Introduction
In the central nervous system, repressor element-1
silencing transcription/neuron-restrictive silencer factor
(REST/NRSF) maintains homeostasis by suppressing the apoptosis of
neurons (1-3).
Its expression increases with age and protects nerves against aging
stress (3). We previously reported
the up-regulated expression of REST/NRSF in neurons in the
peripheral and central nervous systems with aging (4). However, the effects of REST/NRSF on
axon regeneration following peripheral nerve injury currently
remain unclear.
The ability to regenerate axons damaged by
peripheral nerve injury decreases with age (5,6). Our
previous findings, obtained using a mouse peripheral nerve injury
model, suggested that axon regeneration was significantly slower in
aged mice than in young mice (7).
Moreover, ‘angiogenesis’ and ‘Schwann cell migration’, the
processes required for axon regeneration immediately after
peripheral nerve injury, were impaired in aged mice (7).
The up-regulated expression of neurotrophic factors
that occurs within one week of peripheral nerve injury has been
shown to play an important role in Schwann cell migration and
myelination during axon regeneration (8,9).
Based on these findings, the expression of nerve-specific proteins
immediately after peripheral nerve injury needs to be examined in
order to obtain more detailed insights into axon regeneration.
A peripheral nerve injury model in young and aged
mice was used to investigate the effects of aging on axon
regeneration in the present study. The degree of Wallerian
degeneration and the expression of nerve-specific proteins
immediately after peripheral nerve injury were compared between
these groups. Furthermore, the effects of REST/NRSF on axon
regeneration, which currently remain unclear, were discussed based
on the expression of nerve-specific proteins.
Materials and methods
Animal Model
The present study was approved by the Animal Care
Committee of Juntendo University, Tokyo, Japan (registration no.
1555; approval no. 2021312).
Forty male C57BL/6 mice (Young group: 10-week-old
mice, n=20; Aged group: 70-week-old mice, n=20) purchased from
Japan SLC, Inc. (Shizuoka, Japan) were used. Mice were housed at 5
animals/cage in a sterile environment controlled at a temperature
of 22±2˚C, humidity of 40-60%, and 12-h light and dark cycle, and
were given water that was CRF-1 gamma-ray irradiated at 15 kGy
(Oriental Yeast Co., Ltd.) ad libitum. There are some
reports that low estrogen affects peripheral neuropathy (10,11).
Because estrogen decrease with age (12,13),
males with less estrogen fluctuations and susceptible to estrogen
were used in this study.
Peripheral nerve injury model
The Young group (n=20) and Aged group (n=20) were
divided into Control and Crush groups to create four groups (A:
Young control (n=10), B: Young crush (n=10), C: Aged control
(n=10), and D: Aged crush (n=10)). Chronic constriction injury
(CCI) is a partial nerve injury that is mostly used in rodents and
is performed using a hemostatic forceps (14). It induces incomplete nerve injury.
In the present study, this CCI model was used and defined as the
peripheral nerve injury group (Crush group).
Under general anesthesia with isoflurane inhalation
anesthetic solution (4% isoflurane for induction and 2% for
maintenance) (7), a skin incision
was made on the lateral side of the right hindlimb. A light
microscope (Zeiss, Axioskop2, magnification, x40) was used to
manipulate the sciatic nerve. The sciatic nerve was dissected from
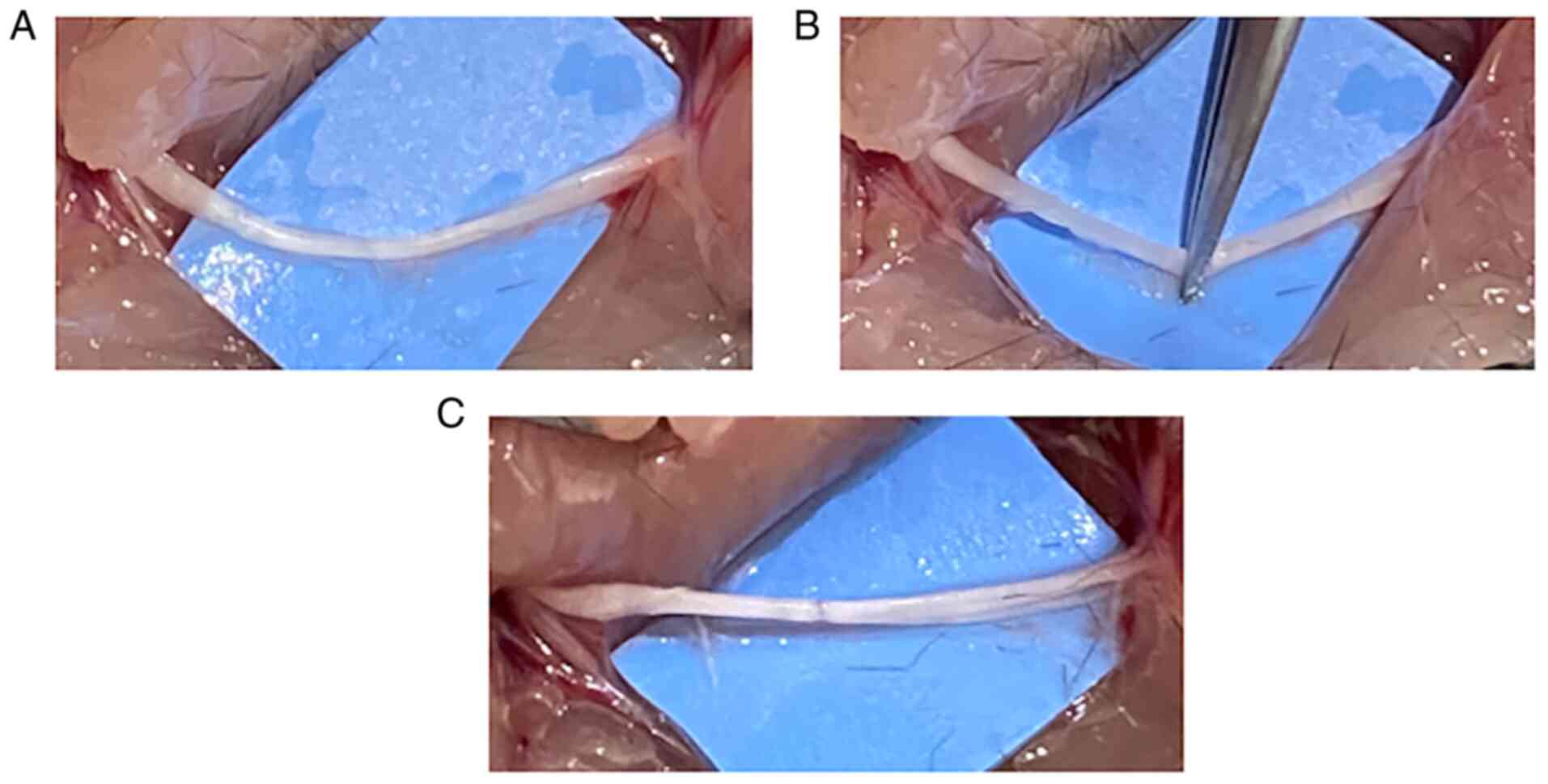
the surrounding tissues (Fig. 1A)
and crushed for 30 sec using the hemostatic forceps with sufficient
strength to flatten the sciatic nerve to caused Wallerian
degeneration (Fig. 1B and C), according to a previously reported
method (14). The postoperative
activity of mice was not limited, and they were maintained in the
same environment as that before the procedure. Under general
anesthesia, the right sciatic nerve and L3-5 dorsal root ganglion
(DRG) of each group were harvested (15). Control group were only harvested
the samples, and samples from the Crush group were harvested one
week after surgery. Mice were sacrificed by cervical dislocation on
the day the sciatic nerves and DRG were harvesting. The harvested
sciatic nerve and DRG were fixed in 4% paraformaldehyde at room
temperature for 72 h and paraffin blocks were prepared.
Histochemical assessment of the degree
of Wallerian degeneration in peripheral nerves
Luxol fast blue (LFB) staining was performed to
assess the degree of Wallerian degeneration after peripheral nerve
injury (16). Tissue sections were
prepared by cutting the sciatic nerve in the longitudinal axis at a
thickness of 3 µm.
Tissue sections of the sciatic nerve in the
longitudinal axis were divided into three areas (the crushed site,
proximal to the crushed site, and distal to the crushed site) in
the Crush groups. Similarly, tissue sections of the sciatic nerve
were divided into three areas in the Control group without
peripheral nerve injury. The area distal to the crushed site was
used in the present study because this was the site at which
Wallerian degeneration occurred. Using a light microscope (Carl
Zeiss, KS400), the percentage of the area stained by LFB to the
total nerve fiber area was calculated in both groups (16,17).
Histochemical assessment of the
expression of nerve-specific proteins after peripheral nerve
injury
Immunofluorescence staining was performed to assess
the expression of nerve-specific proteins after peripheral nerve
injury, as described previous by Goto et al (4). Tissue sections were prepared by
cutting the DRG at a thickness of 3 µm. Samples were deparaffinized
and autoclaved at 121˚C for 10 min for antigen retrieval. After a
treatment with True View™ (SP-8400, Vector) to suppress
autofluorescence, samples were blocked using 2% bovine serum
albumin (A2153; Sigma-Aldrich; Merck KGaA) in PBS containing 0.05%
Tween-20 (PBS-Tween) for 30 min. Samples were then reacted with
antibodies against the target proteins at 4˚C for 15 h. After
washing with PBS-Tween, a goat anti-mouse IgG antibody labeled with
Alexa Fluor 488 (A11001; Thermo Fisher Scientific, Inc.) was used
as a secondary antibody, and a rabbit IgG monoclonal antibody as a
negative control. The intensity of fluorescence in each section was
quantified in the photon counting mode using a fluorescence imaging
microscope (Leica, TCSSP5). The antibodies used in the present
study were against REST/NRSF, a transcription factor that regulates
the expression of nerve-specific proteins, neurotrophin 3 (NT3),
brain-derived neurotrophic factor (BDNF), and nerve growth factor
(NGF), which are neurotrophic factors, and semaphorin 3A (Sema3A),
an axon guidance factor. The following antibodies were obtained
from commercial sources: rabbit polyclonal anti-REST/NRSF (1:200,
22242-1-AP; ProteinTech), rabbit polyclonal anti-NT3 (1:50,
12369-1-AP; ProteinTech), rabbit polyclonal anti-BDNF (1:1,000,
GTX132621; GNT), rabbit polyclonal anti-NGF (1:50, ab6199; Abcam),
and rabbit polyclonal anti-Sema3A (1:50, ab23393; Abcam).
In the photon counting mode, fluorescence intensity
was measured at 20 randomly selected sites from the perikaryon in a
region of interest (ROI) set in a fluorescence-emitting area, and
mean fluorescence intensity was calculated. Fluorescence intensity
measured using each antibody was compared between the four
groups.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) and were analyzed for significant differences using a two-way
ANOVA with age and nerve injury set as two independent variables
(Prism 7; GraphPad Software). After the two-way ANOVA, Turkey's
multiple comparisons test was used as a post hoc test. Differences
were considered to be significant at P<0.05.
Results
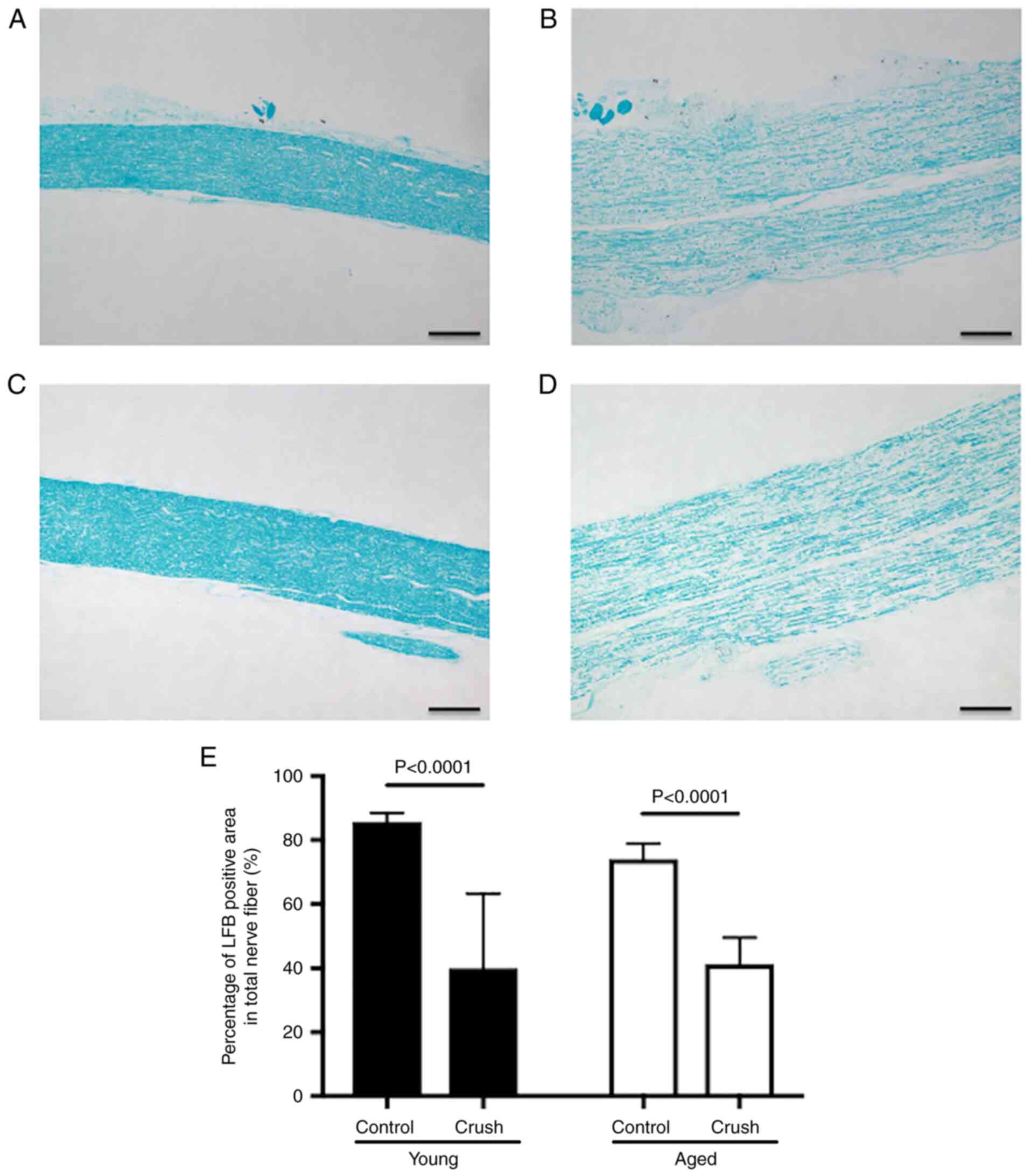
In the sciatic nerve, the percentage of the area of
the myelin sheath to the total nerve fiber area has been used to
assess Wallerian degeneration in peripheral nerve injury (16). LFB stains the myelin sheath blue
and is used to assess the degree of its degeneration (Fig. 2A-D) (17). The percentages of the area of the
myelin sheath to the total nerve fiber area were 85.7±2.6 and
74.0±4.5% in the Young control and Aged control groups,
respectively, and did not significantly differ (P=0.1891).
Following peripheral nerve injury, sciatic nerve fiber swelling,
decreased staining by LFB, and vacuolation were observed in both
the Young and Aged crush groups (Fig.
2B and D). The percentages of
the area of the myelin sheath to the total nerve fiber area were
39.9±22.1 and 41.2±7.8% in the Young and Aged crush groups,
respectively, and were significantly lower in the Crush groups than
in the Control groups in the Young and Aged groups (Young group:
P<0.0001, Aged group: P<0.0001) (Fig. 2B, D and E).
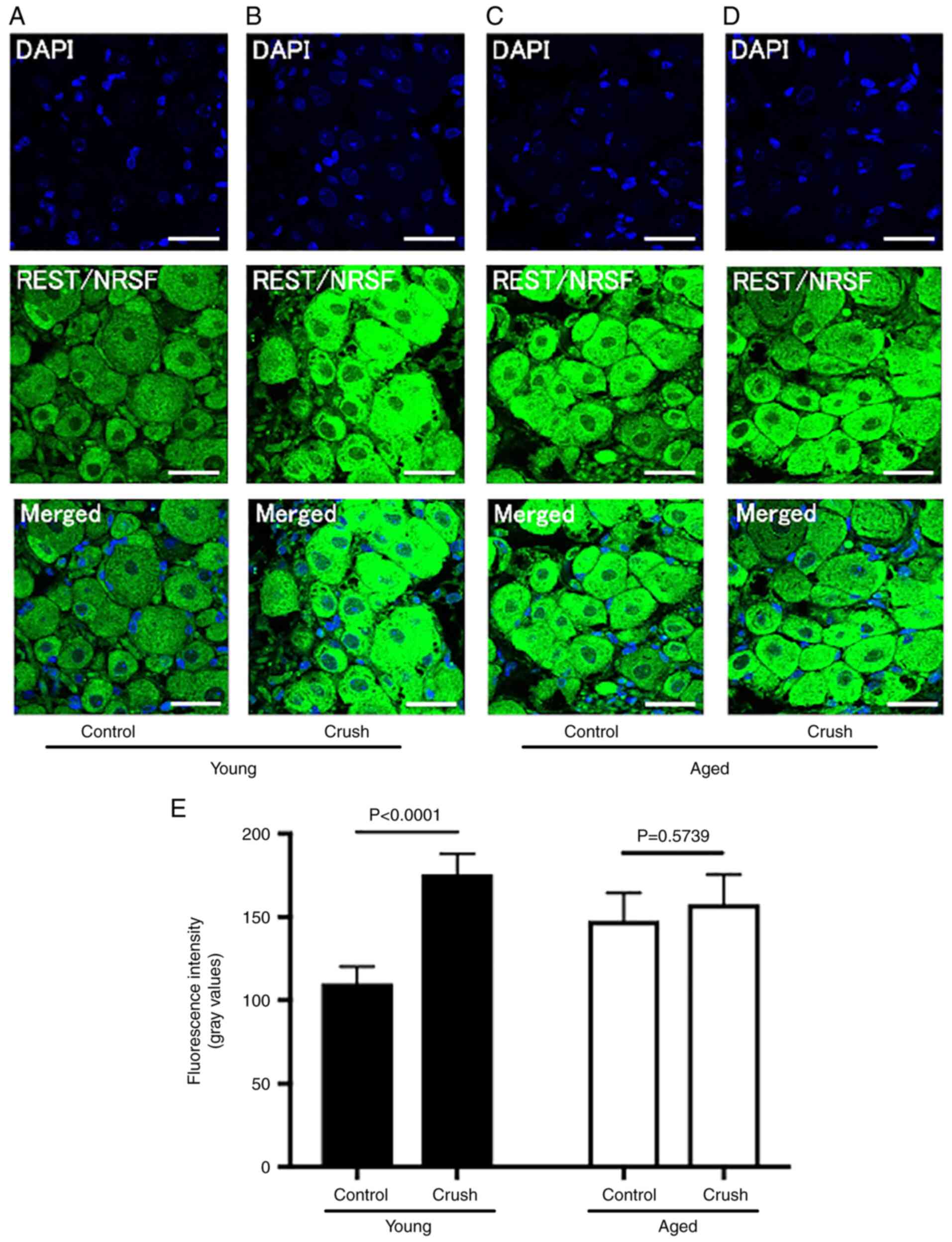
The expression of nerve-specific proteins in DRG was
quantified by immunofluorescence staining. The fluorescence
intensity of REST/NRSF was significantly higher in the Aged control
group (147.8±15.8) than in the Young control group (110.2±9.5)
(P<0.0001) (Table I, Fig. 3A and C). Following peripheral nerve injury, the
fluorescence intensity of REST/NRSF significantly increased in the
Young group (Young crush group: 175.7±11.5, P<0.0001), but not
in the Aged group (Aged crush group: 157.7±16.7, P=0.5739)
(Fig. 3B, D and E).
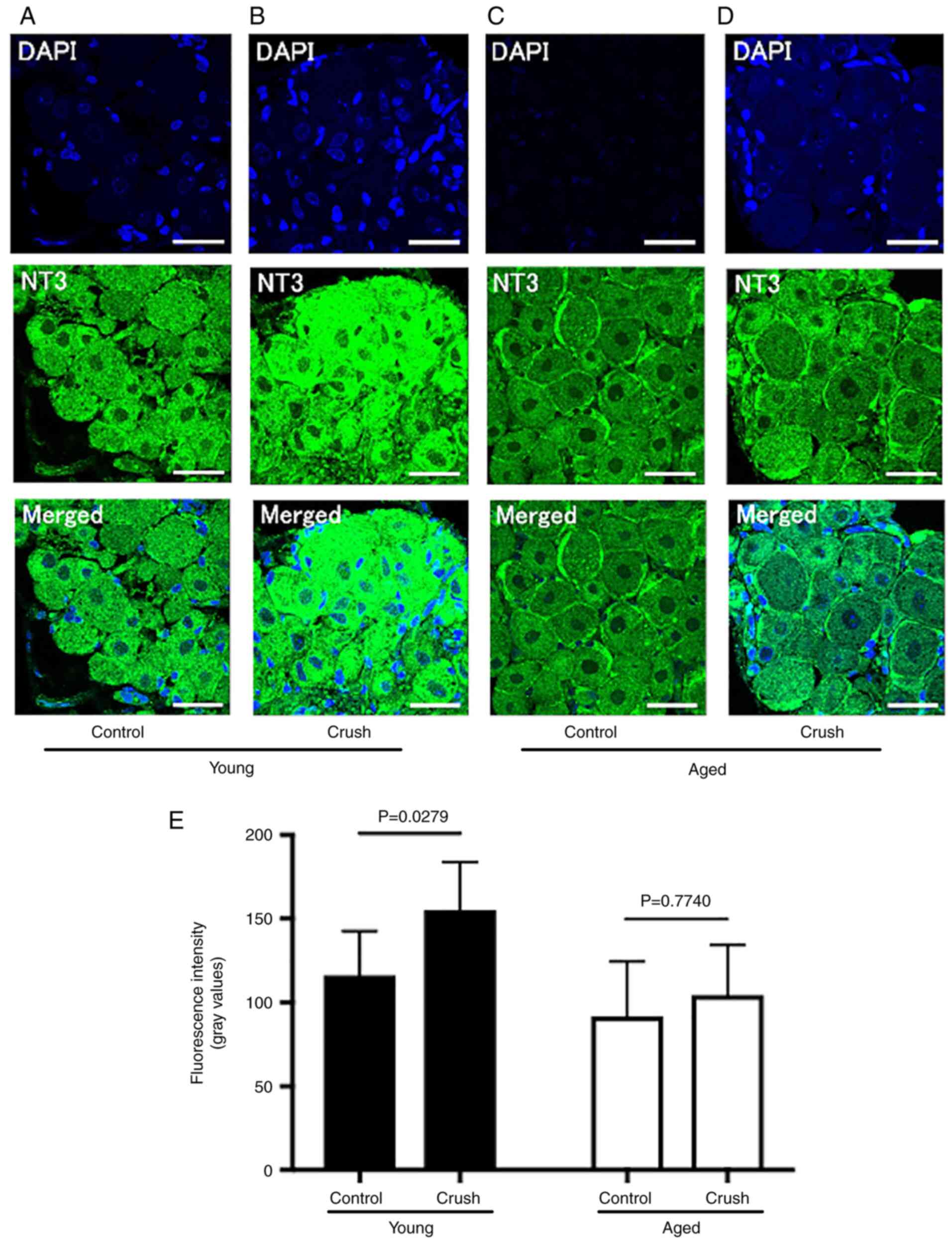
We then investigated the expression of the neurotrophic factors
NT3, BDNF, and NGF. The fluorescence intensity of NT3 was
116.3±24.8 in the Young control group and 91.7±30.9 in the Aged
control group (Fig. 4A and
C), with no significant difference
(P=0.2618) (Table I). Following
peripheral nerve injury, the fluorescence intensity of NT3
significantly increased in the Young group (Young crush group:
155.0±27.1, P=0.0279), but not in the Aged group (Aged crush group:
104.3±28.3, P=0.7740) (Fig. 4B,
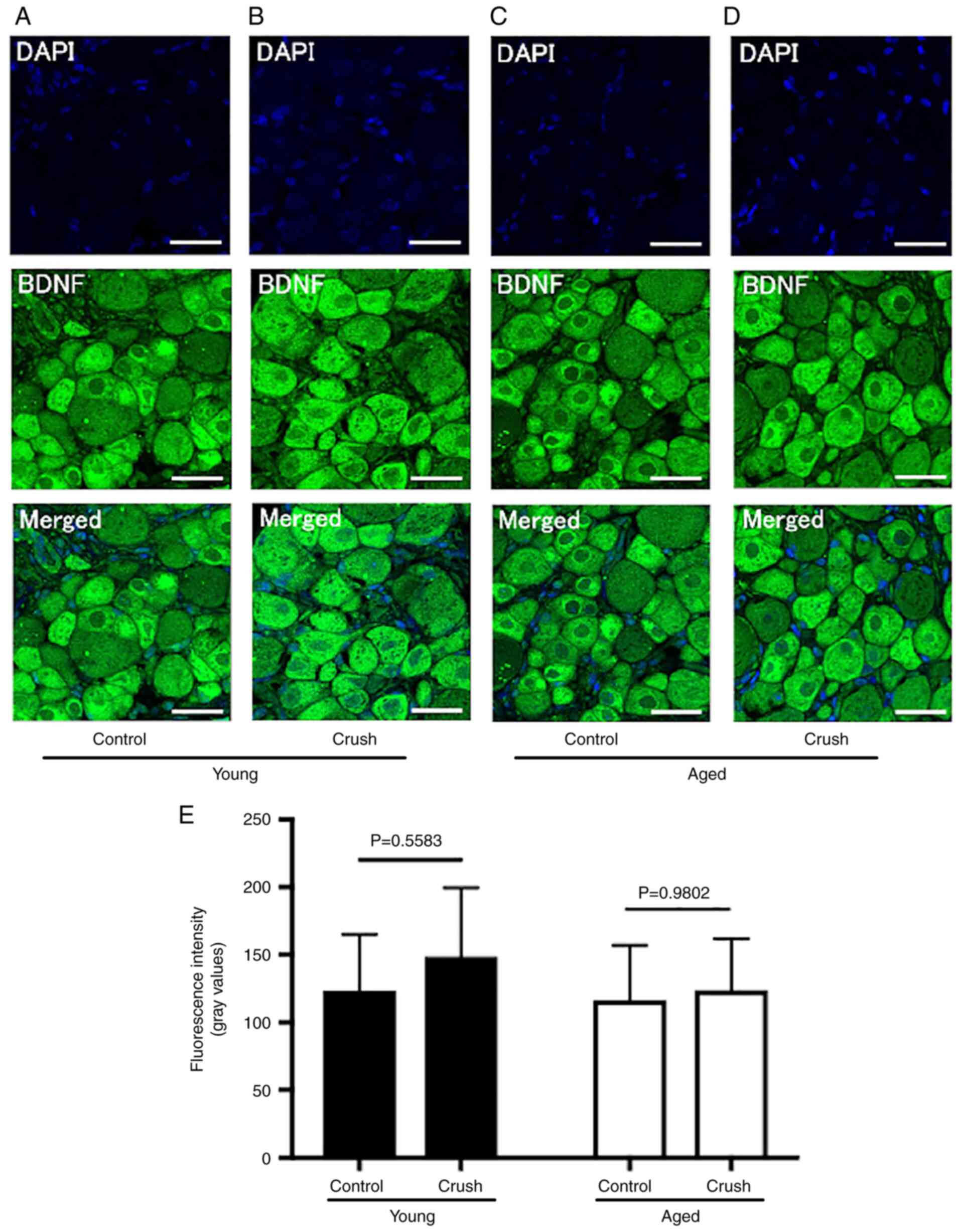
D and E). The fluorescence intensity of BDNF was
123.3±39.6 in the Young control group and 116.4±38.5 in the Aged
control group (Fig. 5A and
C), with no significant difference
(P=0.9836) (Table I). Following
peripheral nerve injury, the fluorescence intensity of BDNF did not
significantly differ in the Young group (Young crush group:
148.6±48.0, P=0.5583) or Aged group (Aged crush group: 123.8±35.9,
P=0.9802) (Fig. 5B, D and E).
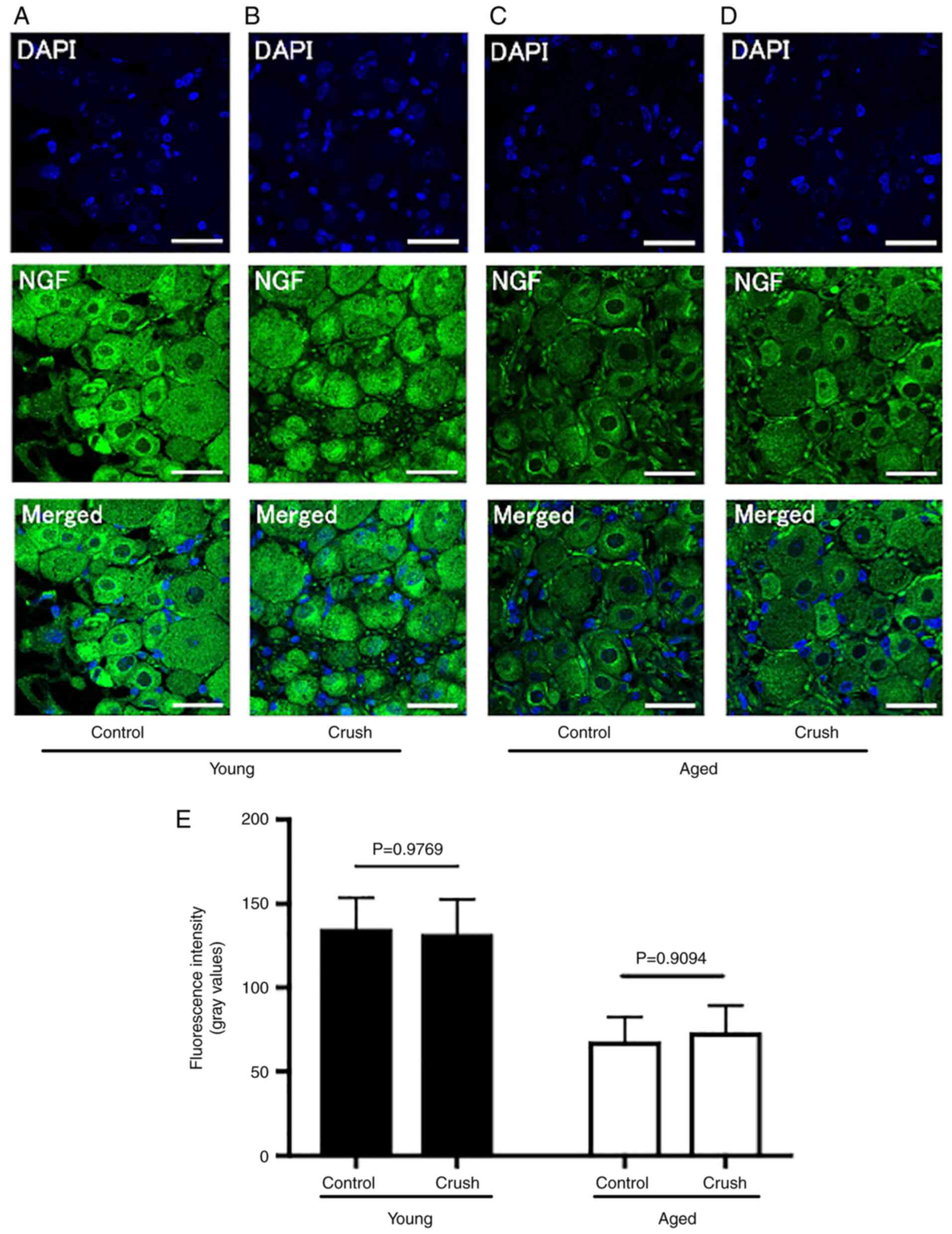
The fluorescence intensity of NGF was significantly higher in the
Young control group (135.1±17.2) than in the Aged control group
(68.1±13.7) (P<0.0001) (Table
I, Fig. 6A and C). On the other hand, following
peripheral nerve injury, the fluorescence intensity of NGF did not
significantly differ in the Young group (Young crush group:
132.0±19.5, P=0.9769) or Aged group (Aged crush group: 73.3±15.1,
P=0.9094) (Fig. 6B, D and E).
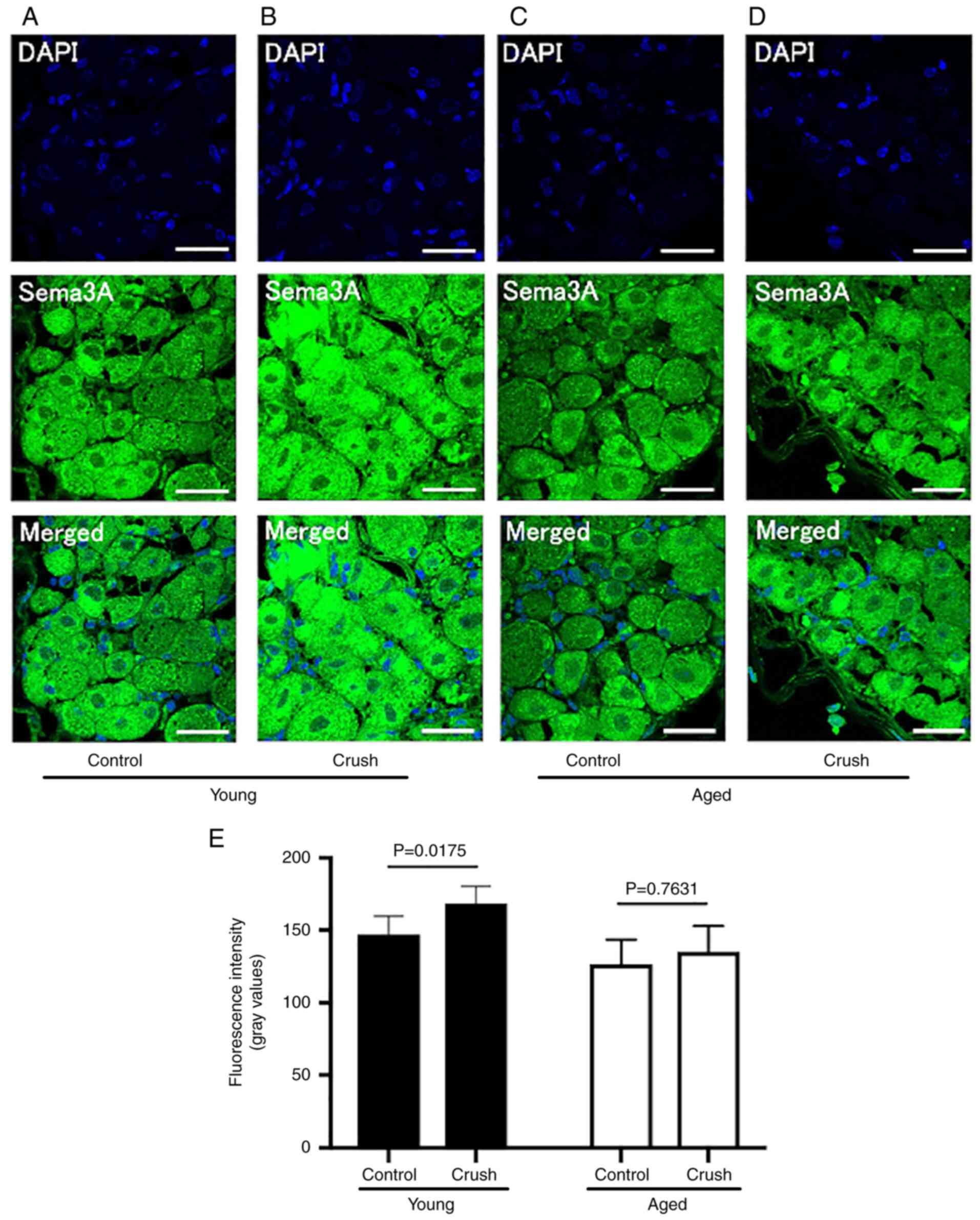
Furthermore, the fluorescence intensity of Sema3A, an axon guidance
factor, was significantly higher in the Young control group
(147.0±12.0) than in the Aged control group (126.5±16.1) (P=0.0266)
(Table I, Fig. 7A and C). Following peripheral nerve injury, the
fluorescence intensity of Sema3A significantly increased in the
Young group (Young crush group: 168.5±11.3, P=0.0175), but remained
unchanged in the Aged group (Aged crush group: 135.1±16.8,
P=0.7631) (Fig. 7B, D and E).
 | Table IComparison of the expression of the
nerve-specific proteins between Young and Aged group. |
Table I
Comparison of the expression of the
nerve-specific proteins between Young and Aged group.
| Nerve-specific
proteins | Young | Aged | P-value |
|---|
| REST/NRSF (gray
values) | 110.2±9.5 | 147.8±15.8 | P<0.0001 |
| NT3 (gray
values) | 116.3±24.8 | 91.7±30.9 | P=0.2618 |
| BDNF (gray
values) | 123.3±39.6 | 116.4±38.5 | P=0.9836 |
| NGF (gray
values) | 135.1±17.2 | 68.1±13.7 | P<0.0001 |
| Sema3A (gray
values) | 147.0±12.0 | 126.5±16.1 | P=0.0266 |
Discussion
In the present study, we investigated whether the
degree of Wallerian degeneration after peripheral nerve injury was
affected by aging using a mouse peripheral nerve injury model. In
peripheral nerves, the number and density of axons decrease with
age (6,18,19).
However, LFB staining in the present study showed that the
percentage of the area of the myelin sheath to the total nerve
fiber area did not significantly differ between the Young and Aged
control groups, and aging did not markedly affect the myelination
of peripheral nerves. Furthermore, following peripheral nerve
injury, sciatic nerve fiber swelling, decreased staining by LFB,
and vacuolation were observed in both the Young and Aged groups,
and the percentage of the area of the myelin sheath was
significantly lower in the Crush group than in the Control group in
both the Young and Aged groups. In other words, Wallerian
degeneration after peripheral nerve injury was detected in both the
Young and Aged groups. However, the effects of aging on axon
regeneration after peripheral nerve injury currently remain unknown
(20-22).
Peripheral nerve injury always induces inflammation.
It is a complex series of molecular and cellular events through the
recruitment of circulating proteins and leukocytes to the injury
site within hours to days after peripheral nerve injury (23). These reactions associated with
inflammation are thought to have a significant effect on axon
regeneration (24,25). To investigate the effects of aging
on axon regeneration after peripheral nerve injury, we quantified
the expression of nerve-specific proteins in young and aged mice
with similarly degenerated peripheral nerves. In peripheral nerve
injury, the expression of most of the genes required for axon
regeneration and synaptogenesis is regulated by REST/NRSF (26-28),
and REST/NRSF possesses a wide number of functions through its
regulation of more than 1000 target genes (3,29-31).
In the central nervous system, the expression of REST/NRSF was
shown to increase with age, and maintained homeostasis by
suppressing the apoptosis of neurons (1-3).
We also previously demonstrated that the expression of REST/NRSF
increased with age in peripheral nerves (4). In the present study, the expression
of REST/NRSF was significantly higher in the Aged control group
than in the Young control group, consistent with the previous
studies. The up-regulated expression of REST/NRSF after peripheral
nerve injury has also been demonstrated (32-35),
and indicates the initiation of axon regeneration in the injured
peripheral nerve (32). In the
peripheral nerve injury model used in the present study, the
expression of REST/NRSF was significantly increased in the Young
group, but not in the Aged group, suggesting that axon regeneration
was initiated immediately after peripheral nerve injury in the
Young group, but not in the Aged group.
The expression of neurotrophic factors has been
shown to play an important role in axon regeneration after
peripheral nerve injury (8,9). NT3
is a necessary factor for Schwann cell survival and differentiation
in the absence of axons (9,36),
BDNF maintains peripheral nerve homeostasis by regulating neuron
survival maintenance, neurite outgrowth promotion, and
synaptogenesis (9,37-40),
and NGF promotes axon elongation in peripheral nerves (41-44).
Therefore, the neurotrophic factors investigated in the present
study are markers for Schwann cell migration (NT3), myelination
(BDNF), and axon elongation (NGF). To investigate the effects of
aging on the expression of these neurotrophic factors, their
expression was compared between the Young and Aged control groups.
The results obtained showed no significant differences in the
expression of NT3 or BDNF between these groups, while the
expression of NGF was significantly lower in the Aged control group
than in the Young control group, suggesting that Schwann cell
migration may not be affected by aging in the Control group in the
presence of axons. Furthermore, the results on the expression of
BDNF suggest that myelination was not affected by aging. However,
the results on the expression of NGF indicate that axon elongation
decreased with aging. These results are consistent with our
previous findings showing that the ability to regenerate axons in
peripheral nerve is decreased with age (7). Moreover, we investigated changes in
the expression of neurotrophic factors characterized by age-related
changes in the Young and Aged groups following peripheral nerve
injury. In comparisons with the respective Control groups, the
expression of BDNF and NGF did not significantly differ between the
Young and Aged crush groups, while the expression of NT3
significantly increased in the Young group, but not in the Aged
group. In other words, Wallerian degeneration occurred and axons
were absent in injured peripheral nerves, axon regeneration was
initiated by the increased expression of REST/NRSF, and the
migration of Schwann cells, which is the initial stage of axon
regeneration, was induced by the upregulation of NT3 in the Young
group. However, based on the results for the expression of
REST/NRSF and NT3, axon regeneration was not initiated in the Aged
group following peripheral nerve injury. The expression of BDNF and
NGF, which have been suggested to play a role in myelination and
axon elongation after Schwann cell migration for axon regeneration,
was not increased in the Young or Aged group one week after
peripheral nerve injury in the present study.
There are several limitations in this study. First,
in creating the CCI model for peripheral nerve injury, the
compression method with a hemostatic forceps was used, so the
degree of nerve injury may be different with each mice. It has been
reported that the expression of neurotrophic factor varies
depending on the degree of nerve injury (Neurapraxia, Axonotmesis,
Neurotmesis) (9). However, there
were no complication (death, disability, etc). Next, in this study,
we performed evaluation the expression of nerve-specific proteins
such as REST/NRSF and discussed how they affect axon regeneration.
On the other hand, although inflammatory cytokines are up-regulated
with aging or nerve injury (45,46),
age-related differences in inflammatory cytokine levels after
peripheral nerve injury are unclear (47). However, the degree of inflammation
after nerve injury was not investigated in this sturdy. In a future
study, to investigate the degree of inflammation and age-related
differences in inflammatory cytokine levels after peripheral nerve
injury may provide a more detailed understanding of the effects of
aging on axon regeneration. Moreover, we also investigated the
expression of nerve-specific proteins in the cytoplasm and the
nucleus in the perikaryon of DRG, but there was no significant
difference among them. Therefore, the fluorescence intensity of the
whole cell was measured by the method of this study. Therefore,
since all DRG samples were used by histochemical assessment in this
study, the quantification of nerve-specific proteins by western
blot were not able to be performed. This is the limitation of this
study and a future issue. Last, since this study is a fixed-point
observation one week after peripheral nerve injury, it only
evaluates the effect of peripheral nerve injury and aging in a
limited manner. It has been reported that the expression of
nerve-specific proteins varies greatly depending on the time after
nerve injury (9,48-51).
We believe that it may be possible to evaluate the effect of
peripheral nerve injury and aging in more detail by evaluating with
time course. However, in assessment of the axon regeneration
process, it is highly meaningful to investigate the expression of
these nerve-specific proteins at one week after peripheral nerve
injury. Because the up-regulated expression of neurotrophic factors
and Schwann cell migration, an early stage of axon regeneration,
occur within one week after nerve injury (52). Therefore, in this study, we
performed evaluation at one week after peripheral nerve injury and
discussed it.
Based on the results of present study, while
compensatory changes for peripheral nerve injury were initiated by
the upregulation of the REST/NRSF, followed by Schwann cell
migration in the Young group, these compensatory changes did not
occur in the Aged group. The regulation of REST/NRSF expression
appears to be essential for axon regeneration when peripheral
nerves are exposed to stress. In peripheral nerves,
aging-associated functional and electrophysiological disorders have
been reported in clinical study (6). This study and our previous study
showed the REST/NRSF expression is increased with age (4), and the expression is also increased
by nerve injury according to the results of this study. Therefore,
focusing on the expression of REST/NRSF is expected to elucidate
the pathology of nerve injury, and is also expected to contribute
significantly to the treatment of age-related peripheral nerve
injury.
In conclusion, the present results suggested that
Wallerian degeneration occurred after peripheral nerve injury in
the Young and Aged groups. On the other hand, compensatory changes
for peripheral nerve injury and Schwann cell migration were
initiated in the Young group, but not in the Aged group.
Acknowledgements
Not applicable.
Funding
Funding: No funding was recieved.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HO mainly wrote the manuscript and acquired,
analyzed and interpretated the data. KN wrote the manuscript and
made substantial contributions to conception and design of the
study, and interpretation of data. SN and KS contributed to
acquisition, analysis and interpretation of data. SK, TS KG, AK, NN
and YS contributed to acquisition of data. SK and TS confirm the
authenticity of all the raw data. IN made substantial contributions
to conception and design. MI contributed to the analysis and
interpretation of data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
Committee of Juntendo University (Tokyo, Japan; registration no.
1555; approval no. 2021312).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen
Y, Yang TH, Kim HM, Drake D, Liu XS, et al: REST and stress
resistance in ageing and Alzheimer's disease. Nature. 507:448–454.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kawamura M, Sato S, Matsumoto G, Fukuda T,
Shiba-Fukushima K, Noda S, Takanashi M, Mori N and Hattori N: Loss
of nuclear REST/NRSF in aged-dopaminergic neurons in Parkinson's
disease patients. Neurosci Lett. 699:59–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mampay M and Sheridan GK: REST: An
epigenetic regulator of neuronal stress responses in the young and
ageing brain. Front Neuroendocrinol. 53(100744)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Goto K, Naito K, Nakamura S, Nagura N,
Sugiyama Y, Obata H, Kaneko A and Kaneko K: Protective mechanism
against age-associated changes in the peripheral nerves. Life Sci.
253(117744)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Büttner R, Schulz A, Reuter M, Akula AK,
Mindos T, Carlstedt A, Riecken LB, Baader SL, Bauer R and Morrison
H: Inflammaging impairs peripheral nerve maintenance and
regeneration. Aging Cell. 17(e12833)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Verdú E, Ceballos D, Vilches JJ and
Navarro X: Influence of aging on peripheral nerve function and
regeneration. J Peripher Nerv Syst. 5:191–208. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kaneko A, Naito K, Nakamura S, Miyahara K,
Goto K, Obata H, Nagura N, Sugiyama Y, Kaneko K and Ishijima M:
Influence of aging on the peripheral nerve repair process using an
artificial nerve conduit. Exp Ther Med. 21(168)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chan JR, Cosgaya JM, Wu YJ and Shooter EM:
Neurotrophins are key mediators of the myelination program in the
peripheral nervous system. Proc Natl Acad Sci USA. 98:14661–14668.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Omura T, Sano M, Omura K, Hasegawa T, Doi
M, Sawada T and Nagano A: Different expressions of BDNF, NT3, and
NT4 in muscle and nerve after various types of peripheral nerve
injuries. J Peripher Nerv Syst. 10:293–300. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim JK, Hann HJ, Kim MJ and Kim JS: The
expression of estrogen receptors in the tenosynovium of
postmenopausal women with idiopathic carpal tunnel syndrome. J
Orthop Res. 28:1469–1474. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Rousan T, Sparks JA, Pettinger M,
Chlebowski R, Manson JE, Kauntiz AM and Wallace R: Menopausal
hormone therapy and the incidence of carpal tunnel syndrome in
postmenopausal women: Findings from the Women's Health Initiative.
PLoS One. 13(e0207509)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee AR, Pechenino AS, Dong H, Hammock BD
and Knowlton AA: Aging, estrogen loss and epoxyeicosatrienoic acids
(EETs). PLoS One. 8(e70719)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shifren JL and Schiff IJ: The aging ovary.
J Womens Health Gend Based Med. 9 (Suppl 1):S3–S7. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schram S, Chuang D, Schmidt G, Piponov H,
Helder C, Kerns J, Gonzalez M, Song F and Loeb JA: Mutant SOD1
prevents normal functional recovery through enhanced glial
activation and loss of motor neuron innervation after peripheral
nerve injury. Neurobiol Dis. 12:469–478. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gallaher ZR and Steward O: Modest
enhancement of sensory axon regeneration in the sciatic nerve with
conditional co-deletion of PTEN and SOCS3 in the dorsal root
ganglia of adult mice. Exp Neurol. 303:120–133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lindborg JA, Mack M and Zigmond RE:
Neutrophils are critical for myelin removal in a peripheral nerve
injury model of Wallerian degeneration. J Neurosci. 37:10258–10277.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Niemi JP, DeFrancesco-Lisowitz A,
Roldán-Hernández L, Lindborg JA, Mandell D and Zigmond RE: A
critical role for macrophages near axotomized neuronal cell bodies
in stimulating nerve regeneration. J Neurosci. 33:16236–16248.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Apel PJ, Ma J, Callahan M, Northam CN,
Alton TB, Sonntag WE and Li Z: Effect of locally delivered IGF-1 on
nerve regeneration during aging: An experimental study in rats.
Muscle Nerve. 41:335–341. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Calkins DJ: Age-related changes in the
visual pathways: Blame it on the axon. Invest Ophthalmol Vis Sci.
54:ORSF37–41. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bland JD: The relationship of obesity,
age, and carpal tunnel syndrome: More complex than was thought?
Muscle Nerve. 32:527–532. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Di Caprio F, Meringolo R, Shehab Eddine M
and Ponziani L: Morton's interdigital neuroma of the foot: A
literature review. Foot Ankle Surg. 24:92–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Martínez-Aparicio C, Jääskeläinen SK,
Puksa L, Reche-Lorite F, Torné-Poyatos P, Paniagua Soto J and Falck
B: Constitutional risk factors for focal neuropathies in patients
referred for electromyography. Eur J Neurol. 27:529–535.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ransohoff RM: Chemokines and chemokine
receptors: Standing at the crossroads of immunobiology and
neurobiology. Immunity. 31:711–721. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Benowitz LI and Popovich PG: Inflammation
and axon regeneration. Curr Opin Neurol. 24:577–583.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vargas ME, Watanabe J, Singh SJ, Robinson
WH and Barres BA: Endogenous antibodies promote rapid myelin
clearance and effective axon regeneration after nerve injury. Proc
Natl Acad Sci USA. 107:11993–11998. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schoenherr CJ and Anderson DJ: The
neuron-restrictive silencer factor (NRSF): A coordinate repressor
of multiple neuron-specific genes. Science. 267:1360–1363.
1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lunyak VV and Rosenfeld MG: No rest for
REST: REST/NRSF regulation of reurogenesis. Cell. 121:499–501.
2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ballas N and Mandel G: The many faces of
REST oversee epigenetic programming of neuronal genes. Curr Opin
Neurobiol. 15:500–506. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bruce AW, Donaldson IJ, Wood IC, Yerbury
SA, Sadowski MI, Chapman M, Göttgens B and Buckley NJ: Genome-wide
analysis of repressor element 1 silencing transcription
factor/neuron-restrictive silencing factor (REST/NRSF) target
genes. Proc Natl Acad Sci USA. 101:10458–10463. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chong JA, Tapia-Ramírez J, Kim S,
Toledo-AraI JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA,
Kraner SD and Mandel G: REST: A mammalian silencer protein that
restricts sodium channel gene expression to neurons. Cell.
80:949–957. 1995.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao Y, Zhu M, Yu Y, Qiu L, Zhang Y, He L
and Zhang J: Brain REST/NRSF is not only a silent repressor but
also an active protector. Mol Neurobiol. 54:541–550.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Oh YM, Mahar M, Ewan EE, Leahy KM, Zhao G
and Cavalli V: Epigenetic regulator UHRF1 inactivates REST and
growth suppressor gene expression via DNA methylation to promote
axon regeneration. Proc Natl Acad Sci USA. 115:E12417–E12426.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang F, Gigout S, Liu Y, Wang Y, Hao H,
Buckley NJ, Zhang H, Wood IC and Gamper N: Repressor element
1-silencing transcription factor drives the development of chronic
pain states. Pain. 160:2398–2408. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang J, Chen SR, Chen H and Pan HL:
RE1-silencing transcription factor controls the acute-to-chronic
neuropathic pain transition and Chrm2 receptor gene expression in
primary sensory neurons. J Biol Chem. 293:19078–19091.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ueda H, Kurita JI, Neyama H, Hirao Y,
Kouji H, Mishina T, Kasai M, Nakano H, Yoshimori A and Nishimura Y:
A mimetic of the mSin3-binding helix of NRSF/REST ameliorates
abnormal pain behavior in chronic pain models. Bioorg Med Chem
Lett. 27:4705–4709. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Richner M, Ulrichsen M, Elmegaard SL, Dieu
R, Pallesen LT and Vaegter CB: Peripheral nerve injury modulates
neurotrophin signaling in the peripheral and central nervous
system. Mol Neurobiol. 50:945–970. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Park H and Poo MM: Neurotrophin regulation
of neural circuit development and function. Nat Rev Neurosci.
14:7–23. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Miranda M, Morici JF, Zanoni MB and
Bekinschtein P: Brain-derived neurotrophic factor: A key molecule
for memory in the healthy and the pathological brain. Front Cell
Neurosci. 13(363)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yang J, Harte-Hargrove LC, Siao CJ,
Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W,
et al: Hempstead, proBDNF negatively regulates neuronal remodeling,
synaptic transmission, and synaptic plasticity in hippocampus. Cell
Rep. 7:796–806. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bhang SH, Jeon O, Choi CY, Kwon YH and Kim
BS: Controlled release of nerve growth factor from fibrin gel. J
Biomed Mater Res A. 80:998–1002. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen ZW and Wang MS: Effects of nerve
growth factor on crushed sciatic nerve regeneration in rats.
Microsurgery. 16:547–551. 1995.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kemp SW, Webb AA, Dhaliwal S, Dhaliwal S,
Syed S, Walsh SK and Midha R: Dose and duration of nerve growth
factor (NGF) administration determine the extent of behavioral
recovery following peripheral nerve injury in the rat. Exp Neurol.
229:460–470. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moattari M, Kouchesfehani HM, Kaka G,
Sadraie SH and Naghdi M: Evaluation of nerve growth factor (NGF)
treated mesenchymal stem cells for recovery in neurotmesis model of
peripheral nerve injury. J Craniomaxillofac Surg. 46:898–904.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill
AH and Meydani SN: Aging up-regulates expression of inflammatory
mediators in mouse adipose tissue. J Immunol. 179:4829–4839.
2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gaudet AD, Popovich PG and Ramer MS:
Wallerian degeneration: Gaining perspective on inflammatory events
after peripheral nerve injury. J Neuroinflammation.
30(110)2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fitzgerald M and McKelvey R: Nerve injury
and neuropathic pain-A question of age. Exp Neurol 275 Pt.
2:296–302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shudo Y, Shimojo M, Fukunaga M and Ito S:
Pituitary adenylate cyclase-activating polypeptide is regulated by
alternative splicing of transcriptional repressor REST/NRSF in
nerve injury. Life Sci. 143:174–181. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rose K, Ooi L, Dalle C, Robertson B, Wood
IC and Gamper N: Transcriptional repression of the M channel
subunit Kv7.2 in chronic nerve injury. Pain. 152:742–754.
2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Uchida S, Hara K, Kobayashi A, Funato H,
Hobara T, Otsuki K, Yamagata H, McEwen BS and Watanabe Y: Early
life stress enhances behavioral vulnerability to stress through the
activation of REST4-mediated gene transcription in the medial
prefrontal cortex of rodents. J Neurosci. 30:15007–15018.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gervasi NM, Dimtchev A, Clark DM, Dingle
M, Pisarchik AV and Nesti LJ: C-terminal domain small phosphatase 1
(CTDSP1) regulates growth factor expression and axonal regeneration
in peripheral nerve tissue. Sci Rep. 11(14462)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yin Q, Kemp GJ, Yu LG, Wagstaff SC and
Frostick SP: Expression of Schwann cell-specific proteins and
low-molecular-weight neurofilament protein during regeneration of
sciatic nerve treated with neurotrophin-4. Neuroscience.
105:779–783. 2001.PubMed/NCBI View Article : Google Scholar
|