Introduction
It is known that 20-40% of patients with infective
endocarditis (IE) present with cerebrovascular complications
(1,2), of which the most severe are
spontaneous intracranial hemorrhage (SICH) and intracranial
infectious aneurysm (IIA) (3). IIA
is a cerebrovascular lesion caused by the microbial infection of
the cerebral arterial vessel wall, accounting for 0.7-5.4% of all
intracranial aneurysms (4). Of
note, ~2-9% of patients with IE have IIAs. In fact, the actual
incidence may be higher, considering that many IIAs are
asymptomatic and resolve with anti-infective therapy (5,6).
Therefore, IIA, as an easily misdiagnosed
complication of IE, possibly results in severe neurological
deficits and even in mortality. Currently, the most common
treatment for ruptured IIAs is endovascular treatment, while
excision surgery is less commonly reported. The present study
describes the case of patient with IIA caused by IE, who
sequentially developed SICH twice, and underwent IIA excision
surgery.
Case report
Primary intracerebral hemorrhage
(ICH)
A 33-year-old male, who had experienced fever and
knee joint pain for 1 week, presented with a sudden headache. The
patient was transported by ambulance to the Emergency Department of
The First People's Hospital of Huzhou (Huzhou, China) in May 2021.
The Glasgow Coma Scale (GCS) score decreased rapidly form E4V5M6 to
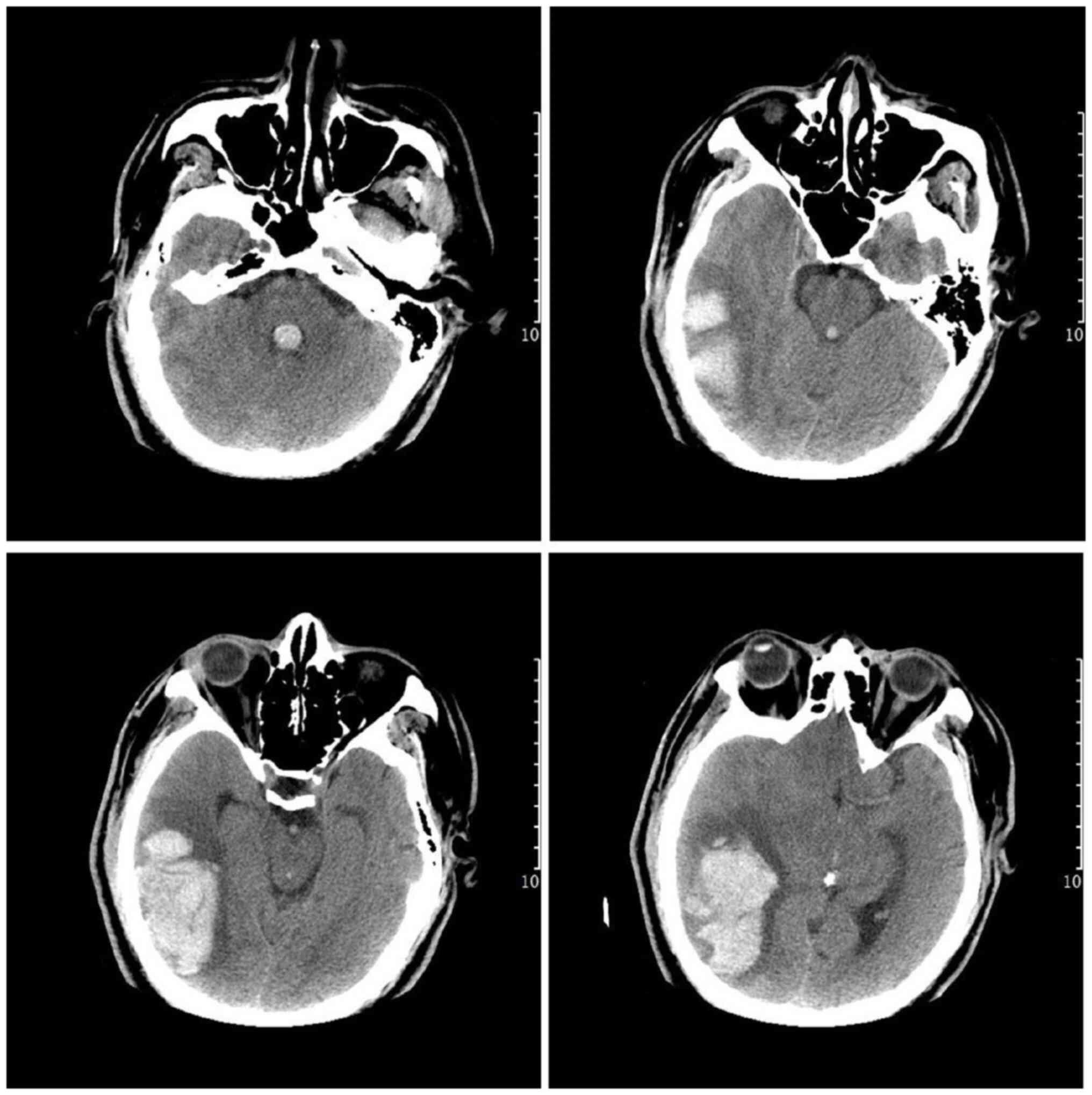
E3V2M5 within 2 h. A computed tomography (CT) scan revealed an
acute hematoma at the temporal and occipital lobe (Fig. 1). At the same time, CT angiography
(CTA) did not reveal any identify aneurysm or arteriovenous
malformation (Fig. 2). The patient
underwent surgery involving external ventricular drainage and
craniotomy for hematoma removal.
A persistent fever occurred after the surgery. An
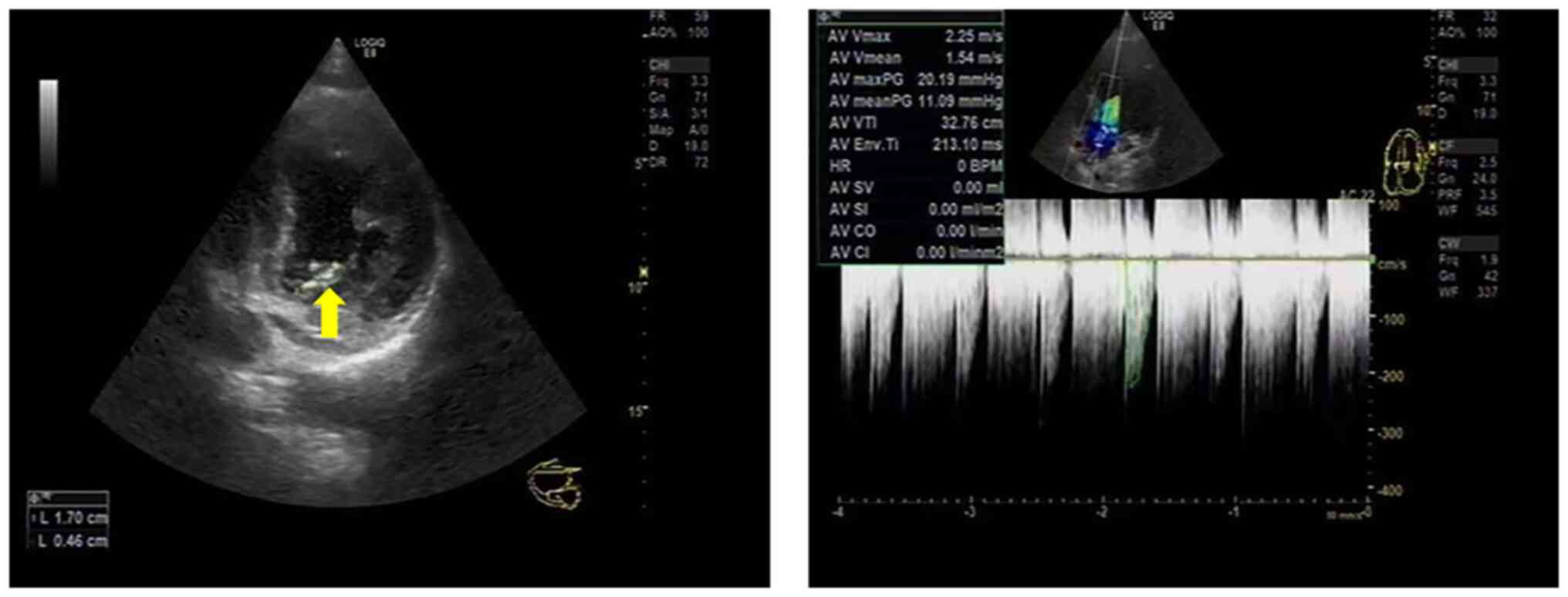
echocardiography demonstrated aortic valve vegetations (Fig. 3). Blood culture suggested that the
pathogen was coagulase-negative Staphylococcus (CoNS).
Combined with the abnormal serological results, the patient was
diagnosed with IE. Based on the drug susceptibility results,
linezolid was selected for anti-infective therapy for 4 weeks. The
dose was 0.6 g administered every 12 h. The patient had a good
post-operative recovery without any neurological dysfunction and
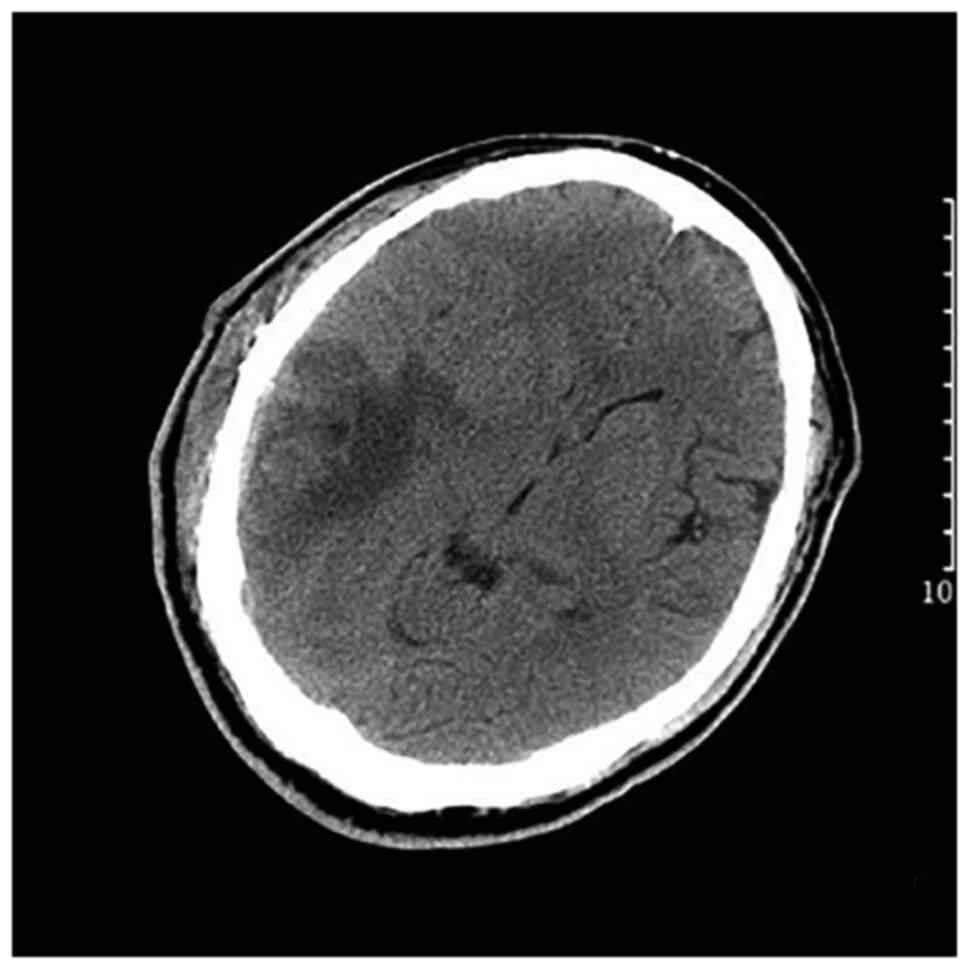
underwent a follow-up CT scan on post-operative day 11 (Fig. 4). At 26 days after the first
presentation, the patient underwent a heart valve operation at The
Second Hospital of Zhejiang University Medical College (Hangzhou,
China). During the surgery, a bicuspid aortic valve was found with
a number of vegetations, the largest of which was ~2.0 cm in
diameter. Another vegetation of 2.0 cm in diameter was also found
in the anterior mitral leaflet near the tendon cord. The aortic and
mitral valves were each removed and replaced with mechanical
valves.
Intracerebral rebleeding
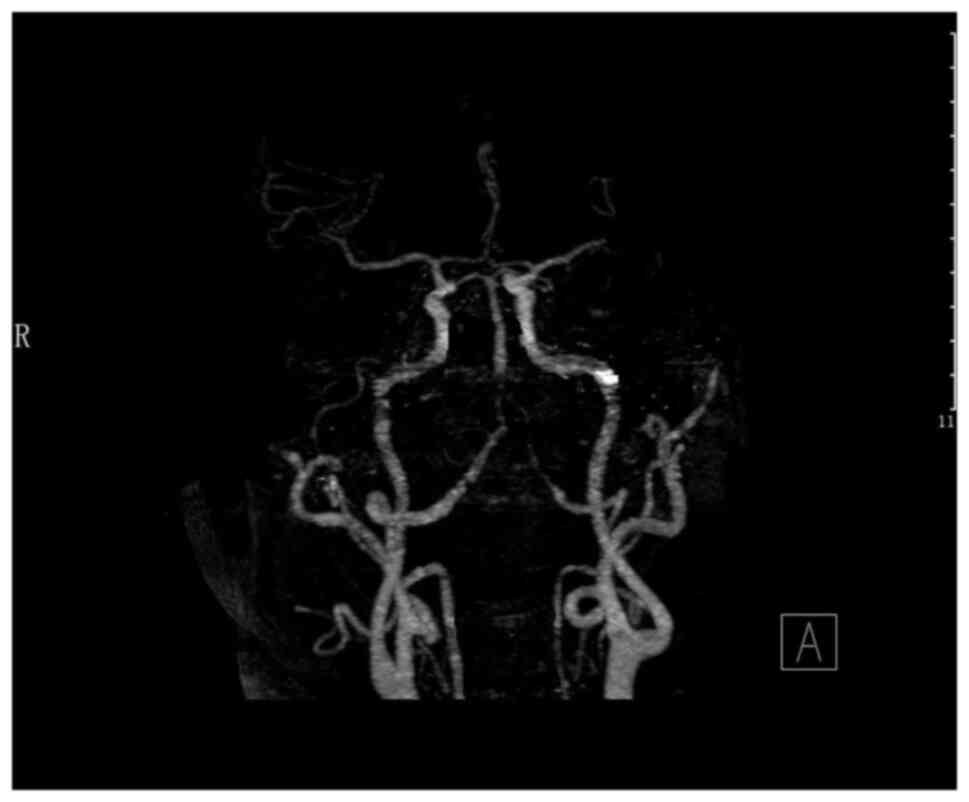
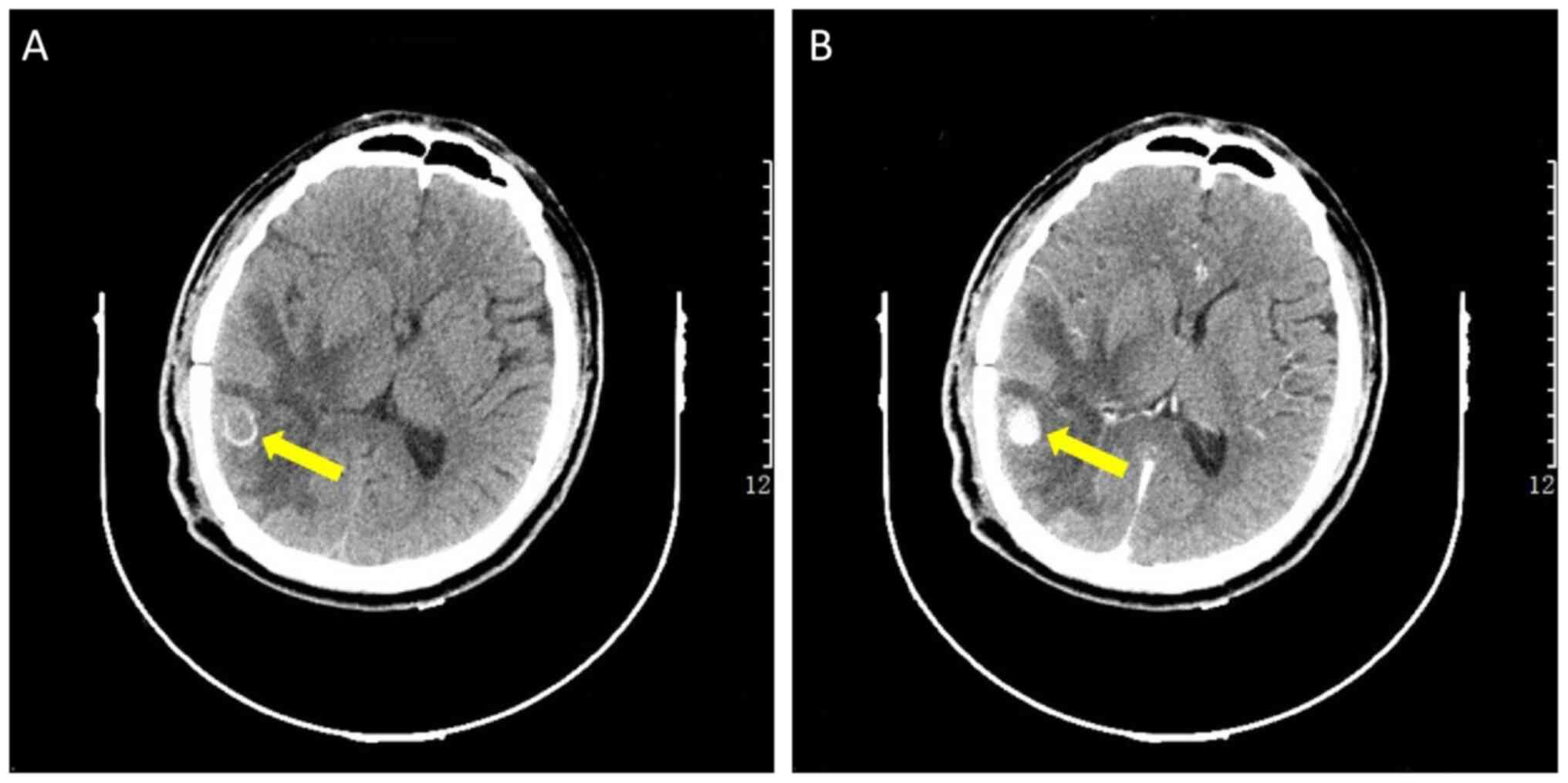
On the 36th day, an enhanced CT scan revealed an 11
mm equal density of the nodule in the right temporal lobe with
surrounding calcification (Fig.
5). These graphic features suggested that the lesion was an IIA
rather than an abscess. On the 66th day, the patient had a severe
headache and fell into a coma with left limb hemiplegia. A CT scan
revealed an acute hematoma in the area of the original hemorrhage,
which was mainly located around the previously identified IIA.
The patient underwent another emergency craniotomy.
The scalp incision approach was selected from the previous surgery.
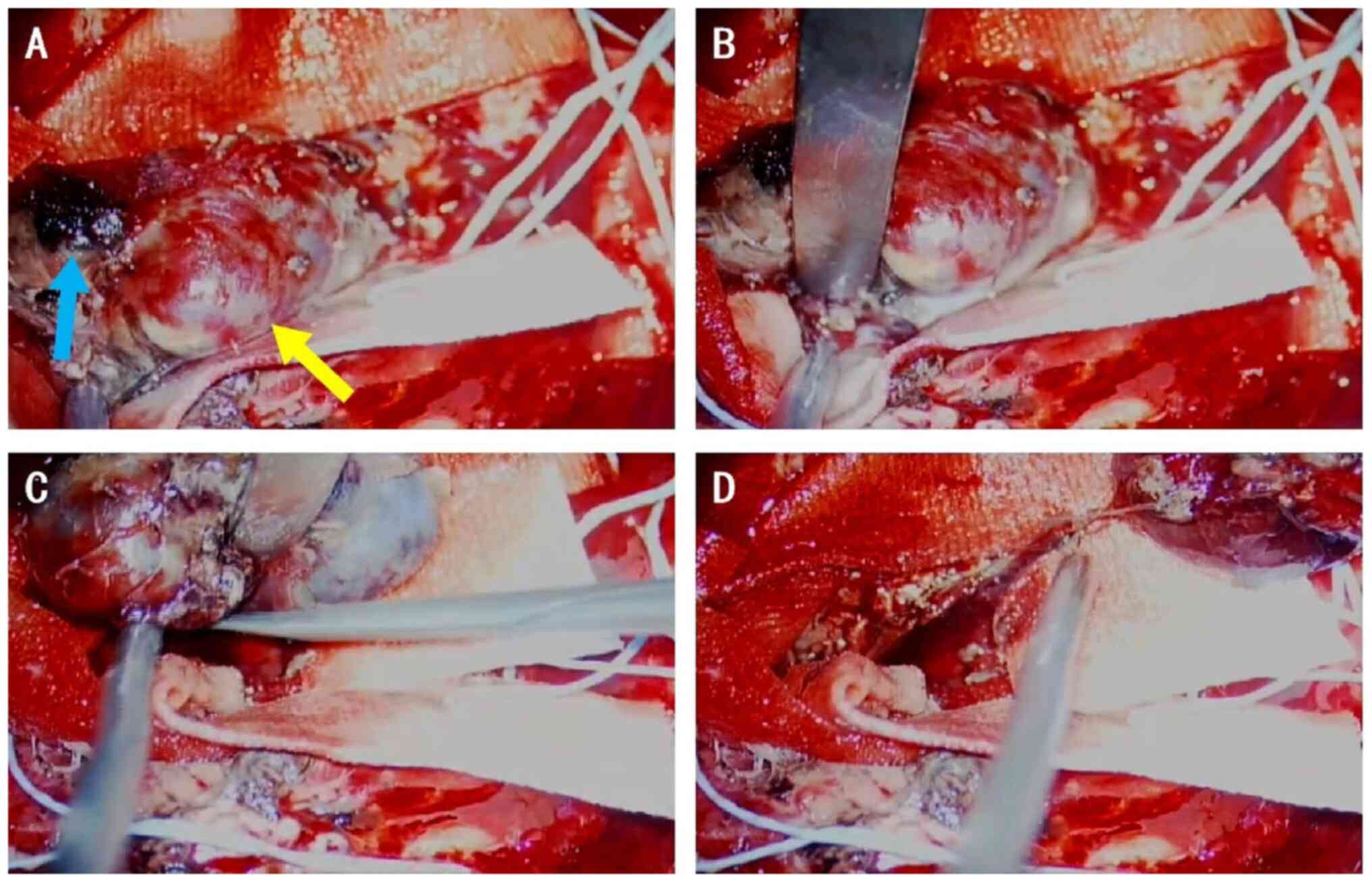
During the surgery, a blind end enlargement of a small artery was
found in the hematoma cavity (Fig.
6). The IIA was completely excised form the supplying artery
(Fig. 6) and pathological analysis
with H&E staining was performed as follows: The IIA was
immersed in 4% paraformaldehyde for 4 h and transferred to 70%
ethanol. Individual lobes of IIA biopsy material were placed in
processing cassettes, dehydrated through a serial alcohol gradient
and embedded in paraffin wax blocks. Tissue sections (5-µm) were
prepared and dewaxed in xylene, rehydrated through decreasing
concentrations of ethanol and washed in PBS. Subsequently, the
slides were stained with H&E and then dehydrated through
increasing concentrations of ethanol and xylene (all steps
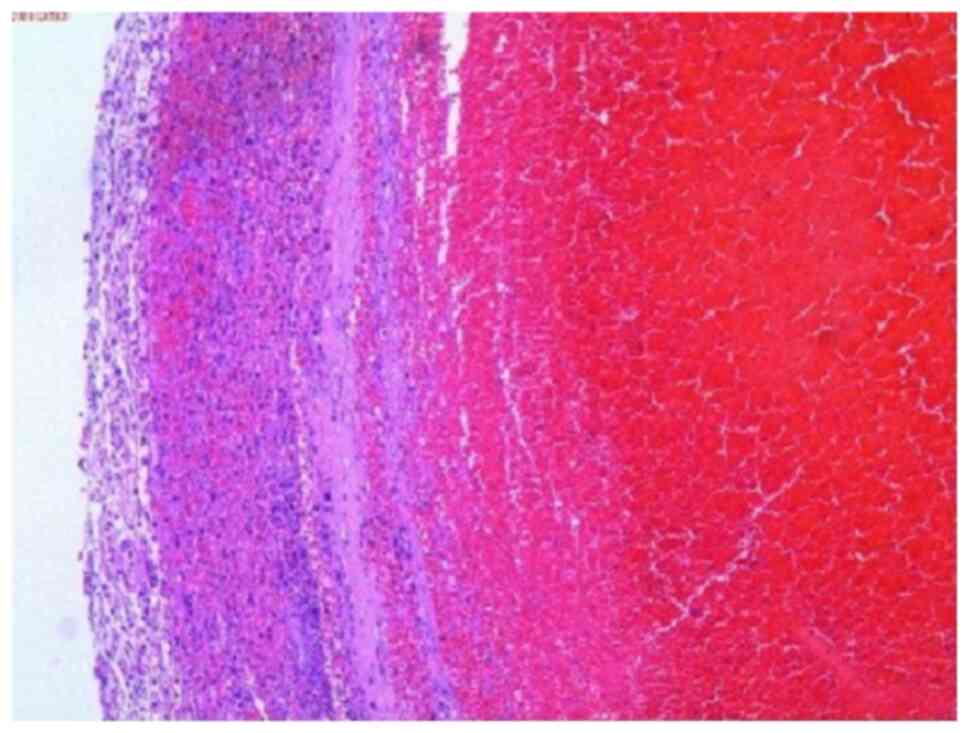
performed at room temperature). Histopathology revealed typical
features of IIA (Fig. 7). The
remaining hematoma was evacuated and the bone flap was removed
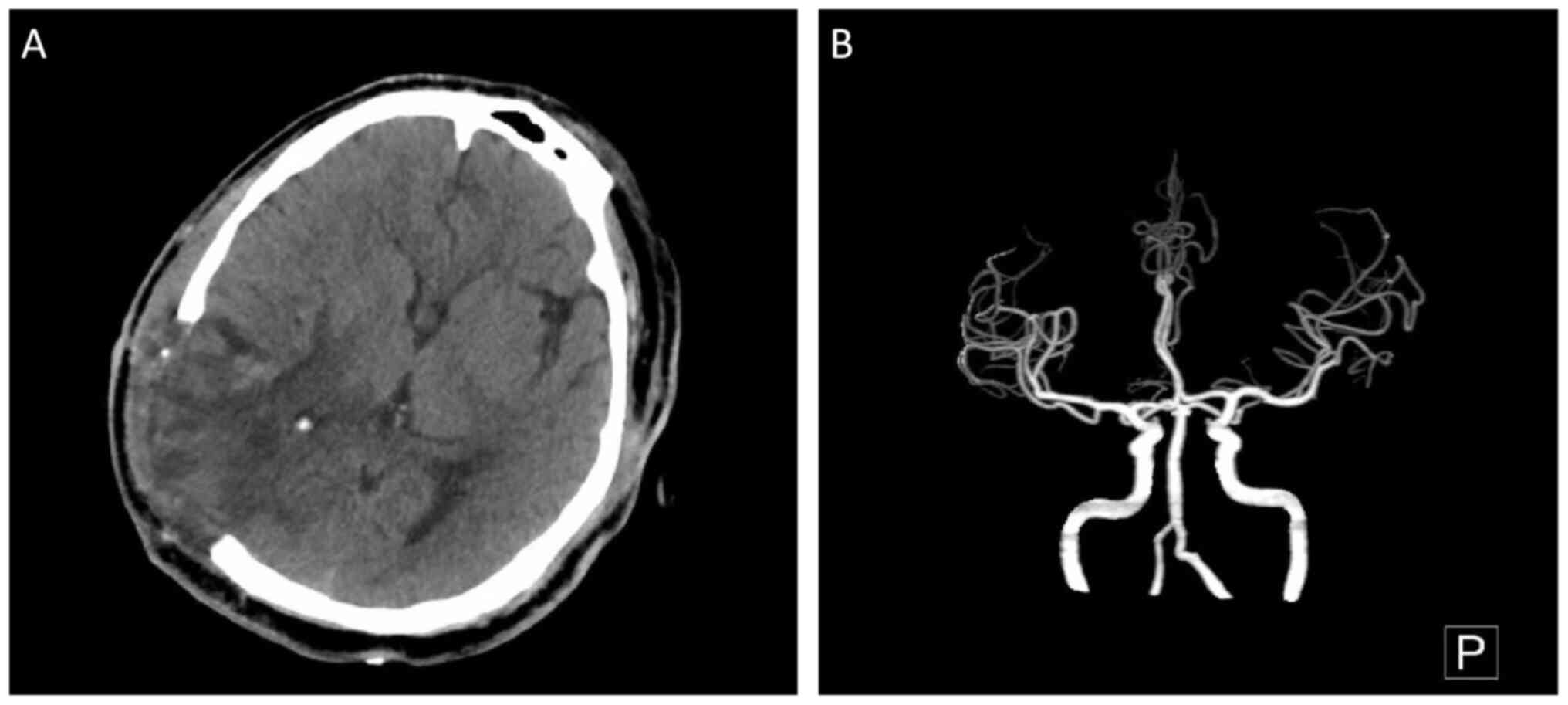
after surgery. A CT scan and CTA reexamination revealed the
absorption of the hematoma and no recurrence of the removed IIA
(Fig. 8). The left limb strength
of the patient returned to grade 5 after 4 weeks. The latest
outpatient review at ~1 year after the first presentation revealed
that the patient had returned to work.
Discussion
An analysis of the pooled cohort of patients from
the literature revealed that 65% of patients with IIAs presented
with bacterial IE (5). In the
pre-antibiotic era, this ratio commonly arises in patients with
prosthetic valves, nosocomial-acquired blood stream infections, or
a history of intravenous drug use (6,7).
Less commonly, IIA can also result from the direct extension of
intracranial bacterial infections, such as meningitis, cavernous
sinus thrombophlebitis and orbital cellulitis, often in patients
who are immunosuppressed (8). The
most common site of IIAs is the middle cerebral artery, which can
account for almost 50% of all cases (5). In order to diagnose IE, two findings
are necessary: A positive blood culture and positive imaging test
results (9). In the case presented
herein, the blood culture of CoNS and the echocardiography led to
the definitive diagnosis of IE. The pre-operative diagnosis for IIA
usually relies on angiography or CTA (10,11),
which were replaced by an enhanced CT scan in the case described
herein. Digital subtraction angiography (DSA) or pathological
results of IIA are considered to be the gold standard for pre- or
post-operative diagnoses (5,11).
However, in the present study, the patient was unable to complete
the DSA as the first ICH was of acute onset. After a proposed
diagnosis of IIA by an enhanced CT scan, the patient had been
scheduled for a DSA. However, a secondary intracerebral rebleeding
occurred prior to the DSA. Fortunately, however, pathological
graphics were obtained, which directly verified the diagnosis for
IIA.
Although the pathogenesis of IIA remains uncertain,
arteritis is generally considered the pathological basis of IIA
(12). The possible underlying
mechanism is that bacteria-containing neoplasms adhere and cause
inflammatory damage to the artery wall. The infectious process
leads to an acute infiltration of both the media and adventitia of
the vessel wall by inflammatory cells, as well as the marked
proliferation of the intima and destruction of the internal elastic
lamina. Hydrostatic pulsation and thrust against the infected
arterial wall then promote aneurysmal development and subsequent
growth (5). The aforementioned
pathophysiological process finally leads to arteritis. Due to the
different pathological structures form other types of aneurysms,
IIAs are generally considered a type of pseudoaneurysm (13). In the case in the present study,
the causes of the twice-occurring intracranial hemorrhages were
presumed not to be the same, since no suspicious aneurysms were
identified through the CTA or during the first surgery. The second
of intracranial hemorrhage with severe clinical symptoms was
confirmed to be caused by an IIA rupturing, while the first one
with milder clinical symptoms, may have been caused by arteritis.
According to the aforementioned analysis, it was found that the
intracranial hemorrhages with IIA may be more severe than those
without IIA caused by IE.
The treatment of unruptured IIAs, according to a
previous study on 16 cases, mainly involves a prolonged course of
antibiotics (14). However,
according to a clinical study published in 2016, IIA has a higher
risk of rupture compared with other aneurysms, of which the
proportion is even as high as 48% (15). Therefore, surgical treatment should
be actively adopted for IIAs that have ruptured or have a higher
risk of rupture. Previous research has indicated that the majority
of ruptured IIAs have received endovascular treatment, such as
coiling and parent artery occlusion (16). The majority of the studies on the
outcomes of surgery have focused on unruptured IIAs (15,16),
while only a limited number of studies have reported on the
surgical prognosis for ruptured IIAs (14). From sporadic case reports, it was
found that both endovascular treatment and surgical clipping have
resulted in equivalent outcomes for ruptured IIAs (5,14,17).
In the case in the present study, treatment with
sensitive antibiotics was continued when the IIAs were found
unruptured following clinical standard treatment (14,15).
However, the aneurysm suddenly ruptured during anti-infective
treatment. In order to remove the intracranial hematoma while
treating the aneurysm, craniotomy surgery was selected as opposed
to endovascular embolization. The patient finally achieved a good
prognosis without severe neurological dysfunction. In a nationwide
database sampled by Singla et al (15), it was found that patients with
ruptured IIAs undergoing neurosurgical treatment had better
outcomes than those who underwent conservative treatment.
Regrettably, the absence of intraoperative and pathological images
is a limitation of the present study, as the heart surgery was
performed at another medical center.
In conclusion, although ICH caused by IIA is rare,
secondary ICH following a previous surgery caused by IIA is more
rare. It can result in severe consequences and requires prompt
surgical treatment. In the case described herein, it was found that
traditional craniotomy may be more appropriate than endovascular
embolization for such specific ruptured IIAs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HG was involved in the writing of the original
draft, in the writing, reviewing and editing of the manuscript, and
in the collection of clinical data of the patient. ZZ was involved
in the writing, reviewing and editing of the manuscript, in the
surgical treatment of the patient, and in the collection of
clinical data of the patient. Both authors have read and approved
the final manuscript. ZZ and HG confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The patient provided signed informed consent for the
inclusion of his data in the present case report.
Patient consent for publication
The patient provided signed informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Horstkotte D, Follath F, Gutschik E,
Lengyel M, Oto A, Pavie A, Soler-Soler J, Thiene G and von
Graevenitz A: Grupo de Trabajo de Endocarditis Infecciosa de la
Sociedad Europea de Cardiología. Guidelines on prevention,
diagnosis and treatment of infective endocarditis, Executive
summary. Rev Esp Cardiol. 57:952–962. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Habib G, Lancellotti P, Antunes MJ,
Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G,
Erba PA, Iung B, et al: 2015 ESC guidelines for the management of
infective endocarditis: The task force for the management of
infective endocarditis of the european society of cardiology (ESC).
Endorsed by: European association for cardio-thoracic surgery
(EACTS), the european association of nuclear medicine (EANM). Eur
Heart J. 36:3075–3128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hemphill JC III, Greenberg SM, Anderson
CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN,
Macdonald RL, Mitchell PH, et al: Guidelines for the management of
spontaneous intracerebral hemorrhage: A guideline for healthcare
professionals from the American heart association/american stroke
association. Stroke. 46:2032–2060. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chapot R, Houdart E, Saint-Maurice JP,
Aymard A, Mounayer C, Lot G and Merland JJ: Endovascular treatment
of cerebral mycotic aneurysms. Radiology. 222:389–396.
2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ducruet AF, Hickman ZL, Zacharia BE,
Narula R, Grobelny BT, Gorski J and Connolly ES Jr: Intracranial
infectious aneurysms: A comprehensive review. Neurosurg Rev.
33:37–46. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Peters PJ, Harrison T and Lennox JL: A
dangerous dilemma: management of infectious intracranial aneurysms
complicating endocarditis. Lancet Infect Dis. 6:742–748.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Avallone SV, Levy AS and Starke RM: A rare
case of Streptococcus anginosus infectious intracranial aneurysm:
Proper management of a poor prognosis. Surg Neurol Int.
12(487)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mincheff TV and Cooler AW: Ruptured
mycotic aneurysm presenting initially with bacterial meningitis. Am
Surg. 74:73–75. 2008.PubMed/NCBI
|
|
9
|
Habib G, Erba PA, Iung B, Donal E, Cosyns
B, Laroche C, Popescu BA, Prendergast B, Tornos P, Sadeghpour A, et
al: Clinical presentation, aetiology and outcome of infective
endocarditis. Results of the ESC-EORP EURO-ENDO (European infective
endocarditis) registry: A prospective cohort study. Eur Heart J.
40:3222–3232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Venkatesh SK, Phadke RV, Kalode RR, Kumar
S and Jain VK: Intracranial infective aneurysms presenting with
haemorrhage: An analysis of angiographic findings, management and
outcome. Clin Radiol. 55:946–953. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kovoor JM, Jayakumar PN, Srikanth SG and
Sampath S: Intracranial infective aneurysms: Angiographic
evaluation with treatment. Neurol India. 49:262–266.
2001.PubMed/NCBI
|
|
12
|
Boukobza M, Naggara O, Duval X and Laissy
JP: Acute enlargement, morphological changes, and rupture of
intracranial infectious aneurysm in infective endocarditis. Serial
imaging. J Clin Neurosci. 82:237–240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumar M and Kitchen ND: Infective and
traumatic aneurysms. Neurosurg Clin N Am. 9:577–586.
1998.PubMed/NCBI
|
|
14
|
Phuong LK, Link M and Wijdicks E:
Management of intracranial infectious aneurysms: A series of 16
cases. Neurosurgery. 51:1145–1151. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Singla A, Fargen K, Blackburn S, Neal D,
Martin TD, Hess PJ, Beaver TM, Klodell CT and Hoh B: National
treatment practices in the management of infectious intracranial
aneurysms and infective endocarditis. J Neurointerv Surg.
8:741–746. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Park W, Ahn JS, Park JC, Kwun BD and Lee
DH: Treatment strategy based on experience of treating intracranial
infectious aneurysms. World Neurosurg. 97:351–359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vieira E, Faquini IV, Silva JL, Griz MFL,
Cezar AB, Almeida NS and Azevedo-Filho HRC: Subarachnoid
neurocysticercosis and an intracranial infectious aneurysm: case
report. Neurosurg Focus. 47(E16)2019.PubMed/NCBI View Article : Google Scholar
|