Introduction
Hashimoto's thyroiditis (HT), an organ-specific
autoimmune disease, is the most common thyroid disease that leads
to hypothyroidism and has a high incidence rate of 5% in women in
China (1). T helper 1 (Th1) cells
were reported to be involved in the development of HT and HT is
initially characterized by lymphocytic infiltration of the thyroid
parenchyma, diffusely enlarged thyroid gland as well as elevated
production of autoantibodies (2).
Therefore, it may be hypothesized that early therapeutic
intervention could potentially prevent the development of this
disease, and maintain the normal structure and function of the
thyroid.
Macrophages are important innate immune cells in the
body that widely exist in various tissues and organs, and serve a
crucial role in the inflammatory response and tissue repair
(3). Stimulated by cytokines, such
as lipopolysaccharide (LPS) and IFN-γ, macrophages polarize into M1
macrophages that serve a pro-inflammatory role via the secretion of
inflammatory factors, such as TNF-α, IL-6 and IL-1(4). M1 macrophages can also aggravate
tissue inflammatory damage. For example, M1 macrophage-derived
exosomes have been reported to aggravate experimental autoimmune
neuritis via modulation of the Th1 response (5). Moreover, in the myocardial infarction
microenvironment, M1 macrophage-derived exosomes can inhibit
angiogenesis and exacerbate cardiac dysfunction (6). Estradiol has been shown to promote
the activation of M1-like macrophages via cadherin-11, which can
worsen the temporomandibular joint inflammation in rats (7). Furthermore, the co-culture of M1
macrophages with articular chondrocytes can exacerbate the
apoptosis of articular chondrocytes (8). However, the role of M1 macrophages in
HT is still unclear. It has previously been reported that
macrophage infiltration occurred in an autoimmune thyroiditis model
(9) and that macrophage migration
inhibitory factor can recruit macrophages to inflammatory injury
sites. Therefore, macrophage levels may be increased in thyroiditis
tissues (10). Macrophage
inflammatory protein-1 (MIP-1) α and MIP-1β expression levels have
been reported to be increased in HT tissues (11). Therefore, the regulation of
macrophages could have an important role in HT research. T-cell
immunoglobulin and mucin domain-containing 4 (TIM4) is expressed in
macrophages and its overexpression can activate the release of
inflammasomes in monocytes/macrophages (12). In liver Kupffer macrophages, TIM4
silencing can reduce C-C motif chemokine ligand 4-induced liver
fibrosis, and in Kupffer macrophages overexpressing TIM4, reactive
oxygen species are produced and mitochondrial autophagy is
activated (13). NOD-, LRR- and
pyrin domain-containing protein 3 (NLRP3) has also been reported to
mediate cytokine secretion and pyroptosis, and is associated with
autoimmune thyroiditis (14).
Moreover, TIM4 was testified to regulate NLRP3 expression in
macrophages (12,15). Despite the fact that the
relationship between TIM4 and NLRP3 has been widely discussed,
their function in HT mediated by macrophages is not clear.
Therefore, the aim of the present study was to investigate whether
TIM4 participated in the underlying mechanism of M1 macrophages in
the inflammation, apoptosis and cell adhesion of thyroid follicular
cells (TFCs), via the regulation of NLRP3.
Materials and methods
Co-culture model of M1 macrophages and
Nthy-ori 3-1 cells
The human monocyte leukemia THP-1 cell line was
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. THP-1 cells were cultured in RPMI-1640
medium (Thermo Fisher Scientific, Inc.) containing 10% FBS (Beijing
Solarbio Science & Technology Co., Ltd.), 100 U/l penicillin
and 100 U/l streptomycin in an incubator at 37˚C with 5%
CO2. When cells reached 80% confluence, they were
sub-cultured. The differentiation of THP-1 cells into macrophages
was induced using 150 nM phorbol 12-myristate 13-acetate
(Sigma-Aldrich; Merck KGaA) for 24 h at room temperature in
RPMI-1640 medium. M0 macrophages were induced according to a
previous study (16).
Subsequently, M1 macrophages were produced via the induction of
macrophage polarization using IFN-γ (20 ng/ml; R&D Systems
China Co., Ltd.) and LPS (10 pg/ml; Sigma-Aldrich; Merck KgaA) for
24 h at room temperature. The immortalized human thyroid follicular
Nthy-ori 3-1 cell line was purchased from the European Collection
of Authenticated Cell Cultures (cat. no. 90011609) and was cultured
in RPMI-1640 medium containing 10% FBS, 100 U/l penicillin and 100
U/l streptomycin in an incubator at 37˚C with 5% CO2.
For co-culture experiments, the cell suspension was prepared using
M1 macrophages and Nthy-ori 3-1 cells. M1 macrophages
(1x106 cells/well) were seeded into the upper Transwell
chamber (pore size, 0.4 µm) which was pre-coated with Matrigel (BD
Biosciences) and added with serum-free medium (Beijing Bitab
Biotechnology Co., Ltd.) at room temperature for 24 h, whereas
Nthy-ori 3-1 cells (1x106 cells/well) were cultured in
the medium with 10% FBS in the lower Transwell chamber at room
temperature for 24 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Nthy-ori 3-1 cells from each group were added into
an Eppendorf tube and lysed using Trizol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The RT reaction
system was prepared using a PrimeScript™ RT Reagent kit (Takara
Bio, Inc.) according to the manufacturer's protocol and cDNA was
produced. qPCR was performed using the 7500 Real-Time PCR System
(Applied Biosystems; Themo Fisher Scientific, Inc.) and
SYBR® Premix Ex Taq™ (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. The required
thermocycling conditions were set as follows: Initial denaturation
at 95˚C for 8 min; denaturation at 95˚C for 25 sec; annealing at
60˚C for 30 sec; extension at 72˚C for 30 sec; and final extension
at 72˚C for 10 min. The primer sequences for qPCR were as follows:
TNF-α, 5'-ATGAGCACTGAAAGCATGATCCG-3' (forward) and
5'-AATGATCCCAAAGTAGACCTGCC-3' (reverse); IL-1β,
5'-GCACGATGCACCTGTACGAT-3' (forward) and
5'-CACCAAGCTTTTTTGCTGTGAGT-3' (reverse); IL-6,
5'-AGACTTGCCTGGTGA-3' (forward) and 5'-GCTCTGGCTTGTTCC-3'
(reverse); C-X-C motif chemokine ligand 10 (CXCL10),
5'-GTGGCATTCAAGGAGTACCTC-3' (forward) and
5'-GCCTTCGATTCTGGATTCAGACA-3' (reverse); TIM4,
5'-ACAGGACAGATGGATGGAATACCC-3' (forward) and
5'-AGCCTTGTGTGTTTCTGCG-3' (reverse); NLRP3,
5'-CTTCTCTGATGAGGCCCAAG-3' (forward) and 5'-GCAGCAAACTGGAAAGGAAG-3'
(reverse); integrin αv, 5'-TGCCAGGGTCTTTCTACCTCT-3' (forward) and
5'-GGGTGCCTAGGAGCATTTGT-3' (reverse); integrin β3,
5'-ACCAGTAACCTGCGGATTGG-3' (forward) and 5'-TCCGTGACACACTCTGCTTC-3'
(reverse); GAPDH, 5'-GAAGGTGAAGGTCGGAGTC-3' (forward) and
5'-GAAGATGGTGATGGGATTTC-3' (reverse). The relative mRNA expression
levels were quantified using the 2-ΔΔCt method (17) with GAPDH serving as an endogenous
control.
Western blotting
Total protein was extracted from Nthy-ori 3-1 cells
with RIPA lysis buffer (Beyotime Institute of Biotechnology) and
then quantified with the use of bicinchoninic acid (BCA) protein
assay kit (Beyotime Institute of Biotechnology). Subsequently, the
proteins (50 µg per lane) were separated via 8% SDS-PAGE. Proteins
were then transferred onto PVDF membranes. The membranes were
blocked using 5% skimmed milk for 2 h at room temperature, which
was followed by incubation with the following primary antibodies at
4˚C overnight: Inducible nitric oxide synthase (iNOS) (1:1,000;
cat. no. ab178945), TIM4 (1:1,000; cat. no. ab47637), NLRP3
(1:1,000; cat. no. ab263899), phosphorylated (p)-p65 (1:1,000; cat.
no. ab86299), p65 (1:1,000; cat. no. ab16502), IκB (1:1,000; cat.
no. ab32518), p-IκB (1:1,000; cat. no. ab133462), Bax (1:1,000;
cat. no. ab32503), cleaved poly (ADP-ribose) polymerase (PARP)
(1:1,000; cat. no. ab32064), PARP (1:1,000; cat. no. ab191217),
Bcl-2 (1:1,000; cat. no. ab32124), GAPDH (1:2,500; cat. no. ab9485)
(all from Abcam) and αvβ3 (1:50; cat. no. #SC7312; Santa Cruz
Biotechnology, Inc.) was detected as an entire protein band under
the conditions used; Molecular Weight of Integrin αvβ3: 125 kDa).
Following the primary antibody incubation, HRP-labeled goat
anti-rabbit (1:2,000; cat. no. ab6721; Abcam) or goat anti-mouse
(1:2,000; cat. no. ab6789; Abcam) secondary antibodies were added
and incubated with the membrane at room temperature for 30 min.
GAPDH was used as the internal control. Finally, the protein band
were visualized with ECL Detection Reagent (Shanghai Yeasen
Biotechnology Co., Ltd.) and ImageJ software (version 7.6.5;
National Institutes of Health) was used for the densitometric
analysis of the protein expression levels.
Immunofluorescence assay
M1 macrophages (1x106 cells/well) that
were inoculated into six-well plates were fixed using 4%
paraformaldehyde for 15 min at room temperature. The cells were
then permeated using 0.5% Triton X-100 for 30 min at room
temperature. Subsequently, cells were rinsed with PBS and were then
incubated with 5% goat serum (Beyotime Institute of Biotechnology)
at room temperature for 30 min. The primary antibody, anti-iNOS
(1:50; cat. no. ab3523; Abcam), was added to the cells at 4˚C
overnight. Following the primary antibody incubation, a goat
anti-rabbit IgG H&L (Alexa Fluor® 488) secondary
antibody (1:200; cat. no. ab150077; Abcam) was added for 1 h at
37˚C. The nuclei were stained using a DAPI solution for 5 min at
room temperature. Finally, the cells were mounted using an
anti-fluorescence quenching agent and the images were observed
using a fluorescence microscope.
Transfection
Short hairpin RNA (shRNA) targeting TIM4
(shRNA-TIM4-1, 5'-GTTCAACGATGTAAAGATA-3'; shRNA-TIM4-2,
5'-GGTACTTTAGAGACCACAA-3'), the corresponding empty vector
[shRNA-negative control (NC), 5'-CCGGCAACAAGATGAAGAGCACCAACTC-3'],
an NLRP3 overexpression plasmid (Ov-NLRP3) or its empty vector
(Ov-NC) were obtained from Shanghai GenePharma Co., Ltd.
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) was
used to transfect the aforementioned vectors into M1 macrophages at
a concentration of 50 ng/ml at 37˚C for 24 h. After 48 h, the cells
were adopted for follow-up experiments.
TUNEL staining
Nthy-ori 3-1 cells (1x106 cells/well)
that were inoculated into six-well plates were fixed using 4%
paraformaldehyde at 4˚C for 25 min. Subsequently, cells were
incubated with proteinase K at room temperature for 5 min. The
apoptotic cells were stained using TUNEL solution (Elabscience
Biotechnology, Inc.) at 37˚C for 1 h according to the
manufacturer's protocol. Subsequently, 1 µg/ml DAPI was applied for
the staining of cell nuclei for 30 min at room temperature in the
dark. A florescent microscope was adopted for the observation of
positive cells.
Cell adhesion assay
Fibronectin (Shanghai Yeasen Biotechnology Co.,
Ltd.) was added to each well of a 96-well plate and incubated at
4˚C overnight. PBS was then used to wash the plate and 1% BSA
(Beyotime Institute of Biotechnology) was added to each well for
incubation for 1 h at room temperature. Subsequently,
2x104 Nthy-ori 3-1 cells were added to each well for
incubation for 30 min at 37˚C. Adherent cells were fixed using 3%
paraformaldehyde for 10 min at room temperature and stained with
0.5% crystal violet for 10 min at room temperature. The number of
cells was analyzed using a spectrophotometer at 540 nm. A total of
five randomly chosen fields were counted for each group.
Statistical analysis
The experimental data are presented as the mean ±
SD. SPSS v21.0 (IBM Corp.) was used for statistical analysis. The
comparison between the two groups was performed using the unpaired
Student's t-test. One-way ANOVA was performed for statistical
comparisons among more than two groups followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
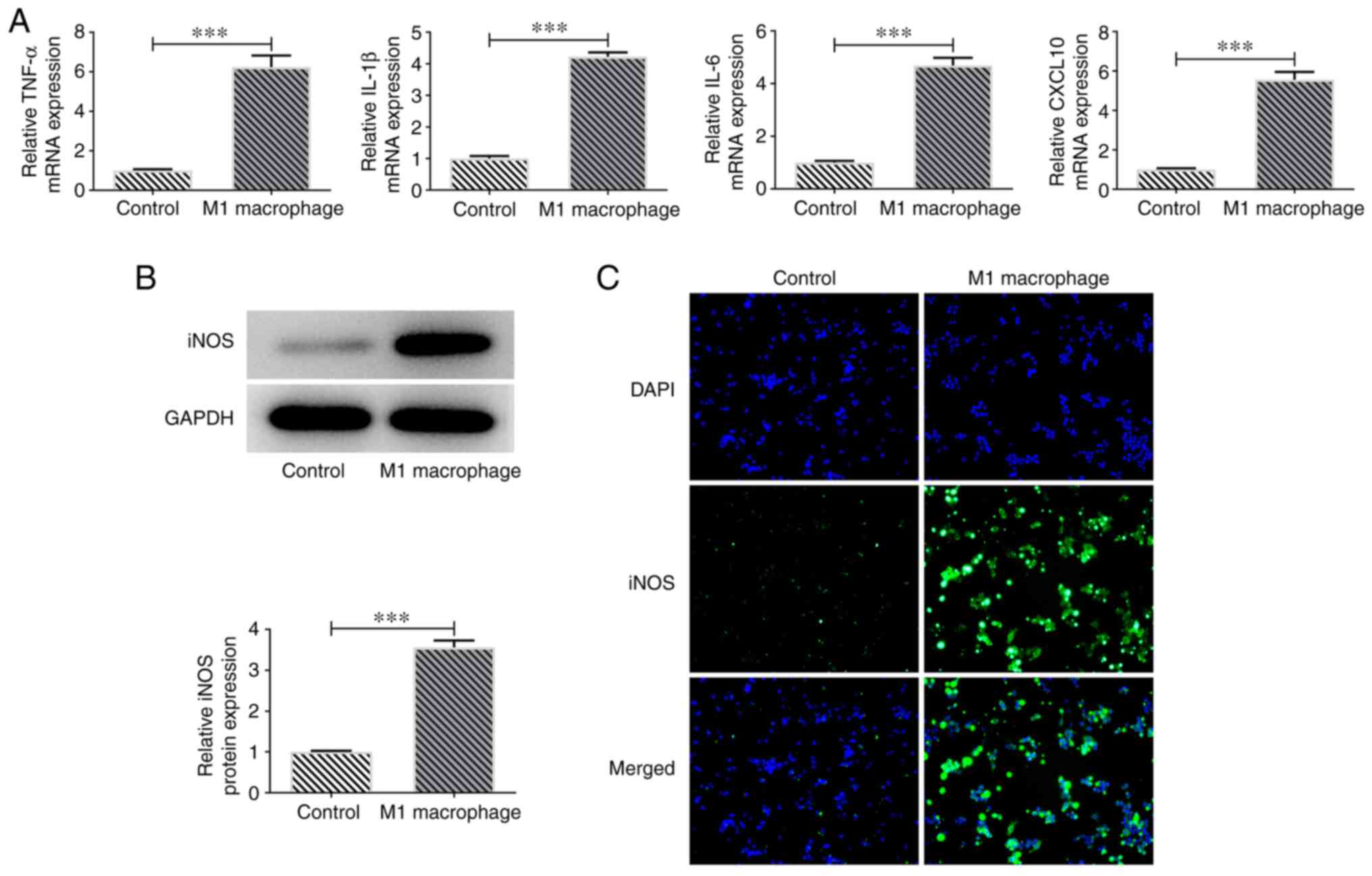
M1 macrophages express decreased NLRP3
levels following TIM4 silencing
M0 macrophages were polarized into M1 macrophages
using 10 pg/ml LPS and 20 ng/ml IFN-γ. The mRNA expression levels
of M1 macrophage markers, including TNF-α, IL-1β, IL-6 and CXCL10
were assessed via RT-qPCR. Their levels were significantly
increased compared with in the M0 macrophage control group
(Fig. 1A). Furthermore, western
blotting and immunofluorescence staining determined that iNOS
protein expression levels were markedly increased in the M1
macrophage group (Fig. 1B and
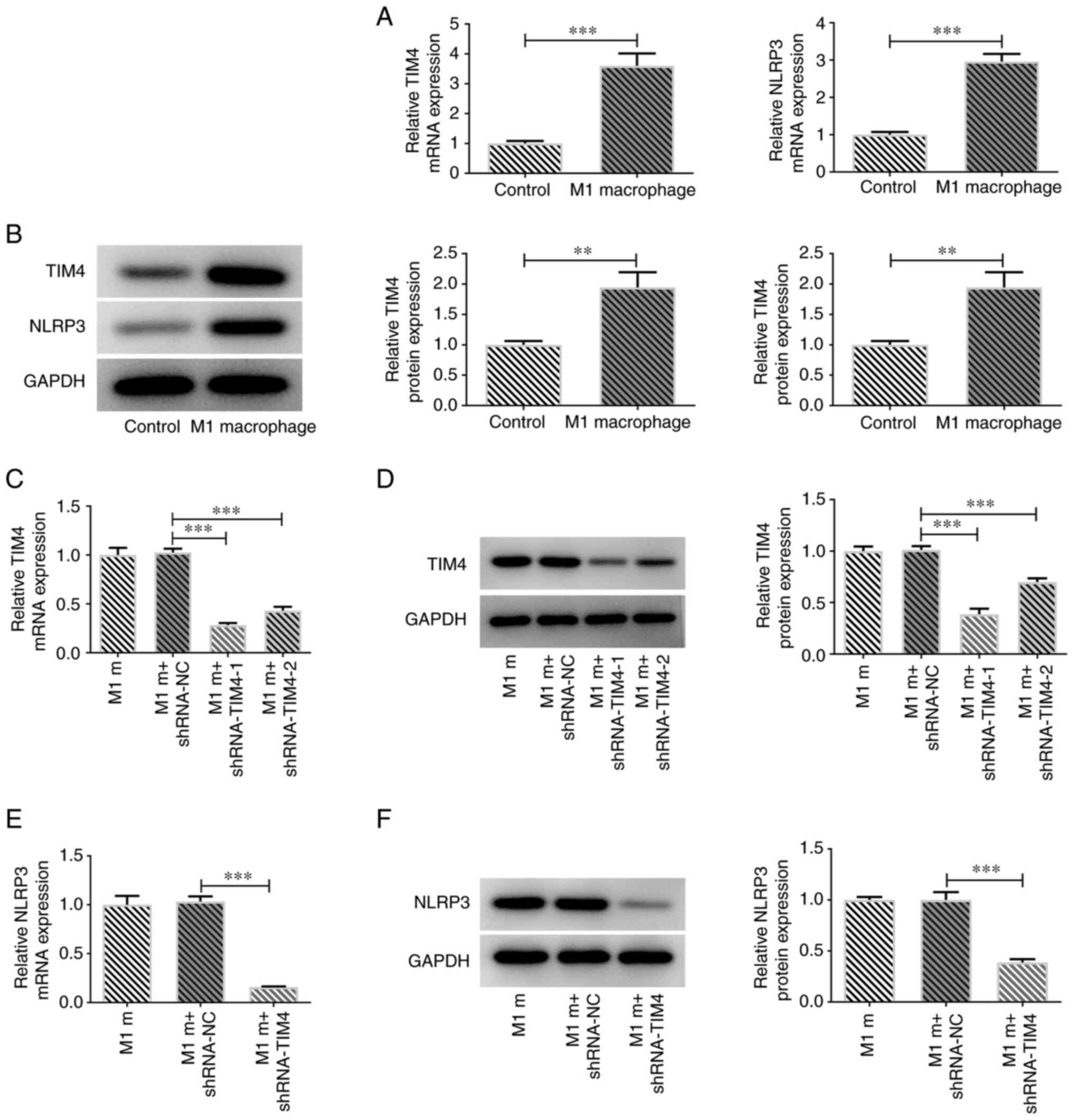
C). Subsequently, the results
demonstrated that the expression levels of TIM4 and NLRP3 were
significantly increased in M1 macrophages compared with in the
control group (Fig. 2A and
B). To further investigate the
role of TIM4, the effects of TIM4 silencing were assessed in M1
macrophages using RT-qPCR and western blotting. Compared with in
the control group, the mRNA and protein expression levels of TIM4
were markedly reduced in the TIM4 knockdown groups (Fig. 2C and D). Moreover, it was observed that
shRNA-TIM4-1 resulted in a more efficient TIM4 knockdown;
therefore, shRNA-TIM4-1 was selected for use in the subsequent
experiments. NLRP3 mRNA and protein expression levels were also
assessed using RT-qPCR and western blotting. The results
demonstrated that NLRP3 expression levels were significantly
decreased following TIM4 silencing (Fig. 2E and F).
Co-culture of M1 macrophages and
Nthy-ori 3-1 cells leads to increased expression levels of
inflammatory factors and apoptosis
To assess whether inflammatory factor expression
levels in Nthy-ori 3-1 cells following co-culture with M1
macrophages were related to TIM4/NLRP3, the mRNA expression levels
of TNF-α, IL-1β and IL-6, and the protein expression levels of
p-p65, p65, IκB and p-IκB were determined via RT-qPCR and western
blotting, respectively. Furthermore, the apoptotic rate was
determined via TUNEL staining and western blotting. M1 macrophages
were transfected with Ov-NLRP3. The results demonstrated that the
Ov-NLRP3 cell group exhibited increased expression levels of NLRP3
compared with the M1-m+Ov-NC group (Fig. 3A and B). Moreover, the mRNA expression levels
of TNF-α, IL-1β and IL-6 in Nthy-ori 3-1+M1-m group were
significantly increased compared with those in the Nthy-ori 3-1
group (Fig. 3C). Compared with the
Nthy-ori 3-1 + M1-m + shRNA-NC group, the levels of TNF-α, IL-1β
and IL-6 were greatly declined by TIM4 knockdown, which were then
elevated by NLRP3 overexpression. The protein expression levels of
p/t-p65 and p/t-I-κB in Nthy-ori 3-1 + M1-m group were
significantly increased compared with those in Nthy-ori 3-1 group
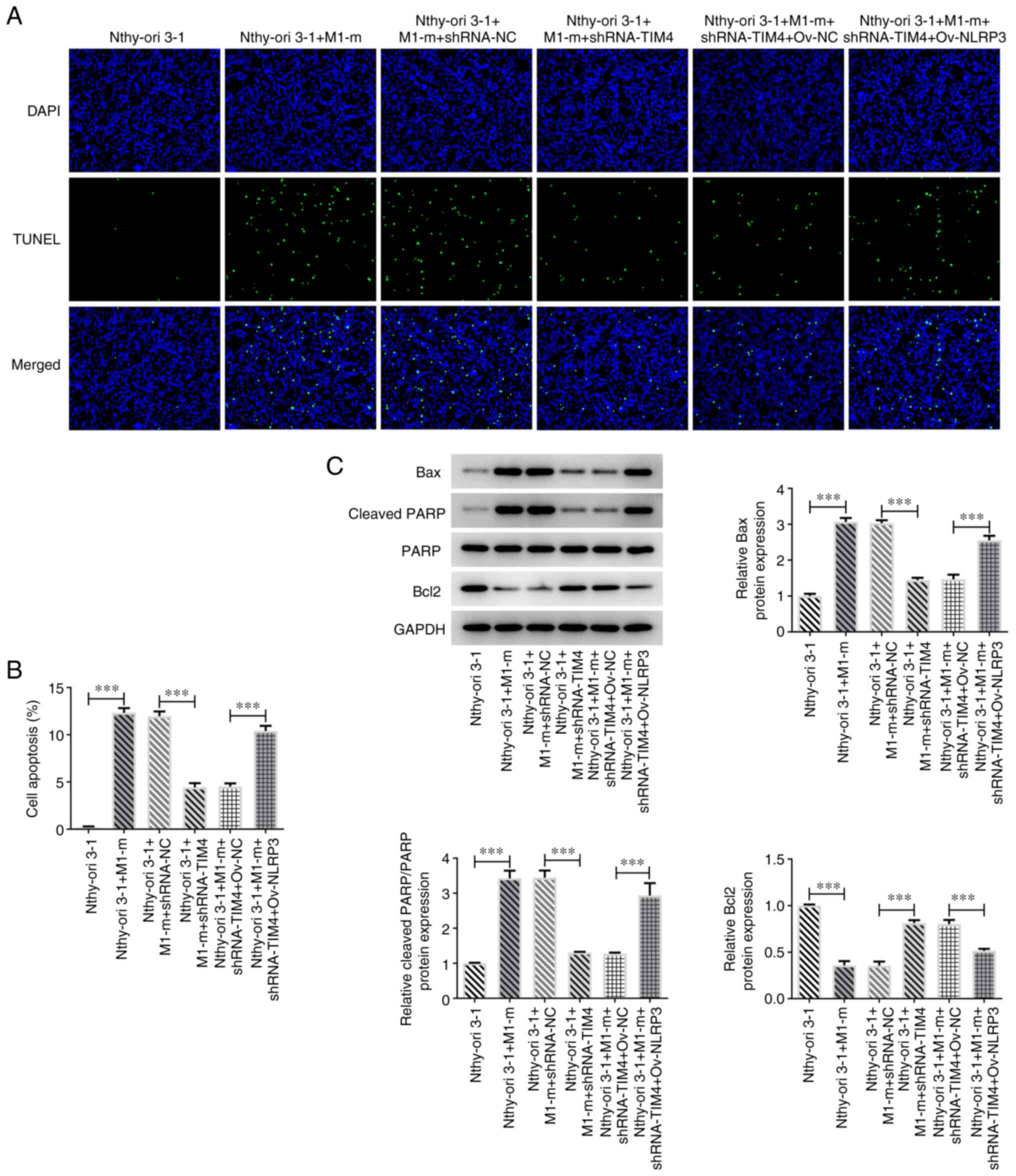
(Fig. 3D). The apoptotic rate was
also significantly increased in Nthy-ori 3-1 + M1-m group when
compared with the Nthy-ori 3-1 group (Fig. 4A and B). Additionally, the reduced apoptosis in
thy-ori 3-1 + M1-m + shRNA-TIM4 group due to TIM4 depletion were
promoted by Ov-NLRP3 in comparison with the thy-ori 3-1 + M1-m +
shRNA-TIM4 + Ov-NC group. The results demonstrated that the protein
expression levels of Bax and cleaved PARP were increased, and Bcl-2
protein expression levels were decreased, following the co-culture
of M1 macrophages with Nthy-ori 3-1 cells compared with the
Nthy-ori 3-1 cells group (Fig.
4C).
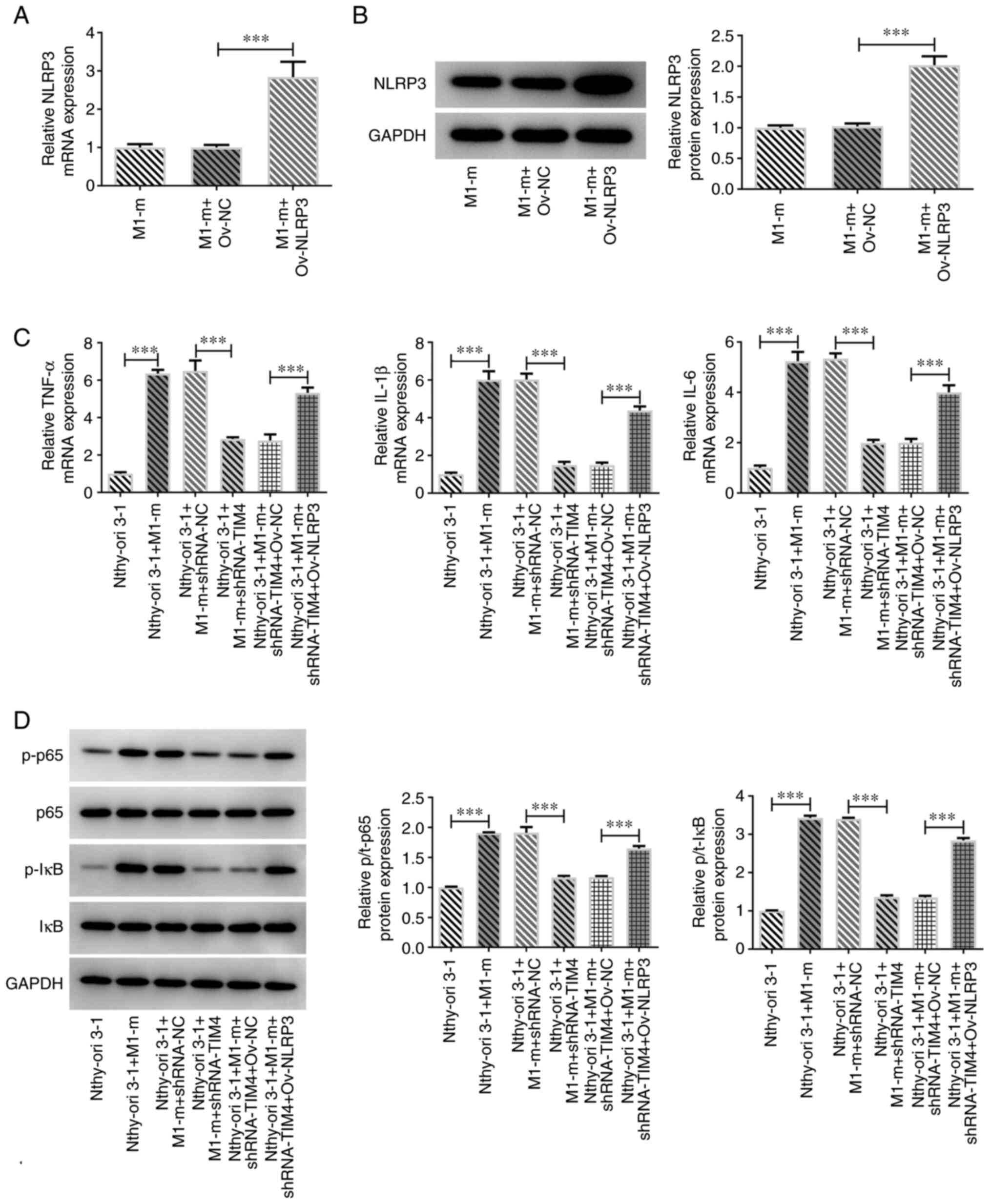 | Figure 3Overexpression of NLRP3 in M1
macrophages affects the expression of inflammatory factors in
Nthy-ori 3-1 cells. NLRP3 expression in M1 macrophages after
transfection with Ov-NLRP3 was determined by (A) RT-qPCR and (B)
western blotting. (C) RT-qPCR analysis showing expression of TNF-α,
IL-1β and IL-6 in Nthy-ori 3-1 cells after transfection. (D)
Expression of p-p65, p65, IκB and p-IκB, detected by western
blotting. Data are shown as the mean ± standard deviation of three
independent experiments. ***P<0.001. TIM4, T-cell
immunoglobulin and mucin domain-containing 4; NLRP3, NOD-, LRR- and
pyrin domain-containing protein 3; Ov, overexpression plasmid;
RT-qPCR, reverse transcription-quantitative PCR; M1-m, M1
macrophage; NC, negative control; shRNA, short hairpin RNA; p,
phosphorylated; t, total. |
A previous study reported that IFN-α can inhibit the
expression of integrin αvβ3 in Nthy-ori 3-1 cells and reduce their
adhesion (18). To investigate
whether the modulation of TIM4/NLRP3 in M1 macrophages regulated
the adhesion of Nthy-ori 3-1 cells, M1 macrophages were transfected
with plasmids inducing TIM4 silencing (shRNA-TIM4) or NLRP3
overexpression (Ov-NLRP3). The number of Nthy-ori 3-1 cells after
co-culture of M1 macrophages and Nthy-ori 3-1 cells was found to be
higher compared with in the Nthy-ori 3-1 group (Fig. 5A). Furthermore, the number of
attachment macrophages in thy-ori 3-1 + M1-m + shRNA-NC group were
reduced by TIM4 deficiency, which was then increased by NLRP3
overexpression (Fig. 5B).
Moreover, RT-qPCR and western blotting demonstrated that TIM4
silencing markedly elevated the mRNA and protein expression levels
of αvβ3 in comparison with the thy-ori 3-1 + M1-m + shRNA-NC group,
while NLRP3 overexpression exhibited opposite impacts on αvβ3,
evidenced by the reduced expression of αvβ3 in thy-ori 3-1 + M1-m +
shRNA-TIM4 + Ov-NC group (Fig. 5C
and D).
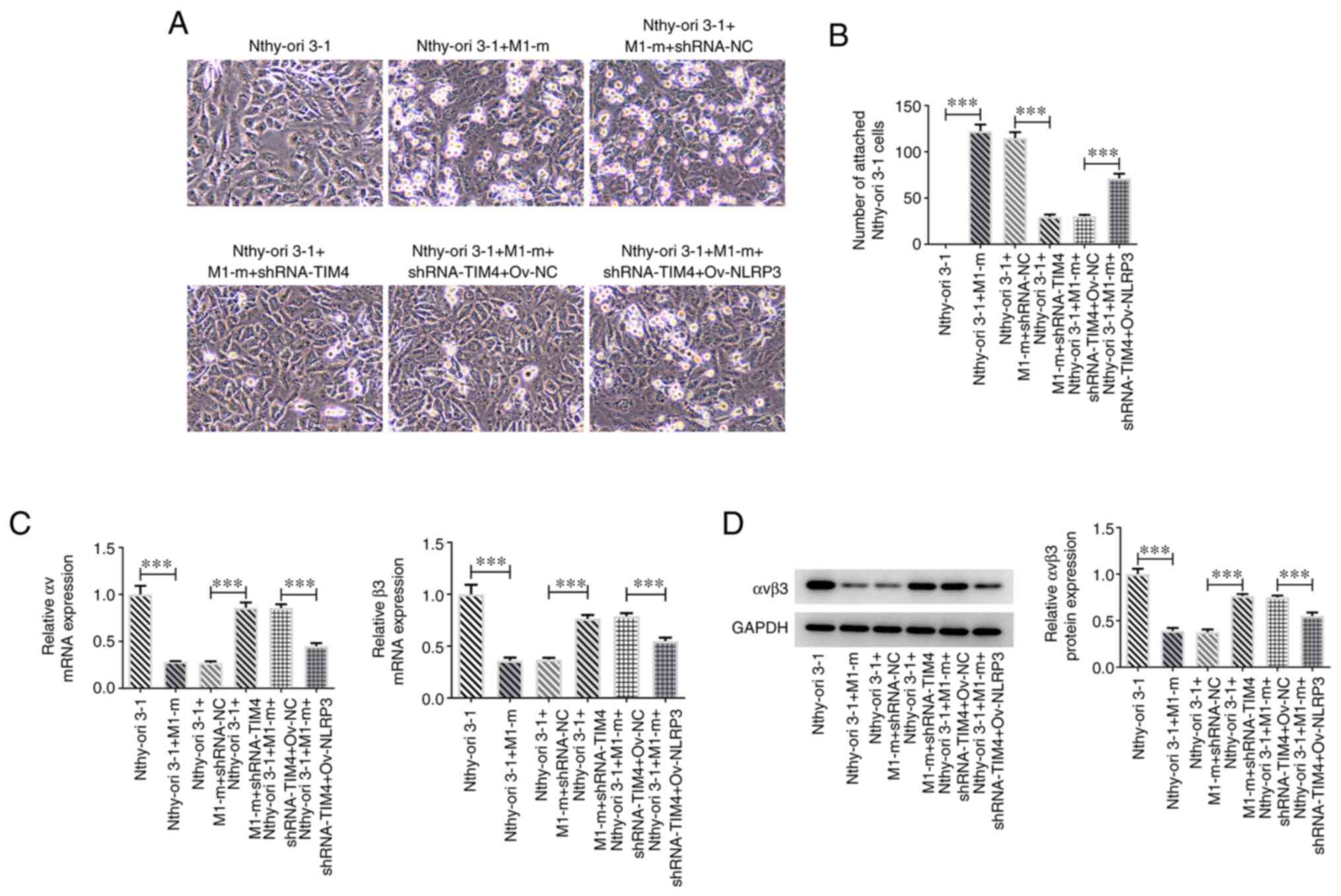 | Figure 5Overexpression of NLRP3 in M1
macrophages affects Nthy-ori 3-1 cell adhesion. (A and B) Adhesive
capacity of Nthy-ori 3-1 cells co-cultured with M1 macrophages
transfected with shRNA-TIM4, with or without Ov-NLRP3. The
expression of integrin αvβ3 in M1 macrophages transfected with
shRNA-TIM4, with or without Ov-NLRP3, was determined by (C) reverse
transcription-quantitative PCR and (D) western blotting. Data are
shown as the mean ± standard deviation of three independent
experiments. ***P<0.001. NLRP3, NOD-, LRR- and pyrin
domain-containing protein 3; shRNA, short hairpin RNA; TIM4, T-cell
immunoglobulin and mucin domain-containing 4; Ov, overexpression
plasmid; M1-m, M1 macrophage; NC, negative control. |
Discussion
HT is an organ-specific autoimmune disease caused by
genetic, environmental and immune tolerance factors (19). Abnormal changes in the thyroid or
immune system can lead to the disruption of immune tolerance, which
can trigger an autoimmune response (20). However, at present, the exact
pathogenesis of HT has not been fully elucidated. The present study
demonstrated that M1 macrophages differentiated and polarized from
THP-1 monocytes, and modulated the inflammatory and apoptotic
response of Nthy-ori 3-1 cells via the TIM4/NLRP3 axis. An in
vitro M1 macrophage and TFC co-culture model was used to
simulate the effects of macrophages observed in clinical or in
vivo studies.
A previous study reported that there was an
increased number of M1 macrophages in the mice model of thyroiditis
relative to the control group and that a decrease in M1 macrophage
polarization could produce a therapeutic effect (21). In the present study, the results
demonstrated that M1 macrophages exhibited increased TIM4 and NLRP3
expression levels, which significantly affected the inflammation
and apoptosis of Nthy-ori 3-1 cells. A previous study demonstrated,
via the analysis of microarray expression profiles of HT and normal
thyroid samples, that genes associated with inflammation and
apoptosis were dysregulated (22).
The present study indicated that this dysregulation was potentially
related to the modulation of M1 macrophages via the TIM4/NLRP3
axis. The present study also explored the effect of M1 macrophages
on the adhesion of Nthy-ori 3-1 cells. TIM4 silencing resulted in a
decreased cell number, whereas NLRP3 overexpression markedly
reduced this effect. Furthermore, it was demonstrated that TIM4
silencing in M1 macrophages significantly increased the protein
expression levels of integrin αvβ3 in Nthy-ori 3-1 cells. However,
its effects were reversed by NLRP3 overexpression, which suggested
that factors secreted by M1 macrophages potentially affected the
adhesion of Nthy-ori 3-1 cells. In addition, the present study
demonstrated that TIM4 silencing resulted in decreased expression
of NLRP3 in M1 macrophages, but the mechanism explaining the
relationship between the two molecules is not clear and should be
explored in the future.
In summary, to the best of our knowledge, the model
used in the present study is novel, as THP-1 cells were used to
produce M1 macrophages, which were then co-cultured with Nthy-ori
3-1 cells. Based on the results in the present study, it could be
speculated that soluble factors secreted by M1 macrophages could
activate inflammatory and apoptotic signaling pathways, and might
be involved in the adhesion of Nthy-ori 3-1 cells to potentially
induce cell injury. The model produced in the present study has
provided a novel approach to study the modulation of the underlying
signaling pathways in the effect of macrophages on Nthy-ori 3-1
cells in co-culture. Therefore, the regulation of M1 macrophages
via targeting of the TIM4/NLRP3 axis may be a potential therapeutic
approach for the treatment of HT.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Grants-in-Aid
for Scientific Research from the Ministry of Nantong Science and
Technology (grant no. JC2019136).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and YS designed the study, performed the
experiments, and drafted and revised the manuscript. XJ, XL, LC and
YQ analyzed the data and searched the literature. YC and YS confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shan Z, Chen L, Lian X, Liu C, Shi B, Shi
L, Tong N, Wang S, Weng J, Zhao J, et al: Iodine status and
prevalence of thyroid disorders after introduction of mandatory
universal salt iodization for 16 years in China: A cross-sectional
study in 10 cities. Thyroid. 26:1125–1130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peng H, Liu Y, Tian J, Ma J, Tang X, Rui
K, Tian X, Mao C, Lu L, Xu H, et al: The long noncoding RNA
IFNG-AS1 promotes T helper type 1 cells response in patients with
Hashimoto's thyroiditis. Sci Rep. 5(17702)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arango Duque G and Descoteaux A:
Macrophage cytokines: Involvement in immunity and infectious
diseases. Front Immunol. 5(491)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Du T, Yang CL, Ge MR, Liu Y, Zhang P, Li
H, Li XL, Li T, Liu YD, Dou YC, et al: M1 Macrophage derived
exosomes aggravate experimental autoimmune neuritis via modulating
Th1 response. Front Immunol. 11(1603)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu S, Chen J, Shi J, Zhou W, Wang L, Fang
W, Zhong Y, Chen X, Chen Y, Sabri A and Liu S: M1-like
macrophage-derived exosomes suppress angiogenesis and exacerbate
cardiac dysfunction in a myocardial infarction microenvironment.
Basic Res Cardiol. 115(22)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kou XX, Li CS, He DQ, Wang XD, Hao T, Meng
Z, Zhou YH and Gan YH: Estradiol promotes M1-like macrophage
activation through cadherin-11 to aggravate temporomandibular joint
inflammation in rats. J Immunol. 194:2810–2818. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li L, Lv G, Wang B and Kuang L:
XIST/miR-376c-5p/OPN axis modulates the influence of
proinflammatory M1 macrophages on osteoarthritis chondrocyte
apoptosis. J Cell Physiol. 235:281–293. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Caturegli P, De Remigis A, Ferlito M,
Landek-Salgado MA, Iwama S, Tzou SC and Ladenson PW: Anatabine
ameliorates experimental autoimmune thyroiditis. Endocrinology.
153:4580–4587. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xue H, Yang Y, Zhang Y, Song S, Zhang L,
Ma L, Yang T and Liu H: . Macrophage migration inhibitory factor
interacting with Th17 cells may be involved in the pathogenesis of
autoimmune damage in Hashimoto's thyroiditis. Mediators Inflamm.
2015(621072)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shi L, Zhou L, Wang J, Li F and Xie L:
Cytokine production of papillary thyroid carcinoma coexisting with
Hashimoto's thyroiditis. Int J Clin Exp Pathol. 10:9567–9574.
2017.PubMed/NCBI
|
|
12
|
Liu Z, Tan K, Bu L, Bo L, Ni W, Fei M,
Chen F, Deng X and Li J: Tim4 regulates NALP3 inflammasome
expression and activity during monocyte/macrophage dysfunction in
septic shock patients. Burns. 46:652–662. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu H, Chen G, Wang J, Deng M, Yuan F and
Gong J: TIM-4 interference in Kupffer cells against CCL4-induced
liver fibrosis by mediating Akt1/Mitophagy signalling pathway. Cell
Prolif. 53(e12731)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo Q, Wu Y, Hou Y, Liu Y, Liu T, Zhang H,
Fan C, Guan H, Li Y, Shan Z and Teng W: Cytokine secretion and
pyroptosis of thyroid follicular cells mediated by enhanced NLRP3,
NLRP1, NLRC4, and AIM2 inflammasomes are associated with autoimmune
thyroiditis. Front Immunol. 9(1197)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu W, Bai F, Wang H, Liang Y, Du X, Liu
C, Cai D, Peng J, Zhong G, Liang X, et al: Tim-4 inhibits NLRP3
inflammasome via the LKB1/AMPKα pathway in macrophages. J Immunol.
203:990–1000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Genin M, Clement F, Fattaccioli A, Raes M
and Michiels C: M1 and M2 macrophages derived from THP-1 cells
differentially modulate the response of cancer cells to etoposide.
BMC Cancer. 15(577)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Russo E, Salzano M, Postiglione L, Guerra
A, Marotta V and Vitale M: Interferon-γ inhibits integrin-mediated
extracellular signal-regulated kinase activation stimulated by
fibronectin binding in thyroid cells. J Endocrinol Invest.
36:375–378. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wiersinga WM: Thyroid autoimmunity. Endocr
Dev. 26:139–157. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Teng W, Shan Z, Teng X, Guan H, Li Y, Teng
D, Jin Y, Yu X, Fan C, Chong W, et al: Effect of iodine intake on
thyroid diseases in China. N Engl J Med. 354:2783–2793.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jia X, Zhai T, Qu C, Ye J, Zhao J, Liu X,
Zhang JA and Qian Q: Metformin reverses Hashimoto's thyroiditis by
regulating key immune events. Front Cell Dev Biol.
9(685522)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Subhi O, Schulten HJ, Bagatian N,
Al-Dayini R, Karim S, Bakhashab S, Alotibi R, Al-Ahmadi A, Ata M,
Elaimi A, et al: Genetic relationship between Hashimoto's
thyroiditis and papillary thyroid carcinoma with coexisting
Hashimoto's thyroiditis. PLoS One. 15(e0234566)2020.PubMed/NCBI View Article : Google Scholar
|