Introduction
Lung cancer is the second most commonly diagnosed
type of cancer and the most common cause of cancer-related death
worldwide (1). Among all types of
lung carcinoma, ~80% are non-small cell lung cancer (NSCLC)
(2,3). Only 19% of patients who are diagnosed
with NSCLC survive after 5 years (4). In the last two decades, as a result
of the discovery of bioindicators with the aim of developing
targeted treatments, survival rates have improved (5). Furthermore, the 5-year survival rate
for metastatic conditions has improved to between 15 and 50%
(6,7). Regarding anaplastic lymphoma kinase
(ALK), which is among these cancer biomarkers, gene reorganization
is reported in 3-7% of cases of NSCLC (8). As a result of phase two single-group
studies in ALK-positive NSCLC cases, crizotinib was approved by the
United States Food and Drug Administration as a treatment for this
group of patients (9). In the
phase three PROFILE 1014 study, progression-free survival (PFS) was
found to be 10.9 months in the ALK-positive group, outperforming
the rates resulting from chemotherapy (10). In the final analysis of the PROFILE
1014 study patients in both the crizotinib and chemotherapy arms
had permanently discontinued treatment due to progression at the
final overall survival (OS) analysis, with a median follow-up
duration for OS of 45.7 months with crizotinib and 45.5 months with
chemotherapy (11). In addition,
in a previous study, the success of crizotinib compared with
chemotherapy in first-line treatment inevitably disappeared with
secondary ALK mutation-based crizotinib resistance, which was
revealed to emerge in most cases within the first year in patients
whose ALK rearrangement was positive (12). The present study reported the case
of a 49-year-old woman with NSCLC for whom, after a complete
response to crizotinib for 6 years, treatment was stopped due to
the patient's own will, followed by the emergence of cranial
metastasis and medical recurrence.
Case report
A 49-year-old, nonsmoking housewife, without
occupational chemical exposure or family history of lung cancer,
and complaining of a cough lasting for 2 months was admitted to the
Sultan II. Abdulhamid Han Training and Research Hospital (Istanbul,
Turkey) in November 2015 As a result of bronchoscopic biopsy,
following the detection of a mass in the lung and mediastinal lymph
node metastases on thoracic tomography, the patient was diagnosed
with moderately differentiated invasive adenocarcinoma. On positron
emission tomography-computed tomography (PET-CT) applied for
staging, the pathological size and metabolic activity of the
primary mass, mediastinal and scalene lymph nodes with were
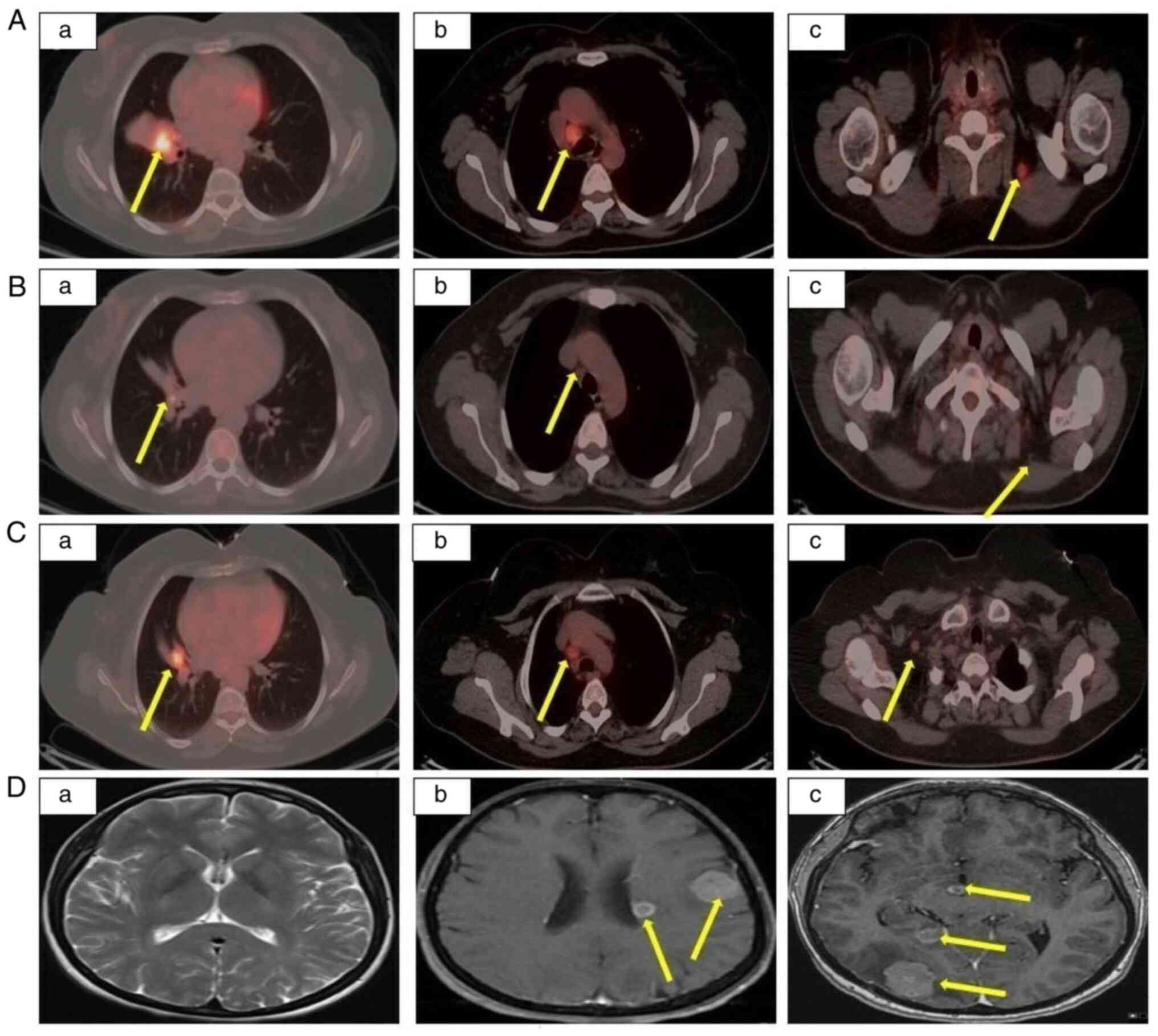
detected (Fig. 1Aa-c). The
patient, whose cranial magnetic resonance did not show any
metastatic lesions, and who was T2N3M0 stage 3B [according to the
7th edition of the tumor, node and metastasis classification
(13)], was diagnosed with
unresectable lung carcinoma. Sanger sequencing method was used for
EGFR detection, and fluorescence in situ hybridization
method was used for ROS-1 and ALK detection (14,15).
Following analysis of EGFR, ALK and ROS1, the patient was
identified as ALK-rearranged variant type 1 (v1) positive. The
patient started treatment with crizotinib in January 2016, at a
daily dosage of 2x250 mg at the Sultan II. Abdulhamid Han
Educational and Research Hospital (Istanbul, Turkey). After three
courses, a complete response was obtained (Fig. 1Ba-c). Adverse events, such as grade
1-2 asthenia, transaminitis and nausea, were reported. Grade 3-4
side effects were not observed. The patient was followed up with a
complete response to crizotinib treatment until February 2022. Upon
the patient's request, the treatment was terminated in February
2022. In May 2022, at the hospital, the patient complained of
headaches and multiple metastatic lesions accompanied by vasogenic
edema were detected in the brain (Fig.
1Db-c). Furthermore, a primary lung mass, metastasis to
mediastinal lymph nodes and suspected right supraclavicular lymph
node metastasis were detected via PET-CT (Fig. 1Ca-c). The patient was initiated on
Alectinib treatment, a second-line ALK inhibitor. The patient's
follow-up and treatment continues.
Discussion
The present study reported the case of a patient
with ALK-rearranged v1 lung adenocarcinoma that showed a complete
response to crizotinib for 6 years and metastatic recurrence after
cessation of treatment. In a phase III study in which crizotinib
was compared with chemotherapy, PFS was revealed to be 10.9 months
(10). In the final analysis of
the PROFILE 1014 study, at the end of the fourth year, the survival
rate in the crizotinib group was 56%, compared with 49% in the
chemotherapy group (11). In the
literature, PFS over 5 years with crizotinib treatment has rarely
been reported (16). The 5-year
estimated PFS rate with crizotinib treatment has previously been
reported as 9% by Rangachari et al (17) in two cases in the metastatic stage.
Kosaka et al (18) reported
that in a patient who developed metastatic recurrence after surgery
and adjuvant chemotherapy, complete response was confirmed after 4
months and was maintained over 5 years after the first
administration of crizotinib. In addition, Gulmez (19) reported on a case of metastatic
recurrence that received crizotinib treatment after postoperative
adjuvant chemotherapy, in which 53-month PFS was obtained.
Regarding the treatment of patients with
ALK-positive NSCLC with crizotinib, two problems must be addressed.
First, it is unclear what the duration of treatment in patients
with locally advanced or metastatic NSCLC who have achieved a
complete response with crizotinib therapy should be. In clinical
practice and randomized controlled trials, in both first-line and
post-treatment patients, the PFS with crizotinib was between 7
months and 1 year (20-22).
In the long-term results of the ALEX study, in which alectinib was
compared with crizotinib, in the fourth year, PFS was 0% in the
crizotinib group (23). As
previously reported, to the best of our knowledge, there is no
evidence-based information in the literature regarding the time
required to continue treatment in patients with long-term and
complete responses. The second issue is that the effect of the
EML4-ALK variant on ALK inhibitor selection has not been clarified.
It has been established that ~20 echinoderm microtubule-associated
protein like 4 (EML4)-ALK fusion subtypes exist (24). Fusion variants are classified
according to their breakpoints (24). The most common EML4-ALK variants
are v1, v2 and v3a/b; the two EML4-ALK variants that together
account for up to 70-80% of all EML4-ALK variants are v1 and v3a/b
(25). Several studies have
explored the potential association between EML4-ALK fusion and the
therapeutic response to crizotinib, but the results are
insufficient to draw a conclusion. These studies reported
differential responses to crizotinib according to ALK variants in
patients. Yoshida et al (26) reported longer responses to
crizotinib with v1 than with non-v1, and the objective response
rate (ORR) and disease control rate of crizotinib-responsive
EML4-ALK v1 were 74 and 95%, respectively, whereas for other ALK
fusions they were 63 and 63%, respectively. Woo et al
(27) demonstrated that patients
with non-v3 EML4-ALK had a longer response to crizotinib than those
with the v3 EML4-ALK, thus suggesting that EML4-ALK v3a/b may be a
major source of ALK inhibitor resistance in the clinical setting.
In another similar study, no statistically significant difference
in PFS was observed between patients with v1 and v3 EML4-ALK that
were treated with crizotinib, although the median PFS was
numerically shorter for v3 than for v1 in all contexts (28). Lei et al (29) did not observe any significant
difference in the efficacy of crizotinib between patients with the
EML4-ALK fusion v3, v1 and the less frequent v2. In a similar
study, Cha et al (30)
found no significant difference in survival between
crizotinib-treated variants. Li et al (31) revealed that PFS in patients with v2
EML4-ALK was significantly higher than that in those with non-v2
EML4-ALK. Notably, although the present case was v1, a complete
response was reached over 6 years. Previous studies have shown that
ORR and PFS obtained with crizotinib treatment vary according to
EML4-ALK variant subtypes (Table
I).
 | Table IDifferences in crizotinib efficacy
according to echinoderm microtubule-associated protein like
4-anaplastic lymphoma kinase variants. |
Table I
Differences in crizotinib efficacy
according to echinoderm microtubule-associated protein like
4-anaplastic lymphoma kinase variants.
| | Variant 1 | Variant 2 | Variant 3a/b | |
|---|
| First author,
year | Total cases, n | n | ORR | PFS | n | ORR | PFS | n | ORR | PFS | (Refs.) |
|---|
| Yoshida et al,
2016 | 35 | 19 | 74% | 11 months | Non-variant | | | | | | (26) |
| Woo et al,
2017 | 51 | Non-variant 3a/b:
n=24; ORR, 83%, 2-year PFSR, 76% | | | 1: n=16; ORR, 63%;
PFS, 4.2 months | | | 20 | 75% | 2 years | (27) |
| Lin et al,
2018 | 129 | 55 | No data | 9.2 months | Number of patients
with non-v1 and non-v3a/b variants was too small | | | 51 | No data | 7.5 months | (28) |
| Lei et al,
2016 | 61 | 22 | 73% | 11 months | No data | No data | No data | 18 | No data | 7.4 months | (29) |
| Cha et al,
2016 | 32 | 10 | 30% | No data | 2 | 100% | No data | 8 | 50% | No data | (30) |
| Li et al,
2018 | 60 | 14 | 46% | 10.7 months | 9 | 67% | 18.5 months | 20 | 65% | 7.9 months | (31) |
As aforementioned, in previous studies, relatively
long-term PFS was observed more frequently in EML4-ALK v1 and v2
subtypes with initial crizotinib treatment. However, it is unclear
if these subtypes should be considered in treatment decisions due
to insufficient evidence. Therefore, the efficacy of EML4-ALK
variants in ALK-positive NSCLC remains an important question to be
answered in the future.
To the best of our knowledge, this is the fifth case
reported in the literature of NSCLC with a long-term complete
response to crizotinib treatment. In addition, the present case is
the first to achieve a complete response for >6 years with
first-line crizotinib treatment in a locally advanced unresectable
condition. Therefore, the present case seems to be valuable from a
clinical standpoint.
In conclusion, prospective studies are needed to
determine target-based agents according to variant subtype in
first-line treatment and on the duration of treatment for patients
with ALK-positive NSCLC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LE and OO contributed to the conceptualization and
design of the study. OO collected clinical information and assisted
with drafting the manuscript. LE searched the literature and wrote
the manuscript. LE and OO confirmed the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent was obtained from the patient for
publication of their data and images included in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Barlesi F, Mazieres J, Merlio JP,
Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I,
Westeel V, et al: Routine molecular profiling of patients with
advanced non-small-cell lung cancer: Results of a 1-year nationwide
programme of the French cooperative thoracic intergroup (IFCT).
Lancet. 387:1415–1426. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rodríguez M, Ajona D, Seijo LM, Sanz J,
Valencia K, Corral J, Mesa-Guzmán M, Pío R, Calvo A, Lozano MD, et
al: Molecular biomarkers in early stage lung cancer. Transl Lung
Cancer Res. 10:1165–1185. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reck M, Rodriguez-Abreu D, Robinson A, Hui
R, Csoszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et
al: Updated analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol.
37:537–546. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johung KL, Yeh N, Desai NB, Williams TM,
Lautenschlaeger T, Arvold ND, Ning MS, Attia A, Lovly CM, Goldberg
S, et al: Extended survival and prognostic factors for patients
with ALK-rearranged non-small-cell lung cancer and brain
metastasis. J Clin Oncol. 34:123–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Crinò L, Kim D, Riely GJ, Janne PA,
Blackhall FH, Hirsh DRC, Mok T, Solomon JB, Park K, Gadgeel SM, et
al: Initial phase II results with crizotinib in advanced
ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J
Clin Oncol. 29 (Suppl 15)(S7514)2011.
|
|
10
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Solomon BJ, Kim DW, Wu YL, Nakagawa K,
Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Tang Y, et al:
Final overall survival analysis from a study comparing first-line
crizotinib versus chemotherapy in ALK-mutation-positive
non-small-cell lung cancer. J Clin Oncol. 36:2251–2258.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dagogo-Jack I and Shaw AT: Crizotinib
resistance: Implications for therapeutic strategies. Ann Oncol. 27
(Suppl 3):iii42–iii50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Conde E, Rojo F, Gómez J, Enguita AB,
Abdulkader I, González A, Lozano D, Mancheño N, Salas C, Salido M,
et al: Molecular diagnosis in non-small-cell lung cancer: Expert
opinion on ALK and ROS1 testing. J Clin Pathol. 75:145–153.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sheikine Y, Rangachari D, McDonald DC,
Huberman MS, Folch ES, VanderLaan PA and Costa DB: EGFR testing in
advanced non-small-cell lung cancer, a mini-review. Clin Lung
Cancer. 17:483–492. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ricciuti B, De Giglio A, Mecca C, Arcuri
C, Marini S, Metro G, Baglivo S, Sidoni A, Bellezza G, Crinò L and
Chiari R: Precision medicine against ALK-positive non-small cell
lung cancer: Beyond crizotinib. Med Oncol. 35(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rangachari D, Le X, Shea M, Huberman MS,
VanderLaan PA, Kobayashi SS and Costa DB: Cases of ALK-rearranged
lung cancer with 5-year progression-free survival with crizotinib
as initial precision therapy. J Thorac Oncol. 12:e175–e177.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kosaka T, Yajima T, Yamaki E, Nakazawa S,
Tomizawa K, Onozato R, Yamazaki A, Hirato J, Yatabe Y, Shimizu K,
et al: Long-term complete response in a patient with postoperative
recurrent ALK-rearranged lung adenocarcinoma treated with
crizotinib: A case report. Mol Clin Oncol. 11:309–312.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gulmez A Dr: Prolonged survival without
progression under crizotinib treatment. Cancer Treat Res Commun.
25(100259)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nakagawa K, Hida T, Nokihara H, Morise M,
Azuma K, Kim YH, Seto T, Takiguchi Y, Nishio M, Yoshioka H, et al:
Final progression-free survival results from the J-ALEX study of
alectinib versus crizotinib in ALK-positive non-small-cell lung
cancer. Lung Cancer. 139:195–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nishio M, Kim DW, Wu YL, Nakagawa K,
Solomon BJ, Shaw AT, Hashigaki S, Ohki E, Usari T, Paolini J, et
al: Crizotinib versus chemotherapy in Asian patients with
ALK-positive advanced non-small cell lung cancer. Cancer Res Treat.
50:691–700. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mok T, Camidge DR, Gadgeel SM, Rosell R,
Dziadziuszko R, Kim DW, Pérol M, Ou SI, Ahn JS, Shaw AT, et al:
Updated overall survival and final progression-free survival data
for patients with treatment-naive advanced ALK-positive
non-small-cell lung cancer in the ALEX study. Ann Oncol.
31:1056–1064. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lei Y, Lei Y, Shi X and Wang J: EML4-ALK
fusion gene in non-small cell lung cancer. Oncol Lett.
24(277)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pan Y, Deng C, Qiu Z, Cao C and Wu F: The
resistance mechanisms and treatment strategies for ALK-rearranged
non-small cell lung cancer. Front Oncol. 11(713530)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yoshida T, Oya Y, Tanaka K, Shimizu J,
Horio Y, Kuroda H, Sakao Y, Hida T and Yatabe Y: Differential
crizotinib response duration among ALK fusion variants in
ALK-positive non-small-cell lung cancer. J Clin Oncol.
34:3383–3389. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Woo CG, Seo S, Kim SW, Jang SJ, Park KS,
Song JY, Lee B, Richards MW, Bayliss R, Lee DH and Choi J:
Differential protein stability and clinical responses ofEML4-ALK
fusion variants to various ALK inhibitors in advancedALK-rearranged
non-small cell lung cancer. Ann Oncol. 28:791–797. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock
AB, Dagogo-Jack I, Jessop NA, Jiang GY, Le LP, Gowen K, et al:
Impact of EML4-ALK variant on resistance mechanisms and clinical
outcomes in ALK-positive lung cancer. J Clin Oncol. 36:1199–1206.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lei YY, Yang JJ, Zhang XC, Zhong WZ, Zhou
Q, Tu HY, Tian HX, Guo WB, Yang LL, Yan HH, et al: Anaplastic
lymphoma kinase variants and the percentage of ALK-positive tumor
cells and the efficacy of crizotinib in advanced NSCLC. Clin Lung
Cancer. 17:223–231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cha YJ, Kim HR and Shim HS: Clinical
outcomes in ALK-rearranged lung adenocarcinomas according to ALK
fusion variants. J Transl Med. 14(296)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li Y, Zhang T, Zhang J, Li W, Yuan P, Xing
P, Zhang Z, Chuai S, Li J and Ying J: Response to crizotinib in
advanced ALK-rearranged non-small cell lung cancers with different
ALK-fusion variants. Lung Cancer. 118:128–133. 2018.PubMed/NCBI View Article : Google Scholar
|