Introduction
Upper tract urothelial carcinoma (UTUC) is a fairly
uncommon cancer worldwide (1) and
it accounts for only 5% of all urothelial carcinoma (2). However, it represents a relatively
higher prevalence in Taiwan than in other countries, especially in
the Southwest coast area (3).
Based on high risk of tumor recurrence and progression, radical
nephroureterectomy (RNU) and bladder cuff excision (BCE) remains
the gold standard of treatment for localized UTUC. Following
surgery, intravesical recurrence is not rare, accounting for 20-50%
of all cases (4,5). So, it is necessary to perform routine
cystoscopy plus urine cytology for early detection of bladder tumor
recurrence. Previous studies have attempted to analyze risk factors
for identifying patients who were at risk of high bladder tumor
recurrence (6). A systemic review
by Seisen et al (7)
reported that male, previous bladder cancer, preoperative renal
impairment, positive preoperative urinary cytology, lower ureteral
tumor, multifocality, invasive pT stage and necrosis significantly
correlated with the poor intravesical recurrence-free rate.
However, the precisely predictive factors for postoperative bladder
recurrence remain to be elucidated.
A large-scale retrospective cohort study revealed
that moderate to severe chronic kidney disorder [CKD; glomerular
filtration rate (eGFR) <45] had a higher risk for urothelial
carcinoma incidence (8). CKD is
also reported to significantly correlate with bladder recurrence in
urothelial carcinoma following surgery (9,10).
It is hypothesized that CKD is closely associated with increased
inflammation (11). Thus, it
results in tumor recurrence and progression based on the important
role of inflammation in cancer development and growth (12). In addition, a number of studies
have shown that neutrophil-to-lymphocyte ratio (NLR) is indicated
as a systemic inflammation marker that is significantly associated
with oncologic outcomes (13,14).
Kishimoto et al (15)
demonstrate that NLR is a prognostic factor affecting intravesical
recurrence following RNU and BCE.
Yoshitomi et al (16) found that the value of NLR is
associated with renal outcomes in patients with CKD. It is
reasonable that NLR and CKD are considered for postoperative
bladder recurrence in patients with UTUC following RNU. No studies,
to the best of the authors' knowledge, suggest any prognostic
association of combining preoperative eGFR and NLR with bladder
recurrence in patients with UTUC. Therefore, the present study
aimed to evaluate the significance of preoperative eGFR and NLR in
predicting bladder recurrence in patients following RNU to treat
pure UTUC that excluded previous/concomitant bladder cancer and
other malignancies.
Patients and methods
Patient population
The present retrospective study was conducted at
National Cheng Kung University medical center, Tainan, Taiwan. It
collected patients with pure UTUC receiving RNU and BCE at the
institute between January 2008 and December 2019 and had approval
from the ethical committee of the National Cheng Kung University
Hospital (IRB number: A-ER-103-036). Pure UTUC referred to no
previous/concomitant bladder cancer or other malignancy. RNU was
laparoscopically performed with the extravesical approach to BCE.
Demographic data included age, sex, renal function status based on
eGFR, hemodialysis, hydronephrosis, hematuria, diabetes mellitus
(DM), hypertension (HTN), tumor location (renal pelvis vs. ureter
vs. both), pathologic tumor stage, lymph node (LN) metastasis,
tumor grade, tumor necrosis and lymphovascular invasion (Table I). Patients diagnosed with distant
metastasis and those with history of kidney transplantation, on
immunosuppressive agent or neoadjuvant/adjuvant chemotherapy, with
a fever episode (>38.3˚C) within 30 days before surgery, or
hematological disorder were excluded from the present study.
 | Table IClinicopathologic characteristics in
patients with upper tract urothelial carcinoma after radical
nephroureterectomy stratified by preoperative eGFR and NLR. |
Table I
Clinicopathologic characteristics in
patients with upper tract urothelial carcinoma after radical
nephroureterectomy stratified by preoperative eGFR and NLR.
| | Renal function
status | eGFR ≥45 | eGFR <45 | |
|---|
| | NLR | <3.8 | >3.8 | <3.8 | >3.8 | |
|---|
| Clinicopathologic
characteristic | Total patients,
n=362 | n=176 | n=46 | n=90 | n=50 | P-value |
|---|
| Mean age (year) | 70.7±10.5 | 69.8±10.4 | 71.1±11.4 | 72.6±8.9 | 70.1±12.3 | |
| Age (year) | | | | | | 0.216 |
|
≤65 | 99 (27%) | 54 (31%) | 14 (30%) | 17 (19%) | 14 (28%) | |
|
>65 | 218 (73%) | 122 (69%) | 32 (70%) | 73 (81%) | 36 (72%) | |
| Sex | | | | | | 0.686 |
|
Male | 151 (42%) | 69 (39%) | 20 (43%) | 42 (47%) | 20 (41%) | |
|
Female | 211 (58%) | 107 (61%) | 26 (57%) | 48 (53%) | 30 (59%) | |
| Hemodialysis | | | | | | <0.001 |
|
No | 329 (90%) | 177 (100%) | 46 (100%) | 72 (80%) | 34 (67%) | |
|
Yes | 35 (10%) | 0 (0%) | 0 (0%) | 18 (20%) | 17 (33%) | |
| DM or HTN | | | | | | 0.223 |
|
Absent | 146 (40%) | 76 (43%) | 22 (48%) | 33 (37%) | 15 (30%) | |
|
Present | 216 (60%) | 100 (57%) | 24 (52%) | 57 (63%) | 35 (70%) | |
| Hematuria | | | | | | 0.376 |
|
No | 47 (13%) | 21 (12%) | 6 (13%) | 16 (18%) | 4 (10%) | |
|
Yes | 315 (87%) | 155 (88%) | 40 (87%) | 74 (82%) | 46 (90%) | |
| Hydronephrosis | | | | | | 0.523 |
|
No | 68 (19%) | 33 (19%) | 12 (26%) | 14 (16%) | 9 (18%) | |
|
Yes | 294 (81%) | 143 (81%) | 34 (74%) | 76 (84%) | 41 (82%) | |
| Tumor location | | | | | | 0.088 |
|
Pelvis | 180 (50%) | 89 (51%) | 30 (65%) | 38 (42%) | 23 (46%) | |
|
Ureter | 121 (33%) | 64 (36%) | 10 (22%) | 30 (34%) | 17 (34%) | |
|
Both | 61 (17%) | 23 (13%) | 6 (13%) | 22 (24%) | 10 (20%) | |
| Pathological T
stage | | | | | | 0.332 |
|
pTa/1 | 147 (40%) | 74 (42%) | 16 (35%) | 38 (42%) | 19 (38%) | |
|
pT2 | 75 (21%) | 40 (23%) | 5 (11%) | 18 (20%) | 12 (24%) | |
|
pT3/4 | 140 (39%) | 62 (35%) | 25 (54%) | 34 (38%) | 19 (38%) | |
| Lymph node
status | | | | | | 0.466 |
|
Nx/0 | 343 (95%) | 169 (96%) | 42 (91%) | 86 (96%) | 46 (92%) | |
|
N+ | 19 (5%) | 7 (4%) | 4 (9%) | 4 (4%) | 4 (8%) | |
| Tumor grade | | | | | | 0.107 |
|
Low | 19 (5%) | 14 (8%) | 4 (4%) | 3 (3%) | 0 (0%) | |
|
High | 343 (95%) | 162 (92%) | 44 (96%) | 87 (97%) | 50 (100%) | |
| Tumor size
(cm) | | | | | | 0.012 |
|
≤3 | 207 (57%) | 115 (65%) | 19 (41%) | 47 (52%) | 26 (52%) | |
|
>3 | 155 (43%) | 61 (35%) | 27 (59%) | 43 (48%) | 24 (48%) | |
| Carcinoma in
situ | | | | | | 0.346 |
|
Absent | 287 (79%) | 143 (81%) | 34 (74%) | 74 (82%) | 36 (72%) | |
|
Present | 75 (21%) | 33 (19%) | 12 (26%) | 16 (18%) | 14 (28%) | |
| Lymphovascular
invasion | | | | | | 0.225 |
|
Absent | 272 (75%) | 139 (79%) | 31 (67%) | 63 (70%) | 39 (78%) | |
|
Present | 90 (25%) | 37 (21%) | 15 (33%) | 27 (30%) | 11 (22%) | |
| Tumor necrosis | | | | | | 0.123 |
|
No | 291 (80%) | 150 (85%) | 36 (78%) | 69 (77%) | 36 (72%) | |
|
Yes | 71 (20%) | 26 (15%) | 10 (22%) | 21 (23%) | 14 (28%) | |
Pathologic tumor grade was determined using the 2004
World Health Organization (WHO) grading system (17) and tumor staging was determined
according to the 7th edition of the American Joint Committee on
Cancer TNM classification (18) by
various urological pathologists. The eGFR was calculated by the
Modification of Diet in Renal Disease (MDRD) Study equation: 186 x
(serum creatinine)-1.154 x (age)-0.203 x
(0.742 if female) (19).
The cut-off value of NLR was determined as 3.8 for
predicting intravesical recurrence following RNU, according to
Kishimoto et al (15).
Basic hemogram data were obtained within 30 days
before the surgery. All patients routinely underwent cystoscopy
during postoperative follow-up to see whether recurrent bladder
tumors. Moreover, the confirmation of bladder cancer recurrence was
diagnosed via pathological report. Other postoperative follow-up
evaluations involved history-taking, physical examination,
urinalysis, urine cytology, cystoscopy, bladder/renal ultrasound
and radiologic imaging every three months during the first two
years, every six months from the third through the fourth year and
annually thereafter.
Statistical analysis
The Chi-square test was used to compare differences
according to eGFR (> and <45 ml/min/1.73 m2) and
NLR (> and <3.8). Intravesical recurrence-free survival
(IVRF) was defined as the interval from RNU until a pathologically
confirmative diagnosis of bladder urothelial malignancy. The
effects of preoperative eGFR and NLR on IVRF were estimated using
Kaplan-Meier survival plots with the log-rank test. Parameters
associated with IVRF were analyzed by univariate and multivariate
Cox proportional hazard regression models, which estimated hazard
ratio (HR) and evaluated the significance of IVRF. All statistical
analyses were performed using SPSS version 21.0 (IBM Corp.).
Additionally, R software version 3.5.3 (http://www.r-project.org) was used to construct a
nomogram predicting the 2- and 5-year IVRF according to variables
resulting from multivariable Cox models. P<0.05 was considered
to indicate a statistically significant difference.
Results
Characteristics of the patients with
different CKD status and NLR level
A total of 362 eligible patients with pure UTUC who
underwent RNU between January 2008 and December 2019 were
collected. All enrolled patients were stratified into four groups
based on eGFR and NLR and analyzed differences in
clinicopathological characteristics between groups, shown in
Table I. Except for tumor size,
there was no difference in age, sex, hematuria, hydronephrosis, DM
or HTN, tumor location, pathological T stage, LN involvement, tumor
grade, CIS, tumor necrosis and LVI.
Association of preoperative renal
function and NLR with bladder recurrence
The present study showed that preoperative poor
renal function and high NLR had a trend of poor IVFR in
Kaplan-Meier analyses (Table SI;
Figs. S1 and S2). In 362 patients with UTUC, 103 (28%)
had bladder recurrence following surgery with a mean and median
follow-up time of 51.1 and 50.1 months, respectively (Table II). Among 103 patients with
postoperative bladder recurrence, 85 (83%) suffered from bladder
recurrence rate within the first two years after RNU. Notably,
patients with eGFR <45 and NLR >3.8 had a relatively higher
bladder recurrence rate compared with other groups. When
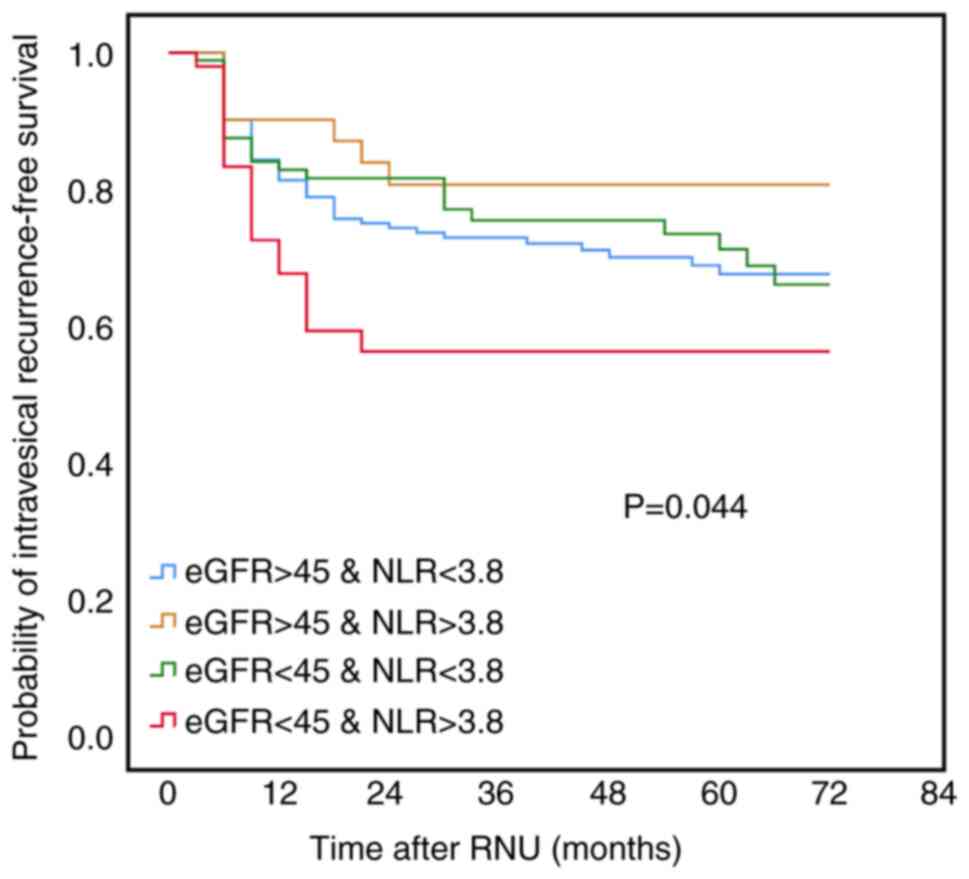
preoperative eGFR and NLR were analyzed together for bladder
recurrence, Kaplan-Meier analyses showed that eGFR <45
synchronous with NLR >3.8 was significantly associated with a
poorer IVRF as compared with eGFR >45 with NLR >3.8, eGFR
>45 with <3.8, or eGFR <45 with NLR <3.8 (Fig. 1).
 | Table IIComparison of bladder recurrence rate
in 362 patients with upper tract urothelial carcinoma after radical
nephroureterectomy according to preoperative renal function status
and NLR. |
Table II
Comparison of bladder recurrence rate
in 362 patients with upper tract urothelial carcinoma after radical
nephroureterectomy according to preoperative renal function status
and NLR.
| | eGFR ≥45 | eGFR <45 |
|---|
| Variable | All patients | NLR <3.8
(n=176) | NLR >3.8
(n=46) | NLR <3.8
(n=90) | NLR >3.8
(n=50) |
|---|
| Postoperative
time | | | | | |
|
Mean time
(month) | 51.1±31.4 | 53.8±30.1 | 43.6±31.8 | 54.9±32.3 | 41.2±31.6 |
|
Median time
(month) | 50.1 | 51.5 | 46.2 | 57.5 | 34.7 |
| BR rate | | | | | |
|
2 years BR,
n (%) | 85 (23%) | 43 (24%) | 7 (15%) | 16 (18%) | 19 (38%) |
|
BR, n
(%) | 103 (28%) | 50 (28%) | 8 (17%) | 26 (29%) | 19 (38%) |
Bladder recurrence according to
preoperative renal function and NLR
As the Kaplan-Meier plot revealed that preoperative
eGFR <45 and NLR >3.8 was significantly associated with a
poor IVRF in patients with UTUC, univariate and multivariate Cox
regression analyses were conducted to evaluate the significance of
each parameter for IVRF (Table
III). In univariate analysis, tumor location involving both
pelvis and ureter (HR: 2.018, 95%CI: 1.231-3.309, P=0.005) and
preoperative eGFR <45 and NLR >3.8 (HR: 1.819, 95%CI:
1.070-3.092, P=0.027) were apparently associated with an inferior
IVRF. In multivariate analysis, tumor location (HR: 1.918, 95%CI:
1.157-3.181, P=0.012) and preoperative eGFR <45 and NLR >3.8
(HR: 1.753, 95%CI: 1.030-2.983, P=0.038) remained significant.
 | Table IIIUnivariate and multivariate Cox
regression analyses for predicting intravesical recurrence-free
survival in patients with upper tract urothelial carcinoma after
radical nephroureterectomy. |
Table III
Univariate and multivariate Cox
regression analyses for predicting intravesical recurrence-free
survival in patients with upper tract urothelial carcinoma after
radical nephroureterectomy.
| | Univariate | Multivariate |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age at RNU | | | | |
|
>65 yr
vs. ≤65 yr | 0.730
(0.485-1.098) | 0.130 | | |
| Sex | | | | |
|
Female vs.
male | 0.747
(0.507-1.101) | 0.140 | | |
| Smoking | | | | |
|
Yes vs.
no | 1.256
(0.672-2.348) | 0.475 | | |
| DM or HTN | | | | |
|
Present vs.
absent | 1.015
(0.684-1.506) | 0.942 | | |
| Hematuria | | | | |
|
Yes vs.
no | 1.784
(0.900-3.537) | 0.097 | 1.893
(0.946-3.790) | 0.071 |
| Hydronephrosis | | | | |
|
Yes vs.
no | 1.615
(0.919-2.839) | 0.096 | 1.684
(0.939-3.017) | 0.080 |
| Tumor location | | | | |
|
Ureter vs.
renal pelvis | 1.082
(0.692-1.692) | 0.730 | 0.994
(0.624-1.586) | 0.981 |
|
Both vs.
renal pelvis | 2.018
(1.231-3.309) | 0.005 | 1.918
(1.157-3.181) | 0.012 |
| Pathological T
stage | | | | |
|
pT2 vs.
<pTa/1 | 1.246
(0.759-2.044) | 0.385 | | |
|
pT3/4 vs.
pTa/1 | 1.096
(0.702-1.712) | 0.687 | | |
| Lymph node
involvement | | | | |
|
N+ vs.
Nx/0 | 0.765
(0.242-2.413) | 0.633 | | |
| Tumor grade | | | | |
|
High vs.
low | 1.638
(0.603-4.452) | 0.333 | | |
| Carcinoma in
situ | | | | |
|
Present vs.
absent | 0.979
(0.606-1.581) | 0.930 | | |
| Lymphovascular
invasion | | | | |
|
Yes vs.
no | 1.277
(0.822-1.984) | 0.276 | | |
| Tumor size | | | | |
|
>3 cm vs.
≤3 cm | 1.015
(0.677-1.523) | 0.942 | | |
| Tumor necrosis | | | | |
|
Present vs.
absent | 0.774
(0.448-1.340) | 0.361 | | |
| Preoperative eGFR
and NLR | | | | |
|
eGFR ≥45 and
high NLR vs. eGFR ≥45 and low NLR | 0.690
(0.327-1.456) | 0.330 | 0.731
(0.344-1.551) | 0.414 |
|
eGFR <45
and low NLR vs. eGFR ≥45 and low NLR | 1.007
(0.627-1.617) | 0.977 | 0.963
(0.597-1.553) | 0.878 |
|
eGFR <45
and high NLR vs. eGFR ≥45 and low NLR | 1.819
(1.070-3.092) | 0.027 | 1.744
(1.024-2.967) | 0.038 |
Discussion
Previously, 22-47% of patients were found to have an
intravesical recurrence (IVRF) within the first two years after the
use of RNU to treat UTUC (20,21).
Similarly, the cohort of the present study showed 24% of patients
with bladder recurrence within the first two years after surgery.
Notably, when preoperative renal function and NLR were analyzed
together, it was found that patients with eGFR <45 and
preoperative NLR >3.8 were significantly associated with a
higher risk of post-RNU bladder recurrence compared with eGFR
<45 or NLR >3.8 alone. Multivariate analysis demonstrated
that moderate to severe CKD combined with NLR >3.8 served as an
independent factor of IVRF in UTUC patients following RNU.
NLR has been reported to be associated with systemic
inflammation (22,23). Studies also suggest that the
development/progression and prognosis of cancers were associated
with systemic inflammation (24,25).
Thus, NLR is hypothesized to serve as a predictive marker for tumor
recurrence and progression and a high NLR is associated with poor
prognoses in several types of malignancy (14). As for UTUC, a number of studies
have noted that preoperative NLR significantly influences
postoperative oncological outcomes (13,26).
However, there has yet to be consensus regarding the optimal value
of NLR. Preoperative NLR has been applied as an indicator for
prediction of bladder recurrence following surgery for UTUC. In
2017, Kishimoto et al (15)
reviewed 192 patients and determined the cut-off value of
preoperative NLR as 3.8, which could identify potential patients at
risk of intravesical recurrence; NLR was demonstrated to be an
independent factor in predicting postoperative bladder recurrence.
In the present study, 28% of patients experienced bladder
recurrence following surgery within a median follow-up duration of
50.1 months; among those with bladder recurrence, 83% occurred
within the first two years after RNU. However, the present study
found that NLR >3.8 alone statistically did not reach
significance concerning high bladder recurrence.
A previous study reported that moderate to severe
CKD patients have a higher bladder incidence (8). Furthermore, a high bladder recurrence
was significantly associated with poor kidney function in UTUC
(9). Similarly, Momota et
al (27) reviewed 456 patients
with UTUC and found that preoperative moderate to severe renal
insufficiency carry a higher risk of disease recurrence, including
bladder recurrence. Some studies suggest that advancing renal
insufficiency contributes to the accumulation of toxic metabolic
products, which result in increased oxidative stress and
inflammation, thus potentially enhancing tumor growth/development
(11,28). The high incidence of malignant
tumor formation in uremia patients is explained by immune system
impairment, incorrect DNA repair mechanisms, reduced antioxidant
defense, accumulation of carcinogenic toxins, decreased renal
elimination and chronic infections and inflammations (29). A more frequent intravesical
recurrence rate in CKD patients with UTUC is suggested to link to
immunosuppressive conditions, exposure to carcinogenic compounds,
lower antioxidant ability and chronic inflammation (9,30,31).
Nevertheless, the present study showed that eGFR
<45 apparently had a trend of poor IVFR compared with eGFR
>45. Yoshitomi et al (16) report that poorer renal function
markedly correlates with the higher NLR value, which reflects the
effect of renal function on the immune inflammation response. Thus,
preoperative renal function status could be considered together
with an inflammation marker, NLR, to predict IVRF. After
stratifying all patients into four risk groups, including eGFR
>45 and NLR <3.8, eGFR >45 and NLR >3.8, eGFR <45
and NLR <3.8 and eGFR <45 and NLR >3.8, Kaplan-Meier plot
showed moderate to severe CKD with high NLR had a significant
association with a high bladder recurrence in UTUC patients
following RNU. In brief, combining preoperative eGFR and NLR could
identify potential patients at high risk of subsequent bladder
recurrence following surgery. Furthermore, a combination of
preoperative eGFR and NLR was shown to be an independent factor for
a poor IVRF in UTUC following RNU. In addition, the present study
attempted to create a nomogram to predict the two- and five-year
IVRF based on variables included in the multivariable Cox
regression models (Fig. S3). This
nomogram may help doctors to distinguish patients at risk of
subsequent bladder recurrence who needed careful follow-up. In the
future, this prediction model should perform the external
validation.
Previously, two clonal hypotheses, including
intraluminal seeding and field cancerization effect, were proposed
to explain bladder tumor multifocality and recurrence (32). The present study cannot confirm
that metachronous bladder tumor recurrence came from UTUC tumor
seeding or cancer field effect through the understanding of the
clinical progress evolution and pathologic characters of tumors.
The present study first minimized the influence of clinical
confounding factors, such systemic chemotherapy, prior bladder
cancer history, concomitant bladder cancer and more, on the
development of metachronous bladder tumors. It focused on exploring
the effect of NLR and preoperative renal function on bladder tumor
recurrence following RNU to treat pure UTUC. Although in the cohort
there were only 14% of UTUC patients with pre-existing renal
insufficiency (eGFR <45) with elevated NLR (>3.8), as high as
38% of these patients developed new bladder urothelial carcinoma.
As aforementioned, it is hypothesized that this phenomenon was due
to systemic inflammation and toxic stress attacks involving
accumulation of biochemical carcinogens and uremic
immunosuppression.
Since decreased eGFR contributed to more oxidative
stress, the resultant NLR elevation reflected the intensity of
stress and systemic inflammation. An elevated NLR indicated
increased serum neutrophil and decreased lymphocyte counts.
Increasing circulating neutrophils had more chances to participate
in tumor-related inflammatory activities in a tumor
microenvironment and then changed to tumor-associated neutrophils
to assist tumor progression through multiple mechanisms (25,33).
Meanwhile, lowering circulating lymphocyte counts leads to weak
anti-tumor immunity (34). Thus,
an active inflammatory and stress-rich status potentially drives
the processing of malignant transformation and also benefits the
viability of seeding tumor cells from the upper urinary tract. This
may explain why incorporating a high NLR and poor renal function
could act as an indicator for predicting bladder recurrence.
However, associations between NLR, CKD and bladder recurrence in
patients with UTUC may be multifactorial. Still more substantial,
biological evidence is required to further elucidate the
pathophysiological mechanism of bladder tumor recurrence.
To the best of the authors' knowledge, the present
study was the first clinical study to evaluate the effect of
combining preoperative renal function status and NLR on IVFR in
patients following RNU to treat UTUC. Physicians should take
preoperative renal function and NLR into account when identifying
patients with high risk of subsequent bladder recurrence following
RNU in clinical practice. Afterwards, intravesical chemotherapy may
be considered for these patients at risk of bladder recurrence
following RNU.
There were some limitations to the present study.
First, this was a retrospective study, which may have led to a
selection bias. Second, all patients were Taiwanese. The high
incidence of both UTUC and CKD was noted in Taiwan. Third, the
present study failed to assess the duration of renal insufficiency
and lacked other reliable serum immune-inflammation markers, which
may have affected the results. Thus, further multicenter and
prospective studies are necessary. Moreover, external validation
which involves other ethnic groups is needed.
The present study showed that moderate to severe CKD
synchronous with high NLR was significantly associated with a poor
IVRF in UTUC following RNU. Furthermore, combining preoperative
eGFR and NLR could be considered as an independent factor for
predicting high risk of bladder recurrence in patients with UTUC.
Thus, one should be more cautious of UTUC patients with
preoperative renal insufficiency and high NLR during post-surgery
follow-up.
Supplementary Material
Kaplan-Meier survival curves for IVRF
in patients with UTUC according to preoperative NLR. IVRF,
intravesical recurrence-free survival; UTUC, upper tract urothelial
carcinoma; NLR, neutrophil-to-lymphocyte ratio; RNU, radical
nephrouretectomy.
Kaplan-Meier survival curves for IVRF
in patients with UTUC according to preoperative eGFR. IVRF,
intravesical recurrence-free survival; UTUC, upper tract urothelial
carcinoma; eGFR, estimated glomerular filtration rate; RNU, radical
nephrouretectomy.
Nomogram to predict the two. and
five-year IVRF in patients with UTUC after RNU. IVRF, intravesical
recurrence-free survival; UTUC, upper tract urothelial carcinoma;
RNU, radical nephrouretectomy; NLR, neutrophil-to-lymphocyte
ratio.
Association of subsequent bladder
recurrence post radical nephroureterectomy with interrelated NLR
and renal function.
Acknowledgements
The authors would like to thank Dr Sheng-Hsiang Lin
and Ms. Wan-Ni Chen (Biostatistics Consulting Center, Clinical
Medicine Research Center, National Cheng Kung University Hospital,
Tainan, Taiwan) for providing statistical consulting services.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
THC and HCJ were responsible for the conception and
design of the current study. CYH, KYW, TYT, HYW, WHY and CHO were
involved in the collection of medical records, and acquisition and
curation of data. THC and HCJ performed formal analysis and data
interpretation. CYH and HCJ developed the methodology. THC and HCJ
drafted the manuscript. CYH, KYW, TYT, HYW, WHY and CHO provided
resources and revised the manuscript critically for important
intellectual content. THC and HCJ confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the institutional review
board of National Cheng-Kung University Hospital (IRB number:
A-ER-103-036; Tainan, Taiwan), which waived the requirement for
informed consent from participants and allowed access to the
follow-up clinical records. It was conducted based on the
guidelines of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Green DA, Rink M, Xylinas E, Matin SF,
Stenzl A, Roupret M, Karakiewicz PI, Scherr DS and Shariat SF:
Urothelial carcinoma of the bladder and the upper tract: Disparate
twins. J Urol. 189:1214–1221. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rouprêt M, Zigeuner R, Palou J, Boehle A,
Kaasinen E, Sylvester R, Babjuk M and Oosterlinck W: European
guidelines for the diagnosis and management of upper urinary tract
urothelial cell carcinomas: 2011 update. Eur Urol. 59:584–594.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
RJ, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 update. Eur
Urol. 68:868–879. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Terakawa T, Miyake H, Muramaki M, Takenaka
A, Hara I and Fujisawa M: Risk factors for intravesical recurrence
after surgical management of transitional cell carcinoma of the
upper urinary tract. Urology. 71:123–127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Novara G, De Marco V, Dalpiaz O, Galfano
A, Bouygues V, Gardiman M, Martignoni G, Patard JJ, Artibani W and
Ficarra V: Independent predictors of contralateral metachronous
upper urinary tract transitional cell carcinoma after
nephroureterectomy: Multi-institutional dataset from three European
centers. Int J Urol. 16:187–191. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xylinas E, Kluth L, Passoni N, Trinh QD,
Rieken M, Lee RK, Fajkovic H, Novara G, Margulis V, Raman JD, et
al: Prediction of intravesical recurrence after radical
nephroureterectomy: Development of a clinical decision-making tool.
Eur Urol. 65:650–658. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Seisen T, Granger B, Colin P, Leon P,
Utard G, Renard-Penna R, Compérat E, Mozer P, Cussenot O, Shariat
SF and Rouprêt M: A systematic review and meta-analysis of
clinicopathologic factors linked to intravesical recurrence after
radical nephroureterectomy to treat upper tract urothelial
carcinoma. Eur Urol. 67:1122–1133. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lowrance WT, Ordoñez J, Udaltsova N, Russo
P and Go AS: CKD and the risk of incident cancer. J Am Soc Nephrol.
25:2327–2334. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chung SD, Huang KH, Lai MK, Huang CY, Chen
CH, Pu YS, Yu HJ and Chueh SC: CKD as a risk factor for bladder
recurrence after nephroureterectomy for upper urinary tract
urothelial carcinoma. Am J Kidney Dis. 50:743–753. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li CE, Chien CS, Chuang YC, Chang YI, Tang
HP and Kang CH: Chronic kidney disease as an important risk factor
for tumor recurrences, progression and overall survival in primary
non-muscle-invasive bladder cancer. Int Urol Nephrol. 48:993–999.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oberg BP, McMenamin E, Lucas FL, McMonagle
E, Morrow J, Ikizler TA and Himmelfarb J: Increased prevalence of
oxidant stress and inflammation in patients with moderate to severe
chronic kidney disease. Kidney Int. 65:1009–1016. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rausch S, Hennenlotter J, Todenhöfer T,
Aufderklamm S, Schwentner C, Sievert KD, Stenzl A and Gakis G:
Impaired estimated glomerular filtration rate is a significant
predictor for non-muscle-invasive bladder cancer recurrence and
progression-introducing a novel prognostic model for bladder cancer
recurrence. Urol Oncol. 32:1178–1183. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marchioni M, Cindolo L, Autorino R,
Primiceri G, Arcaniolo D, De Sio M and Schips L: High
Neutrophil-to-lymphocyte Ratio as Prognostic Factor in patients
affected by upper tract urothelial cancer: A systematic review and
meta-analysis. Clin Genitourin Cancer. 15:343–349.e1.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106(dju124)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kishimoto N, Takao T, Kuribayashi S,
Yamamichi G, Nakano K, Kawamura M, Tsutahara K, Tanigawa G and
Yamaguchi S: The neutrophil-to-lymphocyte ratio as a predictor of
intravesical recurrence in patients with upper urinary tract
urothelial carcinoma treated with radical nephroureterectomy. Int J
Clin Oncol. 22:153–158. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoshitomi R, Nakayama M, Sakoh T, Fukui A,
Katafuchi E, Seki M, Tsuda S, Nakano T, Tsuruya K and Kitazono T:
High neutrophil/lymphocyte ratio is associated with poor renal
outcomes in Japanese patients with chronic kidney disease. Ren
Fail. 41:238–243. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA (eds): World Health Organisation Classification of
Tumours. Pathology and genetics of tumours of the urinary system
and male genital organs. IARC Press, Lyon, 2004.
|
|
18
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A (eds): AJCC cancer staging manual (7th
edition). New York, NY, Springer, 2010.
|
|
19
|
Levey AS, Bosch JP, Lewis JB, Greene T,
Rogers N and Roth D: A more accurate method to estimate glomerular
filtration rate from serum creatinine: A new prediction equation.
Modification of diet in renal disease study group. Ann Intern Med.
130:461–470. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xylinas E, Rink M, Margulis V, Karakiewicz
P, Novara G and Shariat SF: Upper Tract Urothelial Carcinoma
Collaboration (UTUCC). Multifocal carcinoma in situ of the upper
tract is associated with high risk of bladder cancer recurrence.
Eur Urol. 61:1069–1070. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kates M, Badalato GM, Gupta M and
McKiernan JM: Secondary bladder cancer after upper tract urothelial
carcinoma in the US population. BJU Int. 110:1325–1329.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ohno Y, Nakashima J, Ohori M, Hatano T and
Tachibana M: Pretreatment neutrophil-to-lymphocyte ratio as an
independent predictor of recurrence in patients with nonmetastatic
renal cell carcinoma. J Urol. 184:873–848. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Imtiaz F, Shafique K, Mirza SS, Ayoob Z,
Vart P and Rao S: Neutrophil lymphocyte ratio as a measure of
systemic inflammation in prevalent chronic diseases in Asian
population. Int Arch Med. 5(2)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33 (Suppl 1):S79–S84. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Azuma T, Matayoshi Y, Odani K, Sato Y,
Sato Y, Nagase Y and Oshi M: Preoperative neutrophil-lymphocyte
ratio as an independent prognostic marker for patients with upper
urinary tract urothelial carcinoma. Clin Genitourin Cancer.
11:337–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Momota M, Hatakeyama S, Tokui N, Sato T,
Yamamoto H, Tobisawa Y, Yoneyama T, Yoneyama T, Hashimoto Y, Koie
T, et al: The impact of preoperative severe renal insufficiency on
poor postsurgical oncological prognosis in patients with urothelial
carcinoma. Eur Urol Focus. 5:1066–1073. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Panieri E and Santoro MM: ROS homeostasis
and metabolism: A dangerous liason in cancer cells. Cell Death Dis.
7(e2253)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vamvakasa S, Bahnerb U and Heidlandb A:
Cancer in end-stage renal disease: Potential factors
involved-editorial-. Am J Nephrol. 18:89–95. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hafner C, Knuechel R, Zanardo L, Dietmaier
W, Blaszyk H, Cheville J, Hofstaedter F and Hartmann A: Evidence
for oligoclonality and tumor spread by intraluminal seeding in
multifocal urothelial carcinomas of the upper and lower urinary
tract. Oncogene. 20:4910–4915. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yamashita S, Ito A, Mitsuzuka K, Tochigi
T, Namima T, Soma F, Aizawa M, Ioritani N, Kaiho Y and Arai Y:
Clinical implications of intravesical recurrence after radical
nephroureterectomy for upper urinary tract urothelial carcinoma.
Int J Urol. 23:378–384. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Harris AL and Neal DE: Bladder
cancer-field versus clonal origin. N Engl J Med. 326:759–761.
1992.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu L, Saxena S, Awaji M and Singh RK:
Tumor-associated neutrophils in cancer: Going Pro. Cancers (Basel).
11(564)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fogar P, Sperti C, Basso D, Sanzari MC,
Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, et
al: Decreased total lymphocyte counts in pancreatic cancer: An
index of adverse outcome. Pancreas. 32:22–28. 2006.PubMed/NCBI View Article : Google Scholar
|