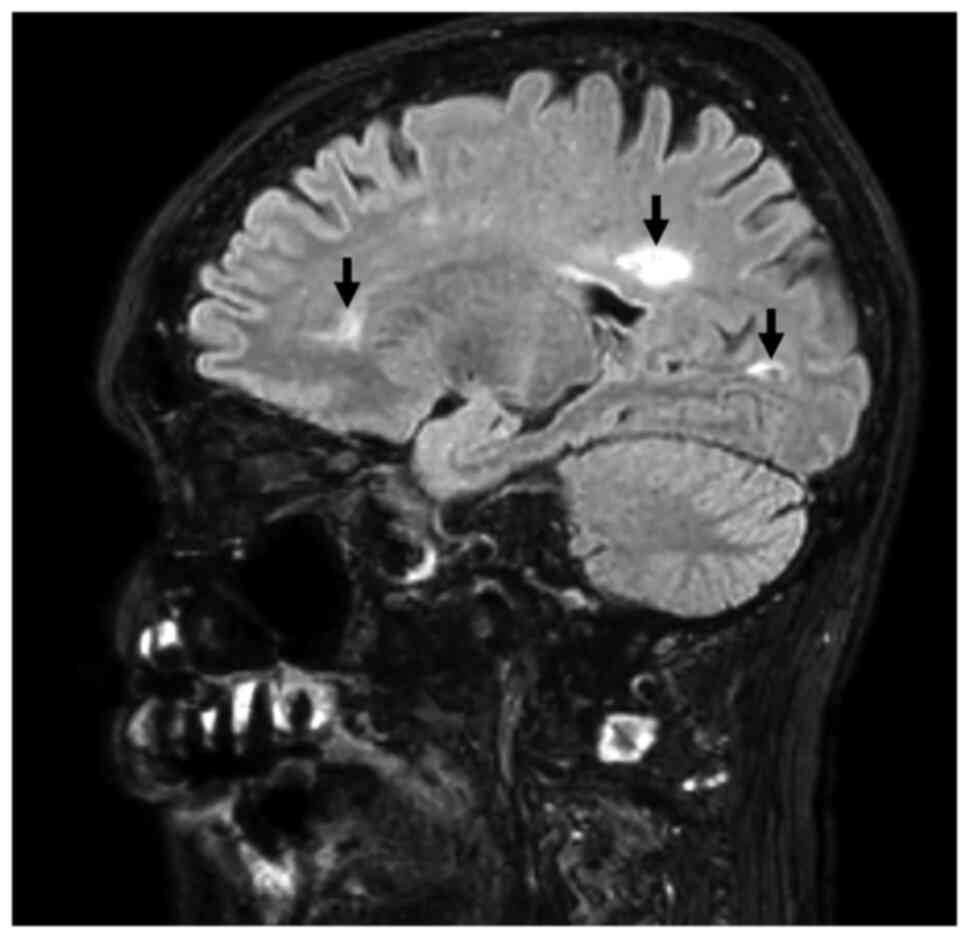
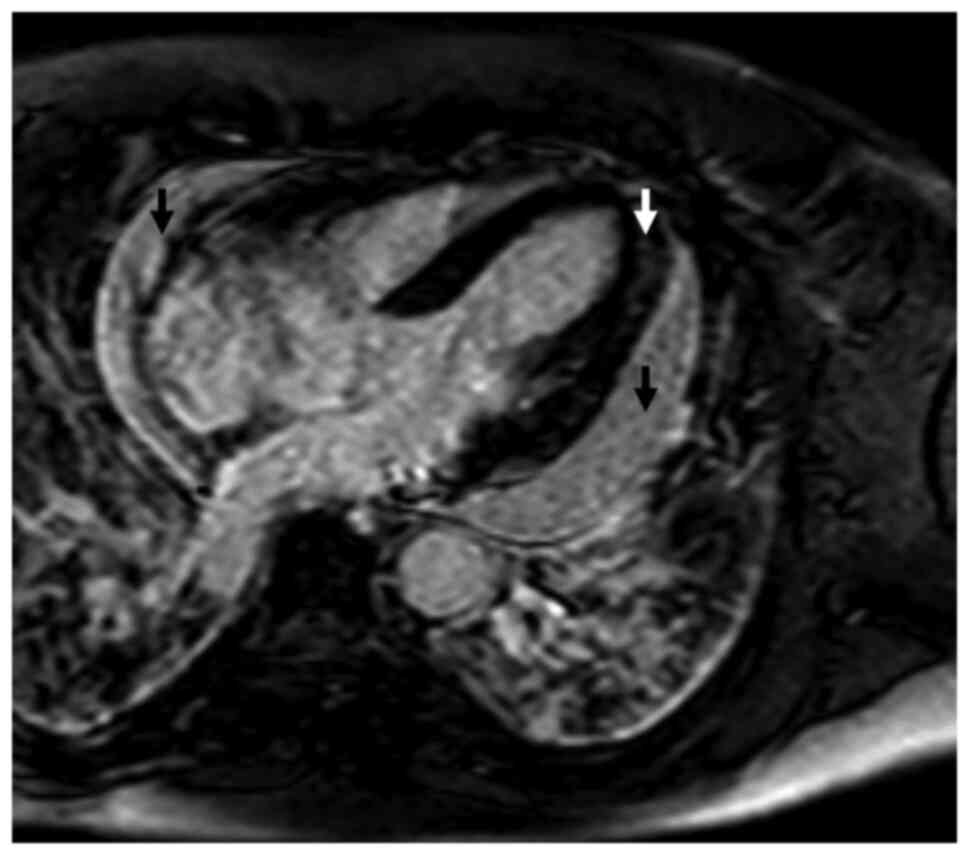
Combined brain/heart MRI images of a case patient
with sarcoidosis and doubtful CD tests, are shown in Figs. 1 and 2.
Rheumatoid arthritis
Patients with rheumatoid arthritis (RA) present an
increased risk of stroke, as a consequence of accelerated
atherosclerosis (70). Among
psychiatric manifestations, depression and anxiety have been found
in 2/3 of RA patients and have been associated with disease
activity (70). RA patients
usually underperform on CD tests, compared to controls (71). Even mild CD may influence the
functional capacity and quality of life of these patients by
affecting reactivity to pain and compliance to treatment (71,72).
CD may occur even in the early stages of the disease (73).
Inflammation affecting the brain (72) and accelerated atherosclerosis, as a
result of systemic inflammation (74) are the main causes of CD in RA.
Furthermore, RA disease activity is an important stimulus of CD,
depression and anxiety (72).
Finally, pain, stress, fatigue and sleep disturbances, may be also
involved in the development of RA-associated CD. Anti-rheumatic
drugs, such as methotrexate (MTX) and corticosteroids are also
related with CD. Both MTX and corticosteroids suppress systemic
inflammation and may have beneficial effects on CD. However, MTX
has been associated with CD, mood disturbances and confusion, while
corticosteroids may influence memory and hippocampal function
(75).
Systemic lupus erythematosus
A wide range of syndromes in systemic lupus
erythematosus (SLE) including stroke, acute confusional state,
headaches and mood disorders may lead to CD (76), which affects 3-81% of SLE patients.
This is due to the absence of standardized diagnostic criteria and
screening tools. Additionally, the neuropsychiatric manifestations
often develop insidiously, can present independently of other SLE
signs of disease activity and often do not respond to standard
immunosuppression. Therefore, CD in SLE patients often remains
underdiagnosed and undertreated in clinical practice (77).
The treatment with neurotoxic/psychoactive
medications such as corticosteroids and cyclophosphamide, and the
neuropsychiatric manifestations of SLE (NPSLE) such as strokes,
seizures, depression or anxiety, can all independently contribute
to CD. However, there is an early presentation of NPSLE, with 40%
of SLE patients diagnosed with neuropsychiatric symptoms at the
time of diagnosis or within the first 3 years post diagnosis
(78,79).
Antiphospholipid (aPL) antibodies, often coexisting
with SLE, are a strong risk factor for NPSLE, due to a stroke,
structural damage and associated CD, as a consequence of
hypercoagulation (80,81). However, aPL antibodies have also
been correlated to NPSLE syndromes that are not directly related to
thrombotic or ischaemic events (82). Neurotoxic auto-antibodies,
pro-inflammatory cytokines and cell-mediated agents together with
abnormalities in neuroimmune structures including the choroid
plexus and blood-brain barrier, allow systemic autoimmune factors
to penetrate into the central nervous system in NPSLE (80,83).
Finally, increased levels of IL-6 and neurotoxic
anti-N-methyl-D-aspartate receptor (NMDAR) antibodies have been
found in SLE with CD (84-86).
Systemic sclerosis
Although anxiety, depression and mood changes are
common, in contrast to other ARDs, CD is rare in systemic sclerosis
(SSc) (87). However, under
special circumstances vascular damage can be of great importance,
as in a renal crisis. This is a due to thrombotic microangiopathy
with concurrent hypertension that occurs almost exclusively in
early-stage diffuse SSc and is strongly associated with the
anti-RNA polymerase autoantibodies. The systemic micro-vasculopathy
of SSc renal crisis may lead to generalized seizures and
potentially significant CD (88).
This disorder generally recovers after several weeks without other
consequences. Furthermore, one in five patients with SSc will also
develop another ARD, such as SLE or Sjögren's syndrome (89) and the CD of these diseases will be
an additional factor for CD development in SSc patients.
Immune-mediated encephalitis may occasionally occur in some SSc
patients (90). SSc is associated
with Raynaud's phenomenon and there are several reports of CD after
cold exposure (91), due to
changes in cerebral perfusion (91). Finally, in localized scleroderma,
especially linear morphoea, structural abnormalities affecting the
cranium or brain can be associated with epilepsy and possibly CD in
some patients.
ANCA-associated vasculitis
According to a recent study, the prevalence of CD in
patients with ANCA-associated vasculitis (AAV) was similar to RA
and those with CD had high disease activity. Abnormal performance
was more frequent in the executive functions, followed by language.
Furthermore, psychomotor functions were more frequently affected in
AAV patients (92).
4. Currently used approach for CD
diagnosis
The currently used diagnostic approach for CD
diagnosis includes a) assessment of problems with memory or another
mental function; b) mental decline over time; c) if overall mental
function and daily activities are affected; and d) mental status
testing. A neural clinical and laboratory examination is necessary.
Finally, cognitive testing and brain CT/MRI provide an integrated
image of mental status.
The great diversity of causes and outcomes of CD has
motivated a wide search for effective therapies. These include
specific neurological medications, interventions to achieve better
brain perfusion, occupational therapy and environmental
approaches.
Future research includes the development of
neuroimaging and genetic testing that help the identification of
individuals at increased risk for CD.
5. Pro and contra of cognitive tests
MoCA is a quick test, taking only 10 to 15 min to be
completed. Various studies have shown that MoCA correctly
identifies dementia in approximately 94% of cases. Furthermore,
while people in the early or mild stages of dementia might score
high enough on other tests (including the MMSE), and the score
would indicate that no dementia is present, MoCA has been proven
effective for showing early-stage dementia, or mild cognitive
impairment. Additionally, MoCA is better than the MMSE at
indicating if people with Parkinson's disease present signs of
Parkinson's disease dementia. However, between the disadvantages of
MoCA is the fact that it must be administered and graded by a
healthcare professional and therefore an appointment with a nurse,
doctor, or therapist is required, in opposition to other tests that
can be taken at home. Furthermore, it does not provide a diagnosis,
so it must be evaluated together with other tests including brain
scans and a neurological testing, before the final diagnosis will
be made (93). Finally, in an
aging stroke population, hearing loss and visual impairment are
problematic for administering a valid MoCA. This limits the
generalizability of conclusions that can be drawn from
epidemiological studies of CD and its evolution after stroke,
although it is recognized that other performance-based cognitive
assessment tools suffer from the same limitation (94).
According to published data, cognitive tests are the
more clinically useful screening tools for use in community mental
health centers. However, because of their poor sensitivity for
detecting CD in this patient population, alternative screening
methods should be explored. Therefore, there is great clinical need
for an objective tool that can assess early the lesion
pathophysiology and can predict the future evolution of the
patient's CD.
6. From cognitive tests to combined
brain/heart magnetic resonance imaging
Currently, the role of brain magnetic resonance
(BMR) in patients with CD is primarily supportive than diagnostic.
American and European guidelines recommend brain imaging to exclude
treatable causes, such as tumor, hydrocephalus or intracranial
hemorrhage, but also to distinguish between different dementia
subtypes. However, this approach depends not only on guidelines,
but also on the availability of imaging techniques at individual
centers. Advanced MRI techniques, such as functional connectivity
MRI, diffusion tensor imaging and magnetic resonance spectroscopy
are now becoming available for clinical use in many specialized
centers. The increasing research on CD identification at early
stages will definitely increase the use of more objective
approaches, such as BMR.
BMR is a robust, versatile modality capable to
detect early brain alterations without radiation. In patients with
long-standing, uncontrolled type 2 DM, white matter hyperintensity
(WMH) in BMR has been associated with decreased processing speed
(95). WMH is a brain lesion
detected as a high-intensity area in MRI T2 and fluid-attenuated
inversion recovery images (FLAIR) and reflects damage of small
vessels in periventricular and subcortical areas. Although WMH was
initially linked to the development of stroke, recently it was
clarified that it is also associated with CD. In addition to
hypertension, which is the classical risk factor for WMH, there is
increasing evidence that DM could also be associated with WMH
progression and CD. Although the factors that accelerate WMH
formation in elderly diabetic patients are poorly clarified, it is
currently known that insulin resistance is an exacerbating factor.
However, hypertension, dyslipidemia and/or other vascular risk
factors can also play an important role (96). Furthermore, brain atrophy is
present in dementia-free middle-aged adults with type 2 DM. It
seems that regional brain atrophy can be developed even in type 2
DM patients with no clinical evidence of microvascular disease.
This is probably due to the fact that brain is particularly
vulnerable to metabolic alterations prior to peripheral
microvascular abnormalities, associated with other target organs
(97).
Multiple vascular risk factors, including smoking,
hypertension, DM and dyslipidemia are associated with CD. Among
them, hypertension is more strongly associated with WMH, possibly
due to cerebral microcirculation damage. There is currently
evidence that intensive treatment of patients with cardiovascular
risk factors, including hypertension and dyslipidemia, can slow
down the progression of WMH, compared to untreated controls
(98). Furthermore, both atrial
fibrillation and HF present high burden of WMH with consequent
poorer cognitive performances and depression (92). Finally, complex congenital heart
disease, including transposition of the great arteries or
univentricular hearts, are associated with neonatal WMH (98).
In Neurology, volumetric MRI is becoming an
increasingly important tool in the early detection and monitoring
of people suspected to have mild CD and/or AD (93). Furthermore, the BMR evaluation of
patients with AD and mild cognitive impairment has revealed early
structural changes in the hippocampus, entorhinal cortex, and gray
matter structures in the medial temporal lobe. The microstructural
integrity of white matter can be studied with diffusion tensor
imaging. Increased mean diffusivity and decreased fractional
anisotropy are also found in subjects with white matter damage.
Recently, magnetization transfer imaging was found to allow the
assessment of ongoing global and regional brain damage, independent
of atrophy, in patients with AD (99). Finally, WMH distributed in anterior
brain regions is related to decline in executive abilities, typical
of normal aging, whereas WMH distributed in more posterior brain
regions is common in AD. Although epidemiological, observational
and pathological studies suggest that WMH may be ischemic in origin
and caused by consistent or variable hypoperfusion, there is
emerging evidence that it may also reflect vascular deposition of
β-amyloid, particularly when it is distributed in posterior areas
and is found in patients with AD (100).
In Rheumatology, significant negative correlations
between cognitive test scores on verbal memory and number/volume of
WMH has been found in SLE, while no significant differences in the
number or volume of WMH have been identified between subgroups of
SLE patients (101). Furthermore,
brain damage due to stroke either ischemic or hemorrhagic and
silent vascular damage such as WMH is increased in ARDs (102). The risk of any stroke and BMR
findings is worse in inflammatory arthropathies (RA, SLE, AS, gout,
psoriatic arthritis) than in noninflammatory arthropathies (OA)
(102). Plasma markers of
inflammation (C-reactive protein, TNF-α, IL-6) are associated with
stroke and increased WMH burden (102).
Although there is an increasing interest about
incorporating BMR in the diagnostic algorithm of CD, there is no
published evidence about the role of cardiovascular magnetic
resonance (CMR) in the evaluation of CD. However, CMR can reveal
silent cardiac lesions in both ischemic and nonischemic diseases
that may lead to brain hypoperfusion with consequent CD. In this
context, CMR was found to detect various phenotypes in diabetic CVD
including myocardial scar, ischemia and non-ischemic cardiomyopathy
(103). Using the unique
capability of CMR to provide sequences dedicated to myocardial
function, structure, perfusion, cardiac energetics, lipid
metabolism and diffuse extracellular volume expansion, CMR can
offer a comprehensive, quantitative and reproducible tool to detect
early on silent cardiac lesions in diabetic patients (104,105).
In the Cardiology literature, CMR is represented in
the majority of the ESC guidelines (106). It is considered as the modality
of choice to perform tissue characterization (differentiation of
various types of scars, acute or chronic myocardial inflammation,
iron overload), evaluate congenital heart diseases, quantify
valvular regurgitation and assess great vessels (106). Furthermore, it is of great
clinical significance in the evaluation of arrhythmogenic substrate
in patients with ventricular tachycardia and sudden cardiac death
(107).
In the Neurology literature, CMR with late
gadolinium enhancement (LGE) is the modality of choice for the
detection of early cardiac involvement in patients with Duchenne
muscular dystrophy (DMD) and Becker muscular dystrophy (BMD)
(105). The early detection of
cardiac involvement allows the initiation of various drugs
including corticosteroids, beta-blockers, angiotensin-converting
enzyme (ACE) inhibitors and mineralocorticoid receptor antagonists
(106).
CMR has been also successfully used to identify
vulnerable plaques in the thoracic aorta using 3D multi-contrast
CMR and estimate the risk of cerebral embolization using 4D flow
CMR in cryptogenic stroke patients (101). Although the literature about the
use of CMR in the diagnostic algorithm of ischemic stroke is
sparse, there are studies demonstrating its potential role in the
diagnostic evaluation of cryptogenic stroke to identify potential
causes such as cardiac thrombi, cardiac tumours, aortic arch
disease and other rare cardiac anomalies (107). CMR can also provide information
about functional and structural details of the left atrium and the
left atrial appendage that are associated with ischemic stroke risk
(108). Finally, cardiac
involvement was assessed in amyotrophic lateral sclerosis (ALS)
without clinical cardiac symptoms and with a normal cardiac routine
assessment. These findings may account for reported cardiac deaths
in late-stage ALS patients (109,110).
In the domain of Rheumatology, there is recently a
considerable increase in literature regarding the use of CMR for
early detection/quantification and disease acuity assessment
(oedema/fibrosis imaging) of cardiac involvement in various ARDs.
In this context, proposals about the use of CMR in Rheumatology
have been already published by a panel of specialists in both
Cardiology and Rheumatology (111). Additionally, CMR has been
successfully used to clarify the arrhythmogenic substrate in ARDs
(111,112).
In contrast to CF testing, a combined MRI of
brain/heart can reveal early pathophysiological changes that are
potentially clinically silent but may seriously affect CF. Using
this approach, subclinical brain involvement was found to be highly
prevalent in ARD patients with cardiac symptoms and was mostly
independent of the severity of cardiac involvement (113). It seems that a combined
brain/heart MRI can be of value in the detection of CD
pathophysiology and potentially facilitate therapeutic risk
stratification. However, availability, doctors' familiarity with
this approach and high cost should be taken into serious
consideration. Finally, studies correlating cognitive tests with
brain-heart MRI findings are needed to clarify their interaction in
the assessment of CD. Additionally, studies regarding the
cost/benefit ratio of this approach are also needed before final
conclusions will be drawn. A comparison between CD testing and
combined brain-heart MRI in the detection of CD is presented in
Table I.
 | Table IComparison between testing and
combined brain-heart MRI in the detection of CD. |
Table I
Comparison between testing and
combined brain-heart MRI in the detection of CD.
| CD evaluation
method | Sensitivity | Specificity | Location of
lesion | Availability | Cost |
|---|
| CD testing | +++ | + | - | ++++ | Low |
| Combined brain and
heart MRI | ++++ | ++++ | ++++ | ++ | Very high |
7. Conclusion
CD is the end-point of cardiovascular, neural,
metabolic, and immune system impairment. CD testing, which is the
most commonly used diagnostic approach, cannot always identify
subclinical cases and the underlying pathophysiologic background of
CD. A combined brain/heart MRI has the ability to diagnose these
patients at an early stage and facilitate the individualization of
risk stratification and early intervention. Furthermore, a combined
brain-heart MRI is an objective approach that has no limitations of
currently used CD tests. However, equipment availability, doctors'
familiarity with this approach, and cost effectiveness are at the
moment serious obstacles and should be taken into consideration,
before brain/heart MRI in the diagnosis of CD is recommended in
every day clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
GMM, FB and SIM recognized the scientific need for a
review article on this topic and conceived the study. GMM, FB, GK,
MRP, AG, AP, GDK, GPC and SIM designed the study structure,
performed the literature search, screened and interpreted the
findings. GMM wrote the initial draft. FB and SIM revised the
original draft. GK, MRP, AG, AP, GDK and GPC enriched the
manuscript and substantially revised the final draft. All authors
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent was obtained from the patient for
publication of the brain/heart MRI images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stedman TL: Stedman's medical dictionary
for the health professions and nursing. Wolters Kluwer
Health/Lippincott Williams & Wilkins, Philadelphia, PA,
2012.
|
|
2
|
Livingston G, Sommerlad A, Orgeta V,
Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A,
Cohen-Mansfield J, et al: Dementia prevention, intervention, and
care. Lancet. 390:2673–2734. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McIntyre RS, Cha DS, Soczynska JK,
Woldeyohannes HO, Gallaugher LA, Kudlow P, Alsuwaidan M and
Baskaran A: Cognitive deficits and functional outcomes in major
depressive disorder: Determinants, substrates, and treatment
interventions. Depress Anxiety. 30:515–527. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Tarumi T and Zhang R: Cerebral blood flow
in normal aging adults: Cardiovascular determinants, clinical
implications, and aerobic fitness. J Neurochem. 144:595–608.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou D, Meng R, Li SJ, Ya JY, Ding JY,
Shang SL, Ding YC and Ji XM: Advances in chronic cerebral
circulation insufficiency. CNS Neurosci Ther. 24:5–17.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mergenthaler P, Lindauer U, Dienel GA and
Meisel A: Sugar for the brain: The role of glucose in physiological
and pathological brain function. Trends Neurosci. 36:587–597.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barnes DE, Beiser AS, Lee A, Langa KM,
Koyama A, Preis SR, Neuhaus J, McCammon RJ, Yaffe K, Seshadri S, et
al: Development and validation of a brief dementia screening
indicator for primary care. Alzheimers Dement. 10:656–665.e1.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nasreddine ZS, Phillips NA, Bédirian V,
Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H:
The montreal cognitive assessment, MoCA: A brief screening tool for
mild cognitive impairment. J Am Geriatr Soc. 53:695–699.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Molloy W and Clarnette R: Standardized
mini-mental state examination: A user's guide. Troy: Newgrange
Press, 1999.
|
|
10
|
Borson S, Scanlan JM, Chen P and Ganguli
M: The mini-cog as a screen for dementia: Validation in a
population-based sample. J Am Geriatr Soc. 51:1451–1454.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sbordone RJ: Limitations of
neuropsychological testing to predict the cognitive and behavioral
functioning of persons with brain injury in real-world settings.
NeuroRehabilitation. 16:199–201. 2001.PubMed/NCBI
|
|
12
|
Cullen B, O'Neill B, Evans JJ, Coen RF and
Lawlor BA: A review of screening tests for cognitive impairment. J
Neurol Neurosurg Psychiatry. 78:790–799. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
David AK, Taylor RB and Fields SA:
Taylor's cardiovascular diseases: A handbook. Springer, New York,
NY, 2004.
|
|
14
|
Carbonara R, Giardinelli F, Zito A,
Scicchitano P, Dentamaro I, Cortese F, Armenise A, Manca F, De Caro
MF, Nazzaro P, et al: Neuro-psychological pattern in patients
suffering from primitive dilated cardiomyopathy with impairment in
executive function. Curr Neurovasc Res. 14:39–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Martis A, Gusetu G, Cismaru G, Zdrenghea
D, Leucuta DC and Pop D: Improvement of cognitive function and
interleukin 1 beta serum concentrations following cardiac pacemaker
implantation in patients with symptomatic bradycardia. J Pers Med.
11(770)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Duschek S, Matthias E and Schandry R:
Essential hypotension is accompanied by deficits in attention and
working memory. Behav Med. 30:149–158. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qiu C, von Strauss E, Fastbom J, Winblad B
and Fratiglioni L: Low blood pressure and risk of dementia in the
Kungsholmen project: A 6-year follow-up study. Arch Neurol.
60:223–228. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Waldstein SR, Giggey PP, Thayer JF and
Zonderman AB: Nonlinear relations of blood pressure to cognitive
function: The baltimore longitudinal study of aging. Hypertension.
45:374–379. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hebert LE, Scherr PA, Bennett DA, Bienias
JL, Wilson RS, Morris MC and Evans DA: Blood pressure and late-life
cognitive function change: A biracial longitudinal population
study. Neurology. 62:2021–2024. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gomez CR, McLaughlin JR, Njemanze C and
Nashed A: Effect of cardiac dysfunction upon diastolic cerebral
blood flow. Angiology. 43:625–630. 1992.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ettorre E, Cicerchia M, De Benedetto G,
Fossati C, Guglielmi S, Manzon L, Servello A, Petrillo A and
Marigliano V: A possible role of atrial fibrillation as a risk
factor for dementia. Arch Gerontol Geriatr. 49 (Suppl 1):S71–S76.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Beeri MS, Ravona-Springer R, Silverman JM
and Haroutunian V: The effects of cardiovascular risk factors on
cognitive compromise. Dialogues Clin Neurosci. 11:201–212.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Waldstein SR and Wendell CR:
Neurocognitive function and cardiovascular disease. J Alzheimers
Dis. 20:833–842. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jerskey BA, Cohen RA, Jefferson AL, Hoth
KF, Haley AP, Gunstad JJ, Forman DE, Sweet LH and Poppas A:
Sustained attention is associated with left ventricular ejection
fraction in older adults with heart disease. J Int Neuropsychol
Soc. 15:137–141. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jefferson AL, Poppas A, Paul RH and Cohen
RA: Systemic hypoperfusion is associated with executive dysfunction
in geriatric cardiac patients. Neurobiol Aging. 28:477–483.
2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hoth KF, Poppas A, Ellison KE, Paul RH,
Sokobin A, Cho Y and Cohen RA: Link between change in cognition and
left ventricular function following cardiac resynchronization
therapy. J Cardiopulm Rehabil Prev. 30:401–408. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dixit NK, Vazquez LD, Cross NJ, Kuhl EA,
Serber ER, Kovacs A, Dede DE, Conti JB and Sears SF: Cardiac
resynchronization therapy: A pilot study examining cognitive change
in patients before and after treatment. Clin Cardiol. 33:84–88.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tisher A and Salardini A: A comprehensive
update on treatment of dementia. Semin Neurol. 39:167–178.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gay BE, Taylor KI, Hohl U, Tolnay M and
Staehelin HB: The validity of clinical diagnoses of dementia in a
group of consecutively autopsied memory clinic patients. J Nutr
Health Aging. 12:132–137. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brunnström H and Englund E:
Clinicopathological concordance in dementia diagnostics. Am J
Geriatr Psychiatry. 17:664–670. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Roman G: Diagnosis of vascular dementia
and Alzheimer's disease. Int J Clin Pract Suppl. 120:9–13.
2001.PubMed/NCBI
|
|
32
|
Jongen PJ, Ter Horst AT and Brands AM:
Cognitive impairment in multiple sclerosis. Minerva Med. 103:73–96.
2012.PubMed/NCBI
|
|
33
|
Mavrogeni S, Pons R, Nikas I, Papadopoulos
G, Verganelakis DA, Kolovou G and Chrousos GP: Brain and heart
magnetic resonance imaging/spectroscopy in duchenne muscular
dystrophy. Eur J Clin Invest. 47:2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vojinovic D, Adams HH, van der Lee SJ,
Ibrahim-Verbaas CA, Brouwer R, van den Hout MC, Oole E, van Rooij
J, Uitterlinden A, Hofman A, et al: The dystrophin gene and
cognitive function in the general population. Eur J Hum Genet.
23:837–843. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jaaro-Peled H and Sawa A:
Neurodevelopmental factors in schizophrenia. Psychiatr Clin North
Am. 43:263–274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rolls ET: The cingulate cortex and limbic
systems for emotion, action, and memory. Brain Struct Funct.
224:3001–3018. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bressi S, Miele L, Bressi C, Astori S,
Gimosti E, Linciano AD, Sessini M and Invernizzi G: Deficit of
central executive component of working memory in schizophrenia. New
Trends Exp Clin Psychiatry. 12:243–252. 1996.
|
|
38
|
Gold JM, Carpenter C, Randolph C, Goldberg
TE and Weinberger DR: Auditory working memory and wisconsin card
sorting test performance in schizophrenia. Arch Gen Psychiatry.
54:159–165. 1997.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Buchsbaum MS and Hazlett EA: Positron
emission tomography studies of abnormal glucose metabolism in
schizophrenia. Schizophr Bull. 24:343–364. 1998.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Spitzer M: The psychopathology,
neuropsychology, and neurobiology of associative and working memory
in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 243:57–70.
1993.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stone M, Gabrieli JD, Stebbins GT and
Sullivan EV: Working and strategic memory deficits in
schizophrenia. Neuropsychology. 12:278–288. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Goldberg TE, Saint-Cry JA and Weinberger
DR: Assessment of procedural learning and problem solving in
schizophrenic patients by Tower of Hanoi type tasks. J
Neuropsychiatry Clin Neurosci. 2:165–173. 1990.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Braff DL, Heaton R, Kuck J, Cullum M,
Moranville J, Grant I and Zisook S: The generalized pattern of
neuropsychological deficits in outpatients with chronic
schizophrenia with heterogeneous wisconsin card sorting test
results. Arch Gen Psychiatry. 48:891–898. 1991.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Voruganti LN, Heslegrave RJ and Awad AG:
Neurocognitive correlates of positive and negative syndromes in
schizophrenia. Can J Psychiatry. 42:1066–1071. 1997.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Young DA, Davila R and Scher H:
Unawareness of illness and neuropsychological performance in
chronic schizophrenia. Schizophr Res. 10:117–124. 1993.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Carrion C, Folkvord F, Anastasiadou D and
Aymerich M: Cognitive therapy for dementia patients: A systematic
review. Dement Geriatr Cogn Disord. 46:1–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Clark LD, Iversen SD and Goodwin GM:
Sustained attention deficit in bipolar disorder. Br J Psychiatry.
180:313–319. 2002.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Drubach DA: Obsessive-compulsive disorder.
Continuum (Minneap Minn). 21 (3 Behavioral Neurology and
Neuropsychiatry):783–788. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zielinski CM, Taylor MA and Juzwin KR:
Neuropsychological deficits in obsessive-compulsive disorder.
Neuropsychiatry Neuropsychol Behav Neurol. 4:110–126. 1991.
|
|
50
|
Loades ME, Chalder T, Smakowski A and
Rimes KA: Anticipation of and response to exercise in adolescents
with CFS: An experimental study. J Psychosom Res.
146(110490)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee CH and Giuliani F: The role of
inflammation in depression and fatigue. Front Immunol.
10(1696)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Abbey SE and Garfenkel PE: Chronic fatigue
syndrome and depression: Cause, effect, or covariate. Rev Infect
Dis. 13 (Suppl 1):S73–S83. 1991.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Woods SP, Lovejoy DW and Ball JD:
Neuropsychological characteristics of adults with ADHD: A
comprehensive review of initial studies. Clin Neuropsychol.
16:12–34. 2002.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Magnin E and Maurs C:
Attention-deficit/hyperactivity disorder during adulthood. Rev
Neurol (Paris). 173:506–515. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Blume AW, Schmaling KB and Marlatt GA:
Memory, executive cognitive function, and readiness to change
drinking behavior. Addict Behav. 30:301–314. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mohammadian J and Miladi-Gorji H: Age- and
sex-related changes in the severity of physical and psychological
dependence in morphine-dependent rats. Pharmacol Biochem Behav.
187(172793)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Allen DN and Landis RKB:
Neuropsychological correlates of substance use disorders. In:
Clinical neuropsychology: A pocket handbook for assessment. Snyder
PJ and Nassbaum PD (eds). American Psychological Association,
Washington, DC, pp591-612, 1998.
|
|
58
|
Bates ME, Bowden SC and Barry D:
Neurocognitive impairment associated with alcohol use disorders:
Implications for treatment. Exp Clin Psychopharmacol. 10:193–212.
2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wessels AM, Scheltens P, Barkhof F and
Heine RJ: Hyperglycaemia as a determinant of cognitive decline in
patients with type 1 diabetes. Eur J Pharmacol. 585:88–96.
2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Munshi MN: Cognitive dysfunction in older
adults with diabetes: What a clinician needs to know. Diabetes
Care. 40:461–467. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gonder-Frederick LA, Zrebiec JF,
Bauchowitz AU, Ritterband LM, Magee JC, Cox DJ and Clarke WL:
Cognitive function is disrupted by both hypo- and hyperglycemia in
school-aged children with type 1 diabetes: A field study. Diabetes
Care. 32:1001–1006. 2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Strachan MW: R D Lawrence Lecture 2010.
The brain as a target organ in type 2 diabetes: Exploring the links
with cognitive impairment and dementia. Diabet Med. 28:141–147.
2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Maric-Bilkan C: Sex differences in micro-
and macro-vascular complications of diabetes mellitus. Clin Sci
(Lond). 131:833–846. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Diabetes Control and Complications
Trial/Epidemiology of Diabetes Interventions and Complications
Study Research Group. Jacobson AM, Musen G, Ryan CM, Silvers N,
Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, et al:
Long-term effect of diabetes and its treatment on cognitive
function. N Engl J Med. 356:1842–1852. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Jacobson AM, Ryan CM, Cleary PA, Waberski
BH, Weinger K, Musen G and Dahms W: Diabetes Control and
Complications Trial/EDIC Research Group. Biomedical risk factors
for decreased cognitive functioning in type 1 diabetes: An 18 year
follow-up of the diabetes control and complications Trial (DCCT)
cohort. Diabetologia. 54:245–255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mukherjee P, Mazumdar S, Goswami S,
Bhowmik J, Chakroborty S, Mukhopadhyay S, Jana S, Chakraborty A,
Pal S, Das SK and Mukhopadhyay J: Cognitive dysfunction in diabetic
patients with special reference to age of onset, duration and
control of diabetes. Act Nerv Super. 54:263–275. 2012.
|
|
67
|
Luchsinger JA, Ryan C and Launer LJ:
Diabetes and Cognitive Impairment. In: Diabetes in America. Cowie
CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB,
Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, et al
(eds). 3rd edition. National Institute of Diabetes and Digestive
and Kidney Diseases (US), Bethesda, MD, Chapter 24, 2018.
|
|
68
|
Cukierman-Yaffe T: Diabetes, dysglycemia
and cognitive dysfunction. Diabetes Metab Res Rev. 30:341–345.
2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sato N and Morishita R: Roles of vascular
and metabolic components in cognitive dysfunction of Alzheimer
disease: Short- and long-term modification by non-genetic risk
factors. Front Aging Neurosci. 5(64)2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Joaquim AF and Appenzeller S:
Neuropsychiatric manifestations in rheumatoid arthritis. Autoimmun
Rev. 14:1116–1122. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Shin SY, Katz P, Wallhagen M and Julian L:
Cognitive impairment in persons with rheumatoid arthritis.
Arthritis Care Res (Hoboken). 64:1144–1150. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Gorelick PB: Role of inflammation in
cognitive impairment: Results of observational epidemiological
studies and clinical trials. Ann N Y Acad Sci. 1207:155–162.
2010.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Simos P, Ktistaki G, Dimitraki G,
Papastefanakis E, Kougkas N, Fanouriakis A, Gergianaki I, Bertsias
G, Sidiropoulos P and Karademas EC: Cognitive deficits early in the
course of rheumatoid arthritis. J Clin Exp Neuropsychol.
38:820–829. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Szekanecz Z, Kerekes G, Dér H, Sándor Z,
Szabó Z, Végvári A, Simkovics E, Soós L, Szentpétery A, Besenyei T,
et al: Accelerated atherosclerosis in rheumatoid arthritis. Ann N Y
Acad Sci. 1108:349–358. 2007.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Coluccia D, Wolf OT, Kollias S, Roozendaal
B, Forster A and de Quervain DJ: Glucocorticoid therapy-induced
memory deficits: Acute versus chronic effects. J Neurosci.
28:3474–3478. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hanly JG, Kozora E, Beyea SD and Birnbaum
J: Review: Nervous system disease in systemic lupus erythematosus:
Current status and future directions. Arthritis Rheumatol.
71:33–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kello N, Anderson E and Diamond B:
Cognitive dysfunction in systemic lupus erythematosus: A case for
initiating trials. Arthritis Rheumatol. 71:1413–1425.
2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hanly JG, Urowitz MB, Su L, Bae SC, Gordon
C, Wallace DJ, Clarke A, Bernatsky S, Isenberg D, Rahman A, et al:
Prospective analysis of neuropsychiatric events in an international
disease inception cohort of patients with systemic lupus
erythematosus. Ann Rheum Dis. 69:529–535. 2010.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Steup-Beekman GM, Zirkzee EJ, Cohen D,
Gahrmann BM, Emmer BJ, Steens SC, Bollen EL, van Buchem MA and
Huizinga TW: Neuropsychiatric manifestations in patients with
systemic lupus erythematosus: Epidemiology and radiology pointing
to an immune-mediated cause. Ann Rheum Dis. 72 (Suppl 2):ii76–ii79.
2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Schwartz N, Stock AD and Putterman C:
Neuropsychiatric lupus: New mechanistic insights and future
treatment directions. Nat Rev Rheumatol. 15:137–152.
2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Bertsias GK, Ioannidis JP, Aringer M,
Bollen E, Bombardieri S, Bruce IN, Cervera R, Dalakas M, Doria A,
Hanly JG, et al: EULAR recommendations for the management of
systemic lupus erythematosus with neuropsychiatric manifestations:
Report of a task force of the EULAR standing committee for clinical
affairs. Ann Rheum Dis. 69:2074–2082. 2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Brey RL, Gharavi AE and Lockshin MD:
Neurologic complications of antiphospholipid antibodies. Rheum Dis
Clin N Am. 19:833–850. 1993.PubMed/NCBI
|
|
83
|
Stock AD, Gelb S, Pasternak O, Ben-Zvi A
and Putterman C: The blood brain barrier and neuropsychiatric
lupus: New perspectives in light of advances in understanding the
neuroimmune interface. Autoimmun Rev. 16:612–619. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Barraclough M, McKie S, Parker B, Jackson
A, Pemberton P, Elliott R and Bruce IN: Altered cognitive function
in systemic lupus erythematosus and associations with inflammation
and functional and structural brain changes. Ann Rheum Dis.
78:934–940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ploran E, Tang C, Mackay M, Small M,
Anderson E, Storbeck J, Bascetta B, Kang S, Aranow C, Sartori C, et
al: Assessing cognitive impairment in SLE: Examining relationships
between resting glucose metabolism and anti-NMDAR antibodies with
navigational performance. Lupus Sci Med. 6(e000327)2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Mackay M, Vo A, Tang CC, Small M, Anderson
EW, Ploran EJ, Storbeck J, Bascetta B, Kang S, Aranow C, et al:
Metabolic and microstructural alterations in the SLE brain
correlate with cognitive impairment. JCI Insight: Jan 10, 2019
(Epub ahead of print).
|
|
87
|
Mouthon L, Alami S, Boisard AS, Chaigne B,
Hachulla E and Poiraudeau S: Patients' views and needs about
systemic sclerosis and its management: A qualitative interview
study. BMC Musculoskelet Disord. 18(230)2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Penn H, Howie AJ, Kingdon EJ, Bunn CC,
Stratton RJ, Black CM, Burns A and Denton CP: Scleroderma renal
crisis: Patient characteristics and long-term outcomes. QJM.
100:485–494. 2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Pakozdi A, Nihtyanova S, Moinzadeh P, Ong
VH, Black CM and Denton CP: Clinical and serological hallmarks of
systemic sclerosis overlap syndromes. J Rheumatol. 38:2406–2409.
2011.PubMed/NCBI View Article : Google Scholar
|
|
90
|
De Stefano P, Chizzolini C, Lalive PH and
Lascano AM: Limbic encephalitis associated with systemic sclerosis.
Mult Scler Relat Disord. 24:142–144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Giuliodori G, Fraticelli P, Bartolini M,
Cagnetti C, Baruffaldi R, Rocchi MB, Provinciali L, Gabrielli A and
Silvestrini M: Cognitive and cerebral hemodynamic impairment in
scleroderma patients. Eur J Neurol. 16:1285–1290. 2009.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Alcocer-Castillejos N, Jiménez-González A
and Hinojosa-Azaola A: No difference in cognitive dysfunction among
patients with ANCA-associated vasculitis, rheumatoid arthritis or
chronic kidney disease. J Int Neuropsychol Soc. 25:595–602.
2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Athilingam P, King KB, Burgin SW,
Acker-man M, Cushman LA and Chen L: Montreal cognitive assessment
and mini-mental status examination compared as cognitive screening
tools in heart failure. Heart Lung. 40:521–529. 2011.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Koski L: Validity and applications of the
montreal cognitive assessment for the assessment of vascular
cognitive impairment. Cerebrovasc Dis. 36:6–18. 2013.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Mankovsky B, Zherdova N, van den Berg E,
Biessels GJ and de Bresser J: Cognitive functioning and structural
brain abnormalities in people with type 2 diabetes mellitus. Diabet
Med. 35:1663–1670. 2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Tamura Y and Araki A: Diabetes mellitus
and white matter hyperintensity. Geriatr Gerontol Int. 15 (Suppl
1):S34–S42. 2015.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Fang F, Zhan YF, Zhuo YY, Yin DZ, Li KA
and Wang YF: Brain atrophy in middle-aged subjects with type 2
diabetes mellitus, with and without microvascular complications. J
Diabetes. 10:625–632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Moroni F, Ammirati E, Rocca MA, Filippi M,
Magnoni M and Camici PG: Cardiovascular disease and brain health:
Focus on white matter hyperintensities. Int J Cardiol Heart Vasc.
19:63–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Yin C, Li S, Zhao W and Feng J: Brain
imaging of mild cognitive impairment and Alzheimer's disease.
Neural Regen Res. 8:435–444. 2013.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Brickman AM, Muraskin J and Zimmerman ME:
Structural neuroimaging in Altheimer's disease: Do white matter
hyperintensities matter? Dialogues Clin Neurosci. 11:181–190.
2009.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cannerfelt B, Nystedt J, Jönsen A, Lätt J,
van Westen D, Lilja A, Bengtsson A, Nilsson P, Mårtensson J and
Sundgren PC: White matter lesions and brain atrophy in systemic
lupus erythematosus patients: Correlation to cognitive dysfunction
in a cohort of systemic lupus erythematosus patients using
different definition models for neuropsychiatric systemic lupus
erythematosus. Lupus. 27:1140–1149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wiseman SJ, Ralston SH and Wardlaw JM:
Cerebrovascular disease in rheumatic diseases: A systematic review
and meta-analysis. Stroke. 47:943–950. 2016.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Liu X, Yang ZG, Gao Y, Xie LJ, Jiang L, Hu
BY, Diao KY, Shi K, Xu HY, Shen MT, et al: Left ventricular
subclinical myocardial dysfunction in uncomplicated type 2 diabetes
mellitus is associated with impaired myocardial perfusion: A
contrast-enhanced cardiovascular magnetic resonance study.
Cardiovasc Diabetol. 17(139)2018.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Shah RV, Abbasi SA and Kwong RY: Role of
cardiac MRI in diabetes. Curr Cardiol Rep. 16(449)2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Mavrogeni SI, Markousis-Mavrogenis G,
Papavasiliou A, Papadopoulos G and Kolovou G: Cardiac involvement
in duchenne muscular dystrophy and related dystrophinopathies.
Methods Mol Biol. 1687:31–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
von Knobelsdorff-Brenkenhoff F and
Schulz-Menger J: Role of cardiovascular magnetic resonance in the
guidelines of the European society of cardiology. J Cardiovasc Magn
Reson. 18(6)2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Mavrogeni S, Petrou E, Kolovou G,
Theodorakis G and Iliodromitis E: Prediction of ventricular
arrhythmias using cardiovascular magnetic resonance. Eur Heart J
Cardiovasc Imaging. 14:518–525. 2013.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Wehrum T, Dragonu I, Strecker C,
Schuchardt F, Hennemuth A, Drex J, Reinhard T, Böhringer D, Vach W,
Hennig J and Harloff A: Aortic atheroma as a source of
stroke-assessment of embolization risk using 3D CMR in stroke
patients and controls. J Cardiovasc Magn Reson.
19(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Yaghi S, Liberman AL, Atalay M, Song C,
Furie KL, Kamel H and Bernstein RA: Cardiac magnetic resonance
imaging: A new tool to identify cardioaortic sources in ischaemic
stroke. J Neurol Neurosurg Psychiatry. 88:31–37. 2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Rosenbohm A, Schmid B, Buckert D,
Rottbauer W, Kassubek J, Ludolph AC and Bernhardt P: Cardiac
findings in amyotrophic lateral sclerosis: A magnetic resonance
imaging study. Front Neurol. 8(479)2017.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Mavrogeni SI, Kitas GD, Dimitroulas T,
Sfikakis PP, Seo P, Gabriel S, Patel AR, Gargani L, Bombardieri S,
Matucci-Cerinic M, et al: Cardiovascular magnetic resonance in
rheumatology: Current status and recommendations for use. Int J
Cardiol. 217:135–148. 2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Mavrogeni S, Gargani L, Pepe A, Monti L,
Markousis-Mavrogenis G, De Santis M, De Marchi D,
Koutsogeorgopoulou L, Karabela G, Stavropoulos E, et al: Cardiac
magnetic resonance predicts ventricular arrhythmias in scleroderma:
The scleroderma arrhythmia clinical utility study (SAnCtUS).
Rheumatology (Oxford). 59:1938–1948. 2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Mavrogeni SI, Sfikakis PP,
Markousis-Mavrogenis G, Bournia VK, Poulos G, Koutsogeorgopoulou L,
Karabela G, Stavropoulos E, Katsifis G, Boki K, et al:
Cardiovascular magnetic resonance imaging pattern in patients with
autoimmune rheumatic diseases and ventricular tachycardia with
preserved ejection fraction. Int J Cardiol. 284:105–109.
2019.PubMed/NCBI View Article : Google Scholar
|