Introduction
Disseminated intravascular coagulation (DIC) is a
common and morbid complication of streptococcal toxic shock
syndrome (STSS). For instance, a retrospective study reported
coagulation abnormalities in 71% of the patients (median age, 47
years) who had blood tests upon admission (1). Another children-centered study also
reported that 78% of the patients developed coagulopathy (2). Accurate, quick administration of care
is needed in DIC with STSS because patients tend to present with a
rapidly developing clinical course. However, there is no standard
anticoagulant therapy for managing DIC with STSS. Recombinant human
soluble thrombomodulin (rhTM) and danaparoid are administered to
patients with DIC. For example, in a retrospective study of 2663
patients with sepsis, 1247 received anticoagulants, out of which
717 received rhTM and 144 received either heparin or danaparoid
(3). However, to the best of our
knowledge, there have been no clinical studies regarding
combination anticoagulant therapy of rhTM and danaparoid, nor are
there reports regarding combination therapy for managing DIC with
STSS. A concerningly high mortality rate as well as a prevalence of
long-term sequelae due to STSS make exploring and suggesting new
therapies necessary. A retrospective study reported a 58% mortality
rate among 29 patients with STSS (1), while another study reported a 34.2%
mortality rate; among those who survived, 26.8% suffered sequelae
(2). Moreover, a third study
reported a mortality rate of 30% among 20 patients with STSS
(median age, 36) (4). Therefore,
we present the case of a 10-year-old boy who survived without
sequelae after treatment with combination anticoagulant therapy for
managing DIC with STSS.
Case report
A 10-year-old boy presented to our hospital with a
fever that had persisted for 7 days. He developed impetigo on the
second day and diarrhea on the sixth day since fever onset. Medical
history and family history were unremarkable.
On physical examination, the patient appeared
acutely ill, uncomfortable, and irritable. He was unable to stand
and converse normally. His vital signs were as follows: heart rate,
147 beats/min; blood pressure, 101/61 mmHg; temperature, 40.5˚C;
respiratory rate, 42 breaths/min; and oxygen saturation, 100%. He
presented with no nuchal rigidity, and lung fields were clear.
Further examination revealed cheek and pharyngeal erythema,
impetigo on the forehead and jaw, and abdominal tenderness. He had
swelling, redness, and tenderness in his left elbow and left ankle
joints (Fig. 1Ca).
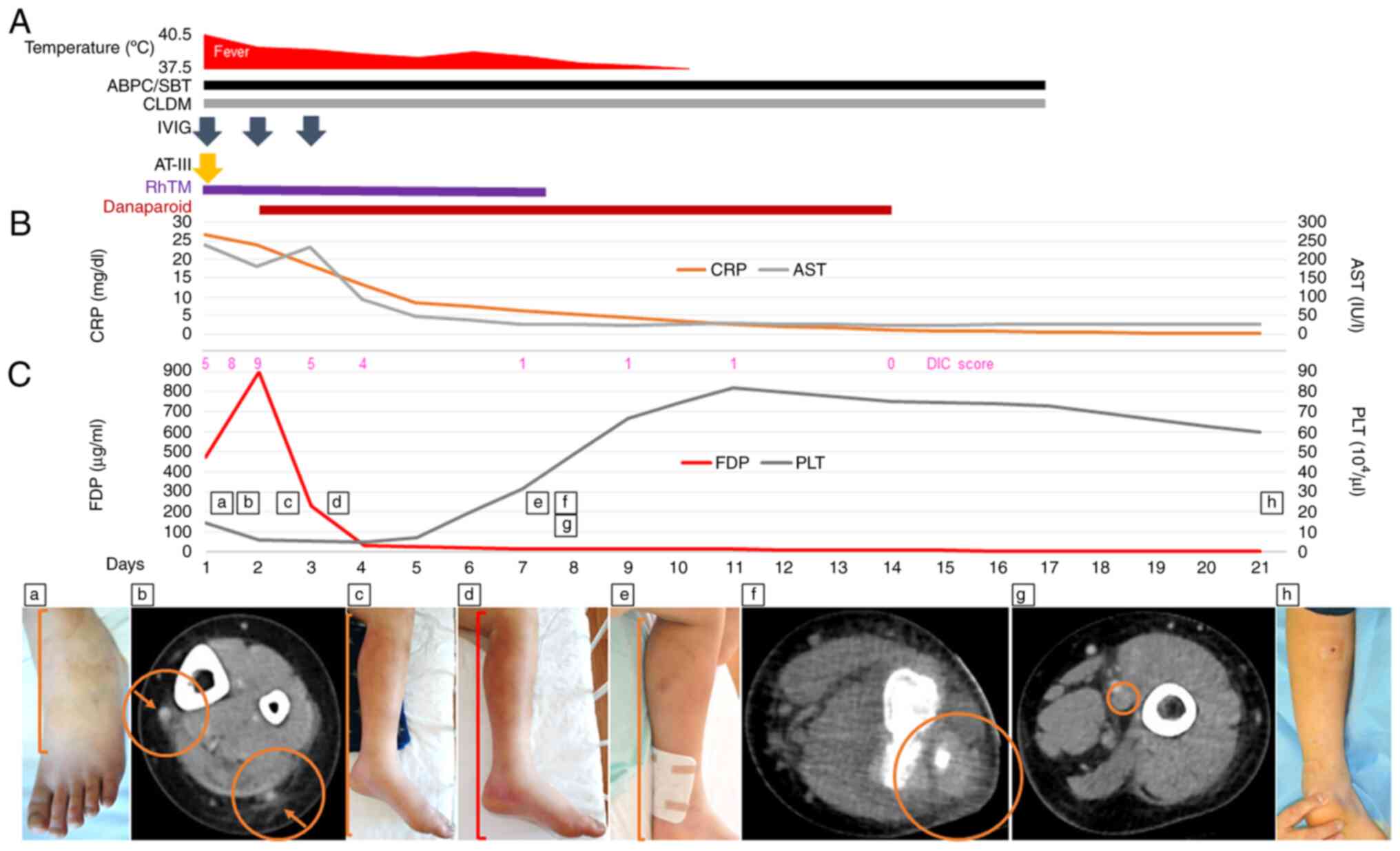 | Figure 1Clinical course of the patient with
streptococcal toxic shock syndrome. (A) Time course of administered
medications and the corresponding patient's temperature. (B) Time
course of CRP and AST measurements. (C) Time course of FDP and PLT
measurements with the corresponding DIC score and clinical
findings. (a) Swelling and redness of the patient's left ankle
joint upon admission. The line indicates swelling and redness. (b)
Contrast-enhanced CT scan showing an increased fat concentration
around the cutaneous veins of the patient's left lower leg upon
admission. The right arrow indicates the cutaneous vein, and the
right circle indicates the increased fat concentration around the
vein. The left arrow indicates the cutaneous vein, and the left
circle indicates the increased fat concentration around the vein.
(c) Swelling and redness of the patient's left lower leg on day 2.
The line indicates swelling and redness. (d) Swelling and redness
of the patient's left lower leg on day 3. The line indicates
swelling and redness. (e) Swelling and redness of the patient's
left lower leg on day 7. The line indicates swelling and redness.
(f) Contrast-enhanced CT scan showing a progressive increase in fat
concentration of the patient's left lower leg on day 7. The circle
indicates a progressive increase in fat concentration. (g)
Contrast-enhanced CT scan showing a ring-shaped contrast
enhancement of the popliteal vein of the patient's left lower leg
on day 7. The circle indicates a ring-shaped contrast enhancement
of the vein. (h) Almost no swelling and redness of the patient's
left lower leg on day 21. ABPC/SBT, ampicillin/sulbactam; CLDM,
clindamycin; IVIG, intravenous immunoglobulin; AT-III,
antithrombin-III; RhTM, recombinant human soluble thrombomodulin;
CRP, C-reactive protein; AST, aspartate aminotransferase; DIC,
disseminated intravascular coagulation; FDP, fibrinogen and fibrin
degradation products; PLT, platelet. |
Laboratory findings are shown in Table I. The rapid antigen detection test
result for Group A Streptococcus pharyngitis was positive.
The blood, throat, and impetigo pus cultures taken upon admission
were positive for Streptococcus pyogenes, and the minimum
inhibitory concentrations against the bacterium were as follows:
ampicillin: ≤0.06 µg/ml; clindamycin: ≤0.12 µg/ml. A
contrast-enhanced computed tomography (CT) scan revealed an
increased fat concentration around the cutaneous veins of his left
lower leg (Fig. 1Cb).
 | Table ILaboratory data on day 1. |
Table I
Laboratory data on day 1.
| | Time after admission,
h |
|---|
| Variable | 0 | 8 | 16 |
|---|
| White blood cell
count, cells/µl | 10,200 | 11,100 | 12,500 |
| Neutrophils, % | 92.0 | 97.0 | 94.3 |
| Band forms, % | 29.0 | 40.0 | 53.0 |
| Platelet count,
platelets/µl | 145,000 | 67,000 | 63,000 |
| International
normalized ratio of prothrombin time | 1.93 | 2.37 | 2.20 |
| Activated
partial-thromboplastin time, sec | 49.1 | 61.4 | 59.0 |
| Fibrinogen,
mg/dl | 487 | 257 | 267 |
| Fibrin degradation
products, µg/ml | 471.1 | 801.8 | 896.1 |
| D-dimer, µg/ml | 183.0 | 290.3 | ≥300 |
| Antithrombin-III,
% | 102 | 78 | 91 |
|
Thrombin-antithrombin-III complexes,
ng/ml | ND | ND | 85.1 |
| Plasmin-α2-plasmin
inhibitor complex, µg/ml | ND | ND | 16.7 |
| Total bilirubin,
mg/dl | 1.52 | 2.28 | 1.75 |
| Aspartate
aminotransferase, U/l | 240 | 275 | 182 |
| Alanine
aminotransferase, U/l | 109 | 102 | 93 |
| Creatine kinase,
U/l | 143 | 153 | ND |
| Creatinine,
mg/dl | 0.59 | 0.70 | 0.47 |
| Ferritin, ng/ml | 2,905.0 | ND | ND |
| C-reactive protein,
mg/dl | 26.67 | 25.00 | 24.08 |
| DIC score,
points | 5 | 8 | 9 |
A diagnosis of STSS was made because this case met
the criteria proposed by The Working Group on Severe Streptococcal
Infections: coagulopathy, liver involvement, generalized
erythematous macular rash, and isolation of Group A
Streptococcus (5).
Furthermore, he fulfilled the revised diagnostic criteria for DIC
by the Japanese Society on Thrombosis and Hemostasis; his DIC score
was 6 points, and since infectious-type DIC is diagnosed if the
score is ≥5 points, a diagnosis of infectious-type DIC was made
(6).
As the patient's state was worsening, he was
immediately admitted to our intensive care unit (ICU). Since his
blood pressure was within the normal range, aggressive intravenous
rehydration was performed up to the maximum recommended maintenance
limit (60 ml/kg/day) to avoid potential hypotension. Intravenous
amoxicillin-sulbactam (180 mg/kg/day in 4 divided doses) and
clindamycin (40 mg/kg/day in 4 divided doses) were administered to
treat the invasive Group A Streptococcus infection.
Intravenous immunoglobulins (150 mg/kg/day) were also administered.
As a result, blood cultures were negative on the third day (day 3)
of ICU admittance, whereas the fever thought to be caused by
hypercytokinemia persisted until day 10 (Fig. 1A). RhTM (380 U/kg/day) and
antithrombin-III (30 U/kg/day) were administered for managing DIC.
Despite this, on day 2 after admission, the patient's general
condition was poor, his fever persisted, and his DIC score was
found to have increased from 5 points (PT/INR, 2; fibrinogen and
fibrin degradation products, 3) upon admission to 9 points
(platelet count, +3 points [≥30% decrease within 24 h];
thrombin-antithrombin-III complexes [TAT], +1 point). Thus,
danaparoid (2,500 U/day in 2 divided doses), in addition to rhTM,
was administered on the same day (day 2) after we obtained informed
consent from his family. After danaparoid + rhTM administration,
his general condition improved, and his laboratory findings became
less alarming (Fig. 1B and
C). From day 3 to day 4, the
patient's DIC score decreased to 4 points from 5 points (fibrin
degradation products, -1 point), indicating that the DIC was
resolved.
Unfortunately, the redness, swelling, and pain in
his left lower leg and elbow joint were gradually worsening
(Fig. 1Cc-e); hence, a
contrast-enhanced CT scan was performed on day 7. It revealed a
progressive increase in fat concentration and ring-shaped contrast
enhancement of his left popliteal vein, suggesting inflammation and
coagulopathy (Fig. 1Cf and
g). The patient's Laboratory Risk
Indicator for Necrotizing Fasciitis (LRINEC) score was 8. If the
LRINEC score is ≥8, a strong possibility of necrotizing fasciitis
is suggested (7). Skin biopsy was
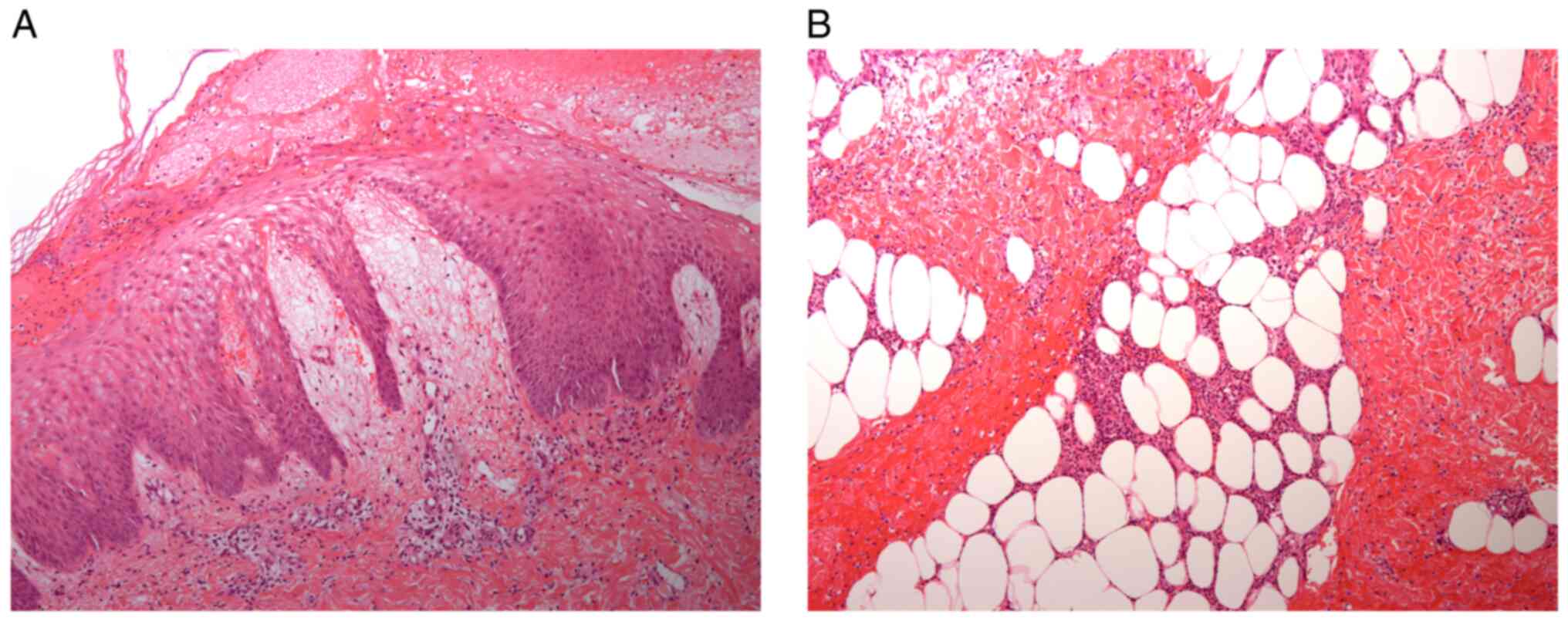
performed for differential diagnosis, and although a diagnosis of
spongiotic dermatitis and suppurative panniculitis was made, his
soft tissue did not progress to necrosis (Fig. 2A and B). Because the range of motion of the
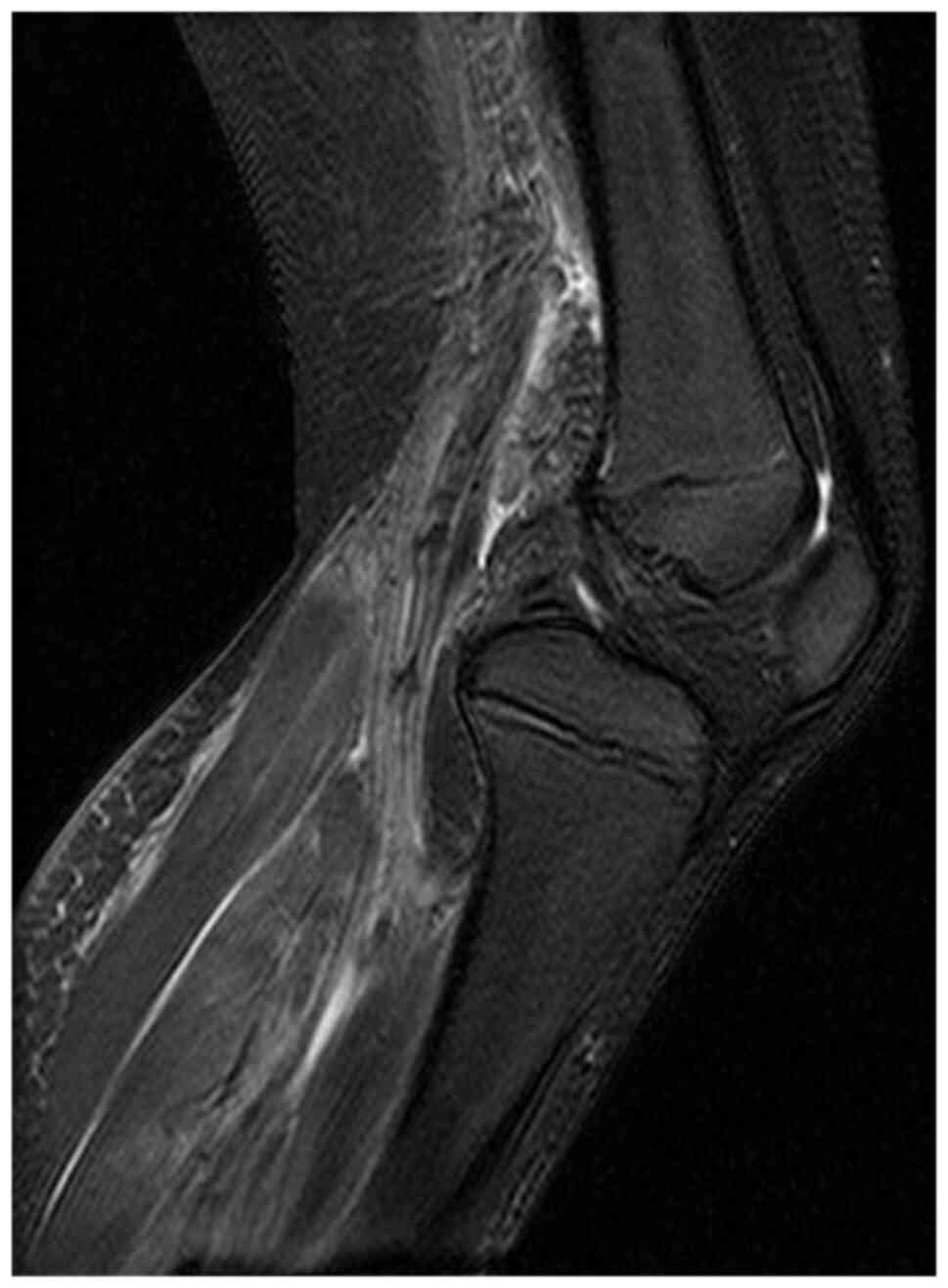
joint had not improved, magnetic resonance imaging (MRI) was
performed on day 9. However, the MRI scan only showed inflammatory
findings in the regions of the left soleus, left gastrocnemius, and
between the two muscles; arthritis or osteomyelitis was not evident
(Fig. 3). His condition improved
gradually with sufficient recovery in his legs, enabling him to
walk, and he was finally discharged on day 20 without sequelae
(Fig. 1Ch). No medication-related
adverse events were observed during or after treatment with the
combination therapy. During four years of follow-up, he did not
show any signs of disease, and his life seemed to have returned to
its regular rhythm prior to hospital admission.
Discussion
This case presents two important clinical findings.
First, the combination anticoagulant therapy of rhTM and danaparoid
for managing DIC in a child with STSS was effective. Several
reports have suggested that organ dysfunction, such as acute
respiratory distress syndrome and acute renal failure, is a
frequent complication in STSS (1,2,4).
Coagulopathy, which is the cause of organ dysfunction, is found in
approximately 70% of STSS cases (1,2). A
previous case report has described anticoagulant therapy
administered to a child for STSS. Intensive care was provided along
with combined administration of rhTM and heparin for managing STSS
in the 3-year-old boy, who, except for the amputation of his left
distal metatarsal, survived with normal development and no sequelae
(8). The mechanism of action of
the anticoagulant therapy in this reported case is similar to that
in ours (Fig. 4). The difference
lies in the selection of heparin rather than danaparoid as the
heparinoid. Unlike the anticoagulant profile of unfractionated
heparin (1:1), that of danaparoid is characterized by a high ratio
of anti-factor Xa to anti-factor IIa activity (≥20:1) (9); the clinical significance of this
difference in anticoagulant effect is yet to be elucidated.
Furthermore, the effective plasma concentration of rhTM estimated
in phase II clinical trials was 500 ng/ml. It took 72 h after
initial administration for this effective plasma concentration to
persist and remain steady in patients with DIC under 65 years of
age and patients with DIC with normal renal function (creatinine
clearance ≥70 ml/min) (10). A
prospective, clinical pharmacological study has reported the
evolution of rhTM plasma concentrations over time in patients with
DIC with acute renal dysfunction. Even when rhTM was administered
to patients with acute renal dysfunction at a normal dosage (380
U/kg/day), it took 72 h after initial administration for this
effective plasma concentration to persist (11). In our case, the progression of
coagulation laboratory findings was observed on day 2, his fever
had persisted as well, and his general condition was not improving.
This was when additional danaparoid was administered. Because this
combination therapy had a stronger anticoagulant effect than the
single-agent therapy, he recovered without requiring mechanical
ventilation or renal replacement therapy, which could have been
necessary if he was not treated accordingly.
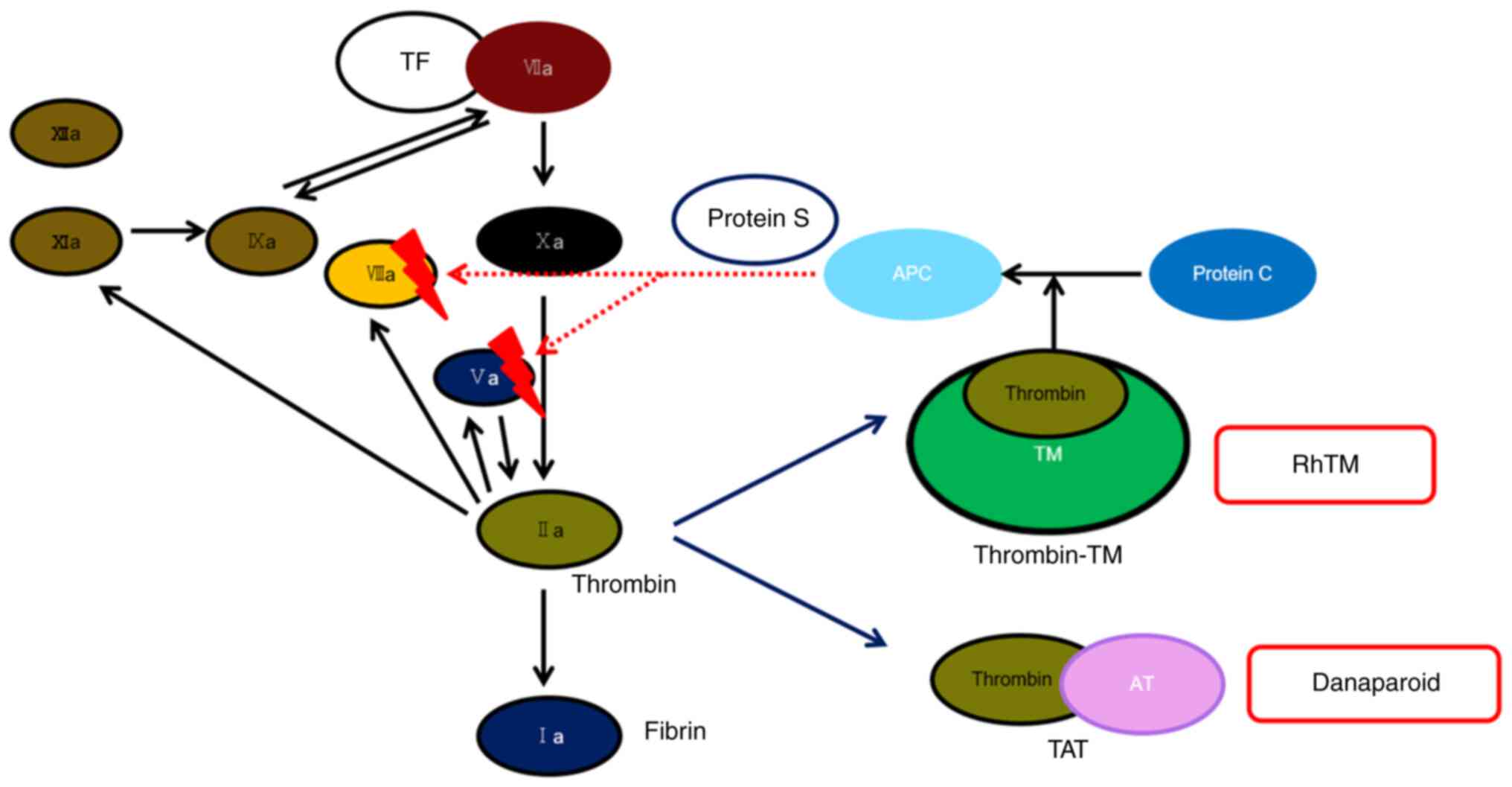 | Figure 4Conceptual diagram of the mechanism of
action of anticoagulant therapy in this case. RhTM binds to
thrombin and inhibits its functions. In addition, rhTM activates
protein C, which degrades and inactivates Va and VIIIa. The arrow
from rhTM indicates that protein C was activated and changed to
APC. Danaparoid binds to AT and changes its structure, causing AT
to bind coagulation factors, thrombin, VIIa, IXa, Xa, XIa, and
XIIa, and inhibit their reaction. Darkening of VIIa, IXa, Xa, XIa
and XIIa indicates their inactivation and the subsequent inhibition
of their reactions. This figure was created with reference to
several papers (17-20).
RhTM, recombinant human soluble thrombomodulin; Ia, fibrin; IIa,
thrombin; TF, tissue factor; Va, activated factor five; VIIa,
activated factor seven; VIIIa, activated factor eight; AT,
antithrombin; IXa, activated factor nine; Xa, activated factor ten;
XIa, activated factor eleven; XIIa, activated factor twelve; TM,
thrombomodulin; TAT, thrombin-antithrombin complex; APC, activated
protein C. |
Second, the combination anticoagulant therapy of
rhTM and danaparoid for managing DIC in a child with STSS was safe.
Safety of rhTM for managing DIC in adults has been established
(12). A retrospective cohort
study has analyzed the efficacy and safety of rhTM for managing DIC
in pediatric patients by comparing pediatric patients with DIC with
adult patients with DIC, evaluating post-marketing surveillance
study data. Newborn infants were excluded, after which data from
210 pediatric patients were analyzed and compared with data from
3786 adult patients. The results of this study have suggested that
the efficacy and safety of rhTM for managing DIC in pediatric
patients were not significantly different from those in adults. In
addition, 21.9% of pediatric patients with DIC who were
administered rhTM were also administered unfractionated heparin
(13). It can be assumed from
these data that the safety of the combined administration of rhTM
and heparin is not significantly low. In addition, safety can be
enhanced by using danaparoid instead of unfractionated heparin, as
demonstrated in this case. Danaparoid has a much higher
anti-Xa/anti-IIa ratio and shows minimal effects on platelet
function and thus has a relatively low risk of bleeding (9,14,15).
Therefore, this combination therapy could be used as an option for
disease states with rapid progression, concerning mortality rates,
and a high risk for sequelae, as is the case in DIC with STSS.
A retrospective cohort study has reported the
efficacy of anticoagulant therapies in sepsis. The results of this
study have suggested that anticoagulant therapies are significantly
associated with reduced mortality in subsets of patients diagnosed
with DIC. In addition, anticoagulant therapies have been
significantly associated with reduced mortality in the high-risk
subset stratified according to the Sequential Organ Failure
Assessment (SOFA) score: SOFA score 13-17(3). Because organ failure and coagulopathy
are common complications in STSS, anticoagulant therapies are an
important avenue. Furthermore, this retrospective cohort study has
reported the efficacy of rhTM in sepsis. As a result of this study,
DIC scores were significantly decreased in the group in which rhTM
was administered when compared with the scores from the no-rhTM
control group on day 3 after the administration of rhTM. In
addition, there was no difference in platelet counts between the
two groups on day 3, and the recovery of platelet counts in the
rhTM group was greater than that in the control group on day
7(16). In this case, additional
administration of danaparoid was started on day 2, and the DIC
score was decreased to 5 points on day 3. In our case, it is
possible that similar favorable outcomes could have been achieved
by the single-agent therapy of rhTM for managing DIC. However,
since several reports indicate high probabilities of mortality and
sequelae with monotherapy (1,2,4,8),
danaparoid addition to rhTM, and its potential improvement of
clinical outcomes, cannot be dismissed. Taken together, it was
quite possible that the combined administration of rhTM and
danaparoid at an early stage, i.e., on day 2, led to positive
outcomes in this case. However, future studies are required to
further examine this possibility due to the current lack of
research on the combination anticoagulant therapy of rhTM and
danaparoid in sepsis-related DIC. Since fibrinolysis is suppressed
in DIC with STSS or sepsis and the risk of coagulation-induced
organ damage is higher than that of fibrinolysis-induced bleeding,
this combination therapy may be considered in patients at high risk
of sequelae or death.
In conclusion, combination anticoagulant therapy of
rhTM and danaparoid for managing DIC in a child with STSS was
effective and safe. This combination therapy could possibly be used
as an option for rapidly progressive disease cases which present
with high risks for sequelae and death, such as DIC with STSS.
Furthermore, it is quite possible that this combination therapy
reduces the risk of serious complications, such as organ failure,
and improves prognosis, not only in children with STSS but also in
cases of sepsis in general.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YK was responsible for the patient’s treatment as
the attending physician, acquired, analyzed and interpreted the
data and drafted the manuscript. HKa and SO analyzed and
interpreted the data, critically revised the manuscript for
intellectual content and are accountable for all aspects of the
manuscript. SI and HKi contributed to the patient's treatment as
medical managers and analyzed and interpreted the data. YK, SI and
HKi confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Since rhTM and heparins are routinely used together
and due to the urgency of this case, ethics approval was not
required for this case study. Oral informed consent was obtained
from the patient's parent.
Patient consent for publication
Oral informed consent was obtained from the
patient's parent for the publication of the data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Demers B, Simor AE, Vellend H, Schlievert
PM, Byrne S, Jamieson F, Walmsley S and Low DE: Severe invasive
group A streptococcal infections in Ontario, Canada: 1987-1991.
Clin Infect Dis. 16:792–800; discussion 801-2. 1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rodríguez-Nuñez A, Dosil-Gallardo S and
Jordan I: ad hoc Streptococcal Toxic Shock Syndrome collaborative
group of Spanish Society of Pediatric Intensive Care. Clinical
characteristics of children with group A streptococcal toxic shock
syndrome admitted to pediatric intensive care units. Eur J Pediatr.
170:639–644. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yamakawa K, Umemura Y, Hayakawa M, Kudo D,
Sanui M, Takahashi H, Yoshikawa Y, Hamasaki T and Fujimi S: Japan
Septic Disseminated Intravascular Coagulation (J-Septic DIC) study
group. Benefit profile of anticoagulant therapy in sepsis: A
nationwide multicentre registry in Japan. Crit Care.
20(229)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stevens DL, Tanner MH, Winship J, Swarts
R, Ries KM, Schlievert PM and Kaplan E: Severe group A
streptococcal infections associated with a toxic shock-like
syndrome and scarlet fever toxin A. N Engl J Med. 321:1–7.
1989.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Defining the group A streptococcal toxic
shock syndrome. Rationale and consensus definition. The Working
Group on Severe Streptococcal Infections. JAMA. 269:390–391.
1993.PubMed/NCBI
|
|
6
|
Wada H, Takahashi H, Uchiyama T, Eguchi Y,
Okamoto K, Kawasugi K, Madoiwa S and Asakura H: DIC subcommittee of
the Japanese Society on Thrombosis and Hemostasis. The approval of
revised diagnostic criteria for DIC from the Japanese Society on
Thrombosis and Hemostasis. Thromb J. 15(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wong CH, Khin LW, Heng KS, Tan KC and Low
CO: The LRINEC (Laboratory Risk Indicator for Necrotizing
fasciitis) score: A tool for distinguishing necrotizing fasciitis
from other soft tissue infections. Crit Care Med. 32:1535–1541.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Okuzono S, Ishimura M, Kanno S, Sonoda M,
Kaku N, Motomura Y, Nishio H, Oba U, Hanada M, Fukushi JI, et al:
Streptococcus pyogenes-purpura fulminans as an invasive form
of group A streptococcal infection. Ann Clin Microbiol Antimicrob.
17(31)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meuleman DG: Orgaran (Org 10172): Its
pharmacological profile in experimental models. Haemostasis.
22:58–65. 1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tsuruta K, Yamada Y, Serada M and
Tanigawara Y: Model-based analysis of covariate effects on
population pharmacokinetics of thrombomodulin alfa in patients with
disseminated intravascular coagulation and normal subjects. J Clin
Pharmacol. 51:1276–1285. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hayakawa M, Kushimoto S, Watanabe E, Goto
K, Suzuki Y, Kotani T, Kiguchi T, Yatabe T, Tagawa J, Komatsu F and
Gando S: Pharmacokinetics of recombinant human soluble
thrombomodulin in disseminated intravascular coagulation patients
with acute renal dysfunction. Thromb Haemost. 117:851–859.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saito H, Maruyama I, Shimazaki S, Yamamoto
Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M
and Aoki N: Efficacy and safety of recombinant human soluble
thrombomodulin (ART-123) in disseminated intravascular coagulation:
Results of a phase III, randomized, double-blind clinical trial. J
Thromb Haemost. 5:31–41. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shirahata A, Mimuro J, Takahashi H, Tsuji
H, Kitajima I, Matsushita T, Eguchi Y, Kitamura N, Honda G and
Sakata Y: Postmarketing surveillance of recombinant human soluble
thrombomodulin (thrombomodulin α) in pediatric patients with
disseminated intravascular coagulation. Clin Appl Thromb Hemost.
20:465–472. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bradbrook ID, Magnani HN, Moelker HC,
Morrison PJ, Robinson J, Rogers HJ, Spector RG, Van Dinther T and
Wijnand H: ORG 10172: A low molecular weight heparinoid
anticoagulant with a long half-life in man. Br J Clin Pharmacol.
23:667–675. 1987.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hobbelen PM, Vogel GM and Meuleman DG:
Time courses of the antithrombotic effects, bleeding enhancing
effects and interactions with factors Xa and thrombin after
administration of low molecular weight heparinoid Org 10172 or
heparin to rats. Thromb Res. 48:549–558. 1987.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yamakawa K, Ogura H, Fujimi S, Morikawa M,
Ogawa Y, Mohri T, Nakamori Y, Inoue Y, Kuwagata Y, Tanaka H, et al:
Recombinant human soluble thrombomodulin in sepsis-induced
disseminated intravascular coagulation: A multicenter propensity
score analysis. Intensive Care Med. 39:644–652. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gando S, Levi M and Toh CH: Disseminated
intravascular coagulation. Nat Rev Dis Primers.
2(16037)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Weitz JI and Bates SM: New anticoagulants.
J Thromb Haemost. 3:1843–1853. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hirsh J, Warkentin TE, Shaughnessy SG,
Anand SS, Halperin JL, Raschke R, Granger C, Ohman EM and Dalen JE:
Heparin and low-molecular-weight heparin: Mechanisms of action,
pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest.
119 (Suppl):64S–94S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wilde MI and Markham A: Danaparoid. A
review of its pharmacology and clinical use in the management of
heparin-induced thrombocytopenia. Drugs. 54:903–924.
1997.PubMed/NCBI View Article : Google Scholar
|