Introduction
Rathke cleft cysts (RCCs) are remnants originating
from Rathke's pouch, which is usually located near the sellar
region. According to the specific location, RCCs may be divided
into the intrasellar, intrasellar to suprasellar and the
suprasellar type (1). The
detection rate of RCCs in routine autopsy may reach 12-33%
(2). RCCs may occur in individuals
of all ages, but mostly occur above the age of 30, and it is more
common in middle-aged individuals than in children and the elderly
(3). The status of RCCs is
variable, most of them remain stable, certain cases exhibit
spontaneous regression and others expand gradually and continuously
(4). Among them, patients with
RCCs with stable and spontaneous regression are usually not
symptomatic and such RCCs are classified as asymptomatic RCCs.
However, gradually expanding RCCs have a great impact on the
surrounding structures (such as the pituitary, hypothalamus and
optic chiasm), so that patients exhibit clinical symptoms and
complaints, such as headache, impaired vision or pituitary
dysfunction, and these RCCs are classified as symptomatic RCCs
(5-7).
Barkhoudarian et al (8)
indicated that ~60% of RCCs were asymptomatic. For asymptomatic
RCCs, follow-up is usually recommended, while for symptomatic RCCs,
surgical treatment is necessary (9). In recent years, with the rapid
development of neuro endoscope technology and hardware equipment,
the endoscopic endonasal transsphenoidal approach (EETA) has been
widely used, with the advantages of a clear field of vision deep
into the sellar region, direct vision of lesions, less trauma,
minimal post-operative discomfort and rapid recovery. In the
present study, 61 cases of symptomatic RCCs treated by EETA were
retrospectively analyzed. Information regarding the clinical
symptoms, MRI features, tumor location and characteristics,
intraoperative conditions, postoperative outcomes, postoperative
complications and long-term follow-up was collected and analyzed in
detail. In addition, the effectiveness and safety of surgical
strategies based on intracapsular decompression, total resection of
cyst contents, partial resection of the cyst wall and no filling of
the cyst cavity were evaluated and verified based on the relief of
clinical symptoms, postoperative complications and recurrence
rate.
Materials and methods
Patient selection
All cases included in the present study were
required to meet the inclusion criteria. The specific conditions
for inclusion were as follows: The RCCs were symptomatic and their
diagnosis was made according to the general criteria (10); the patients underwent EETA at the
Department of Neurosurgery, Chongqing General Hospital (Chongqing,
China) between April 2014 and August 2021; postoperative pathology
confirmed RCCs (when the resected part contained the cyst wall and
cyst contents, a pathological examination was performed on both
parts. When there was no cyst wall and only cyst content in the
resected part, the pathological examination was performed on the
cyst content). The specific conditions for exclusion were as
follows: Inflammation in the nasal cavity and paranasal sinuses;
coagulation dysfunction; patients in a poor physical condition and
not able to tolerate the operation. According to the inclusion
criteria, 61 eligible patients were enrolled. The present study was
approved by the ethics committee of the hospital (approval no. KY
S2022-032-01).
Surgical procedure
In general, patients received EETA within 2 weeks
after the diagnosis of symptomatic RCCs. The specific surgical
procedure was as follows: After general anesthesia, the patient was
placed in the supine position and the head was turned 10˚ to the
right. After routine nasal disinfection and towel laying, the
endoscope was placed along the right nasal cavity. The IMAGE1 S
camera system (Karl Storz) was used during surgery. Under the
guidance of the endoscope, first, the opening of the right sphenoid
sinus was located, then the mucosa above the opening of the
sphenoid sinus was cut, then the sphenoid sinus and sellar base
bone were opened, and finally, the dura mater was exposed. The dura
was cut and enlarged to expose the pituitary gland and lesions. At
the weakest part of the cyst wall, the RCCs were opened for
intracapsular decompression. The outflow cystic fluid was a
yellow-white chylous fluid, a colloidal translucent substance or a
mucus-like substance. The curette and aspirator were used to remove
the cyst fluid and normal saline was used to repeatedly flush the
cyst cavity until the residual cyst contents were resected. Whether
the cyst wall of RCCs is removed depends on the specific situation.
When the cyst wall of RCCs is close to the pituitary and difficult
to remove from the pituitary structure, it is not recommended to
remove the cyst wall of RCCs. When the cyst wall of RCCs is not
closely connected with the pituitary tissue, it is easy to peel the
cyst wall from the surrounding pituitary tissue, which is
recommended to resect. Finally, the skull base was repaired and the
specific steps were as follows: The skull base was directly
repaired with artificial dura with no filling in the cyst cavity.
Hemostatic gauze was used for external filling and iodoform gauze
strips were inserted into the right nasal tract. The operation time
was ~40 to 100 min, with an average of 61 min. In conclusion, the
surgical strategy for EETA resection of RCCs was intracapsular
decompression, total resection of cyst contents, partial resection
of cyst wall and no filling of the cyst cavity.
Histopathological examination
After the operation, a pathological examination was
performed to observe the histopathological characteristics and
confirm the diagnosis. At room temperature, tissue samples from
patients were fixed in 10% neutral formalin solution for 12 h, then
embedded in paraffin, cut into 3-µm sections and subjected to
H&E staining. Pathological examination was performed under a
light microscope (BX43; Olympus Corporation), including analysis of
the type and degree of cystic fibrosis and the presence of
inflammatory infiltration.
Results
Patient demographics and clinical
characteristics
The characteristics of the 61 patients are provided
in Table I. Combined with
preoperative MRIs and intraoperative conditions and according to
the location of the RCCs, 61 patients with RCC were divided into
groups of intrasellar RCCs (n=38), intra- and supra-sellar RCCs
(n=14) and suprasellar RCCs (n=9). Patient age ranged from 13 to 69
years, with a median age of 47 years. Specifically, for patients
with RCCs in different locations, there were certain differences in
age range and median age, which were 13-69 and 47 years
(intrasellar), 17-64 and 40 years (intra- and supra-sellar) and
18-54 and 52 years (suprasellar). The cohort comprised 22 males and
39 females, including 17 males and 21 females in the intrasellar, 4
males and 10 females in intra- and supra-sellar, and 1 male and 8
females in the suprasellar group. The main clinical symptoms of the
patients with RCC were headache, visual impairment and pituitary
dysfunction. Among all patients, 88.5% (n=54 cases) suffered from
headaches, including 35 cases in the intrasellar, 11 cases in the
intra- and supra-sellar and 8 cases in the intrasellar group, with
proportions of as high as 92.1, 78.6 and 88.9%, respectively.
Furthermore, 30 patients had visual impairment, accounting for
49.2%, including 17 cases in the intrasellar, 8 cases in the intra-
and supra-sellar and 5 cases in the suprasellar groups, accounting
for 44.7, 57.1 and 55.6%, respectively. In addition, there were 8
patients (13.1%) with pituitary dysfunction, including 5 cases
(13.2%) in the intrasellar, 1 case (7.1%) in the intra- and
supra-sellar and 2 cases (22.2%) in the suprasellar group. The RCCs
had a maximum diameter of <1, 1-2, 2-3, 3-4 and >4 cm in 1,
54, 5, 0 and 1 cases, respectively. Furthermore, the average
diameter of these symptomatic RCCs was ~1.55x1.48x1.33 cm, which
was measured by caliper measurement tool of the medical imaging
system. In addition, in only one case it was confirmed that the RCC
was enlarged prior to the operation, while the RCCs of the other
patients were not significantly enlarged.
 | Table ICharacteristics of all patients
(n=61). |
Table I
Characteristics of all patients
(n=61).
| Item | Intrasellar
(n=38) | Intra- and
supra-sellar (n=14) | Supersellar
(n=9) | Total |
|---|
| General
information | | | | |
|
Age,
years | 47 (13-69) | 40 (17-64) | 52 (18-54) | 47 (13-69) |
|
Sex
(male/female) | 17/21 | 4/10 | 1/8 | 22/39 |
| Clinical
symptoms | | | | |
|
Headache | 35 (92.1) | 11 (78.6) | 8 (88.9) | 54 (88.5) |
|
Visual
impairment | 17 (44.7) | 8 (57.1) | 5 (55.6) | 30 (49.2) |
|
Pituitary
dysfunction | 5 (13.2) | 1 (7.1) | 2 (22.2) | 8 (13.1) |
| Clinic symptoms
outcome | | | | |
|
Headache
relief | 35(100) | 11(100) | 8(100) | 54(100) |
|
Vision
improvement | 11 (64.7) | 7 (87.5) | 4 (80.0) | 22 (73.3) |
|
Improvement
of pituitary function | 5(100) | 1(100) | 2(100) | 8(100) |
| Postoperative
complications | | | | |
|
Transient
diabetes insipidus | 5 (13.2) | 8 (57.1) | 1 (11.1) | 14 (23.0) |
|
Transient
hypopituitarism | 3 (7.9) | 4 (28.6) | 0 (0.0) | 7 (11.5) |
|
Follow up,
months | 6 (1-29) | 5 (1-22) | 13 (1-47) | 7 (1-47) |
|
Recurrence | 0 | 0 | 0 | 0 |
Postoperative outcomes
After surgery, headache and pituitary dysfunction of
all patients were improved, while visual ability was improved in 22
cases, accounting for 73.3%. The postoperative complications were
transient diabetes insipidus (DI) and hypopituitarism (HP).
Transient DI occurred in 14 cases (23%), including 5 cases (13.2%)
in the intrasellar, 8 cases (57.1%) in the intra- and supra-sellar
and 1 case (11.1%) in the suprasellar group, while transient HP
occurred in 7 cases (11.5%), including 3 cases (7.9%) in the
intrasellar and 4 cases (28.6%) in the intra- and supra-sellar
group. None of the patients with transient DI received treatment
and automatically returned to normal within ~10 days. Patients with
transient HP returned to normal within ~3 weeks after oral
administration of thyroxine and hydrocortisone. The follow-up time
ranged from 1 to 47 months and there was no recurrence based on MRI
during follow-up. In addition, it is worth noting that 28 patients
did not come to our hospital for follow-up after discharge. Their
follow-up time was recorded as 1 month and their recurrence was
judged by postoperative MRI.
Imaging and histopathological
findings
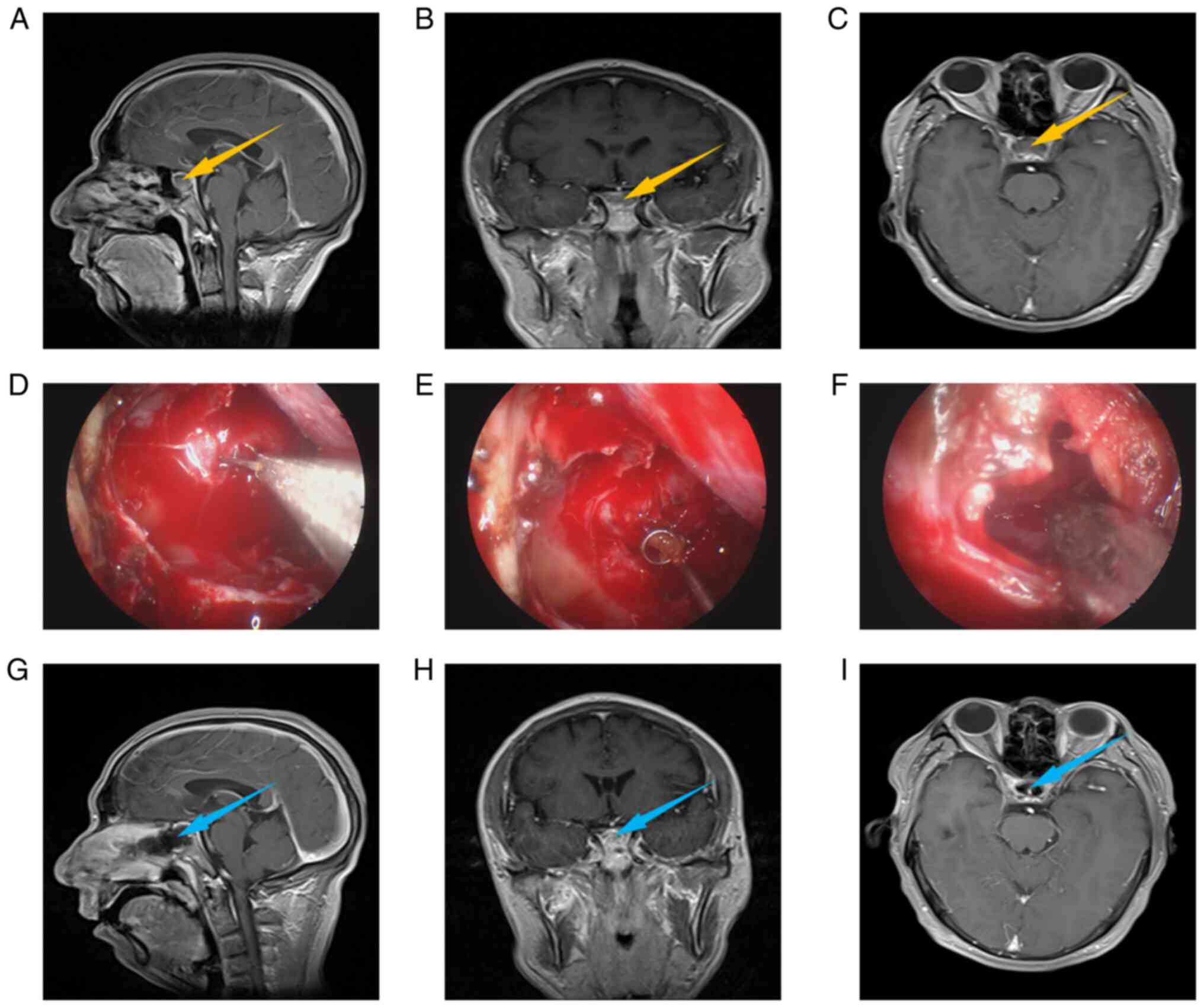
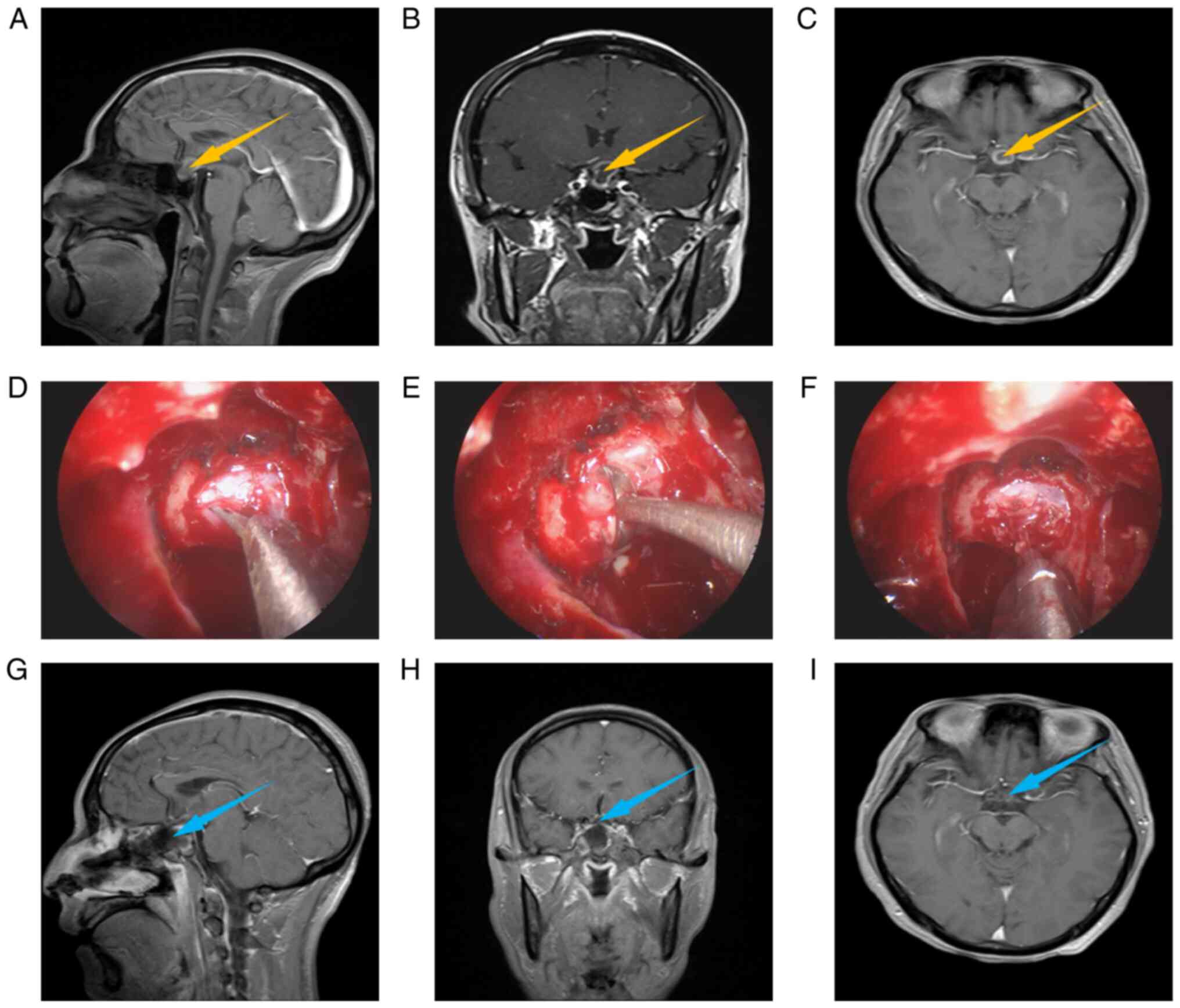
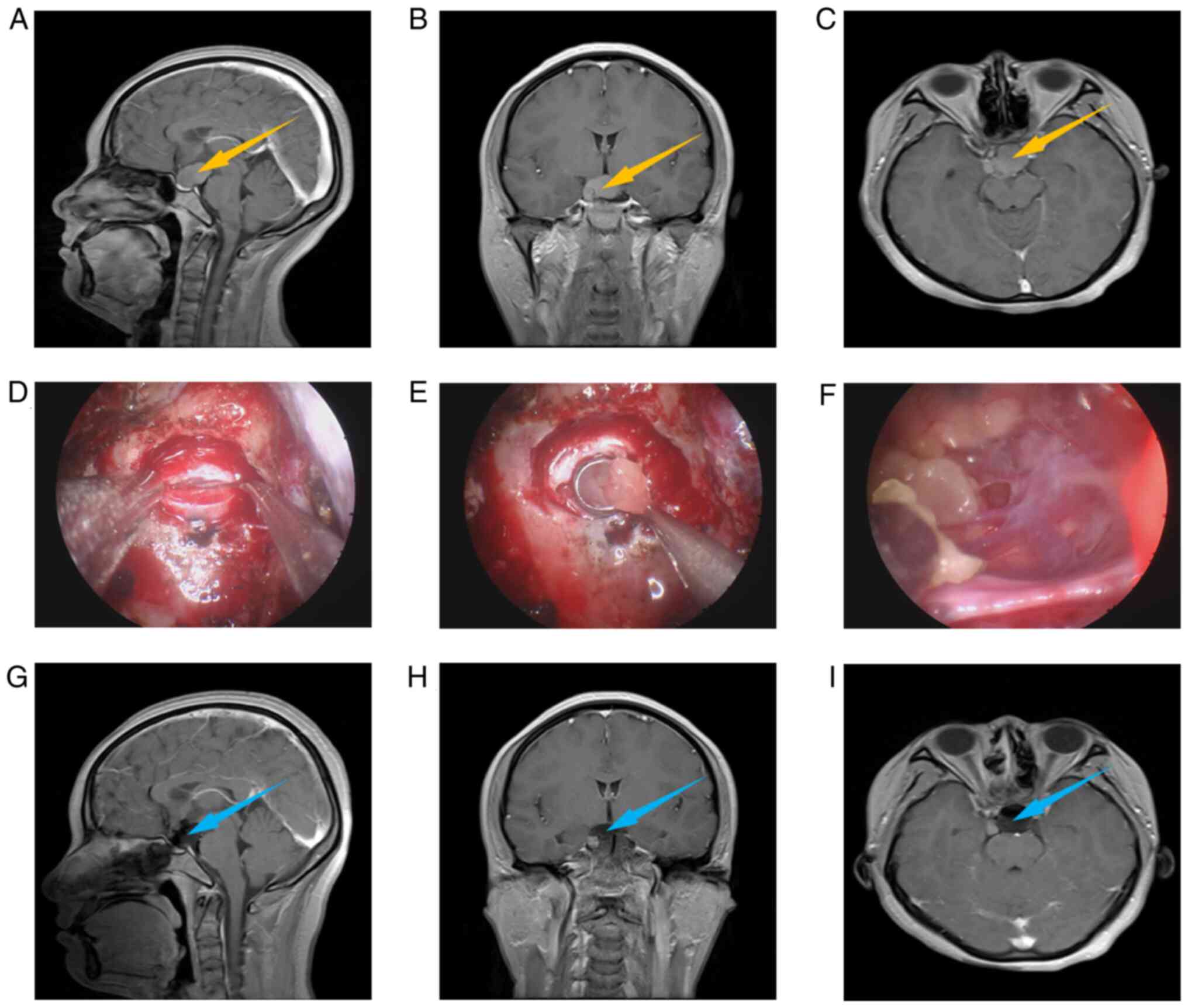
Fig. 1, Fig. 2 and Fig. 3 present the relevant images of an
intrasellar case, an intra- and supra-sellar case, and a
suprasellar case, including preoperative MRI (Fig. 1A-C, Fig. 2A-C and Fig. 3A-C), intraoperative images
(Fig. 1D-F, Fig. 2D-F and Fig. 3D-F) and postoperative MRI (Fig. 1G-I, Fig. 2G-I and Fig. 3G-I). Usually, preoperative MRI is
used to judge the location and type of lesions. From the
preoperative MRI, it may be observed that all 3 cases had obvious
space-occupying lesions, which were located in the intrasellar, the
intra- and supra-sellar and the suprasellar region, respectively,
and were preliminarily diagnosed as RCCs. The intraoperative images
indicate the process of removal of cyst contents. Comparing the
postoperative with the preoperative MRI, it may be observed that
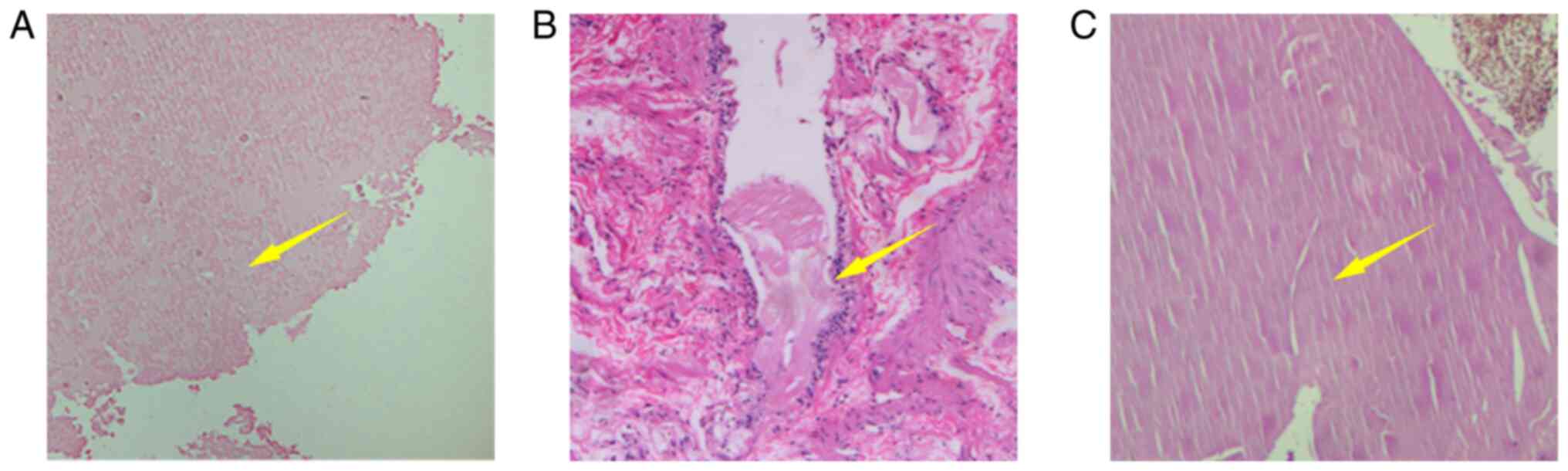
the space-occupying lesions were successfully removed. Fig. 4A-C provides histopathological
images of three cases, patients 1, 2 and 3, respectively. From the
pathological image of patient 1, pink unstructured cystic fluid may
be observed. The pathological image of patient 2 indicates that the
cyst wall is covered by cubic epithelium. The pathological image of
patient 3 is similar to that of patient 1, indicating pink
unstructured cystic fluid, but the difference is that slit-like
structures are visible. In conclusion, according to histopathology
(Fig. 4), all three cases were
diagnosed as RCCs.
Discussion
The preoperative diagnosis of RCCs and their
differentiation from other intracranial lesions are important and
have a guiding role in formulating treatment plans and surgical
strategies. RCCs are usually located in the sellar region and close
to pituitary tissue. In general, round, oval and dumbbell-shaped
thin-walled cystic lesions are displayed on MRI. Furthermore, due
to the different cystic liquid properties of RCCs, RCCs may display
as high, equal and low signals on T1-weighted imaging (T1WI) and
T2WI (10). The above
characteristics of RCCs are similar to those of pituitary adenoma,
craniopharyngioma and meningioma, so RCCs should be differentiated
from these tumors prior to surgery. Pituitary adenoma is the most
common space-occupying lesion in the sellar region. MRI usually
displays isointense to gray matter on TIWI and hyperintense on T2WI
(11). Craniopharyngioma is also a
lesion in the sellar region. The cystic components tend to be
hyperintense on T2WI. Enhancement of the cyst wall and
heterogeneous enhancement of the solid portions are common
(12). Meningiomas may also be
located in the sellar region, presenting as an isosignal on T1WI
and T2WI, usually exhibiting strong uniform enhancement (13). These tumors exhibit similarities on
MRI and it is difficult for MRI to distinguish these tumors. In
addition, these tumors are located in the sellar region and close
to pituitary tissue, which may cause similar clinical symptoms,
such as headache, changes in vision and visual field or endocrine
symptoms. Therefore, it is difficult to distinguish them from their
clinical symptoms. Finally, the differential diagnosis between RCCs
and these tumors is based on the pathological diagnosis.
In general, the size of RCCs is closely related to
the clinical symptoms. Most of the smaller RCCs are scattered and
asymptomatic, while the larger RCCs expand the pressure on the
surrounding tissues, which may lead to clinical symptoms such as
headache, visual field defect and pituitary dysfunction. Therefore,
RCCs are divided into asymptomatic and symptomatic RCCs, and the
symptomatic treatment methods for these two types of RRC are also
different. For asymptomatic RCCs, conservative treatment has been
highly recognized and widely used. The long-term follow-up results
of 75 cases with asymptomatic RCCs accidentally diagnosed by
radiology have indicated that the lesions of most patients remain
unchanged or shrink over time, indicating that conservative
treatment of asymptomatic RCCs is reasonable (14). For symptomatic RCCs, surgery is a
recognized treatment. In recent years, EETA has become a standard
surgical approach for symptomatic RCCs. The specific surgical
strategy is intracapsular decompression, excision of cyst contents,
partial excision of the cyst wall and no filling of the cyst
cavity. In the present study, 61 cases of symptomatic RCCs treated
at our department from 2014 to 2021 were retrospectively analyzed.
Specifically, common clinical symptoms were analyzed, such as
headache, abnormal visual acuity and field and pituitary
insufficiency, and the corresponding possible causes and relief, as
well as the postoperative complications and recurrence. The
surgical results suggested that this surgical method may
effectively relieve symptoms and reduce postoperative complications
and recurrence. As the study is a case series study, there is no
division between any control group and an experimental group;
furthermore, no statistical comparison was performed among the
three different groups and there was a relatively short follow-up
time for certain cases, which are limitations.
The most common clinical manifestation of
symptomatic RCCs is headache, particularly paroxysmal headache in
the forehead, which is a common and characteristic symptom of
symptomatic RCCs (15). In the
cohort of the present study, 88.5% (54 cases) of the patients had
headache symptoms, the rate of which was significantly higher than
that of other clinical symptoms. The headache types were
paroxysmal, persistent, forehead, temporal, parietal, occipital,
tingling and dilative headaches. A total of 39 cases presented with
paroxysmal headache in the forehead, accounting for 72.2%. However,
most of the patients did not go to the hospital for treatment when
they had a headache, but their symptoms were temporarily relieved
after taking medication by themselves. Therefore, their headache
was not diagnosed as a specific type of headache. Only one case was
diagnosed as a headache caused by sinusitis. After the operation,
the headache of these 54 patients was relieved. According to the
literature, the same surgical method is used to treat RCCs and the
relief rate of headache in RCC patients after the operation has
reached 80-93% (16,17). Headaches may be due to the
space-occupying effect or the intermittent inflammatory reaction
caused by the contents of the cyst (17,18).
Therefore, intracapsular decompression may relieve the headache
caused by the space-occupying effect and total resection of the
contents of the cyst may alleviate the inflammatory reaction caused
by the contents.
The abnormal visual acuity and field are
significantly related to RCC size and optic nerve or optic chiasma
compression. The incidence of visual impairment in patients with
RCCs has been reported to be in the range of 11-75% (19,20).
Of the patients in the present study, 30 cases had visual
impairment, accounting for 49.2%. Among the patients with visual
impairment, the maximum diameter was <1, 1-4 and >4 cm in 1,
28 and 1 cases, respectively. The visual acuity of these cases with
visual impairment was documented by formal visual testing,
including 28 cases of blurred vision, 2 cases of visual defect and
4 cases of diplopia. It is worth noting that certain patients may
suffer from two types of visual impairment. From the statistical
results of visual impairment, it may be seen that the intra- and
supra-sellar and suprasellar RCCs had a higher probability of
causing visual impairment, as the intra-and supra-sellar and
suprasellar RCCs are more likely to compress the optic chiasm. It
is considered that the effect of RCCs on visual acuity is mainly
caused by the cyst breaking through the sellar diaphragm and
growing on the sellar, which compresses the optic tract, the optic
chiasm or the related blood vessels supplying the optic nerve,
resulting in changes in visual acuity and visual field. After the
operation, visual acuity was improved in 22 of the 30 cases and the
visual acuity improvement rate reached 73.3%, which is comparable
with the results of a previous study using the same surgical method
(21).
Due to the close relationship between RCCs and the
pituitary, patients with symptomatic RCCs may have pituitary
insufficiency. The cohort of the present study included 8 cases
(13.1%) of pituitary insufficiency, whose gonadal axis was
affected, including 4 cases with decreased testosterone level and 4
cases with increased prolactin, resulting in decreased libido or
menstrual disorder. The maximum diameter of RCCs in 8 cases was in
the range of 1-4 cm, of which 6 cases (11.1%) were 1-2 cm and 2
cases (40%) were 2-3 cm, indicating that the larger-size RCCs may
cause hormone dysfunction. A previous study reported that the
space-occupying features and contents of RCCs may affect the
surrounding pituitary tissue, resulting in abnormal secretion of
pituitary-related hormones (22).
Specifically, the cyst contents leaked from RCCs rupture not only
erode and stimulate the pituitary tissue but may also cause
secondary pituitary inflammation, affecting the surrounding
pituitary tissue. Therefore, the pituitary insufficiency caused by
RCCs is mainly related to the space-occupying effect of RCCs and
the leakage of cyst rupture contents. After the operation, the
hormone levels of 8 patients returned to normal.
The main postoperative complications after EETA were
cerebrospinal fluid leakage, infection, DI and HP (23). In the cohort of the present study,
postoperative complications included transient DI in 14 cases and
transient HP in 7 cases, accounting for 34.5%, which was also
acceptable. Furthermore, patients with transient DI did not receive
treatment and automatically returned to normal within ~10 days.
Furthermore, patients with transient HP returned to normal within
~3 weeks after oral administration of thyroxine and hydrocortisone.
Postoperative DI is mostly caused by the traction of the pituitary
stalk during operation. Excessive cutting and pulling of pituitary
tissue during the operation may lead to damage to pituitary
function and a decrease in pituitary-related hormone levels. In
addition, the incidence of postoperative complications may be
related to the degree of cyst wall resection and whether the cyst
wall is cauterized with alcohol. Fan et al (24) reported that the incidence of
postoperative complications of radical resection for symptomatic
RCCs reached up to 47%. Furthermore, Benveniste et al
(6) indicated that the possibility
of new-onset anterior pituitary defects increased after radical
resection of symptomatic RCCs. Cabuk et al (25) reported that after radical resection
for symptomatic RCCs, there was even new-onset pituitary
dysfunction, such as 1 case of new-onset cortisol decline and
another case of new-onset hypogonadism. It was observed that
radical resection for symptomatic RCCs markedly increased the
probability of postoperative complications. Based on this, it was
reported that radical resection of the cyst wall should be
performed only where it is possible to do so without causing
additional and unnecessary pituitary damage (26). To reduce postoperative
complications, partial resection of the cyst wall for symptomatic
RCCs has been widely adopted and recognized. The incidence of
postoperative complications of partial resection for symptomatic
RCCs was reduced to ~3.8% (20,21).
It was reported that absolute alcohol injected into empty cysts may
increase the incidence of complications (23). Therefore, injection of alcohol into
the empty cyst has no effect, and no filling of the empty cyst is
the simplest and best method.
Recurrence of symptomatic RCCs is considered to be
related to the extent of cyst wall resection. However, the extent
of cyst wall resection in symptomatic RCCs is still controversial
(18). At present, there are two
surgical strategies to deal with the cyst wall of symptomatic RCCs:
One is to completely remove the cyst wall (radical resection) to
prevent recurrence and reduce the recurrence rate, while the other
is to partially remove the cyst wall (partial resection) to protect
pituitary function and reduce postoperative complications (27,28).
The recurrence rate is the gold standard for evaluating these two
surgical strategies. In general, eradication resection is
considered to be an effective measure to reduce the recurrence rate
of symptomatic RCCs, but it is not so in practice. Aho et al
(23) compared the recurrence
rates of radical resection and partial resection for symptomatic
RCCs and the results indicated that 6 of 33 patients who underwent
radical resection had recurrence, with a recurrence rate of 18.2%,
while 18 of 85 patients who underwent partial resection had
recurrence, with a recurrence rate of 21.2% (P=0.473). Higgins
et al (29) reported 61
patients with symptomatic RCCs, including 32 patients in the
radical resection group, of which 5 cases exhibited recurrence,
with a recurrence rate of 9%, and 29 patients in the partial
resection group, of which 3 cases exhibited recurrence, with a
recurrence rate of 17%. Koutourousiou et al (30) reported the outcomes of radical
resection in 14 patients with symptomatic RCCs, of which 2 cases
exhibited recurrence and the recurrence rate was 14.3%. Potts et
al (31) reported that 151
patients with symptomatic RCCs underwent cyst drainage (partial
resection), with a recurrence rate of 11%. Wait et al
(22) reported 8 recurrences in 73
patients and 7 of the 8 recurrences arose after radical resection.
There is increasing evidence that radical resection does not
exhibit greater advantages than partial resection in terms of the
recurrence rate of symptomatic RCCs. The reason for RCC recurrence
is that inflammatory cell infiltration may stimulate squamous cell
metaplasia, thus promoting the formation of cyst wall and leading
to cyst fluid accumulation, which is not directly related to
radical resection and partial resection of the cyst wall of
symptomatic RCCs (16,32). In addition, there are different
opinions regarding whether an empty cyst of symptomatic RCCs should
be dealt with or not. In the past, absolute alcohol was injected
into empty cysts to burn the cyst wall and reduce the chance of
recurrence, but the results were not ideal (33). Lillehei et al (20) reported that in the alcohol-treated
group, the cyst recurred in 8 (12.9%) of the 62 patients over the
follow-up period, while in the no-alcohol treatment group, none
(0%) of the 13 patients had a recurrence, indicting a limited role
for alcohol cauterization in the treatment of symptomatic RCCs. It
was reported that if absolute alcohol was injected into empty
cysts, the recurrence rate of symptomatic cysts was not decreased
(23). Therefore, partial excision
of the cyst wall and no filling of the cyst cavity is indicated to
not increase the recurrence rate of patients, but even reduce the
recurrence rate.
Through the review of 61 patients with symptomatic
RCCs who underwent surgeries, the present study put forward various
suggestions and surgical strategies. First, due to the location of
RCCs, EETA has become a standard treatment for symptomatic RCCs,
which may reduce trauma and improve the recovery rate. Furthermore,
after intracapsular decompression and removal of cyst contents,
partial resection of the cyst wall was adopted, which may
effectively protect pituitary function and reduce postoperative
complications without increasing the recurrence rate. Finally, the
skull base was repaired directly without filling the empty cyst to
reduce inflammation as much as possible and the recurrence rate. In
conclusion, for the treatment of symptomatic RCCs, EETA, partial
removal of cyst wall and no filling of the empty cyst are key to
reducing postoperative complications and recurrence rate.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT, PW, JL, HJ, GZ and NW participated in the
conception and design of the study and data acquisition. CT
participated in drafting and revising the manuscript. PW critically
revised the paper. NW ensured that questions related to the
integrity of any part of the work were appropriately investigated
and resolved. CT, PW, JL, HJ, GZ and NW confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Chongqing General Hospital (approval no. KY S2022-032-01).
Patient consent for publication
Written informed consent was obtained from the
patients for the publication of anonymized data and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jahangiri A, Potts M, Kunwar S, Blevins L,
El-Sayed IH and Aghi MK: Extended endoscopic endonasal approach for
suprasellar Rathke's cleft cysts. J Clin Neurosci. 21:779–785.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Trifanescu R, Ansorge O, Wass JA, Grossman
AB and Karavitaki N: Rathke's cleft cysts. Clin Endocrinol (Oxf).
76:151–160. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoshida J, Kobayashi T, Kageyama N and
Kanzaki M: Symptomatic Rathke's cleft cyst. Morphological study
with light and electron microscopy and tissue culture. J Neurosurg.
47:451–458. 1977.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Amhaz HH, Chamoun RB, Waguespack SG, Shah
K and McCutcheon IE: Spontaneous involution of Rathke cleft cysts:
Is it rare or just underreported? J Neurosurg. 112:1327–1332.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
el-Mahdy W and Powell M: Transsphenoidal
management of 28 symptomatic Rathke's cleft cysts, with special
reference to visual and hormonal recovery. Neurosurgery. 42:7–17.
1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Benveniste RJ, King WA, Walsh J, Lee JS,
Naidich TP and Post KD: Surgery for Rathke cleft cysts: Technical
considerations and outcomes. J Neurosurg. 101:577–584.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zada G, Lin N, Ojerholm E, Ramkissoon S
and Laws ER: Craniopharyngioma and other cystic epithelial lesions
of the sellar region: A review of clinical, imaging, and
histopathological relationships. Neurosurg Focus.
28(E4)2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barkhoudarian G, Palejwala SK, Ansari S,
Eisenberg AA, Huang X, Griffiths CF, Cohan P, Rettinger S, Lavin N
and Kelly DF: Rathke's cleft cysts: A 6-year experience of surgery
vs observation with comparative volumetric analysis. Pituitary.
22:362–371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhong W, You C, Jiang S, Huang S, Chen H,
Liu J, Zhou P, Liu Y and Cai B: Symptomatic Rathke cleft cyst. J
Clin Neurosci. 19:501–508. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choi SH, Kwon BJ, Na DG, Kim JH, Han MH
and Chang KH: Pituitary adenoma, craniopharyngioma, and Rathke
cleft cyst involving both intrasellar and suprasellar regions:
Differentiation using MRI. Clin Radiol. 62:453–462. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bresson D, Herman P, Polivka M and
Froelich S: Sellar lesions/pathology. Otolaryngol Clin North Am.
49:63–93. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zacharia BE, Amine M, Anand V and Schwartz
TH: Endoscopic endonasal management of craniopharyngioma.
Otolaryngol Clin North Am. 49:201–212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nowosielski M, Galldiks N, Iglseder S,
Kickingereder P, von Deimling A, Bendszus M, Wick W and Sahm F:
Diagnostic challenges in meningioma. Neuro Oncol. 19:1588–1598.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Culver SA, Grober Y, Ornan DA, Patrie JT,
Oldfield EH, Jane JA Jr and Thorner MO: A case for conservative
management: Characterizing the natural history of radiographically
diagnosed Rathke cleft cysts. J Clin Endocrinol Metab.
100:3943–3948. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nishioka H, Haraoka J, Izawa H and Ikeda
Y: Headaches associated with Rathke's cleft cyst. Headache.
46:1580–1586. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim JE, Kim JH, Kim OL, Paek SH, Kim DG,
Chi JG and Jung HW: Surgical treatment of symptomatic Rathke cleft
cysts: Clinical features and results with special attention to
recurrence. J Neurosurg. 100:33–40. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nishioka H, Haraoka J, Izawa H and Ikeda
Y: Magnetic resonance imaging, clinical manifestations, and
management of Rathke's cleft cyst. Clin Endocrinol (Oxf).
64:184–188. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Laws ER and Kanter AS: Rathke cleft cysts.
J Neurosurg. 101:571–572. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Binning MJ, Liu JK, Gannon J, Osborn AG
and Couldwell WT: Hemorrhagic and nonhemorrhagic Rathke cleft cysts
mimicking pituitary apoplexy. J Neurosurg. 108:3–8. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lillehei KO, Widdel L, Astete CA, Wierman
ME, Kleinschmidt-DeMasters BK and Kerr JM: Transsphenoidal
resection of 82 Rathke cleft cysts: Limited value of alcohol
cauterization in reducing recurrence rates. J Neurosurg.
114:310–317. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xie T, Hu F, Yu Y, Gu Y, Wang X and Zhang
X: Endoscopic endonasal resection of symptomatic Rathke cleft
cysts. J Clin Neurosci. 18:760–762. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wait SD, Garrett MP, Little AS, Killory BD
and White WL: Endocrinopathy, vision, headache, and recurrence
after transsphenoidal surgery for Rathke cleft cysts. Neurosurgery.
67:837–843. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aho CJ, Liu C, Zelman V, Couldwell WT and
Weiss MH: Surgical outcomes in 118 patients with Rathke cleft
cysts. J Neurosurg. 102:189–193. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan J, Peng Y, Qi S, Zhang XA, Qiu B and
Pan J: Individualized surgical strategies for Rathke cleft cyst
based on cyst location. J Neurosurg. 119:1437–1446. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cabuk B, Selek A, Emengen A, Anik I,
Canturk Z and Ceylan S: Clinicopathologic characteristics and
endoscopic surgical outcomes of symptomatic Rathke's cleft cysts.
World Neurosurgery. 132:e208–e216. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frank G, Sciarretta V, Mazzatenta D,
Farneti G, Modugno GC and Pasquini E: Transsphenoidal endoscopic
approach in the treatment of Rathke's cleft cyst. Neurosurgery.
56:124–129. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chotai S, Liu Y, Pan J and Qi S:
Characteristics of Rathke's cleft cyst based on cyst location with
a primary focus on recurrence after resection. J Neurosurg.
122:1380–1389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ratha V, Patil S, Karmarkar VS, Shah NJ
and Deopujari CE: Surgical management of Rathke cleft cysts. World
Neurosurg. 107:276–284. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Higgins DM, Van Gompel JJ, Nippoldt TB and
Meyer FB: Symptomatic Rathke cleft cysts: Extent of resection and
surgical complications. Neurosurg Focus. 31(E2)2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koutourousiou M, Grotenhuis A,
Kontogeorgos G and Seretis A: Treatment of Rathke's cleft cysts:
Experience at a single centre. J Clin Neurosci. 16:900–903.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Potts MB, Jahangiri A, Lamborn KR, Blevins
LS, Kunwar S and Aghi MK: Suprasellar Rathke cleft cysts: Clinical
presentation and treatment outcomes. Neurosurgery. 69:1058–1077.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kinoshita Y, Tominaga A, Usui S, Arita K,
Sakoguchi T, Sugiyama K and Kurisu K: The long-term recurrence of
Rathke's cleft cysts as predicted by histology but not by surgical
procedure. J Neurosurg. 125:1002–1007. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kleinschmidt-DeMasters BK, Lillehei KO and
Stears JC: The pathologic, surgical, and MR spectrum of Rathke
cleft cysts. Surg Neurol. 44:19–27. 1995.PubMed/NCBI View Article : Google Scholar
|