Introduction
Coronavirus disease 2019 (COVID-19) is considered a
systematic disease with an enhanced host inflammatory response
(1) affecting several
extra-pulmonary organs, such as the gastrointestinal system
(2,3). Abnormalities in liver biochemistry
have been recorded in over half of the patients with COVID-19 upon
admission and this proportion is increased during hospitalization
(4,5). These abnormalities are usually
presented as elevations in the levels of aspartate aminotransferase
(AST) and alanine aminotransferase (ALT), while less frequently,
increased levels of cholestatic enzymes [i.e., alkaline phosphatase
(ALP), γ-glutamyl transpeptidase (γ-GT)] and bilirubin have been
observed (4). The development of
acute hepatitis (AH) is defined as AST and/or ALT levels >400
IU/l, and the presence of liver injury (LI) is defined as an
elevation in ALT levels [(>3-fold the upper limit of normal
(ULN)] or at least as a moderate elevation in the levels of ALP or
total bilirubin (>2-fold the ULN) (6). Although the pathogenetic mechanisms
associated with COVID-19-induced LI (usually defined as a
combination of aminotransferases/cholestatic enzymes/bilirubin
abnormalities) (7) have not yet
been elucidated, elevated levels of aminotransaminases have been
shown to be associated with a higher risk of mortality (6) and complications, indicating the
severity of COVID-19 and the intensity of hyperinflammation
syndrome and cytokine storm observed during severe acute
respiratory syndrome coronavirus-2 (SARS-CoV-2) infection (5,8). Of
note, although the majority of patients develop a hepatocellular
pattern of LI (i.e., predominance of AST/ALT elevation, compared to
ALP/γ-GT), in some patients, a cholestatic pattern of LI has been
recorded (i.e., an elevation in the levels of cholestatic enzymes
and bilirubin) without a clear clinical impact of this finding
(9).
It should be mentioned that abnormalities in the
levels of aminotransferase are usually mild during COVID-19
infection; however, in some patients, these can progress to AH.
Although the development of AH has been found to be associated with
a worse prognosis (9), the
baseline risk factors related to AH during hospitalization have not
yet been fully clarified, since large cohorts need to be assessed
to evaluate this rare biochemical phenomenon. Fortunately, only a
few cases of patients progressing to acute liver failure (ALF) have
been recorded in the literature (10), while in the majority of patients
with abnormalities in biochemical liver test results, values
gradually return to normal levels (11). In a previous study, the authors
evaluated the prevalence, clinical impact and the risk factors
associated with the development of LI (5). Using the same cohort of patients with
COVID-19, the present study aimed to evaluate, for the first time
(to the best of our knowledge), the prevalence, clinical impact and
the risk factors associated with the development of AH and LI, as
well as to assess the association of the LI pattern with the course
of COVID-19 infection. In addition, the present study aimed to
estimate the prevalence of patients discharged with abnormal
aminotransferase levels in a single-center study on Greek patients
hospitalized due to COVID-19.
Patients and methods
Patient population
Consecutive adult patients who had been hospitalized
with documented COVID-19 infection in the non-ICU ward at Laiko
General Hospital, Athens, Greece, between March, 2020 and October,
2021, were evaluated retrospectively. The inclusion criteria were
an age ≥18 years at the time of hospitalization and at least one
positive reverse transcription-polymerase chain reaction (RT-PCR)
test result for SARS-CoV-2 performed on a nasopharyngeal swab
specimen. The exclusion criteria were pregnancy, unavailability of
detailed medical records, or a hospitalization period of <3
days. All patients were followed-up until discharge or mortality.
The study protocol was approved by the Data Protection Officer and
Institutional Review Board of Laiko General Hospital (protocol no.
770/17-12-2021) and conformed to the ethical guidelines of the 1975
Declaration of Helsinki (as revised in 2000). All patients provide
written informed consent.
Baseline evaluation
Clinical variables upon admission were recorded,
including age, sex, body mass index (BMI) and the consumption of
alcohol, as well as past medical history, including the use of
anti-hypertensive and anti-diabetic drugs, while severe (or class
II) obesity was defined as a BMI >35 kg/m2 (10). Laboratory variables during the
first 24 h of admission were obtained from the electronic medical
record system, including white blood cell count (WBC) with
differential, platelets (PLTs), protein, albumin, creatinine, total
bilirubin, clotting profile [international normalized ratio (INR),
fibrinogen and D-dimers], aminotransferases (AST and ALT), ALP,
γ-GT, lactate dehydrogenase (LDH), C-reactive protein (CRP) and
ferritin. In addition, the HBsAg/anti-HCV serological status was
recorded whenever available. Elevated serum levels of
aminotransferases at baseline were defined as ALT or AST levels
>40 IU/l, while ULN ranges of total bilirubin, ALP and γ-GT were
1.2 mg/dl, 104 and 55 IU/l, respectively.
During their hospitalization, all included patients
received supportive care with a prophylactic dose of
low-molecular-weight heparin (or therapeutic dose in cases of
confirmed thromboembolic events), fluid and electrolyte replacement
therapy, and oxygen supplementation (delivered by nasal catheters,
Venturi masks, or high-flow nasal cannula) as required, according
to the national guidelines [https://www.hts.org.gr/assets/anatheorimenos%20algorithmos%20nosileyomenon_Feb2022.pdf].
The administration of all medications was at the discretion of the
attending physician. For COVID-19-associated pneumonia, the
standard of care included the administration of remdesivir and
dexamethasone. The administration of baricitinib and tocilizumab
was decided on a case-by-case basis. In addition, during their
hospital stay, the maximum values of AST, ALT, ALP, γ-GT and
bilirubin were recorded. The development of AH was defined as AST
and/or ALT levels >400 IU/l; the presence of LI during hospital
stay was defined as an elevation of ALT levels (>3-fold ULN) or
at least a moderate elevation in ALP or total bilirubin levels
(>2-fold ULN) during hospital stay (6). The definitions for AH and LI were
universal for all patients, irrespectively of the presence of
underlying liver disease. Furthermore, in those patients with LI,
the pattern of LI was further characterized as hepatocellular (R
≥5), cholestatic (R ≤2), or mixed (R between 2 and 5) [R was
calculated according to the formula R=(ALT value/ALT ULN)/(ALP
value/ALP ULN] (12). Finally, in
the subgroup of patients with available discharge laboratory data,
the serum levels of aminotransferases (AST and ALT) were recorded.
The primary outcome of the study was to identify the baseline
factors associated with the development of AH and LI during
hospitalization, as well as the presence of abnormal
aminotransferase levels at discharge. Secondary outcomes included
the evaluation of the impact of AH and LI on in-hospital mortality,
the need for high-flow nasal cannula and/or intubation, as well as
the development of acute kidney injury (AKI) during
hospitalization. The latter was defined as an increase in serum
creatinine levels 2-fold the baseline values.
Statistical analysis
Continuous variables in the present study cohort are
presented as the mean ± standard deviation (normally distributed)
or median with range (non-normally distributed), while categorical
variables are expressed as frequencies or percentages. Comparisons
of variables between patients were performed using Mann-Whitney U
tests for continuous variables, respectively, and the Chi-squared
test for categorical variables. In univariate analysis, the
baseline variables were included, while multivariate Cox regression
analysis was used to identify the baseline variables independently
associated with the development of AH and LI in the total cohort.
The discriminative ability of the independent baseline factors was
evaluated by using the area under the receiver operating
characteristic curve (ROC) (13).
The patient's outcomes were calculated using Kaplan-Meier analysis
and compared with the log rank sum test. A P-value <0.05
(two-tailed) was considered to indicate a statistically significant
difference. Statistical analysis was conducted using SPSS softwre
(IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version
25.0., IBM Corp.) and MedCalc for Windows (MedCalc Software,
version 20.114).
Results
Baseline characteristics
A total of 1,304 patients with COVID-19 infection
(757 males, age 64±17 years) were evaluated. All patients had
clinical manifestations of COVID-19 infection, including fever and
respiratory symptoms. However, radiological findings of lower
respiratory tract infection were not present in all patients. The
number of patients who received antibiotics was too small to
perform a statistical analysis. Furthermore, the doses of
remdesivir and dexamethasone were the same for all patients. In
addition, the majority of the patients received a single dose of
tocilizumab (dose of 400 mg). In total, 37 (2.8%) patients were
ΗΒsAg-positive, while 11 (0.8%) were anti-HCV-positive. Upon
admission, 864 (66.2%) and 975 (74.8%) patients had normal AST and
ALT values (i.e., ≤40 IU/l), respectively, while only six (0.4%)
patients had AST or ALT levels >400 IU/l. Patients with viral
hepatitis compared to those without viral hepatitis had similar
baseline (at admission) aminotransferase levels. In patients with
viral hepatitis, the baseline value of AST was 55±17 IU/l and that
of ALT was 47±15 IU/l. In addition, 49 (3.7%), 153 (11.7%) and 302
(23.1%) of the patients had abnormal levels of total bilirubin
(i.e., >1.2 mg/dl), ALP (i.e., >104 IU/l) and γ-GT (i.e.,
>55 IU/l), respectively. The baseline clinical and laboratory
characteristics of the patients are presented in Table I.
 | Table IBaseline clinical and laboratory
characteristics of the 1,304 patients with COVID-19 infection. |
Table I
Baseline clinical and laboratory
characteristics of the 1,304 patients with COVID-19 infection.
| Variable | Patients
(n=1,304) |
|---|
| Age (mean ± SD,
years) | 64±17 |
| Sex, n (%) | |
|
Male | 757(58) |
|
Female | 547(42) |
| Co-morbidities, n
(%) | |
|
Diabetes
mellitus | 245 (18.7) |
|
Severe
(class II) obesity (BMI ≥35 kg/m²) | 79(6) |
|
Arterial
hypertension | 422(32) |
|
Gastrointestinal
symptoms | 144(11) |
| AST (median, range,
IU/l) | 33 (4-957) |
| ALT (median, range,
IU/l) | 25 (3-993) |
| ALP (median, range,
IU/l) | 65 (12-1335) |
| γ-GT (median, range,
IU/l) | 35 (6-1304) |
| Total bilirubin
(median, range, mg/dl) | 0.46 (0.11-33) |
| LDH (median, range,
IU/l) | 324 (9-3552) |
| Albumin (median,
range, g/dl) | 3.9 (1.8-5.4) |
| CRP (median, range,
mg/l) | 55 (0.7-478) |
| INR (median,
range) | 1.0 (0.7-11.3) |
| D-Dimers (median,
range, mg/dl) | 0.9 (0.09-52) |
| Fibrinogen (median,
range, mg/dl) | 536 (40-1074) |
| Ferritin (median,
range, ng/ml) | 535 (10-2940) |
| WBC (median, range,
x109/l) | 6.1 (1.2-45) |
| PLTs (mean ± SD,
x109/l) | 211±94 |
Baseline variables associated with the
development of AH
In total, 35 patients (2.7%) developed AH during
hospitalization after 5 (range, 3-40) days. In addition, 4 patients
with viral hepatitis developed AH. However, none of the patients
developed acute liver failure with an increase in the INR or
encephalopathy. In the univariate analysis, AST (HR, 1.008; 95% CI,
1.006-1.009; P<0.001), ALT (HR, 1.007; 95% CI, 1.006-1.009;
P<0.001), LDH (HR, 1.002; 95% CI, 1.001-1.003; P<0.001),
ferritin (HR, 1.003; 95% CI, 1.001-1.004; P=0.007), the
administration of remdesivir (HR, 0.41; 95% CI, 0.19-0.89;
P=0.025), and HBsAg or anti-HCV positivity (HR, 4.05; 95% CI,
1.18-13.9; P=0.026) were the baseline factors associated with the
development of AH (Table II). In
the multivariate analysis, AST at baseline (HR, 1.008, 95% CI,
1.006-1.011; P<0.001) was the only independent factor for AH
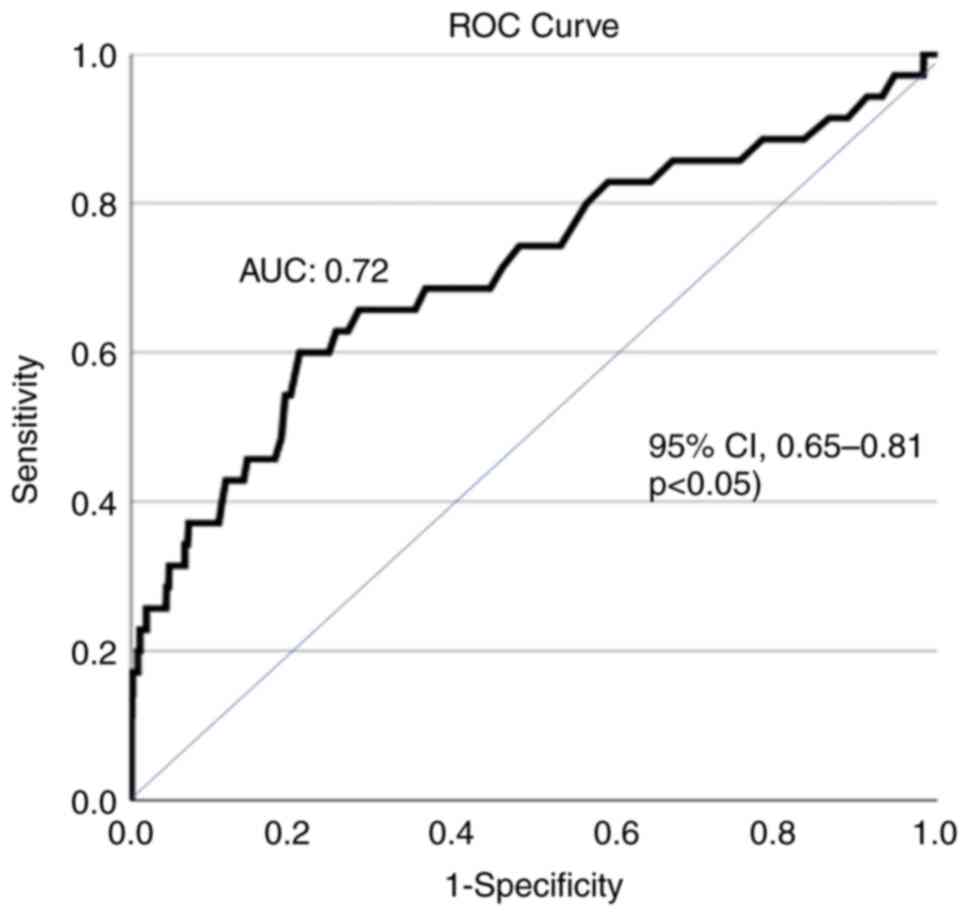
during hospital stay with efficient discriminative ability [area
under the curve (AUC), 0.72; 95% CI, 0.65-0.81, P<0.05)
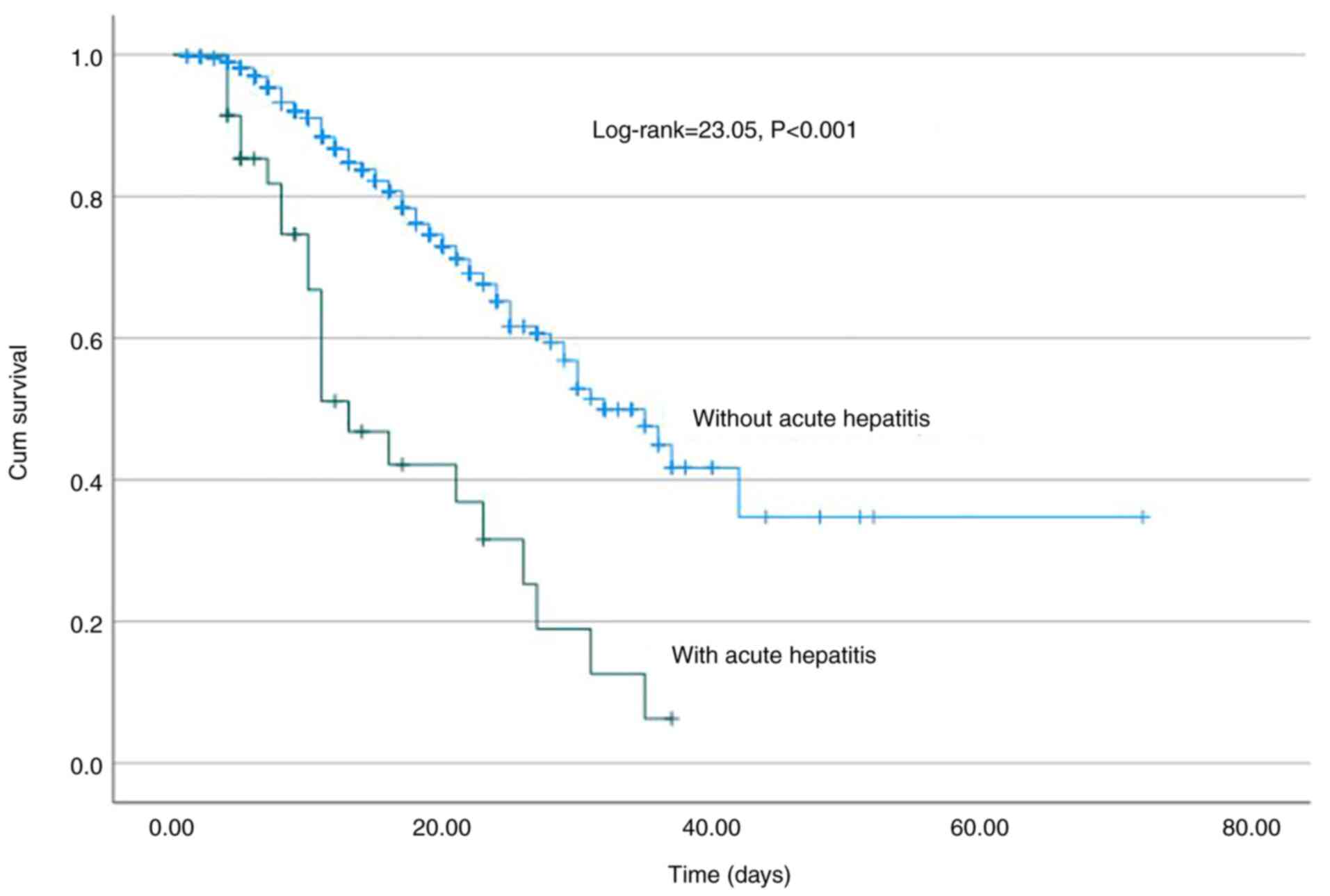
(Fig. 1). Of note, the development
of AH was associated with a higher risk of mortality (log-rank,
23.05, P<0.001) (Fig. 2) and
AKI (log-rank, 33.5, P<0.001) (data not shown); however, it was
not associated with the need for oxygen via high-flow nasal cannula
and/or intubation and mechanical ventilation (log-rank, 0.92,
P=0.34) (data not shown).
 | Table IIBaseline risk factors associated with
acute hepatitis during hospital stay in 1,304 patients with
COVID-19 infection (univariate and multivariate analysis). |
Table II
Baseline risk factors associated with
acute hepatitis during hospital stay in 1,304 patients with
COVID-19 infection (univariate and multivariate analysis).
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | Hazard ratio | P-value | 95% Confidence
interval Lower limit | 95% Confidence
interval Upper limit | Hazard ratio | P-value | 95% Confidence
interval Lower limit | 95% Confidence
interval Upper limit |
|---|
| Age, years | 0.99 | 0.61 | 0.97 | 1.054 | | | | |
| Sex, male | 0.89 | 0.75 | 0.45 | 1.72 | | | | |
| Co-morbidities | | | | | | | | |
|
Diabetes
mellitus | 1.36 | 0.44 | 0.62 | 2.99 | | | | |
|
Severe
(class II) obesity (BMI ≥35 kg/m²) | 1.42 | 0.56 | 0.43 | 4.71 | | | | |
|
Arterial
hypertension | 1.31 | 0.47 | 0.63 | 2.68 | | | | |
|
Gastrointestinal
symptoms | 1.97 | 0.17 | 0.74 | 5.33 | | | | |
| AST (IU/l) | 1.008 | <0.001 | 1.006 | 1.009 | 1.008 | <0.001 | 1.006 | 1.011 |
| ALT (IU/l) | 1.007 | <0.001 | 1.006 | 1.009 | | | | |
| ALP (IU/l) | 1.002 | 0.51 | 0.99 | 1.006 | | | | |
| γ-GT (IU/l) | 1.002 | 0.19 | 0.99 | 1.005 | | | | |
| Total bilirubin
(mg/dl) | 0.99 | 0.34 | 0.908 | 1.095 | | | | |
| LDH (IU/l) | 1.002 | <0.001 | 1.001 | 1.003 | | | | |
| Albumin (g/dl) | 0.99 | 0.64 | 0.97 | 1.017 | | | | |
| CRP (mg/l) | 1.00 | 0.81 | 0.99 | 1.001 | | | | |
| INR | 0.98 | 0.85 | 0.85 | 1.10 | | | | |
| D-Dimers
(mg/dl) | 1.00 | 0.99 | 0.99 | 1.001 | | | | |
| Fibrinogen
(mg/dl) | 1.001 | 0.27 | 0.99 | 1.003 | | | | |
| Ferritin
(ng/ml) | 1.003 | 0.007 | 1.001 | 1.004 | | | | |
| WBC
(x109/l) | 0.99 | 0.85 | 0.93 | 1.06 | | | | |
| PLTs
(x109/l) | 0.99 | 0.26 | 0.99 | 1.002 | | | | |
| HBsAg (+) or
anti-HCV (+) | 4.05 | 0.026 | 1.18 | 13.9 | | | | |
| COVID-19
medication | | | | | | | | |
|
Remdesivir | 0.41 | 0.025 | 0.19 | 0.89 | | | | |
|
Dexamethasone | 1.21 | 0.76 | 0.36 | 3.98 | | | | |
|
Tocilizumab | 1.98 | 0.09 | 0.88 | 4.47 | | | | |
Baseline variables associated with the
development of LI
A total of 336 (25.8%) developed LI during
hospitalization after 3 (1-36) days. In the univariate analysis,
AST (HR, 1.005; 95% CI, 1.004-1.006; P<0.001), ALT (HR, 1.005;
95% CI, 1.004-1.005; P<0.001), ALP (HR, 1.003; 95% CI,
1.002-1.004; P<0.001), γ-GT (HR, 1.003; 95% CI, 1.002-1.003;
P<0.001), LDH (HR, 1.002; 95% CI, 1.001-1.003; P<0.001),
ferritin (HR, 1.002; 95% CI, 1.001-1.003; P<0.001) and D-dimers
(HR, 1.001; 95% CI, 1.00-1.002; P=0.015) were the baseline factors
associated with the development of LI (Table III). In the multivariate
analysis, AST (HR, 1.004; 95% CI, 1.002-1.003; P<0.001), ALP
(HR, 1.003; 95% CI, 1.002-1.004; P<0.001) and ferritin (HR,
1.001; 95% CI, 1.00-1.002; P=0.005) were the independent baseline
factors for LI development, while ALP exhibited the optimal
discriminative ability (AUC, 0.73; 95% CI, 0.68-0.78) followed by
AST (AUC, 0.63; 95% CI, 0.59-0.67) and ferritin (AUC, 0.61; 95% CI,
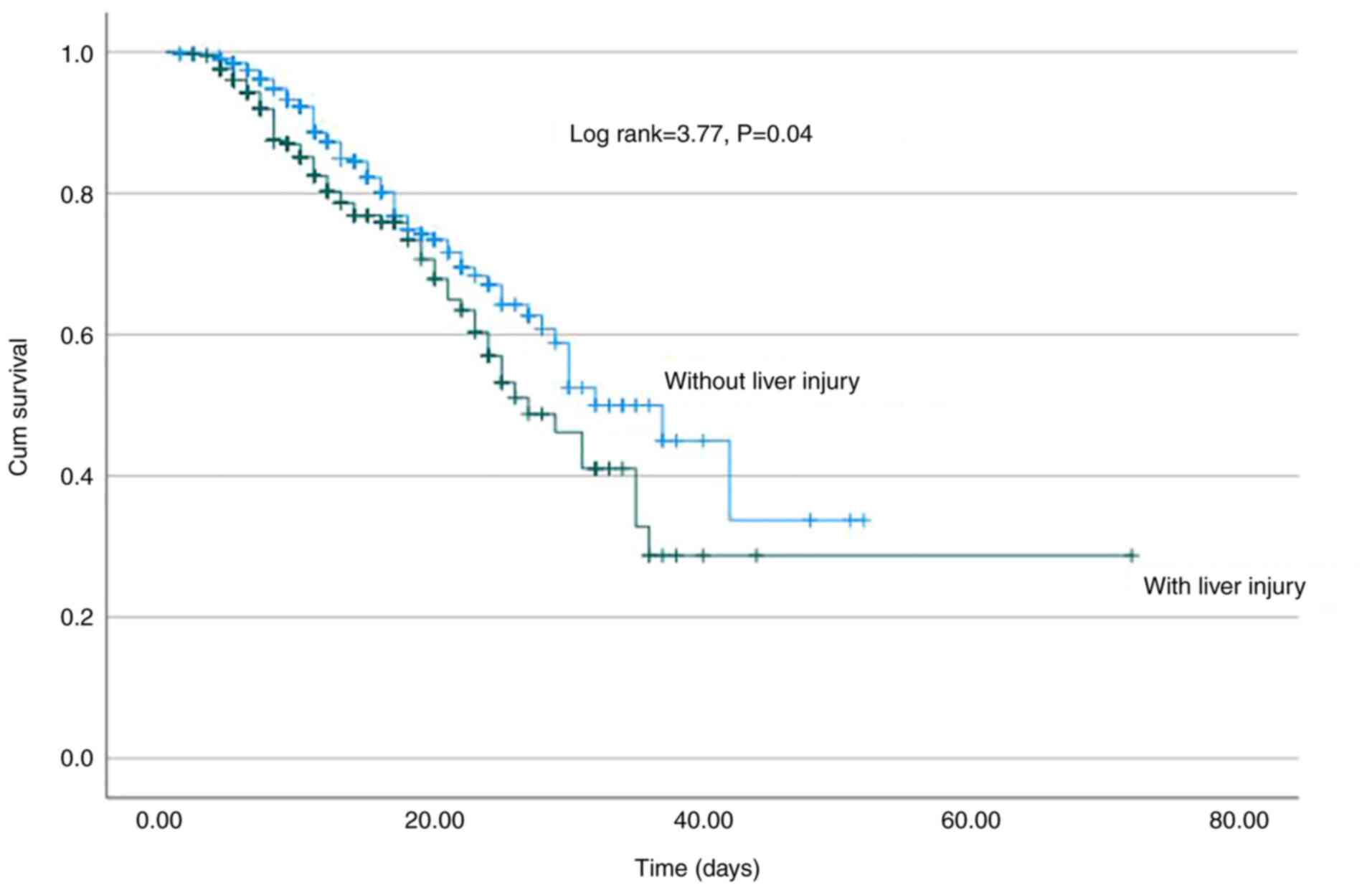
0.57-0.65) (Fig. 3). Of note, LI
development was associated with a higher risk for mortality
(log-rank, 3.77; P=0.04) (Fig. 4),
but neither with AKI (log-rank, 0.83; P=0.36) nor the need for
oxygen via high-flow nasal cannula and/or mechanical ventilation
(log-rank 1.31; P=0.25) (Table
III).
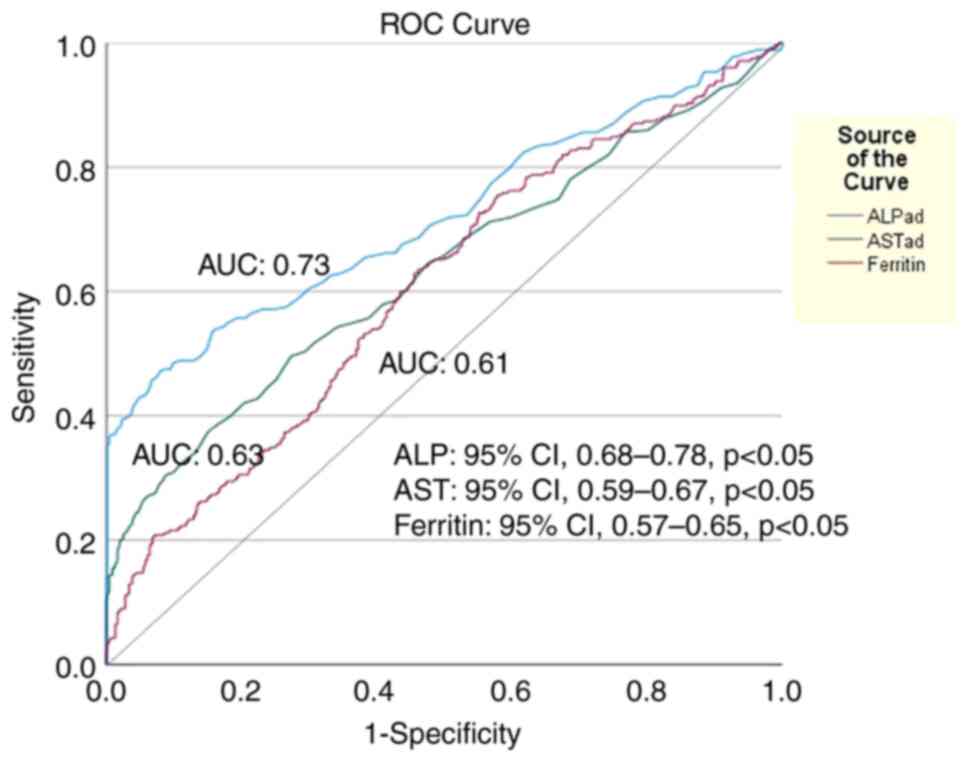 | Figure 3Area under the receiver operating
characteristic curves (AUC) illustrating that (ALP) at baseline had
the best discriminative ability (AUC, 0.73; 95% CI, 0.68-0.78,
P<0.05) followed by AST (AUC, 0.63; 95% CI, 0.59-0.67,
P<0.05) and ferritin (AUC, 0.61; 95% CI, 0.57-0.65, P<0.05)
for the development of acute liver injury during hospital stay in
1,304 patients with COVID-19 infection. The optimal cut-off value
for ALP was 84.5 IU/l (sensitivity, 55%; specificity, 82%). ALP,
alkaline phosphatase; AST, aspartate aminotransferase; AUC, area
under the curve. |
 | Table IIIBaseline risk factors associated with
liver injury (LI) during hospital stay in 1.304 patients with
COVID-19 infection (univariate and multivariate analysis). |
Table III
Baseline risk factors associated with
liver injury (LI) during hospital stay in 1.304 patients with
COVID-19 infection (univariate and multivariate analysis).
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | Hazard ratio | P-value | 95% Confidence
interval Lower limit | 95% Confidence
interval Upper limit | Hazard ratio | P-value | 95% Confidence
interval Lower limit | 95% Confidence
interval Upper limit |
|---|
| Age, years | 0.992 | 0.013 | 0.985 | 0.998 | | | | |
| Sex, male | 0.83 | 0.12 | 0.669 | 1.049 | | | | |
| Co-morbidities | | | | | | | | |
|
Diabetes
mellitus | 0.91 | 0.49 | 0.67 | 1.21 | | | | |
|
Severe
(class II) obesity (BMI ≥35) | 0.82 | 0.38 | 0.53 | 1.27 | | | | |
|
Arterial
hypertension | 0.87 | 0.24 | 0.68 | 1.11 | | | | |
|
Gastrointestinal
symptoms | 1.13 | 0.46 | 0.81 | 1.59 | | | | |
| AST (IU/l) | 1.005 | <0.001 | 1.004 | 1.006 | 1.002 | <0.001 | 1.001 | 1.003 |
| ALT (IU/l) | 1.005 | <0.001 | 1.004 | 1.006 | | | | |
| ALP (IU/l) | 1.003 | <0.001 | 1.002 | 1.004 | 1.003 | <0.001 | 1.002 | 1.004 |
| γ-GT (IU/l) | 1.003 | <0.001 | 1.002 | 1.004 | | | | |
| Total bilirubin
(mg/dl) | 0.979 | 0.93 | 0.88 | 1.016 | | | | |
| LDH (IU/l) | 1.001 | <0.001 | 1.00 | 1.002 | | | | |
| Albumin (g/dl) | 0.99 | 0.63 | 0.99 | 1.003 | | | | |
| CRP (mg/l) | 1.0 | 0.98 | 0.99 | 1.002 | | | | |
| INR | 0.99 | 0.86 | 0.97 | 1.018 | | | | |
| D-Dimers
(mg/dl) | 1.001 | 0.015 | 1.0 | 1.002 | | | | |
| Fibrinogen
(mg/dl) | 1.001 | 0.33 | 0.99 | 1.002 | | | | |
| Ferritin
(ng/ml) | 1.01 | <0.001 | 1.001 | 1.02 | 1.001 | 0.005 | 1.00 | 1.002 |
| WBC
(x109/l) | 0.995 | 0.62 | 0.99 | 1.001 | | | | |
| PLTs
(x109/l) | 1.0 | 0.79 | 0.99 | 1.001 | | | | |
| HBsAg (+) or
anti-HCV (+) | 1.42 | 0.21 | 0.83 | 2.43 | | | | |
| COVID-19
medication | | | | | | | | |
|
Remdesivir | 0.79 | 0.13 | 0.59 | 1.068 | | | | |
|
Dexamethasone | 1.05 | 0.79 | 0.72 | 1.51 | | | | |
|
Tocilizumab | 0.91 | 0.56 | 0.67 | 1.24 | | | | |
Baseline variables associated with the
development of hepatocellular or cholestatic LI
Among the patients with LI (n=336), 129 patients
(38.4%) developed hepatocellular LI (R factor ≥5), 159 patients
(47.3%) cholestatic LI (R factor ≤2) and 48 patients (14.3%)
developed mixed LI (R factor 2-5). The patients with
hepatocellular, compared to those with cholestatic LI, exhibited
similar survival rates (log-rank, 0.37; P=0.54), as well as
similarities in AKI development (log-rank, 0.1; P=0.77) and the
need for oxygen via high-flow nasal cannula and/or mechanical
ventilation (log-rank, 1; P=0.99) (data not shown) The viral
hepatitis status was not significantly associated with LI.
Baseline variables associated with
persistent abnormal aminotransferases at discharge
In the subgroup of patients who were alive with
available data at discharge (n=686), 372 patients had AST and ALT
levels within normal ranges, while 314 patients had abnormal values
of AST and/or ALT after 8 (range, 3-40) days of hospitalization. In
the univariate analysis, age (HR, 0.98; 95% CI, 0.97-0.99;
P<0.001), sex (HR, 0.63; 95% CI, 0.51-0.79; P<0.001), AST
(HR, 1.006; 95% CI, 1.004-1.008; P<0.001), ALT (HR, 1.004; 95%
CI, 1.002-1.005; P<0.001), total bilirubin (HR, 1.006; 95% CI,
1.0-1.012; P=0.04) and ferritin (HR, 1.002; 95% CI, 1.001-1.003;
P=0.003) were the baseline factors associated with the presence of
abnormal AST/ALT at discharge (Table
IV). In the multivariate analysis, age (HR, 0.98; 95% CI,
0.97-0.99; P<0.001) and ALT (HR, 1.005; 95% CI, 1.003-1.007;
P<0.001) were the independent risk factors. In addition,
baseline ALT levels had a notable discriminative ability (AUC,
0.78; 95% CI, 0.74-0.82), better than the discriminative ability of
age (AUC, 0.53; 95% CI, 0.48-0.58; P=0.02) for the presence of
abnormal AST/ALT at discharge (data not shown).
 | Table IVBaseline risk factors of 686 patients
for the presence of normal ALT/AST at discharge after
hospitalization for COVID-19 infection (univariate and multivariate
analysis). |
Table IV
Baseline risk factors of 686 patients
for the presence of normal ALT/AST at discharge after
hospitalization for COVID-19 infection (univariate and multivariate
analysis).
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | Hazard ratio | P-value | 95% Confidence
interval Lower limit | 95% Confidence
interval Upper limit | Hazard ratio | P-value | 95% Confidence
interval Lower limit | 95% Confidence
interval Upper limit |
|---|
| Age, years | 0.98 | <0.001 | 0.97 | 0.99 | 0.98 | <0.001 | 0.97 | 0.99 |
| Sex, male | 0.63 | <0.001 | 0.51 | 0.79 | | | | |
| Co-morbidities | | | | | | | | |
|
Diabetes
mellitus | 0.61 | 0.001 | 0.45 | 0.82 | | | | |
|
Severe
(class II) obesity (BMI ≥35) | 1.15 | 0.45 | 0.79 | 1.69 | | | | |
|
Arterial
hypertension | 0.82 | 0.71 | 0.65 | 1.02 | | | | |
|
Gastrointestinal
symptoms | 1.26 | 0.14 | 0.92 | 1.73 | | | | |
| AST (IU/l) | 1.006 | <0.001 | 1.004 | 1.008 | | | | |
| ALT (IU/l) | 1.004 | <0.001 | 1.002 | 1.005 | 1.005 | <0.001 | 1.003 | 1.007 |
| ALP (IU/l) | 1.00 | 0.66 | 0.99 | 1.002 | | | | |
| γ-GT (IU/l) | 1.001 | 0.12 | 0.99 | 1.002 | | | | |
| Total bilirubin
(mg/dl) | 1.006 | 0.048 | 1.00 | 1.012 | | | | |
| LDH (IU/l) | 1.001 | 0.27 | 0.98 | 1.002 | | | | |
| Albumin (g/dl) | 1.002 | 0.16 | 0.99 | 1.004 | | | | |
| CRP (mg/l) | 1.00 | 0.85 | 0.99 | 1.001 | | | | |
| INR | 0.93 | 0.54 | 0.75 | 1.16 | | | | |
| D-Dimers
(mg/dl) | 0.97 | 0.09 | 0.94 | 1.002 | | | | |
| Fibrinogen
(mg/dl) | 1.00 | 0.98 | 0.99 | 1.001 | | | | |
| Ferritin
(ng/ml) | 1.002 | 0.003 | 1.001 | 1.003 | | | | |
| WBC
(x109/l) | 1.00 | 0.13 | 0.99 | 1.001 | | | | |
| PLTs
(x109/l) | 1.00 | 0.68 | 0.99 | 1.001 | | | | |
| HBsAg (+) or
anti-HCV (+) | 0.88 | 0.76 | 0.39 | 2.01 | | | | |
| COVID-19
medication | | | | | | | | |
|
Remdesivir | 0.93 | 0.59 | 0.71 | 1.22 | | | | |
|
Dexamethasone | 0.93 | 0.69 | 0.67 | 1.29 | | | | |
|
Tocilizumab | 0.69 | 0.07 | 0.48 | 1.01 | | | | |
Discussion
In the present single-center study including a large
cohort of patients with confirmed COVID-19 infection, the
prevalence and the baseline factors associated with the development
of AH (defined as AST and/or ALT >400 IU/l) were evaluated. In
addition, the impact of LI development (defined as an elevation of
ALT levels >3-fold the ULN or ALP or total bilirubin >2-fold
the ULN) and its pattern (hepatocellular or cholestatic) on
survival during hospital stay were assessed. Previous studies have
demonstrated that abnormalities in aminotransferase levels, derived
through direct SARS-CoV-2 or drug-induced liver injury, ischemic
damage, or non-hepatic mechanisms, are frequently observed during
COVID-19 infection and they have been shown to be associated with
the need for intubation and a higher mortality rate (4,5). In
the large cohort in the present study, literature data were
confirmed, since a relatively high prevalence of AST/ALT
abnormalities were observed upon admission, while a lower
proportion of patients with ALP/γ-GT or bilirubin above the ULN
were recorded.
Although these cases very rarely progress to ALF, it
was recently reported that out 624 patients infected with COVID-19,
43 (6.9%) developed ALF during their hospitalization (14). However, this proportion appears to
be very high. To the best of our knowledge, no other study to date
has yet confirmed this finding, and it is outside of the common
daily clinical experience. In fact, in the literature, only a few
cases of ALF upon admission or during hospital stay in patients
with COVID-19 have been reported (10,15).
In the present study, 35 (2.7%) patients developed AH during
hospital stay after 5 (1-40) days, while none of the patients
progressed to ALF failure with INR prolongation or encephalopathy.
In the literature, several studies have recorded AST/ALT
abnormalities of 3- to 5-fold higher than ULN during
hospitalization (6,16,17),
while only a few studies have evaluated the prevalence of AH (i.e.,
AST or ALT >10-fold the ULN) development during COVID-19
hospitalization (5,18). In a recent study (7), AH was recorded in 87 (5.6%) of 1,555
patients with COVID-19, while in another study (19), none of the 554 patients developed
aminotransferase levels >15-fold the ULN, while no data were
provided for patients with AST/ALT levels >10-fold the ULN. In
two studies (5,18), similar to the present study, none
of the patients developed ALF. Nevertheless, the prognostic impact
of severe AST/ALT abnormalities was confirmed in the present study
cohort, since AH was associated with higher risk of mortality
(log-rank 23.05; P<0.001) and AKI (log-rank, 33.5; P<0.001).
To the best of our knowledge, the present study is the first in
which the baseline factors associated with AH development were
evaluated: In multivariate analysis, AST at baseline (HR, 1.008;
95% CI, 1.006-1.011; P<0.001) was the only independent factor
for AH during hospital stay with a notable discriminative ability
(AUC, 0.72; 95% CI, 0.65-0.81) (Fig.
1).
In the present study cohort, it was found that 25.8%
of patients developed LI after 3 (range 1-36) days of
hospitalization. In addition, AST (HR, 1.002; 95% CI, 1.001-1.003;
P<0.001), ALP (HR, 1.003; 95% CI, 1.002-1.004; P<0.001) and
ferritin (HR, 1.001, 95% CI, 1.00-1.002; P=0.005) were the
independent baseline factors for LI development, while similar to a
previous study, LI was associated with a higher risk of mortality
(log-rank, 3.77; P=0.04) (5).
Although in a previous study (19)
the LI pattern was associated with mortality, this was not
confirmed in the present study cohort, since the patients with
hepatocellular, compared to those with cholestatic LI, had similar
survival rates (log-rank 0.37, P=0.54), as well as AKI development
and the need for high-flow oxygen/mechanical ventilation. Although
the patients with hepatocellular, compared to those with
cholestatic LI, had significantly higher baseline inflammatory
markers (CRP and ferritin), possibly indicating a more intense
inflammatory response, this difference was not associated with a
worse outcome (data not shown).
Finally, based on the available data and similar to
a previous study (10), 46%
(314/686) of the patients were discharged with abnormal AST and/or
ALT after 8 (1-40) days of hospitalization. Interestingly, age (HR,
0.98; 95% CI, 0.97-0.99; P<0.001) and ALT (HR, 1.005; 95% CI,
1.003-1.007, P<0.001) were the independent risk factors, while
ALT had a notable discriminative ability (AUC, 0.78; 95% CI,
0.74-0.82) (data not shown).
The present study had several limitations, including
the fact that it was a single-center retrospective study. However,
all eligible patients were included based on the electronic medical
record system of the hospital. Another limitation of the study was
that patients in the intensive care unit with COVID-19 were not
included. The incidence of liver injury is higher in patients with
more severe COVID-19 than in patients with mild disease. Finally,
an additional limitation of the study is that the association
between hypoxia or shock, that are most frequently observed in
critical COVID-19, and the development of LI and AH was not
evaluated. However, the present study evaluated the association
between AH and LI with the need for mechanical ventilation and
AKI.
In conclusion, to the best of our knowledge, the
present study is the first in which the prevalence and the baseline
factors associated with the development of AH, LI and its pattern,
as well as the presence of aminotransferase abnormalities at
discharge were evaluated in the same cohort. AST levels at baseline
were the only independent factor for AH during hospital stay, while
AST, ALP and ferritin levels were the independent baseline factors
for LI development. Patients with hepatocellular, compared to those
with cholestatic LI, had similar survival rates, similarities in
AKI development and the need for oxygen via high-flow nasal cannula
and/or mechanical ventilation. Apart from age, and ALT was an
independent risk factor for the presence of abnormal AST/ALT at
discharge.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EC, MS and NVS conceptualized the study. TB, VEG,
SM, MT, SS, PMV, DB, IE, PP, GK, MAd, SA, OC, EA, AA, MAt and KT
advised on patient care and medical treatment, made substantial
contributions to conception and design and acquisition of data, and
wrote and prepared the draft of the manuscript. EC, MS, DAS and NVS
analyzed the data and provided critical revisions. EC and NVS
confirm the authenticity of all the data. All authors contributed
to manuscript revision, and have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Data
Protection Officer and Institutional Review Board of Laiko General
Hospital (Athens, Greece) and conformed to the ethical guidelines
of the 1975 Declaration of Helsinki (as revised in 2000).
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bhurwal A, Minacapelli CD, Orosz E, Gupta
K, Tait C, Dalal I, Zhang C, Zhao E and Rustgi VK: COVID-19 status
quo: Emphasis on gastrointestinal and liver manifestations. World J
Gastroenterol. 27:7969–7981. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Georgakopoulou VE, Gkoufa A, Damaskos C,
Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A,
Asimakopoulou S, Chlapoutakis S, et al: COVID-19-associated acute
appendicitis in adults. A report of five cases and a review of the
literature. Exp Ther Med. 24(482)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Weber S, Hellmuth JC, Scherer C,
Muenchhoff M, Mayerle J and Gerbes AL: Liver function test
abnormalities at hospital admission are associated with severe
course of SARS-CoV-2 infection: A prospective cohort study. Gut.
70:1925–1932. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cholongitas E, Bali T, Georgakopoulou VE,
Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D,
Basoulis D, Eliadi I, Karamanakos G, et al: Prevalence of abnormal
liver biochemistry and its impact on COVID-19 patients' outcomes: A
single-center Greek study. Ann Gastroenterol. 35:290–296.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y,
Li Z, Zhou G, Gou J, Qu J, et al: COVID-19: Abnormal liver function
tests. J Hepatol. 73:566–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sobotka LA, Esteban J, Volk ML, Elmunzer
BJ and Rockey DC: North American Alliance for the Study of
Digestive Manifestation of COVID-19*. Acute liver injury in
patients hospitalized with COVID-19. Dig Dis Sci. 67:4204–4214.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ekpanyapong S, Bunchorntavakul C and Reddy
KR: COVID-19 and the liver: Lessons learnt from the EAST and the
WEST, a year later. J Viral Hepat. 29:4–20. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bernstein D, Roth N, Kim A, Epstein M,
Hirschwerk D, Kvasnovsky CL and Satapathy SK: Presentation,
patterns and predictive value of baseline liver tests on outcomes
in COVID-19 patients without chronic liver disease. World J
Gastroenterol. 27:7350–7361. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gurala D, Al Moussawi H, Philipose J and
Abergel JR: Acute liver failure in a COVID-19 patient without any
preexisting liver disease. Cureus. 12(e10045)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gan Q, Gong B, Sun M, Cao Z, Zheng Y,
Zhang Y, Wen P, Shen Y, Hong L, Hou T, et al: A high percentage of
patients recovered from COVID-19 but discharged with abnormal liver
function tests. Front Physiol. 12(642922)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Robles-Diaz M, Lucena MI, Kaplowitz N,
Stephens C, Medina-Cáliz I, González-Jimenez A, Ulzurrun E,
Gonzalez AF, Fernandez MC, Romero-Gómez M, et al: Use of Hy's law
and a new composite algorithm to predict acute liver failure in
patients with drug-induced liver injury. Gastroenterology.
147:109–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hanley JA and McNeil BJ: A method of
comparing the areas under receiver operating characteristic curves
derived from the same cases. Radiology. 148:839–843.
1983.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pellegrini JR Jr, Sabbula B, Russe-Russe
JR, Munshi RF, Meshoyrer D, Sajid N, Gutierrez A, Munnangi S,
Szydziak E and Akella J: A retrospective analysis of
COVID-19-infected patients with acute hepatitis who develop acute
liver failure in a safety net hospital. BMJ Open Gastroenterol.
8(e000738)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Melquist S, Estepp K, Aleksandrovich Y,
Lee A, Beiseker A, Hamedani FS and Bassett J: COVID-19 presenting
as fulminant hepatic failure: A case report. Medicine (Baltimore).
99(e22818)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chew M, Tang Z, Radcliffe C, Caruana D,
Doilicho N, Ciarleglio MM, Deng Y and Garcia-Tsao G: Significant
liver injury during hospitalization for COVID-19 is not associated
with liver insufficiency or death. Clin Gastroenterol Hepatol.
19:2182–2191. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Paštrovic F, Lucijanic M, Atic A, Stojic
J, Barisic Jaman M, Tjesic Drinkovic I, Zelenika M, Milosevic M,
Medic B, Loncar J, et al: Prevalence and prognostic impact of
deranged liver blood tests in COVID-19: Experience from the
regional COVID-19 center over the cohort of 3812 hospitalized
patients. J Clin Med. 10(4222)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Krishnan A, Prichett L, Tao X, Alqahtani
SA, Hamilton JP, Mezey E, Strauss AT, Kim A, Potter JJ, Chen PH and
Woreta TA: Abnormal liver chemistries as a predictor of COVID-19
severity and clinical outcomes in hospitalized patients. World J
Gastroenterol. 28:570–587. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Medetalibeyoglu A, Catma Y, Senkal N,
Ormeci A, Cavus B, Kose M, Bayramlar OF, Yildiz G, Akyuz F,
Kaymakoglu S and Tukek T: The effect of liver test abnormalities on
the prognosis of COVID-19. Ann Hepatol. 19:614–621. 2020.PubMed/NCBI View Article : Google Scholar
|