Introduction
Ovarian carcinosarcoma (OCS), also known as
malignant mixed Müllerian tumor (MMMT), is a rare malignant tumor
that postmenopausal females are prone to develop, accounting for
1-4% of ovarian tumors (1). Most
patients are diagnosed in the advanced stage with poor prognosis
and most of them had elevated cancer antigen (CA)125 levels.
Imaging findings usually indicate pelvic masses, mostly with
ascites. OCS consists of malignant epithelial components and
sarcoma components. According to the sarcoma components, it may be
classified as homologous (the sarcoma component was derived from
the stromal component of the primary tumor site, e.g., endometrial
stromal sarcoma) or heterologous (the sarcoma component was not
derived from the interstitial component of the primary tumor site,
such as rhabdomyosarcoma component, osteosarcoma component)
(2). Immunohistochemical (IHC)
analysis of markers including cytokeratin (CK), cytokeratin pan
monoclonal antibody (MNF116), epithelial membrane antigen,
vimentin, S100, chromogranin, synaptophysin, desmin, myogenin
(MYF4) and p53 were used to suggest the presence of the
heterologous component (3). The
current treatment methods mainly include ovarian tumor
cytoreduction surgery, followed by platinum-based chemotherapy. OCS
is rare and patients with advanced OCS are prone to metastases.
Cytoreductive surgery may only remove local lesions. Chemotherapy
and radiotherapy are not able to completely eliminate cancer cells;
in addition, they weaken the patients and decrease their immune
function. Targeted drugs would only kill tumor cells with gene
targets. For those tumor cells without gene targets, targeted drugs
are generally ineffective. It was reported that patients with OCS
had an obviously increased risk of death compared with those with
epithelial ovarian cancer (4). The
majority of patients relapsed within one year following treatment
and survived nearly two years after the initial diagnosis (4,5). Due
to its rarity, there is a lack of large-scale, prospective studies
for this disease and there is still no consensus on a standardized
diagnosis and treatment strategy for OCS. The following report
presents a case of OCS treated with optimal cytoreductive surgery
and chemotherapy.
Patients and methods
Methods
For the examination of the present case, the ARIETTA
70 ultrasound machine (Hitachi, Ltd.) with a 2-10 Hz resolution
transvaginal ultrasound probe (C41V1) was used. Blood flow signals,
including resistive index (RI), peak systolic velocity (PSV) and
end-diastolic velocity (EDV) were detected by color Doppler flow
imaging (CDFI). The patient underwent MRI scanning on a 1.5 T
Philips Achieva MRI system (Philips Healthcare). The parameters of
the echo planar imaging diffusion-weighted imaging (DWI) sequence
[echo time (TE), 60 msec; repetition time (TR), 1,118 msec;
multiband factor, 2; b-values, 0 and 600 sec/mm2;
acquisition time, 1:04 for a single b-value). Fat-suppressed
gradient-echo enhanced T1-weighted imaging (T1WI) were obtained
with the following settings: TE, 7 msec; TR, 497 msec; flip angle,
10; matrix size, 256x256; field of view (FOV), 400 mm; and slice
thickness, 5 mm. Images were available in the transverse, coronal
and sagittal planes. Intravenous contrast medium (Omniscan; GE
Healthcare) was administered at a dose of 0.2 ml/kg.
In the present study, 4 ml of venous blood was
collected from the patient and centrifuged at 1,509.3 x g at 25˚C
for 6 min to separate serum. Serum tumor marker levels of CA125,
alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), CA199 and
CA153 were measured by DXI-800 autoanalysers (Beckman Coulter,
Inc.).
The excised specimens were fixed using
neutral-buffered 10% formalin, dehydrated in a series of ethanols
and embedded in paraffin. Serial sections of 4-µm thickness were
made and then subjected to hematoxylin-eosin staining. IHC staining
was performed following the EnVision two-step l protocol (6,7).
3-3'diaminobenzidine was used for colour development. These steps
were performed with a Ventana automated immunostainer (Ventana
Medical Systems, Inc.) using an UltraView Universal DAB Detection
Kit (Ventana Medical Systems, Inc.). Primary antibodies applied in
the IHC analysis were mainly as follows: Monoclonal mouse
anti-human cytokeratin (CK) (AE1/AE3), mouse anti-human tumor
protein p53 monoclonal antibody (DO-7), rabbit polyclonal
anti-human S100 protein, monoclonal mouse anti-vimentin (V9; all
from Dako; Agilent Technologies, Inc.), mouse anti-human tumor
protein P40 monoclonal antibody (cat. no. 66622-1-Ig) and MYOD1
rabbit polyclonal antibody (cat. no. 18943-1-AP; both from
ProteinTech Group, Inc.). The dilution ratio of CK, p53, S100 and
P40 antibodies was 1:300, 1:800, 1:1,000 and 1:100, respectively.
The different secondary antibodies for IHC were examined through
the Ventana automated immunostainer (Ventana Medical Systems,
Inc.). The ready-to-use secondary antibody was included in the kit.
In addition, a systematic review focusing on the treatment of this
disease was performed. The elaborate literature search was
undertaken through the PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/) and Web of Science
(https://webofscience.com/WOS) database
to identify the studies examining OCS treatment options from
database inception to September, 2021, using the following key
words: Ovarian carcinosarcoma, malignant mixed Mullerian tumor,
carcinosarcoma of the ovary, cytoreduction, chemotherapy,
radiotherapy and targeted therapy in various combinations. The
inclusion criteria were as follows: Patients with OCS, MMMT or
carcinosarcoma of the ovary, who received one or more treatments of
cytoreduction, chemotherapy, radiotherapy and targeted therapy.
Those studies that did not include patients with OCS were excluded.
Other potential studies were retrieved by reviewing the reference
lists within publications.
Case presentation
A 61-year-old post-menopausal female complained of
abdominal distension for one week and presented at Ningbo Women and
Children's Hospital (Ningbo, China). The patient had no history of
vaginal bleeding after menopause. The patient had received radical
mastectomy for left breast cancer at our hospital 10 years
previously and had overgone laparoscopic cholecystectomy 7 years
ago. Approval for the present study was acquired from the
institutional review board of Ningbo Women and Children's Hospital
(Ningbo, China) and informed consent was obtained from the
patient.
Preoperative ultrasonography (US) examination
revealed a mixed cystic-solid sonographic appearance of 37x26x35 mm
in the left adnexal area and the solid area was 17x14x22 mm (yellow
arrow). In the left adnexal area, CDFI indicated the following: RI,
0.68; PSV, 12.9 cm/sec; and EDV, 4.1 cm/sec (Fig. 1A). In the right adnexal area, a
low-echo area of 54x26x41 mm with an irregular shape (yellow
arrows) was present and CDFI indicated the following: RI, 0.48;
PSV, 22.9 cm/sec; and EDV, 11.8 cm/sec (Fig. 1B). A certain amount of pelvic fluid
collection and a small amount of effusion in the uterine cavity
were detected.
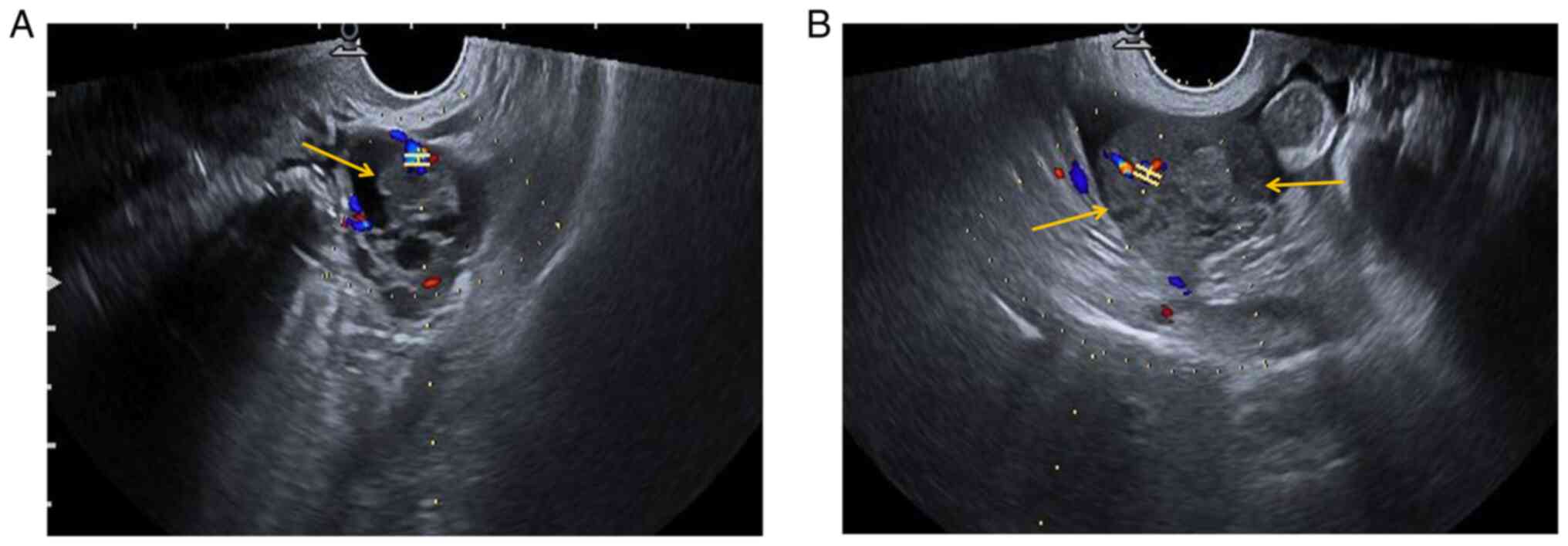 | Figure 1Preoperative US findings. (A)
Preoperative US examination indicated a mixed cystic-solid area of
37x26x35 mm in the left adnexal region, and the solid area was
~17x14x22 mm (yellow arrow). CDFI of the left adnexal area
indicated the following: RI, 0.68; PSV, 12.9 cm/sec; and EDV4.1
cm/sec. (B) A low echo area of 54x26x41 mm with an irregular shape
was observed in the right adnexal area (yellow arrows). CDFI
indicated the following: RI, 0.48; PSV, 22.9 cm/sec; and EDV, 11.8
cm/sec. H-shaped structure indicates the sample gate. The color
represents the direction of blood flow. Red indicates blood flow
towards the probe and blue means blood flow away from the probe. A
certain amount of pelvic fluid collection and a small amount of
effusion in the uterine cavity were observed. US, ultrasonography;
RI, resistive index; PSV, peak systolic velocity; EDV,
end-diastolic velocity; CDFI, color Doppler flow imaging. |
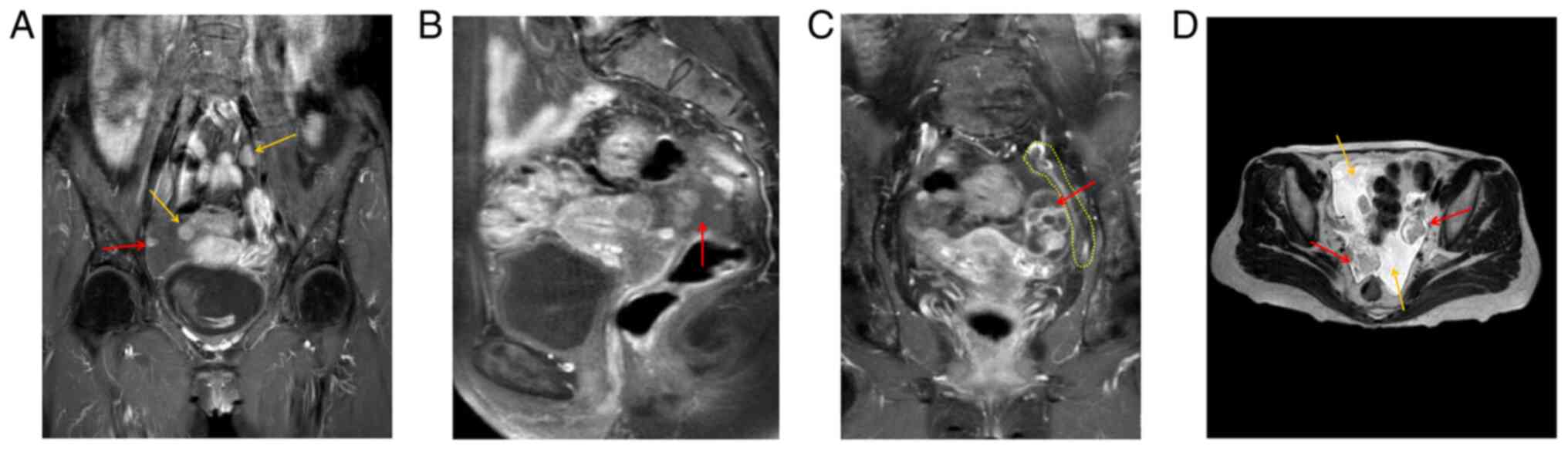
Preoperative MRI indicated two enlarged lymph nodes
in the pelvic cavity (yellow arrows), and peritoneal lesions (red
arrow) (Fig. 2A). The right
adnexal area was irregular in shape, 27x43x28 mm in size, and DWI
indicated high signal intensity, with uneven enhancement of the
right lesion (red arrow) (Fig.
2B). An elliptical cystic solid lesion was present in the left
adnexal area of the pelvis, the size of which was 31x26x40 mm (red
arrow) and the peritoneum was thickened (yellow dotted line)
(Fig. 2C). T2WI indicated two
lesions (red arrows) in the left and right adnexa area and a
certain amount of pelvic fluid collection (yellow arrows) (Fig. 2D).
In the present case, homologous recombination DNA
repair deficiency (HRD) was positive, while negative results were
obtained in BRCA1/2 mutation testing. This suggested cells with HRD
may be more sensitive to platinum drugs and poly ADP-ribose
polymerase inhibitors (8).
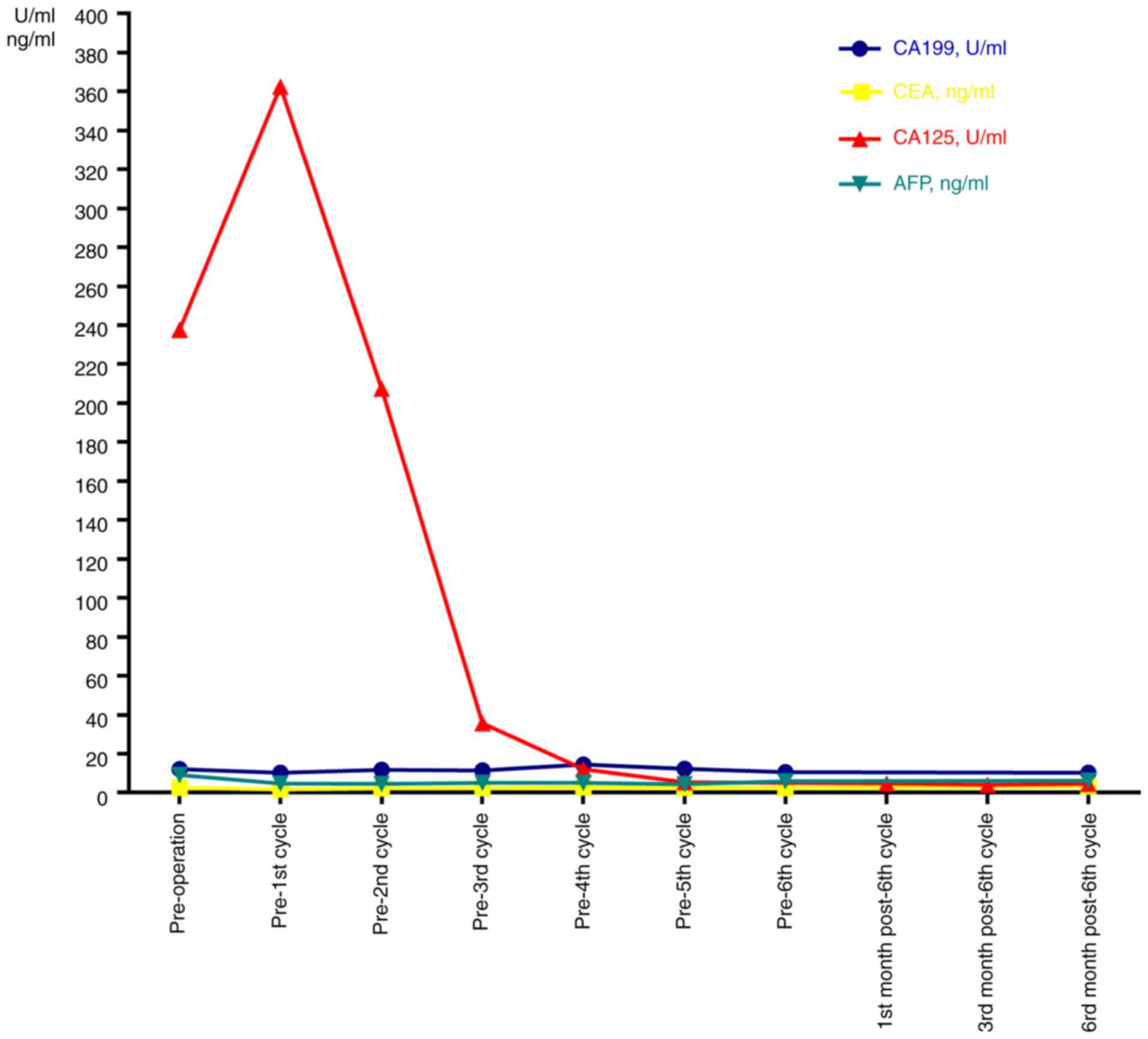
Preoperative CA125 levels were elevated above the normal range
(237.70 U/ml; normal, 0-35 U/ml) and AFP levels were slightly
beyond the normal range (9.13 ng/ml; normal, 0-9 ng/ml). CEA, CA199
and CA153 levels were in the normal ranges (CEA, 0-5 ng/ml; CA199,
0-35 U/ml; CA153, 0-31.3 U/ml) (Fig.
3).
The patient underwent an exploratory laparotomy.
Tumor cells were detected in 300 ml of ascites. After separation of
the adhesions, the left infundibulopelvic ligament, the proper
ovarian ligament, isthmus of the left fallopian tube and left ovary
were removed. Part of the ovarian lesions and mesenteric neoplasms
were used for fast frozen pathology and the results suggested
poorly differentiated cancer. The patient then underwent
cytoreductive surgery, including sub-extensive hysterectomy,
bilateral adnexectomy, sigmoid colon and partial rectal resection,
as well as lymph node dissection.
Postoperative pathology of the bilateral adnexal
masses confirmed carcinosarcoma. The main carcinomatous components
included high-grade serous carcinoma. Tumor cells were arranged in
glandular, papillary and solid patterns. The ducts were slit-like
or irregular, differentiation was poor and cells were arranged in
sheets. The cells had obvious atypia, with large hyperchromatic
pleomorphic nuclei, in which prominent nucleoli and numerous
mitotic figures were present (yellow arrows) (Fig. 4A). Carcinomatous components
included a small amount of squamous cell carcinoma. Tumor cells
were arranged in nested, expansile, polygonal, paving stone-like
patterns. There were intercellular bridges and intracellular
keratinization may be seen in central cells. Nuclei are large,
hyperchromatic or vacuolated with visible nucleoli and mitoses
(yellow arrow) (Fig. 4B).
Sarcomatous components contained fibrosarcoma. The tumor cells were
spindle-shaped, with a cord-like distribution. Nuclei were
hyperchromatic and mitoses were seen (yellow arrow) (Fig. 4C). Sarcomatous components contained
a small amount of chondrosarcoma. Chondrosarcoma was observed to be
scattered in the cartilage lobules. The chondrocytes were located
in the cartilage lacuna, with large hyperchromatic pleomorphic
nuclei, in which binucleated cells and mitotic figures were present
(yellow arrow) (Fig. 4D).
Sarcomatous components also contained a small proportion of
rhabdomyosarcoma. The tumor cells were scattered in round and oval
shapes. The cytoplasm was abundant and eosinophilic. The nuclei
were eccentric and vacuolated with obvious nucleoli (yellow arrow)
(Fig. 4E). Infiltrating cells in
cancer tissues or metastasis were observed on the serosal surface,
muscular and subserosal layers of the uterus, as well as sigmoid
colon and part of the rectum. The final diagnosis of OCS was
obtained by histopathological examination of the resected specimen.
The biphasic component was further clarified by IHC analysis.
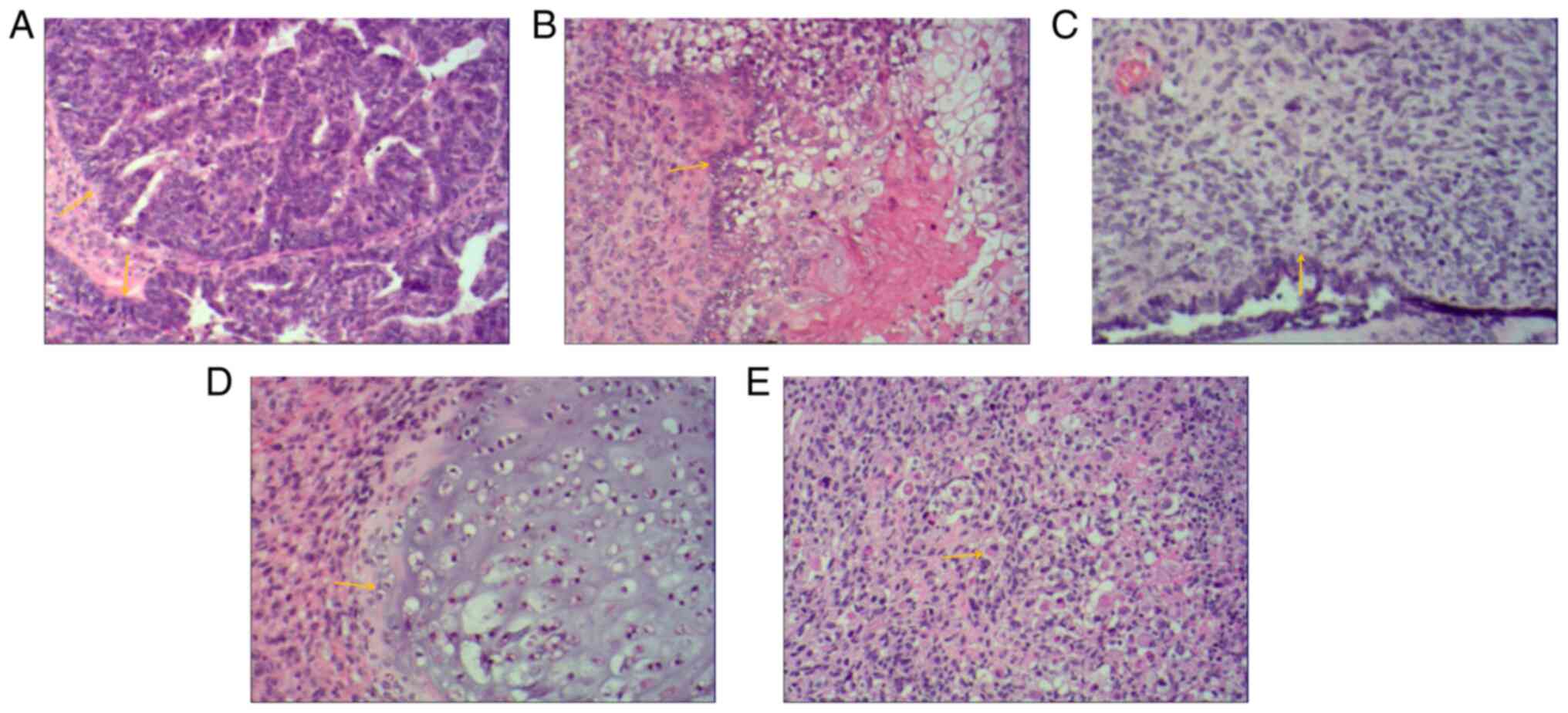 | Figure 4Histological images of bilateral
adnexal masses. Extensive metastases were observed throughout the
pelvis and abdomen. Histological images of bilateral adnexal masses
revealed the carcinosarcoma and sarcomatous elements of ovarian
carcinosarcoma. (A) The main carcinomatous components included
high-grade serous carcinoma. Tumor cells were arranged in
glandular, papillary and solid patterns. The ducts were slit-like
or irregular and poorly differentiated, and cells were arranged in
sheets. The cells had obvious atypia, with large hyperchromatic
pleomorphic nuclei, in which prominent nucleoli and numerous
mitotic figures were present (yellow arrows). (B) Carcinomatous
components included a small amount of squamous cell carcinoma.
Tumor cells were arranged in nested, expansile, polygonal, paving
stone-like patterns. Intercellular bridges were present and
intracellular keratinization was observed in central cells. Nuclei
were large, hyperchromatic or vacuolated with visible nucleoli and
mitoses (yellow arrow). (C) Sarcomatous components contained
fibrosarcoma. The tumor cells were spindle-shaped, with a cord-like
distribution. Nuclei were hyperchromatic and mitotic bodies were
present (yellow arrow). (D) Sarcomatous components contained a
small amount of chondrosarcoma. Scattered chondrosarcoma was
present in the cartilage lobules. The chondrocytes were located in
the cartilage lacuna, with large hyperchromatic pleomorphic nuclei,
in which binucleated cells and mitotic figures were present (yellow
arrow). (E) Sarcomatous components also contained a small amount of
rhabdomyosarcoma. The tumor cells were scattered in round and oval
shapes. The cytoplasm was abundant and eosinophilic. Nuclei were
eccentric and vacuolated with obvious nucleoli (yellow arrow)
(H&E; magnification, x100). |
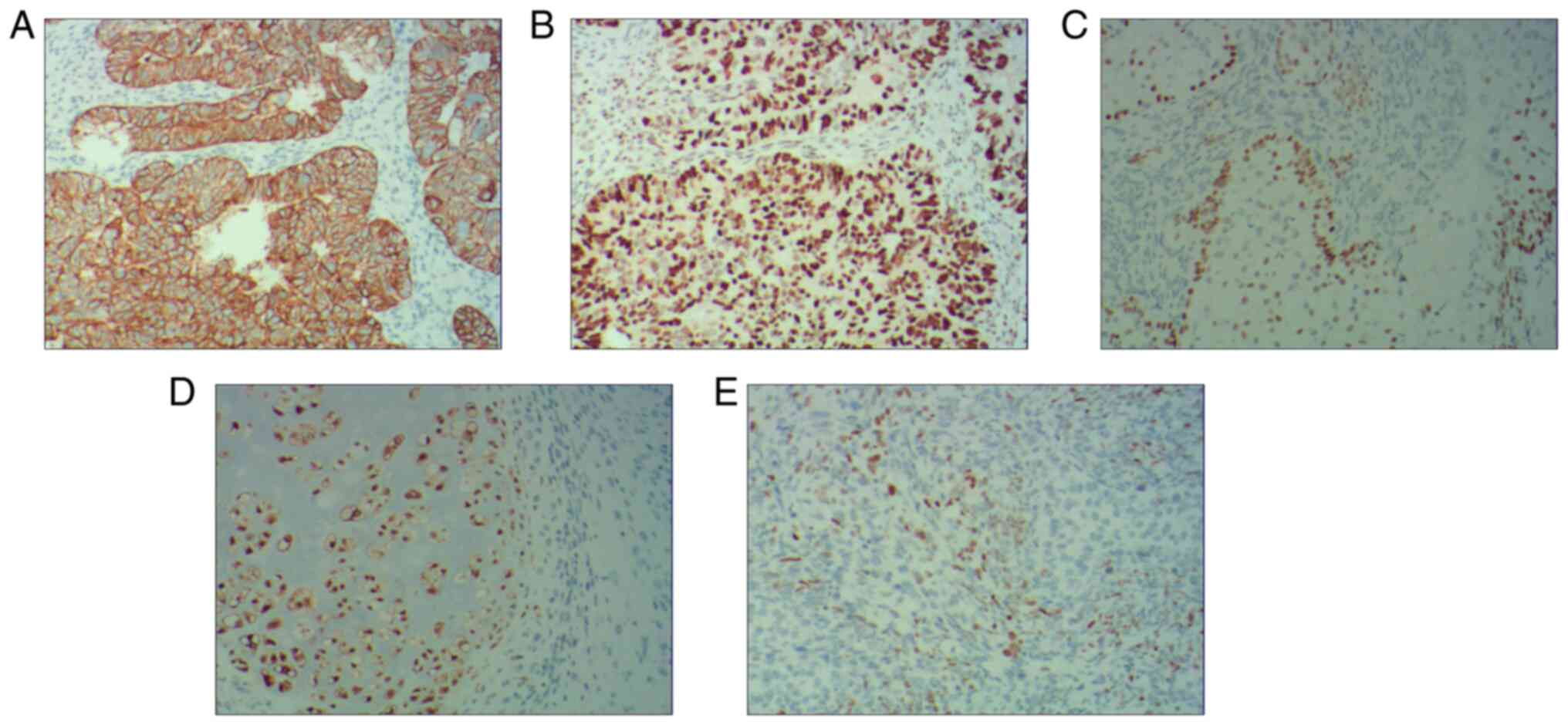
The IHC results indicated that the serous carcinoma
component was positive for cytokeratin (Fig. 5A) and P53 (Fig. 5B), squamous cell carcinoma was
positive for P40 (Fig. 5C),
chondrosarcoma was positive for S100 (Fig. 5D) and rhabdomyosarcoma was positive
for MyoD1 (Fig. 5E). The patient
was diagnosed postoperatively with International Federation of
Gynecology and Obstetrics stage IIIC OCS and T3cN1M0 based on the
TNM system.
After the surgery, the patient was given 6 cycles of
chemotherapy consisting of carboplatin (60 to 75 mg/m2)
plus paclitaxel (135-175 mg/m2) on day 1 in all cycles,
plus bevacizumab (7.5 mg/kg), on day 1 in cycles 3-6. The dose of
carboplatin was calculated using the Calvert formula: Dose
(mg)=area under curve (AUC; min x mg/ml) [glomerular filtration
rate (ml/min) + 25 ml/min] (9);
the AUC was 5 mg/ml/min. The HRD result was positive. The patient
should have received bevacizumab plus niraparib maintenance therapy
after chemotherapy. As severe myelosuppression occurred during and
after chemotherapy and bevacizumab was expensive, bevacizumab
therapy was not continued after chemotherapy, but oral maintenance
therapy with niraparib was given. Initially, the patient received
oral niraparib 0.1 g twice daily for half a month. However, the
patient then discontinued niraparib for one month due to severe
myelosuppression. Subsequently, niraparib was reduced to 0.1 g
orally once daily until now.
Follow-up on the 19th day after surgery indicated
that the CA125 levels had increased to 362.4 U/ml. After 6 cycles
of chemotherapy, the CA125 levels exhibited a gradual reduction. At
six months after the sixth chemotherapy, the CA125 levels dropped
to 4.55 U/ml, which was in the normal range (Fig. 3). At the six-month follow-up, no
tumor recurrence or metastasis was detected in the breast bilateral
axillary lymph nodes or in the pelvic cavity on US. No tumor
recurrence or metastasis was detected on chest CT and pelvic
enhanced MRI.
Discussion
Physicians usually find it difficult to make a
specific diagnosis of OCS prior to surgery. OCS is frequently
asymptomatic in the early stage and initial
signs/symptoms/complaints are usually of a gastrointestinal nature,
including abdominal distension, abdominal pain and abdominal lumps
at the advanced stage. In the present case, the final diagnosis of
OCS was obtained by histopathological examination of the resected
specimen. The biphasic component was further clarified by IHC
analysis. The present study indicated that the serous carcinoma
component was positive for CK and P53, squamous cell carcinoma was
positive for P40, chondrosarcoma was positive for S100 and
rhabdomyosarcoma was positive for MyoD1. The findings of the
present study were similar to those of previous studies (3,10,11).
In a study of 8 cases with OCS (3), the carcinomatous components exhibited
positive staining for epithelial markers, including cytokeratin
MNF116. All sarcomatous components had positive staining for
vimentin and all rhabdomyosarcomas were positive for myogenic
markers, including MYF4. All chondrosarcomas were positive for
S100. All tumors displayed were positive for p53(3). Pankaj et al (10) indicated that CK and vimentin were
positive, indicating the likely presence of carcinosarcoma and
epithelioid sarcoma. Another study reported that tumor cells were
positive for the skeletal muscle marker myoD1 for rhabdomyosarcomas
(11). The above IHC results
suggested that these indicators may be of great significance for
the diagnosis of OCS and further large-scale studies will be
required to confirm these findings in the future.
Regarding imaging characteristics of OCS, a previous
study reported that US displayed large solid tumors with irregular
edges and uneven echo of solid tissues with cystic areas (12). MRI displayed a multicystic tumor of
a multilobulated shape located in the ovary. T1WI indicated
iso-signal and slightly low signal intensity, while T2WI displayed
high signal intensity. When cystic necrosis is combined in the
mass, it displays with a low signal on T1WI and a high signal on
T2WI. When bleeding is present, the T1WI and T2WI has high signal
intensity and the solid part of the tumor is obviously enhanced
(13).
In terms of the possible pathogenesis, three
different hypotheses have been made to explain the origin of
carcinosarcoma tissue. First, the collision theory revealed that
the malignant epithelial component and the sarcoma component are
derived from two different types of stem cell. According to the
combination theory, both the malignant epithelial component and the
sarcoma component came from pluripotent stem cells that have
divergent differentiation. Finally, the conversion theory suggested
that the sarcoma component of the tumor resulted from the evolution
of the carcinomatous component of the tumor (14,15).
Regarding the therapeutic strategy, as OCS is rare,
it is difficult to perform prospective clinical studies on
treatment. Small case series studies reported on the efficacy of
OCS treatment (16,17), but no detailed guidelines for the
diagnosis and treatment of OCS currently exist. At present, the
treatment of OCS mainly includes one therapy alone or a combination
of two or more therapies of debulking surgery, chemotherapy,
radiotherapy and targeted therapies (17-19).
In terms of surgical treatment, a comprehensive
staging operation should be performed in the early stage of the
tumor and cytoreductive surgery for advanced tumors is still the
most important treatment method. There are numerous factors that
may affect the prognosis of patients with OCS, such as residual
disease after surgery, disease stage (20,21)
and p53 overexpression (22).
Most of the retrospective studies indicated that
there may be better survival after optimal debulking surgery.
Satisfactory cytoreductive surgery is usually defined as the
diameter of the largest residual lesion being >1 cm after
surgery (23). It was reported
that cytoreductive surgery for tumors with residual tumor burden ≤1
cm was able to improve the prognosis (16). For patients with OCS of all stages,
patients with no visible disease had an overall survival (OS) of 57
months, vs. 32 months in those with ≤1 cm of residual disease and
those with >1 cm of residual disease. Furthermore, another
analysis of patients with stage 3 OCS was performed. It was
reported that patients with no visible tumor residue had a
significantly longer median OS (57 months) than those with ≤1 cm of
residual tumor (median OS of 31 months) and those with >1 cm of
residual tumor (median OS of 3 months) (23). Another study of 70 patients with
OCS indicated that patients with residual lesions <2 cm after
initial surgery had a longer survival time than those with ≥2 cm
residual tumor (24). Wang et
al (25) analyzed a total of
363 females with OCS and obtained no statistically significant
difference in OS between lymph node dissection and no lymph node
dissection for early-stage disease (hazard ratio, 0.88; 95% CI:
0.56-1.38), indicating that early lymph node dissection may not be
associated with prognosis. However, since only a small number of
studies have explored early lymph node dissection for OCS, further
research is required.
In addition, preoperative CA125 levels were raised
(>35 U/ml) in 93% of patients with OCS. The preoperative mean
CA125 level was 447 U/ml. Preoperative CA125 <75 U/ml may be
associated with a favorable survival prognosis (P=0.01) (16), which may also be an indicator for
the efficacy evaluation and follow-up of patients with OCS. This
was similar to the observation in the present study.
In terms of chemotherapy, as OCS is highly
malignant, there is a high risk of local and distant recurrences,
even for early-stage patients, and thus, adjuvant systemic therapy
is generally considered. However, due to the lack of large-scale
clinical studies, no consensus has been reached regarding the
first-line chemotherapy regimen for OCS. The latest National
Comprehensive Cancer Network Clinical Practice Guidelines in
Oncology for Ovarian Cancer suggest paclitaxel/carboplatin as the
first choice for chemotherapy of carcinosarcoma (26). The chemotherapy regimens mainly
refer to the treatment of epithelial ovarian cancer, including
carboplatin/paclitaxel (27),
ifosfamide/cisplatin (28) and
ifosfamide/paclitaxel (29). A
study by Tate Thigpen et al (30) reported on 136 patients with OCS who
received cisplatin (50 mg/m2) every 3 weeks until
disease progression or unacceptable toxicity occurred. The median
progression-free survival was 5.2 months. The overall median
survival time was 11.7 months. In 27 patients with OCS using the
combination therapy of radiotherapy, melphalan or chemotherapy
after surgical cytoreduction, the four patients in Stages I or II
were disease-free after at least 5 years. The median survival time
and 5-year survival rate for patients with Stage III or IV OCS was
18 months and 8%, respectively (31).
In the Surveillance, Epidemiology and End Results
database 1,763 patients with OCS were registered between 1988 and
2007; they had poor prognosis and it was reported that the
five-year survival rate for these patients for each stage was low
(Stage I, 65.2%; Stage II, 34.6%; Stage IIIC, 18.2%; and Stage IV,
11.2%) (32). However, these
studies did not account for chemotherapy application and
comorbidities. For 2,886 female patients with chemotherapy for OCS
in the National Cancer Data Base between 2003 and 2011, it was
indicated that the five-year survival rate for the entire
population was 26.63%. However, with the increase in the stage, the
survival prognosis also deteriorated (5-year survival rate for
Stage I, 59.07%; Stage II, 46.78%; Stage III, 20.14%; and Stage IV,
13.68%) (19). Thus, these studies
may suggest that platinum-based combination chemotherapy followed
by surgery is associated with better survival.
Adjuvant radiotherapy may have a better effect in
terms of local control. The survival rate of patients with combined
radiotherapy and chemotherapy after surgery may be higher than that
of patients with radiotherapy or chemotherapy alone. Adjuvant
radiotherapy (external beam irradiation and/or vaginal
brachytherapy) was not able to improve OS but it may decrease local
recurrences (33). A previous
study reported a case of recurrence of OCS with a large pelvic mass
(14 cm) after surgery. After chemotherapy combined with lattice
radiation therapy, the tumor size was significantly reduced with
favorable clinical and imaging-based follow-up prognosis for >4
years (34).
Although cytoreductive surgery is the most important
treatment for OCS, due to the high recurrence rate and the poor
survival, it is necessary to determine effective adjuvant
therapies. In recent years, targeted therapy has gradually been
regarded as an important way to cause tumor cell death whilst
minimizing injury to healthy cells. Numerous studies have explored
molecular biomarkers that may be utilized for targeted therapy.
Numerous studies suggested that human EGFR-2 (HER2)/neu may be a
potential target for immunotherapy in gynecological
carcinosarcomas. In OCS refractory to salvage chemotherapy,
HER2/neu expression was detected in two primary OCS cell lines,
which identified the amplification of the c-erbB2 gene by
fluorescent in situ hybridization analysis and high
sensitivity to antibody-dependent cell-mediated cytotoxicity (mean
killing rate, 45.6%; range, 32.3-72.6%) (35). SYD985 is a novel duocarmycin-based
HER2-targeting antibody-drug conjugate, which caused efficient
bystander killing of HER2/neu 0/1+ tumor cells mixed with HER2/neu
3+ cells, suggesting antitumor activity in OCS with HER2/neu
expression (36).
In addition, a study by Zhu et al (18) estimated the expression of
programmed death ligand 1 (PD-L1) and intratumoral CD8+
T lymphocytes from 19 OCS cases undergoing primary surgery and
determined that positive tumoral CD8+ T lymphocytes and
mesenchymal PD-L1-negative expression was associated with favorable
survival in OCS. Solitomab is an epithelial cell-adhesion molecule
(EpCAM)/CD3 bispecific antibody construct, which is highly active
against OCS cell lines in vitro. Ex vivo incubation
of autologous tumor-associated-T cells with EpCAM-expressing
malignant cells in pleural effusion with solitomab resulted in a
significant increase in T-cell proliferation of both
CD4+ and CD8+ T cells, as well as a reduction
in the number of viable OCS cells in the exudate (37). HRS7 is a humanized monoclonal
anti-trophoblast cell-surface antigen-2 (Trop-2) antibody, which
may represent a potential effective treatment option for patients
with OCS with treatment-refractory carcinosarcomas overexpressing
human Trop-2(17), which was
associated with increased tumor invasiveness and reduced OS of
patients with carcinomas (38).
Thus, targeted therapy focusing on inhibiting molecular
abnormalities or pathways may be an attractive and novel approach
for residual or drug-resistant OCS of refractory chemotherapy.
The present study has certain limitations. The
sample size of the present study was only one. Furthermore, the
removed part of the ovarian lesions and mesenteric neoplasms was
not photographed. Molecular characterization of OCS may indicate
both targetable alterations and predictive biomarkers of response
and there was a lack of detailed molecular analysis of OCS.
In conclusion, there are still no specific clinical
manifestations and serological detection indicators for OCS. The
present study showcased that in patients with OCS undergoing
cytoreduction surgery, adjuvant platinum-based chemotherapy and
targeted therapy may be effective. Owing to the rarity of OCS, in
the future, more studies will be required to explore better
treatment options to improve the prognosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF designed and performed the study. She treated the
patient, wrote the manuscript, performed the literature search and
made critical revisions. The author has read and approved the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
As it is a case report, ethics approval is not
applicable.
Patient consent for publication
Written informed consent to publish the case,
including images and clinical details, was obtained from the
patient.
Competing interests
The author declares that she has no competing
interests.
References
|
1
|
del Carmen MG, Birrer M and Schorge JO:
Carcinosarcoma of the ovary: A review of the literature. Gynecol
Oncol. 125:271–277. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Le T, Krepart GV, Lotocki RJ and Heywood
MS: Malignant mixed mesodermal ovarian tumor treatment and
prognosis: A 20-year experience. Gynecol Oncol. 65:237–240.
1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trento M, Munari G, Carraro V, Lanza C,
Salmaso R, Pizzi S, Santoro L, Chiarelli S, Dal Santo L, Nardelli
GB, et al: Mutational and immunophenotypic profiling of a series of
8 Tubo-ovarian carcinosarcomas revealed a monoclonal origin of the
disease. Int J Gynecol Pathol. 39:305–312. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Barnholtz-Sloan JS, Morris R, Malone JM Jr
and Munkarah AR: Survival of women diagnosed with malignant, mixed
mullerian tumors of the ovary (OMMMT). Gynecol Oncol. 93:506–512.
2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duska LR, Garrett A, Eltabbakh GH, Oliva
E, Penson R and Fuller AF: Paclitaxel and platinum chemotherapy for
malignant mixed müllerian tumors of the ovary. Gynecol Oncol.
85:459–463. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang Y, Li XM, Chen SG, Deng J, Lei Y, Li
WJ, Zhang HZ, Zhang H, Li D and Xie P: Application of antibodies
against Borna disease virus phosphoprotein and nucleoprotein on
paraffin sections. Mol Med Rep. 17:5416–5422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pinheiro C, Roque R, Adriano A, Mendes P,
Praça M, Reis I, Pereira T, Srebotnik Kirbis I and André S:
Optimization of immunocytochemistry in cytology: comparison of two
protocols for fixation and preservation on cytospin and smear
preparations. Cytopathology. 26:38–43. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stover EH, Fuh K, Konstantinopoulos PA,
Matulonis UA and Liu JF: Clinical assays for assessment of
homologous recombination DNA repair deficiency. Gynecol Oncol.
159:887–898. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van Warmerdam LJ, Rodenhuis S, ten Bokkel
Huinink WW, Maes RA and Beijnen JH: The use of the Calvert formula
to determine the optimal carboplatin dosage. J Cancer Res Clin
Oncol. 121:478–486. 1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pankaj S, Nazneen S, Kumari A, Kumari S,
Choudhary V and Roy VK: A rare tumor of the ovary: carcinosarcoma
report and review of literature. J Obstet Gynaecol India.
66:648–650. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McCluggage WG, Lioe TF, McClelland HR and
Lamki H: Rhabdomyosarcoma of the uterus: report of two cases,
including one of the spindle cell variant. Int J Gynecol Cancer.
12:128–132. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ciccarone F, Biscione A, Moro F,
Fischerova D, Savelli L, Munaretto M, Jokubkiene L, Sladkevicius P,
Chiappa V, Fruscio R, et al: Imaging in gynecological disease:
clinical and ultrasound characteristics of ovarian carcinosarcomas.
Ultrasound Obstet Gynecol. 59:241–247. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saida T, Mori K, Tanaka YO, Sakai M, Amano
T, Kikuchi S, Masuoka S, Yoshida M, Masumoto T, Satoh T, et al:
Carcinosarcoma of the ovary: MR and clinical findings compared with
high-grade serous carcinoma. Jpn J Radiol. 39:357–366.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dasgupta S, Bose D, Bhattacharyya NK and
Biswas PK: Carcinosarcoma of ovary with its various
immunohistochemical expression: Report of a rare case. J Cancer Res
Ther. 11(1022)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Carnevali IW, Cimetti L, Sahnane N, Libera
L, Cavallero A, Formenti G, Riva C and Tibiletti MG: Two cases of
carcinosarcomas of the ovary involved in hereditary cancer
syndromes. Int J Gynecol Pathol. 36:64–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sood AK, Sorosky JI, Gelder MS, Buller RE,
Anderson B, Wilkinson EJ, Benda JA and Morgan LS: Primary ovarian
sarcoma: Analysis of prognostic variables and the role of surgical
cytoreduction. Cancer. 82:1731–1737. 1998.PubMed/NCBI
|
|
17
|
Raji R, Guzzo F, Carrara L, Varughese J,
Cocco E, Bellone S, Betti M, Todeschini P, Gasparrini S, Ratner E,
et al: Uterine and ovarian carcinosarcomas overexpressing Trop-2
are sensitive to hRS7, a humanized anti-Trop-2 antibody. J Exp Clin
Cancer Res. 30(106)2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu J, Wen H, Ju X, Bi R, Zuo W and Wu X:
Clinical significance of programmed death Ligand-1 and
intra-tumoral CD8+ T lymphocytes in ovarian carcinosarcoma. PLoS
One. 12(e0170879)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rauh-Hain JA, Gonzalez R, Bregar AJ,
Clemmer J, Hernández-Blanquisett A, Clark RM, Schorge JO and Del
Carmen MG: Patterns of care, predictors and outcomes of
chemotherapy for ovarian carcinosarcoma: A national cancer database
analysis. Gynecol Oncol. 142:38–43. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paulsson G, Andersson S and Sorbe B: A
population-based series of ovarian carcinosarcomas with long-term
follow-up. Anticancer Res. 33:1003–1008. 2013.PubMed/NCBI
|
|
21
|
Ariyoshi K, Kawauchi S, Kaku T, Nakano H
and Tsuneyoshi M: Prognostic factors in ovarian carcinosarcoma: A
clinicopathological and immunohistochemical analysis of 23 cases.
Histopathology. 37:427–436. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zorzou MP, Markaki S, Rodolakis A,
Kastritis E, Bozas G, Dimopoulos MA and Papadimitriou CA:
Clinicopathological features of ovarian carcinosarcomas: A single
institution experience. Gynecol Oncol. 96:136–142. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Doo DW, Erickson BK, Arend RC, Conner MG,
Huh WK and Leath CA III: Radical surgical cytoreduction in the
treatment of ovarian carcinosarcoma. Gynecol Oncol. 133:234–237.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brown E, Stewart M, Rye T, Al-Nafussi A,
Williams AR, Bradburn M, Smyth J and Gabra H: Carcinosarcoma of the
ovary: 19 years of prospective data from a single center. Cancer.
100:2148–2153. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang WP, Li N, Zhang YY, Gao YT, Sun YC,
Ge L and Wu LY: Prognostic significance of lymph node metastasis
and lymphadenectomy in early-stage ovarian carcinosarcoma. Cancer
Manag Res. 10:1959–1968. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M,
Eisenhauer EL, et al: Ovarian Cancer, Version 2.2020, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
19:191–226. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rutledge TL, Gold MA, McMeekin DS, Huh WK,
Powell MA, Lewin SN, Mutch DG, Johnson GA, Walker JL and Mannel RS:
Carcinosarcoma of the ovary-a case series. Gynecol Oncol.
100:128–132. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Silasi DA, Illuzzi JL, Kelly MG,
Rutherford TJ, Mor G, Azodi M and Schwartz PE: Carcinosarcoma of
the ovary. Int J Gynecol Cancer. 18:22–29. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brackmann M, Stasenko M, Uppal S, Erba J,
Reynolds RK and McLean K: Comparison of first-line chemotherapy
regimens for ovarian carcinosarcoma: A single institution case
series and review of the literature. BMC Cancer.
18(172)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tate Thigpen J, Blessing JA, DeGeest K,
Look KY and Homesley HD: Cisplatin as initial chemotherapy in
ovarian carcinosarcomas: A gynecologic oncology group study.
Gynecol Oncol. 93:336–339. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Muntz HG, Jones MA, Goff BA, Fuller AF Jr,
Nikrui N, Rice LW and Tarraza HM: Malignant mixed müllerian tumors
of the ovary: experience with surgical cytoreduction and
combination chemotherapy. Cancer. 76:1209–1213. 1995.PubMed/NCBI View Article : Google Scholar
|
|
32
|
George EM, Herzog TJ, Neugut AI, Lu YS,
Burke WM, Lewin SN, Hershman DL and Wright JD: Carcinosarcoma of
the ovary: natural history, patterns of treatment, and outcome.
Gynecol Oncol. 131:42–45. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Berton-Rigaud D, Devouassoux-Shisheboran
M, Ledermann JA, Leitao MM, Powell MA, Poveda A, Beale P, Glasspool
RM, Creutzberg CL, Harter P, et al: Gynecologic cancer InterGroup
(GCIG) consensus review for uterine and ovarian carcinosarcoma. Int
J Gynecol Cancer. 24 (9 Suppl 3):S55–S60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Blanco Suarez JM, Amendola BE, Perez N,
Amendola M and Wu X: The use of lattice radiation therapy (LRT) in
the treatment of bulky tumors: A case report of a large metastatic
mixed mullerian ovarian tumor. Cureus. 7(e389)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guzzo F, Bellone S, Buza N, Hui P, Carrara
L, Varughese J, Cocco E, Betti M, Todeschini P, Gasparrini S, et
al: HER2/neu as a potential target for immunotherapy in gynecologic
carcinosarcomas. Int J Gynecol Pathol. 31:211–221. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Menderes G, Bonazzoli E, Bellone S, Black
J, Predolini F, Pettinella F, Masserdotti A, Zammataro L, Altwerger
G, Buza N, et al: SYD985, a novel duocarmycin-based HER2-targeting
antibody-drug conjugate, shows antitumor activity in uterine and
ovarian carcinosarcoma with HER2/Neu expression. Clin Cancer Res.
23:5836–5845. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ferrari F, Bellone S, Black J, Schwab CL,
Lopez S, Cocco E, Bonazzoli E, Predolini F, Menderes G, Litkouhi B,
et al: Solitomab, an EpCAM/CD3 bispecific antibody construct
(BiTE®), is highly active against primary uterine and ovarian
carcinosarcoma cell lines in vitro. J Exp Clin Cancer Res.
34(123)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cubas R, Zhang S, Li M, Chen C and Yao Q:
Trop2 expression contributes to tumor pathogenesis by activating
the ERK MAPK pathway. Mol Cancer. 9(253)2010.PubMed/NCBI View Article : Google Scholar
|