Introduction
Sjögren's syndrome (SS) is a complex chronic
inflammatory autoimmune disease. Its etiology is known to be
related to the production of autoantibodies, but the specific
details remain unclear (1).
Previous studies have shown that the pathogenesis of SS is centered
on abnormal accumulation of B lymphocytes (2,3).
However, some studies have shown that T follicular helper cells, T
helper (Th)17 and Th22 cells also serve an important role in the
pathogenesis of SS (3). SS mainly
affects the salivary and lacrimal glands, leading to dry mouth and
eyes, as well as other exocrine glands and extraglandular organs,
resulting in multisystem symptoms that ultimately lead to a
declining quality of life for patients (4). The degree of lymphocyte infiltration
of submandibular glands (SMGs) is often considered as an index to
judge the progress of SS (5).
Previous studies have shown that SS is also associated with an
increased incidence of atherosclerosis, interstitial lung disease
and even lymphoma (5-7).
Currently, there is no effective treatment for SS. The most common
clinical treatment methods are local substitution therapy and
systemic immunotherapy (7).
Nevertheless, glucocorticoids such as dexamethasone (DEX) have been
considered effective therapeutic agents (8). At present, targeted therapy is
thought to delay the progression of SS (9). For example, Gandolfo et al
(10) suggest that targeting
B-lymphocyte activating factor can delay the progression of SS.
Similarly, de Vita et al (11) suggest that sequential treatment
with belimumab to inhibit the proliferation of B lymphocytes may
also be an effective treatment. However, at present, no drug is
considered effective against SS in the long term. Therefore, it is
important to develop novel therapies for SS treatment.
Adipose-derived stem cells (ADSCs) are stem cells
capable of multidirectional differentiation and are produced by
adipose tissue. They have self-renewal ability and can secrete a
variety of cytokines. Therefore, ADSCs are thought to serve
specific roles in the onset and progression of some diseases, such
as the occurrence and development of breast cancer, cervical cancer
and other types of tumors (12,13),
as well as of a variety of autoimmune diseases. At present, the
transplantation of ADSCs for the treatment of autoimmune diseases
is an important topic in academic circles. For example, Zhang et
al (14) observe that
transplantation of ADSCs can improve the balance between Th17 and
regulatory T cells to support the treatment of lupus nephritis.
Another study reported that ADSCs can induce the downregulation of
IL-17 expression, thereby delaying the progression of systemic
lupus erythematosus (SLE) (15).
The Hippo signaling pathway is a core pathway that
regulates organ size, cell proliferation and differentiation. This
signaling pathway is thought to be also related to the occurrence
and development of a number of autoimmune diseases (16). In fact, a previous study suggests
that tRNA-derived small RNA-21109 can inhibit the Hippo signaling
pathway and thus polarization of M1 macrophages, thereby delaying
SLE progression (17). Another
study shows that the inhibition of the Hippo signaling pathway can
inhibit TGF-β accumulation and thus delay the progression of
rheumatoid arthritis (18). In
addition, Enger et al (19)
note that the Hippo signaling pathway is necessary for the
development of the SMG, and its imbalance is related to the
occurrence of Sjögren's syndrome. Transcriptional coactivator with
PDZ-binding motif (TAZ), Yes-associated protein (YAP) and α-catenin
are three proteins involved in the Hippo signaling pathway
(16). Another study reported that
altered expression of these proteins may be related to the
progression and severity of SS (17). E-cadherin can affect the changes of
cell adhesion; E-cadherin-mediated cell adhesion has been shown to
influence the activity of the Hippo pathway (18,19).
However, the possible role of ADSCs in delaying the progression of
SS and their mechanism of action have not yet been examined.
Therefore, the present study investigated the effect and mechanism
of action of ADSCs on SS in order to assess whether ADSC
transplantation can help delay the progression of SS, with the
final aim to contribute to the development of novel methods for SS
treatment.
Materials and methods
Ethics approval and consent to
participate
Experiments were performed under a project license
(approval no. 20201002) granted by the Institutional Ethics Board
of Stomatological Hospital of Shandong University (Shandong,
China), in compliance with Chinese national or institutional
guidelines for the care and use of animals.
Mouse model of SS
All animal experiments were performed according to
the guidelines provided by the National Institutes of Health Guide
for the Care and Use of Laboratory Animals (15) and the study was approved by the
Animal Ethics Committee of Shandong University. A total of 75
female non-obese diabetic (NOD) mice (10-week-old) and 15 female
Control mice (10-week-old) used in the study were purchased from
Huafukang Biotechnology Co., Ltd. Control mice are normal BALB/c
mice. The mice were housed in accordance with animal welfare
regulations, under specific-pathogen-free conditions at 25˚C,
humidity of 50% and a 12-h light/dark cycle. All NOD mice had free
access to food and water. The mice were sacrificed by cervical
dislocation. Inflammatory cells could be detected in the exocrine
glands of NOD mice of ~12 weeks of age and obvious exocrine
dysfunction appeared at ~16 weeks; therefore, these mice were used
as the main model to study SS progression, as previously described
(20). NOD mice were randomly
divided into three groups (n=15 NOD mice/group): i) ADSC group; ii)
DEX group and iii) untreated NOD Control group. A total of
1x105 ADSCs (Guangzhou Saliai Stem Cell Science and
Technology Co., Ltd.) suspended at 200 µl PBS and injected into the
SMG of NOD mice in the ADSC group. According to a previous study,
ADSCs can influence immune responses and hence are key cell sources
for tissue repair and regeneration (21). The DEX group NOD mice were injected
with 200 µl 0.1% DEX (MilliporeSigma) (8). Each Control group NOD mouse was
injected with 200 µl PBS solution.
Collection of tissue
In preliminary experiments, lymphocyte infiltration
in the SMGs of NOD mice appeared at ~13 weeks of age. Therefore,
when the mice reached 12 weeks of age, ADSCs (5x105
cells/ml; 0.2 ml/mouse), 0.1% DEX (4.125-8.25 mg/kg) and PBS were
injected into the SMGs of ADSC, DEX and NOD Control group mice,
respectively. A total of three mice in each group were sacrificed
every week between 13 and 17 weeks of age. All animal experiments
were repeated three times (8).
After sacrificing the mice, SMGs were collected and SMG samples
were fixed in 4% formalin at 37˚C for 72 h. The fixed tissues were
then dehydrated in a series of graded ethanol solutions, immersed
in xylene and embedded in paraffin. Sections were cut at a
thickness of 3-4 µm using a sliding microtome and then kept at 37˚C
overnight.
Hematoxylin and eosin (H&E)
staining
The sections were stained with hematoxylin at room
temperature for 5 min, 1% HCl-alcohol differentiation for 5-30 sec
and then with eosin staining solution for 5 min at room
temperature. The sections were dehydrated with 95% alcohol for 5
min at room temperature, then cleared with xylene for 5 min and
finally sealed with neutral balsam. The tissue sections were
subsequently visualized under a light microscope at a magnification
of x20 and x200 (Leica Microsystems GmbH). All parameters were
assessed by a pathologist (Mr. Guimiao Xing; School and Hospital of
Stomatology, Cheeloo College of Medicine; Shandong, China).
Lymphocyte aggregates (LAs) were defined as sharp-edged dense
groups of at least 50 lymphocytes; the size of these LAs was
irrelevant. Each section was prepared as a 1x1 mm square to ensure
the consistency of the total observation area and the number of LAs
was detected on this basis. The amount of LA represents the degree
of lymphocyte infiltration (8,19,20).
Antibodies and vectors
Anti-E-cadherin (cat. no. 564186; BD Transduction
Laboratories; BD Biosciences), anti-α-catenin (cat. no. 610193; BD
Transduction Laboratories; BD Biosciences), anti-TAZ (cat. no.
ab224239; Abcam), anti-p-TAZ (cat. no. 59971; CST) and anti-GAPDH
(cat. no. ab8245; Abcam) antibodies, as well as DAPI (cat. no.
ab285390; Abcam) were used in western blotting and
immunofluorescence staining assays.
The lentiviral vector (V0009) containing short
hairpin (sh)RNAs targeting human TAZ (sh-TAZ) or a non-targeting
oligonucleotide was bought from Wuhan Miaolingbio Co., Ltd. The
target sequence for sh-TAZ was 5'-AGGTACTTCCTCAATCACA-3' and the
target sequence for the negative control shRNA (sh-NC) was
5'-GAGAACTATCTCATAACCA-3'. Briefly, the lentiviral constructs for
sh-TAZ and sh-NC were constructed into
pLV3-U6-TAZ(human)-shRNA1-EF1a-turboRFP-Puro (cat. no. P37411;
Wuhan Miaolingbio Co., Ltd.). All the plasmids were co-transfected
with pHelper 1.0 (20 µg) and pHelper 2.0 (10 µg) into 293T cells
(National Collection of Authenticated Cell Cultures). The knockdown
efficiency was confirmed by western blotting analysis. A total of 1
µg (50 pmol) shRNA was combined with serum-free diluent to a final
volume of 25 µl. shRNA plasmids (4 µg) were co-transfected with the
packaging vectors using TransLipid Transfection Reagent (Beijing
TransGen Biotech Co., Ltd.) in accordance with the manufacturer's
recommendations. Cells were cultured at 37˚C in a 5% CO2
incubator for 12 h, after which, the culture medium containing
infection mixture was removed and replaced with complete culture
medium. After 48 h incubation, the cell supernatant rich in
lentiviral particles was collected and centrifuged for 10 min at
4˚C and 3,000 x g; then the supernatant was filtered with 0.45 µm
PVDF membrane and stored separately at -80˚C. At 15 weeks, the
sh-TAZ lentiviral particles (0.2 ml/mouse; effective titer,
5x109 TU/ml) were subsequently injected into the tail
vein of NOD mice (n=15) to construct a TAZ-knockdown NOD mouse
model; the sh-NC viral particles (0.2 ml/mouse; effective titer,
5x109 TU/ml) were injected into the tail vein of NOD
mice (n=15); 0.2 ml/mouse PBS was injected into the Control group
(n=15 NOD mice). ADSCs were injected into SMGs of the shRNA-treated
mice at 13 weeks; ADSCs were not injected into Control group mice,
which received PBS. At 17 weeks, the mice were killed and SMG
tissues were collected.
Immunofluorescence staining
For immunofluorescence analysis, 3x105
SMG acinar cells were seeded on 6-well glass slides for 24-48 h,
fixed with 4% paraformaldehyde for 15 min at 25˚C and then washed
with PBS. After blocking with 10% normal goat serum (WGAR1009-5
Wuhan Servicebio Technology Co., Ltd.) 37˚C for 48 h, the cells
were incubated overnight with the primary antibodies against TAZ
and E-cadherin (each 1:1,000) at 4˚C. Then, the slides were washed
with PBS and incubated with FITC-conjugated goat anti-rabbit IgG
(1:1,000; cat. no. ab6717; Abcam) and FITC-conjugated goat
anti-mouse IgG (1:1,000; cat. no. ab6785; Abcam) for 1 h at room
temperature. Thereafter, the slides were washed with PBS, stained
with DAPI and examined under a fluorescence microscope (Olympus
Corporation). ImageJ software v1.8.0.112 (National Institutes of
Health) was used to analysis the average intensity of
expression.
Western blotting
The SMG lysates of were extracted using RIPA lysis
buffer. The homogenates were centrifuged at 12,000 x g for 15 min
at 4˚C, and the protein concentrations were determined using a BCA
kit (Thermo Fisher Scientific, Inc.). Proteins (30 µg) were
separated by 10% SDS-PAGE, transferred to PVDF membranes
(MilliporeSigma) and blocked in 5% non-fat milk at 25˚C for 1.5 h.
The membranes subsequently were incubated at 4˚C overnight with
rabbit E-cadherin, p-TAZ, TAZ, α-catenin and GAPDH (1:1,000 each).
Following three washes with TBS +0.1% Tween-20, the membranes were
incubated with HRP-conjugated goat anti-rabbit and goat anti-mouse
secondary antibodies (1:20,000; cat. nos. G1213-100UL and
G1214-100UL, respectively; Wuhan Servicebio Technology Co., Ltd.)
for 2 h at room temperature and developed with ECL Reagent
(MilliporeSigma). Densitometric analysis was conducted using ImageJ
software (version 1.44p; National Institutes of Health).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 8.0 software (GraphPad Software, Inc.). Two-tailed
unpaired t-tests were used to analyze two groups. One-way analysis
of variance followed by Tukey's test was used to analyze multiple
groups. All results are presented as mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
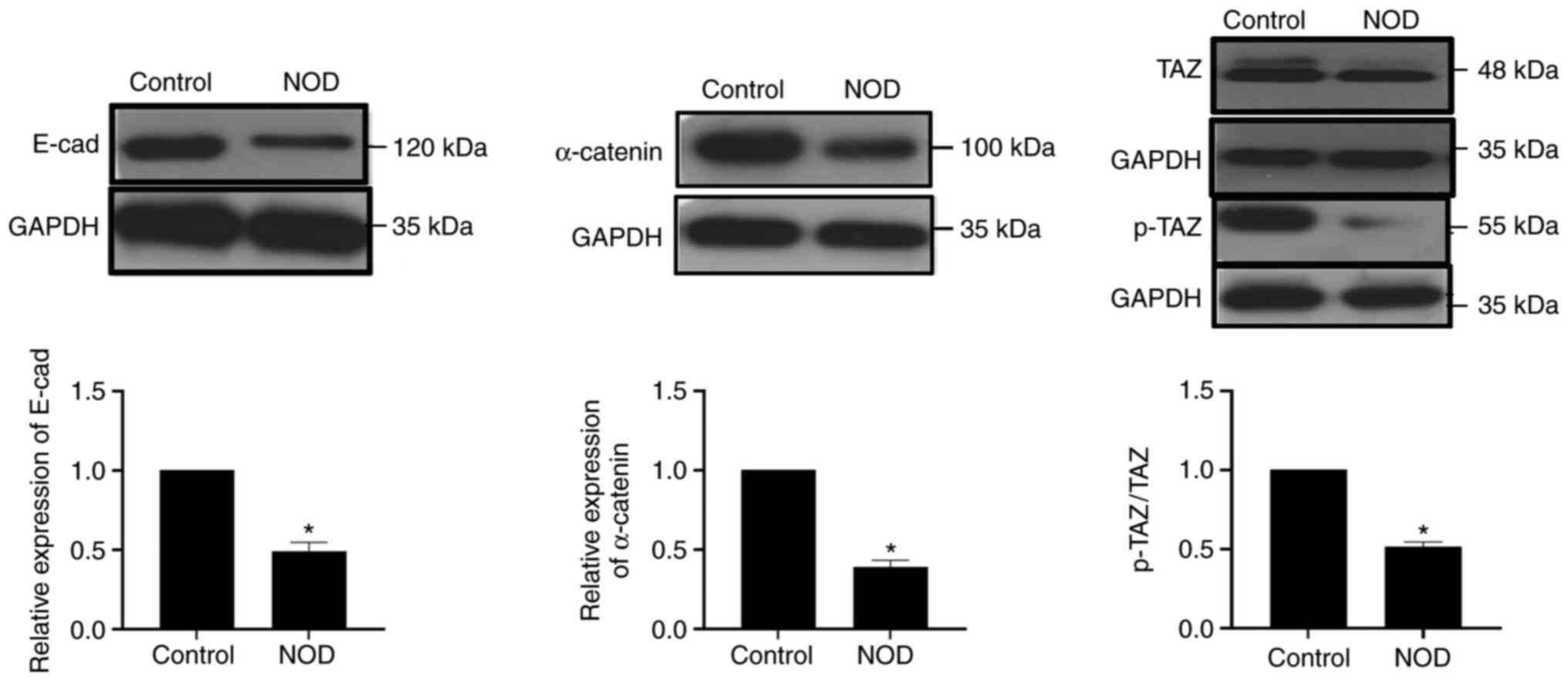
SS occurrence is accompanied by
downregulation of the Hippo signaling pathway
To explore the relationship between the Hippo
signaling pathway and SS occurrence, SMG tissues were collected
from NOD mice and normal BALB/c Control mice of the same age (17
weeks) and proteins extracted for quantification. As expected, the
expression of TAZ, p-TAZ, E-cadherin and α-catenin in NOD mice was
lower compared with that in Control mice. Similarly, the ratio of
p-TAZ/TAZ also decreased (Fig. 1).
The above experiments demonstrated that, along SS occurrence, the
Hippo signaling pathway was inhibited and the expression of its key
proteins decreased.
ADSCs postpone the infiltration of
lymphocytes and thus SS progression
To verify whether ADSCs exert a therapeutic effect
on SS, NOD mice were randomly divided into three groups (Fig. 2A). H&E staining revealed that
the SMGs of the NOD Control (PBS)-, DEX- and ADSC-treated groups
showed obvious lymphocyte infiltration at 13, 14, 15, 16 and 17
weeks of age (Fig. 2B). To clarify
the results, the number of LAs were detected and counted (Fig. 2C). These results suggested that
ADSC injection may postpone the infiltration of lymphocytes and the
progression of SS, with a stronger effect compared with that of DEX
treatment.
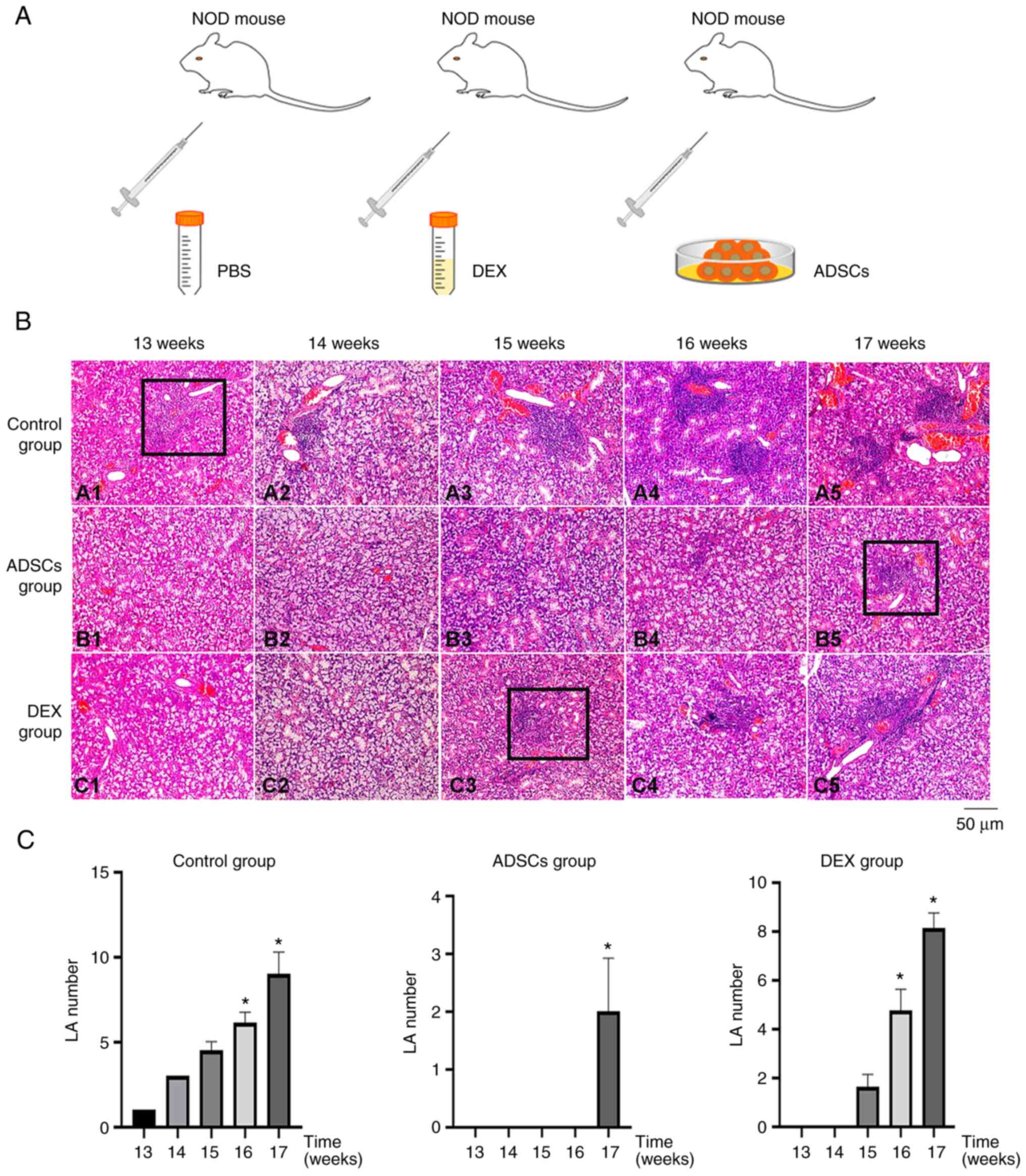 | Figure 2ADSCs may delay the infiltration of
lymphocytes and thus Sjögren's syndrome progression. (A) NOD mice
were randomly divided into Control (PBS-), DEX- and ADSC-treatment
groups (n=15 mice/group). (B) Hematoxylin and eosin staining showed
that, between 13 and 17 weeks, the degree of lymphocyte
infiltration in SMGs of NOD mice in the ADSC group (B1-B5) was
significantly lower compared with the Control group (A1-A5),
whereas the degree of lymphocyte infiltration in SMGs of NOD mice
in the DEX group (C1-C5) was between the two. (C) At the same time,
the number of LAs in the different groups was detected (black
rectangles in B) and counted. *P<0.05 vs. 13 weeks.
ADSCs, adipose-derived stem cells; DEX, dexamethasone; LA,
lymphocyte aggregate; NOD, non-obese diabetic; SMG, submandibular
gland. |
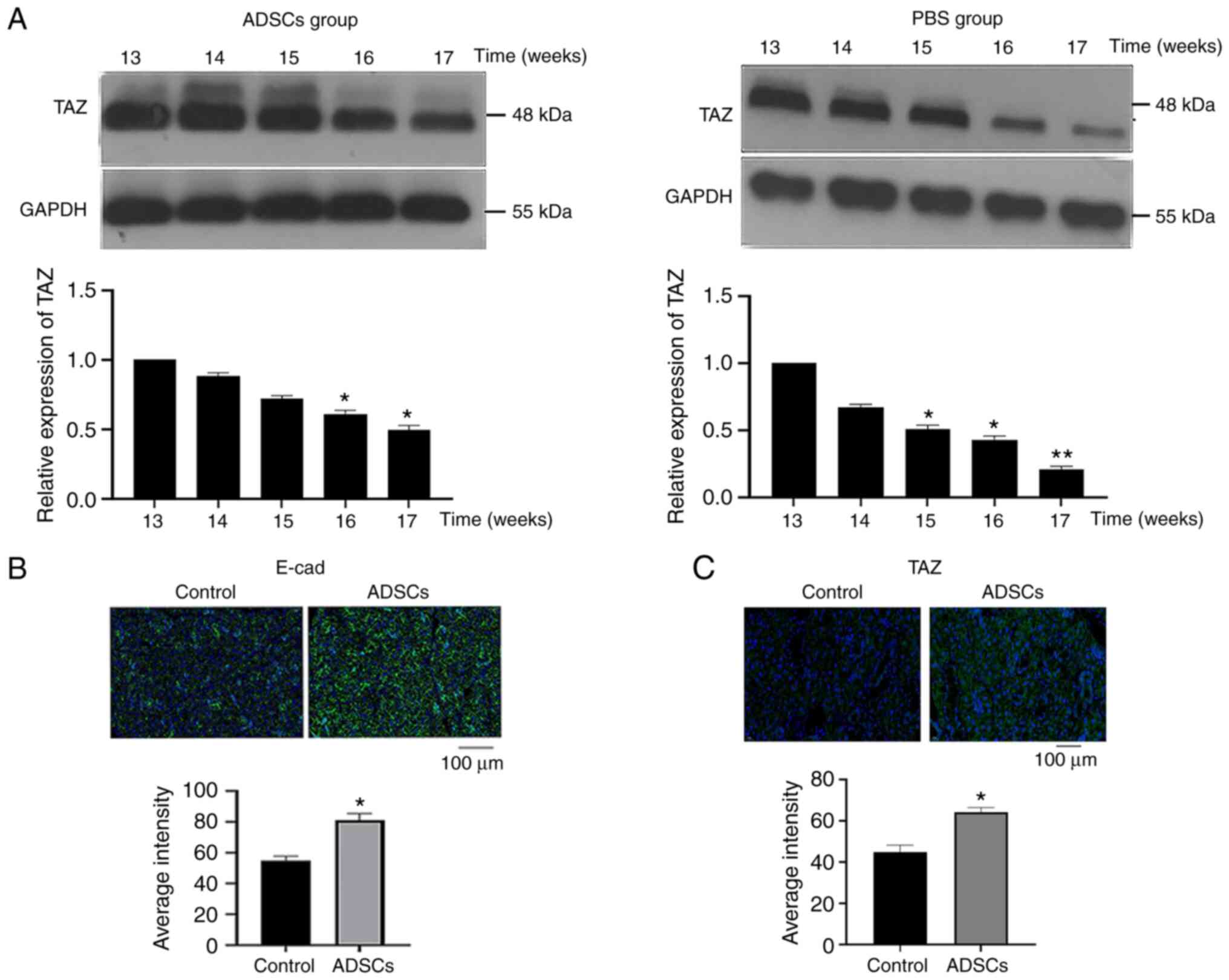
ADSCs postpone the progression of SS
by upregulating the Hippo signaling pathway
In previous experiments, it was found that ADSCs
could postpone the progression of SS (21); however, their molecular mechanism
of action is not clear. Therefore, the present study explored the
mechanism by which ADSCs may trigger such postponement using
western blotting and immunofluorescence. First, altered TAZ protein
expression was observed in the SMGs of mice following
transplantation of ADSCs. In fact, compared with the results shown
in the NOD Control group, the decline of TAZ expression in SMGs was
postponed following ADSC treatment (Fig. 3A). Next, the expression of
E-cadherin and TAZ was assessed in the SMGs of NOD mice treated
with ADSCs and of the NOD Control group by immunofluorescence.
Moreover, at 17 weeks, ADSC treatment was found to increase the
expression of E-cadherin and TAZ compared with the Control group
(Fig. 3B and C).
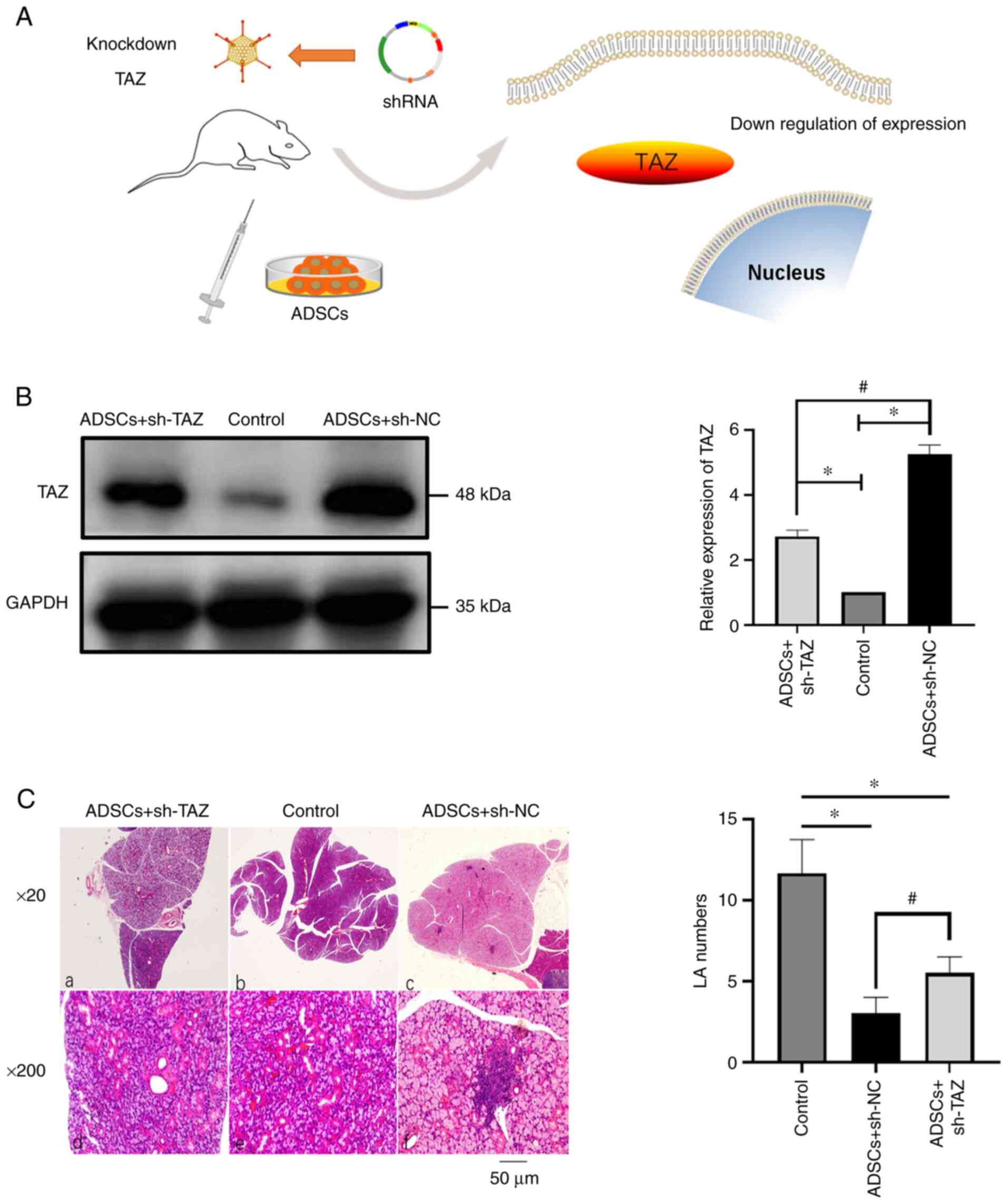
Knocking down the expression of TAZ
may reduce the postponing effect of ADSCs on SS
As aforementioned, the expression of TAZ and
E-cadherin decreased during the pathogenesis of SS, whereas ADSC
treatment seemed to postpone the progression of SS by increasing
the expression of TAZ and E-cad. To further support these results,
a gene knockdown experiment was performed by injecting shRNA
lentiviral particles targeting TAZ into the caudal vein of NOD mice
(with ADSCs treated) at week 15 (Fig.
4A). After the mouse SMG tissue was removed at week 17, the
protein expression levels were detected; the western blotting
results verified that the expression of TAZ was knocked down
(Fig. 4B). Then, the SMGs of NOD
mice were stained to determine the progress of SS by estimating the
degree of lymphocyte infiltration by counting LAs (Fig. 4C). The results suggested that the
effect of ADSCs was weakened after knocking down TAZ, but it was
still stronger compared with that of the sh-NC group. TAZ is an
important protein in Hippo signaling pathway; the above results
suggested that ADSCs may delay the progression of SS by
upregulating the activity of Hippo signaling pathway.
Discussion
SS is a complex autoimmune disease that mainly
causes dry mouth and dry eyes. At present, its etiology has not
been fully elucidated and there is no effective treatment for this
condition (22). Therefore,
exploring SS pathogenesis is required to develop new methods for SS
treatment.
The Hippo signaling pathway is a core pathway that
regulates organ size, cell proliferation and differentiation
(23). In addition, the effector
protein TAZ of the catenin-related Hippo signaling pathway becomes
increasingly phosphorylated, consistent with the activation of the
Hippo signaling pathway (24).
Another previous study reported that E-cadherin-mediated cell
adhesion can affect the activity of the Hippo signaling pathway
(25). The present study results
demonstrated that the progression of SS was accompanied by a
decrease in the expression of TAZ and E-cadherin. Conversely,
transplantation of ADSCs upregulated the Hippo signaling pathway
and increased the expression of Hippo signaling-related proteins.
The TAZ knockdown experiment suggested that TAZ may serve an
important role in the process of ADSCs delaying the progression of
SS. In addition, it has been previously noted that the expression
of E-cadherin will change with the progression of autoimmune
diseases (26,27). The reason for these phenomena may
be the different pathogenesis of the different autoimmune diseases.
It was hypothesized that in addition to E-cad, there remained a
number of proteins that served a role in this process, which will
be the focus of future studies.
ADSCs have been considered good candidates for the
treatment of various autoimmune diseases, such as multiple
sclerosis, osteoarthritis, Crohn's disease and type 1 diabetes,
against which they have shown a good therapeutic effect (28-32).
A previous study suggested that transplantation of ADSCs can
effectively alleviate dry eye symptoms caused by autoimmune factors
(33). The present study found
that ADSCs can reduce the infiltration of lymphocytes in the SMG,
thus ADSCs were considered to delay the progression of SS. The
mechanism of this effect of ADSCs may be to increase the expression
of E-cad and TAZ. It has been proposed that the expression of
E-cadherin is negatively correlated with the activation level of
Hippo signaling pathway (34).
This is contrary to the results of the present study. The changes
in protein expression as the disease progresses may be a potential
explanation; but the reason for this difference will be one of the
focuses of future research. In fact, whether SS or another
autoimmune disease, treatment strategies are still the focus of
modern research. ADSCs have been used in the treatment of a number
of diseases, such as rheumatoid arthritis (35), but its detailed mechanism still
needs to be explored. In different diseases, the mechanism of
action of ADSCs is also different. Exploring these mechanisms is
not only conducive to the development of new treatments, but also
conducive to improving our better understanding of the pathogenesis
of these diseases. Overall, the present study suggested that ADSCs
may delay the progress of SS, which is accompanied by the
upregulation of Hippo signaling pathway activation. Therefore,
transplantation of ADSCs may be a potential method for the
treatment of SS.
The present study had some limitations. For example,
it did not explore how ADSCs upregulated the Hippo signaling
pathway. In addition, it did not explore the adverse effects of its
treatment These limitations will be the focus of future research.
Nevertheless, the present study provided new strategies for the
treatment of SS. Future studies will continue to pursue the search
for effective treatment methods for SS and will also explore other
mechanisms by which ADSCs may delay SS progression.
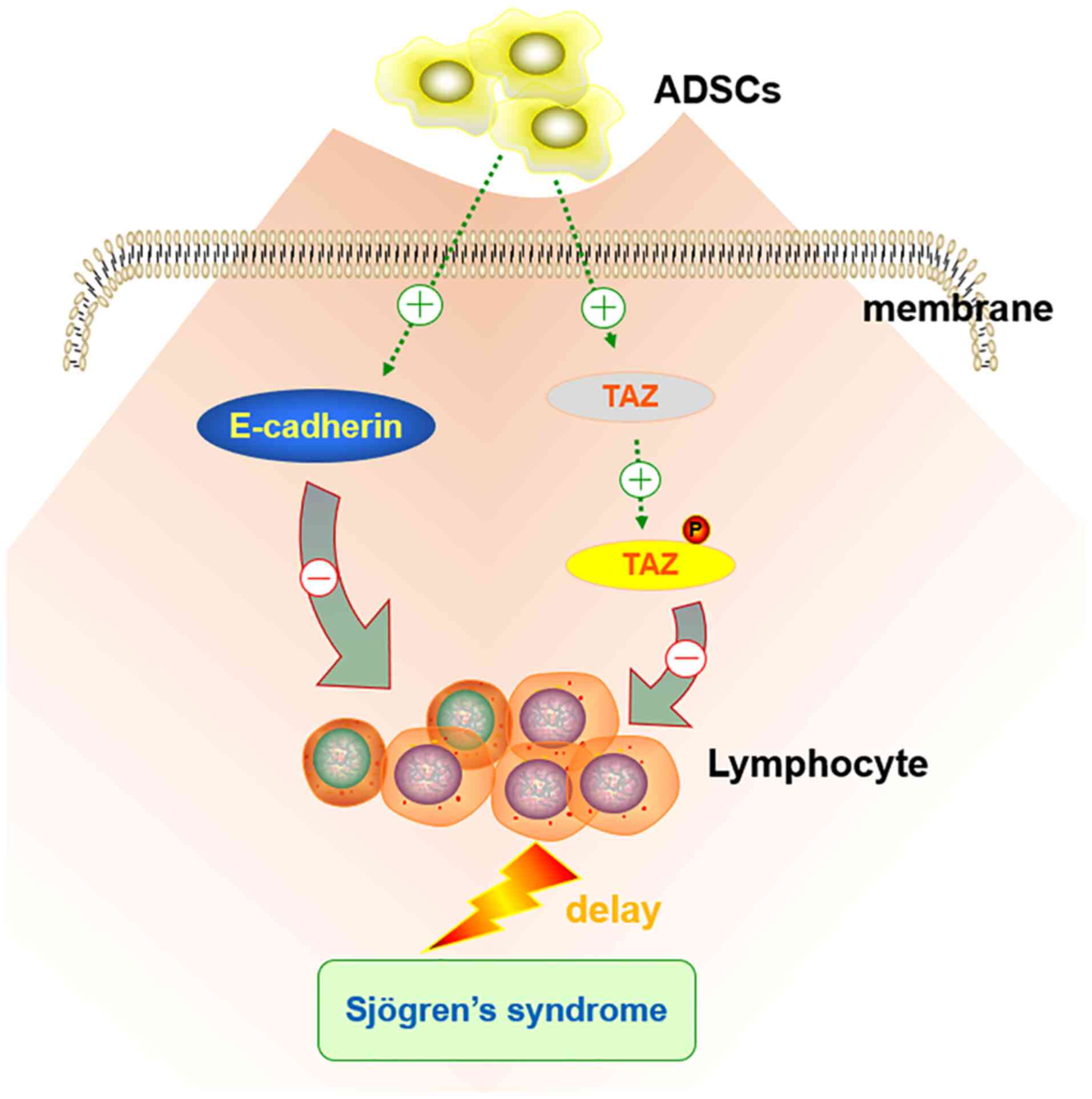
In conclusion, ADSCs may delay the progression of
SS, with a stronger effect compared with that of DEX; this
therapeutic effect is mainly achieved through the upregulation of
the Hippo signaling pathway (Fig.
5). However, our understanding of the role of ADSCs in SS
remains limited. The study of cell-cell interactions between ADSCs
and SS tissues may result in important developments of novel
treatment strategies for SS, which should be the focus of future
research.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The National Natural
Science Foundation of China (grant no. 81402298), The Young
Scholars Program of the Shandong University and the opening project
of The Collaborative Innovation Center for Classic and Famous
Prescriptions of Traditional Chinese Medicine in Shandong Province
entitled ‘Functional mechanism of Chaihu Guizhi Ganjiang Decoction
in the treatment of Sjögren's syndrome’ (grant no. 2019KFY10). The
present study was also supported by the project ‘Research on the
functional mechanism of stem cell exosomes in the treatment of
Sjögren's syndrome’ of Shandong Jiekai Biotechnology Co., Ltd.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and GL guided the project, analyzed the data and
wrote the manuscript. XF and XX conceived the technical details and
designed the experiments. ZL, QZ and GX performed the experiments.
XF, GL and ZL confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Experiments were performed under a project license
(approval no. 20201002) granted by Institutional Ethics Board of
Stomatological Hospital of Shandong University (Shandong, China),
in compliance with Chinese national or institutional guidelines for
the care and use of animals. Informed consent was obtained from
each patient upon their recruitment to the present study, which was
approved by the Institutional Research Ethics Committee of School
of Stomatology, Shandong University (approval no. 20201002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jonsson R, Brokstad KA, Jonsson MV,
Delaleu N and Skarstein K: Current concepts on Sjögren's
syndrome-classification criteria and biomarkers. Eur J Oral Sci.
126 (Suppl 1):S37–S48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stefanski AL, Tomiak C, Pleyer U, Dietrich
T, Burmester GR and Dörner T: The diagnosis and treatment of
Sjögren's syndrome. Dtsch Arztebl Int. 114:354–361. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fasano S, Mauro D, Macaluso F, Xiao F,
Zhao Y, Lu L, Guggino G and Ciccia F: Pathogenesis of primary
Sjögren's syndrome beyond B lymphocytes. Clin Exp Rheumatol. 38
(Suppl 126):S315–S323. 2020.PubMed/NCBI
|
|
4
|
Vivino FB: Sjögren's syndrome: Clinical
aspects. Clin Immunol. 182:48–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Melissaropoulos K, Bogdanos D, Dimitroulas
T, Sakkas LI, Kitas GD and Daoussis D: Primary Sjögren's syndrome
and cardiovascular disease. Curr Vasc Pharmacol. 18:447–454.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Luppi F, Sebastiani M, Silva M,
Sverzellati N, Cavazza A, Salvarani C and Manfredi A: Interstitial
lung disease in Sjögren's syndrome: A clinical review. Clin Exp
Rheumatol. 38 (Suppl 126):S291–S300. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bowman SJ: Primary Sjögren's syndrome.
Lupus. 27 (1 Suppl):S32–S35. 2018.
|
|
8
|
Easley JT, Nelson JW, Mellas RE, Sommakia
S, Wu C, Trump B and Baker OJ: Aspirin-triggered resolvin D1 versus
dexamethasone in the treatment of Sjögren's syndrome-like
NOD/ShiLtJ mice-a pilot study. J Rheum Dis Treat.
1(27)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Srivastava A and Makarenkova HP: Innate
immunity and biological therapies for the treatment of Sjögren's
syndrome. Int J Mol Sci. 21(9172)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gandolfo S and De Vita S: Double anti-B
cell and anti-BAFF targeting for the treatment of primary Sjögren's
syndrome. Clin Exp Rheumatol. 37 (Suppl 118):S199–S208.
2019.PubMed/NCBI
|
|
11
|
De Vita S, Quartuccio L, Salvin S, Picco
L, Scott CA, Rupolo M and Fabris M: Sequential therapy with
belimumab followed by rituximab in Sjögren's syndrome associated
with B-cell lymphoproliferation and overexpression of BAFF:
Evidence for long-term efficacy. Clin Exp Rheumatol. 32:490–494.
2014.PubMed/NCBI
|
|
12
|
Schmid R, Wolf K, Robering JW, Strauß S,
Strissel PL, Strick R, Rübner M, Fasching PA, Horch RE, Kremer AE,
et al: ADSCs and adipocytes are the main producers in the
autotaxin-lysophosphatidic acid axis of breast cancer and healthy
mammary tissue in vitro. BMC Cancer. 18(1273)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhai Y, Wu W, Xi X and Yu R:
Adipose-derived stem cells promote proliferation and invasion in
cervical cancer by targeting the HGF/c-MET pathway. Cancer Manag
Res. 12:11823–11832. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang W, Feng YL, Pang CY, Lu FA and Wang
YF: Transplantation of adipose tissue-derived stem cells
ameliorates autoimmune pathogenesis in MRL/lpr mice: Modulation of
the balance between Th17 and Treg. Z Rheumatol. 78:82–88.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the care and use of laboratory animals, 8th
edition. Washington (DC): National Academies Press (US), 2011.
|
|
16
|
Boki KA, Ioannidis JP, Segas JV,
Maragkoudakis PV, Petrou D, Adamopoulos GK and Moutsopoulos HM: How
significant is sensorineural hearing loss in primary Sjögren's
syndrome? An individually matched case-control study. J Rheumatol.
28:798–801. 2001.PubMed/NCBI
|
|
17
|
Dou R, Zhang X, Xu X, Wang P and Yan B:
Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus
erythematosus by inhibiting macrophage M1 polarization. Mol
Immunol. 139:106–114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bottini A, Wu DJ, Ai R, Le Roux M, Bartok
B, Bombardieri M, Doody KM, Zhang V, Sacchetti C, Zoccheddu M, et
al: PTPN14 phosphatase and YAP promote TGFβ signalling in
rheumatoid synoviocytes. Ann Rheum Dis. 78:600–609. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Enger TB, Samad-Zadeh A, Bouchie MP,
Skarstein K, Galtung HK, Mera T, Walker J, Menko AS, Varelas X,
Faustman DL, et al: The Hippo signaling pathway is required for
salivary gland development and its dysregulation is associated with
Sjogren's syndrome. Lab Invest. 93:1203–1218. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Inoue H, Kishimoto A, Ushikoshi-Nakayama
R, Hasaka A, Takahashi A, Ryo K, Muramatsu T and Ide F: Resveratrol
improves salivary dysfunction in a non-obese diabetic (NOD) mouse
model of Sjögren's syndrome. J Clin Biochem Nutr. 59:107–122.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang X, Liu C, Li S, Xu Y, Chen P, Liu Y,
Ding Q, Wahapu W, Hong B and Yang M: Effects of continuous passage
on immunomodulatory properties of human adipose-derived stem cells.
Cell Tissue Bank. 16:143–150. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jonsson R, Bolstad AI, Brokstad KA and
Brun JG: Sjögren's syndrome-a plethora of clinical and
immunological phenotypes with a complex genetic background. Ann N Y
Acad Sci. 1108:433–447. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Szymaniak AD, Mi R, McCarthy SE, Gower AC,
Reynolds TL, Mingueneau M, Kukuruzinska M and Varelas X: The Hippo
pathway effector YAP is an essential regulator of ductal progenitor
patterning in the mouse submandibular gland. Elife.
6(e23499)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miyachi Y, Nishio M, Otani J, Matsumoto S,
Kikuchi A, Mak TW, Maehama T and Suzuki A: TAZ inhibits acinar cell
differentiation but promotes immature ductal cell proliferation in
adult mouse salivary glands. Genes Cells. 26:714–726.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Varelas X and Wrana JL: Coordinating
developmental signaling: Novel roles for the Hippo pathway. Trends
Cell Biol. 22:88–96. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sisto M, Ribatti D and Lisi S: Cadherin
signaling in cancer and autoimmune diseases. Int J Mol Sci.
22(13358)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang C, Xu X, Jin H and Liu G: Nicotine
may promote tongue squamous cell carcinoma progression by
activating the Wnt/β-catenin and Wnt/PCP signaling pathways. Oncol
Lett. 13:3479–3486. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Naderi N, Combellack EJ, Griffin M,
Sedaghati T, Javed M, Findlay MW, Wallace CG, Mosahebi A, Butler
PE, Seifalian AM and Whitaker IS: The regenerative role of
adipose-derived stem cells (ADSC) in plastic and reconstructive
surgery. Int Wound J. 14:112–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li Z, Wang S, Fang S, Li X, Li T and Liu
G: Adipose-derived stem cells promote the proliferation, migration,
and invasion of oral squamous cell carcinoma cells by activating
the Wnt/planar cell polarity signaling pathway. Transl Cancer Res.
11:306–315. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bora P and Majumdar AS: Adipose
tissue-derived stromal vascular fraction in regenerative medicine:
A brief review on biology and translation. Stem Cell Res Ther.
8(145)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lv W, Graves DT, He L, Shi Y, Deng X, Zhao
Y, Dong X, Ren Y, Liu X, Xiao E and Zhang Y: Depletion of the
diabetic gut microbiota resistance enhances stem cells therapy in
type 1 diabetes mellitus. Theranostics. 10:6500–6516.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chang Q, Li C, Lu Y, Geng R, Wei JN and Hu
JZ: Adipose-derived mesenchymal stromal cells suppress
osteoclastogenesis and bone erosion in collagen-induced arthritis.
Scand J Immunol. 92(e12877)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li X, Lu X, Sun D, Wang X, Yang L, Zhao S,
Nian H and Wei R: Adipose-derived mesenchymal stem cells reduce
lymphocytic infiltration in a rabbit model of induced autoimmune
dacryoadenitis. Invest Ophthalmol Vis Sci. 57:5161–5170.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim NG, Koh E, Chen X and Gumbiner BM:
E-cadherin mediates contact inhibition of proliferation through
Hippo signaling-pathway components. Proc Natl Acad Sci USA.
108:11930–11935. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao Y, Gao C, Liu H, Liu H, Feng Y, Li Z,
Liu H, Wang J, Yang B and Lin Q: Infliximab-based self-healing
hydrogel composite scaffold enhances stem cell survival,
engraftment, and function in rheumatoid arthritis treatment. Acta
Biomater. 121:653–664. 2021.PubMed/NCBI View Article : Google Scholar
|