Introduction
As of July 14, 2022, the severe acute respiratory
syndrome coronavirus-2 (SARS-CoV-2) has caused 6,356,812 deaths
globally (1). The mortality rates
associated with SARS-CoV-2 infection vary according to the
geographical region and are associated with age, comorbidities and
vaccination status (2). Organ
damage is considered to be caused by the cytokine release syndrome,
which is crucial during the course of coronavirus disease 2019
(COVID-19) infection. Organ damage is also caused by septic shock,
thrombosis and oxidative stress (3). Excessive cytokine release in patients
with COVID-19 is induced by the stimulation of the innate and
adaptive immune systems. An unbalanced immune response and
excessive inflammation are key pathogenic factors in
COVID-19(4).
Interleukin (IL)-6) is secreted by macrophages in
response to specific microbial molecules known as
pathogen-associated molecular patterns. These patterns bind to a
key type of innate immune system detection molecules known as
pattern recognition receptors, which include Toll-like receptors.
These are found on the cell surface and in intracellular
compartments, and they initiate intracellular signaling cascades,
resulting in the release of inflammatory cytokines (5). IL-6 has been found to be implicated
in severe SARS-CoV-2 infection (6). IL-6 levels of 80 pg/ml suggest an
increased risk of respiratory failure and mortality, and
immunomodulatory therapy is an area of urgent research (6).
Tocilizumab is an anti-IL-6 receptor antagonist that
is used in the treatment of connective tissue disorders, such as
rheumatoid arthritis, giant cell arteritis, polyarticular juvenile
idiopathic arthritis and systemic juvenile idiopathic arthritis
(7). This agent has exhibited
efficacy against COVID-19 (8-12).
The RECOVERY trial reported a decrease in the mortality rate from
35 to 31% when corticosteroids were used simultaneously in
hospitalized patients with moderate, severe or critical COVID-19
infection, and with evidence of inflammation (8). Another study demonstrated that
patients who were critically ill with COVID-19 who received
tocilizumab or sarilumab presented with a mortality rate of 27% in
the REMAP-CAP trial, compared to 36% in the control group receiving
only standard care (9).
Furthermore, another three meta-analyses (10-12)
all agreed that tocilizumab should be used in the treatment of
patients with severe COVID-19 infection. The first revealed a
pooled mortality rate of 19% in the tocilizumab group (10), the second revealed a lower 28-day
mortality rate with 32 fewer individuals per 1,000 who succumbed
when treated with tocilizumab plus standard care, compared with
standard care alone or placebo (11), and the third revealed a 22% 28-day
mortality rate (12).
On the other hand, there is evidence to indicate
that tocilizumab is ineffective in some cases of COVID-19, as it
has been hypothesized that early drug administration is probably
more beneficial (13). The World
Health Organization (WHO) recommends the use of tocilizumab if
inflammation is evident and a patient has severe or critical
COVID-19(14). More specifically
tocilizumab is recommended in patients who have rapidly increasing
oxygen needs and systemic inflammation (14). Tocilizumab is a potent
anti-inflammatory drug that has been shown to reduce C-reactive
protein (CRP) levels, although not always with a therapeutic
effect. Other clinical parameters, such as the degree of hypoxia,
may be a crucial factor in the decision of whether to escalate
treatment or in determining prognosis (15). The present study aimed to describe
in detail the characteristics of patients who received this agent
and to identify determinants of mortality and other unfavorable
outcomes.
Patients and methods
Study design
The present study was a single-center retrospective
study on patients with COVID-19 admitted to the Department of
Infectious Diseases-COVID-19 Unit of Laiko General Hospital
(Athens, Greece) between September 21, 2020 and April 15, 2022. The
study was conducted in line with the Declaration of Helsinki and
obtained approval by the Institutional Review Board of Laiko
General Hospital (protocol no. 765/12-2021). Written informed was
obtained from all patients. The following criteria were required
for inclusion in the study: A polymerase chain reaction diagnosis
of COVID-19, a WHO clinical progression scale score ≥5, and
tocilizumab treatment in accordance with the WHO recommendations
(13). Some of the
participants had a follow-up appointment 3 months after their
admission to the post-COVID-19 outpatient clinic of Laiko General
Hospital, and if that was not possible, a telephone call was made
to determine the 90-day mortality rate. The exclusion criteria were
an age <18 years and a lack of available data on survival at 3
months post-diagnosis.
Investigations
Demographics, vaccination status against SARS-CoV-2
and the Charlson comorbidity index (CCI) were recorded. Hemoglobin
levels, white blood cell (WBC) count, blood neutrophil, lymphocyte
and immature granulocyte counts, neutrophil-to-lymphocyte ratio,
the number of platelets (PLTs), platelet-to-lymphocyte ratio, CRP
and serum albumin levels, CRP-to-albumin ratio (CAR), serum lactate
dehydrogenase (LDH), d-dimer, ferritin, aspartate aminotransferase
(AST), alanine aminotransferase (ALT), alkaline phosphatase and
gamma glutamyl-transferase (GGT) levels were recorded upon
admission. In addition, the Modified Chest X-ray Scoring System was
calculated for chest X-ray upon admission by two experienced
radiologists, as previously described (16). Charts were evaluated for the
implementation of intubation and all-cause mortality rates at 90
days.
Statistical analysis
Continuous variables are presented as the mean
(standard deviation). The assessment of the normal distribution of
variables was performed with the use the Kolmogorov-Smirnov test.
The comparison of normally distributed variables was performed
using an independent samples Student's t-test on variables with two
groups and not normally distributed variables were examined using
an unpaired non-parametric two-tailed Mann-Whitney test.
Categorical variables were examined using the Fisher's exact test
or the Chi-squared test and are presented as absolute numbers
(frequency, percentage). The CCI data were numerically recorded. To
find predictors of event(s) (event=intubation, or mortality at 90
days), statistically significant factors were subsequently examined
using Cox proportional hazards multivariate regression analysis.
The quality of fit of the log-likelihood ratio was evaluated. The
Kaplan-Meier method with log-rank (Mantel-Cox) test was used to
plot and analyze survival curves utilizing significant variables
and specific cut-offs. The discriminative ability of significant
variables was evaluated by using the area under the receiver
operating characteristic curve (ROC). Participants were censored at
90 days. Values of P<0.05 were considered to indicate
statistically significant differences. Statistical analysis was
conducted using IBM SPSS-Statistics version 26.0 (IBM Corp.).
Results
In total, 174 subjects (121 males; mean age,
62.43±13.47 years) fulfilling the inclusion criteria were included.
Among the 174 participants, 58 (33.3%) were intubated. From the 174
individuals analyzed, 113 were alive after 90 days (survivors), and
61 had succumbed (non-survivors). The mortality rate was 35.1%
(61/174). The demographics and baseline data of the study
population are presented in Table
I.
 | Table IDemographics of the study
population. |
Table I
Demographics of the study
population.
| Parameter | Mean/no. of
patients | SD/% |
|---|
| Age (mean ±
SD) | 62.43 | 13.47 |
| Sex, number and
percentage | 174 | |
|
Male | 121 | 69.5 |
|
Female | 53 | 30.5 |
| Type of
treatment | | |
|
Remdesivir | 174 | |
|
Yes | 172 | 98.9 |
|
No | 2 | 1.1 |
|
Dexamethasone | 174 | |
|
Yes | 173 | 99.4 |
|
No | 1 | 0.6 |
|
Anticoagulants | 174 | |
|
No | 5 | 2.9 |
|
Yes | 169 | 97.1 |
| Anticoagulants | 169 | |
|
Prophylactic
dose | 161 | 95.2 |
|
Therapeutic
dose | 8 | 4.8 |
| Outcome | | |
|
Intubation | 174 | |
|
Yes | 58 | 33.3 |
|
No | 116 | 66.7 |
|
Mortality at
90 days | 174 | |
|
Yes | 61 | 35.1 |
|
No | 113 | 64.9 |
| Vaccination
status | 174 | |
|
Fully
vaccinated | 20 | 11.5 |
|
Unvaccinated | 154 | 88.5 |
The non-survivors were older, mostly females and had
a higher CCI score. At the evaluation upon admission to the
hospital unit, the survivors presented with higher levels of ALT
and GGT and with a greater number of PLTs (P<0.05; Table II). The patients that were
intubated were also older, mostly females, and had a higher CCI
score (P<0.05; Table
III).
 | Table IIUnivariate analysis (outcome,
mortality). |
Table II
Univariate analysis (outcome,
mortality).
| Parameter | Survivors | Non-survivors | P-value |
|---|
| Age (years) | 57.88±12.86 | 70.84±10.17 | 0.01 |
| Sex | | | 0.01 |
|
Male | 86 | 35 | |
|
Female | 27 | 26 | |
| Type of
treatment | | | |
|
Remdesivir | | | 0.999 |
|
Yes | 112 | 60 | |
|
No | 1 | 1 | |
|
Dexamethasone | | | 0.999 |
|
Yes | 112 | 61 | |
|
No | 1 | 0 | |
|
Anticoagulants | | | 0.65 |
|
Yes | 109 | 60 | |
|
No | 4 | 1 | |
| Vaccination
status | | | 0.07 |
|
Fully
vaccinated | 9 | 11 | |
|
Unvaccinated | 104 | 50 | |
| CCI | 1.96±1.71 | 3.49±1.46 | 0.01 |
| Hb (g/dl) | 13.77±1.38 | 13.66±1.89 | 0.67 |
| WBC (k/µl) | 8.44±8.03 | 9.50±14.58 | 0.67 |
| Neutrophils
(k/µl) | 6.54±3.61 | 6.42±2.92 | 0.73 |
| Lymphocytes
(k/µl) | 1.62±6.45 | 2.31±10.80 | 0.57 |
| IGs (k/µl) | 0.11±0.26 | 0.09± 0.16 | 0.97 |
| PLTs (k/µl) | 227.71±83.86 | 195.56±66.72 | 0.01 |
| D-dimers
(µg/ml) | 2.18±4.40 | 2.67±4.86 | 0.19 |
| Creatinine
(mg/dl) | 1.20±1.69 | 1.33±1.73 | 0.22 |
| AST (U/l) | 52.43±36.82 | 48.36±26.65 | 0.72 |
| ALT (U/l) | 50.69±49.64 | 35.67± 27.09 | 0.02 |
| ALP (U/l) | 72.96± 35.97 | 72.42±28.58 | 0.40 |
| GGT (U/l) | 77.34 ±80.91 | 60.78±72.16 | 0.03 |
| LDH (U/l) | 447.38±175.60 | 442±185.39 | 0.82 |
| CRP (mg/l) | 127.83 ±82.96 | 108.74 ±79.66 | 0.09 |
| Fibrinogen
(mg/dl) | 634.16±162.36 | 602.92±155.85 | 0.22 |
| Ferritin
(ng/ml) |
1,258.25±1,629.29 |
1,527.11±1,821.12 | 0.42 |
| Albumin (g/l) | 37.77±4.63 | 36.48±5.61 | 0.15 |
| NLR | 9.19±11.89 | 9.25±8.53 | 0.75 |
| PLR | 329.07±526.24 | 273.04±201.45 | 0.52 |
| CAR | 3.47±2.51 | 3.05±2.55 | 0.15 |
| Chest X-ray
score | 9.15±2.99 | 9.64±3.17 | 0.13 |
 | Table IIIUnivariate analysis (outcome,
intubation). |
Table III
Univariate analysis (outcome,
intubation).
| Parameter | Non-intubated | Intubated | P-value |
|---|
| Age (years) | 59.80±14.36 | 67.67±9.62 | 0.01 |
| Sex | | | 0.03 |
|
Male | 86 | 34 | |
|
Female | 30 | 24 | |
| Type of
treatment | | | |
|
Remdesivir | | | 0.55 |
|
Yes | 114 | 58 | |
|
No | 2 | 0 | |
|
Dexamethasone | | | 0.33 |
|
Yes | 116 | 57 | |
|
No | 0 | 1 | |
|
Anticoagulants | | | 0.66 |
|
Yes | 112 | 57 | |
|
No | 4 | 1 | |
| Vaccination
status | | | 0.23 |
|
Fully
vaccinated | 11 | 9 | |
|
Unvaccinated | 105 | 49 | |
| CCI | 2.15±1.82 | 3.19±1.49 | 0.01 |
| Hb (g/dl) | 13.71±1.45 | 13.78±1.82 | 0.92 |
| WBC (k/µl) | 8.34±7.95 | 9.74±14.91 | 0.28 |
| Neutrophils
(k/µl) | 6.42±3.58 | 6.66±2.95 | 0.24 |
| Lymphocytes
(k/µl) | 1.63±6.36 | 2.33±11.08 | 0.10 |
| IGs (k/µl) | 0.12±0.27 | 0.09±0.11 | 0.38 |
| PLTs (k/µl) | 225.16±84.33 | 198.91±66.39 | 0.06 |
| D-dimers
(µg/ml) | 2.24±4.36 | 2.58±4.98 | 0.82 |
| Creatinine
(mg/dl) | 1.22±1.67 | 1.30±1.76 | 0.49 |
| AST (U/l) | 52.25±36.86 | 48.52±25.92 | 0.91 |
| ALT (U/l) | 50.28±49.98 | 35.71±24.22 | 0.08 |
| ALP (U/l) | 73.44±35.96 | 71.45±28.25 | 0.62 |
| GGT (U/l) | 69.47±62.97 | 75.81±102.39 | 0.55 |
| LDH (U/l) | 439.87±174.25 | 457±188.01 | 0.51 |
| CRP (mg/l) | 122.67±83.17 | 118.07±80.52 | 0.70 |
| Fibrinogen
(mg/dl) | 619.58±166.74 | 631.20±147.58 | 0.65 |
| Ferritin
(ng/ml) |
1,307.16±1,716.38 |
1,444.05±1,675.10 | 0.44 |
| Albumin (g/l) | 37.69±4.54 | 36.57±5.84 | 0.22 |
| NLR | 8.77±11.69 | 10.09±8.80 | 0.09 |
| PLR | 320.92±519.88 | 286.16±206.57 | 0.93 |
| CAR | 3.34±2.52 | 3.28±2.55 | 0.63 |
| Chest X-ray
score | 9.28±3.30 | 9.41±2.94 | 0.43 |
All parameters with significant differences in the
univariate analysis were analyzed using the Cox proportional
hazards multivariate regression analysis. The outcome was all-cause
mortality, and cases were censored at 90 days. The only independent
predictor of mortality found was age (P<0.05; Table IV).
 | Table IVCox regression multivariable analysis
(outcome, mortality). |
Table IV
Cox regression multivariable analysis
(outcome, mortality).
| | 95% CI for
Exp(B) |
|---|
| Parameter | P-value | Exp(B) | Lower | Upper |
|---|
| Age | 0.01 | 1.045 | 1.010 | 1.081 |
| Sex | 0.17 | 1.436 | 0.851 | 2.425 |
| PLTs (k/µl) | 0.07 | 0.996 | 0.992 | 1.000 |
| ALT (U/l) | 0.82 | 0.999 | 0.990 | 1.008 |
| GGT (U/l) | 0.88 | 1.000 | 0.996 | 1.003 |
| CCI | 0.75 | 1.038 | 0.820 | 1.316 |
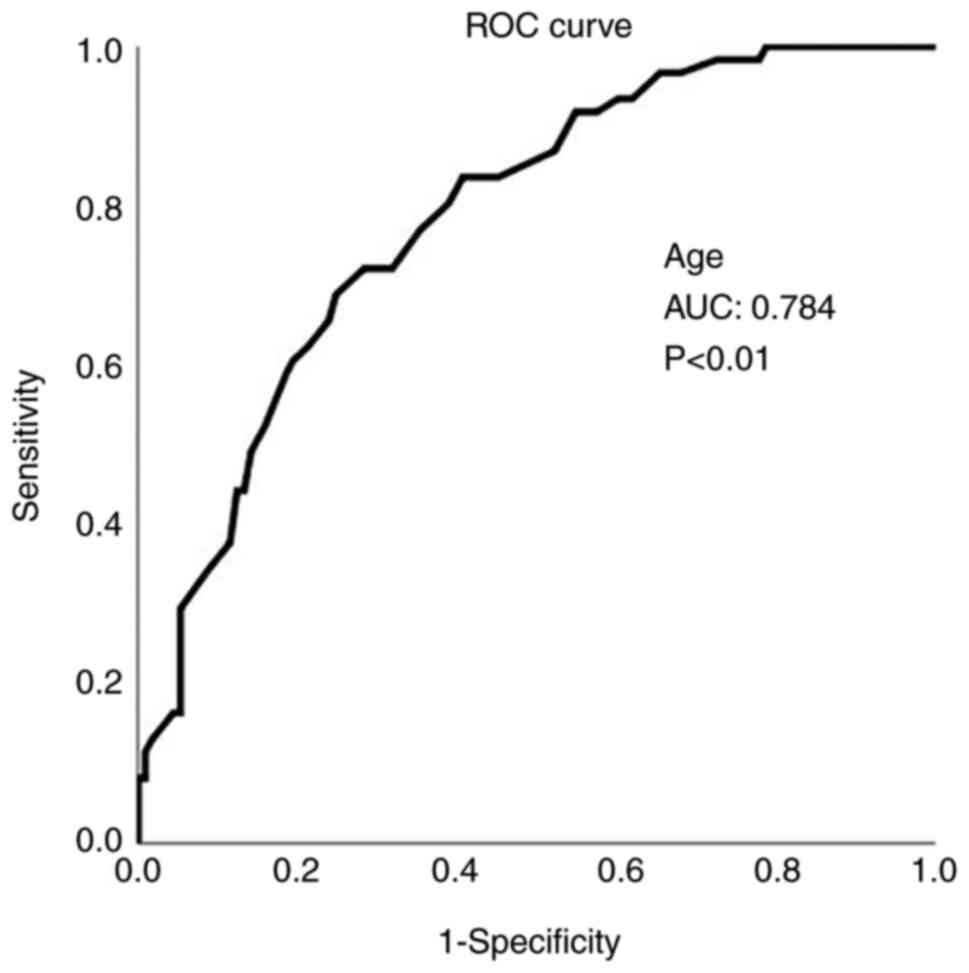
In addition, age was also found to be a significant
predictor of mortality using ROC analysis (Fig. 1). An age >64.5 years predicted
mortality with 72.1% sensitivity and 71.7% specificity (AUC,
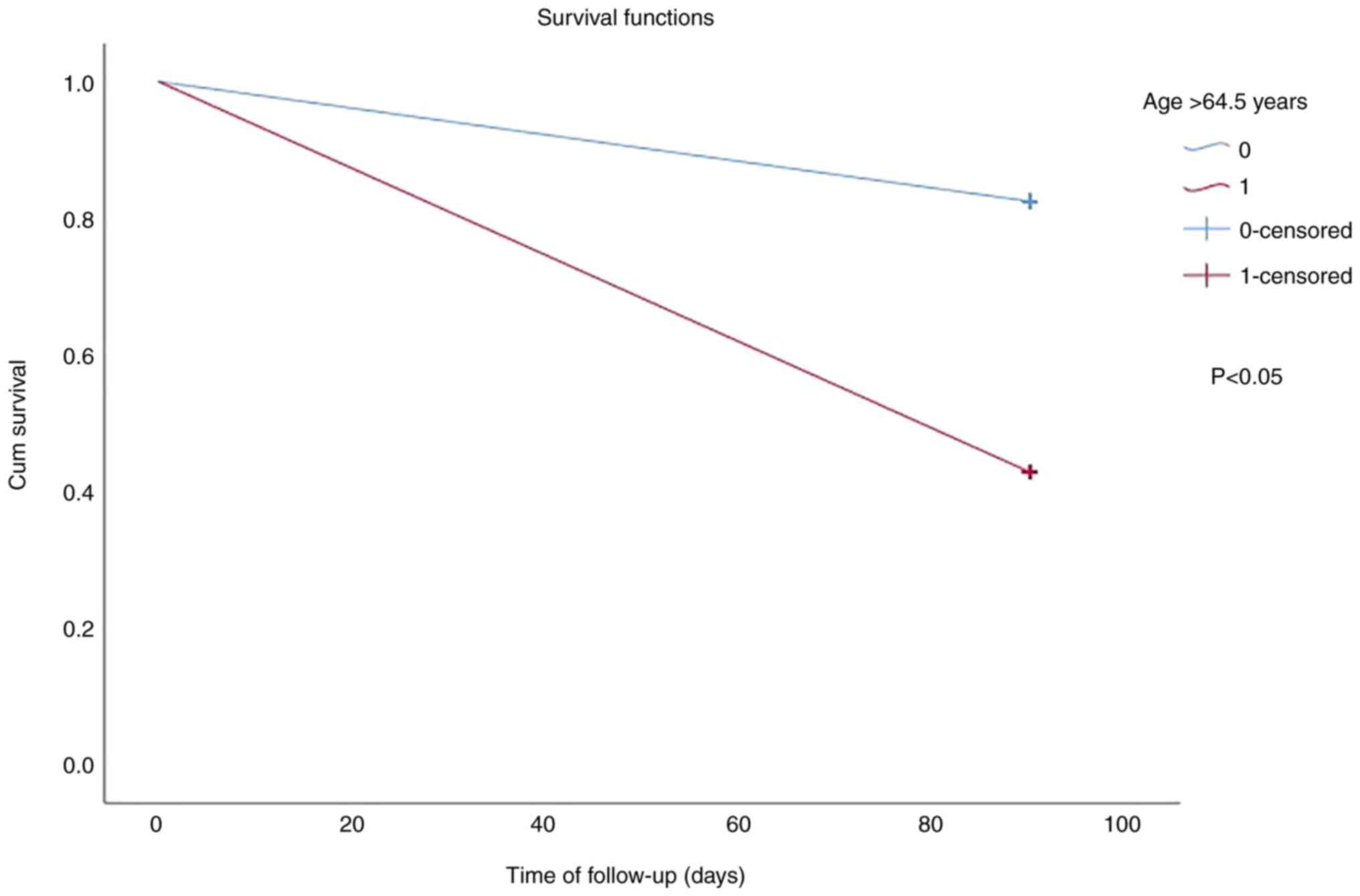
0.784). Kaplan-Meier survival analysis based on cut-off values for
age (>64.5 years and ≤64.5 years) revealed a worse survival in
subjects with an age >64.5 years (log-rank test for trend,
P<0.05; Fig. 2). Furthermore,
Cox proportional hazards multivariate regression analysis with
intubation as the outcome did not identify any independent factors
predicting intubation (Table
V).
 | Table VCox regression multivariable analysis
(outcome, intubation). |
Table V
Cox regression multivariable analysis
(outcome, intubation).
| | 95% CI for
Exp(B) |
|---|
| Parameter | P-value | Exp(B) | Lower | Upper |
|---|
| Age | 0.286 | 1.018 | 0.985 | 1.052 |
| CCI | 0.372 | 1.108 | 0.885 | 1.388 |
| Sex | 0.231 | 1.382 | 0.814 | 2.348 |
Of note, a sub-analysis regarding sex was performed,
which revealed that the male survivors were younger, had lower
chest X-ray scores, greater PLTs values and serum albumin, and
lower values of CCI and creatinine (P<0.05; Table VI).
 | Table VIUnivariate analysis for male
patients. |
Table VI
Univariate analysis for male
patients.
| Outcome,
mortality |
|---|
| Parameter | Survivors | Non-survivors | P-value |
|---|
| Age (years) | 57.06±12.61 | 71.09±10.47 | 0.001 |
| CCI | 1.92±1.75 | 3.49±1.54 | 0.001 |
| Hb (g/dl) | 14.09±1.32 | 13.88±2.11 | 0.58 |
| WBC (k/µl) | 7.82±3.64 | 10.93±19.06 | 0.60 |
| Neutrophils
(k/µl) | 6.60±3.63 | 6.62±2.95 | 0.98 |
| Lymphocytes
(k/µl) | 1.00±0.97 | 3.27±14.26 | 0.50 |
| IGs (k/µl) | 0.11±0.26 | 0.11±0.18 | 0.34 |
| PLTs (k/µl) | 231.98±84.80 | 182.94±59.26 | 0.002 |
| D-dimers
(µg/ml) | 2.22±4.57 | 3.68±6.23 | 0.056 |
| Creatinine
(mg/dl) | 1.35±1.91 | 1.45±1.33 | 0.005 |
| AST (U/l) | 52.60±37.66 | 51.66±30.19 | 0.95 |
| ALT (U/l) | 53.19±53.94 | 38.91±30.24 | 0.12 |
| ALP (U/l) | 69.91±33.88 | 71.74±31.85 | 0.52 |
| GGT (U/l) | 78.05±83.05 | 62.09±55.64 | 0.30 |
| LDH (U/l) | 443.36±180.05 | 452.50±202.18 | 0.82 |
| CRP (mg/l) | 130.06±80.86 | 118.26±71.19 | 0.59 |
| Fibrinogen
(mg/dl) | 650.70±152.52 | 626.38±146.08 | 0.42 |
| Ferritin
(ng/ml) |
1,377.47±1,760.41 |
1,929.71±1,999.20 | 0.90 |
| Albumin (g/l) | 37.96±4.71 | 35.78±5.56 | 0.047 |
| NLR | 9.98±13.25 | 10.11±7.83 | 0.97 |
| PLR | 359.62±592.21 | 273.01±190.27 | 0.40 |
| CAR | 3.52±2.37 | 3.68±2.61 | 1.00 |
| Chest X-ray
score | 8.80±3.01 | 9.71±3.11 | 0.04 |
| Outcome,
intubation |
| Parameter | Non-intubated | Intubated | P-value |
| Age (years) | 58.60±13.71 | 67.56±11.00 | 0.001 |
| CCI | 2.05±1.81 | 3.21±3.62 | 0.001 |
| Hb (g/dl) | 14.02±1.40 | 14.06±2.00 | 0.88 |
| WBC (k/µl) | 7.69±3.60 | 11.36±19.30 | 0.19 |
| Neutrophils
(k/µl) | 6.45±3.57 | 7.01±3.05 | 0.42 |
| Lymphocytes
(k/µl) | 1.00±0.76 | 3.32±14.47 | 0.26 |
| IGs (k/µl) | 0.11±0.28 | 0.09±0.10 | 0.07 |
| PLTs (k/µl) | 230.47±84.85 | 185.32±60.69 | 0.002 |
| D-dimers
(µg/ml) | 2.26±4.54 | 3.61±6.36 | 0.29 |
| Creatinine
(mg/dl) | 1.38±1.90 | 1.37±1.34 | 0.023 |
| AST (U/l) | 53.14±37.80 | 50.26±29.40 | 0.85 |
| ALT (U/l) | 53.80±54.41 | 36.91±25.75 | 0.09 |
| ALP (U/l) | 71.02±33.71 | 68.94±32.24 | 0.65 |
| GGT (U/l | 68.94±58.84 | 82.85±109.53 | 0.89 |
| LDH (U/l) | 439.13±178.75 | 463.94±204.95 | 0.57 |
| CRP (mg/l) | 125.50±81.12 | 129.57±70.76 | 0.79 |
| Fibrinogen
(mg/dl) | 641.11±157.8 | 650.91±133.66 | 0.75 |
| Ferritin
(ng/ml) |
1,374.27±1,765.06 |
1,954.02±1,990.64 | 0.036 |
| Albumin (g/l) | 37.72±4.57 | 36,.20±6.05 | 0.17 |
| NLR | 9.69±13.15 | 10.88±7.98 | 0.62 |
| PLR | 358.09±589.22 | 274.33±190.30 | 0.41 |
| CAR | 3.41±2.38 | 3.95±2.58 | 0.40 |
| Chest X-ray
score | 8.81±3.04 | 9.71±3.04 | 0.066 |
Cox proportional hazards multivariate regression
analysis with mortality as the outcome identified PLTs as an
independent factor predicting mortality in males (Table VII). In addition, the male
patients that were intubated were older, had higher values of CCI,
creatinine and ferritin and lower values of platelets (P<0.05;
Table VI). Moreover, Cox
proportional hazards multivariate regression analysis with
intubation as the outcome, identified PLTs as an independent factor
predicting intubation in male patients (Table VII).
 | Table VIICox regression multivariable analysis
for male patients. |
Table VII
Cox regression multivariable analysis
for male patients.
| Outcome,
mortality |
|---|
| | 95% CI for
Exp(B) |
|---|
| Parameter | P-value | Exp(B) | Lower | Upper |
|---|
| Age (years) | 0.052 | 1.056 | 0.999 | 1.115 |
| X-ray score | 0.393 | 1.055 | 0.933 | 1.193 |
| CCI | 0.959 | 1.010 | 0.686 | 1.488 |
| PLTs (K/µl) | 0.048 | 0.994 | 0.987 | 0.999 |
| Creatinine
(mg/dl) | 0.868 | 0.981 | 0.778 | 1.236 |
| Albumin (g/l) | 0.828 | 1.009 | 0.929 | 1.096 |
| Outcome,
intubation |
| | 95% CI for
Exp(B) |
| Parameter | P-value | Exp(B) | Lower | Upper |
| Age (years | 0.244 | 1.027 | 0.982 | 1.074 |
| CCI | 0.781 | 1.049 | 0.750 | 1.467 |
| PLTs (K/µl) | 0.025 | 0.994 | 0.988 | 0.999 |
| Creatinine
(mg/dl) | 0.686 | 0.947 | 0.727 | 1.233 |
| Ferritin
(ng/ml) | 0.367 | 1.000 | 0.997 | 0.999 |
As regards the female patients, the survivors were
younger, had lower values of CCI, and greater values of GGT and CAR
compared to the non-survivors (P<0.05). In addition, female
patients that were intubated had lower values of lymphocytes
compared to those that were not intubated (P<0.05; Table VIII). Furthermore, Cox
proportional hazards multivariate regression analysis with
mortality as the outcome did not identify any independent factor
predicting mortality in female patients (Table IX).
 | Table VIIIUnivariate analysis for female
patients. |
Table VIII
Univariate analysis for female
patients.
| Outcome,
mortality |
|---|
| Parameter | Survivors | Non-survivors | P-value |
|---|
| Age (years) | 60.52±13.55 | 71.00±10.10 | 0.02 |
| CCI | 2.07±1.61 | 3.59±1.44 | 0.001 |
| Hb (g/dl) | 12.76±1.07 | 13.26±1.64 | 0.18 |
| WBC (k/µl) | 10.39±15.13 | 7.49±3.00 | 0.98 |
| Neutrophils
(k/µl) | 6.36±3.64 | 6.06±2.90 | 0.97 |
| Lymphocytes
(k/µl) | 3.60±13.11 | 1.02±0.48 | 0.41 |
| IGs (k/µl) | 0.14±0.23 | 0.07±0.13 | 0.18 |
| PLTs (k/µl) | 213.77±80.72 | 211.78±72.07 | 0.90 |
| D-dimers
(µg/ml) | 2.05±3.86 | 1.36±1.43 | 0.60 |
| Creatinine
(mg/dl) | 0.74±0.19 | 1.24±2.13 | 0.26 |
| AST (U/l) | 51.89±34.66 | 43.33±20.53 | 0.56 |
| ALT (U/l) | 42.74±31.88 | 30.59±21.87 | 0.15 |
| ALP (U/l) | 82.70±41.11 | 73.58±23.41 | 0.97 |
| GGT (U/l) | 75.07±75.15 | 56.96±90.43 | 0.04 |
| LDH (U/l) | 460.19±163.15 | 418.96±167.67 | 0.14 |
| CRP (mg/l) | 120.73±90.55 | 92.91±89.32 | 10.13 |
| Fibrinogen
(mg/dl) | 579.47±184.11 | 562,42±168.31 | 0.72 |
| Ferritin
(ng/ml) |
882.96±1,062.10 |
953.92±1,393.28 | 0.52 |
| Albumin (g/l) | 37.22±4.45 | 37.53±5.56 | 0.84 |
| NLR | 6.67±5.11 | 7.93±9.29 | 0.53 |
| PLR | 229.21±162.91 | 270.22±215.71 | 0.36 |
| CAR | 3.23±2.75 | 1.92±2.14 | 0.03 |
| Chest X-ray
score | 10.31±2.69 | 9.56±3.25 | 0.29 |
| Outcome,
intubation |
| Parameter | Non-intubated | Intubated | P-value |
| Age (years) | 64.10±16.03 | 67.83±7.45 | 0.26 |
| CCI | 2.57±1.94 | 3.17±1.30 | 0.16 |
| Hb (g/dl) | 12.71±1.26 | 13.39±1.48 | 0.07 |
| WBC (k/µl) | 10.14±14.42 | 7.44±2.76 | 0.78 |
| Neutrophils
(k/µl) | 6.24±3.64 | 6.17±2.8 | 0.67 |
| Lymphocytes
(k/µl) | 3.42±12.43 | 0.93±0.42 | 0.043 |
| IGs (k/µl) | 0.13±0.22 | 0.08±0.13 | 0.51 |
| PLTs (k/µl) | 208.28±80.66 | 218.17±70.56 | 0.32 |
| D-dimers
(µg/ml) | 2.11±3.73 | 1.21±1.17 | 0.37 |
| Creatinine
(mg/dl) | 0.78±0.23 | 1.25±2.26 | 0.81 |
| AST (U/l | 48.87±34.01 | 46.04±20.37 | 0.65 |
| ALT (U/l | 38.80±31.68 | 34.00±22.31 | 0.92 |
| ALP (U/l) | 80.90±41.24 | 75.00±21.55 | 0.52 |
| GGT (U/l) | 66.48±75.10 | 65.83±92.7 | 0.79 |
| LDH (U/l) | 433.27±167.32 | 447.46±165.69 | 0.68 |
| CRP (mg/l | 110.84±90.31 | 101.78±91.72 | 0.56 |
| Fibrinogen
(mg/dl) | 545.59±181.49 | 602.91±164.46 | 0.24 |
| Ferritin
(ng/ml) |
1,075.93±1,577.99 | 721.58±582.44 | 0.70 |
| Albumin (g/l) | 37.43±4.55 | 37.25±5.57 | 0.91 |
| NLR | 5.96±4.42 | 8.97±9.90 | 0.10 |
| PLR | 206.39±139.84 | 302.93±230.84 | 0.06 |
| CAR | 2.93±2.76 | 2.20±2.21 | 0.21 |
| Chest X-ray
score | 10.69±2.02 | 9.00±3.68 | 0.08 |
 | Table IXCox regression multivariable analysis
for female patients. |
Table IX
Cox regression multivariable analysis
for female patients.
| Outcome,
mortality |
|---|
| | 95% CI for
Exp(B) |
|---|
| Parameter | P-value | Exp(B) | Lower | Upper |
|---|
| CCI | 0.891 | 0.960 | 0.533 | 1.728 |
| Age (years) | 0.317 | 1.040 | 0.963 | 1.123 |
| CAR | 0.413 | 0.904 | 0.710 | 1.151 |
| GGT (U/l) | 0.843 | 0.999 | 0.994 | 1.005 |
Discussion
The mortality rate found in the present study was
relatively higher compared to the rates demonstrated in randomized
controlled trials of tocilizumab and in meta-analyses mentioning a
pooled mortality prevalence of 20-30% (17-21).
One of the main findings of the present study was that among all
patients treated with tocilizumab for COVID-19-associated
pneumonia, age was the only independent factor predicting 90-day
mortality.
Previous research has indicated that age is a major
risk factor for mortality in SARS-CoV-2 infection (22-24).
As a result of age-related hematopoietic mosaic chromosomal changes
that decrease immunity, age has been revealed to be the main risk
factor for infections and the accompanying mortality (25). Moreover, differences in lung
structure, muscular atrophy, poor airway clearance, diminished lung
reserve, diminished resistance to infections, the increased
expression of angiotensin converting enzyme (ACE)-2, particularly
among the elderly receiving ACE inhibitors and angiotensin II
receptor blockers, along with prior exposure to circulating
coronaviruses with reduced neutralizing capacity to SARS-CoV-2, may
all contribute to an increased vulnerability of elderly individuals
to this infection and poor outcomes (26). Similar to the findings of the
present study, age has been reported as a predictor of mortality in
patients receiving tocilizumab for SARS-CoV-2 infection in other
studies (27-33).
Another notable finding of the present study was
that the mortality rate was higher among female patients compared
to male patients. However, sex was not identified as an independent
factor predicting mortality according to the multivariate analysis,
as has been shown in previous research (34). It has been reported that males have
significantly higher rates of adverse events and mortality due to
COVID-19(35). Biological sex
differences manifest as differences in the balance between
inflammation and tissue healing following the resolution of
infection, differences in the time of pathogenesis, differences in
innate viral control and adaptive immune responses, and a
difference in vulnerability to infection (36). These disparities in sex are most
likely pathogen-specific and complex in nature. Thus far, changes
in immune function linked with the X chromosome, the impact of sex
hormones and sex-related behavioral and sociocultural variables
have been hypothesized to explain male-female discrepancies in
SARS-CoV-2 infection. For example, the existence of monoallelic vs.
biallelic ACE2 and Toll-like receptor 7 genes on the X chromosome
may help to explain the greater risk of COVID-19 infection in males
compared to females (37).
Several studies have reported laboratory parameters,
such as eosinophils, lymphocytes, PLTs, immature granulocytes,
ferritin and liver enzymes as biomarkers of poor outcomes in
patients with COVID-19 (36,38-42).
Of note, only a few studies have evaluated laboratory data as
biomarkers of poor outcomes in tocilizumab-treated patients with
COVID-19 (29-34,43-45).
Some studies have examined the role of laboratory parameters on
specific days following the tocilizumab administration as potential
markers of mortality (31,45). According to the aforementioned
studies, d-dimer levels (29), the
WBC count (30), LDH levels
(32,45), procalcitonin levels (33), ferritin (43), AST levels (44), CRP levels (34), lymphocyte count (44,45)
and the number of PLTs (31,34,44),
have all been identified as predictors of poor outcomes in
tocilizumab-treated patients with COVID-19. Of note, the value of
PTLs upon admission and following the tocilizumab administration
was found to be significantly associated with mortality in the
study by Sarabia De Ardanaz et al (31).
The present study did not identify any independent
laboratory factors predicting intubation or mortality in the
population examined. However, when analyzing females and males who
had a difference in mortality rate separately, it was identified
that the value of PLTs was the only independent factor predicting
intubation and 90-day mortality in male patients, and the
lymphocyte count was the only factor associated with intubation in
female patients. Several clinical studies have found that increased
platelet activation leads to platelet deposition in injured
pulmonary blood arteries, and thrombocytopenia is a common
characteristic of SARS-CoV-2 infection (46,47).
Thrombocytopenia is a major predictor of a poor prognosis. PLTs in
patients with SARS-CoV-2 are inversely linked with soluble vascular
cell adhesion molecule-1 (sVCAM-1) levels. sVCAM-1 is involved in
adhesion and chemotaxis, and it leads to early vascular damage and
T-cell inhibition. The poor outcome observed may be explained by
vascular damage or immunosuppression (48).
The present study is one of a handful of studies
evaluating laboratory data as biomarkers of poor outcomes in
tocilizumab-treated patients with COVID-19 and, to the best of our
knowledge, the first to mention PLTs as an independent factor
predicting intubation and 90-day mortality in male patients treated
with tocilizumab and the lymphocyte count as the only factor
associated with intubation in female patients treated with
tocilizumab.
The present study has certain limitations which
should be mentioned. It was of a retrospective design, and there
was no control group. Furthermore, it is possible that the negative
results obtained in the present study (all-cause mortality) may be
attributable to other etiologies in addition to severe COVID-19
(thromboembolism, sepsis, or coexisting diseases). The advantages
of the present study were the relatively large number of
tocilizumab-treated patients, the reliable follow-up data and the
availability of 90-day data. Another strong point of the study was
that participants were patients with COVID-19 admitted between
September 21, 2020 and April 15, 2022, covering the periods of
alpha, delta and omicron variant predominance.
In conclusion, in the present retrospective study,
mortality occurred in 35.1% of the tocilizumab-treated COVID-19
patients, with a greater rate of mortality observed among females.
The only independent prognosticator of mortality in the study
population was age. In addition, the value of PLTs was an
independent factor predicting intubation and 90-day mortality in
male patients treated with tocilizumab, and the lymphocyte count
was the only factor associated with intubation in female patients
treated with tocilizumab. These data may be used to identify
patient subpopulations responding to therapy in prospective
clinical trials investigating the efficacy of treatment with
tocilizumab.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DB, GK and VEG conceptualized the study. VEG, DB,
PMV, GK, IrE, SS, SM, OK, IoE, MT, AB, CVP and AA advised on
patient care and medical treatment, obtained patient data, wrote
and prepared the draft of the manuscript and made substantial
contributions to the acquisition and interpretation of data. DAS,
PP, AG and NVS analyzed the data and provided critical revisions.
VEG and NVS confirm the authenticity of all the data. All authors
contributed to manuscript revision. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in line with the
Declaration of Helsinki and obtained approval by the Institutional
Review Board of Laiko General Hospital (protocol no. 765/12-2021).
Written informed was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: WHO coronavirus
(COVID-19) dashboard. https://covid19.who.int/. Accessed August 28,
2022.
|
|
2
|
Naleway AL, Groom HC, Crawford PM, Salas
SB, Henninger ML, Donald JL, Smith N, Thompson MG, Blanton LH,
Bozio CH and Azziz-Baumgartner E: Incidence of SARS-CoV-2
infection, emergency department visits, and hospitalizations
because of COVID-19 among persons aged ≥12 years, by COVID-19
vaccination status-oregon and washington, July 4-September 25,
2021. MMWR Morb Mortal Wkly Rep. 70:1608–1612. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Paidas MJ, Sampath N, Schindler EA, Cosio
DS, Ndubizu CO, Shamaladevi N, Kwal J, Rodriguez S, Ahmad A, Kenyon
NS and Jayakumar AR: Mechanism of Multi-organ injury in
experimental COVID-19 and its inhibition by a small molecule
peptide. Front Pharmacol. 13(864798)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Darif D, Hammi I, Kihel A, El Idrissi Saik
I, Guessous F and Akarid K: The pro-inflammatory cytokines in
COVID-19 pathogenesis: What goes wrong? Microb Pathog.
153(104799)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mogensen TH: Pathogen recognition and
inflammatory signaling in innate immune defenses. Clin Microbiol
Rev. 22:240–273. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen LYC, Hoiland RL, Stukas S, Wellington
CL and Sekhon MS: Confronting the controversy: Interleukin-6 and
the COVID-19 cytokine storm syndrome. Eur Respir J.
56(2003006)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fraenkel L, Bathon JM, England BR, St
Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK,
Kerr G, et al: 2021 American college of rheumatology guideline for
the treatment of rheumatoid arthritis. Arthritis Care Res
(Hoboken). 73:924–939. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
RECOVERY Collaborative Group. Tocilizumab
in patients admitted to hospital with COVID-19 (RECOVERY): A
randomised, controlled, open-label, platform trial. Lancet.
397:1637–1645. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
REMAP-CAP Investigators, Gordon AC,
Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D,
Beane A, van Bentum-Puijk W, et al: Interleukin-6 receptor
antagonists in critically ill patients with Covid-19. N Engl J Med.
384:1491–1502. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Berardicurti O, Ruscitti P, Ursini F,
D'Andrea S, Ciaffi J, Meliconi R, Iagnocco A, Cipriani P and
Giacomelli R: Mortality in tocilizumab-treated patients with
COVID-19: A systematic review and meta-analysis. Clin Exp
Rheumatol. 38:1247–1254. 2020.PubMed/NCBI
|
|
11
|
Ghosn L, Chaimani A, Evrenoglou T,
Davidson M, Graña C, Schmucker C, Bollig C, Henschke N, Sguassero
Y, Nejstgaard CH, et al: Interleukin-6 blocking agents for treating
COVID-19: A living systematic review. Cochrane Database Syst Rev.
3(CD013881)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
WHO Rapid Evidence Appraisal for COVID-19
Therapies (REACT) Working Group. Shankar-Hari M, Vale CL, Godolphin
PJ, Fisher D, Higgins JPT, Spiga F, Savovic J, Tierney J, Baron G,
et al: Association between administration of IL-6 antagonists and
mortality among patients hospitalized for COVID-19: A
meta-analysis. JAMA. 326:499–518. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
San-Juan R, Fernández-Ruiz M,
López-Medrano F, Carretero O, Lalueza A, Maestro de la Calle G,
Pérez-Jacoiste Asín MA, Bueno H, Caro-Teller JM, Catalán M, et al:
Analysis of the factors predicting clinical response to tocilizumab
therapy in patients with severe COVID-19. Int J Infect Dis.
117:56–64. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
World Health Organization: Therapeutics
and COVID-19. Living guideline, July 6, 2021. https://apps.who.int/iris/bitstream/handle/10665/342368/WHO-2019-nCoV-therapeutics-2021.2-eng.pdf.
Accessed August 28, 2022.
|
|
15
|
Salvarani C, Dolci G, Massari M, Merlo DF,
Cavuto S, Savoldi L, Bruzzi P, Boni F, Braglia L, Turrà C, et al:
Effect of tocilizumab vs standard care on clinical worsening in
patients hospitalized with COVID-19 pneumonia: A randomized
clinical trial. JAMA Intern Med. 181:24–31. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Setiawati R, Widyoningroem A, Handarini T,
Hayati F, Basja AT, Putri ARDS, Jaya MG, Andriani J, Tanadi MR and
Kamal IH: Modified chest X-Ray scoring system in evaluating
severity of COVID-19 patient in Dr. Soetomo general hospital
Surabaya, Indonesia. Int J Gen Med. 14:2407–2412. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang J, Chen C, Yang Y and Yang J:
Effectiveness of tocilizumab in the treatment of hospitalized
adults COVID-19: A systematic review and meta-analysis. Medicine
(Baltimore). 101(e28967)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peng J, She X, Mei H, Zheng H, Fu M, Liang
G, Wang Q and Liu W: Association between tocilizumab treatment and
clinical outcomes of COVID-19 patients: A systematic review and
meta-analysis. Aging (Albany NY). 14:557–571. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Luo L, Luo T, Du M, Mei H and Hu Y:
Efficacy and safety of tocilizumab in hospitalized COVID-19
patients: A systematic review and meta-analysis. J Infect.
84:418–467. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vela D, Vela-Gaxha Z, Rexhepi M, Olloni R,
Hyseni V and Nallbani R: Efficacy and safety of tocilizumab versus
standard care/placebo in patients with COVID-19; a systematic
review and meta-analysis of randomized clinical trials. Br J Clin
Pharmacol. 88:1955–1963. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Piscoya A, Parra Del Riego A,
Cerna-Viacava R, Rocco J, Roman YM, Escobedo AA, Pasupuleti V,
White CM and Hernandez AV: Efficacy and harms of tocilizumab for
the treatment of COVID-19 patients: A systematic review and
meta-analysis. PLoS One. 17(e0269368)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kokkoris S, Gkoufa A, Maneta E, Doumas G,
Mizi E, Georgakopoulou VE, Sigala I, Dima E, Papachatzakis I,
Ntaidou TK, et al: Older adults with severe coronavirus disease
2019 admitted to intensive care unit: Prevalence, characteristics
and risk factors for mortality. Minerva Anestesiol: Apr 13, 2022
(Epub ahead of print).
|
|
24
|
Gkoufa A, Maneta E, Ntoumas GN,
Georgakopoulou VE, Mantelou A, Kokkoris S and Routsi C: Elderly
adults with COVID-19 admitted to intensive care unit: A narrative
review. World J Crit Care Med. 10:278–289. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zekavat SM, Lin SH, Bick AG, Liu A,
Paruchuri K, Wang C, Uddin MM, Ye Y, Yu Z, Liu X, et al:
Hematopoietic mosaic chromosomal alterations increase the risk for
diverse types of infection. Nat Med. 27:1012–1024. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Georgakopoulou VE, Papalexis P, Sanos C,
Bitsani A, Garmpi A, Damaskos C, Garmpis N, Gkoufa A, Chlapoutakis
S, Sklapani P, et al: Asymptomatic SARS-CoV-2 infection in an
unvaccinated 97-year-old woman: A case report. Biomed Rep.
15(107)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
AlQahtani H, AlBilal S, Mahmoud E,
Aldibasi O, Alharbi A, Shamas N, Alsaedy A, Owaidah K, Alqahtani
FY, Aleanizy FS, et al: Outcomes associated with tocilizumab with
or without corticosteroid versus dexamethasone for treatment of
patients with severe to critical COVID-19 pneumonia. J Infect
Public Health. 15:36–41. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Duarte-Millán MA, Mesa-Plaza N,
Guerrero-Santillán M, Morales-Ortega A, Bernal-Bello D,
Farfán-Sedano AI, García de Viedma-García V, Velázquez-Ríos L,
Frutos-Pérez B, De Ancos-Aracil CL, et al: Prognostic factors and
combined use of tocilizumab and corticosteroids in a Spanish cohort
of elderly COVID-19 patients. J Med Virol. 94:1540–1549.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Desai HD, Sharma K, Parikh A, Patel K,
Trivedi J, Desai R, Patel PP, Patel Z, Patel S and Kini S:
Predictors of mortality amongst tocilizumab administered COVID-19
asian indians: A predictive study from a tertiary care centre.
Cureus. 13(e13116)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ercan S, Ergan B, Özuygur SS, Korkmaz P,
Taşbakan MS, Basoglu ÖK, Kerget B, Akgün M, Elbek O, Sayıner A and
Kılınç O: Clinical predictors of response to tocilizumab: A
retrospective multicenter study. Turk Thorac J. 23:225–230.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sarabia De Ardanaz L, Andreu-Ubero JM,
Navidad-Fuentes M, Ferrer-González MÁ, Ruíz Del Valle V,
Salcedo-Bellido I, Barrios-Rodríguez R, Cáliz-Cáliz R and Requena
P: Tocilizumab in COVID-19: Factors associated with mortality
before and after treatment. Front Pharmacol.
12(620187)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pagkratis K, Chrysikos S, Antonakis E,
Pandi A, Kosti CN, Markatis E, Hillas G, Digalaki A, Koukidou S,
Chaini E, et al: Predictors of mortality in tocilizumab-treated
severe COVID-19. Vaccines (Basel). 10(978)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Masotti L, Landini G, Panigada G, Grifoni
E, Tarquini R, Cei F, Cimolato BMA, Vannucchi V, Di Pietro M, Piani
F, et al: Predictors of poor outcome in tocilizumab treated
patients with Sars-CoV-2 related severe respiratory failure: A
multicentre real world study. Int Immunopharmacol.
107(108709)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mussini C, Cozzi-Lepri A, Menozzi M,
Meschiari M, Franceschini E, Milic J, Brugioni L, Pietrangelo A,
Girardis M, Cossarizza A, et al: Development and validation of a
prediction model for tocilizumab failure in hospitalized patients
with SARS-CoV-2 infection. PLoS One. 16(e0247275)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Biolè C, Bianco M, Núñez-Gil IJ, Cerrato
E, Spirito A, Roubin SR, Viana-Llamas MC, Gonzalez A, Castro-Mejía
AF, Eid CM, et al: Gender differences in the presentation and
outcomes of hospitalized patients with COVID-19. J Hosp Med.
16:349–352. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Brook R, Lim HY, Ho P and Choy KW: Risk
factors and early prediction of clinical deterioration and
mortality in adult COVID-19 inpatients: An Australian tertiary
hospital experience. Intern Med J. 52:550–558. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fortunato F, Martinelli D, Lo Caputo S,
Santantonio T, Dattoli V, Lopalco PL and Prato R: Sex and gender
differences in COVID-19: An Italian local register-based study. BMJ
Open. 11(e051506)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Georgakopoulou VE, Garmpis N, Damaskos C,
Valsami S, Dimitroulis D, Diamantis E, Farmaki P, Papageorgiou CV,
Makrodimitri S, Gravvanis N, et al: The impact of peripheral
eosinophil counts and eosinophil to lymphocyte ratio (ELR) in the
clinical course of COVID-19 patients: A retrospective study. In
Vivo. 35:641–648. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Georgakopoulou VE, Lembessis P, Skarlis C,
Gkoufa A, Sipsas NV and Mavragani CP: Hematological abnormalities
in COVID-19 disease: Association with type I interferon pathway
activation and disease outcomes. Front Med (Lausanne).
9(850472)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Georgakopoulou VE, Vlachogiannis NI,
Basoulis D, Eliadi I, Georgiopoulos G, Karamanakos G, Makrodimitri
S, Samara S, Triantafyllou M, Voutsinas PM, et al: A simple
prognostic score for critical COVID-19 derived from patients
without comorbidities performs well in unselected patients. J Clin
Med. 11(1810)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Georgakopoulou VE, Makrodimitri S,
Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A,
Papageorgiou CV, Spandidos DA, Gkoufa A, Papalexis P, et al:
Immature granulocytes: Innovative biomarker for SARS-CoV-2
infection. Mol Med Rep. 26(217)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cholongitas E, Bali T, Georgakopoulou VE,
Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D,
Basoulis D, Eliadi I, Karamanakos G, et al: Prevalence of abnormal
liver biochemistry and its impact on COVID-19 patients' outcomes: A
single-center Greek study. Ann Gastroenterol. 35:290–296.
2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tom J, Bao M, Tsai L, Qamra A, Summers D,
Carrasco-Triguero M, McBride J, Rosenberger CM, Lin CJF, Stubbings
W, et al: Prognostic and predictive biomarkers in patients with
coronavirus disease 2019 treated with tocilizumab in a randomized
controlled trial. Crit Care Med. 50:398–409. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lohse A, Klopfenstein T, Balblanc JC,
Royer PY, Bossert M, Gendrin V, Charpentier A, Bozgan AM, Badie J,
Bourgoin C, et al: Predictive factors of mortality in patients
treated with tocilizumab for acute respiratory distress syndrome
related to coronavirus disease 2019 (COVID-19). Microbes Infect.
22:500–503. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lakatos B, Szabo BG, Bobek I, Gopcsa L,
Beko G, Kiss-Dala N, Petrik B, Gaspar Z, Farkas BF, Sinko J, et al:
Laboratory parameters predicting mortality of adult in-patients
with COVID-19 associated cytokine release syndrome treated with
high-dose tocilizumab. Acta Microbiol Immunol Hung: Aug 6, 2021
(Epub ahead of print).
|
|
46
|
Bone RC, Francis PB and Pierce AK:
Intravascular coagulation associated with the adult respiratory
distress syndrome. Am J Med. 61:585–589. 1976.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Peiris JS, Yuen KY, Osterhaus AD and Stöhr
K: The severe acute respiratory syndrome. N Engl J Med.
349:2431–2441. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao
S, Long D and Yu L: Association between platelet parameters and
mortality in coronavirus disease 2019: Retrospective cohort study.
Platelets. 31:490–496. 2020.PubMed/NCBI View Article : Google Scholar
|