Introduction
Cervical cancer is one of the most common
gynecological malignant tumors (1). In recent years, its incidence rate
has tended towards an increase in younger women, and the mortality
rate associated with cervical cancer is increasing year by year,
posing a serious threat to health and life of women (1). Around 570,000 newly diagnosed cases
and 311,000 deaths worldwide are reported in 2018(2). At present, the most commonly used
treatment methods in the clinic are surgery and chemoradiotherapy
(3,4). However, one adverse effect of using
chemoradiotherapy is the damage caused by radiotherapy to the
ovarian function of young patients, which is not negligible;
furthermore, the commonly used chemotherapy drugs, such as platinum
drugs, are far from ideal when applied in the treatment of cervical
cancer due to their toxicity and other side effects, and also
because of the development of drug resistance (5,6).
Therefore, an exploration of the pathogenesis and targeting factors
of cervical cancer would be conducive to the development of
effective tumor suppressor drugs with low toxicity.
p53 apoptosis-stimulating protein 2 (ASPP2) is a
member of the ASPP family, which has characteristic sequences of
ankyrin repeats, an SH3 domain and a proline-rich region (7). ASPP2 can directly interact with p53
family members and selectively enhance their transcriptional
activities toward pro-apoptosis genes (8). ASPP2 is commonly considered as a
tumor suppressor by promoting cancer cell apoptosis and inhibiting
cell migration and growth (9-11).
Previous studies have shown that the expression of ASPP2 is
downregulated in breast cancer, liver cancer and other malignant
tumors, and its downregulated level is associated with an advanced
tumor stage and poor prognosis, suggesting that this gene may be an
important target for tumor therapy (12-14).
It has been previously observed that ASPP2 is also downregulated in
cervical cancer (15); however, to
the best of the authors' knowledge, the direct molecular regulation
and underlying mechanism of ASPP2 in cervical cancer have yet to be
completely elucidated. In addition, previous studies have revealed
the involvement of ASPP2 in the regulation of autophagy in
hepatocellular carcinoma, colorectal cancer and which was
associated with BECN1-dependent autophagy initiation (16-18),
thus, whether ASPP2 got involved in cervical cancer development by
regulating autophagy raised our interest.
Therefore, the aim of the present study was to
perform a systematic analysis of ASPP2 in order to unravel the
underlying mechanism of cervical carcinogenesis, and to provide new
leads for novel treatment strategies.
Materials and methods
Patient samples
Tissue samples from patients were collected between
March 2018 and September 2019 at Yancheng First People's Hospital
(Yancheng, China). Those who received preoperative chemotherapy or
radiotherapy or had concurrent or successive primary malignancies
were excluded. The patients were aged 30-69 years old. A total of
30 patients diagnosed with cervical cancer were included in the
present study, and tumor tissues and para-tumor tissues were
collected for the subsequent investigation. All experiments were
approved [approval no. (2018)-(k022)] by the ethics committee of
Yancheng First People's Hospital (Yancheng, China). All
participants signed informed consent forms and gave their approval
for this article to be published.
Cell culture and treatment
Human cervical squamous cell carcinoma cell lines
(Ca-Ski, Hela, SiHa and c-33A) were obtained from Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). A
non-cancerous ectocervical epithelial cell line (Ect1/E6E7) was
obtained from American Type Culture Collection (ATCC) and
maintained in Gibco® Keratinocyte-Serum Free medium with
0.1 ng/ml human recombinant epidermal growth factor, 0.05 mg/ml
bovine pituitary extract, and 0.4 mM calcium chloride. Ca-Ski cells
were cultured in the Gibco® RPMI-1640 medium and the
other cells were cultured in the Gibco® DMEM medium
(Thermo Fisher Scientific, Inc.) supplemented with 10% Gibco FBS
(Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin.
All cell lines were incubated at 37˚C in an incubator in an
atmosphere containing 5% CO2. The cells were treated
with 30 µM Tat-BECN1 (Selleck Chemicals), a potent and specific
autophagy inducer via activating Beclin1 (19,20),
or 200 ng/ml TNF-related apoptosis-inducing ligand (TRAIL; Merck
KGaA).
Cell transfection
The ASPP2 overexpression vector (Ov-ASPP2) was
constructed by inserting the full length of ASPP2 coding sequences
into pcDNA3.1, which was obtained from Shanghai GenePharma Co.,
Ltd. The empty vector was regarded as the negative control of
overexpression vectors (Ov-NC). The vectors (1 µg) were transfected
into c-33A cells at 37˚C for 48 h using Invitrogen™
Lipofectamine 2000™ (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After 48 h, the transfection efficiency was assessed using western
blotting analysis.
Cell Counting Kit (CCK)-8 assay
C-33A cells (at a density of 2,000 cells/well) were
seeded into a 96-well plate and divided into the control, Ov-NC,
Ov-ASPP2 and Ov-ASPP2 + Tat-BECN1 groups. CCK-8 reaction solution
(10 µM) was added to the cells at 24, 48 and 72 h after
transfection or treatment, and after having incubated the cells for
a further 2 h at 37˚C, the absorbance of each well was measured at
450 nm, and the cell proliferation curves were plotted.
Immunofluorescence
C-33A cells were fixed in 4% formaldehyde at room
temperature for 10 min, before being washed twice with PBS buffer
solution. 0.5% Triton X-100 was adopted to permeabilize cells.
After being blocked with 5% bovine serum albumin (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature, cells were incubated
with anti-LC3B antibody (1:50; cat. no. ab192890; Abcam) at 4˚C
overnight. After washing with PBS, cells were incubated with Alexa
Fluor 488-conjugated secondary antibody (1:200; cat. no. ab150077;
Abcam) at room temperature for 2 h. The nuclei were stained by DAPI
(0.01 mg/ml; Vector Laboratories, Inc.). The images were observed
under a fluorescence microscope (Nikon Corporation).
EdU staining
The cell proliferation level was measured using EdU
staining. An EdU staining kit (cat. no. CA1170) was purchased from
Beijing Solarbio Science & Technology Co., Ltd., and the
experiments were performed according to the instructions provided
with the kit. Briefly, cells at the exponential growth stage were
collected and seeded into 96-well plates at a density of
1x104 cells per well. The cells were cultured to the
normal growth stage. Subsequently, 100 µl EdU culture medium (50 µM
concentration) was added to each well, and the cells were incubated
for 2 h at 37˚C. The cells were then washed 1 or 2 times, and 4%
paraformaldehyde (50 µl) was added to the cells for an incubation
for 30 min at 37˚C. Subsequently, 100 µl 1X Apollo®
staining solution was added to each well, prior to the cells being
incubated in the dark at room temperature for 30 min; the staining
solution was then discarded. Subsequently, the cells were washed
2-3 times with 100 µl 0.5% Triton X-100/PBS, and 0.01 mg/ml DAPI
solution was added to each well; the plates were then incubated in
the dark at room temperature for 30 min, before discarding the
staining solution. Finally, the cells were observed under a
fluorescence microscope (Olympus FV500; Olympus Corporation).
TUNEL assay
C-33A cells were fixed in 4% formaldehyde at room
temperature for 10 min, before being washed twice with PBS buffer
solution. Apoptosis of the c-33A cells was detected using a TUNEL
assay kit (cat. no. ab206386; Abcam), according to the
manufacturer's protocol. Finally, the sections were mounted with
Vectashield® mounting medium containing 50 µg/ml DAPI
for nuclear labeling for 5 min at room temperature (Vector
Laboratories, Inc.). TUNEL-positive cells presented with green
nuclei following visualization under a fluorescence microscope
(Olympus FV500; Olympus Corporation). For quantification, five
randomly selected fields of view were selected.
Reverse transcription-quantitative
(RT-q) PCR analysis
TRIzol® isolation reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was added to lyse the cells and
tissues. Total RNA of the c-33A cells and tissues was extracted
using the phenol-chloroform method after complete lysis had
occurred. The molecular weight and concentration of the extracted
RNAs were analyzed using 1% agarose gel electrophoresis, and by
measuring the ratio of the absorbance at 260-280 nm with an
ultraviolet spectrophotometer (Nanodrop2000; Thermo Fisher
Scientific, Inc.) respectively. The RNA was reverse-transcribed
into complementary (c)DNA according to the poly-A tailing method
using a miScript SYBR® Green PCR kit (Qiagen GmbH)
according to the manufacturer's instructions. The cDNA samples were
then assessed using RT-qPCR with an Applied Biosystems®
7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.), as
recommended by the manufacturer. The PCR thermocycling conditions
were as follows: 95˚C for 5 min; 95˚C for 30 sec and 60˚C for 30
sec, for a total of 40 cycles. The relative amount of mRNA was
quantified using the 2-ΔΔCq method (21), and β-actin was used as an internal
control. The primer sequences were as follows: ASPP2 forward,
5'-GAAGACTCGGTGAGCATGCG-3' and reverse, 5'-GCGATACGCTCTGAGCCAGT-3';
β-actin forward, 5'-AGCGAGCATCCCCCAAAGTT-3' and reverse,
5'-GGGCACGAAGGCTCATCATT-3'. The RT-qPCR experiments were
independently performed three times.
Western blot analysis
The samples (c-33A cells or tissues) were denatured
in a boiling water bath for 10 min. Protein concentrations were
measured using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories,
Inc.). Proteins (30 µg) from each sample were separated using
SDS-PAGE (10%). The protein bands were transferred onto a
polyvinylidene difluoride membrane at a constant current of 300 mA
for 2 h on an ice bath, and the membranes were then blocked with 50
mg/ml skimmed milk for 1 h at room temperature. Primary antibodies
including anti-ASPP2 (1:10,000; cat. no. ab181377), Beclin1
(1:2,000; cat. no. ab207612), p62 (1:1,000; cat. no. ab207305), LC3
II/I (1:2,000; cat. no. ab192890), Ki67 (1:5,000; cat. no.
ab92742), proliferating cell nuclear antigen (PCNA; 1:1,000; cat.
no. ab92552), Bcl-2 (1:1,000; cat. no. ab32124), Bax (1:1,000; cat.
no. ab32503; all from Abcam), cleaved caspase-3 (1:1,000; cat. no.
9661), caspase-3 (1:1,000; cat. no. 9662), cleaved poly ADP-ribose
polymerase (PARP; 1:1,000; cat. no. 5625), PARP (1:1,000; cat. no.
9542, all from Cell Signaling Technology, Inc.), Death receptor 4
(DR4; 1: 500; cat. no. ab216662), DR5 (1:1,000; cat. no. ab8416)
and GAPDH (1:2,500; cat. no. ab9485; all from Abcam) were added,
followed by incubation overnight at 4˚C on a shaker. The membrane
was washed five times with Tris-buffered saline containing 0.1%
Tween 20 (TBST) (5 min each wash). HRP-conjugated secondary
antibodies (1:20,000; cat. no. ab205718; Abcam) were added,
followed by incubation for 1 h at room temperature. The membrane
was then washed five times with TBST for 5 min each wash and
developed with enhanced chemiluminescent liquid (cat. no. P0018A;
Beyotime Institute of Biotechnology). The images were analyzed
using Quantity One 4.62 analysis software (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All experiments were independently performed three
times, and the data are presented as the mean ± standard deviation
(SD). Statistical analysis was performed using GraphPad Prism 8.0.1
software (GraphPad Software, Inc.). Comparison between two groups
were performed with unpaired Student's t-test, while comparisons
among three or more groups were performed with one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
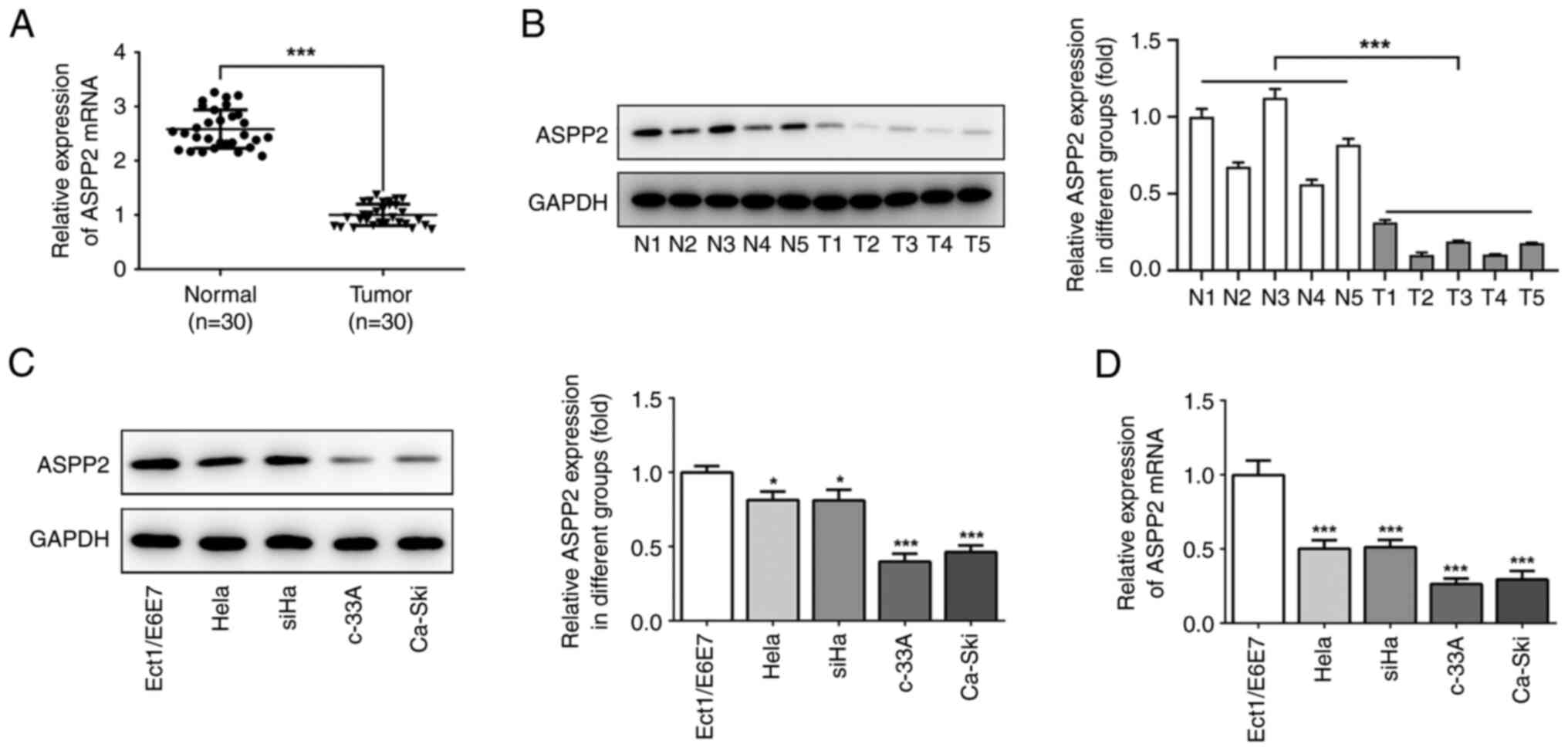
ASPP2 is downregulated in cervical
cancer
To explore the roles of ASPP2 in the process of
cervical cancer, the expression level of ASPP2 in cervical cancer
tissues were first examined using RT-qPCR and western blot
analyses, respectively. As shown in Fig. 1A and B, ASPP2 expression in the cancer tissues
was decreased compared with normal tissues. To further investigate
the expression of ASPP2, a range of cervical cancer cell lines,
including HeLa, SiHa, c-33A and Ca-ski cells, were examined. The
results obtained revealed that ASPP2 expression was decreased in
the human cervical cancer cell lines, particularly in c-33A cells,
compared with Ect1/E6E7 cells, thus c-33A cells were used for the
subsequent experiments (Fig. 1C
and D).
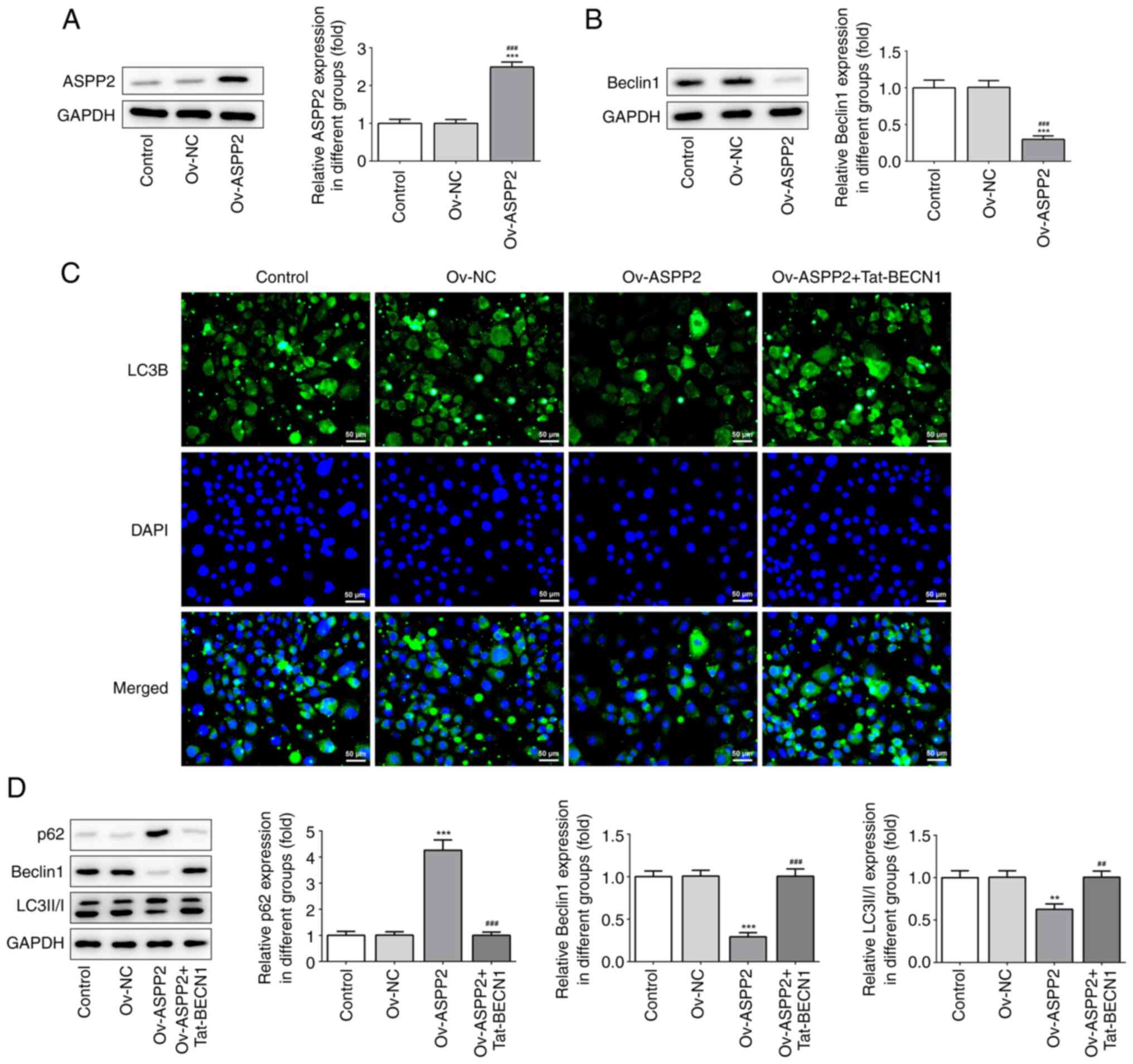
Overexpression of ASPP2 suppresses
Beclin1 expression and autophagy in cervical cancer cells
To further investigate the effects of ASPP2 in
cervical cancer cells, western blot analysis was used to determine
the expression level of ASPP2 after cell transfection. Transfection
with Ov-ASPP2 led to a significant upregulation of ASPP2 expression
(Fig. 2A). Given that a previous
study demonstrated that ASPP2 is able to bind to Beclin1 and to
inhibit Beclin1 expression (15),
the western blotting results in the present study also indicated
that overexpressing ASPP2 caused a significant downregulation of
Beclin1 expression (Fig. 2B).
Subsequently, immunofluorescence assay was subsequently used to
investigate the expression of LC3B. Overexpression of ASPP2 led to
a decrease of LC3B-positive cells; these effects were partly
weakened through additional treatment with Tat-BECN1, the inducer
of autophagy (Fig. 2C). These
results were further confirmed by detecting the expression levels
of LC3II/I, p62 and Beclin1. As revealed in Fig. 2D, Ov-ASPP2 upregulated p62
expression in the c-33A cells, whereas the expression levels of
LC3II/LC3I and Beclin1 were suppressed. By contrast, these trends
were reversed following treatment of the cells with Tat-BECN1.
Collectively, these findings demonstrated that overexpression of
ASPP2 inhibited autophagy.
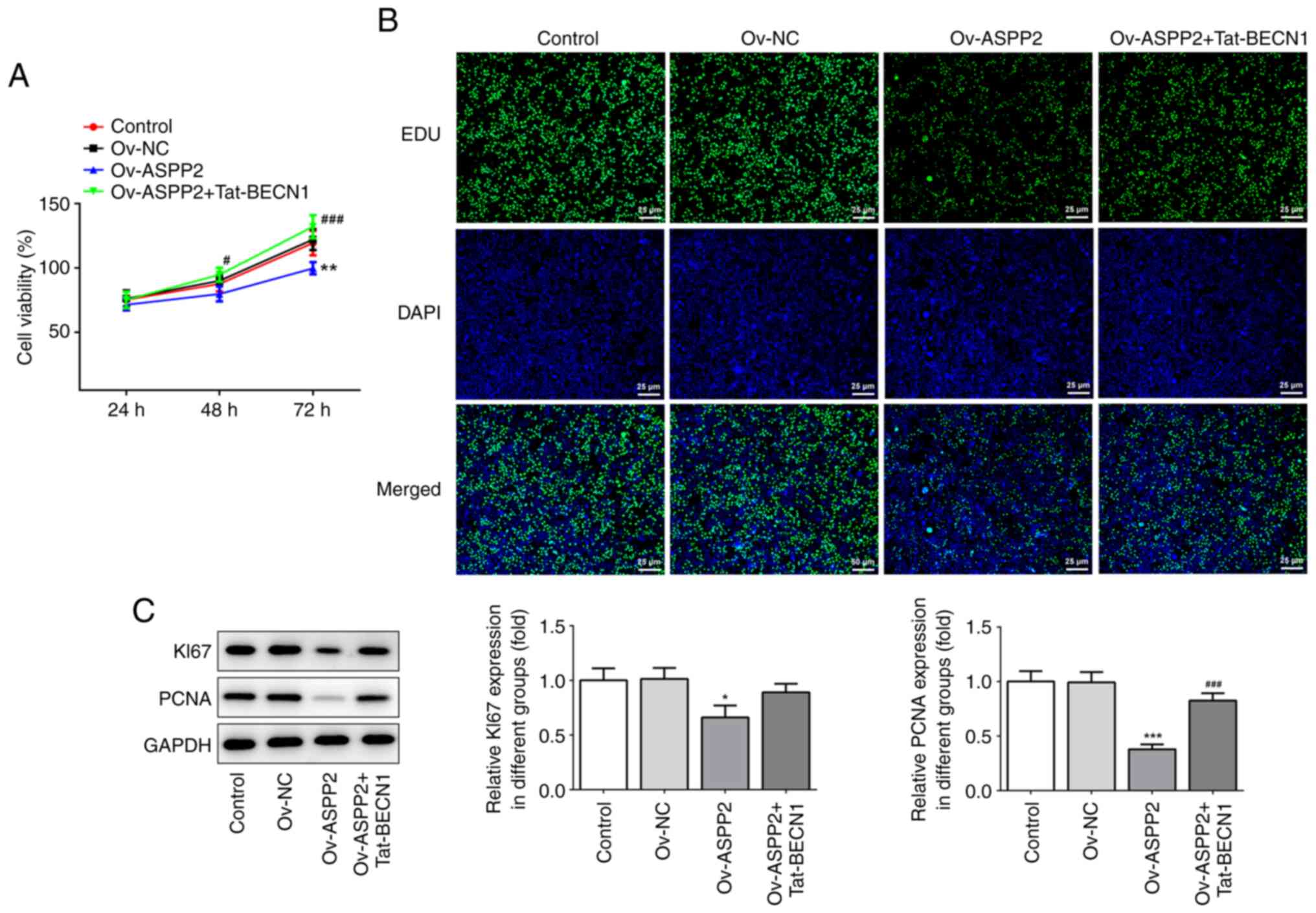
Overexpression of ASPP2 ameliorates
the proliferation of cervical cancer cells through inhibiting
autophagy
Subsequently, CCK-8 assay was performed to assess
the level of cell proliferation. The results obtained showed that
Ov-ASPP2 led to an inhibition of the proliferation of the c-33A
cells at 24, 48 and 72 h compared with the Ov-NC group (Fig. 3A). Additionally, the results of the
EdU staining revealed that overexpression of ASPP2 ameliorated
proliferation of the cervical cancer cells via inhibiting autophagy
(Fig. 3B). Moreover, western blot
assay revealed that the expression levels of Ki67 and PCNA were
significantly decreased in the Ov-ASPP2 group compared with the
Ov-NC group, whereas these trends were reversed following treatment
of the cells with Tat-BECN1 (Fig.
3C).
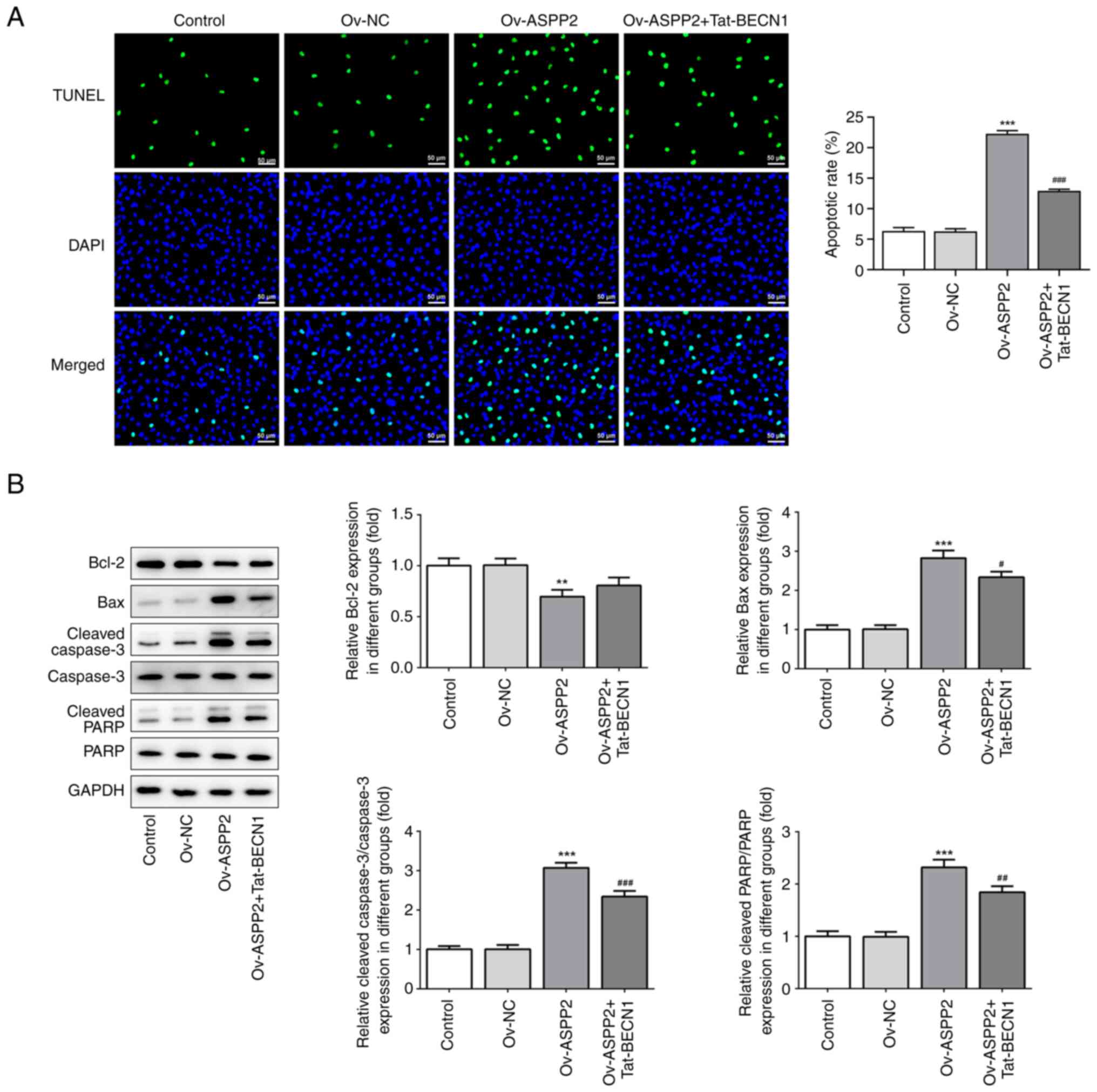
Overexpression of ASPP2 promotes
apoptosis through inhibiting autophagy
To verify the effect of ASPP2 on the apoptosis of
c-33A cells, TUNEL staining assay was performed. These experiments
revealed that the ratio of apoptosis (where green staining
represents the apoptotic cells) in the Ov-ASPP2 group was higher
compared with that in the Ov-NC and control groups, whereas
Tat-BECN1 treatment led to a clear reduction in the extent of cell
apoptosis (Fig. 4A). These results
were further confirmed by detecting the expression of
apoptosis-associated proteins. As demonstrated in Fig. 4B, Ov-ASPP2 led to an upregulation
of the expression levels of Bax, cleaved-caspase3/caspase3 and
cleaved-PARP/PARP in the c-33A cells, although the expression of
the anti-apoptosis protein, Bcl-2, was suppressed; these findings
were found to be reversed upon treating the cells with Tat-BECN1.
Collectively, these results demonstrated that overexpression of
ASPP2 promoted apoptosis through inhibiting autophagy.
Overexpression of ASPP2 leads to an
enhancement in the apoptosis of TRAIL-induced cervical cancer cells
via inhibiting autophagy
The results of the aforementioned studies have
strongly suggested that ASPP2 could promote apoptosis through
inhibiting autophagy, and the inhibition of autophagy may lead to
an increase in TRAIL-induced cell sensitivity and the promotion of
apoptosis of cancer cells (22).
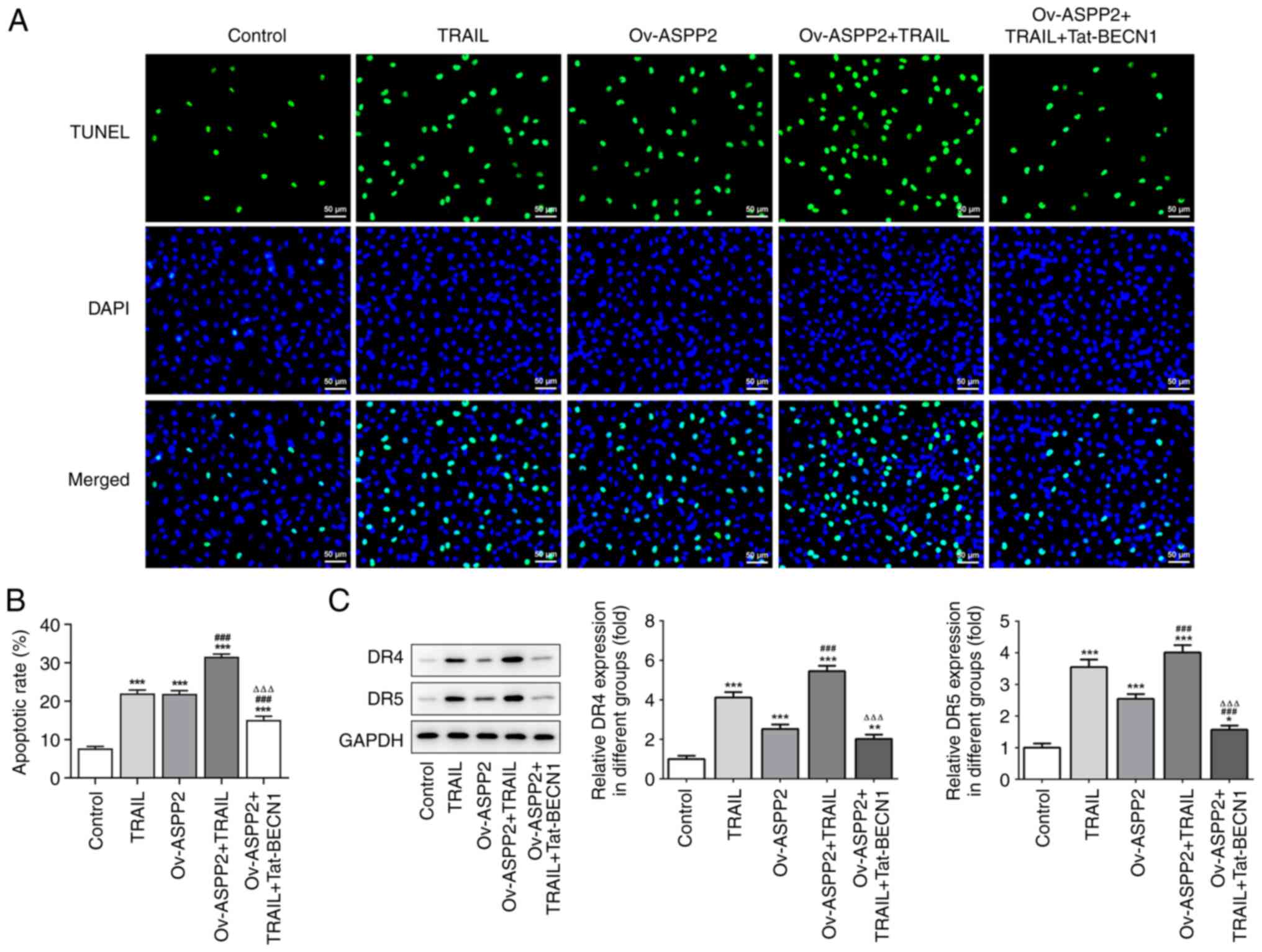
Therefore, TUNEL staining was performed to verify the effects of
ASPP2 on the apoptosis of TRAIL-induced c-33A cells. The ratio of
apoptosis in the Ov-ASPP2 or TRAIL group was found to be higher
than that in the control group, and the combined treatment of TRAIL
and Ov-ASPP2 transfection presented a synergistic effect, whereas
additional treatment with Tat-BECN1 caused a clear reduction in
cell apoptosis in the TRAIL-induced c-33A cells (Fig. 5A and B). The western blot assay results showed
that the expression levels of DR4 and DR5 were upregulated in the
TRAIL and Ov-ASPP2 + TRAIL treatment groups, whereas these trends
were reversed in the Ov-ASPP2 + TRAIL + Tat-BECN1 group (Fig. 5C).
Discussion
In the current study, the data obtained have implied
that ASPP2 is a key factor involved in the apoptosis of cervical
cancer cells. ASPP2 was downregulated in cervical cancer. ASPP2
overexpression could suppress cervical cancer cell autophagy,
proliferation and promote apoptosis, which was partly abolished by
Tat-BECN1, an inducer of autophagy via activating Beclin1, thus it
was suggested that ASPP2 may exert its anti-tumor effect partly via
regulating autophagy. In addition, the present study also addressed
that ASPP2 could enhance the sensitivity of TRAIL-induced cervical
cancer cells to apoptosis, disclosing the specific role of ASPP2 in
cervical cancer.
Patients presenting with cervical cancer have shown
a trend towards women of a younger age in recent years, and herpes
simplex virus type II, human papilloma virus, human cytomegalovirus
and fungal infection are all known to cause cervical cancer
(23). ASSP2 is a p53-selective
stimulator (24). Binding of the
protein to its relevant binding sites on wild-type p53 induces
transactivation of the pro-apoptotic promoter region and promotes
cell apoptosis, thereby inhibiting the occurrence of cervical
cancer. When ASSP2 expression is low, however, the binding ability
of wild-type p53 to DNA is altered, and the ability of wild-type
p53 to induce apoptosis is consequently inhibited (11,25).
The synergistic effects of ASSP2 and p53 have been shown to have a
certain influence on the occurrence of apoptosis in various types
of malignant tumor cells (10).
From the perspective of the mechanism underlying ASSP2's influence
on cervical cancer, enhancing the binding of ASSP2 to p53 and
increasing the stimulatory role of ASSP2, and thereby its ability
to induce the p53 gene, are promising strategies that may lead to
the development of novel methods for treating cervical cancer.
Liang et al (26) showed that Beclin-1 is highly
expressed in normal breast epithelial tissues, although the
expression level was decreased, or the protein was not expressed,
in breast cancer tissues. Beclin-1 transfected into the human
breast cancer MCF7 cell line was shown to stimulate autophagy of
the MCF7 cells; furthermore, the proliferation of the MCF7 cells
was inhibited in vitro, and the tumorigenicity of the cell
line was inhibited. It is also discovered that Beclin 1 is not only
an autophagy gene essential for early embryonic development, but
also a haploinsufficient tumor suppressor, as the heterozygous
disruption of the beclin 1 gene will promote tumorigenesis
(27,28). In the present study, overexpression
of ASPP2 led to an inhibition of autophagy, which was reversed by
Tat-BECN1, the inducer of autophagy via activating Beclin1,
indicating that ASPP2 may inhibit autophagy partly via regulating
Beclin1, which subsequently contributing to the suppression of cell
apoptosis; however, it needs to be further verified in future
studies.
TRAIL is a signaling molecule involved in the
regulation of apoptosis, as well as being a type II transmembrane
protein, which can interact with DRs, thereby activating the
caspase cascade protease family and inducting apoptosis (29,30).
Recent studies have shown that the combination of TRAIL and
chemotherapeutic drugs leads to an improvement in the sensitivity
of tumor cells to TRAIL. TRAIL is a member of the tumor necrosis
factor superfamily, which is able to induce tumor cell apoptosis
through binding to DRs on cell membranes with low toxicity to
normal tissues and cells (31-33).
TRAIL has an intracellular death domain, which is able to
effectively transmit apoptotic signals and induce apoptosis. When
the concentration of TRAIL is low, TRAIL can competitively bind to
the inducible receptor, and under these circumstances, the ability
of TRAIL to induce death of the cells is not obvious. When the
concentration of TRAIL is high, however, TRAIL can bind to the DRs
and induce apoptosis. In our experiments, it was shown that
overexpression of ASPP2 was able to enhance the apoptosis of
TRAIL-induced cervical cancer cells via inhibiting autophagy.
In conclusion, the present study has shown that
ASPP2 expression was decreased in cervical cancer, and that the
mechanism of ASPP2 action is dependent on inhibiting autophagy and
promoting the apoptosis of cervical cancer. Although the underlying
mechanism has not been fully delineated and the clinical
information of these patients are limited such as prognosis, the
present study has at least suggested that the mechanism involves
ASPP2 regulating cervical cancer progression via the targeting of
Beclin1. These findings may lead to the identification of novel
targets for the diagnosis of cervical cancer, and novel strategies
for the treatment of cervical cancer.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the General Project
of the National Natural Science Foundation of China (grant no.
81974404).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL designed the present study. FJ and GB collected,
analyzed and interpreted the data. FJ and GB confirm the
authenticity of all the raw data. FJ wrote the article. JL
critically revised the article. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experiments were approved [approval no.
(2018)-(k022)] by the ethics committee of Yancheng First People's
Hospital (Yancheng, China). Written informed consent was provided
by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen G, Zhang M, Zhu J, Chen F, Yu D,
Zhang A, He J, Hua W and Duan P: Common genetic variants in
pre-microRNAs are associated with cervical cancer susceptibility in
southern Chinese women. J Cancer. 11:2133–2138. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gardner AB, Charo LM, Mann AK, Kapp DS,
Eskander RN and Chan JK: Ovarian, uterine, and cervical cancer
patients with distant metastases at diagnosis: Most common
locations and outcomes. Clin Exp Metastasis. 37:107–113.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Printz C: Rethinking a common surgery
technique for early cervical cancer: Experts are reconsidering the
use of minimally invasive radical hysterectomy as a treatment for
early cervical cancer after multiple studies found that patients
who undergo the procedure by either laparoscopy or robotic surgery
have poorer outcomes. Cancer. 125:3485–3487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mendez LC, Moraes FY, Castilho MS, Louie
AV and Qu XM: Lives and economic loss in brazil due to lack of
radiotherapy access in cervical cancer: A cost-effectiveness
analysis. Clin Oncol (R Coll Radiol). 31:e143–e148. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
You KY, Peng HH, Jiang YH, Bi ZF and Qiu
XS: Selective use of concurrent chemotherapy in elderly cervical
cancer patients treated with definitive radiotherapy: Experience
from two institutions. Cancer Manag Res. 11:4815–4823.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vives V, Slee EA and Lu X: ASPP2: A gene
that controls life and death in vivo. Cell Cycle. 5:2187–2190.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bergamaschi D, Samuels Y, Jin B,
Duraisingham S, Crook T and Lu X: ASPP1 and ASPP2: Common
activators of p53 family members. Mol Cell Biol. 24:1341–1350.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Konno T, Kohno T, Okada T, Shimada H,
Satohisa S, Kikuchi S, Saito T and Kojima T: ASPP2 suppression
promotes malignancy via LSR and YAP in human endometrial cancer.
Histochem Cell Biol. 154:197–213. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang T, Gao Y, Liu D, Wang Y, Wu J, Liu X,
Shi Y and Chen D: ASPP2 enhances chemotherapeutic sensitivity
through the down-regulation of XIAP expression in a p53 independent
manner in hepatocellular carcinoma. Biochem Biophys Res Commun.
508:769–774. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liang B, Chen R, Song S, Wang H, Sun G,
Yang H, Jing W, Zhou X, Fu Z, Huang G and Zhao J: ASPP2 inhibits
tumor growth by repressing the mevalonate pathway in hepatocellular
carcinoma. Cell Death Dis. 10(830)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu T, Song H, Xie D, Zhao B, Xu H, Wu C,
Hua K, Deng Y, Ji C, Hu J and Fang L: Silencing of ASPP2 promotes
the proliferation, migration and invasion of triple-negative breast
cancer cells via the PI3K/AKT pathway. Int J Oncol. 52:2001–2010.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xie F, Jia L, Lin M, Shi Y, Yin J, Liu Y,
Chen D and Meng Q: ASPP2 attenuates triglycerides to protect
against hepatocyte injury by reducing autophagy in a cell and mouse
model of non-alcoholic fatty liver disease. J Cell Mol Med.
19:155–164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang W, Li X and Cai L: Effects of ASPP2
on proliferation and apoptosis of malignant spinal tumor cells. Int
J Clin Exp Pathol. 10:8023–8030. 2017.PubMed/NCBI
|
|
15
|
Wang X, Yu M, Zhao K, He M, Ge W, Sun Y,
Wang Y, Sun H and Hu Y: Upregulation of MiR-205 under hypoxia
promotes epithelial-mesenchymal transition by targeting ASPP2. Cell
Death Dis. 7(e2517)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen R, Wang H, Liang B, Liu G, Tang M,
Jia R, Fan X, Jing W, Zhou X, Wang H, et al: Downregulation of
ASPP2 improves hepatocellular carcinoma cells survival via
promoting BECN1-dependent autophagy initiation. Cell Death Dis.
7(e2512)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shi Y, Han Y, Xie F, Wang A, Feng X, Li N,
Guo H and Chen D: ASPP2 enhances oxaliplatin (L-OHP)-induced
colorectal cancer cell apoptosis in a p53-independent manner by
inhibiting cell autophagy. J Cell Mol Med. 19:535–543.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song B, Bian Q, Zhang YJ, Shao CH, Li G,
Liu AA, Jing W, Liu R, Zhou YQ, Jin G and Hu XG: Downregulation of
ASPP2 in pancreatic cancer cells contributes to increased
resistance to gemcitabine through autophagy activation. Mol Cancer.
14(177)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gund R and Christiano AM: Impaired
autophagy promotes hair loss in the C3H/HeJ mouse model of alopecia
areata. Autophagy: Jun 2, 2022 (Epub ahead of print).
|
|
20
|
Follo C, Cheng Y, Richards WG, Bueno R and
Broaddus VC: Autophagy facilitates the release of immunogenic
signals following chemotherapy in 3D models of mesothelioma. Mol
Carcinog. 58:1754–1769. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zinnah KMA and Park SY: Duloxetine
enhances TRAIL-mediated apoptosis via AMPK-mediated inhibition of
autophagy flux in lung cancer cells. Anticancer Res. 39:6621–6633.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hailu HE, Mondul AM, Rozek LS and Geleta
T: Descriptive Epidemiology of breast and gynecological cancers
among patients attending Saint Paul's hospital millennium medical
college, Ethiopia. PLoS One. 15(e0230625)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schittenhelm MM, Walter B, Tsintari V,
Federmann B, Bajrami Saipi M, Akmut F, Illing B, Mau-Holzmann U,
Fend F, Lopez CD and Kampa-Schittenhelm KM: Alternative splicing of
the tumor suppressor ASPP2 results in a stress-inducible, oncogenic
isoform prevalent in acute leukemia. EBioMedicine. 42:340–351.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schittenhelm MM, Illing B, Ahmut F, Rasp
KH, Blumenstock G, Döhner K, Lopez CD and Kampa-Schittenhelm KM:
Attenuated expression of apoptosis stimulating protein of p53-2
(ASPP2) in human acute leukemia is associated with therapy failure.
PLoS One. 8(e80193)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676.
1999.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hartung F, Krüwel T, Shi X, Pfizenmaier K,
Kontermann R, Chames P, Alves F and Pardo LA: A novel anti-Kv10.1
nanobody fused to single-chain TRAIL enhances apoptosis induction
in cancer cells. Front Pharmacol. 11(686)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cao Y, Kong S, Xin Y, Meng Y, Shang S and
Qi Y: Lestaurtinib potentiates TRAIL-induced apoptosis in glioma
via CHOP-dependent DR5 induction. J Cell Mol Med. 24:7829–7840.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Saparbay J, Tanaka Y, Tanimine N, Ohira M
and Ohdan H: Everolimus enhances TRAIL-mediated anti-tumor activity
of liver resident natural killer cells in mice. Transpl Int.
33:229–243. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cao TM and King MR: Supercharged
eGFP-TRAIL decorated NETs to ensnare and kill disseminated tumor
cells. Cell Mol Bioeng. 13:359–367. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhuo FF, Zhang C, Zhang H, Xia Y, Xue GM,
Yang L and Kong LY: Chrysanthemulide A induces apoptosis through
DR5 upregulation via JNK-mediated autophagosome accumulation in
human osteosarcoma cells. J Cell Physiol. 234:13191–13208.
2019.PubMed/NCBI View Article : Google Scholar
|