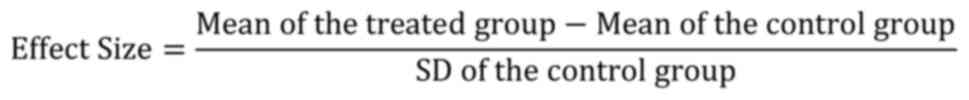Introduction
Drug-induced cardiotoxicity is a primary cause of
failure in drug development, with approximately one-third of cases
of drug development failure attributed to safety problems
associated with cardiovascular toxicity (1-4).
Failure in the development of candidate drugs is an economic loss
for pharmaceutical companies, whereas side effects and toxicity
threaten the health of patients (3,5).
Therefore, successfully predicting drug-induced cardiotoxicity in
non-clinical studies is important in decreasing drug development
failure and clinical adverse reactions.
Current non-clinical safety evaluation methods for
drug-induced myocardial injury in experimental animals include
pathomorphological, clinicopathological and safety pharmacological
examination (such as assessment of blood pressure, heart rate and
electrocardiography) (S6 (R1): Preclinical Safety Evaluation of
Biotechnology-Derived Pharmaceuticals, S7A: Safety Pharmacology
Studies for Human Pharmaceuticals) (6,7). In
non-clinical safety studies, cardiac pathomorphological
examinations are the gold standard for evaluating myocardial injury
in experimental animals (8,9).
However, this evaluation system is insufficient because certain
drugs are still eliminated owing to cardiotoxicity in clinical
trials, while others are withdrawn from the market owing to
cardiotoxicity in clinical application. Thus, predicting the
occurrence of drug-induced cardiotoxicity at the early stage of
drug development is important. Furthermore, the lack of sensitive
and specific markers prevents prediction at an early stage of the
injury, which can lead to irreversible myocardial damage; thus,
drug-induced chronic myocardial injury remains difficult to
evaluate. More sensitive and specific evaluation methods are
needed, among which use of predictive biomarkers is most valuable
to predict cardiotoxicity in non-clinical safety evaluations and to
monitor clinical drug-induced myocardial injury (10).
Traditional cardiotoxicity markers, such as CK and
lactate dehydrogenase (LDH), have poor specificity (11). cTn is a sensitive and specific
indicator of myocardial necrosis, and the increase in cTn levels is
associated with the degree of myocardial injury (12). Therefore, cTn (cTnI and cTnT) is
considered a good indicator for myocardial injury in experimental
animals in non-clinical safety evaluations (12). However, there are limitations to
the use of cTn. First, the detection methods have been developed
and optimized for humans rather than for animals (13). Second, cTn is cleared rapidly in
rat plasma (14) and blood
collection time points are restricted, blood must be collected
before cTn is cleared. Third, the cTn complex comprises three
subunits that bind to thin myofilaments of striated muscles,
troponin I, T, and C (15). A
previous study suggested that cTnI and cTnT have strong predictive
power for myocardial necrosis and are markers of structural damage,
rather than early myocardial injury (16). FABP3 is a low molecular weight
protein involved in lipid transport, storage, signal transduction,
oxidation and transcriptional regulation (17), FABP3 are abundant in myocyte
cytoplasm and rapidly released with cell injury (18), so it is another early biomarkers
for assessing cardiomyocyte degeneration and necrosis.
DOX is an anthracycline antibiotic and its toxic
effect on cardiomyocytes is an important research area (19). Although the specific mechanism
remains unclear, DOX toxicity primarily involves oxidative stress,
DNA/RNA damage, endoplasmic reticulum-mediated apoptosis and
disturbance of calcium homeostasis (20). Apoptosis or necrosis of
cardiomyocytes causes a release of enzymes and structural proteins,
such as CK or cTn, into the blood. Over production of reactive
oxygen species (ROS) occurs during oxidative stress; ROS activate a
variety of signaling kinases and transcription factors, such as
MAPK and NF-κB (21), which may be
responsible for changes in miRNA levels.
MicroRNAs (miRNAs) are short non-coding RNAs (~22
nucleotides long) that are relatively highly conserved (22). Most miRNAs in circulation originate
from blood and endothelial cells and are found in the plasma of
humans. The levels of cardio-specific miRNAs are low and an
increase suggests that myocardial injury has occurred, such as
miR-29a (23). miRNAs serve
important roles in cardiac function and cardiovascular disease
(24) and have emerged as key
regulators of cardiac injury (25). miR-31 has been reported to
participate in cardiac disorders, such as ischaemic heart diseases
and arrhythmia (26). Yang et
al (27) found that abnormal
expression of miRNA (miR-499) leads to irreversible myocardial
damage; that study also reported that DOX significantly increases
the expression of miR-140-5p in rat heart tissue, leading to
increased myocardial oxidative damage (28). miR-208a is a cardio-specific miRNA,
the level of which increase significantly following repeated
administration of isoprenaline (29). miR-208a silencing alleviates
DOX-induced myocardial apoptosis in Balb/c mice (30). These reports demonstrate that
miRNAs are potential biomarkers for cardiotoxicity.
The present study aimed to analyze changes in the
levels of seven biomarkers [CK, LDH, cTnI, cTnT, FABP3, miR-146b,
and miR-208a)] in DOX-induced rat models of acute and chronic
myocardial injury. The change in expression patterns of these
markers and their predictive value for myocardial injury, as well
as the correlation between cardiotoxicity risk and biomarker levels
were also analyzed.
Materials and methods
Animals and experimental design
Male 7-week-old Sprague-Dawley rats (weight, 174-213
g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. They were maintained at 2 or 3 rats/cage, with
unrestricted standard diet and sterilized water, temperature of
21.7-24.4˚C, relative humidity of 44.1-72.2% and a 12/12-h
light/dark cycle.
In the acute myocardial injury model, 29 rats were
randomly divided into control group and DOX-treated group (Table I). The animals in the treated group
were intraperitoneally injected with 40 mg/kg (10 ml/kg) DOX (lot
no. H44024359; Shenzhen Main Luck Pharmaceuticals, Inc.); whereas
the control group received the same volume of normal saline. The
dosage was set according to preliminary experiments (data not
shown). The chronic myocardial injury model included 16 groups, as
outlined in Table I). DOX was
administered at 1, 2 and 3 mg/kg, named as low-, medium-, and
high-dose groups, respectively. All animals received DOX or normal
saline once/week via caudal vein injection; the dosages were used
as previously described (29,31,32).
The clinical symptoms of the animals from both acute and chronic
myocardial injury models were observed twice a week. All
experiments were approved by the Institutional Animal Care and Use
Committee (approval nos. IACUC-2015-P13 and IACUC-2017-K007 for
acute and chronic myocardial injury model, respectively) of
National Center for Safety Evaluation of Drugs (Beijing, China).
Acute and chronic experiment animals were tested once each.
 | Table IOverview of experimental design. |
Table I
Overview of experimental design.
| A, Myocardial
injury model (acute) |
|---|
| Group | n | Compound | Dose, mg/kg | Volume, ml/kg | Treatment time,
h | Blood/tissue
collection time, h |
|---|
| 1 | 5 | Saline | 0 | 10 | 24 | 24 |
| 2 | 5 | DOX | 40 | 10 | 2 | 2 |
| 3 | 5 | DOX | 40 | 10 | 4 | 4 |
| 4 | 5 | DOX | 40 | 10 | 8 | 8 |
| 5 | 5 | DOX | 40 | 10 | 24 | 24 |
| 6 | 4 | DOX | 40 | 10 | 48 | 48a |
| B, Myocardial
injury model (chronic) |
| Group | n | Compound | Doseb mg/kg | Volume ml/kg | Treatment time
(weeks) | Blood/tissue
collection (weeks) |
| 1 | 5 | Saline | 0 | 2 | 2 | 2 |
| 2 | 5 | DOX | 1 | 2 | 2 | 2 |
| 3 | 5 | DOX | 2 | 2 | 2 | 2 |
| 4 | 5 | DOX | 3 | 2 | 2 | 2 |
| 5 | 5 | Saline | 0 | 2 | 4 | 4 |
| 6 | 5 | DOX | 1 | 2 | 4 | 4 |
| 7 | 5 | DOX | 2 | 2 | 4 | 4 |
| 8 | 5 | DOX | 3 | 2 | 4 | 4 |
| 9 | 5 | Saline | 0 | 2 | 6 | 6 |
| 10 | 5 | DOX | 1 | 2 | 6 | 6 |
| 11 | 5 | DOX | 2 | 2 | 6 | 6 |
| 12 | 5 | DOX | 3 | 2 | 6 | 6 |
| 13 | 5 | Saline | 0 | 2 | 7 | 8 |
| 14 | 5 | DOX | 1 | 2 | 7 | 8 |
| 15 | 5 | DOX | 2 | 2 | 7 | 8 |
| 16 | 5 | DOX | 3 | 2 | 7 | 8 |
In the acute myocardial injury study, one animal
died 48 h after DOX was administered. The time point of 48 h was
used to confirm whether the acute myocardial injury model was
successfully established; therefore, animals at this time point
only underwent histopathological examination. In the chronic
myocardial injury model, after 7 weeks, one animal died in groups
15 and 16; therefore, further administration was terminated for all
the remaining animals, which were dissected after 8 weeks.
At the designated timepoints, the rats were
anesthetized with 4.5% pentobarbital sodium (45 mg/kg) and 3-4 ml
blood was collected from the vena cava caudalis, after which the
animals were euthanized by exsanguination; death was confirmed by
lack of nerve reflex and muscle relaxation. Whole blood was
centrifuged at 4˚C and 2,000 x g for 10 min; the serum was
collected and frozen at -80˚C for later use. The heart was cut
longitudinally. One part of the left ventricle was frozen in liquid
nitrogen and stored at -80˚C for detection of miRNA, whereas the
remaining part was fixed with 10% neutral formalin at room
temperature for 2 weeks for pathomorphological examination. In the
acute myocardial injury model, small RNA sequencing and reverse
transcription-quantitative qPCR (RT-qPCR) analysis of heart samples
were performed at 24 h after administration.
Serum biochemical detection
CK and LDH activity was quantified using CK(Cat#:
990-64293/996-64393, Wako Pure Chemical Industries, Ltd) and LDH
(Cat#: 994-63093/990-63193, Wako Pure Chemical Industries, Ltd)
test kits on HITACHI 7180 biochemical analyzer (Hitachi, Ltd.).
Serum cTnI, cTnT and FABP3 levels were detected using the
MILLIPLEX® MAP Rat Cardiac Injury Magnetic Bead Panel 1
kit (Cat#: RCI 1MAG-87K, EMD Millipore Corporation, Merck KGaA) on
a Luminex® 200 platform (Luminex Corporation). cTnI,
cTnT and FABP3 concentrations were analyzed using MILLIPLEX
software (version 5.1; Merck KGaA).
Sequence analysis of miRNAs
Five animals from the control group and five from
the DOX-treated acute myocardial injury model group at 24 h after
administration were selected for cardiac miRNA sequencing. A
sufficient amount (~50 mg) of myocardial tissue was ground with
liquid nitrogen to extract RNA. Following dilution (10-20 times),
the concentration, integrity were detected using Agilent RNA 6000
Nano Kit (Cat. no. 5067-1511; Agilent) on a Agilent 2100
Bioanalyzer (Agilent Technologies, Inc.), and purity were evaluated
on micro-spectrophotometer (K5500, Chongqing Keao Biotechnology).
The sequencing results were validated by RT-qPCR, the extracted RNA
was partly used for sequencing and partly used for RT-qPCR
validation. A library was constructed using the TruSeq Small RNA
Library Prep kit (Cat#: RS-200-0024, Illumina), qPCR was used to
accurately quantify the effective concentration of the libraries
(effective library concentration >2 nM) to ensure the quality of
the libraries. The files were pooled and sequenced on the Hiseq
2500 system to generate 50 bp long single-end reads. Clean data
(18-30 nt) were obtained from high-throughput sequencing through
data processing, such as joint, low quality and pollution removal.
The sequence length distribution and common sequences between
samples were analyzed. The clean data were classified and annotated
to obtain information on RNA components and expression levels in
the samples. All clean data were annotated according to priority
(rRNAetc> known miRNA > repeat > exon > intron).
Unannotated fragments were screened for novel miRNA prediction and
differential miRNA expression. Differential miRNA analysis was
performed using edgeR software (V3.3, bioconductor.org/packages/3.3/bioc/html/edgeR.html).
LogFC >1 or LogFC <-1 &&
P-value<0.05 were considered to indicate a statistically
significant difference.
Quantitative detection of miRNAs by
RT-qPCR
miRNAs were extracted and purified from 50-100 mg
heart tissue using the EasyPure miRNA kit (TransGen Biotech Co.,
Ltd.) according to the manufacturer's instructions, and small RNA
(≤200 nucleotides) was collected. miRNAs were specifically adsorbed
on the silica gel membrane of the spin columns provided in the kit;
miRNAs were then collected by eluting with 50 µl RNase-free water.
RT was performed using TransScript miRNA First-Strand cDNA
Synthesis SuperMix kit (TransGen Biotech Co., Ltd.). qPCR detection
was performed using TransStart Top Green qPCR SuperMix kit
(TransGen Biotech Co., Ltd.) on the CFX96 Real-Time System
Detection Platform (Bio-Rad Laboratories, Inc.). Thermocycling
condiitions were as follows: Initial denaturation at 95˚C, 2 min;
stage 2, reaction, repeats: 40, 95˚C, 10 sec, 60˚C, 15 sec, 72˚C,
20 sec. RNU6B (U6) was used as the loading control; sequence
specific primers are listed in Table
SI. Cq values were calculated using Bio-Rad CFX Manager
software (V3.1; Bio-Rad Laboratories, Inc.); the relative
expression of miRNAs was calculated using the 2-ΔΔCq
method (33).
Histopathological examination
The portion of rat heart fixed with formalin,
aforementioned, was embedded in paraffin, sliced into sections (3-5
µm) and stained with hematoxylin for 5 min and 0.5% eosin for 2 min
in the room temperature. Non-blinded examination was performed
using a light microscope by two independent pathologists. The
degree and extent of lesion were recorded as follows: ‘-’ was
defined as lesion not observed; ‘+’ was defined as minimal severity
of lesion; and ‘++’ was defined as mild severity of lesion.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Normality of the data was tested using the Shapiro-Wilk normality
test. Nonparametric data with multiple comparisons were analyzed by
Kruskal-Wallis; the Mann-Whitney U test was used for comparisons
between two groups. Data with normal distribution were analyzed by
one-way ANOVA with Dunnett's post hoc test (homogeneous) and
Games-Howell (heterogeneous) for multiple comparisons. SPSS 19.0
(version 19.0; IBM Corp) was used to analyze data. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism 8 (GraphPad Software, Inc.) was used to plot data
and to perform receiver operating characteristic (ROC) curve and
Spearman's correlation analysis. The increase in markers is
expressed as effect size, effect size was calculated based on
standardized difference (Cohen effect size d) between treatment and
control groups using the standard deviation of control groups,
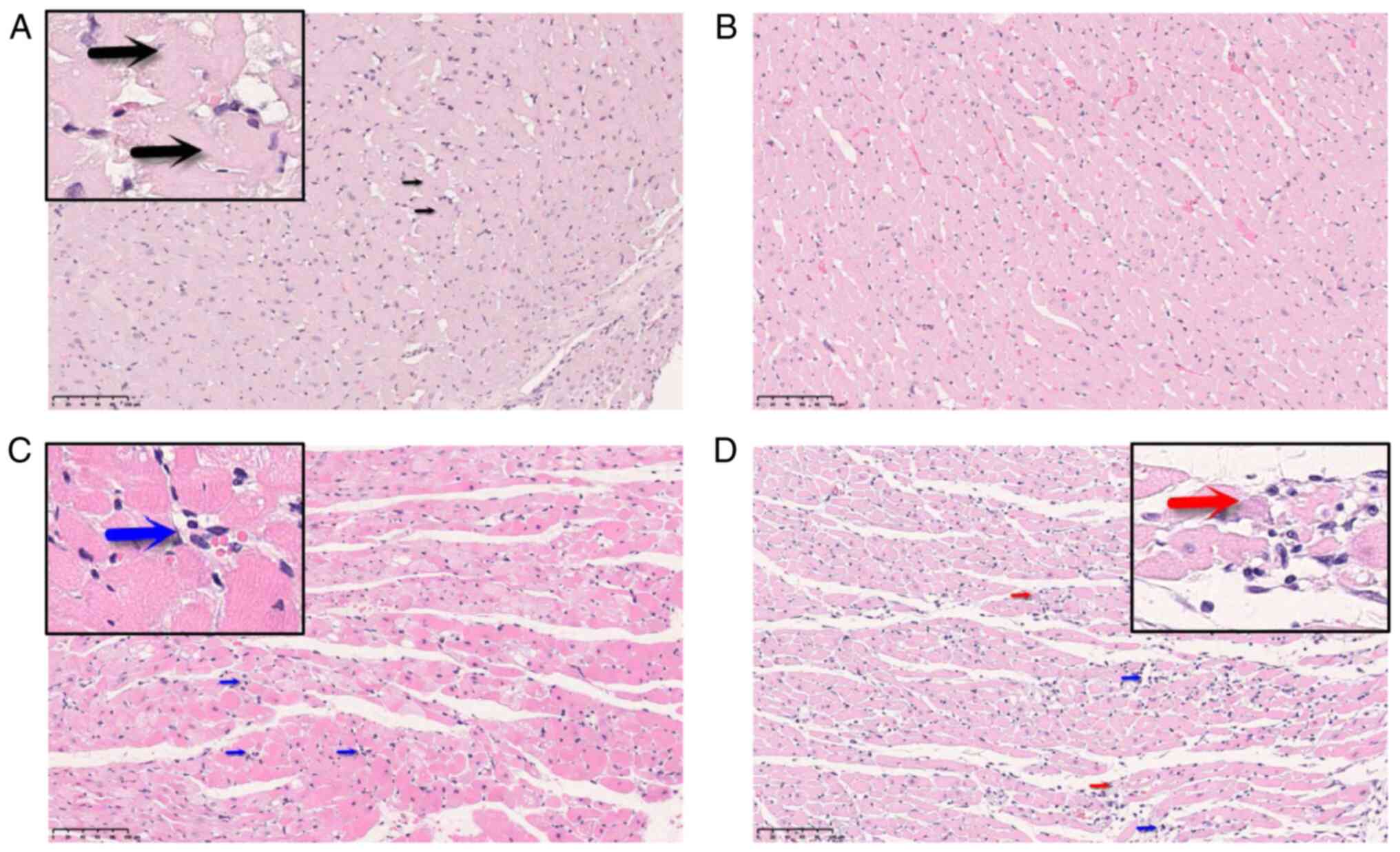
which is calculated as follows:
Results
Histopathological examination and
changes in serum biochemical indicators
In the acute myocardial injury model, pathological
changes occurred 48 h after administration, including inflammatory
cell infiltration, extensive cardiomyocyte degeneration and
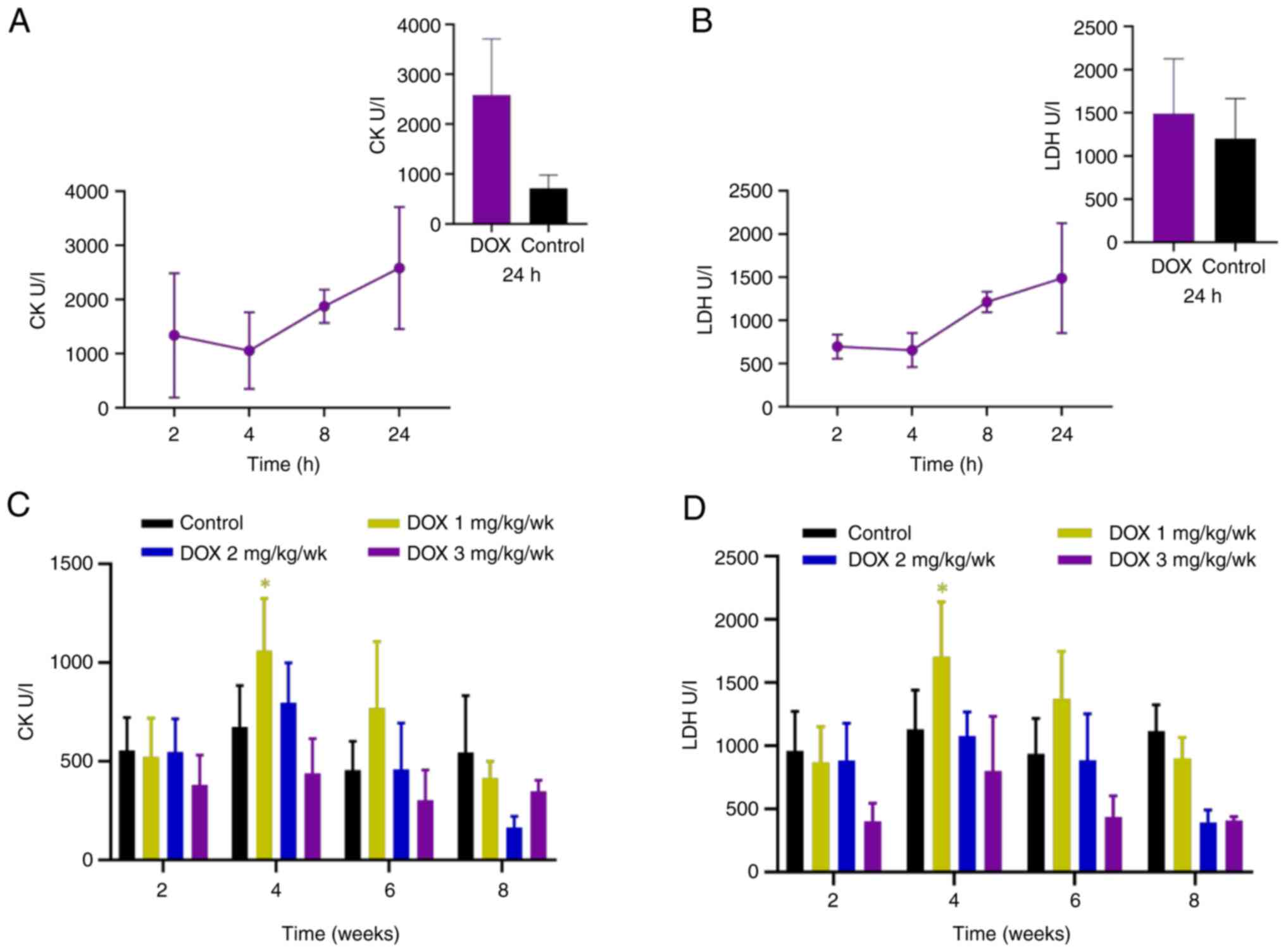
necrosis (cardiomyocyte degeneration was shown in Fig. 1A; Table II). CK increased between 8 and 24
h after administration (Fig. 2A);
neither CK nor LDH was significantly elevated compared to the
control group at 24 h.
 | Table IIHistopathology of DOX-treated
rats. |
Table II
Histopathology of DOX-treated
rats.
| A, Myocardial
injury model (acute) |
|---|
| | Interval, h |
|---|
| DOX, mg/kg | 2-4 | 8 | 24 | 48 |
|---|
| 0 | n/a | n/a | - (5/5) | n/a |
| 40 | - (5/5) | - (5/5) | - (5/5) | Inflammatory cell
infiltration, cardiomyocyte degeneration and necrosis; ++
(3/3)a |
| B, Myocardial
injury model (chronic) |
| | Interval,
weeks |
| DOX, mg/kg | 2 | 4 | 6 | 8 |
| 0 | - (5/5) | -(5/5) | - (5/5) | - (5/5) |
| 1 | - (5/5) | - (5/5) | Inflammatory cell
infiltration; + (1/5) | Cardiomyocyte
degeneration; + (2/5) Cardiomyocyte degeneration and necrosis; +
(1/5) |
| 2 | - (5/5) | - (5/5) | Inflammatory cell
infiltration; + (1/5) Cardiomyocyte degeneration; + (4/5) | Inflammatory cell
infiltration, cardiomyocyte degeneration and necrosis; ++
(4/4)a |
| 3 | - (5/5) | Inflammatory cell
infiltration; + (1/5) Cardiomyocyte degeneration; + (2/5) | Cardiomyocyte
degeneration; ++ (3/5) Cardiomyocyte degeneration and necrosis; ++
(2/5) | Inflammatory cell
infiltration, cardiomyocyte degeneration and necrosis; ++
(4/4)a |
In the chronic myocardial injury model, the
morphological changes were mainly as follows: after 4 weeks of
administration, monocyte infiltration and cardiomyocyte necrosis
(Fig. 1C; Table II), whereas the pathological
changes appearing after 6 weeks were cardiomyocyte degeneration and
necrosis (Fig. 1D; Table II). CK and LDH levels in the
low-dose group increased significantly after 4 weeks of
administration and decreased to levels comparable with those of the
control group after 8 weeks (Fig.
2C and D). No significant
elevation in CK and LDH was observed in the medium- and high-dose
groups. The increase in CK was smaller in the chronic injury model
(Maximum effect size=2.15 ) compared in the acute injury model
(effect size=7.04, Table III).
Based on the pathological findings, the acute and chronic
myocardial injury models were fully established.
 | Table IIIEffect sizea of biomarkers. |
Table III
Effect sizea of biomarkers.
| A, Myocardial
injury model (acute) |
|---|
| Interval, h | DOX, mg/kg | CK | LDH | cTnI | cTnT | FABP3 | miR-146b | miR-208a |
|---|
| 24 | 40 | 7.04 | 0.62 | -0.25 | 0.34 | 6.09b | -0.42 | 0.24 |
| B, Myocardial
injury model (chronic) |
| Interval,
weeks | DOX, mg/kg | CK | LDH | cTnI | cTnT | FABP3 | miR-146b | miR-208a |
| 2 | 1 | 0.79 | 0.62 | 0.00 | 0.61 | 0.67 | -0.81 | -0.65 |
| | 2 | -0.34 | -0.61 | 9.56c | -0.15 | -0.44 | 1.38 | 3.27b |
| | 3 | -1.10 | -1.86 | 11.04c | -0.23 | -0.36 | 3.33 | 3.54 |
| 4 | 1 | 1.33b | 1.37c | -0.13 | -0.41 | -0.52 | -0.20 | 0.34 |
| | 2 | 2.05 | 0.32 | 3.56c | 0.30 | 3.08 | 0.76 | 0.53 |
| | 3 | -0.74 | -0.74 | -6.06 | -0.22 | 1.09 | -0.41 | 0.27 |
| 6 | 1 | 2.15 | 1.56c | 0.95 | 0.36 | 0.18 | 0.18 | -0.89 |
| | 2 | 0.02 | -0.18 | 1.46 | 0.01 | 1.06 | -0.45 | -0.87 |
| | 3 | -1.05 | -1.80 | 3.86 | -0.12 | 1.99 | 0.21 | 0.17 |
| 8 | 1 | -0.46 | -1.04 | 13.14c | 0.27 | 0.02 | -1.94 | 0.97 |
| | 2 | -1.32 | -3.49 | 30.24 | -0.60 | 5.28b | 4.64b | -1.40 |
| | 3 | -0.69 | -3.41 | 6.92 | -1.17 | 0.37 | 1.60 | -1.22 |
Changes in serum cTn and FABP3
levels
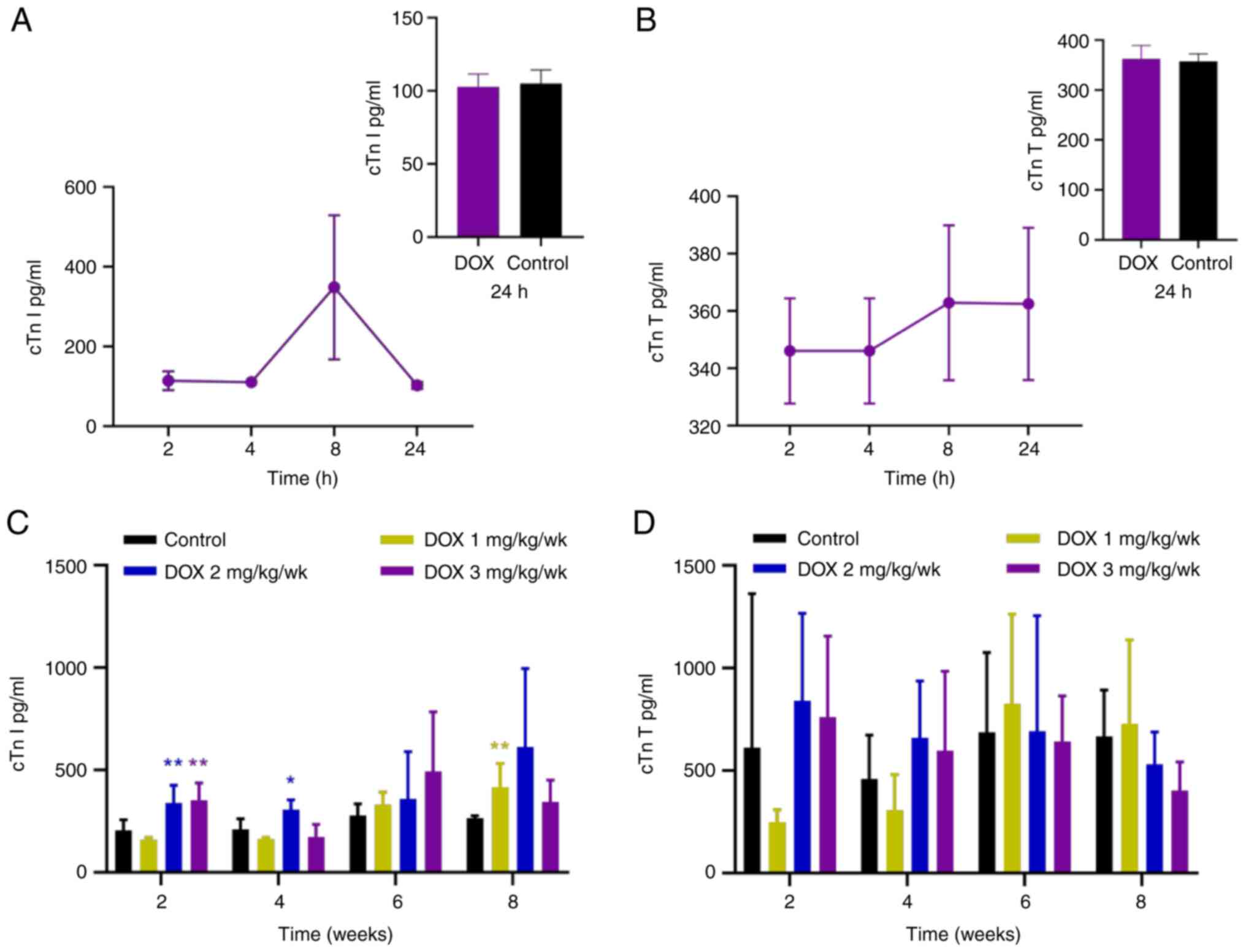
In the acute myocardial injury model, cTnI peaked at
8 h after administration, followed by a decrease to control levels
at 24 h (Fig. 3A); cTnT increased
at 4 h and continued to rise up to 8 h after administration
(Fig. 3B). Neither cTnI nor cTnT
was significantly elevated compared to the control group at 24 h.
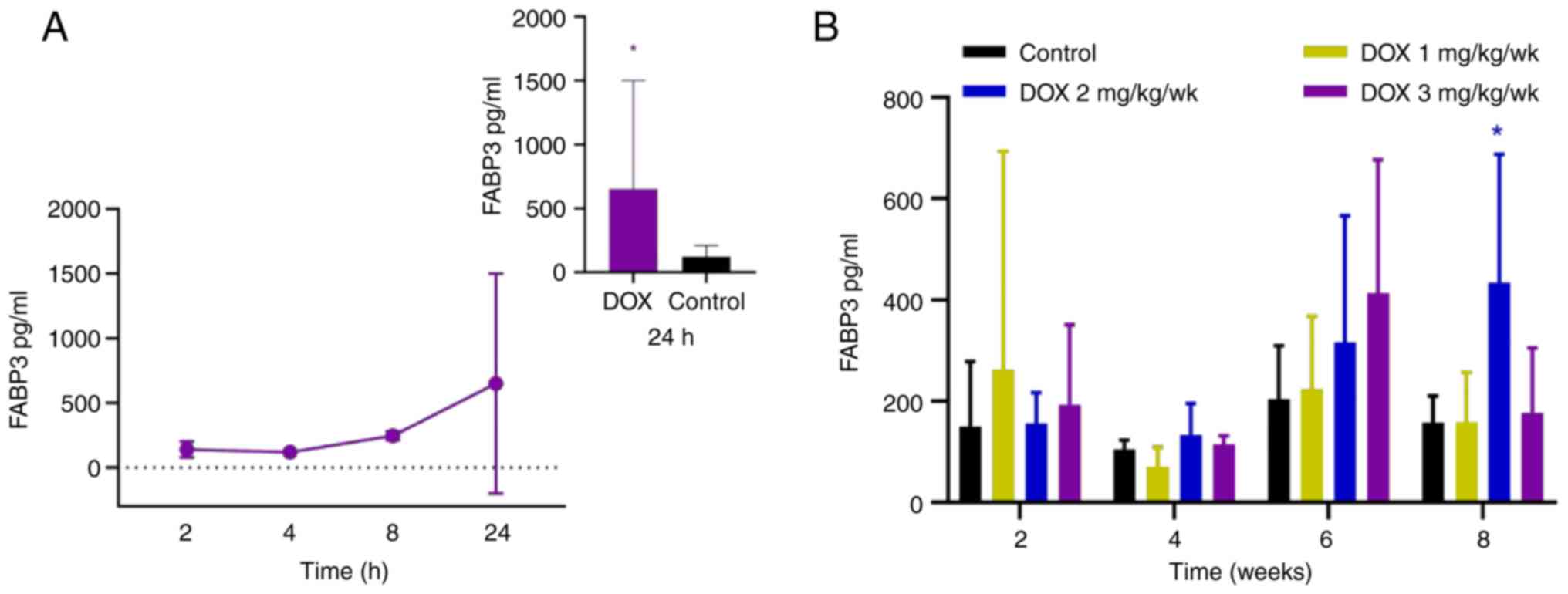
FABP3 increased at 8 h and continued to rise up to 24 h after
administration, and significantly elevated at 24 h (Fig. 4A). In the chronic myocardial injury
model, cTnI in the low-dose group increased significantly at 8
weeks (effect size=13.14; Fig. 3C
and Table III). In the medium-
and high-dose groups, cTnI increased significantly at 2 weeks
compared with the control (Fig.
3C). However, compared with control, cTnT did not increase
significantly in any of the treatment groups (Fig. 3D). A significant increase in FABP3
was only observed in the medium-dose group at 8 weeks compared with
the control (Fig. 4B).
Changes in miRNA expression
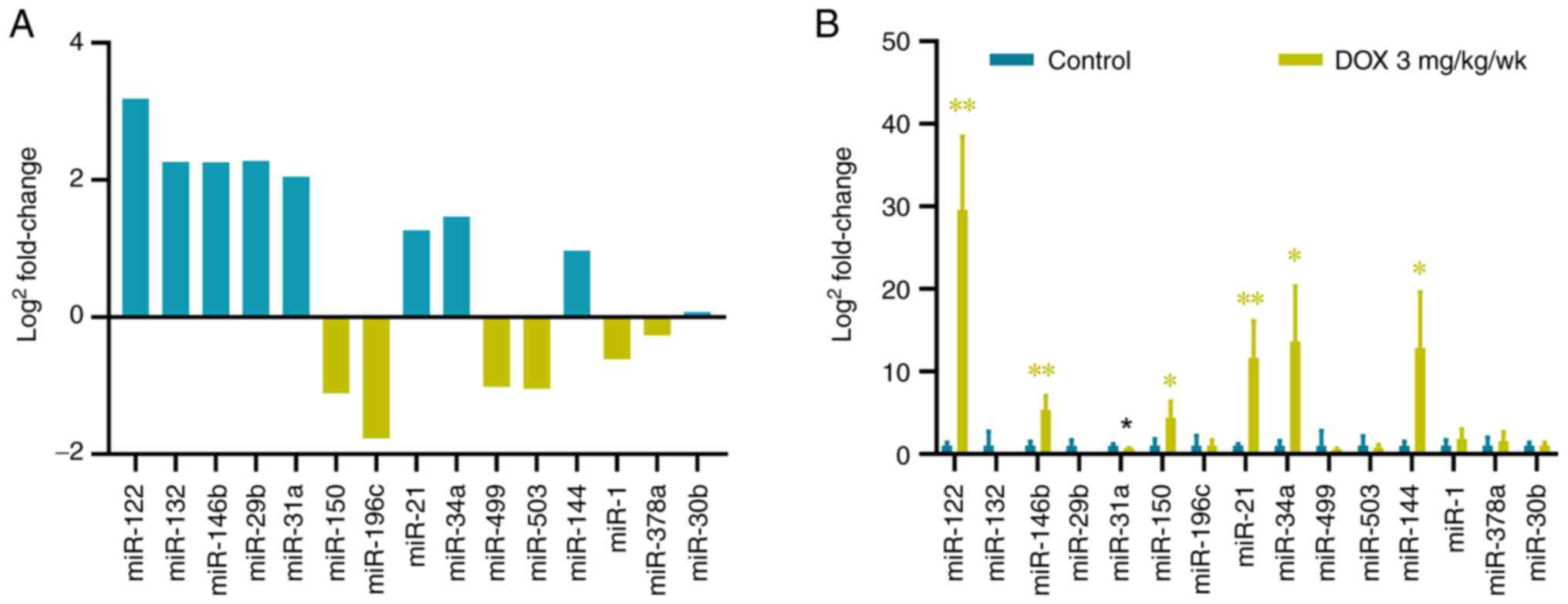
The sequencing results showed a total of 15
differentially expressed miRNAs were screened (Fig. S1) and five miRNAs were
significantly upregulated: miR-122, miR-132, miR-146b, miR-29b and
miR-31a (Fig. 5A). As verified by
RT-qPCR, miR-122 and miR-146b expression were upregulated and
results were consistent with the sequencing results. In the chronic
myocardial injury model, 15 differentially expressed miRNAs were
analyzed by RT-qPCR in the control and high-dose groups (3 mg/kg)
at 6 weeks. A total of six miRNAs were significantly upregulated:
miR-122, miR-146b, miR-150, miR-21, miR-34a and miR-144, whereas
miR-31a was significantly downregulated (Fig. 5B). miR-146b, which is associated
with cardiotoxicity (34), was
chosen as a candidate marker; the other candidate marker was
miR-208a, which is reported to be associated with cardiotoxicity
(29).
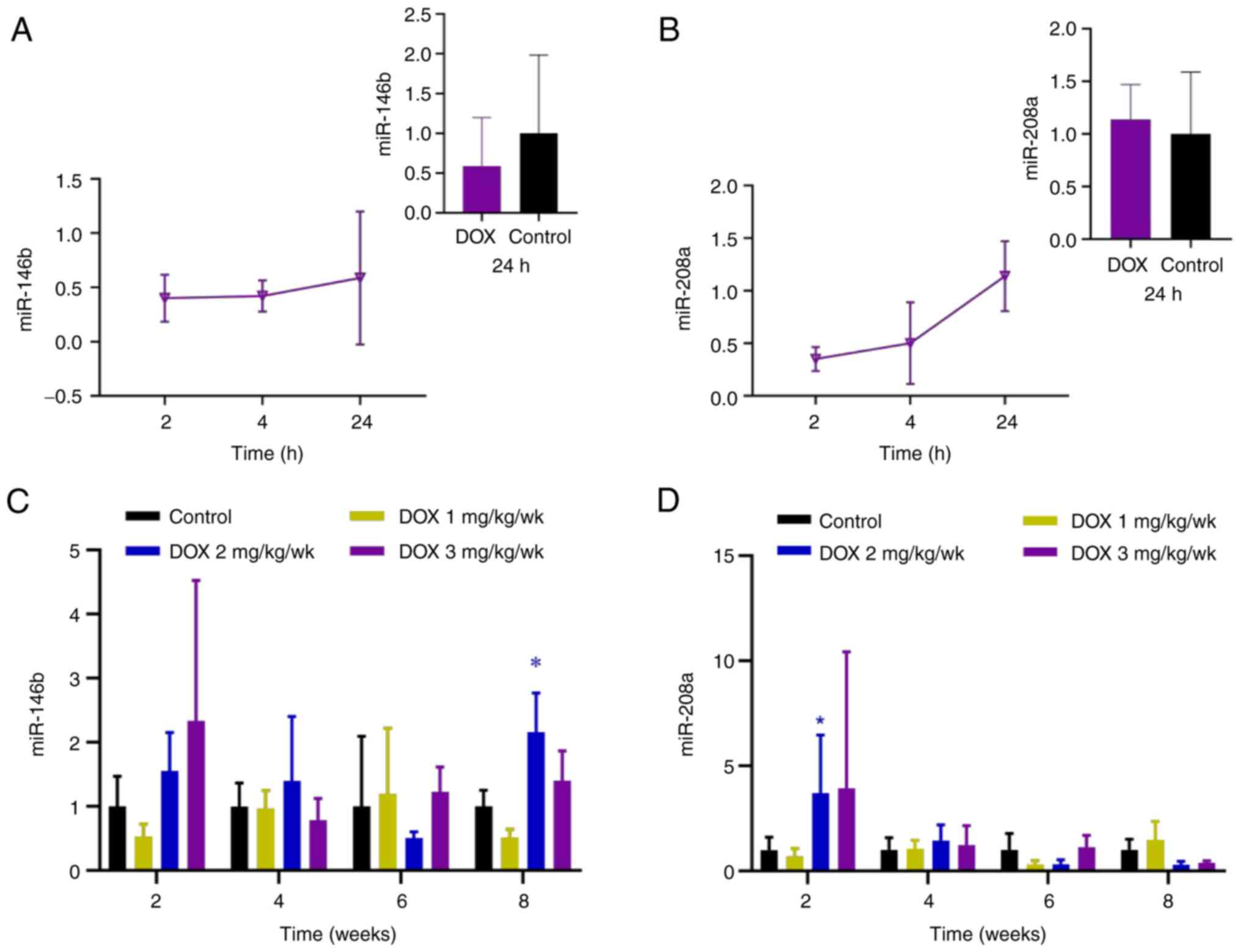
In the acute myocardial injury model, relative
expressions of miR-146b and miR-208a increased between 4 and 24 h
(Fig. 6A and B), however, expression of miR-146b was
lower than that in the control group at 24 h, and the expression of
miR-208a was slightly higher than that in the control group. In the
chronic myocardial injury models, the miR-146b and miR-208a levels
in the low-dose group were lower compared with those in the control
group at 2 weeks; the relative expression of miR-146b in the
low-dose group increased at 6 weeks, whereas relative expression of
miR-208a increased at 4 weeks. In the medium- and high-dose groups,
miR-146b and miR-208a increased at 2 weeks. The relative expression
of miR-146b in the medium-dose group increased significantly at 8
weeks compared with the control group (Fig. 6C); while expression of miR-208a in
the medium-dose group was significantly elevated at 2 weeks
(Fig. 6D).
ROC and correlation analysis
In ROC analyses, pathological changes were
considered to indicate toxicity; absence of pathological changes
was considered non-toxic. ROC and Spearman's correlation analyses
were performed only for chronic myocardial injury at weeks 6 and 8.
Biomarkers were compared by ROC analysis using area under the curve
(AUC) to evaluate the diagnostic performance (Fig. 7). CK (AUC=0.71), LDH (AUC=0.76) and
FABP 3 (AUC=0.70) exhibited high predictive value at 6 weeks, of
which, CK and LDH were negatively correlated with myocardial
pathological injury (ρ=-0.43 and ρ=-0.49; Fig. S2), and FABP 3 was positively
correlated with myocardial pathological injury (ρ=0.24). At 8
weeks, cTnI (AUC=0.83; ρ=0.55) and miR-146b (AUC=0.71; ρ=0.50)
showed a relatively high predictive value and strong correlation
with myocardial pathological injury. The predictive value of FABP 3
at 8 weeks was also relatively high (AUC=0.79) and exhibited a
moderate correlation with pathological injury (ρ=0.38), other
markers negatively correlated with myocardial injury.
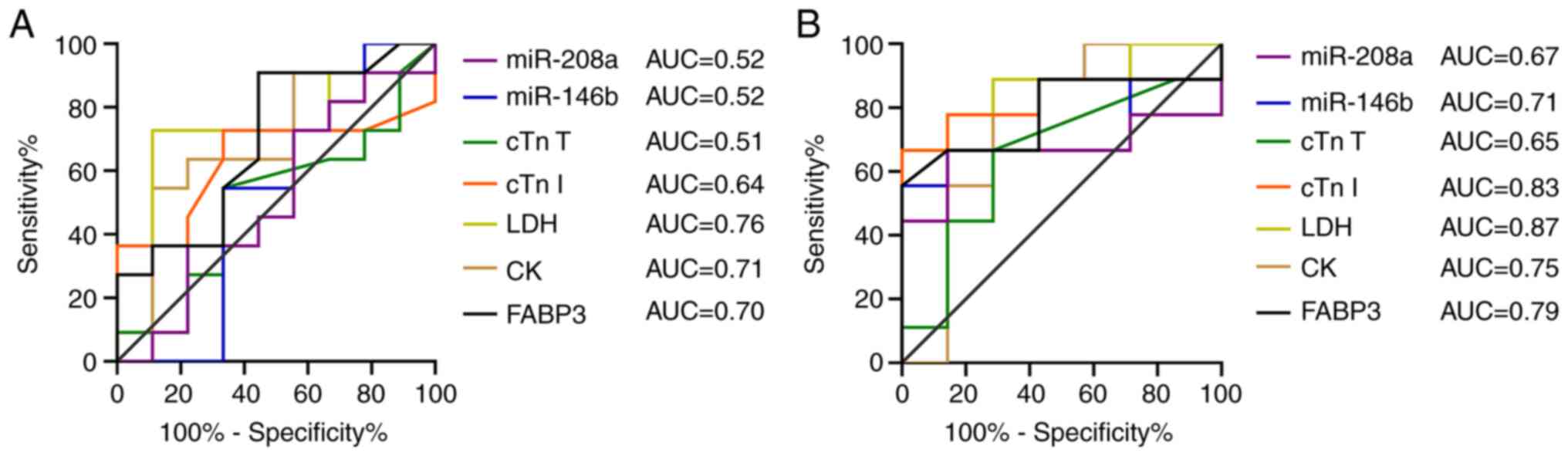 | Figure 7ROC curves for CK, LDH, cTn I, cTnT,
FABP3, miR-146b and miR-208a. ROC curves were plotted for chronic
myocardial injury at (A) 6 and (B) 8 weeks. AUC, area under the
curve; CK, creatine kinase; cTn, cardiac troponin; FABP3, fatty
acid-binding protein 3; LDH, lactate dehydrogenase; miR, microRNA;
ROC, receiver operating characteristic. |
Discussion
One of the primary side effects of DOX is
cardiotoxicity (32). Therefore,
DOX is used to establish experimental models of cardiotoxicity
(32,35). DOX causes acute, as well as
chronic, myocardial injury. The present study identified five
biomarkers to evaluate these injuries, including CK, LDH, cTn,
FABP3 and miRNA. To the best of our knowledge, most reports
(8,12,29)
have only evaluated changes in one or two of these markers in a
single injury model. Markers vary in sensitivity and specificity to
different toxicity models and should therefore be evaluated in
detail. The present study evaluated changes in myocardial injury
markers in both acute and chronic myocardial injury models.
Pathological changes, such as inflammatory cell
infiltration, cardiomyocyte degeneration and necrosis, are
considered to indicate successful induction of a rat myocardial
injury model. The present study aimed to determine changes in
miRNA, as well as pathological injury at different time points.
Therefore, expression levels of miRNA markers were investigated
before the occurrence of pathological injury, therefore, sequencing
analysis was performed 24 h after administration in the acute
myocardial injury model, the aim was to find potential markers for
the early predictiong of the myocardial injury. P-value was used to
reflect the statistical significance of toxicity, whereas the
effect size was used to reflect the degree of toxicity (36). The LDH level did not elevated in
the high-dose group, but was lower than the control group at 2
weeks; cTnT, miR-146b and miR-208a levels did not elevated in the
low-dose group, but were lower compared with those in the control
group at 2 weeks, which may be influenced by inter-individual
differences in the animals.
When cardiomyocytes are injured, intracellular
enzymes are released into the blood, therefore, these enzymes serve
as markers for myocardial injury (37,38).
Numerous studies (39-41)
have shown that DOX treatment leads to increased serum CK and LDH
levels. In the present acute myocardial injury model, CK began to
rise at 8 h following DOX administration, which was earlier than
cardiac histopathological changes, which occurred 48 h after
administration. In the chronic injury model, CK and LDH in the
low-dose group increased significantly after 4 weeks, but the
increase in CK and LDH levels was not observed in the high-dose
group. Fredericks et al (42) showed that the half-life of CK in
rats is 0.6 h, suggesting that the increase in CK level in the
high-dose group may occur 2 weeks earlier than that in the chronic
injury model. The increase in CK in the acute injury model was
larger than that in the chronic myocardial injury model. Therefore,
CK was relatively sensitive to acute myocardial injury. In
addition, owing to the short half-life of CK and its rapid
clearance in vivo, the blood must be taken prior to
clearance.
Although the diagnostic performance of CK and LDH
were good, they were negatively correlated with myocardial injury
and showed poor specificity. Other tissue damage, such as skeletal
muscle, can also lead to an increase in CK and LDH levels (43,44).
cTn is a regulatory globular protein found in thin
myofilaments that is involved in myocardial contraction (16). In the acute injury model, cTnI
peaked at 8 h then decreased. However, the time taken to peak by
cardiotoxic compounds varies. A previous study showed that cTnI
rises 1 h after administration of isoproterenol (1.5 mg/kg), peaks
at 2 h and returns to baseline level at 72 h (45). By comparison, cTnI increases
significantly at 72 h after DOX (20 mg/kg) administration.
Therefore, cTnI may be an early indicator of DOX-induced acute
myocardial injury. However, the elevation of cTnI is drug-specific
(46).
In the chronic myocardial injury model, the increase
in cTnI in the low dose-group was noted at 6 weeks, whereas the
increase in the middle- and high-group occurred at 2 weeks after
administration, indicating that a higher dose induced an earlier
increase in cTnI. Reagan et al (32) showed that the degree of cTn I
elevation and the incidence of cTn I elevation increased with
increasing dose, as well as with longer dosing cycles, and some
animals showed a decrease in cTnI after discontinuing DOX
administration. Serum cTnI levels are maintained for 5-7 days after
elevation before being cleared (47). In the present study, at 2 weeks,
circulating cTnI increased significantly but no histopathological
changes were noted, suggesting that cardiomyocytes were damaged
which led to release of intracellular free or partially-conjugated
cTnI into the blood. At 4 weeks, cTnI levels in some animals (3/5
in the medium- and 5/5 in the high-dose group) decreased,
potentially owing to faster clearance rate. At 6 weeks,
pathological changes in cardiomyocytes worsened and the lesions
were enlarged. Massive cardiomyocyte necrosis (mild severity of
lesion) followed release of numerous conjugated cTnI into the
blood, along with a corresponding increase in circulating cTnI. The
largest increase in cTnI was observed in the medium-dose group and
was greater than that of cTnT. Reagan et al (32) showed that the rate of cTn I
production in rat serum was greater than that of cTn T. At 8 weeks,
the most serious pathological injury of cardiomyocytes was noted
and cTnI showed the best predictive potential for myocardial
injury. Furthermore, it was positively correlated with myocardial
pathological injury, indicating a higher sensitivity to structural
damage of cardiomyocytes. Because free cTnI in the cytoplasm is low
(48), a significant increase
indicates structural damage. cTnI increased earlier than cTnT and
FABP 3 in the chronic myocardial injury model, the degree of change
was greater and elevated for a longer duration, suggesting that
good sensitivity and a wide detection window for a marker.
In the present study, cTnT increased 8 h following
treatment with a single high dose of DOX. Wu and Feng (49) hypothesized that the first peak in
the increase in cTn in peripheral blood is due to cTnT in acute
coronary syndrome, because 6-8% of free cTnT exists in the
cytoplasm, whereas cTnI seldom exists in the free form (2.8-4.1%)
and thus increases later than cTnT (48). In the present study, the increase
in cTnT was not initially observed because compared with conjugated
cTnT, free cTnT accounted for a small proportion (48). In addition, owing to the
insufficient sensitivity of the assay, a relatively small increase
could not be identified. In the chronic injury model, no
significant increase of cTnT was observed. In the study by Herman
et al (50), repeated
administration of DOX (1 mg/kg) with cumulative doses of 2 and 4
mg/kg did not increase cTnT. However, when the cumulative dose
reached 7 mg/kg, cTnT increased significantly. In the present
study, no significant increase in cTnT was observed, which may have
resulted from an insufficient administration period.
FABP3, also known as heart-type FABP, is released
rapidly (within 1 h) following myocardial injury (51). In the acute myocardial injury
model, FABP3 was increased at 8-24 h after administration, and
significantly elevated at 24 h. In the chronic myocardial injury
model, FABP3 showed a dose-dependent increase at 6 weeks of DOX
administration. In the middle-dose group, FABP3 showed a
significant increase at 8 weeks; there were no significant
time-dependent changes and the dose-dependent change in FABP3 was
not obvious. These results indicated that FABP3 was more sensitive
to acute myocardial injury.
In the chronic injury model, the relative expression
of miR-146b in the high-dose group increased at 2 weeks, then
declined, the results were generally consistent with literature
(52). In a previous study of male
mice treated with DOX weekly (5 mg/kg), miR-146a expression in
myocardial tissue and plasma was upregulated, reaching a peak after
3 days and lasting for 1 week before being cleared (52). The relative expression of miR-146b
showed a dose-dependent increase at 2 weeks of DOX administration,
this result was consistent with Horie's report (34). Horie et al showed that miR-146a
expression increases in a dose-dependent manner following DOX
treatment in neonatal rat cardiomyocytes, reaching a peak at 16 h
after administration (34,52). The pyroptotic marker IL-1β also
increases following DOX exposure and is involved in NLRP3-mediated
pyroptosis in H9c2 cells (53).
IL-1 receptors are activated by IL-1β, which activate MAPKs, such
as MEK-1/2 and JNK-1/2, which mediate transcription of miR-146b
(54,55). Although the mechanism is not clear,
IL-1β may induce miR-146b expression (54,55).
miR-146a mediates maintenance of normal function of mature
cardiomyocytes by inhibiting the neuregulin/ErbB pathway; it also
inhibits DOX-induced cardiotoxicity by inhibiting the TATA-box
binding protein-associated factor 9b/p53 signaling pathway
(34,52). In addition, knocking out the
miR-146a gene in mice aggravates DOX-induced myocardial injury
(52). In the present study, after
8 weeks of DOX administration, miR-146b showed a relatively high
predictive value and was correlated with myocardial pathological
injury. Therefore, miR-146b may serve as a marker for DOX-induced
chronic cardiotoxicity. However, the significance of the increase
in miR-146b needs further study. In the present study, both
sequencing results and RT-qPCR validation results showed miR-146b
as up-regulated (compared with the control), while the expression
of miR-146b in the acute injury model was lower than that in the
control group, this may be due to the fact that the RNA used for
the PCR assay was not the same batch as the sequencing sample,
resulting in a slightly lower expression level in the DOX-treated
group (Mean=1.00) than in the control group (Mean=1.07).
miR-208a is specifically expressed in the heart and
is a regulator of cardiac hypertrophy and the cardiac conduction
system (45,56). In the present study, acute
myocardial injury mode, miR-208a increased slightly at 24 h after
administration compared with the control group. The changes of
miR-208a for acute myocardial injury is also compound-specific.
Isoproterenol (1.5 mg/kg intraperitoneal) significantly increases
miR-208a within 1 h, with a peak at 4 h (45). miR-208a reaches peak value at 24 h
after administration of allylamine (100 mg/kg) (45). In the chronic myocardial injury
mode, miR-208a in the medium-dose group increased significantly at
2 weeks, The changes of miR-208a for chronic myocardial injury may
be compound-specific. In the report of nishimura's, following
repeated administration of isoprenaline in rats (0.5 mg/kg), the
relative expression of miR-208a in plasma increases significantly
after 2 days and decreases after 4 days (29). Sadek et al (57) showed that fibrogenic factors, such
as α smooth muscle actin, TGF-β1 and p16INK4A, are
upregulated following DOX-induced cardiac injury. TGF-β1 binds to
type II TGF-β receptor, subsequently activating type I TGF-β
receptor. The activated type I TGF-β receptor phosphorylates SMAD
proteins, which transduce the signal to the nucleus, thereby
increasing activity of the miR-208a promoter (58). The upregulation of miR-208a is
induced by DOX. One target of miR-208a is GATA4; increased miR-208a
expression downregulates GATA4, resulting in cardiomyocyte
apoptosis and heart dysfunction (34). Furthermore, miR-208a silencing
attenuates myocardial apoptosis (30). These results suggested that
miR-208a could serve as a potential biomarker for DOX-induced
cardiotoxicity.
One limitation of the present study is the small
sample size, which limits the generalizability of findings and ROC
analysis. Here, only DOX-induced cardiotoxicity was evaluated.
Whether the seven biomarkers evaluated were DOX-specific or not and
whether they have predictive value for other compounds needs
further investigation.
In conclusion, the present study showed that CK,
cTnI and FABP3 were relatively sensitive markers for DOX-induced
acute myocardial injury, of which CK and FABP3 were elevated to a
greater extent than in the chronic injury model. cTnI was
relatively sensitive to DOX-induced chronic myocardial injury, cTnI
and miR-146b showed relatively high predictive values for
late-stage myocardial injury (following occurrence of myocardial
pathological changes). Therefore, CK, cTnI and FABP3 may serve as
toxicity endpoints for compounds with expected acute myocardial
injury, whereas cTnI and miR-146b may serve as toxicity endpoints
for compounds with expected chronic myocardial injury.
Supplementary Material
Cluster analysis of differentially
expressed miRs. miR, microRNA.
Correlation analysis of
histopathological changes and changes in biomarkers. Curves were
plotted for chronic myocardial injury at (A) 6 and (B) 8 weeks.
Score indicated the degree and extent of lesion. miR, microRNA;
cTn, cardiac troponin; CK, creatine kinase; FABP3, fatty
acid-binding protein 3; LDH, lactate dehydrogenase.
Primer sequences.
Acknowledgements
The authors would like to thank Dr. Chao Wang, Dr.
Ming Li, Dr. Chao Qin, Dr. Yanwei Yang and Dr. Guitao Huofor
technical assistance with animal and pathology experiments; (all
from National Institutes for Food and Drug Control).
Funding
Funding: The present study was supported by The National Major
Scientific and Technological Special Project for Significant New
Drugs Development (grant no. 2018ZX09201017-001) from the Ministry
of Science and Technology of the People's Republic of China.
Availability of data and materials
The raw sequencing data in this study have been
deposited in NBCI's Sequence Read Archive (accession no.
PRJNA839543); other data generated or analyzed during this study
are included in this manuscript published article.
Authors' contributions
DP, SW and BL contributed to the conception of the
study. DP and SW performed the experiments. DP analyzed data and
wrote the manuscript. DP and SW confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of National Center for Safety
Evaluation of Drugs (Beijing, China; approval nos. IACUC-2015-P13
and IACUC-2017-K007).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Laverty H, Benson C, Cartwright E, Cross
M, Garland C, Hammond T, Holloway C, McMahon N, Milligan J, Park B,
et al: How can we improve our understanding of cardiovascular
safety liabilities to develop safer medicines? Br J Pharmacol.
163:675–693. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharma A, McKeithan WL, Serrano R, Kitani
T, Burridge PW, Del Álamo JC, Mercola M and Wu JC: Use of human
induced pluripotent stem cell-derived cardiomyocytes to assess drug
cardiotoxicity. Nat Protoc. 13:3018–3041. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chaudhari U, Nemade H, Gaspar JA,
Hescheler J, Hengstler JG and Sachinidis A: MicroRNAs as early
toxicity signatures of doxorubicin in human-induced pluripotent
stem cell-derived cardiomyocytes. Arch Toxicol. 90:3087–3098.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Onakpoya IJ, Heneghan CJ and Aronson JK:
Post-marketing withdrawal of 462 medicinal products because of
adverse drug reactions: A systematic review of the world
literature. BMC Med. 14(10)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Volkova M and Russell R III: Anthracycline
cardiotoxicity: Prevalence, pathogenesis and treatment. Curr
Cardiol Rev. 7:214–220. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jin SA, Lim BK, Seo HJ, Kim SK, Ahn KT,
Jeon BH and Jeong JO: Elevation of serum APE1/Ref-1 in experimental
murine myocarditis. Int J Mol Sci. 18(2664)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ferdinandy P, Baczkó I, Bencsik P, Giricz
Z, Görbe A, Pacher P, Varga ZV, Varró A and Schulz R: Definition of
hidden drug cardiotoxicity: Paradigm change in cardiac safety
testing and its clinical implications. Eur Heart J. 40:1771–1777.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Koh E, Nakamura T and Takahashi H:
Troponin-T and brain natriuretic peptide as predictors for
adriamycin-induced cardiomyopathy in rats. Circ J. 68:163–167.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gallay-Lepoutre J, Bélanger MC and Nadeau
ME: Prospective evaluation of Doppler echocardiography, tissue
Doppler imaging and biomarkers measurement for the detection of
doxorubicin-induced cardiotoxicity in dogs: A pilot study. Res Vet
Sci. 105:153–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sandhu H and Maddock H: Molecular basis of
cancer-therapy-induced cardiotoxicity: Introducing microRNA
biomarkers for early assessment of subclinical myocardial injury.
Clin Sci (Lond). 126:377–400. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Boyd JW: The mechanisms relating to
increases in plasma enzymes and isoenzymes in diseases of animals.
Vet Clin Pathol. 12:9–24. 1983.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bertinchant JP, Robert E, Polge A,
Marty-Double C, Fabbro-Peray P, Poirey S, Aya G, Juan JM, Ledermann
B, de la Coussaye JE and Dauzat M: Comparison of the diagnostic
value of cardiac troponin I and T determinations for detecting
early myocardial damage and the relationship with histological
findings after isoprenaline-induced cardiac injury in rats. Clin
Chim Acta Int J Clin Chem. 298:13–28. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Apple FS, Murakami MM, Ler R, Walker D and
York M: HESI Technical Committee of Biomarkers Working Group on
Cardiac Troponins. Analytical characteristics of commercial cardiac
troponin I and T immunoassays in serum from rats, dogs, and monkeys
with induced acute myocardial injury. Clin Chem. 54:1982–1989.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tonomura Y, Matsushima S, Kashiwagi E,
Fujisawa K, Takagi S, Nishimura Y, Fukushima R, Torii M and
Matsubara M: Biomarker panel of cardiac and skeletal muscle
troponins, fatty acid binding protein 3 and myosin light chain 3
for the accurate diagnosis of cardiotoxicity and musculoskeletal
toxicity in rats. Toxicology. 302:179–189. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Katrukha IA: Human cardiac troponin
complex. Structure and functions. Biochemistry (Mosc).
78:1447–1465. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Christenson RH and Azzazy HME: Biomarkers
of myocardial necrosis-past, present, and future. In: Morrow DA,
ed. Cardiovascular Biomarkers: Pathophysiology and Disease
Management. Morrow DA (ed.) Humana Press: pp. 3-25, 2006.
|
|
17
|
Zhuang L, Li C, Chen Q, Jin Q, Wu L, Lu L,
Yan X and Chen K: Fatty acid-binding protein 3 contributes to
ischemic heart injury by regulating cardiac myocyte apoptosis and
MAPK pathways. Am J Physiol Heart Circ Physiol. 316:H971–H984.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim K, Chini N, Fairchild DG, Engle SK,
Reagan WJ, Summers SD and Mirsalis JC: Evaluation of cardiac
toxicity biomarkers in rats from different laboratories. Toxicol
Pathol. 44:1072–1083. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Songbo M, Lang H, Xinyong C, Bin X, Ping Z
and Liang S: Oxidative stress injury in doxorubicin-induced
cardiotoxicity. Toxicol Lett. 307:41–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Abdel-Daim MM, Kilany OE, Khalifa HA and
Ahmed AAM: Allicin ameliorates doxorubicin-induced cardiotoxicity
in rats via suppression of oxidative stress, inflammation and
apoptosis. Cancer Chemother Pharmacol. 80:745–753. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rababa'h AM, Guillory AN, Mustafa R and
Hijjawi T: Oxidative stress and cardiac remodeling: An updated
edge. Curr Cardiol Rev. 14:53–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vacchi-Suzzi C, Hahne F, Scheubel P,
Marcellin M, Dubost V, Westphal M, Boeglen C, Büchmann-Møller S,
Cheung MS, Cordier A, et al: Heart structure-specific
transcriptomic atlas reveals conserved microRNA-mRNA interactions.
PLoS One. 8(e52442)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Williams Z, Ben-Dov IZ, Elias R,
Mihailovic A, Brown M, Rosenwaks Z and Tuschl T: Comprehensive
profiling of circulating microRNA via small RNA sequencing of cDNA
libraries reveals biomarker potential and limitations. Proc Natl
Acad Sci U S A. 110:4255–4260. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen Y, Xu Y, Deng Z, Wang Y, Zheng Y,
Jiang W and Jiang L: MicroRNA expression profiling involved in
doxorubicin-induced cardiotoxicity using high-throughput
deep-sequencing analysis. Oncol Lett. 22(560)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo L, Zheng X, Wang E, Jia X, Wang G and
Wen J: Irigenin treatment alleviates doxorubicin (DOX)-induced
cardiotoxicity by suppressing apoptosis, inflammation and oxidative
stress via the increase of miR-425. Biomed Pharmacother.
125(109784)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stepicheva NA and Song JL: Function and
regulation of microRNA-31 in development and disease. Mol Reprod
Dev. 83:654–674. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang Y, Yu T, Jiang S, Zhang Y, Li M, Tang
N, Ponnusamy M, Wang JX and Li PF: miRNAs as potential therapeutic
targets and diagnostic biomarkers for cardiovascular disease with a
particular focus on WO2010091204. Expert Opin Ther Pat.
27:1021–1029. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L
and Peng J: MicroRNA-140-5p aggravates doxorubicin-induced
cardiotoxicity by promoting myocardial oxidative stress via
targeting Nrf2 and Sirt2. Redox Biol. 15:284–296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nishimura Y, Kondo C, Morikawa Y, Tonomura
Y, Torii M, Yamate J and Uehara T: Plasma miR-208 as a useful
biomarker for drug-induced cardiotoxicity in rats. J Appl Toxicol.
35:173–180. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tony H, Yu K and Qiutang Z: MicroRNA-208a
silencing attenuates doxorubicin induced myocyte apoptosis and
cardiac dysfunction. Oxid Med Cell Longev.
2015(597032)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Desai VG, Kwekel JC, Vijay V, Moland CL,
Herman EH, Lee T, Han T, Lewis SM, Davis KJ, Muskhelishvili L, et
al: Early biomarkers of doxorubicin-induced heart injury in a mouse
model. Toxicol Appl Pharmacol. 281:221–229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Reagan WJ, York M, Berridge B, Schultze E,
Walker D and Pettit S: Comparison of cardiac troponin I and T,
including the evaluation of an ultrasensitive assay, as indicators
of doxorubicin-induced cardiotoxicity. Toxicol Pathol.
41:1146–1158. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Horie T, Ono K, Nishi H, Nagao K,
Kinoshita M, Watanabe S, Kuwabara Y, Nakashima Y, Takanabe-Mori R,
Nishi E, et al: Acute doxorubicin cardiotoxicity is associated with
miR-146a-induced inhibition of the neuregulin-ErbB pathway.
Cardiovasc Res. 87:656–664. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yin J, Xie J, Guo X, Ju L, Li Y and Zhang
Y: Plasma metabolic profiling analysis of cyclophosphamide-induced
cardiotoxicity using metabolomics coupled with UPLC/Q-TOF-MS and
ROC curve. J Chromatogr B Analyt Technol Biomed Life Sci.
1033-1034:428–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sullivan GM and Feinn R: Using effect
size-or why the P value is not enough. J Grad Med Educ. 4:279–282.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liao DH, Zhang C, Liu N, Cao LZ, Wang CS,
Feng QY, Yao DW, Long MH and Jiang P: Involvement of neurotrophic
signaling in doxorubicin-induced cardiotoxicity. Exp Ther Med.
19:1129–1135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Boshra S: Resveratrol modulates miR-34a in
cardiotoxicity induced by isoproterenol. J Med Food. 23:593–599.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Prasanna PL, Renu K and Gopalakrishnan AV:
New molecular and biochemical insights of doxorubicin-induced
hepatotoxicity. Life Sci. 250(117599)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bredahl EC, Najdawi W, Pass C, Siedlik J,
Eckerson J and Drescher K: Use of creatine and creatinine to
minimize doxorubicin-induced cytotoxicity in cardiac and skeletal
muscle myoblasts. Nutr Cancer. 73:2597–2604. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mihm MJ, Yu FS, Weinstein DM, Reiser PJ
and Bauer JA: Intracellular distribution of peroxynitrite during
doxorubicin cardiomyopathy: Evidence for selective impairment of
myofibrillar creatine kinase. Br J Pharmacol. 135:581–588.
2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fredericks S, Merton GK, Lerena MJ,
Heining P, Carter ND and Holt DW: Cardiac troponins and creatine
kinase content of striated muscle in common laboratory animals.
Clin Chim Acta. 304:65–74. 2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang CC, Fang CC, Lee YH, Yang MT and Chan
KH: Effects of 4-week creatine supplementation combined with
complex training on muscle damage and sport performance. Nutrients.
10(1640)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fonseca LB, Brito CJ, Silva RJ,
Silva-Grigoletto ME, da Silva WMJ and Franchini E: Use of
cold-water immersion to reduce muscle damage and delayed-onset
muscle soreness and preserve muscle power in jiu-jitsu athletes. J
Athl Train. 51:540–549. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Glineur SF, De Ron P, Hanon E, Valentin
JP, Dremier S and Nogueira da Costa A: Paving the route to plasma
miR-208a-3p as an acute cardiac injury biomarker: Preclinical rat
data supports its use in drug safety assessment. Toxicol Sci.
149:89–97. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jasim ST, Al-Kuraishy HM and Al-Gareeb AI:
Gingko Biloba protects cardiomyocytes against acute doxorubicin
induced cardiotoxicity by suppressing oxidative stress. JPMA J Pak
Med Assoc. 69 (Suppl 3):S103–S107. 2019.PubMed/NCBI
|
|
47
|
Maynard SJ, Menown IB and Adgey AA:
Troponin T or troponin I as cardiac markers in ischaemic heart
disease. Heart. 83:371–373. 2000.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Park KC, Gaze DC, Collinson PO and Marber
MS: Cardiac troponins: From myocardial infarction to chronic
disease. Cardiovasc Res. 113:1708–1718. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu AH and Feng YJ: Biochemical differences
between cTnT and cTnI and their significance for diagnosis of acute
coronary syndromes. Eur Heart J. 19 (Suppl N):N25–N29.
1998.PubMed/NCBI
|
|
50
|
Herman EH, Lipshultz SE, Rifai N, Zhang J,
Papoian T, Yu ZX, Takeda K and Ferrans VJ: Use of cardiac troponin
T levels as an indicator of doxorubicin-induced cardiotoxicity.
Cancer Res. 58:195–197. 1998.PubMed/NCBI
|
|
51
|
Goel H, Melot J, Krinock MD, Kumar A,
Nadar SK and Lip GYH: Heart-type fatty acid-binding protein: An
overlooked cardiac biomarker. Ann Med. 52:444–461. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pan JA, Tang Y, Yu JY, Zhang H, Zhang JF,
Wang CQ and Gu J: miR-146a attenuates apoptosis and modulates
autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced
cardiotoxicity. Cell Death Dis. 10(668)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tavakoli Dargani Z and Singla DK:
Embryonic stem cell-derived exosomes inhibit doxorubicin-induced
TLR4-NLRP3-mediated cell death-pyroptosis. Am J Physiol Heart Circ
Physiol. 317:H460–H471. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Perry MM, Williams AE, Tsitsiou E,
Larner-Svensson HM and Lindsay MA: Divergent intracellular pathways
regulate interleukin-1beta-induced miR-146a and miR-146b expression
and chemokine release in human alveolar epithelial cells. FEBS
Lett. 583:3349–3355. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Callis TE, Pandya K, Seok HY, Tang RH,
Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, et al:
MicroRNA-208a is a regulator of cardiac hypertrophy and conduction
in mice. J Clin Invest. 119:2772–2786. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sadek KM, Mahmoud SFE, Zeweil MF and
Abouzed TK: Proanthocyanidin alleviates doxorubicin-induced cardiac
injury by inhibiting NF-kB pathway and modulating oxidative stress,
cell cycle, and fibrogenesis. J Biochem Mol Toxicol.
35(e22716)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Shyu KG, Wang BW, Wu GJ, Lin CM and Chang
H: Mechanical stretch via transforming growth factor-β1 activates
microRNA208a to regulate endoglin expression in cultured rat
cardiac myoblasts. Eur J Heart Fail. 15:36–45. 2013.PubMed/NCBI View Article : Google Scholar
|