Introduction
Iatrogenic bile duct injury is a serious and
potentially life-threatening complication of cholecystectomy
(1). Improper management of bile
duct injury and traumatic bile duct stricture leads to severe
consequences, such as repeated cholangitis, formation of
hepatolithiasis and biliary cirrhosis (2). Bile duct injury seriously affects the
quality of life of patients (3).
At present, surgery is the main treatment for the injured bile
duct. Roux-en-Y hepaticojejunostomy has been the most commonly used
approach for biliary reconstruction. However, because this
operation changes the physiological structure of the biliary tract,
its long-term outcome is still far from satisfactory due to the
high incidence of reflux cholangitis, choledocholithiasis, biliary
cirrhosis, anastomotic stenosis and even oncogenesis (4,5).
Tissue-engineered bile ducts can potentially be used
for repairing bile duct injury while preserving the function of the
Oddi sphincter. New techniques in bile duct epithelial organoids
culture and cell reprogramming provide a new opportunity in the
regenerative medicine of bile duct (6). Sampaziotis et al (7) seeded human cholangiocyte organoids
(ECOs) on a biodegradable scaffold to form artificial structure and
to repair the gallbladder wall or bile duct in immunodeficiency
mice. They proved the potential of ECOs in bile duct repair.
However, if the exogenous cells seeded in the tissue-engineered
bile ducts could survive in healthy animals but not
immunodeficiency animals still needs evidence. Several studies in
pigs or dogs have shown that transplanted acellular artificial bile
duct scaffolds could be re-epithelialized but lack experimental
details (8,9). In clinical studies, the application
of autologous graft to repair bile duct defects also failed to
demonstrate whether biliary re-epithelialization occurred due to
ethical issues (10). However,
preliminary evidence demonstrates that recipient-derived cells are
observed in the peribiliary glands and biliary epithelium of the
large donor bile ducts after liver transplantation (11). Notably, it is worth investigating
whether it is possible to perform bile duct allografts, as in
vascular surgery (12,13).
Mice are the most commonly used laboratory animals
and various transgenic and gene knockout mice can provide
beneficial technical means for in-depth research on the repair
mechanism of biliary tract injury. To establish a reproducible
mouse model that can be used for bile duct repair research, the
present study used the partial bile duct ligation technique
(14) to cause common bile duct
(CBD) distal stenosis and proximal dilation (i.e., bile duct
dilation, BDD). Then, it developed a microsurgical operation for
bile duct injury and repair of the dilated bile duct. The present
study proved the feasibility and safety of this novel model for
bile duct injury and repair research in mice.
Materials and methods
Animals
Adult male C57BL/6 mice (n=85; age, 8 to 10 weeks;
weight, 20-24 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. Twenty adult male C57BL/6-Tg
[CAG-enhanced green fluorescence protein (EGFP)]/Nju mice (age, 8
to 10 weeks; weight, 20-24 g) were purchased from Nanjing
University Model Animal Center. Animals were fed standard chow with
water ad libitum and kept at 24˚C and 40% humidity with a 12
h light/dark cycle. Experiments were approved by the Committee on
Ethics of Animal Experiments of the Chinese PLA General Hospital
(approval no. 2017-X13-65) and in compliance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Research Council (US) Committee, 8th
edition, 2011 (https://nap.nationalacademies.org/read/12910).
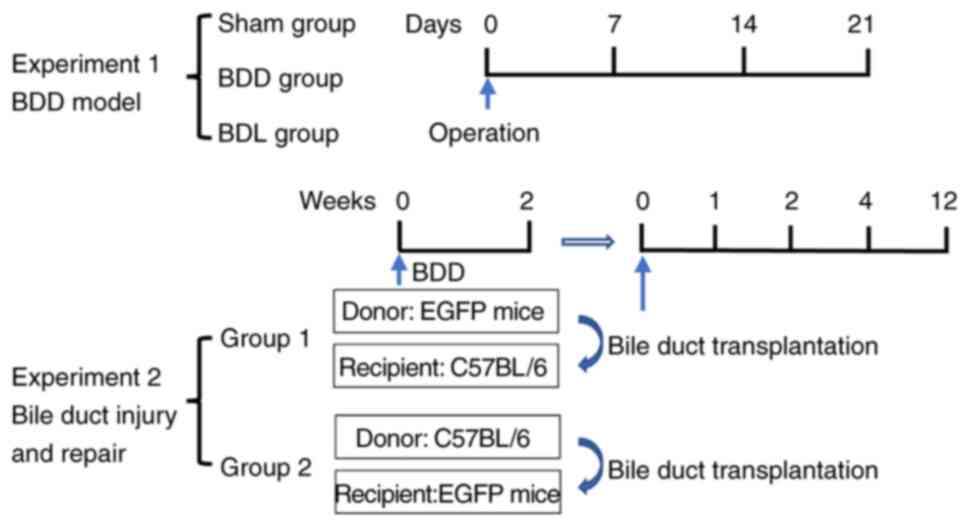
Experimental design
In the BDD model experiment (Fig. 1), C57BL/6 mice were randomly
divided into three groups: A control group with a sham operation
(Sham group; n=6), a bile duct dilation group with a CBD partial
ligation to cause CBD distal stenosis and proximal dilation (BDD
group; n=24) and a bile duct ligation group with CBD ligated (BDL
group; n=24). The BDD and BDL groups were set to three time points:
7, 14 and 21 days after the operation (n=8 for each time point in
each group). Each mouse was weighed preoperatively (day 0) and on
days 7, 14 and 21 after the operation. The mouse with body weight
loss >20% was sacrificed by exsanguination under 3% isoflurane
anesthesia before the planned time point (15). All mice were monitored every day
for their health and behavior in our Experimental Animal
Centre.
In the bile duct injury and repair experiment
(Fig. 1), an elliptical incision
was made in the anterior wall of the dilated CBD of recipient BDD
mice and then repaired by transplanting a bile duct patch from
donor BDD mice. The patches from EGFP mice were transplanted into
wild-type mice (EGFP donors: n=14; C57BL/6 recipients: n=28) and
the patches from wild-type mice were transplanted into EGFP mice
(C57BL/6 donors: n=3; EGFP recipients: n=6). One donor could
provide bile duct patches for two recipients. Four time points (1,
2, 4 and 12 weeks after transplantation) were set to observe the
outcomes of recipient mice.
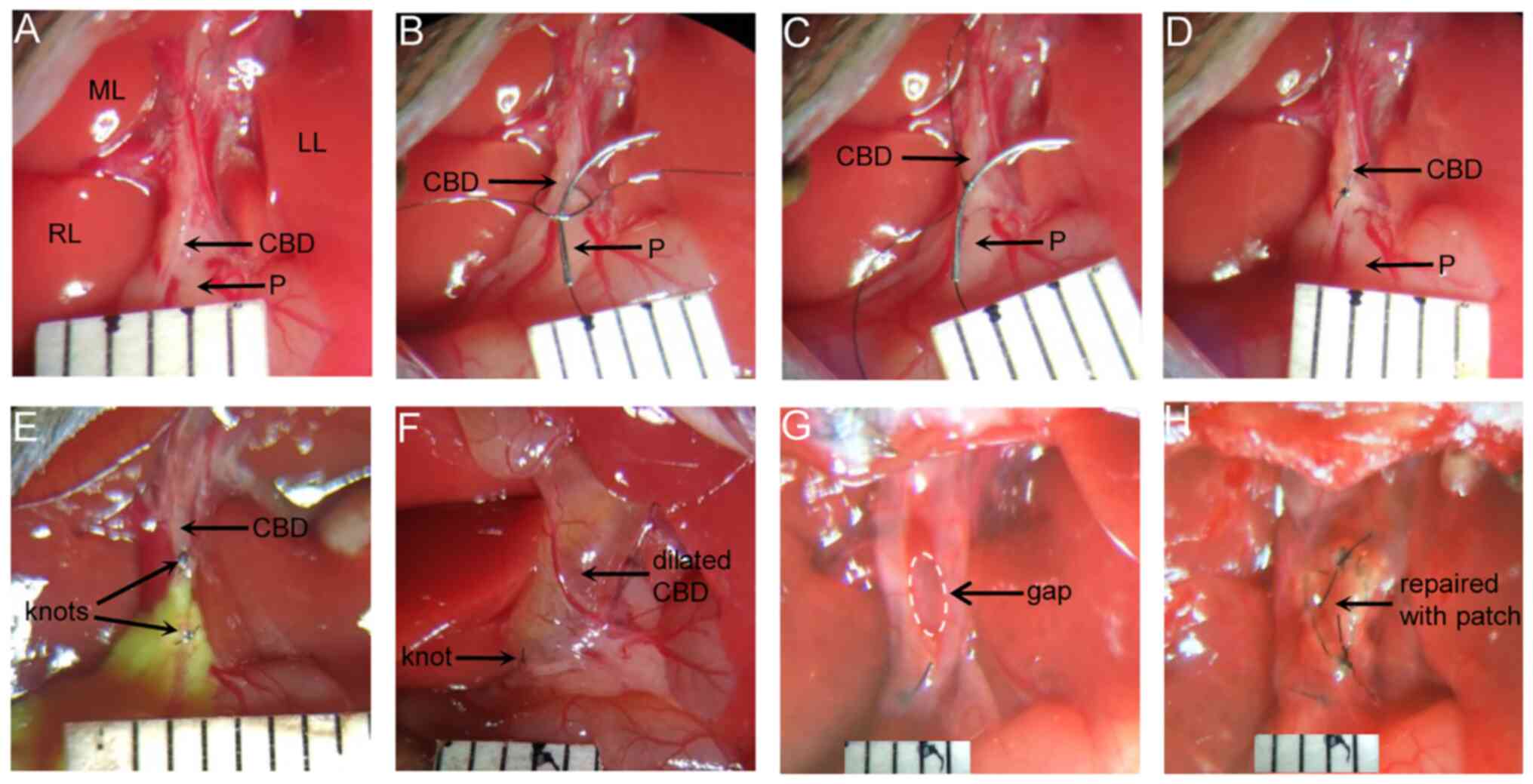
Surgery for the BDD mouse model
All mice were fasted overnight with free access to
water before surgery. Anesthesia was performed under
isoflurane/oxygen inhalation (3% isoflurane for induction, 1.5% for
maintenance of anesthesia). Following a midline incision, the CBD
was exposed and separated carefully by a microtweezer under a
surgical microscope. In the Sham group, the abdomen was closed
after the CBD was separated (Fig.
2A). In the BDD group, an 8-0 nylon suture was placed around
the CBD at the upper edge of the pancreas and tied with one lax
surgical knot. An 8-0 surgical needle (Φ=0.15 mm, Nylon suture;
Lingqiao) was placed into the lax surgical knot (Fig. 2B) and then the knot was tied
tightly (Fig. 2C). The needle was
removed, leaving a defined lumen between the bile duct and the
ligation suture (Fig. 2D). The
superfluous sutures were removed with a microscissor and the
abdomen was closed by a 4-0 surgical suture. In the BDL group, the
CBD was ligated tightly with two surgical knots using 8-0 nylon
suture and then the CBD was transected between the two knots
(Fig. 2E).
Surgery for bile duct injury and
repair
At 14 days after the modelling operation, BDD mice
were used as donors and recipients. First, the dilated CBD of donor
mice was excised, divided into two patches and stored in a
histidine-tryptophan-ketoglutarate solution (Custodiol, Koehler
Chemi) at 4˚C for transplantation. Second, the dilated CBD of
recipient mice was exposed (Fig.
2F). An elliptical incision of ~2x3 mm was made with a
microscissor on the ventral side of the CBD (Fig. 2G). The bile was absorbed with a
cotton swab. A donor patch was taken and trimmed to be slightly
larger than the incision. The patch was first fixed to the CBD at
the upper and lower ends with two sutures using two 8-0 nylon
threads with needles and then continuous suture was performed from
the top to the bottom at one side and from the bottom to the top at
the other side of the patch (Fig.
2H). After ensuring no bile leakage, the abdominal cavity was
rinsed with normal saline and the abdomen was closed with a 4-0
suture. Antibiotics (cefoperazone sulbactam sodium; Pfizer, Inc.;
60 mg/kg, once per day) were given for 3 days postoperatively and
20% glucose in their drinking water was given for 24 h except for
the standard laboratory chow ad libitum (16).
Sample collection and CBD diameter
measurement
The mice were anaesthetized with isoflurane (3%
isoflurane for induction, 1.5% for maintenance of anesthesia) and
the abdomen was opened by a midline incision. The diameter of the
CBD was measured in situ with a Vernier caliper. Blood was
collected via the vena cava with a 1 ml syringe. The CBD was
perfused with 4% paraformaldehyde via the gallbladder. Samples of
liver tissue were fixed with 10% buffered formalin at 4˚C for 24 h.
Finally, the mice were sacrificed by exsanguination. Prior to
sample collection, a high-resolution small animal ultrasound system
(Vevo 2100, VisualSonics) was also used for the noninvasive
measurement of CBD diameter in the BDD group mice 21 days after the
operation.
Blood biochemical analysis
Levels of serum alanine aminotransferase (ALT),
aspartate aminotransferase (AST) and total bilirubin (TBil) were
measured using the Cobas 8000 serum analyzer (Roche
Diagnostics).
Histology and immunofluorescence
detection
The fixed bile duct and liver tissue were embedded
in paraffin after dehydration in an ascending ethanol series in
turn (from 75 to 100% ethanol), cleared by dimethylbenzene and cut
into 5-µm thick sections. For hematoxylin and eosin (HE) staining,
sections were stained for 3 min with hematoxylin and 1 min with
eosin at room temperature. For assessment of the presence of
collagen, the sections were stained with Masson's Trichrome
including staining in Weigert hematoxylin for 8 min, Ponceau for 10
min and aniline blue for 2 min at 25˚C. For immunofluorescence
detection, the bile duct was further fixed with 4% paraformaldehyde
for 4 h at 4˚C, dehydrated with 30% sucrose solution and then
embedded with OCT for 8-µm thick frozen sections. Tissue sections
were incubated at 4˚C overnight with primary antibodies against the
myofibroblast (activated fibroblast) marker α smooth muscle actin
(α-SMA; cat. no. D4K9N; 1:200; Cell Signaling Technology, Inc.),
proliferative cell nuclear antigen (Ki67; cat. no. D3B5; 1:400;
Cell Signaling Technology, Inc.) and cholangiocyte-specific marker
cytokeratin 19 (CK19; cat. no. ab52625; 1:400; Abcam). Then, the
sections were incubated with fluorescent secondary antibodies
(1:400; Alexa 594; cat. no. 711-585-152; Jackson ImmunoResearch
Laboratories, Inc.) at 25˚C for 2 h. Cell nuclei were stained with
DAPI at 25˚C for 10 min (MilliporeSigma).
Indocyanine green (ICG) fluorescence
imaging
Bile excretion of ICG can be used for real-time
visualization of biliary tract structure. The mice were
anaesthetized with isoflurane (3% isoflurane for induction, 1.5%
for maintenance). Following a midline incision, 0.5 mg/kg ICG was
injected into the inferior vena cava. Fluorescence imaging of the
liver, biliary tract and duodenum was observed intermittently with
an ICG imager (Beijing Digital Precision Medicine Technology Co.
Ltd.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed by SPSS 17.0 (SPSS,
Inc.). The values between the two groups were compared with
unpaired Student's t test. Comparisons of multiple groups were
performed with Student-Newman-Keuls test for three groups and
Turkey test for more than three groups after analysis of variance
(ANOVA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Changes in body weight and CBD
diameter of the model mice
The body weight of both BDD mice (19.39±1.64 g) and
BDL mice (19.30±0.79 g) decreased significantly during the first
week postoperation compared with their baseline levels (BDD:
22.28±0.79 g; BDL: 21.89±1.08 g) or the Sham group (24.25±1.26 g;
P<0.001). However, during the second and third weeks, the BDL
mice continued to lose weight (day 14: 18.00±0.78 g; day 21:
17.40±0.53 g) and four mice had a body weight loss of >20% on
day 21 and were sacrificed after sample collection. Meanwhile, the
BDD mice began to gain weight (day 14: 21.46±1.18 g; day 21:
24.41±1.04 g) as did the Sham group mice (day 14: 25.75±0.82 g; day
21: 26.98±0.75 g; Fig. 3A). The
CBD diameter of sham mice was 0.51±0.08 mm. The CBD diameters of
BDD mice were 3.11±0.76, 2.89±0.76 and 2.93±0.66 mm on days 7, 14
and 21 postoperation, respectively, while the CBD diameters of BDL
mice were 2.95±0.39, 4.71±0.64 and 4.92±0.95 mm, respectively
(Fig. 3B). The CBD remained
slightly dilated in BDD mice but continued to expand in BDL mice
from days 7 to 21 postoperation. These results indicated that the
effect of partial CBD ligation on mouse body weight was transient
and the degree of CBD dilation in BDD mice was mild and stable
compared with that in BDL mice. To provide a noninvasive method to
assess the degree of CBD dilation, the CBD diameter of BDD mice on
day 21 was also measured with ultrasonic Doppler for small animals.
As shown in Fig. 3C, the maximum
bile duct diameter measured by ultrasonic Doppler was 2.82±0.77 mm,
which was not significantly different from the diameter measured by
Vernier calipers in vivo under laparotomy (2.93±0.66 mm,
P=0.265). The bile duct diameters measured by the two methods were
also comparable on days 7 and 14.
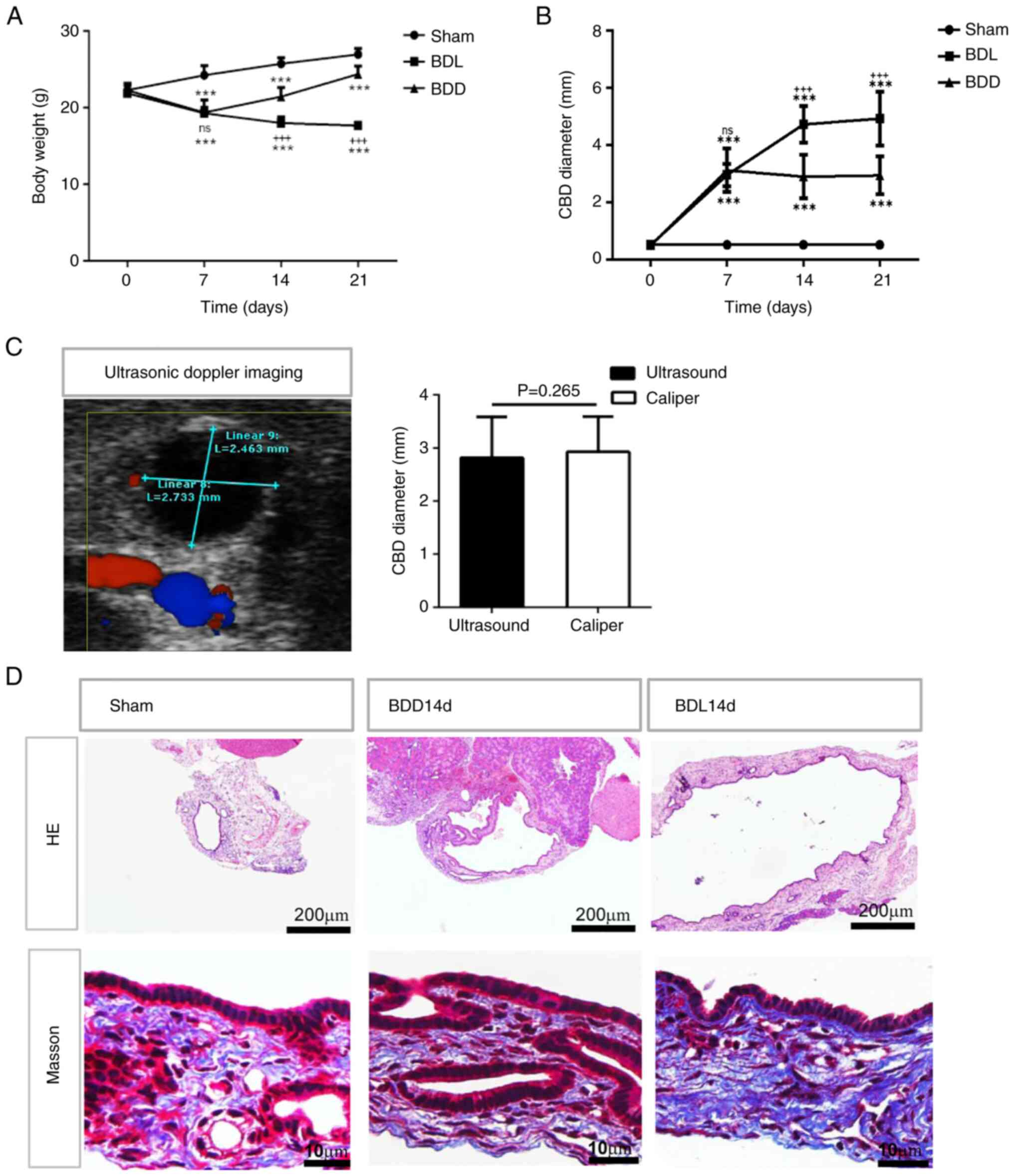 | Figure 3The changes in body weight and CBD of
the mice that underwent different modelling operations. (A) The
body weight of the Sham, BDL and BDD groups. (B) The CBD diameter
of the Sham, BDL and BDD groups. (C) Representative ultrasonic
Doppler imaging for the measurement of CBD diameter in the BDD mice
on day 21 post operation compared with using the Vernier caliper
method under laparotomy. (D) HE-stained CBD cross-sections and
Masson staining for collagen in CBD cross-sections from sham, BDL
and BDD mice on day 14 after the operation (magnification, x100).
n=6 for the sham group; n=8 for the BDL and BDD groups.
***P<0.001 vs. Sham; +++P<0.001 vs.
BDD; ns, P>0.05 vs. BDD. CBD, common bile duct; BDL, bile duct
ligation; BDD, bile duct dilation; HE, hematoxylin and eosin; ns,
not significant. |
HE staining of the CBD cross sections also showed
the mild expansion of CBD in the BDD group compared with the BDL
group on day 14 (Fig. 3D). Masson
staining showed a similar signal intensity for collagen in the Sham
and BDD groups but much more compact collagen fiber deposition in
the CBD wall of the BDL group. The thickness of the CBD wall was
similar in the three groups and all had peribiliary glands within
the CBD walls.
Liver function of the model mice
Complete CBD obstruction in BDL mice resulted in
severe liver damage with markedly elevated ALT, AST and TBil serum
levels and increased intrahepatic bile duct hyperplasia (as shown
by HE staining and CK19 immunostaining) on days 7, 14 and 21
postoperation (Fig. 4).
Nevertheless, the liver damage was very mild in BDD mice, with much
lower ALT, AST and TBil serum levels and slight intrahepatic bile
duct hyperplasia due to appropriate partial CBD ligation (Fig. 4). As the CBD diameter and liver
damage of BDD mice became stable from day 14 after the operation,
Day 14 was chosen as the time point to perform bile duct injury and
repair in BDD mice in the following experiments.
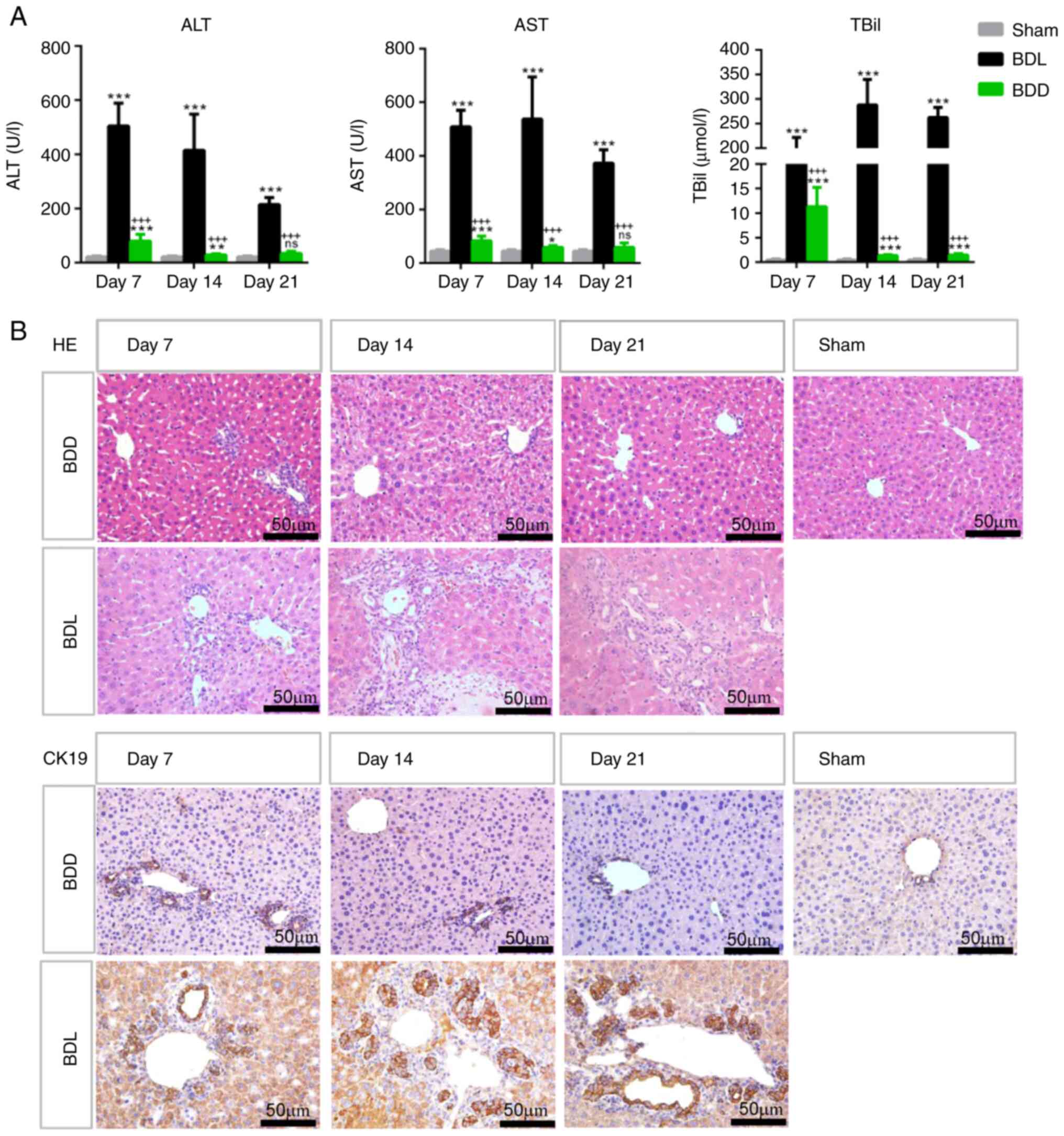 | Figure 4The liver function of the mice that
underwent different modelling operations. (A) ALT, AST and TBil
serum levels in the Sham, BDL and BDD groups on days 7, 14 and 21
after the operation. n=6 for the Sham group, n=8 for the BDL and
BDD groups. *P<0.05 vs. sham; **P<0.01
vs. sham; ***P<0.001 vs. Sham;
+++P<0.001 vs. BDL; ns, P>0.05 vs. Sham. (B) HE
staining and CK19 immunostaining of liver sections from Sham, BDL
and BDD mice (magnification, x400). ALT, alanine aminotransferase;
AST, aspartate aminotransferase; TBil, total bilirubin; BDD, bile
duct dilation; BDL, bile duct ligation; HE, hematoxylin and
eosin. |
Outcomes of the mice with bile duct
transplantation
The size of the elliptical incision made on the
dilated CBD was 2.53±0.48 mm in length and 1.7±0.41 mm in width
(n=6). The trimmed patch was 3.53±0.48 mm in length and 2.73±0.46
mm in width (n=6). Altogether, 34 BDD mice underwent bile duct
transplantation (referred to as Tp mice); of these, 30 Tp mice
survived to the scheduled time points without obvious morbidity or
body weight loss. Three mice died of abdominal bleeding on the
second day and one mouse succumbed on the seventh day due to bile
leakage. These mice were excluded from the study.
The present study first examined the mice that
survived 12 weeks after CBD repair. Under a surgical microscope,
the position of the grafted patch could be roughly identified by
the surgical suture (Fig. 5A). The
patch was covered with abundant blood vessels and fused with the
native CBD. The border was indistinguishable except for the suture.
The CBD retained the same degree of dilation as when being
repaired. There were no signs of bile leakage or intraabdominal
abscesses in the Tp mice. Fluorescence imaging showed that ICG
could be excreted from the liver through the CBD into the duodenum,
indicating that the CBD was unobstructed (Fig. 5B).
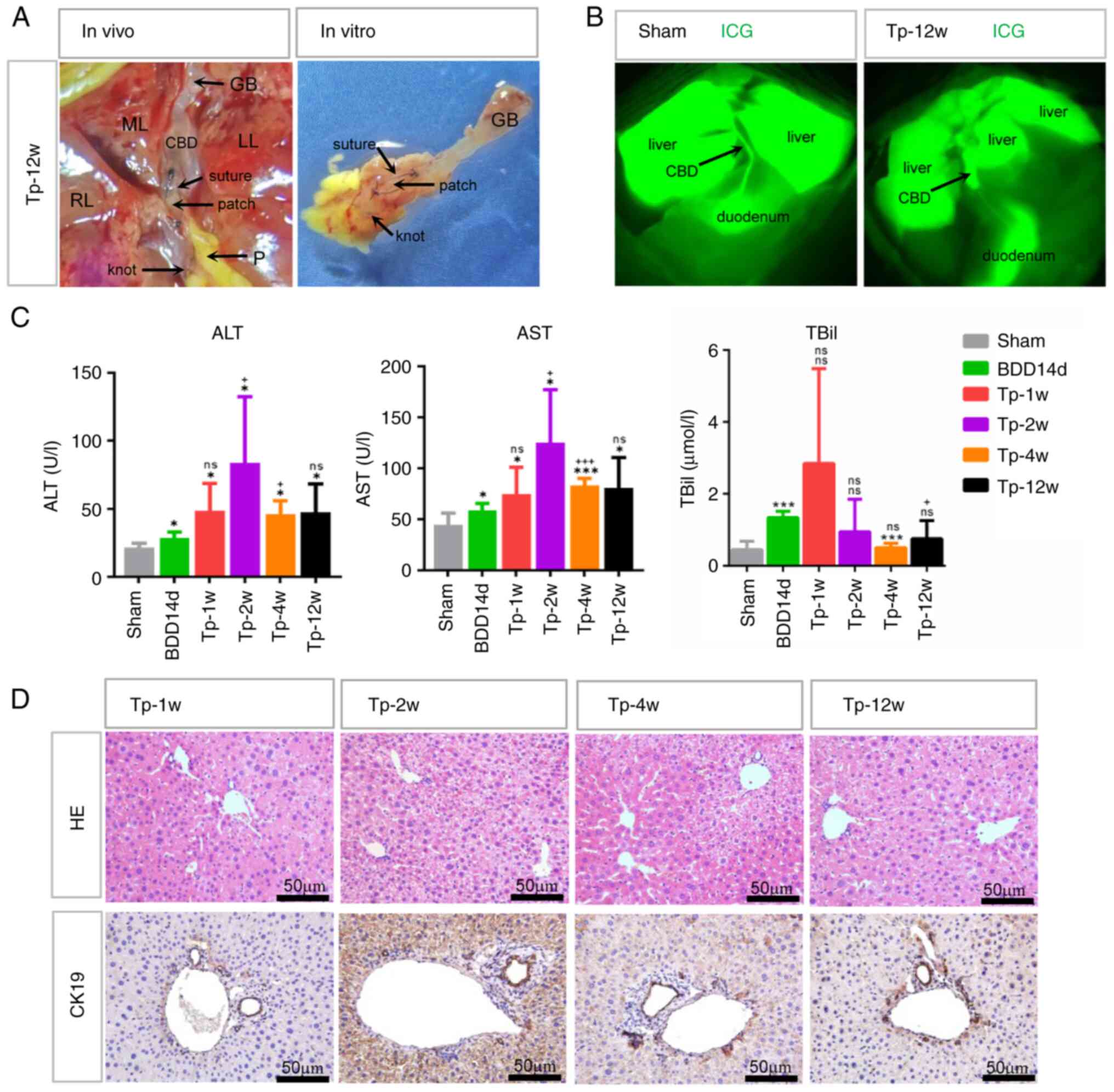 | Figure 5The macroscopic finding of repaired
CBD and liver function of BDD mice following transplantation of
bile duct patches (Tp mice). (A) Images of the repaired CBD in
situ and in vitro from the Tp mice 12 weeks after
transplantation under a surgical microscope (magnification, x10).
(B) ICG fluorescence imaging of sham mice and Tp mice 12 weeks
after transplantation showing that ICG was excreted from the liver
through the CBD into the duodenum. (C) ALT, AST and TBil serum
levels in BDD mice before (BDD14d) and 1, 2, 4 and 12 weeks after
transplantation. n=6 for each time point of each group.
*P<0.05 vs. sham; ***P<0.001 vs. Sham;
+P<0.05 vs. BDD14d; +++P<0.001 vs.
BDD14d; ns, P>0.05 vs. sham or BDD14d. (D) HE staining and CK19
immunostaining of representative liver sections showing mild bile
duct hyperplasia in Tp mice (magnification, x400). CBD, common bile
duct; BDD, bile duct dilation; ICG, indocyanine green; ALT, alanine
aminotransferase; AST, aspartate aminotransferase; TBil, total
bilirubin; HE, hematoxylin and eosin; ns, non significant. |
Liver injury in Tp mice was examined at 1, 2, 4 and
12 weeks after CBD repair. Although their ALT and AST serum levels
were slightly higher than those of the Sham group, they were
comparable to or slightly higher than those before CBD repair
(BDD14d). The TBil levels of the Tp mice at 1, 2 and 4 weeks after
CBD repair were not significantly different from those before CBD
repair (BDD14d) (Fig. 5C). HE
staining showed that the hepatic sinus structure remained intact
and there was slight bile duct hyperplasia in Tp mice, as shown by
CK19 immunostaining (Fig. 5D).
However, mild liver injury did not affect the long-term survival of
Tp mice.
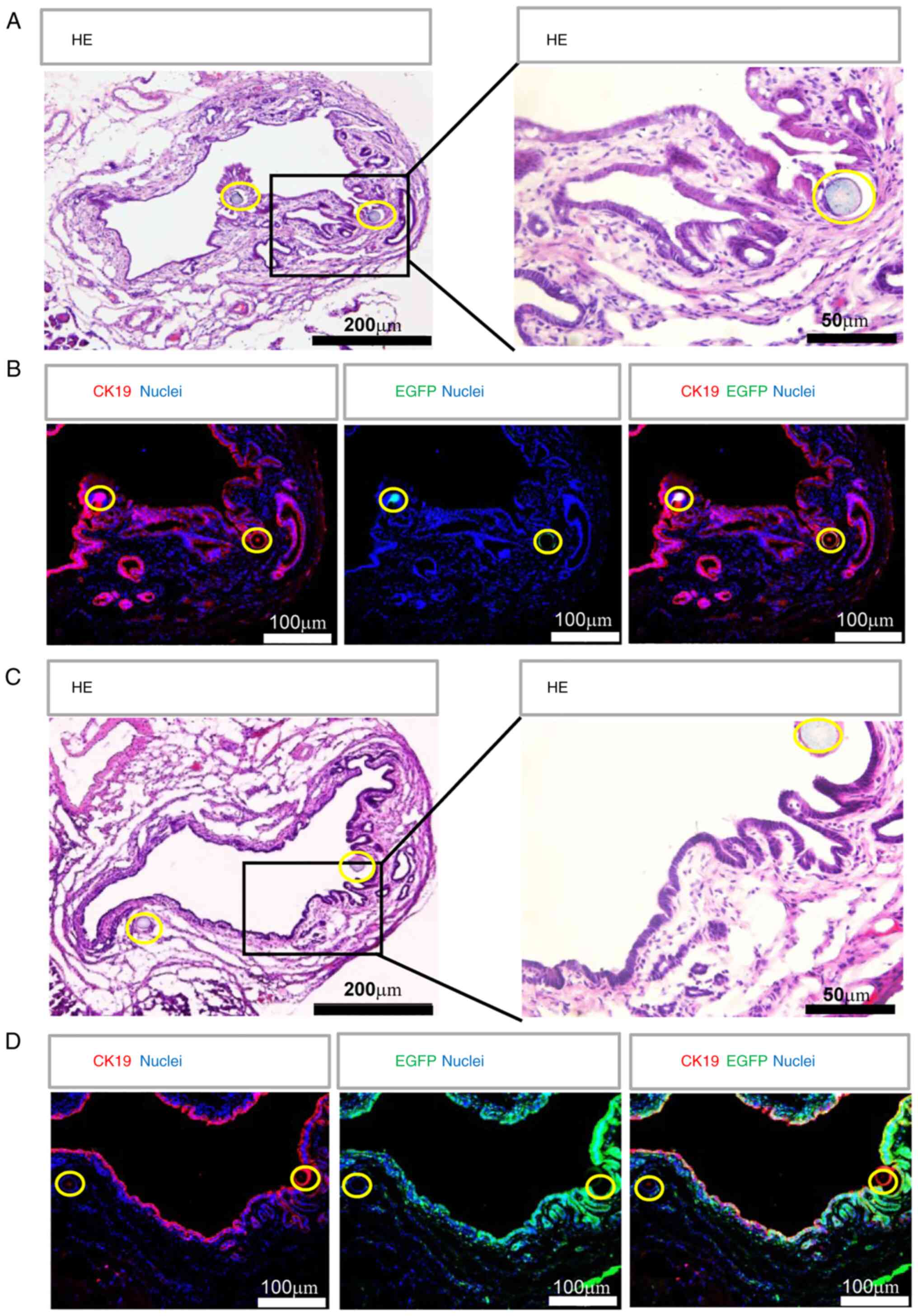
Histologic examination of the
transplanted patches
The long-term outcomes of repaired CBD were examined
by cross transplantation of bile duct patches from EGFP mice to
wild-type mice and vice versa at 12 weeks after transplantation. As
shown in Fig. 6, HE staining
showed that the CBD structure was intact and its lumen was free
from sludge. Immunofluorescence detection of cholangiocyte marker
CK19 showed that the CK19+ biliary epithelium on the
bile duct patch, which was identified by the surgical suture, was
arranged in an orderly manner. There were peribiliary glands (PBGs)
that were CK19+ within the bile duct wall of the patch
and the native bile duct (Fig. 6A
and B). However, no
EGFP+ cells were preserved within the patch from EGFP
BDD mice (Fig. 6B) and vice versa,
the patches became EGFP positive when the patches from wild-type
mice were transplanted into EGFP BDD mice at 12 weeks after
transplantation (Fig. 6C and
D). These results demonstrated
that the transplanted bile duct patch, including biliary epithelial
cells, was replaced by recipient-derived cells.
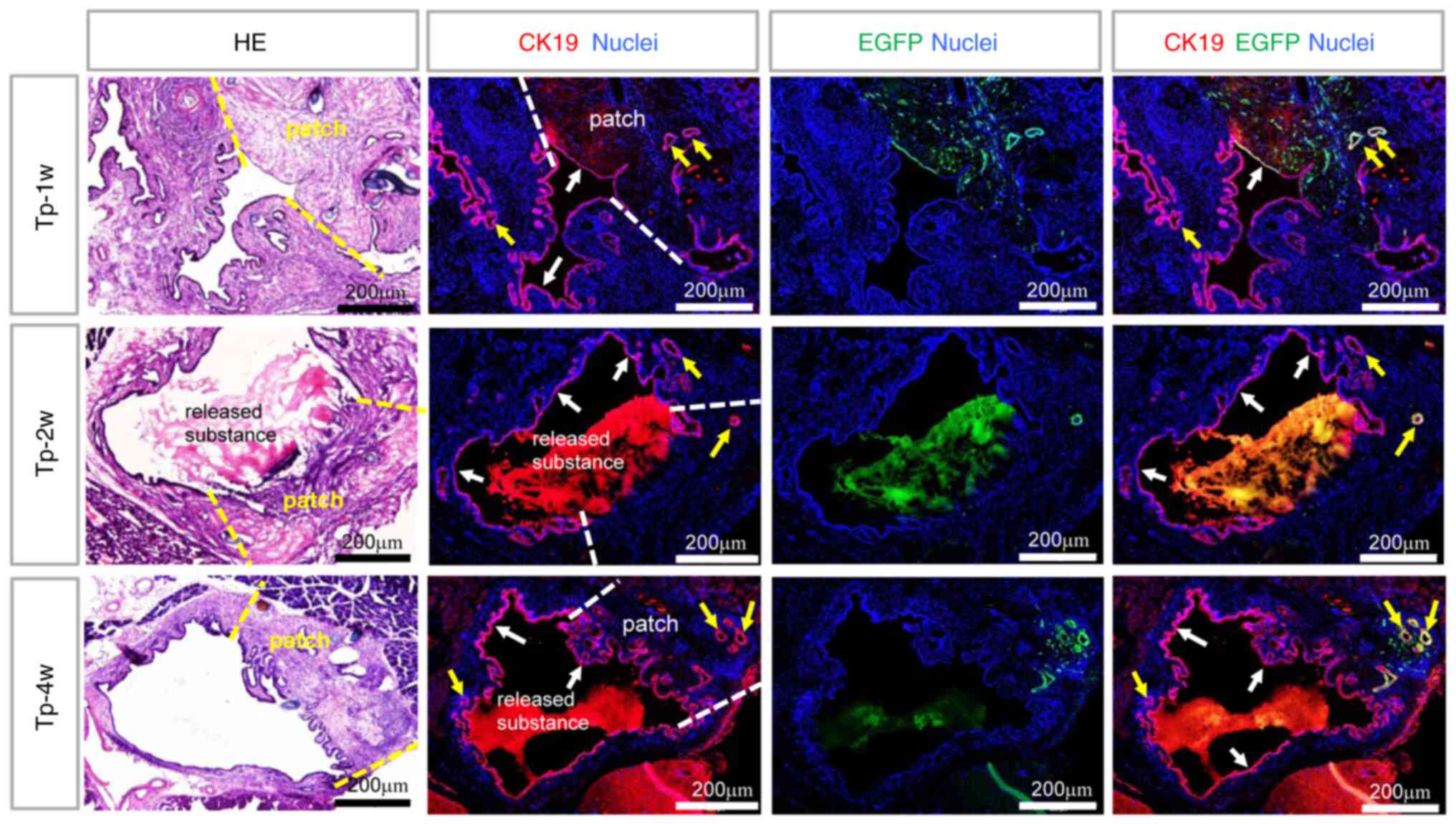
Regeneration process of the repaired
CBD
The regeneration process of the repaired CBD with
EGFP patches was observed at 1, 2 and 4 weeks after
transplantation. As shown in Fig.
7, at 1 week, there were still numerous EGFP-positive cells
within the patch and the wall structure of the patch seemed intact
with CK19+ and EGFP+ epithelium (indicated
with a white arrow) and PBGs (indicated with a yellow arrow). At 2
weeks, the patch lost its CK19+ epithelium and connected
with a mass of loosely released substance protruding into the lumen
of the CBD. Meanwhile, the structure of the patch seemed disordered
in HE staining. However, at 4 weeks, the patch was again covered
with CK19+ biliary epithelium and the wall structure of
the patch became ordered again. Only a few EGFP+ and
CK19+ PBGs were still present within the bile duct wall
in some mice. The loose material released from the patch into the
lumen was obviously reduced or missing. During this repair process,
the native CBD did not seem to change much and was always covered
with CK19+ biliary epithelium. These results suggested
that the bile duct patch went through a process from destruction to
regeneration and its cells were replaced by recipient-derived
cells, including biliary epithelial cells.
Regeneration mechanism of the
transplanted CBD patch
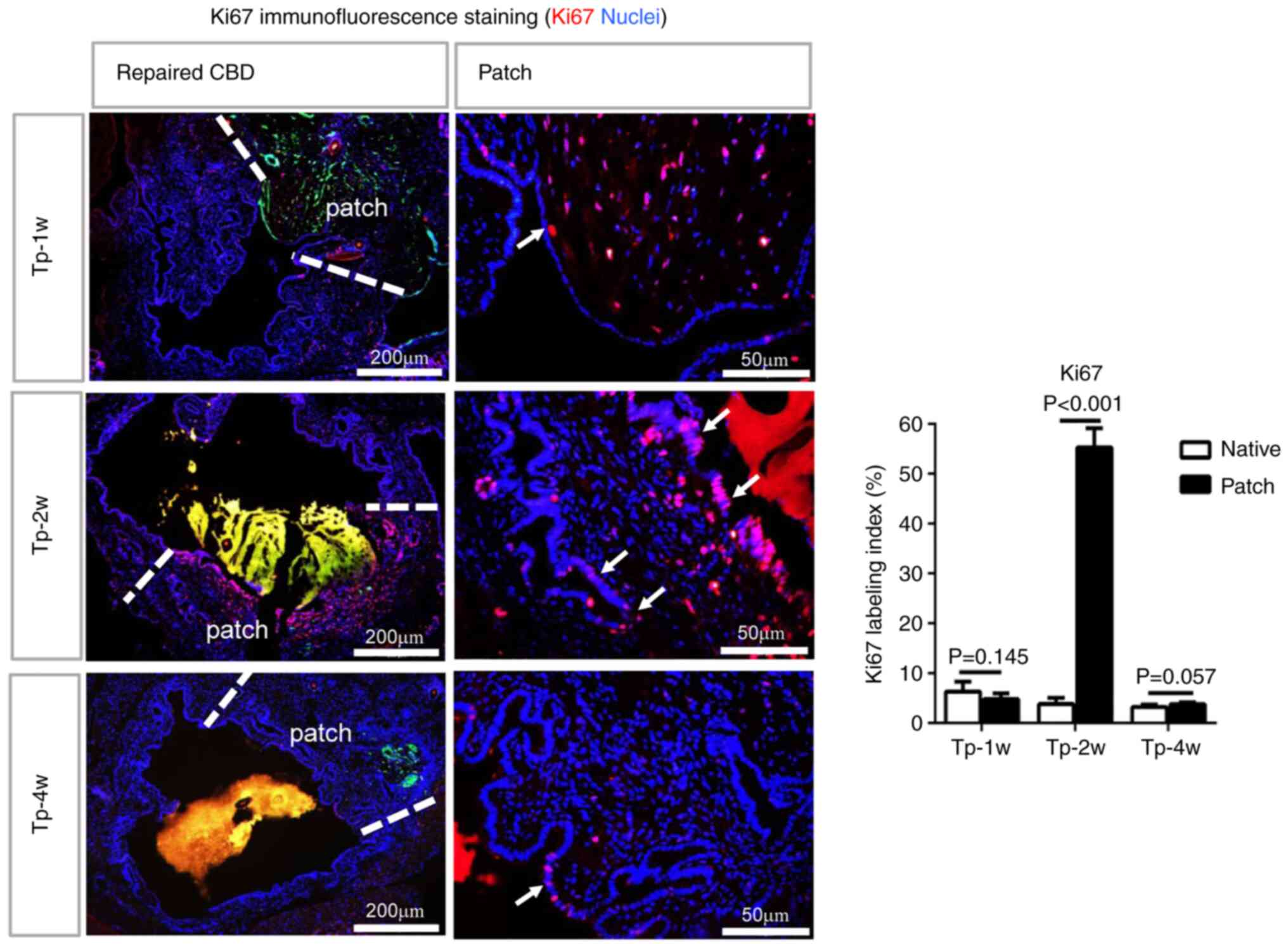
Ki67 is a commonly used sensitive indicator of cell
proliferation. Immunofluorescence staining of the repaired CBD
indicated that a significantly increased number of Ki67+
cells was observed within and adjacent to the patch compared with
the native CBD at 1, 2 and 4 weeks following transplantation,
demonstrating the activation of cell proliferation during the
repair process (Fig. 8). In the
patch, Ki67+ cuboidal columnar epithelial cells were
found within the biliary epithelium and peribiliary glands
identified by neatly arranged tall columnar nuclei (indicated with
a white arrow). The cell proliferation of the biliary epithelium
was most significant at 2 weeks after transplantation. There were
almost no Ki67+ cells in the patch at 12 weeks after
transplantation.
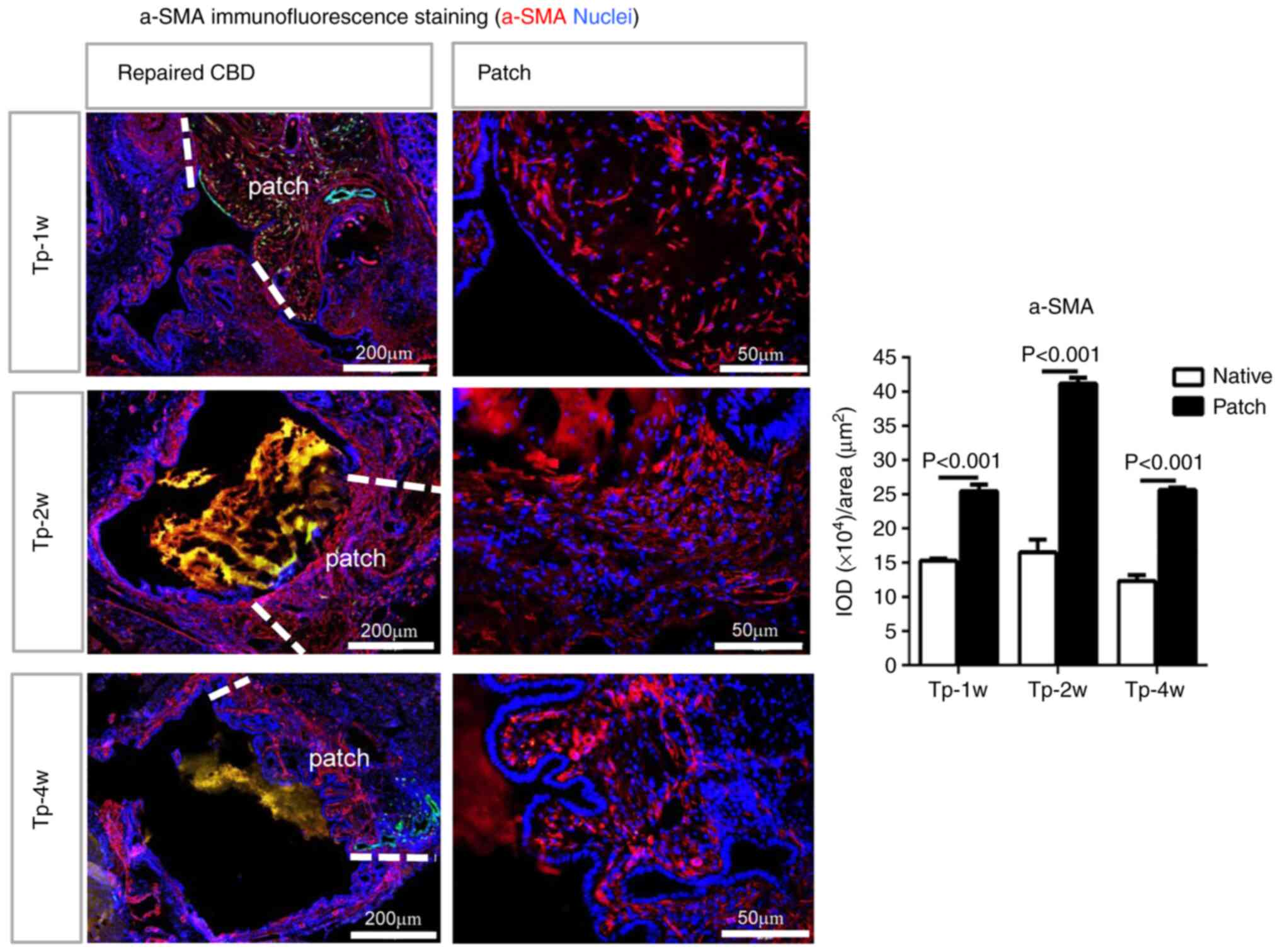
α-SMA is usually used as a marker of myofibroblasts
or activated fibroblasts. An increase in α-SMA+ cells
was also observed during the regeneration process of repaired CBD
(Fig. 9). Immunofluorescence
staining for α-SMA indicated significantly upregulated expression
in the patch compared with the native CBD at 1, 2 and 4 weeks after
transplantation. The increase in α-SMA expression was highest at 2
weeks after transplantation. At 12 weeks, the α-SMA expression
level in the patch was comparable to that in the native CBD. These
results suggested that the regeneration of the muscular layer and
epithelia occurred at the same rate to complete the CBD repair
process.
Discussion
The outcomes of the current treatment strategies for
bile duct injury are not always favorable (2,3).
Much remains to be clarified with regard to the process of bile
duct healing or regeneration following severe injury. The present
study investigated whether a bile duct defect and repair model
could be established in mice, which are the most commonly used
animal models for studying mechanisms. The present study
successfully established a novel mouse model for the study of bile
duct injury and repair. To the best of the authors' knowledge, this
is the first study to report the histological changes and healing
process of repaired CBD.
For BDL model, it is important to ensure that the
CBD is ligated by two surgical knots and then transected between
the two ligations (17,18). One of the key points of BDD mouse
model is the proper induction of CBD dilation by partial ligation
of the CBD, which makes the slender bile duct of the mouse
moderately dilated for easier surgical repair. Although the BDD
mice experienced obvious but mild liver injury because of biliary
stenosis compared with the Sham group, its effect on body weight
was transient and the BDD mice did not show obvious morbidity. In
BDL mice where the CBD was completely obstructed, their body weight
decreased dramatically, their liver damage was very serious and
their ALT, AST and TBil levels were 10 times higher than those of
BDD mice. BDL caused a decrease in body weight by ~20% and obvious
liver injury which were also observed in previous studies (19,20).
In the study by Heinrich et al (14), a similar method was used to
establish a model of acute cholestasis in mice; however, the
gallbladder was removed and the mice were observed for only 2 weeks
in their study. In the present BDD model, the gallbladder was
preserved to maintain the physiological state as much as possible
and the gallbladders of BDD mice did not show obvious expansion, in
contrast to the extensively expanded gallbladder in BDL mice. These
BDD mice survived at least 6 months without significant symptoms of
morbidity.
The CBD diameter of normal mice was ~0.5 mm. After
BDD, the CBD became 4-7-fold of its normal size and reached a
steady state from day 14 after surgery. HE and Masson staining
showed that the CBD wall of BDD mice was similar to that of normal
mice, but there was more collagen fiber in the CBD wall of BDL
mice. This might be due to the high biliary pressure because of
complete obstruction in the BDL mice. These results suggested that
the degree of bile duct dilation in the BDD model was very mild and
had little effect on its survival.
The present study then demonstrated that the dilated
CBD could be injured and repaired easily by suturing a bile duct
patch onto the damaged region under surgical microscopy. It is
almost impossible to perform these procedures on normal mice.
Biliary fistula was the main reason for early mortality and usually
occurred during the learning period for the microsurgical
performer. After a short time of training, >90% of the mice were
able to survive to the predetermined time points.
In addition, the CBD diameter remained almost the
same as before repair during the observation period of 12 weeks,
although it was not accurately measured because of tissue adhesion.
There were no signs of bile leakage or intraabdominal abscesses. At
12 weeks, the site of the patch was covered with abundant blood
vessels and was indistinguishable from the native duct. Its lumen
was free from sludge. Histological examination also demonstrated
that the bile duct injury was completely repaired and could not be
distinguished from the surrounding CBD wall. Only the suture silk
could show the approximate position of the patch. The suture silk
was even extruded into the lumen from the bile duct wall after 6
months (data not shown).
By cross transplantation of the bile duct patch
between wild-type mice and EGFP mice, it was found that no
EGFP+ cells were observed within the patch at 12 weeks
after transplantation when the EGFP patch was transplanted into
wild-type mice and vice versa, the site of the patch became
EGFP+ when the wild-type mouse patch was transplanted
into EGFP mice. At 1 week after transplantation, the biliary
epithelium of the patch seemed intact and there were still numerous
EGFP+ cells in the patch. However, at 2 weeks after
transplantation, EGFP+ cells were destroyed and reduced
and the biliary epithelium became imperfect. At 4 weeks, the
biliary epithelium regenerated and became intact. CK19+
EGFP- cholangiocytes with tall columnar nuclei were
neatly arranged on the inner side of the CBD, although a few
EGFP+ peribiliary glands were still present within the
CBD wall in some mice. These results demonstrated that the
transplanted bile duct patch, including the biliary epithelium, was
replaced by recipient-derived cells. The injured CBD was
regenerated and repaired. Recently, de Jong et al (11) also demonstrated that
recipient-derived cholangiocytes were present in the large bile
ducts of the donor liver after liver transplantation. The presence
of chimaerism in the large bile ducts suggested that
recipient-derived cells may play a role in biliary regeneration
following ischemia-induced injury.
The regeneration of transplanted artificial bile
ducts at the graft site after the tube had been degraded has been
reported in several experimental studies in pigs (21,22),
but the detailed process was not disclosed. The present study
demonstrated that the transplanted bile duct patch did not survive
but went through a process of destruction and regeneration. It is
known that epithelial cells and periductal myofibroblasts are
closely linked because damage to the former leads to activation and
proliferation of the latter (23,24).
Ki67+ cuboidal columnar epithelial cells were found
within the columnar biliary epithelial and peribiliary glands. An
increase in α-SMA+ myofibroblasts was also observed in
the bile duct wall of the patch and the surrounding area. The
proliferation was most significant at 1-4 weeks after the repair
operation. At 12 weeks, the number of Ki67-positive cells was
minimal and the number of α-SMA+ cells was reduced to
the level observed in the surrounding bile duct wall. These results
were consistent with the process of graft re-epithelialization
observed by HE and CK19 staining. Although it was not clear whether
the extracellular matrix was also completely replaced by new
extracellular matrix, synthesis of extracellular matrix by
α-SMA+ cells was suggested.
Long-term outcomes are key clinical settings for the
surgical treatment of bile duct injury. A satisfactory follow-up
period of 2-5 years is necessary to assess the long-term outcome of
repair surgery (2,3). In the present study, 12 weeks is a
relatively long time considering the lifespan of mice. Creation of
artificial bile ducts functionally identical to natural organs is a
final goal in the research area of artificial bile ducts. In the
study of Sampaziotis et al (7), the bile ducts were repaired using
specially made very fine artificial tissue-engineered bile ducts
and the microsurgical procedure was extremely difficult and lacked
universality. In the present study, a BDD model was used to achieve
bile duct injury and repair in mice, which significantly reduced
the difficulty of microsurgery. It was hypothesized that the mouse
model established in the present study can be used to evaluate the
biocompatibility, repairing effect and mechanism of
tissue-engineered bile ducts (8-10).
Notably, if the survival of the grafted bile duct patch is not as
important as proven in the present study, cryopreserved bile duct,
allogeneic or even heterogenic decellular bile duct might be
superior to synthetic materials for the recipient-derived cells to
engraft after transplanting (25,26).
The limitation of the present study was that bile
duct injury and repair were not performed in healthy mice but in an
experimental model of partial bile duct ligation. However, when the
bile duct needs to be repaired due to injury or stricture, it would
also be carried out in a pathologic environment. Therefore, the BDD
model has clinical significance. As the dilated CBD was short and
stuck tightly together to the portal vein below, it was very easy
to injure the portal vein when attempting to resect the stenosis at
the beginning to establish this model. Therefore, due to technical
reasons, the stenosis was not removed when the CBD was repaired in
the present study. It is hoped to perform this with more elaborate
microsurgical procedures or in other experimental animals, such as
rats, in future studies. In addition, due to the small size of the
bile duct patch, a limited number of tissue sections were available
for immunofluorescence and IHC staining and the presence of EGFP
fluorescence limited the double staining capabilities. Therefore,
more detailed cellular and molecular analyses were not performed in
this study.
To the best of the authors' knowledge, this is the
first study to show a replicable and successful model for the study
of bile duct injury and repair in mice. With the help of surgical
microscopy, a good postoperative survival rate (>90%) was
obtained in the present study without serious complications.
Preliminary results suggested that the recipient-derived cells were
responsible for bile duct repair through epithelial cell
proliferation and extracellular matrix synthesis. More elaborate
molecular mechanisms of bile duct injury and repair will be
explored using genetically engineered mice or cell tracer
techniques in the future.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the Nonprofit Industry
Research Project of National Health Commission (grant no.
201502014); National Natural Science Foundation of China
(81670590); National Key R&D Program of China (grant nos.
2017YFA0103003 and 2017YFA0103002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG and DX participated in the experiments and were
responsible for the data acquisition and analysis. KP and GM were
responsible for literature searches and statistical analysis. XG
and CL drafted the manuscript. CL and SL designed the study. XG and
CL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Experiments were approved by the Committee on Ethics
of Animal Experiments of the Chinese PLA General Hospital (approval
no. 2017-X13-65) and in compliance with the recommendations of the
Guide for the Care and Use of Laboratory Animals of the National
Research Council (US) Committee, 8th edition, 2011 (https://nap.nationalacademies.org/read/12910).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pesce A, Palmucci S, La Greca G and Puleo
S: Iatrogenic bile duct injury: Impact and management challenges.
Clin Exp Gastroenterol. 12:121–128. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Booij KAC, Coelen RJ, de Reuver PR,
Besselink MG, van Delden OM, Rauws EA, Busch OR, van Gulik TM and
Gouma DJ: Long-term follow-up and risk factors for strictures after
hepaticojejunostomy for bile duct injury: An analysis of surgical
and percutaneous treatment in a tertiary center. Surgery.
163:1121–1127. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Booij KAC, de Reuver PR, van Dieren S, van
Delden OM, Rauws EA, Busch OR, van Gulik TM and Gouma DJ: Long-term
impact of bile duct injury on morbidity, mortality, quality of
life, and work related limitations. Ann Surg. 268:143–150.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stilling NM, Fristrup C, Wettergren A,
Ugianskis A, Nygaard J, Holte K, Bardram L, Sall M and Mortensen
MB: Long-term outcome after early repair of iatrogenic bile duct
injury. A national Danish multicentre study. HPB (Oxford).
17:394–400. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tocchi A, Mazzoni G, Liotta G, Lepre L,
Cassini D and Miccini M: Late development of bile duct cancer in
patients who had biliary-enteric drainage for benign disease: A
follow-up study of more than 1,000 patients. Ann Surg. 234:210–214.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Buisson EM, Jeong J, Kim HJ and Choi D:
Regenerative medicine of the bile duct: Beyond the myth. Int J Stem
Cells. 12:183–194. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sampaziotis F, Justin AW, Tysoe OC, Sawiak
S, Godfrey EM, Upponi SS, Gieseck RL III, de Brito MC, Berntsen NL,
Gómez-Vázquez MJ, et al: Reconstruction of the mouse extrahepatic
biliary tree using primary human extrahepatic cholangiocyte
organoids. Nat Med. 23:954–963. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Justin AW, Saeb-Parsy K, Markaki AE,
Vallier L and Sampaziotis F: Advances in the generation of
bioengineered bile ducts. Biochim Biophys Acta Mol Basis Dis.
1864:1532–1538. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miyazawa M, Aikawa M, Okada K, Toshimitsu
Y, Okamoto K, Koyama I and Ikada Y: Regeneration of extrahepatic
bile ducts by tissue engineering with a bioabsorbable polymer. J
Artif Organs. 15:26–31. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zeng J, Wang J, Dong J, Huang X, Xia H and
Xiang X: The application of vascularized stomach flap to repair
postoperative biliary stricture. Medicine (Baltimore).
97(e11344)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Jong IEM, Sutton ME, van den Heuvel MC,
Gouw ASH and Porte RJ: Evidence for recipient-derived cells in
peribiliary glands and biliary epithelium of the large donor bile
ducts after liver transplantation. Front Cell Dev Biol.
8(693)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee J, Chan MC, James C and Lantis JC II:
Cryopreserved allograft use in vascular surgery. Surg Technol Int.
37:237–243. 2020.PubMed/NCBI
|
|
13
|
Furlough CL, Jain AK, Ho KJ, Rodriguez HE,
Tomita TM and Eskandari MK: Peripheral artery reconstructions using
cryopreserved arterial allografts in infected fields. J Vasc Surg.
70:562–568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heinrich S, Georgiev P, Weber A,
Vergopoulos A, Graf R and Clavien PA: Partial bile duct ligation in
mice: A novel model of acute cholestasis. Surgery. 149:445–451.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Talbot SR, Biernot S, Bleich A, van Dijk
RM, Ernst L, Häger C, Helgers SOA, Koegel B, Koska I, Kuhla A, et
al: Defining body-weight reduction as a humane endpoint: A critical
appraisal. Lab Anim. 54:99–110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Emond J, Capron-Laudereau M, Meriggi F,
Bernuau J, Reynes M and Houssin D: Extent of hepatectomy in the
rat. Evaluation of basal conditions and effect of therapy. Eur Surg
Res. 21:251–259. 1989.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chilvery S, Bansod S, Saifi MA and Godugu
C: Piperlongumine attenuates bile duct ligation-induced liver
fibrosis in mice via inhibition of TGF-β1/Smad and EMT pathways.
Int Immunopharmacol. 88(106909)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tang G, Seume N, Häger C, Kumstel S,
Abshagen K, Bleich A, Vollmar B, Talbot SR, Zhang X and Zechner D:
Comparing distress of mouse models for liver damage. Sci Rep.
10(19814)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gäbele E, Froh M, Arteel GE, Uesugi T,
Hellerbrand C, Schölmerich J, Brenner DA, Thurman RG and Rippe RA:
TNFalpha is required for cholestasis-induced liver fibrosis in the
mouse. Biochem Biophys Res Commun. 378:348–353. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ezure T, Sakamoto T, Tsuji H, Lunz JG III,
Murase N, Fung JJ and Demetris AJ: The development and compensation
of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol.
156:1627–1639. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aikawa M, Miyazawa M, Okamoto K,
Toshimitsu Y, Torii T, Okada K, Akimoto N, Ohtani Y, Koyama I and
Yoshito I: A novel treatment for bile duct injury with a
tissue-engineered bioabsorbable polymer patch. Surgery.
147:575–580. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zong C, Wang M, Yang F, Chen G, Chen J,
Tang Z, Liu Q, Gao C, Ma L and Wang J: A novel therapy strategy for
bile duct repair using tissue engineering technique: PCL/PLGA
bilayered scaffold with hMSCs. J Tissue Eng Regen Med. 11:966–976.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Siqueira OH, Herani Filho B, Paula RE,
Ascoli FO, Nóbrega AC, Carvalho AC, Pires AR, Gaglionone NC, Cunha
KS and Granjeiro JM: Tamoxifen decreases the myofibroblast count in
the healing bile duct tissue of pigs. Clinics (Sao Paulo).
68:101–106. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kosmidis C, Efthimiadis C, Anthimidis G,
Basdanis G, Apostolidis S, Hytiroglou P, Vasiliadou K, Prousalidis
J and Fahantidis E: Myofibroblasts and colonic anastomosis healing
in Wistar rats. BMC Surg. 11(6)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guruswamy Damodaran R and Vermette P:
Tissue and organ decellularization in regenerative medicine.
Biotechnol Prog. 34:1494–1505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roelofs LA, Oosterwijk E, Kortmann BB,
Daamen WF, Tiemessen DM, Brouwer KM, Eggink AJ, Crevels AJ, Wijnen
RM, van Kuppevelt TH, et al: Bladder regeneration using a smart
acellular collagen scaffold with growth factors VEGF, FGF2 and
HB-EGF. Tissue Eng Part A. 22:83–92. 2016.PubMed/NCBI View Article : Google Scholar
|