Introduction
Second-generation antipsychotics (SGAs) are widely
used for the treatment of schizophrenia. These agents are
antagonists of dopamine D2 and various neuroreceptors,
such as 5-hydroxytryptamine receptors (5-HT2A and
5-HT2C) and adrenaline receptors (α1 and α2) (1,2).
Extrapyramidal side effects of SGAs are less frequent than those
observed in patients taking first-generation antipsychotics (FGAs)
(2). However, SGAs frequently
cause metabolic dysfunctions, such as abnormal weight gain,
hyperglycemia, and dyslipidemia (1). Olanzapine is an SGA classified as a
multi-acting receptor-targeted antipsychotic (MARTA). Although
olanzapine is widely used for the treatment of schizophrenia, the
frequency of abnormal weight gain associated with its
administration is the highest among SGAs (1,3).
Abnormal body weight gain increases the risk of hyperlipidemia and
type 2 diabetes and reduces patient compliance (4). Although an increase in food intake is
a major cause of weight gain during olanzapine therapy (5), some patients still become obese while
taking the drug even if they do not increase their food intake.
Obesity is caused by enhanced energy uptake that is
not balanced by energy expenditure. Energy sources, such as glucose
and lipids, are stored as triacylglycerols in adipocytes. During
this process, preadipocytes differentiate into adipocytes.
Thereafter, adipocytes accumulate triacylglycerols as lipid
droplets via maturation (6).
3T3-L1 murine preadipocytes are an established in-vitro
model for exploring various facets of adipogenesis (6,7). In
this model, the differentiation of preadipocytes into adipocytes is
induced by stimulation with dexamethasone (DEX),
isobutyl-methyl-xanthine (IBMX), and insulin; subsequently, the
differentiated cells are cultured in insulin-supplemented culture
medium for maturation (6).
Peroxisome proliferator-activated receptor γ (PPARγ) is a crucial
regulator of these processes (6,8,9).
Perilipin is expressed during adipocyte maturation, and it
localizes to the surface of lipid droplets (10). Under normal conditions, perilipin
suppresses triacylglycerol hydrolysis catalyzed by adipose
triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) in
lipid droplets (10-14).
Stimulation of the adrenaline β receptor expressed in adipocytes
leads to perilipin phosphorylation mediated by cyclic AMP-dependent
protein kinase (PKA), which then accelerates ATGL activation and
induces HSL translocation into lipid droplets (10,11,13).
It is assumed that patients administered olanzapine who do not
increase food intake have a low glucose (LG) concentration and weak
adipocyte differentiation and maturation stimulation conditions
compared with patients with increased food intake. In this study,
we investigated the effects of olanzapine on adipogenesis and
lipolysis in 3T3-L1 cells under low-glucose and weak
differentiation and maturation conditions.
Materials and methods
Materials
Murine preadipocytes (3T3-L1 cells) were purchased
from the Japanese Cancer Research Resources Bank (JCRB; Osaka,
Japan). Dulbecco's modified Eagle's medium (DMEM), fetal bovine
serum (FBS), insulin (I6634), DEX (D4902), and IBMX (I5879) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Olanzapine
(150-03071) was purchased from FUJIFILM Wako Pure Chemical
Corporation (Osaka, Japan). Isoprenaline hydrochloride (I0260) was
purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). The
Phosphatase Inhibitor Cocktail (EDTA-free; 07575-51) was purchased
from Nacalai Tesque Inc. (Kyoto, Japan).
Antibodies against β-actin (Sigma-Aldrich, A5441),
PPARγ [Cell Signaling Technology (CST), Massachusetts, USA, 2435],
perilipin (D1D8) XP (CST, 9349), and p-perilipin 1 (Vala Science,
CA, USA, 4856; specificity/target: Recognizes human perilipin 1
phosphorylated at serine 522; equivalent to serine 517 of the
murine sequence) were used as primary antibodies. Anti-mouse
IgG-HRP (Santa Cruz Biotechnology, Texas, USA, sc-2005) and
anti-rabbit IgG-HRP (CST, 7074) were used as the secondary
antibodies. Block Ace powder was obtained from DS Pharma
Biochemical Co., Ltd (Osaka, Japan). The ECL Prime Western Blotting
Detection Reagent was purchased from Cytiva (Tokyo, Japan,
RPN2232).
Adipogenesis of 3T3-L1 cells
3T3-L1 cells were maintained in DMEM supplemented
with 10% FBS at 37˚C in a 5% CO2 atmosphere. The cells
were devoid of Mycoplasma contamination based on results of
the Takara PCR Mycoplasma Detection Set (Takara Bio Inc.,
Shiga, Japan). The cells were seeded at a density of
1.0x105 cells/mL in a 24-well plate. After 5 days, the
medium was changed to DMEM supplemented with 1.6 µM insulin, 1 µM
DEX, and 500 µM IBMX, and cells were cultured for 48 h to induce
differentiation. Next, the medium was changed to DMEM containing
1.6 µM insulin every 48 h, and the cells were cultured for 8 days
for maturation.
Olanzapine was dissolved in dimethyl sulfoxide (100
mM) and added to cells at concentrations ranging between 2.5 and 10
µM when the culture medium for differentiation and/or maturation
was changed. Glucose was either added at 5.5 mM (LG) or 25 mM (high
glucose, HG). To examine the effects of olanzapine on adipogenesis
under conditions of LG and weak stimulation during differentiation
and maturation (1/10-fold conditions), cells were treated with 10
µM olanzapine every 2 days for 10 days. When the effects of
olanzapine on differentiation were observed, differentiation was
induced under 1´ and 1/10´ conditions. In this case, maturation was
induced in the presence of insulin alone, without olanzapine.
However, when the effects of olanzapine on maturation were
assessed, maturation was induced under 1x or 1/10 and 1/100´
conditions. In this case, differentiation was induced in the
presence of insulin, DEX, and IBMX, without olanzapine.
Oil red O staining
Ten days after differentiation, the 3T3-L1 cells
were stained with oil red O to check the lipid content. Briefly,
the cells were fixed with 10% formaldehyde for 1 h. After washing
with water, they were immersed in 0.3% oil red O solution prepared
in 60% isopropanol for 1 h. The non-specific staining was removed
by washing with 60% isopropanol and images were acquired.
Effects of olanzapine on
lipolysis
3T3-L1 cells were differentiated and matured for 10
days under HG conditions. The medium was changed to LG medium
without insulin, and the cells were treated with 10 µM olanzapine
for 1 h. The cells were then treated with 10 µM isoprenaline for 1
h. The cells were lysed in sodium dodecyl sulfate (SDS) sample
buffer [25 mM tris-HCl (pH 6.8), 0.8% SDS, 5% glycerol] in the
presence of the Phosphatase Inhibitor Cocktail and boiled.
Perilipin phosphorylation was examined by western blot
analysis.
Western blotting
Western blotting was performed using previously
described methods (15,16). Briefly, the samples were separated
using acrylamide gels and transferred to nitrocellulose membranes.
The membranes were blocked with 4% Block Ace solution for 1 h.
Subsequently, the membrane was incubated with the primary
antibodies specific to β-actin (1:5,000), PPARγ (1:1,000),
perilipin (1:5,000), and p-perilipin (pS517) (1:1,000). Next, the
membrane was incubated with the relevant HRP-linked secondary
antibodies, and immunoreactive signals were detected using the ECL
Prime Western Blotting Detection Reagent.
Statistical analysis
Each experiment was repeated twice, and thus,
statistical analysis was not performed.
Results
Olanzapine enhances lipid droplet
accumulation in 3T3-L1 adipocytes under low-glucose conditions
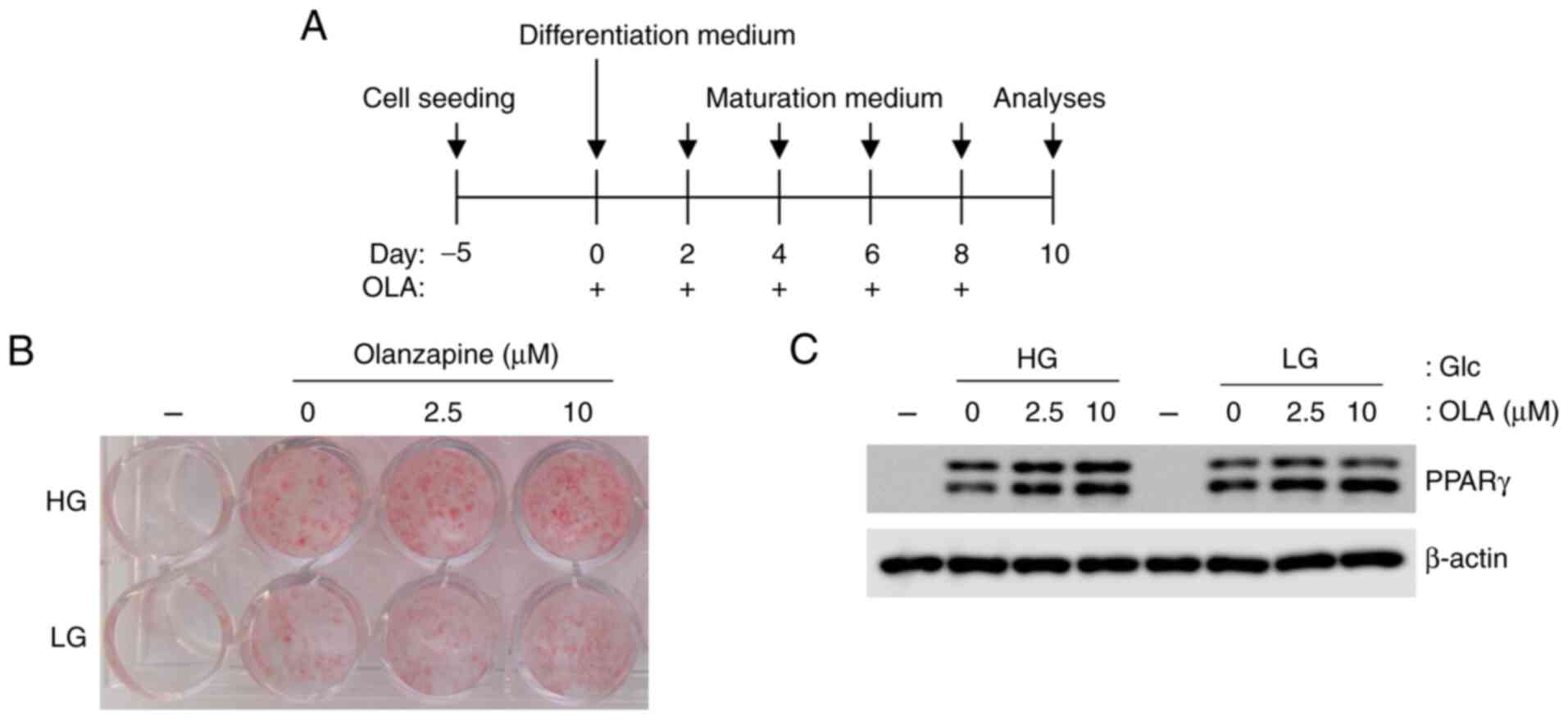
To verify whether olanzapine promotes the
accumulation of lipid droplets in adipocytes under LG conditions,
3T3-L1 cells were differentiated and matured under high- and
low-glucose conditions in the presence of olanzapine (0-10 µM). Oil
red O staining showed that olanzapine enhanced lipid droplet
accumulation in adipocytes under both HG and LG conditions
(Fig. 1A). Although lipid
accumulation was lower under LG conditions than under HG
conditions, olanzapine increased adipogenesis under LG conditions
compared with observations made in the absence of olanzapine
(Fig. 1B). The expression of PPARγ
also increased in response to olanzapine under both conditions
(Fig. 1C). In addition,
adipogenesis with olanzapine at 10 µM was increased compared with
that with olanzapine at 2.5 µM. Therefore, olanzapine at 10 µM was
used in the following experiments.
Olanzapine induces adipogenesis under
weak differentiation and maturation stimulation conditions
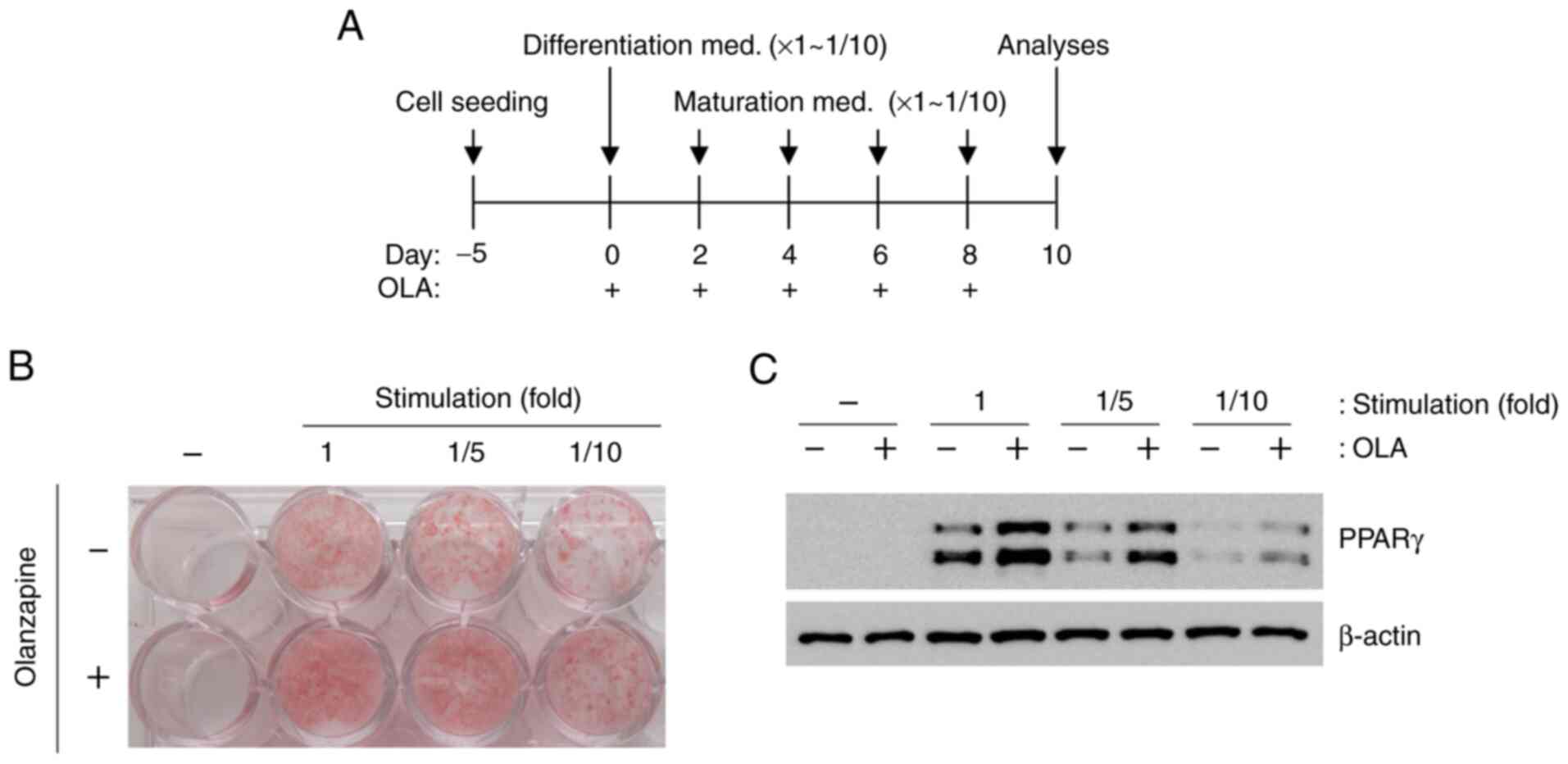
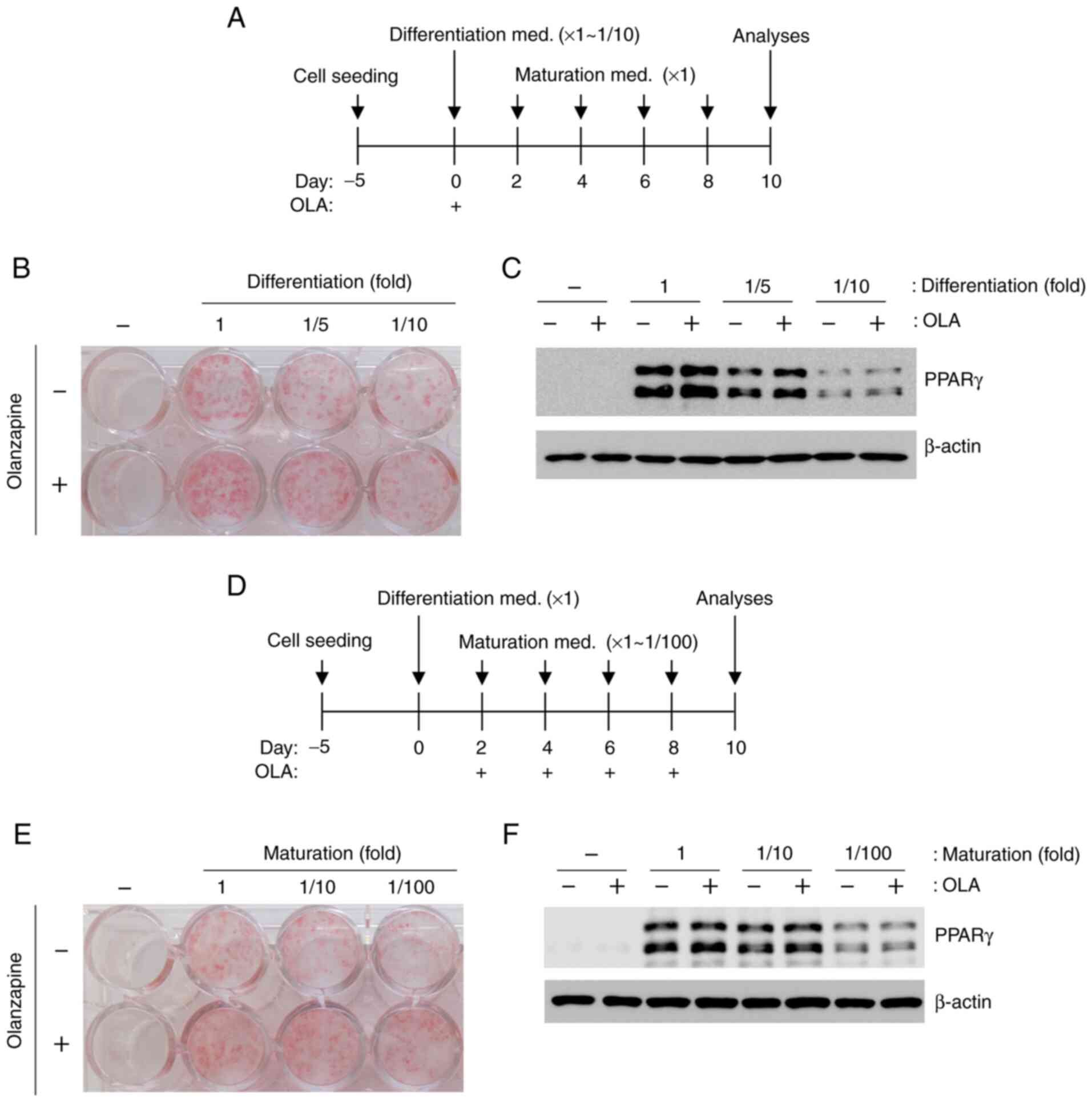
To examine the effects of olanzapine on adipogenesis
under LG and weak stimulation conditions, differentiation and
maturation were induced by weak stimulation (reduced by 1/10-fold)
(Fig. 2A). However, olanzapine
treatment led to enhanced oil red O staining and PPARγ expression
under all conditions (Fig. 2B and
C), although these changes were
not robust in response to weak stimulation conditions. When
olanzapine was added to stimulated 3T3-L1 cells only during
differentiation, oil red O staining and PPARγ expression increased
under all conditions (Fig. 3A-C).
In addition, adipogenesis increased following olanzapine
stimulation during maturation (Fig.
3D-F).
Effect of olanzapine on lipolysis
To determine the effect of olanzapine on lipolysis,
we analyzed perilipin phosphorylation in mature 3T3-L1 cells
stimulated with isoprenaline. Our results showed that olanzapine
treatment suppressed perilipin phosphorylation (Fig. 4).
Discussion
The number of patients with psychiatric disorders
continues to increase owing to various reasons. Schizophrenia is a
psychiatric disorder that typically occurs in late adolescence and
early adulthood (1). The symptoms
of the condition are classified as either positive symptoms,
including hallucinations and delusions following an increase in
dopamine levels, or negative symptoms, such as apathy and avolition
owing to functional decline of the glutamine acid nerve (1). Medications for schizophrenia are
classified as first- or second-generation antipsychotics. FGAs
strongly inhibit dopamine D2 receptors and are useful
for treating positive symptoms; however, extrapyramidal disorders
frequently result from using these agents (1). SGAs inhibit not only the dopamine
D2 receptor but also other neuroreceptors, such as the
serotonin 5-HT2 receptor and noradrenalin receptors (α1
and α2) (1,2). SGAs are classified as serotonin
dopamine antagonists, MARTAs, and dopamine partial agonists.
Although the extrapyramidal effects caused by these medications are
less frequent than those caused by FGAs, SGAs induce metabolic
dysfunctions, such as abnormal weight gain (1,2). One
reason for this is the increase in food intake following increased
appetite (1). However, some
patients without enhanced appetite are also prone to weight gain,
and the mechanism underlying this effect remains unknown.
Although lack of quantification (owing to n=2) was a
limitation of this study, olanzapine showed the tendency to promote
adipogenesis in 3T3-L1 cells, even under LG and weak
differentiation and maturation conditions. LG (5.5 mM glucose)
represents the global age-standardized mean fasting plasma glucose
concentration (17). Accumulated
triacylglycerols in adipocytes are hydrolyzed by ATGL and HSL
(10,11,13).
Perilipin is located on the surface of lipid droplets and binds to
comparative gene identification-58 (CGI-58), an ATGL activating
factor, and regulates ATGL activity (11,14).
Perilipin also prevents HSL from approaching lipid droplets. When
adipocytes are stimulated by β-adrenaline, perilipin is
phosphorylated by PKA (10,11,13).
CGI-58 detaches from phospho-perilipin and binds to ATGL, which
then accelerates the activation of ATGL. Furthermore, HSL can then
approach lipid droplets and hydrolyze triacylglycerols. Olanzapine
reportedly suppressed isoprenaline-induced lipolysis in 3T3-L1
cells (18). In our study,
olanzapine suppressed perilipin phosphorylation, which might
decrease triacylglycerol hydrolysis. These results possibly explain
the weight gain observed in patients who do not present with an
increased appetite during olanzapine therapy. However, the
olanzapine concentrations used in this study were higher than those
present in the blood in vivo. Previous studies reported that
5 µM olanzapine increased the viability of 3T3-L1 cells by 10%. On
other hand, the growing rates were inhibited by olanzapine
treatments from 10 to 20 µM (19).
They showed that 5 µM olanzapine induced apoptosis (<1%) and
increased cell growth. Lv et al (19) demonstrated the phenomenon is one of
the reasons of olanzapine-induced obesity, but they did not show
the direct effects of olanzapine on adipogenesis. Moreover, the
glucose concentration of medium was not mentioned, making it
difficult to compare our results with their findings (19). Additionally, adipogenesis are
regulated by various transcription factors such as
CCAAT/enhancer-binding proteins (C/EBPs), signal transducers and
activators of transcriptions (STATs) (20,21),
and Yanjie et al (22)
reported olanzapine induced AMP-activated protein kinase-α
(AMPKα)-Sterol regulatory element binding protein (SREBP) pathway,
which is involved in lipogenesis and cholesterogenesis, in 3T3-L1
cells. Therefore, further studies, including in vivo
experiments and analyses of various factors are necessary to
clarify the detailed mechanisms underlying abnormal weight gain in
patients who do not present with olanzapine-induced increased
appetite.
In conclusion, this study demonstrated that
olanzapine enhances adipogenesis and reduces lipolysis in
adipocytes, even when the cells are cultured under LG and weak
differentiation and maturation stimulatory conditions. These
results provide new insights to elucidate the mechanism underlying
abnormal weight gain without increased appetite in patients taking
olanzapine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM designed the study, conducted the experiments,
analyzed and interpreted the data, and wrote the manuscript. YO
conducted the experiments and analyzed and interpreted the data.
Data interpretation was performed by TT and YS. TM and TT confirmed
the authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferreira V, Grajales D and Valverde ÁM:
Adipose tissue as a target for second-generation (atypical)
antipsychotics: A molecular view. Biochim Biophys Acta Mol Cell
Biol Lipids. 1865(158534)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lieberman JA, Stroup TS, McEvoy JP, Swartz
MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE,
Lebowitz BD, et al: Effectiveness of antipsychotic drugs in
patients with chronic schizophrenia. N Engl J Med. 353:1209–1223.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nimura S, Yamaguchi T, Ueda K, Kadokura K,
Aiuchi T, Kato R, Obama T and Itabe H: Olanzapine promotes the
accumulation of lipid droplets and the expression of multiple
perilipins in human adipocytes. Biochem Biophys Res Commun.
467:906–912. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Milano W, Grillo F, Del Mastro A, De Rosa
M, Sanseverino B, Petrella C and Capasso A: Appropriate
intervention strategies for weight gain induced by olanzapine: A
randomized controlled study. Adv Ther. 24:123–134. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lord CC, Wyler SC, Wan R, Castorena CM,
Ahmed N, Mathew D, Lee S, Liu C and Elmquist JK: The atypical
antipsychotic olanzapine causes weight gain by targeting serotonin
receptor 2C. J Clin Invest. 127:3402–3406. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rubin CS, Hirsch A, Fung C and Rosen OM:
Development of hormone receptors and hormonal responsiveness in
vitro. Insulin receptors and insulin sensitivity in the
preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem.
253:7570–7578. 1978.PubMed/NCBI
|
|
8
|
Rosen ED, Sarraf P, Troy AE, Bradwin G,
Moore K, Milstone DS, Spiegelman BM and Mortensen RM: PPAR gamma is
required for the differentiation of adipose tissue in vivo and in
vitro. Mol Cell. 4:611–617. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Siersbaek R, Nielsen R and Mandrup S:
PPARgamma in adipocyte differentiation and metabolism-novel
insights from genome-wide studies. FEBS Lett. 584:3242–3249.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sztalryd C and Brasaemle DL: The perilipin
family of lipid droplet proteins: Gatekeepers of intracellular
lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids.
1862:1221–1232. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brasaemle DL: Thematic review series:
Adipocyte biology. The perilipin family of structural lipid droplet
proteins: Stabilization of lipid droplets and control of lipolysis.
J Lipid Res. 48:2547–2559. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shen WJ, Patel S, Miyoshi H, Greenberg AS
and Kraemer FB: Functional interaction of hormone-sensitive lipase
and perilipin in lipolysis. J Lipid Res. 50:2306–2313.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bickel PE, Tansey JT and Welte MA: PAT
proteins, an ancient family of lipid droplet proteins that regulate
cellular lipid stores. Biochim Biophys Acta. 1791:419–440.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Granneman JG, Moore HP, Krishnamoorthy R
and Rathod M: Perilipin controls lipolysis by regulating the
interactions of AB-hydrolase containing 5 (Abhd5) and adipose
triglyceride lipase (Atgl). J Biol Chem. 284:34538–34544.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matsuo T, Fujiwara A, Nakamura K and
Sadzuka Y: The effects of vitamin B6 compounds on cell
proliferation and melanogenesis in B16F10 melanoma cells. Oncol
Lett. 15:5181–5184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matsuo T and Sadzuka Y: Extracellular
acidification by lactic acid suppresses glucose deprivation-induced
cell death and autophagy in B16 melanoma cells. Biochem Biophys Res
Commun. 496:1357–1361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Danaei G, Finucane MM, Lu Y, Singh GM,
Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA,
et al: National, regional, and global trends in fasting plasma
glucose and diabetes prevalence since 1980: Systematic analysis of
health examination surveys and epidemiological studies with 370
country-years and 2·7 million participants. Lancet. 378:31–40.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vestri HS, Maianu L, Moellering DR and
Garvey WT: Atypical antipsychotic drugs directly impair insulin
action in adipocytes: Effects on glucose transport, lipogenesis,
and antilipolysis. Neuropsychopharmacology. 32:765–772.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lv Y, Liu S, Tong J, Zhang Q, Zhang Z,
Peng S, Li S, Yang N and Li W and Li W: Atypical antipsychotic
olanzapine induces obese via an apoptotic feedback pathway. J Clin
Toxicol. 10(1000453)2020.
|
|
20
|
Gretchen JD, Sarah ER and Ormond AM: The
role of C/EBP genes in adipocyte differentiation. J Biol Chem.
273:30057–30060. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng Z and Jacqueline MS: Identification
of STAT target genes in adipocytes. JAKSTAT.
2(e23092)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yanjie L, Xiaomin Z, Xiyu F, Xuemei L,
Chao D and Chang HH: Berberine Alleviates olanzapine-induced
adipogenesis via the AMPKα-SREBP pathway in 3T3-L1 cells. Int J Mol
Sci. 17(1865)2016.PubMed/NCBI View Article : Google Scholar
|