Introduction
As one of the most common types of gastrointestinal
cancer, esophageal cancer has shown an increasing incidence in
previous years (1,2). The detection rate of esophageal
cancer has increased from 3.79% in 2016 to 5.42% in 2020(3). Moreover, the overall survival rate of
esophageal cancer is 15-25% (4).
To date, several available treatments, including chemotherapy,
radiotherapy, surgery and combined therapy, have improved the
survival rate of esophageal cancer (5,6).
Nevertheless, little improvement has been achieved in its mortality
(7). To the best of our knowledge,
the underlying mechanism and progression of esophageal cancer have
not been fully determined.
Angiopoietin-2 (ANGPT2) is a growth factor that
regulates vessel growth and maturation during angiogenesis
(8,9). ANGPT2 expression is associated with
tumor metastasis in numerous types of human cancer (10). For example, Urosevic et al
(11) demonstrated that
upregulation of ANGPT2 mediates liver metastasis in colon cancer.
Moreover, ANGPT2 upregulation is associated with poor prognosis of
patients with non-small cell lung cancer (12). However, the role of ANGPT2 in
esophageal cancer development remains unclear.
Homeobox B5 (HOXB5), a member of the homeobox gene
family, participates in the progression of multiple types of
cancer, such as non-small cell lung (13) and gastric cancer (14) and head and neck squamous cell
carcinoma (15). HOXB5 is reported
to regulate a number of cancer cell functions, such as pancreatic,
colorectal cancer, breast cancer and so on, and its overexpression
is associated with cancer progression and poor patient prognosis
(16-18).
Nevertheless, the role of HOXB5 in esophageal cancer remains
unclear; therefore, the present study aimed to investigate its
underlying mechanism in the malignant progression of esophageal
cancer.
Materials and methods
Cell culture and transfection
Human normal esophageal epithelial cell line (HEEC;
cat. no. CP-H031) was obtained from Procell Life Science &
Technology Co., Ltd. Human umbilical vein endothelial cells
(HUVECs; cat. no. 3571773) and esophageal cancer cell lines,
including KYSE-70 (squamous carcinoma cells; cat. no. ACC 363) and
KYSE-30 (squamous carcinoma cells; cat. no. ACC 351), were obtained
from BioVector NTCC Inc. and EC-9706 cell line (squamous carcinoma
cells; cat. no. ZY6226) was purchased from Shanghai Zeye
Biotechnology Co., Ltd. HEECs and HUVECs were immortalized cell
lines. Dubelcco's modified eagle medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin was used to culture cells at 37˚C with 5%
CO2.
To knock down ANGPT2 and upregulate HOXB5 expression
in esophageal cancer cells, short hairpin RNA (shRNA) against
ANGPT2 (sh-ANGPT2#1 and sh-ANGPT2#2; 50 nM), pcDNA3.1-HOXB5 (2 µg),
as well as corresponding negative control (shRNA-NC; 50 nM) and
pcDNA3.1-NC (2 µg) were obtained from Genscript Biotech
Corporation. EC-9706 cells in logarithmic growth phase were
inoculated into 6-well plates (6x104 cells/well).
EC-9706 cells at 90% confluence were transfected using
Lipofectamine 2000® transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) for 24 h at 37˚C. Following 48 h
incubation, cells were collected for subsequent experiments.
Sequence fragments that interfere with ANGPT2 are not be provided
as the company did not provide them.
Reverse transcription-quantitative PCR
(RT-qPCR)
EC-9706 cells were placed in a 6-well plate
(6x104 cells/well). Following transfection, total RNA
isolated from cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol was
reverse transcribed into complementary DNA using PrimeScript
reverse transcriptase (Takara Biotechnology Co., Ltd.).
Subsequently, qPCR was performed using SYBR Green Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) on the ABI
PRISM 7900 System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR conditions: 95˚C for 10 min for initial
denaturation, 40 cycles of denaturation 15 sec at 95˚C, annealing
30 sec at 60˚C and elongation 30 sec at 72˚C and final extension
for 5 min at 72˚C. Finally, the relative gene expression was
calculated via the 2-ΔΔCq method (19) and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) used as an endogenous control for HOXB5 and
ANGPT2. The following primers were used for qPCR: HOXB5 forward,
5'-AACTCCTTCTCGGGGCGTTAT-3' and reverse,
5'-CATCCCATTGTAATTGTAGCCGT-3'; ANGPT2 forward,
5'-AACTTTCGGAAGAGCATGGAC-3' and reverse,
5'-CGAGTCATCGTATTCGAGCGG-3' and GAPDH forward,
5'-AATGGGCAGCCGTTAGGAAA-3' and reverse
5'-GCGCCCAATACGACCAAATC-3'.
Western blot analysis
The extraction and quantification of total proteins
from cells were conducted with RIPA lysis buffer (Beyotime
Institute of Biotechnology) and BCA kit (Beyotime Institute of
Biotechnology), respectively. After being separated by 10%
SDS-PAGE, the proteins (30 µg/lane) were then transferred onto PVDF
membranes, as previously described (20). Membranes were blocked with 5%
non-fat milk for 2 h at room temperature and then incubated with
primary antibodies against ANGPT2 (1:1,000; cat. no. ab155106;
Abcam), HOXB5 (1:1,000; cat. no. ab109375; Abcam), E-cadherin
(1:10,000; cat. no. ab40772; Abcam), N-cadherin (1:5,000; cat. no.
ab76011; Abcam), Vimentin (1:1,000; cat. no. ab92547; Abcam),
phosphorylated (p)-ERK (1:1,000; cat. no. ab201015; Abcam), p-AKT
(1:1,000; cat. no. 9271; Cell Signaling Technology, Inc.), ERK
(1:1,000; cat. no. ab17942; Abcam), AKT (1:1,000; cat. no. 9272;
Cell Signaling Technology, Inc.) and GAPDH (1:10,000; cat. no.
ab181602; Abcam) at 4˚C overnight. Following primary antibody
incubation, membranes were incubated for 2 h at room temperature
with horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibody (1:20,000; cat. no. ab205718; Abcam). Finally,
the protein signals were detected using enhanced chemiluminescence
kit (Beyotime Institute of Biotechnology). ImageJ 1.50i software
(National Institutes of Health) was used to analyze the blots. All
results were verified using ≥3 independent experiments.
Cell Counting Kit (CCK)-8 assay
EC-9706 cells were inoculated into 96-well plates
(1.5x104 cells/well) and incubated for 24 h at 37˚C.
Subsequently, 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) was added into each well and cells were incubated
for another 3 h. The absorbance at 450 nm was measured using a
microplate reader (Thermo Fisher Scientific, Inc.).
EdU staining assay
EC-9706 cells seeded into 6-well plates
(6x104 cells/well) were incubated at 37˚C overnight.
After exposure to 50 µM EdU solution (Beyotime Institute off
Biotechnology), the cells were incubated at 37˚C for another 4 h.
Then, the working solution was removed, followed by digestion with
trypsin at 37˚C for 3 min, centrifugation at 1,500 x g for 10 min
at 4˚C and fixation with 4% paraformaldehyde for 15 min at room
temperature. Following permeation with 0.5% Triton X-100 at room
temperature for 10 min, the cells were incubated with Click
reaction solution in the dark for 30 min. The nuclei were
counterstained for 15 min at room temperature with 100 ng/ml DAPI.
Finally, the cells were observed under a fluorescence microscope
(Olympus Corporation; magnification, x200).
Wound healing assay
EC-9706 cells were plated in 6-well plates
(6x104 cells/well) and cultured to 90% confluence in
DMEM with 10% FBS at 37˚C for 48 h. To make a straight scratch in
the cell monolayer, a 200-µl pipette tip was applied. After washing
three times with PBS, the cells were then incubated in serum-free
DMEM for 48 h at 37˚C with 5% CO2 and imaged at 0 and 48
h using a light microscope. The migration rate was calculated based
on the formula: (Wound width at 0 h-wound width at 48 h)/wound
width at 0 h x 100%. The images of the scratch areas were processed
using ImageJ 1.50i software (National Institutes of Health).
Transwell assay
EC-9706 cells were inoculated into the upper chamber
(6x104 cells) containing serum-free DMEM of Transwell
plates (EMD Millipore), which were precoated with Matrigel (37˚C
for 30 min) and incubated at 37˚C for 24 h; complete medium with
10% FBS to the lower chamber of 6-well plates. After 24 h
migration, the fixation and staining of EC-9706 cells was performed
using 4% paraformaldehyde at room temperature and 0.1% crystal
violet at room temperature for 30 min each, respectively. The
images of invasion were captured and the number of invading cells
was counted using an inverted light microscope (Eclipse Ti2; Nikon
Corporation).
Colony formation assay
EC-9706 cells were plated in 6-well plates
(1x103 cells/well). After transfection, EC-9706 cells
were continuously cultured for two weeks at 37˚C in DMEM, which was
replaced every 3 days. Then, 4% paraformaldehyde was used to fix
the cell colonies for 20 min at room temperature, followed by
staining using Giemsa (Beyotime Biotechnology Institute) for 20 min
at a room temperature. The colonies containing >50 cells were
imaged using a COOLPIX S520 digital camera (Nikon) and the number
of clones was counted using Image J 1.50i software (National
Institutes of Health).
Tube formation analysis in esophageal
cancer cells
HUVEC cells (100 µl) were seeded into a precooled
96-well plate (1.5x104 cells/well) before addition of
100 µl/well Matrigel at 37˚C for 30 min. Following incubation at
37˚C for 24 h, the tube formation was monitored and imaged using an
inverted light microscope (IX70; Olympus Corporation). Five visual
fields were randomly selected and length of the lumen was
calculated using Image Pro Plus (version 6.0; Media Cybernetics,
Inc.).
Luciferase report assay
JASPAR database (jaspar.genereg.net/) was used to predict the binding
sites of HOXB5 and ANGPT2. Luciferase report assay was performed to
investigate the interaction between HOXB5 and ANGPT2 using
Luciferase Reporter System (Promega Corporation). The cloning
primers designed via Primer3Plus were as follows: ANGPT2 forward,
5'-GCATTTGCTGGAGGTCACAC-3' and reverse, 5'-AGCTGGAAGACATGCTCTGG-3'.
The 3'-untranslated region (UTR) of ANGPT2 containing the seed
sequence of wild-type (WT) or mutated (MUT) binding site of HOXB5
was cloned into pGL3 vectors (Promega Corporation) to generate
pGL3-ANGPT2-3'UTR-WT and MUT luciferase reporter plasmids.
Subsequently, the transfection of EC-9706 cells (2x105
cells/well) was performed with pGL3-based reporter constructs, as
previously described (21).
OPTI-MEM (49 µl; Gibco; Thermo Fisher Scientific, Inc.) was used to
dilute 1 µl Lipofectamine 2000® reagent (Invitrogen;
Thermo Fisher Scientific, Ltd.). Following 36 h transfection at
37˚C, Dual-Luciferase Reporter assay system (Promega Corporation)
was utilized to detect luciferase activity which was measured in
comparison with Renilla luciferase activity using luciferase
reporter assay substrate kit (Abcam).
Chromatin immunoprecipitation (CH-IP)
assay
Formaldehyde (1%; Sigma-Aldrich; Merck KGaA) was
used to crosslink EC-9706 cells for 10 min at 37˚C in PBS; the
reaction was terminated by adding glycine (Beijing Solarbio Science
& Technology Co., Ltd.). A total of 300 µl SDS lysis buffer (1%
SDS, 10 mM EDTA and 50 mM Tris-HCl pH 8.0) was used to lyse
2x106 cells at room temperature for 10 min. The lysed
cells were subjected to sonication (60 Hz) in ice water for 10 min.
The resulting sonicated fragments were 200-1,000 bp in length.
Following sonication, the samples were centrifuged at 13,000 x g
for 10 min at 4˚C. Subsequently, the supernatant (100 µg) were
pre-absorbed by 50 µl protein G beads and was incubated with
magnetic beads conjugated to 5 µg anti-ANGPT2 (1 µl/mg; cat. no.
ab276042; Abcam), anti-HOXB5 (1/100; cat. no. ab229345; Abcam) or
anti-rabbit IgG antibodies (1/100; cat. no. ab172730; Abcam) at 4˚C
for 2 h. The magnetic beads were then rinsed four times with lysis
buffer, twice with LiCl buffer, and three times with Tris-EDTA
buffer. The bound immunocomplex was eluted by adding 300 µl of
fresh elution buffer [10 mM Tris; 1 mM EDTA, (pH 8.0)].
Subsequently, 20 µl 5 M NaCl was mixed with the eluted product,
which was incubated at 65˚C overnight to reverse the crosslinking
and the purification of immunoprecipitated DNA was conducted using
a CH-IP DNA purification kit (cat. no. D0033; Beyotime
Biotechnology Institute) and the enrichment of ANGPT2 was detected
using RT-qPCR. Primer sequences were as follows: ANGPT2 forward,
5'-TGTCCAGAACCTTGGTGGAAT-3' and reverse,
5'-AGTTCTGAGTATTGTGGCAGC-3' and GAPDH forward,
5'-AATGGGCAGCCGTTAGGAAA-3' and reverse
5'-GCGCCCAATACGACCAAATC-3'.
Bioinformatics analysis
Gene Expression Profiling Interactive (GEPIA)
Analysis 2 database (gepia2.cancer-pku.cn/#index) was used to explore the
expression of ANGPT2 and HOXB5 in esophageal cancer and the
association between the expression levels of candidate genes and
survival rate for patients with esophageal cancer. The key words
ANGPT2 and HOXB5 were utilized as input. ‘Expression DIY’ and
‘Survival analysis’ in the Expression Analysis function were chosen
for the analysis of The Cancer Genome Atlas and Genotype-Tissue
expression data.
Statistical analysis
All experiments were repeated three times. All data
collected from experiments are presented as mean ± standard
deviation and were analyzed with SPSS 11.0 software (SPSS, Inc.).
Unpaired Student's t-test was used to analyze differences between 2
groups and one-way analysis of variance followed by Tukey's post
hoc test was adopted to analyze differences among ≥3 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ANGPT2 is upregulated in esophageal
cancer tissue and cell lines and is associated with poor patient
prognosis
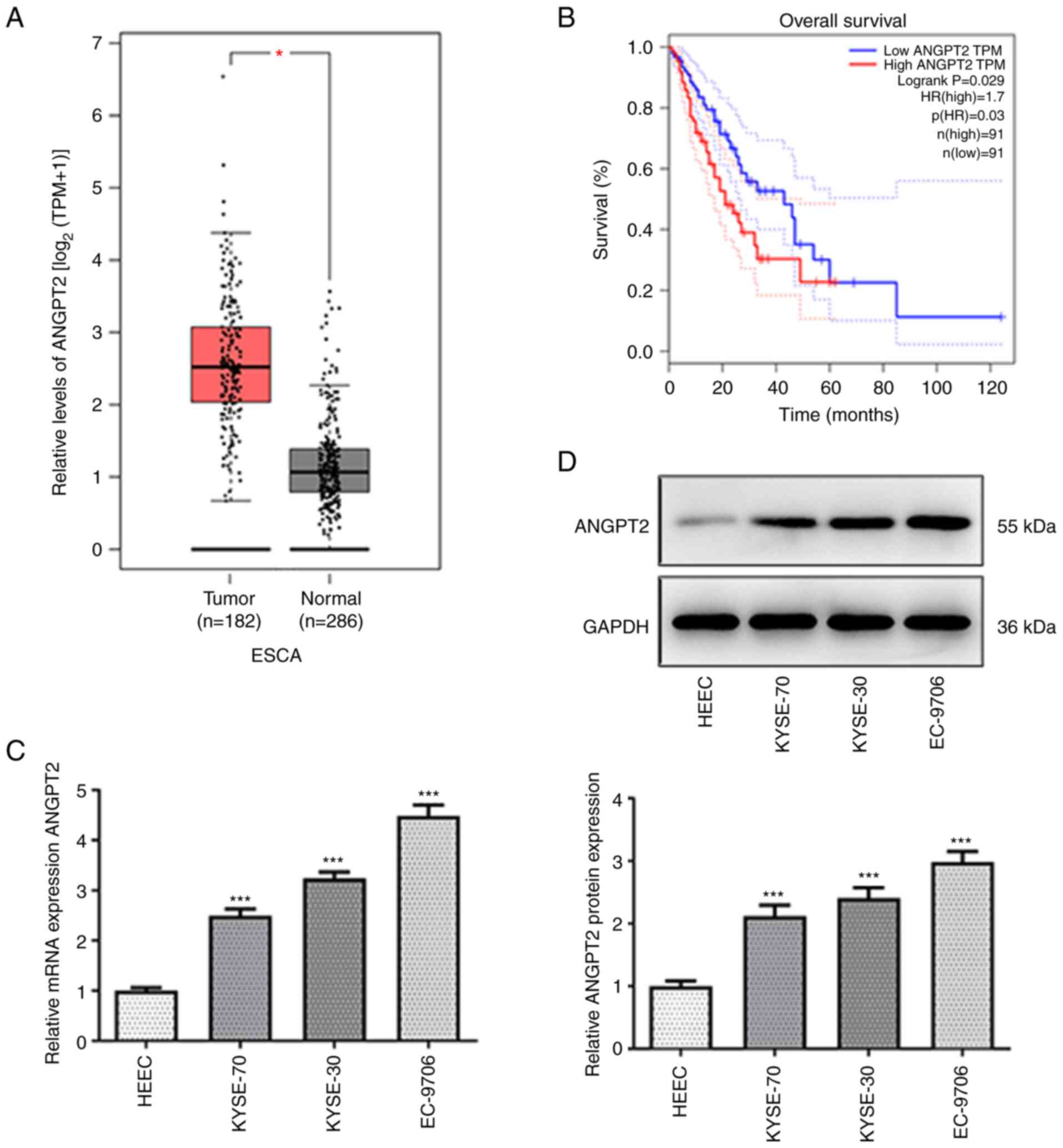
According to GEPIA database, ANGPT2 was upregulated
in patients with esophageal cancer (Fig. 1A). Data from GEPIA database also
demonstrated that ANGPT2 upregulation was significantly associated
with low overall survival rate of patients with esophageal cancer
(Fig. 1B). Compared with HEEC,
mRNA and protein expression levels of ANGPT2 were enhanced in
KYSE-70, KYSE-30 and EC-9706 cells (Fig. 1C and D). EC-9706 cells had the highest
expression of ANGPT2 and were therefore selected for subsequent
experiments. The aforementioned results suggested that ANGPT2 was
upregulated in esophageal cancer cells and this led to lower
overall survival rate.
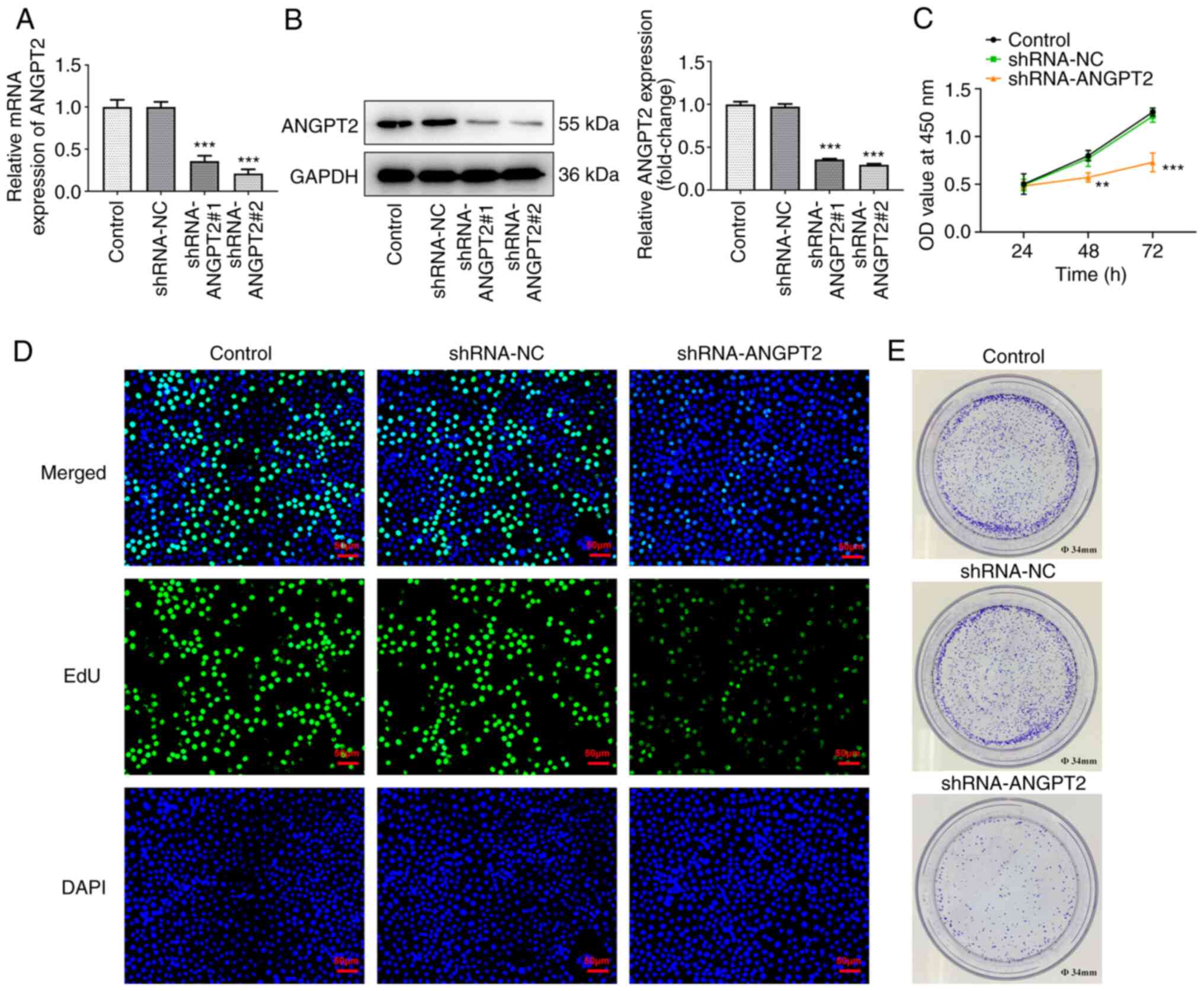
ANGPT2 silencing inhibits
proliferation of esophageal cancer
To knock down ANGPT2, shRNA targeting ANGPT2 was
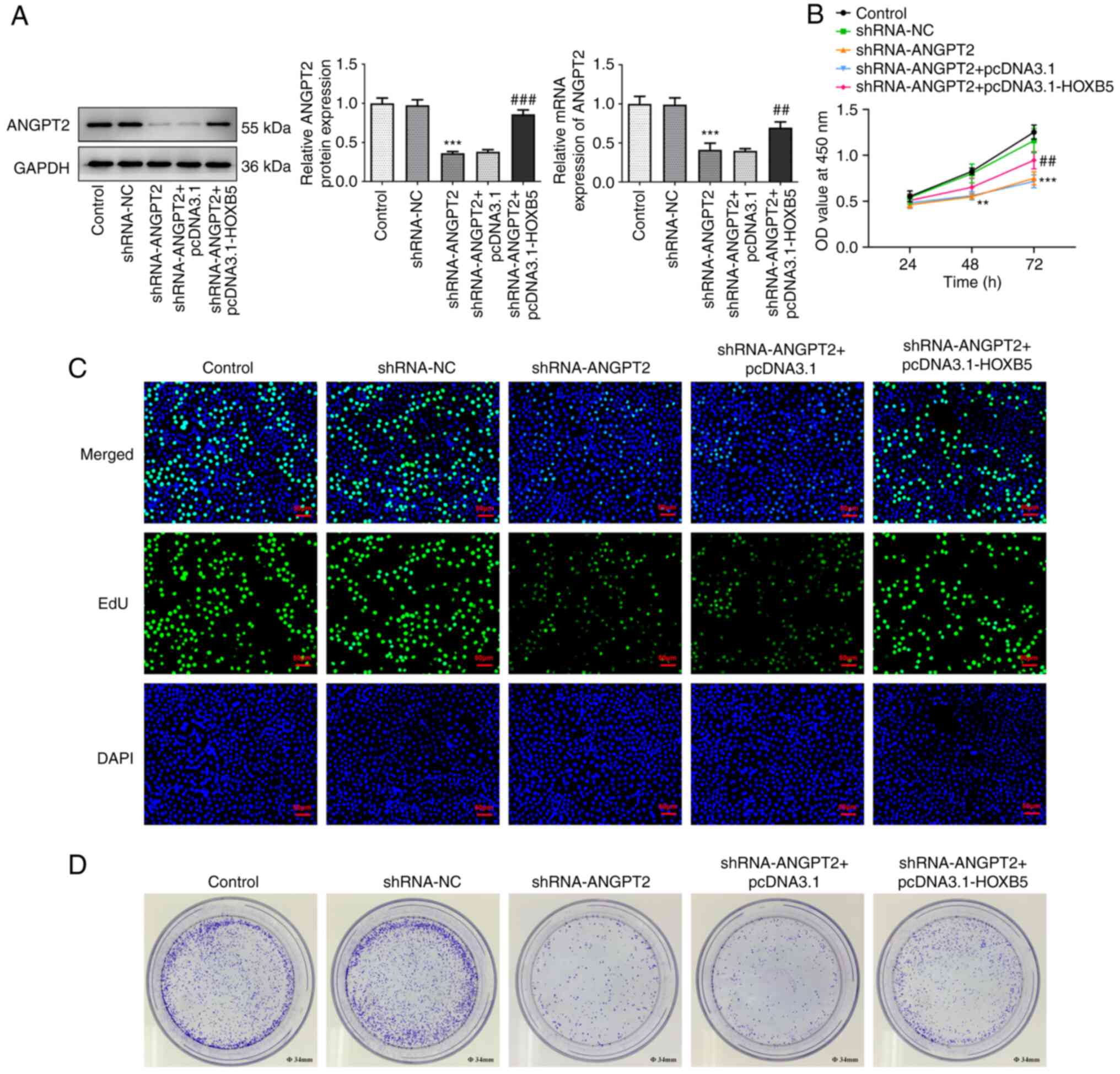
used to transfect EC-9706. RT-qPCR and western blot analysis
indicated that the mRNA and protein expression levels of ANGPT2 in
EC-9706 cells were significantly decreased following transfection
with sh-ANGPT2 plasmids (Fig. 2A
and B). EC-9706 cells transfected
with shRNA-ANGPT2#2 showed low ANGPT2 expression compared with
shRNA-ANGPT2#1. Therefore, subsequent experiments were performed on
EC-9706 cells transfected with shRNA-ANGPT2#2.
Viability, proliferation and colony formation of
esophageal cancer cells were evaluated. The viability of EC-9706
cells was significantly decreased at 48 and 72 h following
transfection with shRNA-ANGPT2 (Fig.
2C). Likewise, ANGPT2 silencing had suppressive effects on
proliferation and colony formation of EC-9706 cells (Fig. 2D and E).
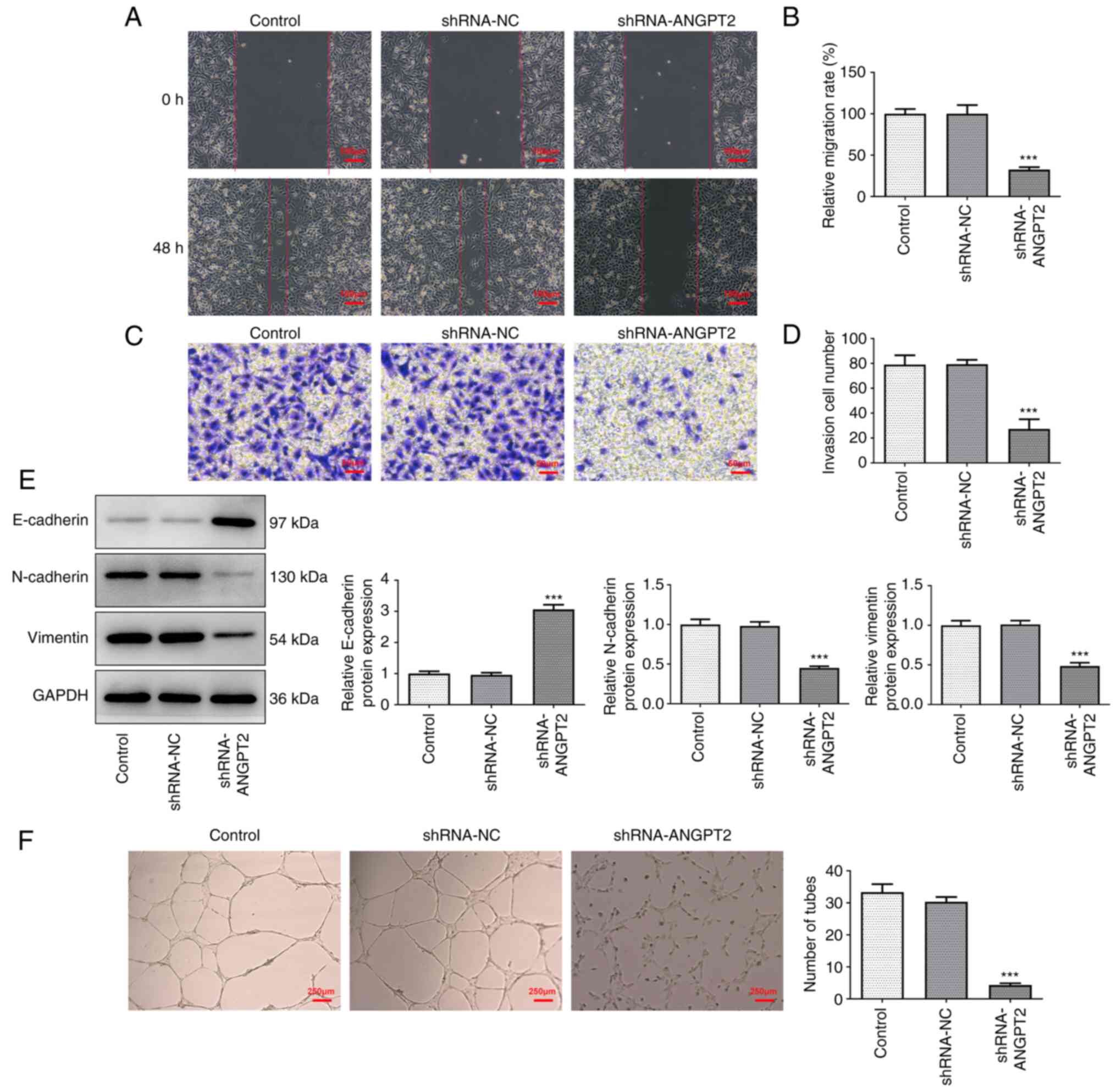
ANGPT2 silencing inhibits metastasis
and angiogenesis of esophageal cancer cells
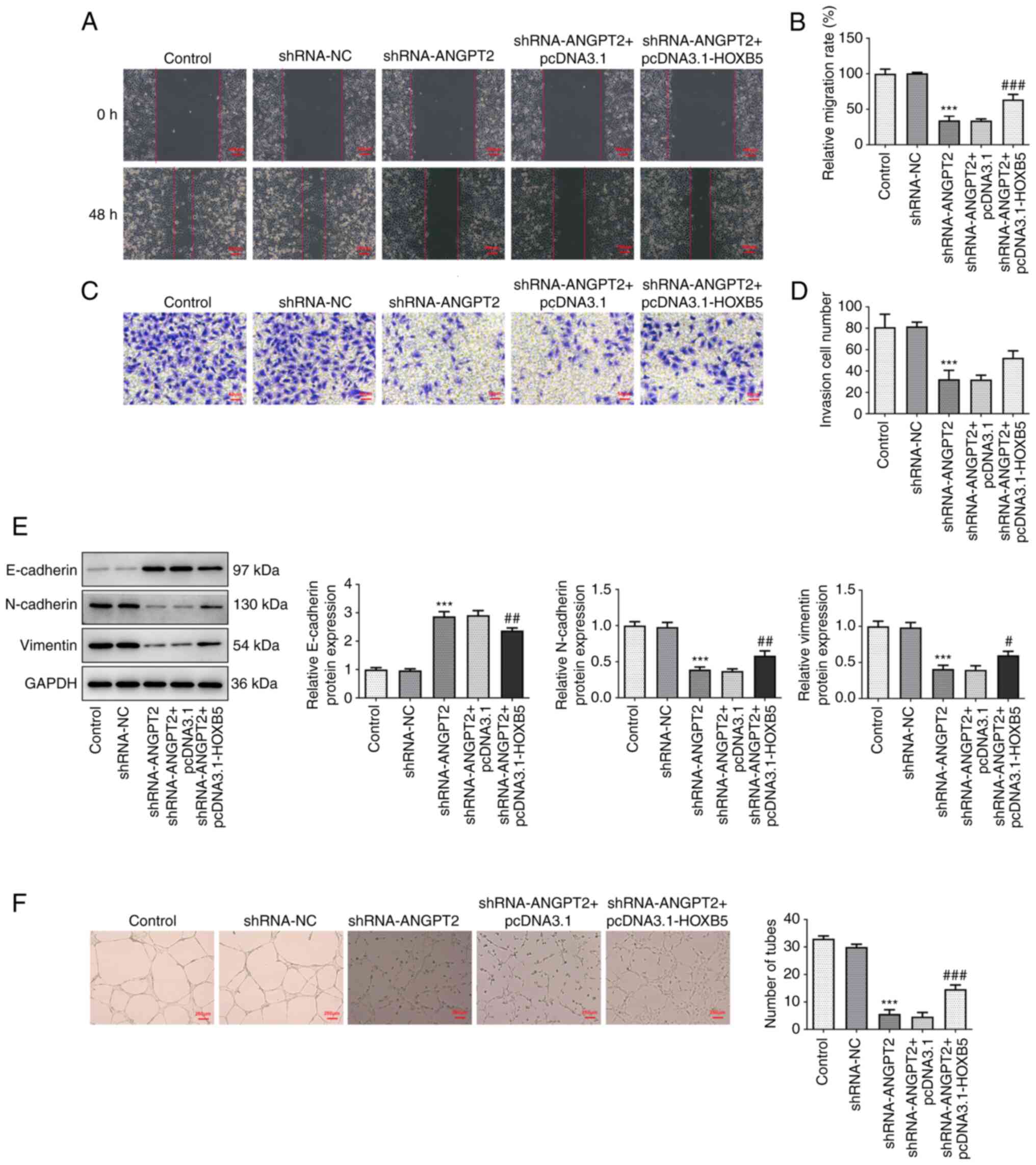
Wound healing and Transwell assays were performed to
investigate the migration and invasion of ANGPT2-silenced EC-9706
cells. The relative migration rate and number of invaded EC-9706
cells were significantly decreased following transfection with
shRNA-ANGPT2, revealing that ANGPT2 silencing inhibited metastasis
of esophageal cancer cells (Fig.
3A-D). In addition, the expression levels of
epithelial-mesenchymal transition (EMT)-associated proteins and
biomarkers (Vimentin) were measured by western blot assay. ANGPT2
silencing upregulated E-cadherin but downregulated N-cadherin and
Vimentin expression (Fig. 3E).
Moreover, tube formation analysis indicated that the number of
tubes was decreased following transfection with shRNA-ANGPT2,
indicating that angiogenesis of esophageal cancer cells was
inhibited by ANGPT2 silencing (Fig.
3F).
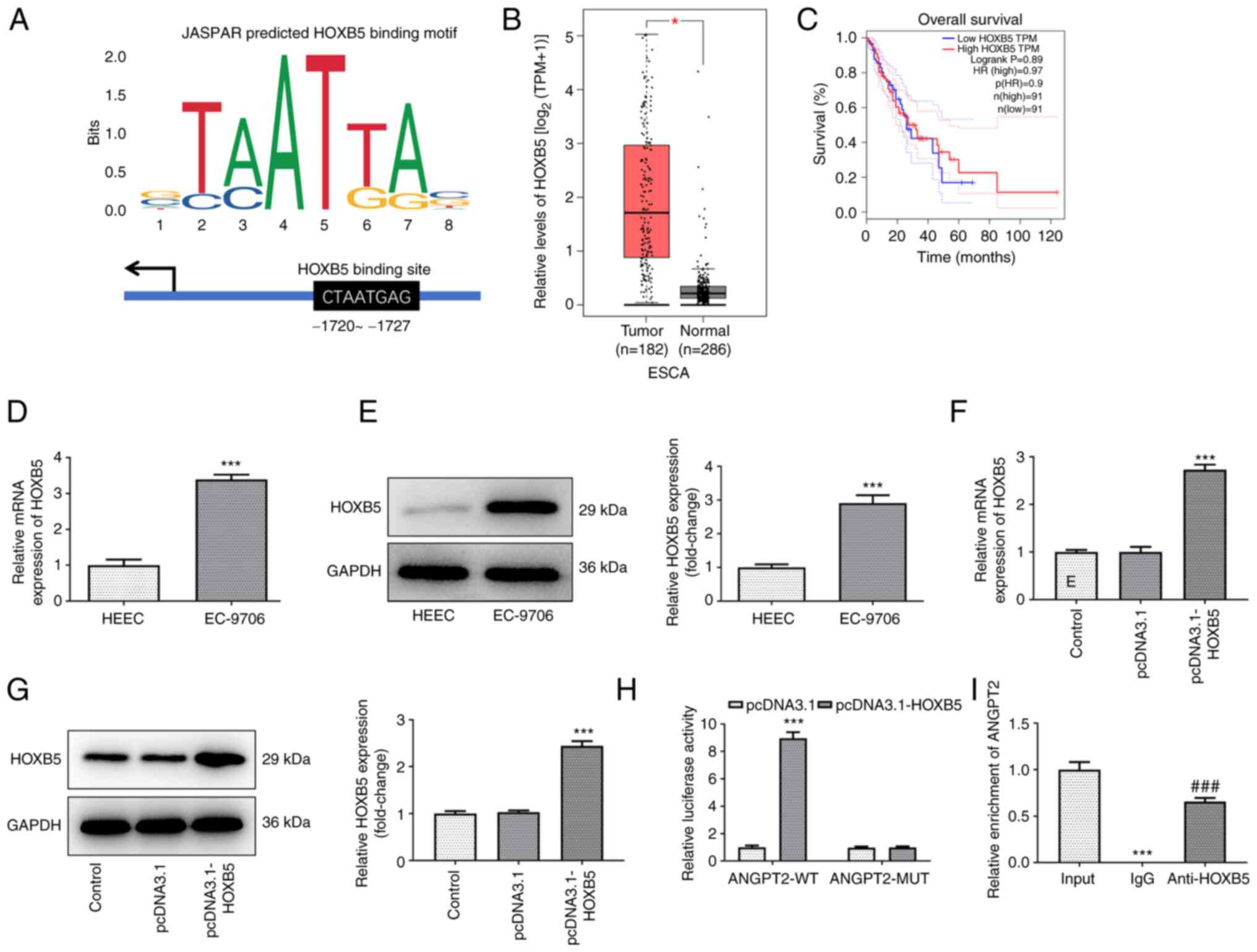
HOXB5 transcription activates
ANGPT2
JASPAR database was used to predict the binding
sites of transcription factor HOXB5 and ANGPT2 promoters (Fig. 4A). According to GEPIA database,
HOXB5 had a high expression in tissue of patients with esophageal
cancer compared with normal tissue, while its upregulation had no
significant association with low overall survival rate of patients
with esophageal cancer (Fig. 4B
and C). In addition, the mRNA and
protein levels of HOXB5 in EC-9706 cells were increased compared
with those in HEECs (Fig. 4D and
E).
To increase expression of HOXB5, EC-9706 cells were
transfected with pcDNA3.1-HOXB5 plasmids. Both mRNA and protein
levels of HOXB5 were enhanced in HOXB5-overexpressing EC-9706 cells
compared with pcDNA3.1 group (Fig.
4F and G). Moreover, ANGPT2
promoters were activated by the transcription factor HOXB5, as
suggested by the strong luciferase activity observed in the
ANGPT2-WT + pcDNA3.1-HOXB5 group (Fig.
4H). To validate the binding ability of HOXB5 and ANGPT2
promoters, CH-IP assay was performed with HOXB5 antibody. ANGPT2
was enriched in anti-HOXB5, indicating that HOXB5 bound to ANGPT2
promoters (Fig. 4I).
Overexpression of transcription factor
HOXB5 reverses effects of ANGPT2 silencing on esophageal cancer
cells
The mRNA and protein expression levels of ANGPT2,
which were decreased in the shRNA-ANGPT2 group, were partly
recovered in the shRNA-ANGPT2 + pcDNA3.1-HOXB5 group (Fig. 5A). The viability, proliferation and
colony formation, which were decreased in the shRNA-ANGPT2 group,
were partially restored in the shRNA-ANGPT2 + pcDNA3.1-HOXB5 group,
revealing that HOXB5 overexpression could reverse the effect of
ANGPT2 silencing (Fig. 5B-D).
The migration and invasion of EC-9706 cells were
diminished following transfection with shRNA-ANGPT2; this effect
was reversed by HOXB5 overexpression (Fig. 6A-D). Moreover, ANGPT2 silencing
upregulated E-cadherin expression and downregulated the expression
levels of N-cadherin and Vimentin, whereas HOXB5 overexpression
partially abolished the aforementioned effects of ANGPT2 silencing
(Fig. 6E). Furthermore, the
decreased number of tubes in ANGPT2-silenced EC-9706 cells was
increased following HOXB5 overexpression, suggesting that HOXB5
overexpression enhanced angiogenesis of esophageal cancer cells
(Fig. 6F).
Overexpression of transcription factor
HOXB5 abolishes the inactivation of ERK/AKT signaling pathway
induced by ANGPT2 silencing
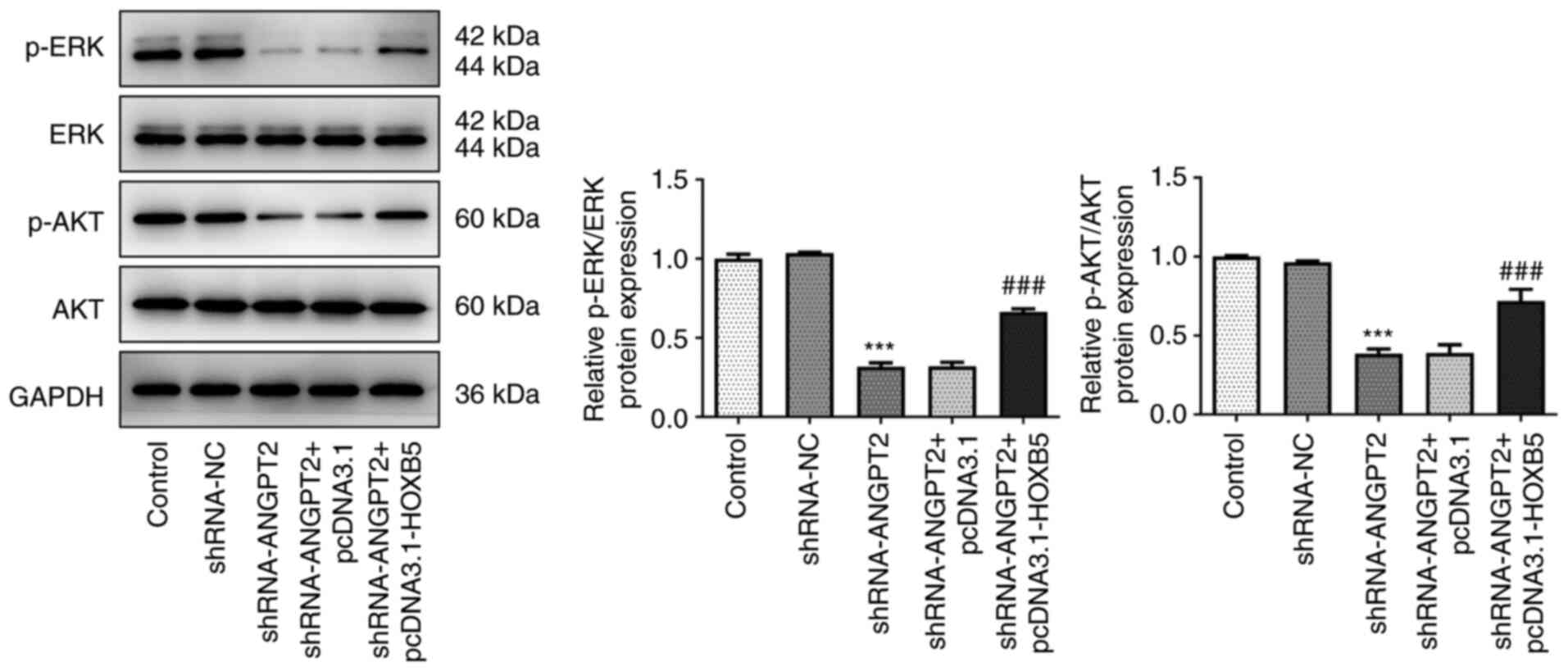
To understand the effects of ANGPT2 silencing on
ERK/AKT signaling pathway, the expression levels of ERK/AKT
signaling pathway-associated proteins, such as p-ERK, p-AKT, ERK
and AKT, were measured using western blotting. The decreased
expression levels of p-ERK and p-AKT in ANGPT2-silenced EC-9706
cells were upregulated after overexpressing HOXB5. However,
expression levels of ERK and AKT remained unchanged following
transfection with shRNA-ANGPT2 and pcDNA3.1-HOXB5 (Fig. 7). The aforementioned results
indicated that HOXB5 overexpression blocked the inhibitory effect
of ANGPT2 silencing on ERK/AKT signaling pathway.
Discussion
To the best of our knowledge, the present study is
the first to investigate the role of HOXB5 and ANGPT2 in the
malignant progression of esophageal cancer. Firstly, the expression
levels of HOXB5 and ANGPT2 in esophageal cancer cells were
detected. Subsequently, functional experiments were conducted to
explore the effects of ANGPT2 silencing on the proliferation and
colony formation of esophageal cancer cells. In the present study,
ANGPT2 and HOXB5 were upregulated in esophageal cancer cells;
ANGPT2 upregulation was significantly associated with the low
overall survival rate of patients with esophageal cancer. Moreover,
ANGPT2 silencing inhibited the viability, proliferation, colony
formation, migration, invasion and angiogenesis of esophageal
cancer cells. In addition, the HOXB5 transcription factor was
demonstrated to activate ANGPT2, whereas HOXB5 overexpression
reversed the effect of ANGPT2 silencing on the proliferation,
metastasis and angiogenesis of esophageal cancer cells.
Furthermore, the inhibition of the ERK/AKT signaling pathway caused
by ANGPT2 silencing was also reversed by HOXB5 overexpression.
In recent years, a number of studies have been
performed to explore the role of ANGPT2 in cancer (22-24).
For example, miR-145-5p overexpression exerts inhibitory effects on
the proliferation, migration and invasion of gastric cancer cells
via the ANGPT2 axis (22). In
addition, the insulin gene enhancer protein ISL2 induces
angiogenesis to promote malignant transformation via regulating
ANGPT2(23). Moreover, ANGPT2 may
serve as a potential therapeutic target for antiangiogenic therapy
(24). In the present study,
ANGPT2 was upregulated in esophageal cancer cells and this was
associated with low overall survival of patients with esophageal
cancer. Additionally, the viability, proliferation, colony
formation, migration, invasion and angiogenesis were inhibited in
ANGPT2-silenced EC-9706 cells.
Several studies have suggested that HOXB5 may serve
a key role in the regulation of tumor progression (25,26).
For example, HOXB5 exerts promotive effects on the proliferation,
migration and invasion of pancreatic cancer cells (27). Lee et al (15) suggested that HOXB5 serves as an
oncogenic driver in head and neck squamous cell carcinoma. In the
present study, HOXB5 was upregulated in esophageal cancer cells.
Data from JASPAR database predicted the binding between
transcription factor HOXB5 and ANGPT2, which was verified by
luciferase reporter and CH-IP assay. Moreover, the effect of ANGPT2
silence on the proliferation, metastasis and angiogenesis of
esophageal cancer cells were reversed following HOXB5
overexpression.
A previous study indicated that stimulation of
ERK/AKT pathway signaling enhances proliferation, survival and
metabolism of cancer cells (28).
Zhou et al (29)
demonstrated that blockade of the ERK/AKT pathway inhibits human
endometriosis progression. Moreover, activation of ERK/AKT pathway
promotes proliferation and migration of renal cancer cells
(30). In the present study,
ERK/AKT signaling was inhibited by ANGPT2 silencing, while HOXB5
overexpression partially abolished the effects of ANGPT2
silencing.
There are some limitations in the present study. The
present study was performed only on the EC-9706 cell line; other
types of esophageal cancer cell should be investigated in future as
the role of HOXB5 may be different in the different types of
esophageal cancer. Moreover, the effect of downregulation of HOXB5
on ANGPT2 in esophageal cancer need to be explored in future
investigations. Furthermore, the EC-9706 cell line displayed the
highest ANGPT2 expression levels and this should also be
investigated in future work. To the best of our knowledge,
HOXB5/ANGPT2 have not been investigated for use in the treatment of
other types of cancer.
In conclusion, ANGPT2 silencing inhibited the
proliferation, migration, invasion and angiogenesis of esophageal
cancer cells via targeting HOXB5 and blocking the ERK/AKT signaling
pathway, suggesting that ANGPT2/HOXB5 may be potential therapeutic
targets for the treatment of angiogenesis abnormality and
metastasis of esophageal cancer.
Supplementary Material
Reverse transcription-quantitative PCR
product electrophoresis obtained from chromatin immunoprecipitation
assay.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG designed the experiments and wrote the paper. JL
performed the experiments, participated in study design and wrote
the manuscript. All authors have read and approved the final
manuscript. JL and SG confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pakzad R, Mohammadian-Hafshejani A,
Khosravi B, Soltani S, Pakzad I, Mohammadian M, Salehiniya H and
Momenimovahed Z: The incidence and mortality of esophageal cancer
and their relationship to development in Asia. Ann Transl Med.
4(29)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tan HZ, Lin WJ, Huang JQ, Dai M, Fu JH,
Huang QH, Chen WM, Xu YL, Ye TT, Lin ZY, et al: Updated incidence
rates and risk factors of esophageal cancer in Nan'ao Island, a
coastal high-risk area in southern China. Dis Esophagus. 30:1–7.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lei S, Yang L and Li Y: Epidemiological
characteristics and changing trends of esophageal cancer diagnosed
by gastroscopy in Xijing Hospital from 2016 to 2020. J Hebei Med
Univ. 43:150–154. 2022.(In Chinese).
|
|
4
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dvoretskii SIu, Levchenko EV, Karachun AM,
Komarov IV, Pelipas' Iu V, Avanesian AA, Khandogin NV and Tiuriaeva
EI: Experience of the use of endovideotechnology in surgical
treatment of esophageal cancer. Vestn Khir Im I I Grek. 173:54–59.
2014.PubMed/NCBI(In Russian).
|
|
6
|
Sugimura K, Miyata H, Tanaka K, Takahashi
T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M and Doki
Y: High infiltration of tumor-associated macrophages is associated
with a poor response to chemotherapy and poor prognosis of patients
undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg
Oncol. 111:752–759. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu Z, Wang H, Pang Y, Hu H, Zhang H and
Wang W: Exosomal long non-coding RNA UCA1 functions as growth
inhibitor in esophageal cancer. Aging (Albany NY). 12:20523–20539.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maisonpierre PC, Suri C, Jones PF,
Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J,
Aldrich TH, Papadopoulos N, et al: Angiopoietin-2, a natural
antagonist for Tie2 that disrupts in vivo angiogenesis. Science.
277:55–60. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saharinen P, Eklund L and Alitalo K:
Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug
Discov. 16:635–661. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ladeira K, Macedo F, Longatto-Filho A and
Martins SF: Angiogenic factors: Role in esophageal cancer, a brief
review. Esophagus. 15:53–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Urosevic J, Blasco MT, Llorente A,
Bellmunt A, Berenguer-Llergo A, Guiu M, Canellas A, Fernandez E,
Burkov I, Clapes M, et al: ERK1/2 signaling induces upregulation of
ANGPT2 and CXCR4 to mediate liver metastasis in colon cancer.
Cancer Res. 80:4668–4680. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lauret Marie Joseph E, Laheurte C, Jary M,
Boullerot L, Asgarov K, Gravelin E, Bouard A, Rangan L, Dosset M,
Borg C and Adotévi O: Immunoregulation and clinical implications of
ANGPT2/TIE2(+) M-MDSC signature in non-small cell lung cancer.
Cancer Immunol Res. 8:268–279. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang B, Li N and Zhang H: Knockdown of
homeobox B5 (HOXB5) inhibits cell proliferation, migration, and
invasion in non-small cell lung cancer cells through inactivation
of the Wnt/β-catenin pathway. Oncol Res. 26:37–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hong CS, Jeong O, Piao Z, Guo C, Jung MR,
Choi C and Park YK: HOXB5 induces invasion and migration through
direct transcriptional up-regulation of β-catenin in human gastric
carcinoma. Biochem J. 472:393–403. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee K, Chang JW, Oh C, Liu L, Jung SN, Won
HR, Kim YI, Rha KS and Koo BS: HOXB5 acts as an oncogenic driver in
head and neck squamous cell carcinoma via EGFR/Akt/Wnt/β-catenin
signaling axis. Eur J Surg Oncol. 46:1066–1073. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li ZX, Wu G, Jiang WJ, Li J, Wang YY, Ju
XM and Yin YT: HOXB5 promotes malignant progression in pancreatic
cancer via the miR-6732 pathway. Cell Cycle. 19:233–245.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Feng W, Huang W, Chen J, Qiao C, Liu D, Ji
X, Xie M, Zhang T, Wang Y, Sun M, et al: CXCL12-mediated HOXB5
overexpression facilitates colorectal cancer metastasis through
transactivating CXCR4 and ITGB3. Theranostics. 11:2612–2633.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang J, Zhang S, Li X, Zhang F and Zhao
L: HOXB5 promotes the progression of breast cancer through
wnt/beta-catenin pathway. Pathol Res Pract.
224(153117)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liang J, Li H, Han J, Jiang J, Wang J, Li
Y, Feng Z, Zhao R, Sun Z, Lv B and Tian H: Mex3a interacts with
LAMA2 to promote lung adenocarcinoma metastasis via PI3K/AKT
pathway. Cell Death Dis. 11(614)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang J, Hu X, Nan J, Zhang X and Jin X:
HOXD9-induced SCNN1A upregulation promotes pancreatic cancer cell
proliferation, migration and predicts prognosis by regulating
epithelialmesenchymal transformation. Mol Med Rep.
24(819)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou K, Song B, Wei M, Fang J and Xu Y:
MiR-145-5p suppresses the proliferation, migration and invasion of
gastric cancer epithelial cells via the ANGPT2/NOD_LIKE_RECEPTOR
axis. Cancer Cell Int. 20(416)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qi L, Wang ZY, Shao XR, Li M, Chen SN, Liu
XQ, Yan S, Zhang B, Zhang XD, Li X, et al: ISL2 modulates
angiogenesis through transcriptional regulation of ANGPT2 to
promote cell proliferation and malignant transformation in
oligodendroglioma. Oncogene. 39:5964–5978. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xie JY, Wei JX, Lv LH, Han QF, Yang WB, Li
GL, Wang PX, Wu SB, Duan JX, Zhuo WF, et al: Angiopoietin-2 induces
angiogenesis via exosomes in human hepatocellular carcinoma. Cell
Commun Signal. 18(46)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee JY, Hur H, Yun HJ, Kim Y, Yang S, Kim
SI and Kim MH: HOXB5 promotes the proliferation and invasion of
breast cancer cells. Int J Biol Sci. 11:701–711. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tucci R, Campos MS, Matizonkas-Antonio LF,
Durazzo M, Pinto Junior Ddos S and Nunes FD: HOXB5 expression in
oral squamous cell carcinoma. J Appl Oral Sci. 19:125–129.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gao Y, Fei X, Kong L and Tan X: HOXB5
promotes proliferation, migration, and invasion of pancreatic
cancer cell through the activation of the GSK3β/β-catenin pathway.
Anticancer Drugs. 31:828–835. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Asati V, Mahapatra DK and Bharti SK:
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as
anticancer agents: Structural and pharmacological perspectives. Eur
J Med Chem. 109:314–341. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou CF, Liu MJ, Wang W, Wu S, Huang YX,
Chen GB, Liu LM, Peng DX, Wang XF, Cai XZ, et al: miR-205-5p
inhibits human endometriosis progression by targeting ANGPT2 in
endometrial stromal cells. Stem Cell Res Ther.
10(287)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu R, Ge J, Ma J and Zheng J:
Carcinoembryonic antigen related cell adhesion molecule 6 promotes
the proliferation and migration of renal cancer cells through the
ERK/AKT signaling pathway. Transl Androl Urol. 8:457–466.
2019.PubMed/NCBI View Article : Google Scholar
|