Introduction
Ischemic stroke is a clinically common and dangerous
disease with the second highest morbidity, mortality and disability
rates worldwide (1). The most
serious hazard of ischemic stroke is neuronal apoptosis (2). Mitochondria are important organelles
that can generate oxidative stress and dysfunction in response to
ischemia and hypoxia events (3-6).
This series of pathological processes decreases the anti-apoptotic
protein Bcl-2 level, increases the pro-apoptotic protein Bax level
and enhances the expression of cleaved caspase-3 (7-10).
Neuronal cells with high energy depletion undergo large-scale
apoptosis (11). While it takes a
long period for cells to undergo apoptosis, drug treatment of
transient focal cerebral ischemia is possible (12).
Gastrodia elata (GE) Blume is a common and
valuable traditional Chinese medicine, mainly produced in central
and south China (13). It has been
used to treat lumbago, headache, epilepsy, paralysis, rheumatism
and other diseases for thousands of years and has been recorded in
the Chinese pharmacopoeia (14).
GE has been widely studied in the central nervous system (CNS)
because it improves ischemia in several in vitro and in
vivo models (15-19).
It has been reported that GE extract can protect the dopaminergic
cell toxicity induced by 1-methyl-4-phenylpyridinium through
antioxidation and anti-apoptosis activities (20). In addition, several compounds
present in GE, such as 4-hydroxybenzyl alcohol, vanillin,
parisonoside C and 3,4-dihydroxybenzaldehyde, have been shown to
have strong neuroprotective effects in cerebral ischemia (21-23).
Therefore, several studies have focused on the effect of different
components of GE on the CNS (24,25).
P-hydroxybenzaldehyde (PHBA) is one of the
compounds isolated from GE and has various pharmacological effects,
such as antioxidant, anti-inflammatory and vasodilation effects
(26-28).
Due to its lipid solubility and small molecule characteristics,
PHBA is likely to pass through the blood-brain barrier (BBB) to
exert a therapeutic role on the CNS (29,30).
Simultaneously, the structure of its C-4-aldehyde and hydroxyl
groups can regulate the neurotransmission function in the brain
(31). Zhu et al (30) replicated the model of oxidative
damage to the BBB by co-culturing mouse brain microvascular
endothelial cells and astrocytes, and confirmed that PHBA has a
protective effect on oxidative stress-induced BBB damage. Moreover,
PHBA increases the expression of antioxidant-related proteins and
improves the endogenous antioxidant capacity of cells (30).
Kim et al (23) revealed that PHBA increases neuronal
survival by 43.21% in the reperfusion model of the common carotid
artery replicated in Mongolian gerbils, although the study did not
clarify the relevant mechanism underlying its neuroprotective
activity. In vitro experiments showed that in the
oxygen-glucose deprivation/reperfusion model, PHBA helps regain the
lost neurons in the ischemic area by promoting the reprogramming
conversion of astrocytes to neurons (32).
Therefore, PHBA has an important potential in the
neuroprotection of cerebral ischemia, although its mechanism of
action remains clear. Mitochondria play a critical role in the
course of transient focal cerebral ischemia (33). Mitochondria-targeted therapy has
shown promising results in the treatment of cerebral ischemia
(34). In contrast to previous
studies, the present study aimed to explore the protective effect
of PHBA on middle cerebral artery occlusion (MCAO) model in rats
based on mitochondrial function.
Materials and methods
Animals
A total of 56 male Sprague-Dawley rats
(5-8-weeks-old; 250-350 g weight), were purchased from the Sichuan
Central Animal Experimental Base, China [Laboratory animal
qualification certificate: scxk (Chuan) 2018-24]. The rats were
given free access to food and water; the rearing environment was
maintained in a 12-h light/dark cycle with temperature (18-25˚C)
and relative humidity (40-60%). All animal experiments were
approved by the Animal Ethics Committee of Yunnan University of
Traditional Chinese Medicine, Yunnan, China (approval no.
R-062019039) and the care and use of experimental animals were
carried out in accordance with the guidelines of the American
National Institutes of Health (35). All efforts are made to reduce the
number and the suffering of the animals.
Experimental schedule and MCAO model
in rats
Rats were equally divided into four groups: Control
(sham operation group), MCAO, PHBA-high dose (PHBA-H; 11.1 mg/kg
PHBA) and PHBA-low dose (PHBA-L; 0.74 mg/kg PHBA) groups by random
number table method. PHBA (cat no. DRE-C14228740; CAS:123-08-0;
J&K Scientific, Ltd.) was administered intragastrically for 5
days (once daily) before the experiment on the MCAO rat model.
Moreover, control and MCAO groups were given an equivalent volume
of normal saline. Then, the cortex area in the ipsilateral
hemisphere was chosen for experimental procedures.
In the present study, the operation method of MCAO
was selected to establish the model, which resulted in cerebral
ischemia injury in rats. Rats were anesthetized using 10% chloral
hydrate intraperitoneal injection (cat. no. 20141012; Damao
Chemical Reagent Factory) at a dose of 300 mg/kg weight (36). After routine disinfection, the
following steps were undertaken: i) The skin was cut along the
midline of the neck; ii) the muscle and fascia were separated layer
by layer; iii) the vagus nerve was separated from the common
carotid artery on the right; iv) the external carotid artery was
ligated with nylon filament; and v) the middle cerebral artery was
blocked by gently introducing 0.26-mm nylon filament (Ruibo
Biotechnology Co., Ltd.) through the right common carotid artery.
After 2 h of occlusion, the blood flow of the anterior cerebral
artery was affected, resulting in local middle artery ischemia.
After removing the occlusion, the blood flow of the middle cerebral
artery was restored and the MCAO model was established in normal
rats. In the sham group, only the right internal and external
carotid branches were separated after anesthesia and the middle
cerebral artery was not blocked. The temperature of the rats was
maintained at 37.0±0.5˚C during and after the operation. The MCAO
model was assessed by laser speckle imaging (RWD Life Science Co.,
Ltd.). The state of the rats was closely observed during and after
the modeling operation. During this period, none of the animals
developed humane endpoint indications, such as non-feeding,
dyspnea, convulsion and hypothermia, or died prematurely. At the
time of sampling, one rat in each group was randomly selected to
dissect the abdomen and no symptoms of peritonitis were
observed.
General state of rats
After 6 and 24 h of MCAO model reperfusion, the
neurological deficit score (Table
I) was evaluated using the improved method of Bederson
(37,38).
 | Table INeurological deficit score
parameters. |
Table I
Neurological deficit score
parameters.
| | Score |
|---|
| Parameters | 0 | 1 | 2 |
|---|
| Flexion degree of
forelimb | No flexion | Wrist flexion | Wrist and elbow
flexion |
| Floor walking | Straight path | Curvilinear
path | Walking in circles
only |
| Response to
vibration | Sensitive
response | Weak response | No response |
| Body rotation | No rotation | Seldom
rotation | Frequent
rotation |
After 24 h of MCAO, anesthesia was induced by 5%
isoflurane inhalation (RWD Life Science Co., Ltd.) and maintained
with 3% isoflurane (39).
Subsequently, rats entered deep anesthesia and had no response to
tail pinching. The animals were rapidly sacrificed using a
decapitation device. Brain tissue samples were collected and
weighed to calculate the cerebral index as follows: Cerebral
index=wet weight of the brain/weight of the body. The body weight
before MCAO and 24 h after MCAO were recorded. The weight loss
percentage=[(weight before modeling-weight after modeling)/weight
before modeling] x100.
To calculate the volume of cerebral infarction,
brain tissue was removed and cut into four coronal sections (2-mm
thick) 24 h after successful modeling and stained with 0.5%
triphenyltetrazolium chloride (TTC; Sigma-Aldrich; Merck KGaA) at
37˚C for 30 min (40). A digital
camera (Canon 600D; Canon, Inc.) was used to record the staining
image and Motic image plus 2.0 (Motica China Group Co., Ltd.) was
used for quantification analysis. Furthermore, brain edema may
greatly affect the volume calculation of cerebral infarction as
follows: The volume of infarcted=total volume of infarcted x left
hemisphere volume/right hemisphere volume. The infarction rate was
calculated as follows: Infarction (%)=(volume of
infarcted/bilateral hemisphere volume) x100(41).
Cerebral neuronal cell apoptosis
assay
H&E staining was performed using a H&E kit
(cat. no. KGA224; KeyGEN Biotech Co., Ltd.). The brain tissue was
fixed in 4% paraformaldehyde at room temperature overnight,
embedded in paraffin and cut into 5-µm paraffin sections of brain
tissue. The paraffin sections were placed in an electric heating,
constant temperature, drying oven and baked at 60˚C for 3 h. The
dried paraffin sections were dewaxed with conventional xylene at
room temperature, hydrated with a descending gradient of ethanol
and washed with distilled water. The nuclei were stained with
hematoxylin for 2 min at room temperature, differentiated with 1%
hydrochloric acid alcohol for several seconds, washed with water
and returned to blue. Then, the slides were stained with eosin
staining solution for 1 min at room temperature, and the residual
staining solution was washed away with water. Subsequently, the
sections were dehydrated with graded alcohol, made transparent in
xylene and sealed with neutral gum. A field of view was randomly
selected using a phase contrast microscope (Olympus Corporation)
and stained specimens were visualized at 400x magnification.
For the TUNEL immunofluorescence assay, the brain
tissue was fixed in 4% paraformaldehyde at room temperature
overnight, embedded in paraffin and cut into 5-µm paraffin sections
of brain tissue. The paraffin sections were placed in an electric
heating, constant temperature, drying oven and baked at 60˚C for 60
min. Then the sections were dewaxed twice with xylene, hydrated
with descending ethanol series (100, 95, 80 and 75% ethanol) for 5
min each and rinsed three times in 1X PBS for 5 min each. Then, 100
µl ready-to-use Proteinase K solution (10 µl of 10X Proteinase K in
90 µl 1X PBS per sample; Takara Biotechnology Co., Ltd.) was added
dropwise to each tissue section and left to react at 37˚C for 30
min. The sections were rinsed three times in 1X PBS for 5 min each
and 50 µl TUNEL detection solution (cat. no. C1090; Beyotime
Institute of Biotechnology), prepared in a ratio of 5 µl terminal
deoxynucleotidyl transferase enzyme and 45 µl fluorescent labeling
solution, was added to each sample and incubated at 37˚C for 60 min
in the dark. After rinsing with PBS for 5 min three times, the
sections were counterstained with DAPI. The sections were rinsed 3
times with PBS again, and dehydrated with graded alcohol, made
transparent in xylene and sealed with neutral gum. The images were
observed and captured under a laser confocal microscope at 400x
magnification (Zeiss LSM; Zeiss AG).
Western blotting analysis
Western blotting was performed to measure the
protein levels of Bc1-2, Bax and caspase-3 in brain tissue. Total
protein was extracted from brain tissue samples using RIPA lysis
buffer (cat. no. P0013C; Beyotime Institute of Biotechnology). The
lysate was centrifugated at 4˚C and 14,300 x g for 10 min and the
supernatant were collected. After determining and adjusting protein
concentration using the Enhanced BCA Protein Assay kit (cat. no.
P0010; Beyotime Institute of Biotechnology), the different mass
fractions of protein (50 µg/lane) were separated via SDS-PAGE on a
10% gel (Criterion™; Trans-Blot®; Bio-Rad
Laboratories, Ltd.). Then, the proteins were transferred onto a
PVDF membrane (Bio-Rad Laboratories, Ltd.) and the membrane was
blocked in 5% skimmed milk at room temperature for 2 h. After
blocking, the membrane was washed two/three times with TBST buffer
(0.1% Tween; cat. no. QN1236; Beijing Biolab Technology Co., Ltd.).
Subsequently, the membrane was incubated with the following primary
antibodies at 4˚C overnight: Anti-Bax (1:5,000; cat. no.
50599-2-Ig; Proteintech Group, Inc.), anti-Bcl-2 (1:1,000; cat. no.
26593-1-AP; Proteintech Group, Inc.) and anti-caspase-3 (1:1,000;
cat. no. 9662; Cell Signaling Technology, Inc.) and β-actin
(1:1,000; cat. no. 4967; Cell Signaling Technology, Inc.).
Following primary antibody incubation, a volume of 200 µl
horseradish peroxidase (HRP) was added to the surface of the
membrane to make it uniformly adhere to the substrate. The membrane
was incubated with the goat anti-rabbit IgG secondary antibody
(1:5,000; cat. no. ab6721; Abcam) at room temperature for 1 h.
Finally, the enhanced chemiluminescence (ECL; cat. no. A38555;
Thermo Fisher Scientific, Inc.) was added to visualize the immune
response bands in the Bio-Rad ChemiDoc™ XRS gel imaging
system (Bio-Rad Laboratories, Ltd.). Image Lab™ V4.0
software (Bio-Rad Laboratories, Ltd.) was used to detect the
optical signal and to quantify the protein levels. All the
experimental groups were divided into three parallel groups and the
experiment was repeated three times and the data were averaged.
Measurement of mitochondrial oxidative
stress indicators
High-purity mitochondria were extracted according to
the instructions of the mitochondrial extraction kit (cat. no.
SM0020; Beijing Solarbio Science & Technology Co., Ltd.), and
assayed using reactive oxygen species (ROS) detection kits (cat.
no. E004-1-1, Nanjing Jiancheng Bioengineering Institute) with an
in situ loading probe. The mitochondrial pellet was
resuspended in diluted (1:1,000) 2,7-dichlorodi-hydrofluorescein
diacetate (DCFH-DA; cat. no. CA1410, Beijing Solarbio Science &
Technology Co., Ltd.) and incubated at 37˚C for 25 min. The
supernatant from probe-labeled mitochondrial suspension was
collected by centrifugation at 1,000 x g for 5-10 min and then
discarded, while the pellet was collected. The mitochondria were
washed one/two times with PBS to adequately remove the excess of
DCFH-DA Fluorescence intensity was measured at an excitation
wavelength of 488 nm and emission wavelength of 525 nm in each well
using on a microplate reader (Thermo Fisher Scientific, Inc.).
Subsequently, the content of peroxidated lipids was
estimated by colorimetry at 532 nm using the micro malondialdehyde
(MDA) assay kit (cat. no. BC0025; Beijing Solarbio Science &
Technology Co., Ltd.).
The right ischemic cerebral tissue was collected 24
h after reperfusion and then homogenized. After centrifugation at
1,000 x g and 4˚C for 10 min, total superoxide dismutase (T-SOD)
activity was measured in the supernatant using the total superoxide
dismutase assay kit (hydroxylamine method; cat. no. A001-1-2;
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions.
Estimation of mitochondrial
dysfunction indicators
The protein concentration of the freshly prepared
mitochondrial suspension was adjusted to 10 mg/ml using the BCA
method (cat. no. PC0020; Beijing Solarbio Science & Technology
Co., Ltd.). The mitochondrial swelling assay was performed using a
purified mitochondrial swelling colorimetric assay kit (cat. no.
GMS10101; Shanghai Genmed Technology Co., Ltd.) according to the
manufacturer's instructions. The 20 µl of fresh mitochondrial
suspension and 170 µl of GENMED buffer from the kit were
proportionally added to a 96-well plate and measured at 520 nm for
0 min absorbance. After 1 min, 10 µl of GENMED expansion solution
was added and measured again after 10 min to obtain 10 min
absorbance. Actual absorbance=0 min absorbance-10 min
absorbance.
High-purity mitochondria were thawed from -80˚C, 400
µl of the extract (a salt-containing buffer) from the kit below was
added to the purified cerebral mitochondria, followed by
ultrasonication (ice bath, 200 W, ultrasonication for 5 sec with an
interval of 10 sec, fifteen times) to measure the enzyme activity
assay of complex IV (cytochrome C oxidase) using a Mito Check
complex IV activity assay kit (cat. no. BC0945, Beijing Solarbio
Science & Technology Co., Ltd.) according to the manufacturer's
instructions.
To measure the ATP content, 5 µl of high-purity
cerebral mitochondria extract was mixed with 80 µl boiling double
distilled water and boiled for 10 min for mitochondria lysis. The
lysate was centrifugated at 3,500 x g and 4˚C for 10 min, the
supernatant was collected and the absorbance was recorded at 570 nm
on a microplate reader to measure the ATP content using an ATP
assay kit (cat. no. A016-1, Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's instructions.
Transmission electron microscopy
Rat brains were removed quickly following euthanasia
and cut into 1-mm3 sections before fixing via immersion
in 4% pre-cooled glutaraldehyde at 4˚C for 4 h. Then, the specimens
were fixed with 1% osmic anhydride (cat. no. GP18456; Beijing
Zhongjingkeyi Technology Co., Ltd.) at 4˚C for 2 h, followed by
stepwise dehydration with gradient alcohol and acetone: 50% Alcohol
for 10 min, 70% alcohol for 10 min, 80% acetone for 10 min two
times, 90% acetone for 10 min two times and anhydrous acetone for
10 min two times. After dehydration, the sections were embedded in
epoxy resin and polymerized at 60˚C incubator for 48 h. Ultra-thin
sections were cut at a thickness of 100-nm using a microtome and
stained with uranium acetate and lead citrate (cat. nos. GZ02625
and GA1070; Beijing Zhongjingkeyi Technology Co., Ltd.) for 15 min
each at room temperature. Images of the ultrastructure of the
mitochondria were observed and captured using transmission electron
microscopy.
Statistical analysis
Data were analyzed using GraphPad Prism 9.0 software
(GraphPad Software, Inc.). Data are presented as mean ± standard
deviation. Data conform to the normal distribution, if the variance
is homogeneous, multiple comparisons were performed using the ANOVA
test followed by Bonferroni's correction. Multiple comparisons
among data with unequal variance were performed using the Welch's
ANOVA test followed by the Dunnett's T3 post hoc test. Multiple
comparisons among abnormally distributed data were performed using
the Kruskal-Wallis test followed by the Dunn's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
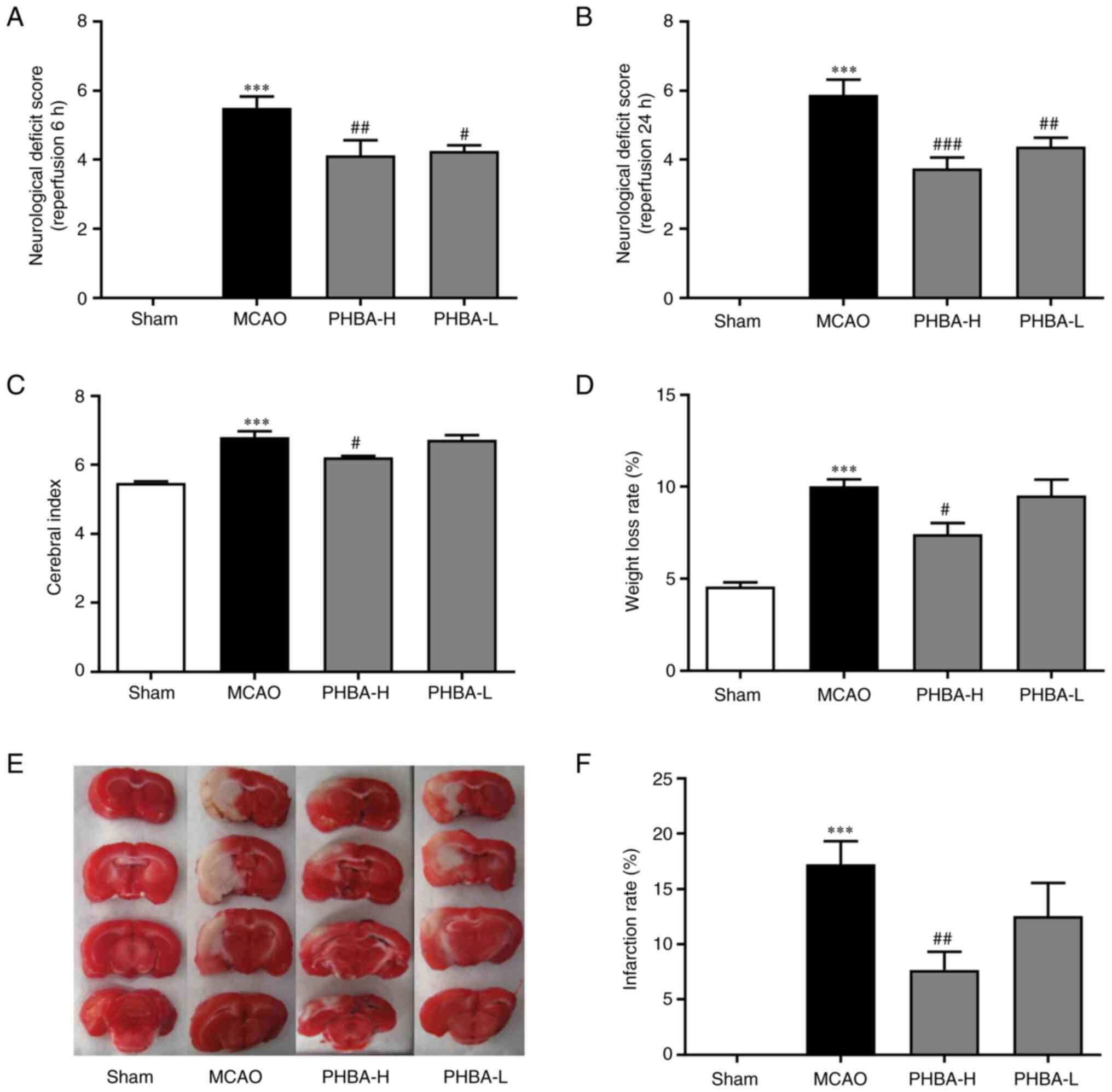
PHBA reduces infarct volume
Compared with the sham group, the MCAO group showed
high neurological deficit scores at 6 and 24 h after reperfusion,
as well as high cerebral index, weight loss rate and infarction
rate (all P<0.001; Fig. 1). The
neurological deficit scores at 6 (P<0.01; Fig. 1A) and 24 h (P<0.001; Fig. 1B) after reperfusion, cerebral index
(P<0.05; Fig. 1C), weight loss
rate (P<0.05, Fig. 1D) and
infarction rate (P<0.01; Fig.
1F) of in the PHBA-H group were lower compared with those in
the MCAO group. With the exception of the neurological deficit
score at 6 (P<0.05 vs. MCAO; Fig.
1A) and 24 h after reperfusion (P<0.01 vs. MCAO; Fig. 1B), no significant differences were
observed between PHBA-L and MCAO groups in all the remaining
indicators.
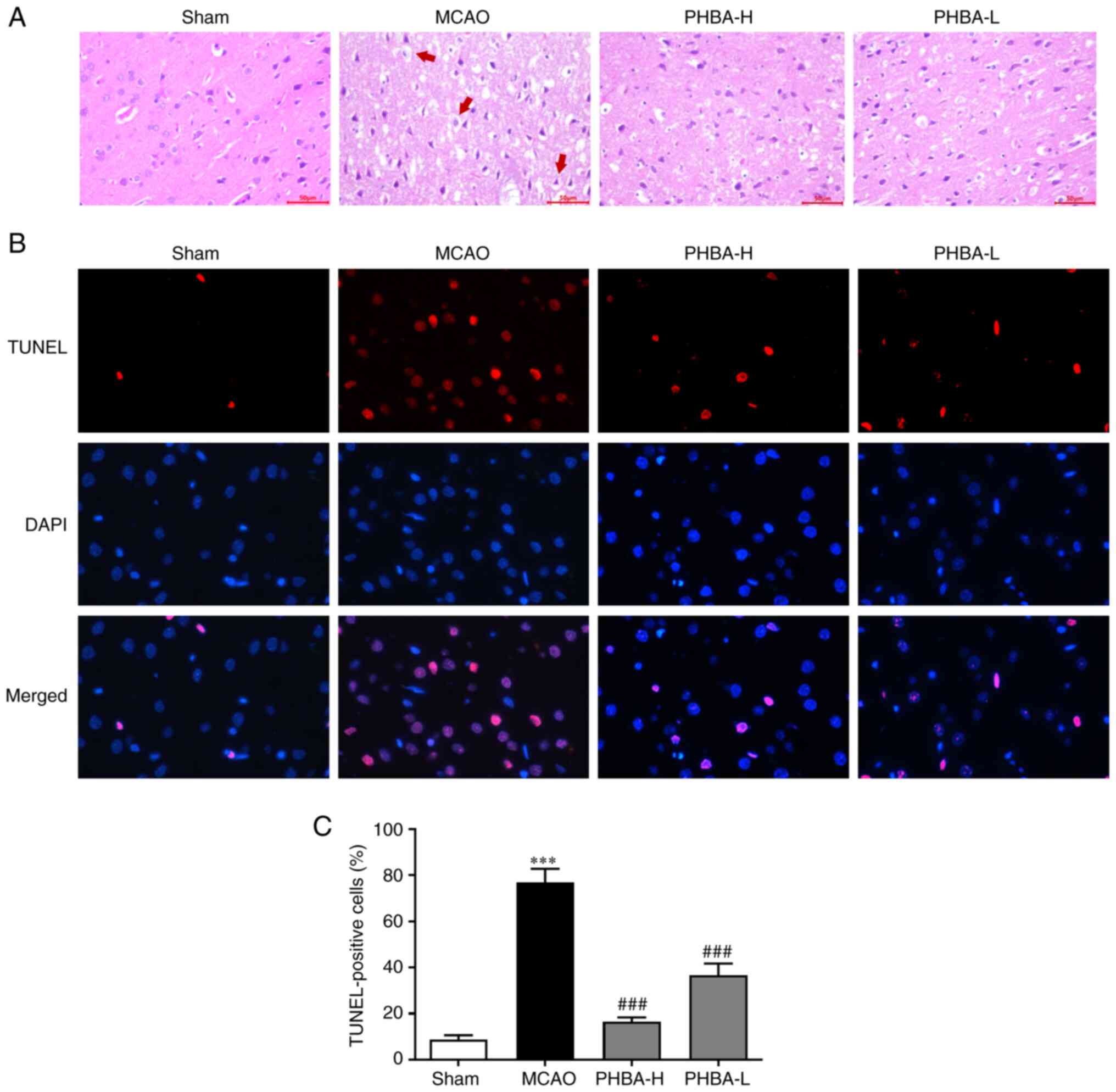
PHBA reduces neuronal apoptosis
induced by MCAO
The results of H&E staining showed that the
brain tissue of the rats in the sham group was intact, the cells
were arranged orderly, the shape was normal and the nuclei were
clear and intact (Fig. 2A). The
brain tissue in the MCAO group had edema, the structure gap was
widened and the cells showed pathological conditions, such as cell
body dissolution, nuclear pyknosis and fragmentation and disordered
arrangement of nerve fibers (Fig.
2A). The pathological conditions of the brain tissue in the
PHBA-H and PHBA-L groups were improved and the changes in cell
morphology were alleviated (Fig.
2A).
According to the results of TUNEL immunofluorescence
assay, the cells showed red fluorescence, nuclei showed blue
fluorescence and the positive cells showed rose-red fluorescence
after the images were merged (Fig.
2B). Compared with the sham group, the number of positive
cells/mm2 in the brain tissue in the MCAO group was
significantly increased (P<0.001 vs. sham; Fig. 2C). The number of positive
cells/mm2 in the PHBA-H and PHBA-L rats was
significantly lower compared with in the MCAO group (P<0.001;
Fig. 2C).
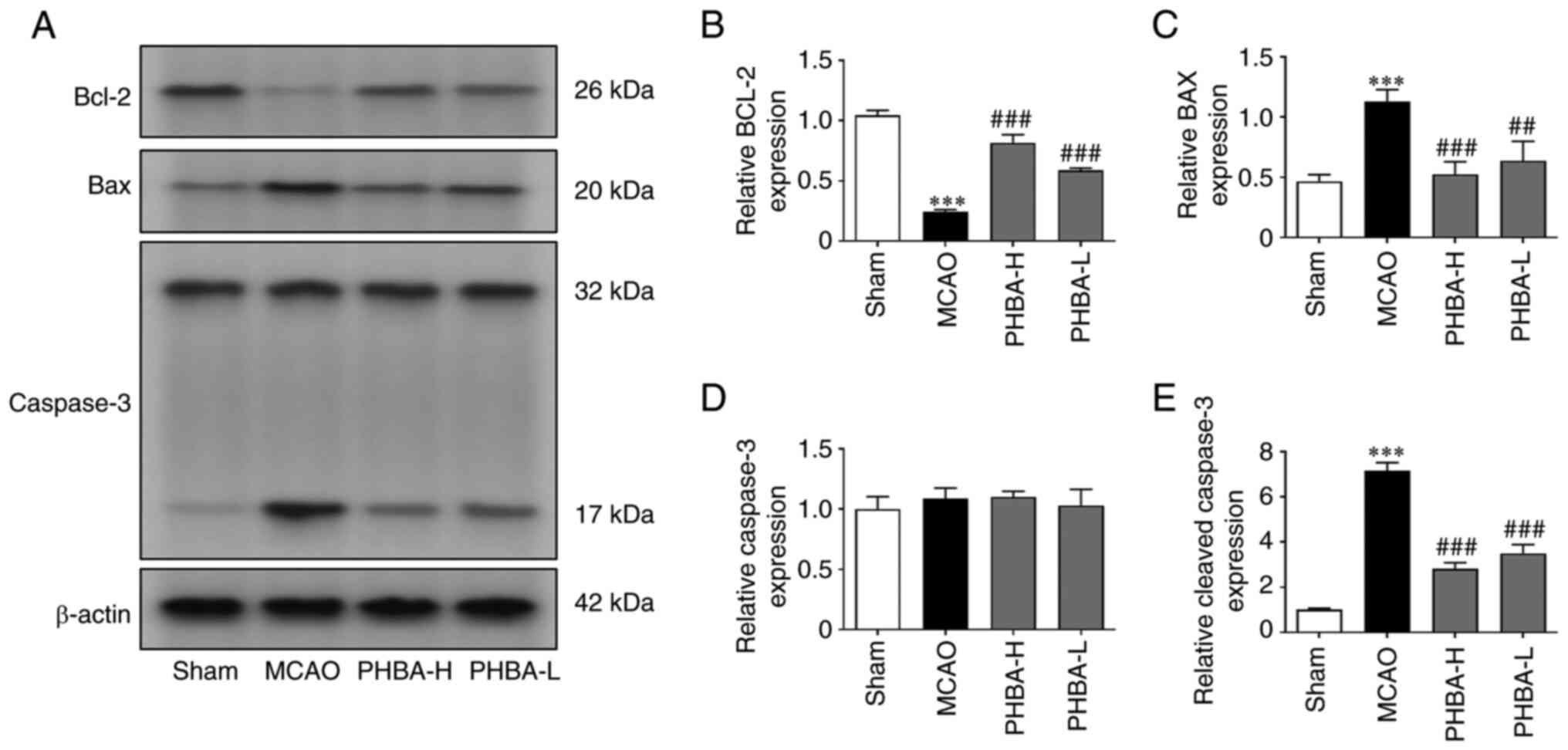
Altered expression of Bcl-2, Bax and
cleaved Caspase-3 in brain mitochondria
Compared with the sham group, the expression levels
of cleaved caspase-3 and Bax were significantly higher in the MCAO
group, while the levels of Bcl-2 were significantly lower
(P<0.001 for all; Fig. 3).
PHBA-H and PHBA-L groups showed significantly higher expression of
Bcl-2 and lower expression of cleaved caspase-3 and Bax compared
with the that in the MCAO group (P<0.001 for all; Fig. 3), while no significant difference
was observed in the protein expression of Caspase-3 between these
groups (Fig. 3D).
Effects of PHBA on indicators of
mitochondrial oxidative stress and dysfunction
Compared with the brain mitochondria in the sham
group, the activities of T-SOD (P<0.001; Fig. 4A), cytochrome C oxidase
(P<0.001; Fig. 4F) and the
content of ATP (P<0.05; Fig.
4E) were significantly decreased in the MCAO group, while the
levels of ROS (P<0.001; Fig.
4B) and MDA (P<0.05; Fig.
4C), as well as the opening degree of mitochondrial
permeability transition pore (MPTP; P<0.001; Fig. 4D) were significantly increased. The
levels of ROS (P<0.001; Fig.
4B) and MDA (P<0.001; Fig.
4C) and the degree of MPTP opening (P<0.001; Fig. 4D) in the PHBA-H group were
significantly lower compared with those in the MCAO group, while
the activities of T-SOD (P<0.05; Fig. 4A) and cytochrome C oxidase
(P<0.05; Fig. 4F), as well as
the content of ATP (P<0.05; Fig.
4E) were significantly higher compared with those in the MCAO
group. Compared with the MCAO group, the activity of T-SOD and
cytochrome C oxidase, as well as the content of ATP and MDA of
PHBA-L, were not significantly different, while the levels of ROS
(P<0.001; Fig. 4B) and the
degree of MPTP opening (P<0.001; Fig. 4D) were significantly improved.
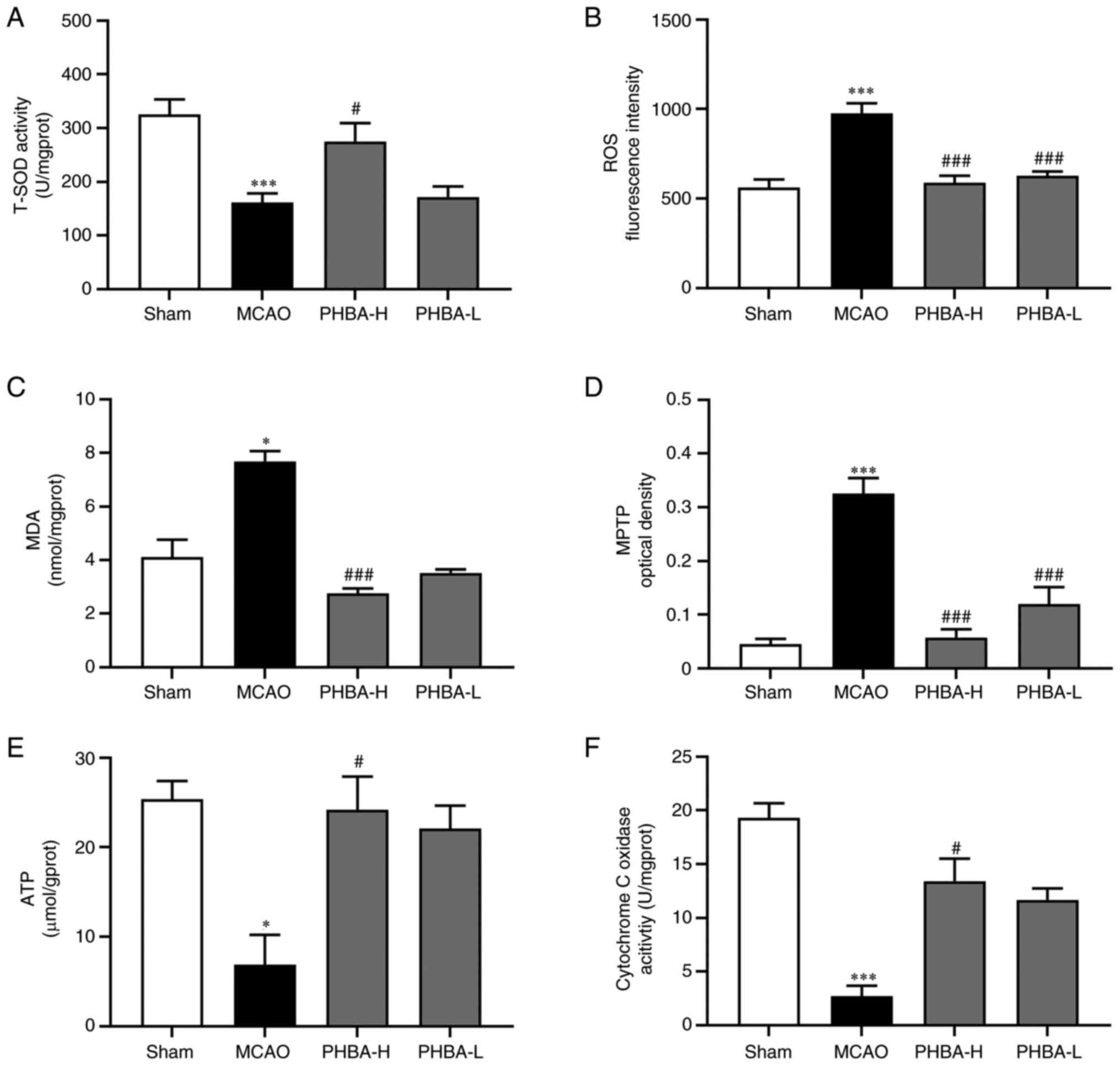 | Figure 4PHBA alleviates oxidative stress and
dysfunction in mitochondria in the MCAO model. Changes in the (A)
T-SOD activity, (B) level of ROS and (C) MDA, (D) the opening
degree of MPTP, (E) ATP level and (F) cytochrome C oxidase activity
after PHBA treatment were detected with the corresponding kits.
Data are presented as indicated by mean ± standard deviation.
*P<0.05 and ***P<0.001 vs. sham;
#P<0.05, ###P<0.001 vs. model. PHBA,
P-hydroxybenzaldehyde; MCAO, middle cerebral artery
occlusion; PHBA-H, PHBA-high dose; PHBA-L, PHBA-low dose; T-SOD,
total superoxide dismutase; ROS, reactive oxygen species; MDA,
malondialdehyde; MPTP, mitochondrial permeability transition
pore. |
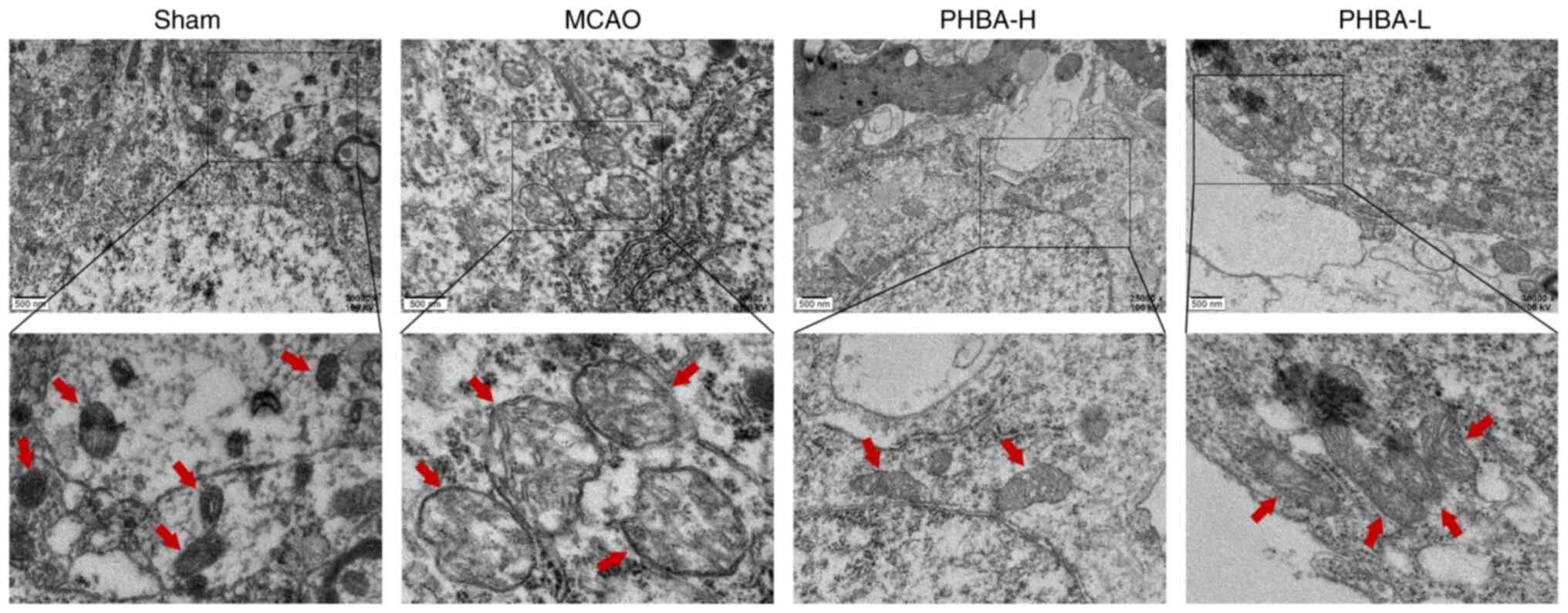
PHBA improves the ultrastructural
changes in the mitochondria
The electron microscopy results (Fig. 5) showed that the morphology of the
mitochondria in the sham group was normal, with intact membranes,
dense mitochondrial cristae and uniform staining of the matrix
pattern. By contrast, the mitochondrial morphology of the MCAO
group was severely impaired. The mitochondrial membrane shrunk, the
cristae were sparse and fractured and the matrix was
electron-lucent. Interestingly, the PHBA-H and PHBA-L groups
improved the ultrastructure of the mitochondria. The mitochondrial
morphology and membrane integrity were restored, the density of the
matrix decreased minimally and the majority of mitochondrial
cristae were intact, especially in the PHBA-H group.
Discussion
A previous study has shown that GE extract has
significant antioxidant and anti-apoptotic properties (42). To date, the majority of studies
have focused on the pharmacological activity and effects of
gastrodin on the CNS (43).
Previous studies, performed on >10 phenolic compounds isolated
from GE, have shown that the abundant phenolic compounds in GE can
enhance the endogenous antioxidants and improve disease prognosis
in transient focal cerebral ischemia (44-46).
The majority of studies have focused on the pharmacological effects
of gastrodin in GE on CNS-related mechanisms (47-49).
However, the effect of the large number of phenolic compounds in GE
on the nervous system remains unclear. Although a previous
pharmacokinetic study showed that PHBA in GE has brain targeting
activity during drug distribution (50), its effect and mechanism on
transient focal cerebral ischemia remains unclear. At present, PHBA
can already be synthesized artificially (51). The preliminary experiment of our
research group confirmed that the commercially available PHBA is
consistent with the PHBA isolated from GE (52). The MCAO model is consistent with
the course of transient focal cerebral ischemia and is often used
to evaluate the efficacy of the treatment of stroke and its
mechanism (53,54). Therefore, the present study aimed
to use the MCAO model to study the effect of PHBA on transient
focal cerebral ischemia, its correlation with mitochondrial
dysfunction and oxidative stress, as well as the effect on the
protein expression of Bcl-2, Bax and cleaved caspase-3.
Cerebral edema in ischemic penumbra leads to severe
consequences and adverse clinical events post-ischemic brain injury
(55). In the present study the
cerebral index was used to show the severity degree of
encephaledema. CNS injury was assessed by neurological deficit
score and infarct area using TTC staining (56). H&E staining revealed the
morphological changes of brain cells during apoptosis after
transient focal cerebral ischemia. TUNEL immunofluorescence was
used to evaluate cell apoptosis during cerebral ischemia.
Mitochondria are the major organelles involved in
the cellular respiration, controlling the oxidation of substances
to generate the energy needed for cell survival (32). Cerebral ischemia triggers
mitochondrial dysfunction leading to oxidative stress, which
stimulates O2 to lose electrons to form a large amount
of ROS (57). Under normal
physiological conditions, ROS regulates the operation of the
mitochondrial redox signaling pathway (58). However, when transient focal
cerebral ischemia occurs, T-SOD, a key antioxidant enzyme, cannot
normally scavenge excess oxygen free radicals due to its reduced
activity, resulting in excessive ROS-oxidized lipids in the
mitochondria (59). MDA is a
product of lipid peroxidation that directly reflects the level of
oxidative stress (60-62).
This change causes oxidative stress, alters mitochondrial membrane
permeability and releases pro-apoptotic factors into the cytoplasm
that in turn promote neuronal apoptosis (63). In addition, excess O2 is
consumed to destroy the supply of the respiratory chain, ATP
content drops sharply and energy metabolism is abnormal. It affects
the structural integrity and function of the mitochondria,
prompting the organelle to function appropriately (64).
MPTP reflects the structure and function of the
mitochondria and it is located between the inner and outer
membranes of the organelle (65).
The lipid oxidative stress damage to the mitochondrial membrane
leads to the abnormal opening of MPTP, which cannot maintain the
normal potential difference of the inner membrane, while the
oxidative phosphorylation on the inner membrane cannot proceed
smoothly (66,67). The cytochrome C oxidase is also
known as respiratory chain complex IV and it is embedded in the
bilayer lipid membrane of the inner mitochondrial membrane
(68). As a key proton pump for
energy generation in the respiratory chain, MPTP transfers the
H+ of cytochrome C to O2 and reduces it to
H2O (69,70). In the MCAO model established in the
present study, MPTP was opened and cytochrome C oxidase activity
was reduced. These two changes resulted in the loss of the
H+ gradient in the inner membrane, disruption of the
oxidative phosphorylation of the respiratory chain and impairment
of the normal synthesis and supply of ATP. Because of the lack of
energy supply, neuronal cells reached apoptosis rapidly.
Previous studies have shown that Bcl-2/Bax mainly
control the apoptosis of neuronal cells by acting on mitochondria
and the protein dimer formed by Bcl-2/Bax is the main component of
MPTP (71). When the expression of
Bax is higher compared with that of Bcl-2 after transient focal
cerebral ischemia, the MPTP is opened, facilitating the passage of
apoptotic factors into the cytoplasm to promote apoptosis, as well
as leading to mitochondrial dysfunction and abnormal transport of
the ion channels (72,73). Bcl-2 family members are a members
of the apoptosis regulatory proteins: Anti-apoptosis protein Bcl-2
and pro-apoptosis protein Bax (74). Bcl-2 is the most widely studied
anti-apoptotic gene (75). Its
high expression can directly alleviate to oxidative stress injury,
inhibit ROS production and regulate mitochondrial membrane
permeability to maintain mitochondrial homeostasis and prevent
apoptosis (76). Finally, it
improves ischemia-induced neuronal apoptosis (77). However, Bax can activate ion
channels on the mitochondrial membrane and some small molecular
substances, such as cytochrome C leak into the cytoplasm, causing
cell damage (78). Under normal
circumstances, the proportion of the Bcl-2/Bax is maintained in a
certain range (79). An imbalance
in the proportion of the two molecules leads to apoptosis (80). The high levels of Bax induce
cytochrome C to enter the cytoplasm and activate the caspase
cascade (81). The caspase protein
family is important for apoptosis and Caspase-3 is an executory
protein as it is the convergence point of multiple apoptosis
signaling pathways (82). When
Caspase-3 is stimulated by apoptosis, it is activated and cleaved
into cleaved Caspase-3, an essential condition for the initiation
of apoptosis (83). Subsequently,
cleaved Caspase-3 will decompose the nuclear DNA repair enzymes,
resulting in nuclear DNA chromatin condensation and damage,
eventually leading to apoptosis (83-85).
The present study demonstrated that PHBA reduces
cerebral index, neurological dysfunction, weight loss, infarction
rate and the number of TUNEL-positive cells. On the other hand,
PHBA reduces mitochondrial oxidative stress and dysfunction.
Moreover, the expression levels of Bcl-2 protein were increased,
while those of Bax and cleaved caspase-3 proteins were decreased.
At present, single-targeted therapy can shorten the onset time of
the drug, make it reach the lesion quickly and gain valuable time
for disease treatment (86). PHBA
can target the brain (87), and
the present study demonstrated that prophylactic administration can
significantly reduce the size of cerebral infarction and
effectively improve neuronal damage caused by cerebral
ischemia.
The present results suggested that PHBA could be
used as a clinical candidate for the prevention and treatment of
transient focal cerebral ischemia (87). However, due to the lack of research
on PHBA, the specific brain targeting mechanism remains unclear.
For clinical applications, additional experiments are required to
address the issues pertaining PHBA. The present study is only a
preliminary study on the effect of PHBA on mitochondria during the
treatment of cerebral ischemia, while mitochondrial dysfunction
also involves several other pathways, such as abnormal energy
metabolism, nitrosative stress, mitophagy and so on (87-89).
Therefore, in vitro experiments will be the focus of future
investigations on mitochondrial energy metabolism to provide a
pharmacological basis for PHBA-targeting mitochondria in the
treatment of cerebral ischemia-related diseases.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant no. 81960733) and the Applied
Basic Research Program of Yunnan Province (grant no.
2019FB120).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XD and LY conceived and designed this study. TX and
LY participated in the experiments. PC and TX analyzed the data. TX
wrote the manuscript. PC and XD reviewed and revised the
manuscript. All authors read and approved the final version of the
manuscript. TX and XD confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Yunnan University of Traditional Chinese
Medicine (approval no. R-062019039) and the care and use of
experimental animals were performed in accordance with the
guidelines of the National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wisen WP, Evans WR, Sure VN, Sperling JA,
Sakamuri SS, Mostany R and Katakam PV: Nitric oxide synthase
inhibitor is an effective therapy for ischemia-reperfusion injury
in mice. FASEB J. 36 (Suppl 1)(R5443)2022.
|
|
2
|
Chen G, Shan X, Li L, Dong L, Huang G and
Tao H: circHIPK3 regulates apoptosis and mitochondrial dysfunction
induced by ischemic stroke in mice by sponging miR-148b-3p via
CDK5R1/SIRT1. Exp Neurol. 355(114115)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang Y, Ye Z, Ma T, Li H, Zhao Y, Chen W,
Wang Y, Yan X, Gao Y and Li Z: Carbon monoxide (CO) modulates
hydrogen peroxide (H2O2)-mediated cellular
dysfunction by targeting mitochondria in rabbit lens epithelial
cells. Exp Eye Res. 169:68–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guan R, Zou W, Dai X, Yu X, Liu H, Chen Q
and Teng W: Mitophagy, a potential therapeutic target for stroke. J
Biomed Sci. 25(87)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duan C, Kuang L, Hong C, Xiang X, Liu J,
Li Q, Peng X, Zhou Y, Wang H, Liu L and Li T: Mitochondrial Drp1
recognizes and induces excessive mPTP opening after hypoxia through
BAX-PiC and LRRK2-HK2. Cell Death Dis. 12(1050)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carinci M, Vezzani B, Patergnani S,
Ludewig P, Lessmann K, Magnus T, Casetta I, Pugliatti M, Pinton P
and Giorgi C: Different roles of mitochondria in cell death and
inflammation: Focusing on mitochondrial quality control in ischemic
stroke and reperfusion. Biomedicines. 9(169)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee HK, Jang JY, Yoo HS and Seong YH:
Neuroprotective effect of 1,3-dipalmitoyl-2-oleoylglycerol derived
from rice bran oil against cerebral ischemia-reperfusion injury in
rats. Nutrients. 14(1380)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pasala PK, Abbas Shaik R, Rudrapal M, Khan
J, Alaidarous MA, Jagdish Khairnar S, Bendale AR, Naphade VD, Kumar
Sahoo R, Zothantluanga JH and Walode SG: Cerebroprotective effect
of Aloe Emodin: In silico and in vivo studies. Saudi J Biol Sci.
29:998–1005. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Uzdensky AB: Apoptosis regulation in the
penumbra after ischemic stroke: Expression of pro- and
antiapoptotic proteins. Apoptosis. 24:687–702. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yan H, Huang W, Rao J and Yuan J: miR-21
regulates ischemic neuronal injury via the p53/Bcl-2/Bax signaling
pathway. Aging (Albany NY). 13:22242–22255. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang M, He Y, Deng S, Xiao L, Tian M, Xin
Y, Lu C, Zhao F and Gong Y: Mitochondrial quality control: A
pathophysiological mechanism and therapeutic target for stroke.
Front Mol Neurosci. 14(786099)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu Q, Zuo T, Deng L, Chen S, Yu W, Liu S,
Liu J, Wang X, Fan X and Dong Z: β-Caryophyllene suppresses
ferroptosis induced by cerebral ischemia reperfusion via activation
of the NRF2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine.
102(154112)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhan HD, Zhou HY, Sui YP, Du XL, Wang WH,
Dai L, Sui F, Huo HR and Jiang TL: The rhizome of Gastrodia
elata blume-an ethnopharmacological review. J Ethnopharmacol.
189:361–385. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang T, Chen H, Xia S, Chen X, Sun H and
Xu Z: Ameliorative effect of parishin C against cerebral
ischemia-induced brain tissue injury by reducing oxidative stress
and inflammatory responses in rat model. Neuropsychiatr Dis Treat.
17:1811–1823. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang ZL, Gao YG, Zang P, Gu PP, Zhao Y,
He ZM and Zhu HY: Research progress on mechanism of gastrodin and
p-hydroxybenzyl alcohol on central nervous system. Zhongguo Zhong
Yao Za Zhi. 45:312–320. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
16
|
Yang F, Li G, Lin B and Zhang K: Gastrodin
suppresses pyroptosis and exerts neuroprotective effect in
traumatic brain injury model by inhibiting NLRP3 inflammasome
signaling pathway. J Integr Neurosci. 21(72)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Y, Lu YY, Huang L, Shi L, Zheng ZY,
Chen JN, Qu Y, Xiao HT, Luo HR and Wu GS: Para-hydroxybenzyl
alcohol delays the progression of neurodegenerative diseases in
models of caenorhabditis elegans through activating multiple
cellular protective pathways. Oxid Med Cell Longev.
2022(8986287)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gong X, Cheng J, Zhang K, Wang Y, Li S and
Luo Y: Transcriptome sequencing reveals Gastrodia elata
Blume could increase the cell viability of eNPCs under hypoxic
condition by improving DNA damage repair ability. J Ethnopharmacol.
282(114646)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duan X, Wang W, Liu X, Yan H, Dai R and
Lin Q: Neuroprotective effect of ethyl acetate extract from
Gastrodia elata against transient focal cerebral ischemia in
rats induced by middle cerebral artery occlusion. J Tradit Chin
Med. 35:671–678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jiang G, Hu Y, Liu L, Cai J, Peng C and Li
Q: Gastrodin protects against MPP(+)-induced oxidative stress by up
regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway
in human dopaminergic cells. Neurochem Int. 75:79–88.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Luo L, Kim SW, Lee HK, Kim ID, Lee H and
Lee JK: Anti-Zn2+-toxicity of 4-hydroxybenzyl alcohol in
astrocytes and neurons contribute to a robust neuroprotective
effects in the postischemic brain. Cell Mol Neurobiol. 38:615–626.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X, Xiang B, Shen T, Xiao C, Dai R, He F
and Lin Q: Anti-neuroinflammatory effect of
3,4-dihydroxybenzaldehyde in ischemic stroke. Int Immunopharmacol.
82(106353)2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
23
|
Kim HJ, Hwang IK and Won MH: Vanillin,
4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent
hippocampal CA1 cell death following global ischemia. Brain Res.
1181:130–141. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu J, Wu B, Tang C and Zhao J: Analytical
techniques and pharmacokinetics of Gastrodia elata blume and
its constituents. Molecules. 22(1137)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu CS, Liang X, Wei XH, Chen FL, Tang QF
and Tan XM: Comparative pharmacokinetics of major bioactive
components from Puerariae radix-gastrodiae rhizome extracts and
their intestinal absorption in rats. J Chromatogr B Analyt Technol
Biomed Life Sci. 1105:38–46. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Loh YC, Oo CW, Tew WY, Wen X, Wei X and
Yam MF: The predominance of endothelium-derived relaxing factors
and beta-adrenergic receptor pathways in strong vasorelaxation
induced by 4-hydroxybenzaldehyde in the rat aorta. Biomed
Pharmacother. 150(112905)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee JY, Jang YW, Kang HS, Moon H, Sim SS
and Kim CJ: Anti-inflammatory action of phenolic compounds from
Gastrodia elata root. Arch Pharm Res. 29:849–858.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ha JH, Lee DU, Lee JT, Kim JS, Yong CS,
Kim JA, Ha JS and Huh K: 4-Hydroxybenzaldehyde from Gastrodia
elata B1. is active in the antioxidation and GABAergic
neuromodulation of the rat brain. J Ethnopharmacol. 73:329–333.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Feng J, Sun H, Xu Y, Yang Q and He F:
Gastrointestinal absorption characteristics of
p-hydroxybenzaldehyde, a constituent of Gastrodia elata.
Pharmacology and Clinics of Chinese Materia Medica. 37:23–28.
2021.
|
|
30
|
Zhu YP, Li X, Du Y, Zhang L, Ran L and
Zhou NN: Protective effect and mechanism of p-hydroxybenzaldehyde
on blood-brain barrier. Zhongguo Zhong Yao Za Zhi. 43:1021–1027.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
31
|
Ha JH, Shin SM, Lee SK, Kim JS, Shin US,
Huh K, Kim JA, Yong CS, Lee NJ and Lee DU: In vitro effects of
hydroxybenzaldehydes from Gastrodia elata and their
analogues on GABAergic neurotransmission, and a structure-activity
correlation. Planta Med. 67:877–880. 2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gorsky A, Monsour M, Nguyen H, Castelli V,
Lee JY and Borlongan CV: Metabolic switching of cultured
mesenchymal stem cells creates super mitochondria in rescuing
ischemic neurons. Neuromolecular Med: Jul 20, 2022 (Epub ahead of
print).
|
|
33
|
Brenza TM, Ghaisas S, Ramirez JEV,
Harischandra D, Anantharam V, Kalyanaraman B, Kanthasamy AG and
Narasimhan B: Neuronal protection against oxidative insult by
polyanhydride nanoparticle-based mitochondria-targeted antioxidant
therapy. Nanomedicine. 13:809–820. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Buchke S, Sharma M, Bora A, Relekar M,
Bhanu P and Kumar J: Mitochondria-targeted, nanoparticle-based
drug-delivery systems: Therapeutics for mitochondrial disorders.
Life (Basel). 12(657)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li N, Qin S, Xie L, Qin T, Yang Y, Fang W
and Chen MH: Elevated serum potassium concentration alleviates
cerebral ischemia-reperfusion injury via mitochondrial
preservation. Cell Physiol Biochem. 48:1664–1674. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang M, Yang JK and Ma J: Regulation of
the long noncoding RNA XIST on the inflammatory polarization of
microglia in cerebral infarction. Exp Ther Med.
22(924)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tian J, Yao H, Liu Y, Wang X, Wu J, Wang
J, Yu D, Xie Y, Gao J, Zhu Y and Yang C: Extracellular vesicles
from bone marrow stromal cells reduce the impact of stroke on glial
cell activation and blood brain-barrier permeability via a putative
miR-124/PRX1 signalling pathway. Eur J Neurosci. 56:3786–3805.
2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lee J, Kang CG, Park CR, Hong IK and Kim
DY: The neuroprotective effects of pregabalin after cerebral
ischemia by occlusion of the middle cerebral artery in rats. Exp
Ther Med. 21(165)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yu SS, Zhao J, Zheng WP and Zhao Y:
Neuroprotective effect of 4-hydroxybenzyl alcohol against transient
focal cerebral ischemia via anti-apoptosis in rats. Brain Res.
1308:167–175. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cui L, Zhang X, Yang R, Liu L, Wang L, Li
M and Du W: Baicalein is neuroprotective in rat MCAO model: Role of
12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic
phospholipase A2. Pharmacol Biochem Behav. 96:469–475.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jang JH, Son Y, Kang SS, Bae CS, Kim JC,
Kim SH, Shin T and Moon C: Neuropharmacological potential of
Gastrodia elata blume and its components. Evid Based
Complement Alternat Med. 2015(309261)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu Y, Gao J, Peng M, Meng H, Ma H, Cai P,
Xu Y, Zhao Q and Si G: A review on central nervous system effects
of gastrodin. Front Pharmacol. 9(24)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xiang B, Xiao C, Shen T and Li X:
Anti-inflammatory effects of anisalcohol on
lipopolysaccharide-stimulated BV2 microglia via selective
modulation of microglia polarization and down-regulation of NF-κB
p65 and JNK activation. Mol Immunol. 95:39–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shi A, Xiang J, He F, Zhu Y, Zhu G, Lin Y
and Zhou N: The phenolic components of Gastrodia elata
improve prognosis in rats after cerebral ischemia/reperfusion by
enhancing the endogenous antioxidant mechanisms. Oxid Med Cell
Longev. 2018(7642158)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li L, Wan G, Han B and Zhang Z:
Echinacoside alleviated LPS-induced cell apoptosis and inflammation
in rat intestine epithelial cells by inhibiting the mTOR/STAT3
pathway. Biomed Pharmacother. 104:622–628. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu B, Li F, Shi J, Yang D, Deng Y and
Gong Q: Gastrodin ameliorates subacute phase cerebral
ischemia-reperfusion injury by inhibiting inflammation and
apoptosis in rats. Mol Med Rep. 14:4144–4152. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mi Y, Mao Y, Cheng H, Ke G, Liu M, Fang C
and Wang Q: Studies of blood-brain barrier permeability of
gastrodigenin in vitro and in vivo. Fitoterapia.
140(104447)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang S, Nan Y, Zhu W, Yang T, Tong Y and
Fan Y: Gastrodin improves the neurological score in MCAO rats by
inhibiting inflammation and apoptosis, promoting revascularization.
Int J Clin Exp Pathol. 11:5343–5350. 2018.PubMed/NCBI
|
|
50
|
Ning L, Sun H, Feng J, Yang Q, Zhou Y and
He F: Tissue distribution of p-hydroxybenzaldehyde metabolite 4-HBA
in rats. J Shanghai Univ Tradit Chin Med. 35:65–70. 2021.
|
|
51
|
Kim HS, Choi JA, Kim BY, Ferrer L, Choi J,
Wendisch VF and Lee JH: Engineered corynebacterium glutamicum as
the platform for the production of aromatic aldehydes. Front Bioeng
Biotechnol. 10(880277)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Duan XH, Li ZL, Yang DS, Zhang FL, Lin Q
and Dai R: Study on the chemical constituents of Gastrodia
elata. Zhong Yao Cai. 36:1608–1611. 2013.PubMed/NCBI(In Chinese).
|
|
53
|
Lemmerman LR, Harris HN, Balch MHH,
Rincon-Benavides MA, Higuita-Castro N, Arnold DW and Gallego-Perez
D: Transient middle cerebral artery occlusion with an intraluminal
suture enables reproducible induction of ischemic stroke in mice.
Bio Protoc. 12(e4305)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yuan Y, Xia F, Gao R, Chen Y, Zhang Y,
Cheng Z, Zhao H and Xu L: Kaempferol mediated AMPK/mTOR signal
pathway has a protective effect on cerebral ischemic-reperfusion
injury in rats by inducing autophagy. Neurochem Res. 47:2187–2197.
2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhu A, Lin Y, Hu X, Lin Z, Lin Y, Xie Q,
Ni S, Cheng H, Lu Q, Lai S, et al: Treadmill exercise decreases
cerebral edema in rats with local cerebral infarction by modulating
AQP4 polar expression through the caveolin-1/TRPV4 signaling
pathway. Brain Res Bull. 188:155–168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Jin M, Kim KM, Lim C, Cho S and Kim YK:
Neuroprotective effects of Korean White ginseng and Red ginseng in
an ischemic stroke mouse model. J Ginseng Res. 46:275–282.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Qi S, Zhang X, Fu Z, Pi A, Shi F, Fan Y,
Zhang J, Xiao T, Shang D, Lin M, et al:
(±)-5-bromo-2-(5-fluoro-1-hydroxyamyl) benzoate protects against
oxidative stress injury in PC12 cells exposed to
H2O2 through activation of Nrf2 pathway.
Front Pharmacol. 13(943111)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Song ZL, Bai F, Zhang B and Fang J:
Synthesis of Dithiolethiones and Identification of Potential
Neuroprotective Agents via Activation of Nrf2-Driven Antioxidant
Enzymes. J Agric Food Chem. 68:2214–2231. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Huang SS, Su HH, Chien SY, Chung HY, Luo
ST, Chu YT, Wang YH, MacDonald IJ, Lee HH and Chen YH: Activation
of peripheral TRPM8 mitigates ischemic stroke by topically applied
menthol. J Neuroinflammation. 19(192)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wu J, Jin Z, Yang X and Yan LJ:
Post-ischemic administration of 5-methoxyindole-2-carboxylic acid
at the onset of reperfusion affords neuroprotection against stroke
injury by preserving mitochondrial function and attenuating
oxidative stress. Biochem Biophys Res Commun. 497:444–450.
2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Narne P, Pandey V and Phanithi PB:
Interplay between mitochondrial metabolism and oxidative stress in
ischemic stroke: An epigenetic connection. Mol Cell Neurosci.
82:176–194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li Y, Zhang X, Ma A and Kang Y: Rational
application of β-hydroxybutyrate attenuates ischemic stroke by
suppressing oxidative stress and mitochondrial-dependent apoptosis
via activation of the Erk/CREB/eNOS pathway. ACS Chem Neurosci.
12:1219–1227. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kaushik P, Ali M, Salman M, Tabassum H and
Parvez S: Harnessing the mitochondrial integrity for
neuroprotection: Therapeutic role of piperine against experimental
ischemic stroke. Neurochem Int. 149(105138)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Morkuniene R, Rekuviene E and
Kopustinskiene DM: Rotenone decreases ischemia-induced injury by
inhibiting mitochondrial permeability transition: A study in brain.
Methods Mol Biol. 2497:63–72. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Huang P, Wu SP, Wang N, Seto S and Chang
D: Hydroxysafflor yellow A alleviates cerebral ischemia reperfusion
injury by suppressing apoptosis via mitochondrial permeability
transition pore. Phytomedicine. 85(153532)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Su F, Yang H, Guo A, Qu Z, Wu J and Wang
Q: Mitochondrial BKCa mediates the protective effect of low-dose
ethanol preconditioning on oxygen-glucose deprivation and
reperfusion-induced neuronal apoptosis. Front Physiol.
12(719753)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Malko P and Jiang LH: TRPM2
channel-mediated cell death: An important mechanism linking
oxidative stress-inducing pathological factors to associated
pathological conditions. Redox Biol. 37(101755)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Xu M, Ma Q, Fan C, Chen X, Zhang H and
Tang M: Ginsenosides Rb1 and Rg1 protect primary cultured
astrocytes against oxygen-glucose deprivation/reoxygenation-induced
injury via improving mitochondrial function. Int J Mol Sci.
20(6086)2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Pozdnyakov DI, Chernikov MV, Sarkisyan KK
and Rybalko IE: Neuroprotective potential of pyrimidine-4-H1-OHa
derivatives in experimental cerebral ischemia. Zh Nevrol Psikhiatr
Im S S Korsakova. 121:63–68. 2021.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
70
|
Morse PT, Goebel DJ, Wan J, Tuck S, Hakim
L, Hüttemann CL, Malek MH, Lee I, Sanderson TH and Hüttemann M:
Cytochrome c oxidase-modulatory near-infrared light penetration
into the human brain: Implications for the noninvasive treatment of
ischemia/reperfusion injury. IUBMB Life. 73:554–567.
2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ma Y, Zhao P, Zhu J, Yan C, Li L, Zhang H,
Zhang M, Gao X and Fan X: Naoxintong protects primary neurons from
oxygen-glucose deprivation/reoxygenation induced injury through
PI3K-Akt signaling pathway. Evid Based Complement Alternat Med.
2016(5815946)2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zeng JW, Chen BY, Lv XF, Sun L, Zeng XL,
Zheng HQ, Du YH, Wang GL, Ma MM and Guan YY: Transmembrane member
16A participates in hydrogen peroxide-induced apoptosis by
facilitating mitochondria-dependent pathway in vascular smooth
muscle cells. Br J Pharmacol. 175:3669–3684. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
He F, Wu Q, Xu B, Wang X, Wu J, Huang L
and Cheng J: Suppression of Stim1 reduced intracellular calcium
concentration and attenuated hypoxia/reoxygenation induced
apoptosis in H9C2 cells. Biosci Rep. 37(BSR20171249)2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Momenabadi S, Vafaei AA, Zahedi Khorasani
M and Vakili A: Pre-ischemic oxytocin treatment alleviated neuronal
injury via suppressing NF-κB, MMP-9, and apoptosis regulator
proteins in a mice model of stroke. Cell J. 24:337–345.
2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Huang LY, Song JX, Cai H, Wang PP, Yin QL,
Zhang YD, Chen J, Li M, Song JJ, Wang YL, et al: Healthy
serum-derived exosomes improve neurological outcomes and protect
blood-brain barrier by inhibiting endothelial cell apoptosis and
reversing autophagy-mediated tight junction protein reduction in
rat stroke model. Front Cell Neurosci. 16(841544)2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
77
|
Zhai Y, Liu B, Wu L, Zou M, Mei X and Mo
X: Pachymic acid prevents neuronal cell damage induced by
hypoxia/reoxygenation via miR-155/NRF2/HO-1 axis. Acta Neurobiol
Exp (Wars). 82:197–206. 2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yan Y, Liu Y, Yang Y, Ding Y and Sun X:
Carnosol suppresses microglia cell inflammation and apoptosis
through PI3K/AKT/mTOR signaling pathway. Immunopharmacol
Immunotoxicol. 1–11. 2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
79
|
Liu X, Zhu X, Chen M, Ge Q, Shen Y and Pan
S: Resveratrol protects PC12 cells against OGD/R-induced apoptosis
via the mitochondrial-mediated signaling pathway. Acta Biochim
Biophys Sin (Shanghai). 48:342–353. 2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Wang PC, Wang SX, Yan XL, He YY, Wang MC,
Zheng HZ, Shi XG, Tan YH and Wang LS: Combination of paeoniflorin
and calycosin-7-glucoside alleviates ischaemic stroke injury via
the PI3K/AKT signalling pathway. Pharm Biol. 60:1469–1477.
2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Khaksar S, Bigdeli M, Samiee A and
Shirazi-Zand Z: Antioxidant and anti-apoptotic effects of
cannabidiol in model of ischemic stroke in rats. Brain Res Bull.
180:118–130. 2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Li Z, Xiao G, Wang H, He S and Zhu Y: A
preparation of Ginkgo biloba L. leaves extract inhibits the
apoptosis of hippocampal neurons in post-stroke mice via regulating
the expression of Bax/Bcl-2 and caspase-3. J Ethnopharmacol.
280(114481)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Yang L, Tao Y, Luo L, Zhang Y, Wang X and
Meng X: Dengzhan Xixin injection derived from a traditional Chinese
herb Erigeron breviscapus ameliorates cerebral ischemia/reperfusion
injury in rats via modulation of mitophagy and mitochondrial
apoptosis. J Ethnopharmacol. 288(114988)2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Nhu NT, Li Q, Liu Y, Xu J, Xiao SY and Lee
SD: Effects of mdivi-1 on neural mitochondrial dysfunction and
mitochondria-mediated apoptosis in ischemia-reperfusion injury
after stroke: A systematic review of preclinical studies. Front Mol
Neurosci. 14(778569)2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Gan L, Liao S, Tong Y, Li W, Peng W and
Deng S: Long noncoding RNA H19 mediates neural stem/progenitor
cells proliferation, differentiation and apoptosis through the p53
signaling pathway after ischemic stroke. Biochem Biophys Res
Commun. 597:8–15. 2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
86
|
MacDougall G, Anderton RS, Mastaglia FL,
Knuckey NW and Meloni BP: Mitochondria and neuroprotection in
stroke: Cationic arginine-rich peptides (CARPs) as a novel class of
mitochondria-targeted neuroprotective therapeutics. Neurobiol Dis.
121:17–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Shefa U, Jeong NY, Song IO, Chung HJ, Kim
D, Jung J and Huh Y: Mitophagy links oxidative stress conditions
and neurodegenerative diseases. Neural Regen Res. 14:749–756.
2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Awooda HA, Lutfi MF, Sharara GG and Saeed
AM: Oxidative/nitrosative stress in rats subjected to focal
cerebral ischemia/reperfusion. Int J Health Sci (Qassim). 9:17–24.
2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Chen Y: Disturbed cerebral circulation and
metabolism matters: A preface to the special issue ‘stroke and
energy metabolism’: A preface to the special issue ‘stroke and
energy metabolism’. J Neurochem. 160:10–12. 2022.PubMed/NCBI View Article : Google Scholar
|