Introduction
Surgery is the standard treatment for most early to
intermediate stages of solid cancers (1). The surgical excision of the primary
tumor, along with adjuvant therapy, reduces the risk of disease
recurrence and distant metastasis for cancers (2,3).
Adjuvant treatment options, including chemotherapy, radiotherapy,
and endocrine therapy (4-6),
have been widely used in resected cancers for several decades.
Targeted agents such as imatinib (7) and osimertinib (8) are also found to significantly improve
the survival of patients with high-risk primary gastrointestinal
stromal tumor and EGFR mutation-positive non-small-cell lung
cancer, respectively. However, despite the advent of adjuvant
therapies, the severe toxicities of chemotherapy usually cause
significant pain to patients with cancer. Although some patients
can benefit from adjuvant treatment with targeted agents, this type
of treatment is only for patients with specific and sensitive gene
mutations, which occur in a small number of patients. In addition,
due to the drug resistance of tumors, disease recurrence and
distant metastasis are also unavoidable problems (9). Therefore, more effective and safer
adjuvant treatment strategies are needed to improve survival for
patients with cancer.
In recent years, adjuvant immunotherapy with
anti-programmed cell death protein-1 (anti-PD-1)/programmed cell
death 1 ligand 1 (PD-L1) antibodies, such as pembrolizumab (a
humanized IgG4 monoclonal anti-PD-1 antibody), nivolumab (a fully
human IgG4 anti-PD-1 antibody), and atezolizumab (MPDL3280A, an IgG
anti-PD-L1 antibody), has shown promising anti-tumor activity and
safety in various solid cancers (10-12).
Eggermont et al (13)
report that adjuvant pembrolizumab significantly improves
recurrence-free survival for patients with completely resected
high-risk stage III melanoma. The one-year recurrence-free survival
was 75.4% for pembrolizumab vs. 61.0% for placebo [hazard ratio
(HR)=0.57; 98.4% confidence interval (CI): 0.43-0.74; P<0.001].
In the CheckMate 238 trial (11),
adjuvant nivolumab resulted in significantly prolonged
recurrence-free survival and a lower rate of grade 3 or higher
adverse events (AEs) than ipilimumab among patients with resected
stage IIIB-IV melanoma. In the KEYNOTE-564 trial (14), adjuvant treatment with
pembrolizumab was associated with a significantly longer
disease-free survival (DFS) compared with placebo after nephrectomy
among patients with renal cell carcinoma who were at high risk for
recurrence. In the CheckMate 274 trial (15) and IMMUNED trial (16), DFS was longer with adjuvant
nivolumab than with placebo in patients with high-risk
muscle-invasive urothelial carcinoma. In the CheckMate 577 trial
(17), nivolumab adjuvant therapy
showed a significantly longer DFS than placebo in patients with
resected esophageal or gastroesophageal junction cancer who
received neoadjuvant chemoradiotherapy in previous treatments.
Clinical trials (13-18)
have shown that adjuvant therapy with PD-1/PD-L1 inhibitors has
promising efficacy and safety in solid cancers and this type of
treatment strategy may become a new standard of care for malignant
tumors. However, despite encouraging results, current treatment
guidance for adjuvant therapy with PD-1/PD-L1 inhibitors in solid
cancers is lacking. Therefore, a meta-analysis is warranted to
present evidence regarding the clinical efficacy and safety of
adjuvant therapy with PD-1/PD-L1 inhibitors in solid cancers.
Materials and methods
Strategy of study screening
Potentially relevant studies were obtained by
searching the databases of PubMed, Embase, and Cochrane Library
from inception until April 2022. For the literature search, the
present study used any of the following key words: ‘Immune
checkpoint blockade, immune checkpoint inhibitor, immune therapy,
immunotherapy, PD-1, PD-L1, pembrolizumab, nivolumab, atezolizumab,
tremelimumab, avelumab, durvalumab, adjuvant, postoperative and
randomized controlled trial’. It also manually searched relevant
references to identify other relevant clinical trials. The
inclusion criteria were as follows: i) Participants-patients
diagnosed as cancers by post-operative pathology; ii)
intervention-adjuvant therapy with PD-1/PD-L1 inhibitors; iii)
comparison-placebo, observation, or other adjuvant treatments, such
as chemotherapy, target therapy, and endocrine therapy; iv)
outcomes-reporting data of DFS, overall survival (OS), and grade 3
or higher AEs; v) randomized controlled trials (RCTs). The
exclusion criteria were as follows: i) Non-English articles; ii)
non-RCTs, reviews, meta-analysis, letters, or case reports; and
iii) animal studies or basic experiments. The trials identified via
the search were independently screened for inclusion by two
authors; MDC and LZY. Any disagreements were arbitrated by a third
author; CL.
Data extraction and quality
assessment
The key information in the included articles was
independently extracted by two reviewers; HJF and LPH. The key
information included the study details, year, phase, tumors, sample
size, age, sex, and regimens. Clinical outcomes including DFS, OS
and grade 3 or higher treatment-related AEs were recorded for
further analysis. The DFS data of patients with PD-L1-negative
(<1%) and PD-L1-positive (≥1%) tumors were also recorded in
detail. When multiple papers of the same trial were identified,
data were extracted and recorded as a single trial. If any
discrepancy occurred, problems were resolved via discussions and
consensus. Two authors; CL and WHL, used the Cochrane Collaboration
risk of bias assessment tool to assess the risk of bias of the
included RCTs (19).
Statistical analysis
The meta-analysis was conducted with Stata 15.0
software (Stata Corporation). HRs with 95% CIs were used to
evaluate the influence of anti-PD-1/PD-L1 treatments on the DFS and
OS of patients with cancer. Odds ratios (ORs) with 95% CI
represented the effects of anti-PD-1/PD-L1 treatments on AEs.
Between-study heterogeneity was analyzed using I-squared
(I2) tests in the meta-analysis. If the heterogeneity
was considered high (either I2 >50% or P<0.1), the
randomized-effects model was applied; otherwise, the fixed-effects
model was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Search results and study
characteristics
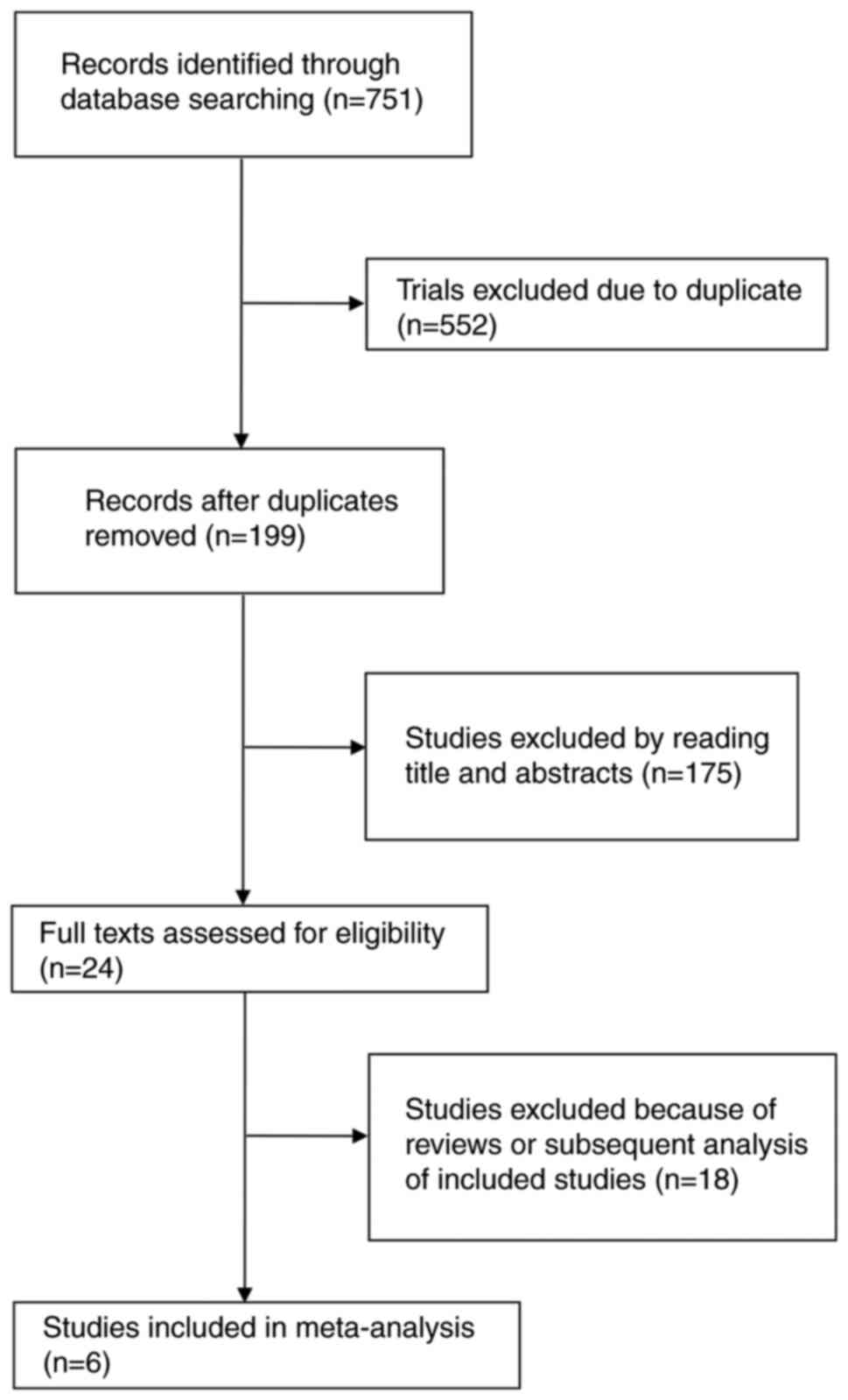
Fig. 1 shows the
flowchart of the selection process and detailed identification.
After screening, six RCTs with a total of 4,436 patients were
included (13-18).
Among the six global, multi-center RCTs, five were phase 3 studies
(13-15,17,18),
and the remaining study was a phase 2 study (16). All patients were diagnosed with
solid cancers by post-operative pathology and received a PD-1/PD-L1
inhibitor in the adjuvant setting. Across these six trials, four
types of malignancies were included: Melanoma, urothelial
carcinoma, esophageal and gastroesophageal junction cancer, and
renal cell carcinoma. All articles were published between 2018 and
2021. All anti-PD-1/PD-L1 agents were identified in the systematic
evaluation, including three doses of nivolumab (15-17),
two doses of pembrolizumab (13,14),
and one dose of atezolizumab (18). Table
I shows the main characteristics of the included studies.
 | Table IThe main characteristics of included
studies. |
Table I
The main characteristics of included
studies.
| Study (author,
year) | Phase | Tumor | Sample size, n | Median age
(years) | Male (%) | Regimens | Median DFS
(months) | Grade 3 or higher
immune-related AEs of Exp, % | (Refs.) |
|---|
| EORTC 1325 (Eggermont
et al 2018) | 3 | Melanoma | Exp: 514; Con:
505 | 54 vs. 54 | 63 vs. 60 | Pembrolizumab vs.
placebo | NR vs. 20.0 | 7.1 | (13) |
| KEYNOTE-564 (Choueiri
et al 2021) | 3 | Renal-cell
carcinoma | Exp: 496; Con:
498 | 60 vs. 60 | 70 vs. 72 | Pembrolizumab vs.
placebo | NR in both
groups | 18.9 | (14) |
| CheckMate 274
(Bajorin et al 2021) | 3 | Urothelial
carcinoma | Exp: 353; Con:
356 | 65 vs. 66 | 75 vs. 77 | Nivolumab vs.
placebo | 22.9 vs. 13.7 | 17.9 | (15) |
| IMMUNED (Zimmer et
al 2020) | 2 | Melanoma | Exp: 59; Con: 52 | 57 vs. 59 | 53 vs. 63 | Nivolumab vs.
placebo | 12.4 vs. 6.4 | 26.7 | (16) |
| CheckMate 577 (Kelly
et al 2021) | 3 | Esophageal or
gastroesophageal junction cancer | Exp: 532; Con:
262 | 62 vs. 61 | 84 vs. 85 | Nivolumab vs.
placebo | 22.4 vs. 11.0 | 13.3 | (17) |
| IMvigor010
(Bellmunt et al 2021) | 3 | Urothelial
carcinoma | Exp: 406; Con:
403 | 67 vs. 66 | 79 vs. 78 | Atezolizumab vs.
observation | 19.4 vs. 16.6 | 16 | (18) |
Efficacy
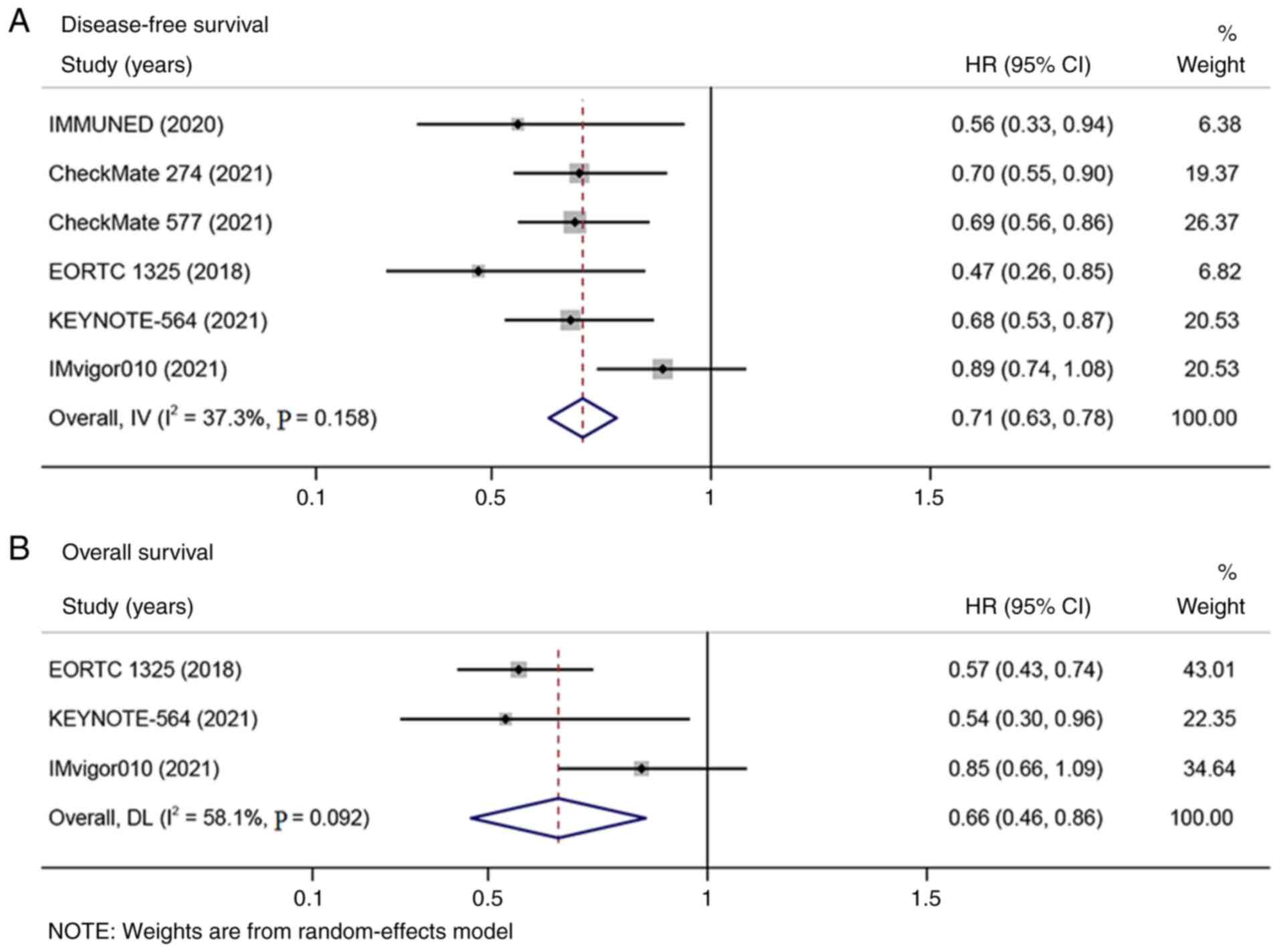
DFS data were extracted from the six included
studies and three publications reported the data of OS. In the DFS
analysis, no heterogeneity was observed (I2 <50%)
(Fig. 2A). In the OS analysis,
clear heterogeneity was observed (I2 >50%; Fig. 2B). The meta-analysis indicated that
adjuvant therapy with PD-1/PD-L1 inhibitors significantly improved
DFS (HR=0.71; 95% CI: 0.63-0.78; P<0.001; Fig. 2A) and OS (HR=0.66; 95% CI:
0.46-0.86; P<0.001; Fig. 2B)
compared with placebo or observation.
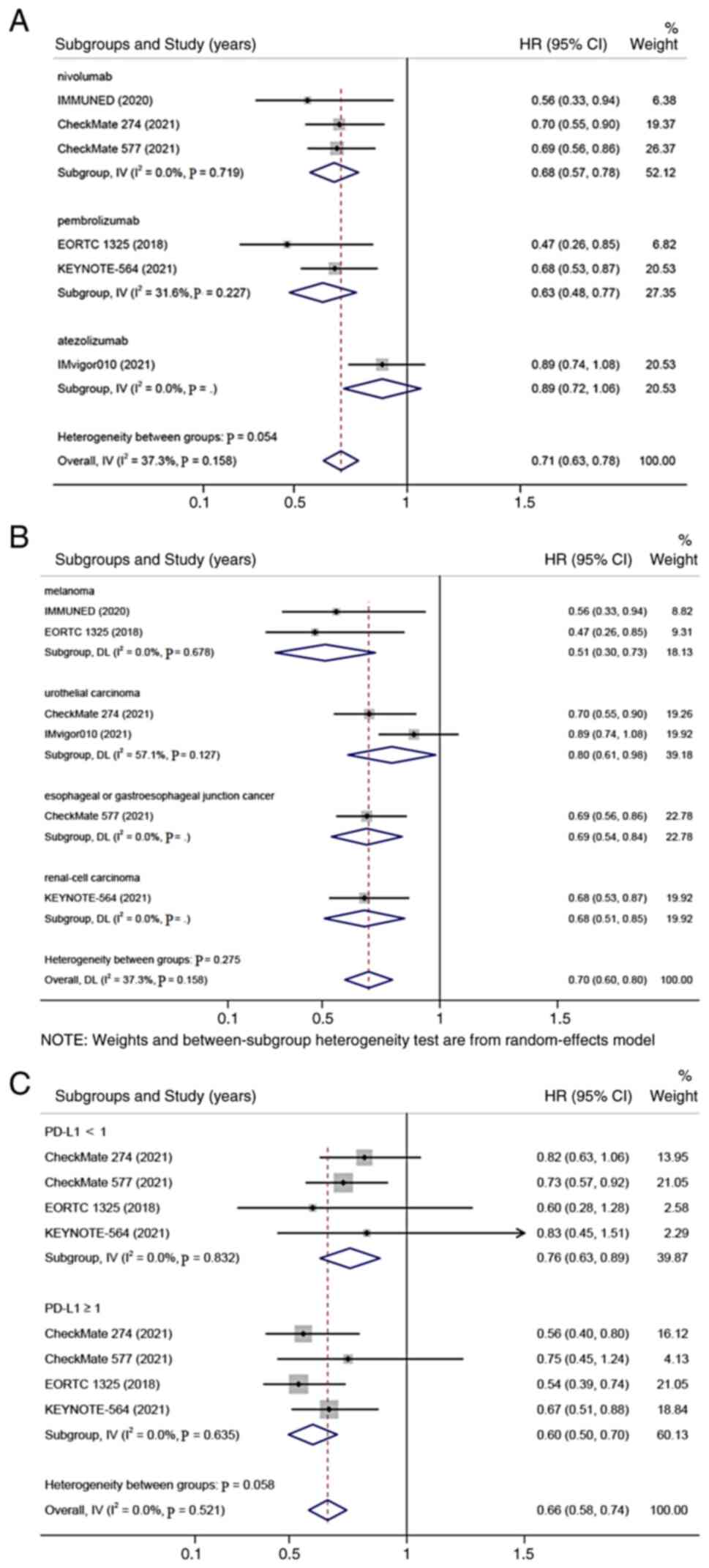
On the basis of the anti-PD-1/PD-L1 drugs
(nivolumab, pembrolizumab, or atezolizumab), tumors (melanoma,
urothelial carcinoma, esophageal or gastroesophageal junction
cancer, or renal cell carcinoma), and PD-L1 status [negative
(<1%) or positive (≥1%)], a subgroup analysis of DFS was
performed. It was found that patients receiving adjuvant therapy
with PD-1/PD-L1 inhibitors was associated with significantly longer
DFS than those receiving placebo in all subgroups (all P<0.001;
Fig. 3A-C).
Safety
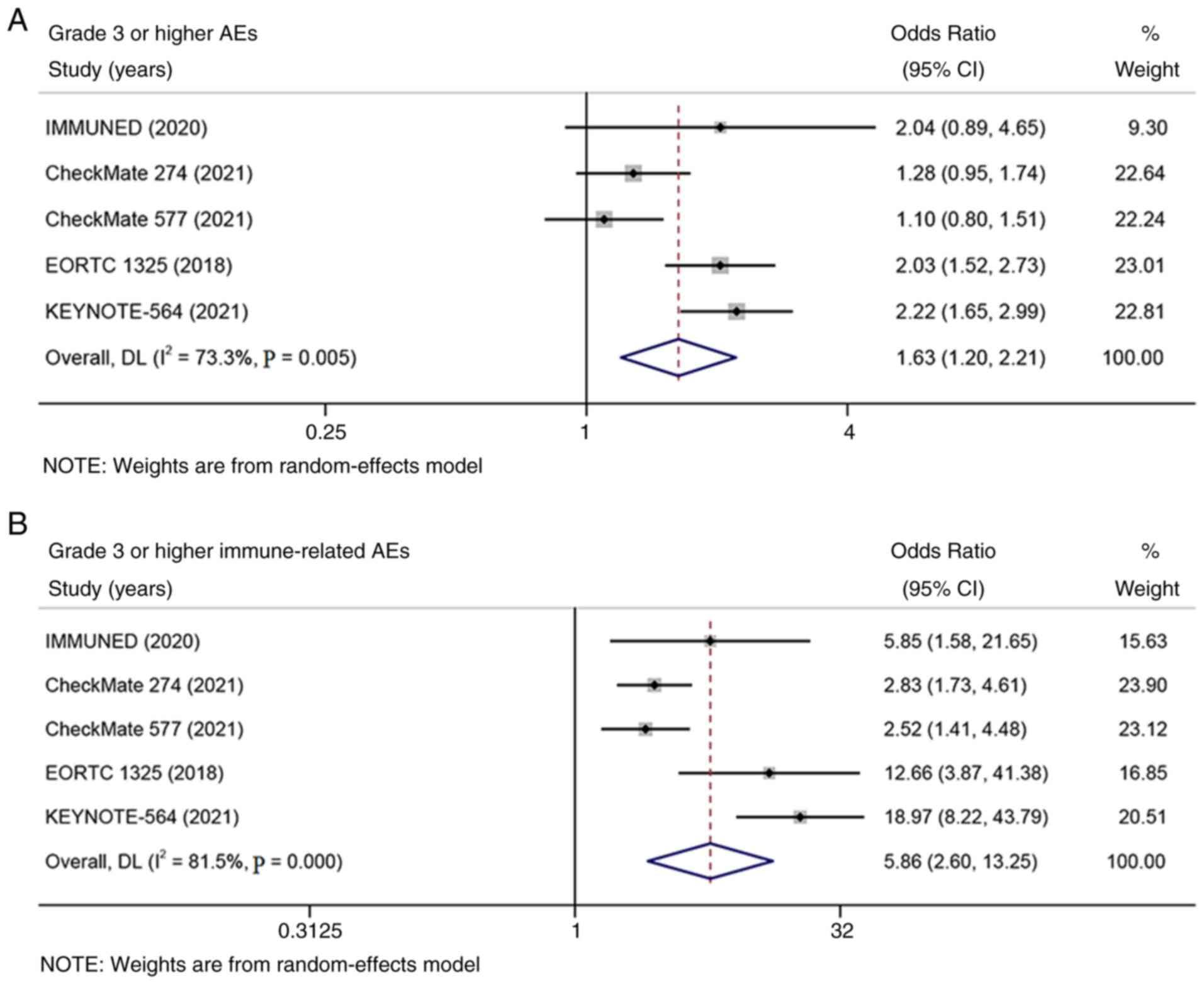
The rates of grade 3 or higher treatment-related AEs
and immune-related AEs were extracted from five of the six included
studies. Significant heterogeneity was found in the analysis of AEs
(I2 > 50%; Fig. 4).
The meta-analysis suggested that adjuvant therapy with PD-1/PD-L1
inhibitors cause more grade 3 or higher treatment-related AEs and
immune-related AEs than placebo [OR=1.63; 95% CI: 1.20-2.21; and
P=0.002 (Fig. 4A) and OR=5.86; 95%
CI: 2.60-13.25; and P<0.001 (Fig.
4B), respectively].
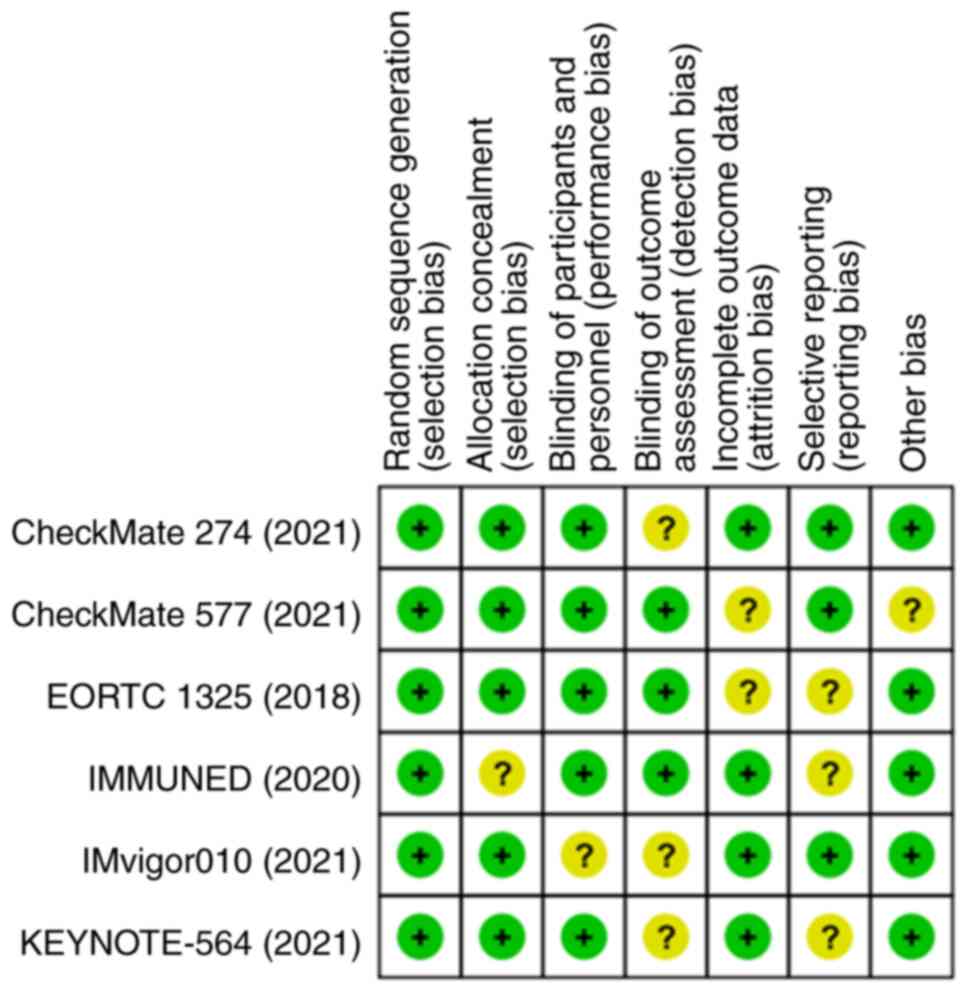
Quality of the included studies
Fig. 5 shows the
risks of bias of the included studies in this meta-analysis. The
results demonstrated that the six eligible studies were of high
quality and that the pooled analysis results were credible.
Discussion
Immune checkpoint inhibitors play an important role
in the treatment of cancers (20)
and anti-PD-1/PD-L1 treatments are widely used in advanced or
metastatic cancers (21-23).
However, the use of PD-1/PD-L1 inhibitors in the adjuvant setting
for patients with cancer is still a tentative approach. Clinical
trials show that adjuvant therapy with PD-1/PD-L1 inhibitors offers
a better survival benefit than placebo in various solid cancers
(11,13-18).
On the basis of the results of the CHECKMATE-238 and
EORTC1325/KEYNOTE-054 trials, the US Food and Drug Administration
approved PD-1 blocking agents nivolumab and pembrolizumab as
adjuvant treatments for patients with high-risk melanoma (11,24).
Although adjuvant therapy with PD-1/PD-L1 inhibitors has
demonstrated promising efficacy in many resected solid cancers, a
treatment guidance regarding adjuvant immunotherapy for cancers is
lacking. Furthermore, whether this treatment strategy could be
generalizable to more malignant tumors remain uncertain.
The present meta-analysis showed that adjuvant
therapy with PD-1/PD-L1 inhibitors significantly improved DFS
(HR=0.71; 95% CI: 0.63-0.78; P<0.001) and OS (HR=0.66; 95% CI:
0.46-0.86; P<0.001) compared with placebo or observation in
solid cancers, thus suggesting that adjuvant therapy with
PD-1/PD-L1 inhibitors is more effective than placebo as adjuvant
treatments for some types of solid cancers. Similar results were
observed for adjuvant immunotherapy with ipilimumab, which is a
cytotoxic T lymphocyte antigen 4 inhibitor. In the phase 3 EORTC
18071 trial (25), adjuvant
ipilimumab resulted in a significantly longer recurrence-free
survival than placebo (HR=0.75; 95% CI; 0.64-0.90; P=0.0013) for
patients with completely resected high-risk stage III melanoma. In
the IMMUNED trial (16), adjuvant
therapy with nivolumab plus ipilimumab significantly improved
recurrence-free survival compared with placebo (HR=0.23; 97.5% CI,
0.12-0.45; P<0.0001) in patients with stage IV melanoma with no
evidence of disease. Moreover, the present analysis showed that
significantly improved DFS was observed in subgroups including
anti-PD-1/PD-L1 drugs (nivolumab, pembrolizumab, or atezolizumab),
types of tumors (melanoma, urothelial carcinoma, esophageal or
gastroesophageal junction cancer, or renal cell carcinoma), and
PD-L1 status [negative (<1%) or positive (≥1%)], thus indicating
that adjuvant therapy with PD-1/PD-L1 inhibitors is efficacious for
various solid cancers and is likely to benefit patients regardless
of PD-L1 expression levels. Although the exact anti-tumor mechanism
remains to be elucidated, the possible reason for the enhanced
benefit of adjuvant immunotherapy for patients with cancer may be
related to the immune reconstruction by anti-PD-1/PD-L1 treatments
after the removal of tumors by surgery (26,27).
The findings of the present study supported the use of
anti-PD-1/PD-L1 treatments in an adjuvant setting for some types of
solid cancers.
Regarding toxicity, the safety and tolerability
profile of PD-1/PD-L1 inhibitor monotherapy are well-established in
advanced or metastatic cancers (28,29).
In the current study, the meta-analysis showed that adjuvant
therapy with PD-1/PD-L1 inhibitors was associated with more grade 3
or higher treatment-related AEs and immune-related AEs than placebo
(OR=1.63; 95% CI: 1.20-2.21; and P=0.002 and OR=5.86; 95% CI:
2.60-13.25; and P<0.001, respectively). These results were
consistent with those of Galsky et al (30), who report that pembrolizumab shows
more treatment-emergent grade 3-4 AEs (59 vs. 38%) than placebo
after first-line chemotherapy in patients with metastatic
urothelial cancer. Grade 3 or higher AEs occurred in 59% of
patients receiving pembrolizumab and 38% of patients receiving
placebo. Naidoo et al (28)
report that the commonest immune-related AEs of anti-PD-1/PD-L1
antibodies were fatigue, rash, pruritus, pneumonitis, infusion
reaction and hypothyroidism and that PD-1/PD-L1 inhibitor
monotherapy were well-tolerated. Compared with anti-cytotoxic T
lymphocyte antigen 4 antibodies, anti-PD-1/PD-L1 agents were
associated with significantly less toxicity. In the current study,
although the general safety profile of anti-PD-1/PD-L1 treatments
was found to be worse than that of placebo, the rates of grade 3 or
higher immune-related AEs were low (7.1-26.7%; Table I) and treatment-related deaths were
rarely reported; these findings were consistent with the known AEs
reported in previous studies (28-30).
The toxicities of PD-1/PD-L1 inhibitors were manageable and
well-tolerated.
Despite encouraging results, the present study has
several limitations. First, patients in the included trials were
diagnosed with different types of tumors (melanoma, urothelial
carcinoma, esophageal or gastroesophageal junction cancer and renal
cell carcinoma) and tumor stages, thus adding heterogeneity to the
analysis. Second, the number of included studies is small
(unpublished papers had not been considered) and a limited number
of PD-1/PD-L1 inhibitors was included; these may lead to a
limitation in the evaluation of results in the current study.
Third, in the present study, patients treated with or without prior
neoadjuvant chemo-radiotherapy were both eligible. However, because
of limited data, a subgroup analysis according to neoadjuvant
treatments was not performed. Considering the possible impact of
neoadjuvant treatments on survival, future research is needed.
Finally, the follow-up time among each trial is different, and the
data of OS from some included trials are not mature enough because
of the limited follow-up time. Therefore, the analysis of OS needs
further investigations.
The current meta-analysis demonstrated that compared
with placebo or observation, PD-1/PD-L1 inhibitors greatly enhanced
DFS and OS in the adjuvant setting for solid cancers. Although
grade 3 or higher treatment-related AEs increased, the toxicities
were manageable. The results supported the use of adjuvant therapy
with PD-1/PD-L1 inhibitors in some types of solid cancers and this
treatment strategy is worth popularizing in clinics. Given the
limitations of the present study, further investigations are
required.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded in part by the Self-Raised
Scientific Research Fund of the Ministry of Health of Guangxi
Province (grant no. Z20200080).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
DM and ZL contributed to the study design and
writing. JH and PL performed the data collection and selection. LC
and HW performed the data analysis. DM and ZL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wyld L, Audisio RA and Poston GJ: The
evolution of cancer surgery and future perspectives. Nat Rev Clin
Oncol. 12:115–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hortobagyi GN and Buzdar AU: Current
status of adjuvant systemic therapy for primary breast cancer:
Progress and controversy. CA Cancer J Clin. 45:199–226.
1995.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pondé NF, Zardavas D and Piccart M:
Progress in adjuvant systemic therapy for breast cancer. Nat Rev
Clin Oncol. 16:27–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maehara Y, Baba H and Sugimachi K:
Adjuvant chemotherapy for gastric cancer: A comprehensive review.
Gastric Cancer. 4:175–184. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Minsky BD: Adjuvant radiation therapy for
colon cancer. Cancer Treat Rev. 21:407–414. 1995.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Osborne CK: Tamoxifen in the treatment of
breast cancer. N Engl J Med. 339:1609–1618. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Joensuu H, Eriksson M, Sundby Hall K,
Reichardt A, Hermes B, Schütte J, Cameron S, Hohenberger P, Jost
PJ, Al-Batran SE, et al: Survival outcomes associated with 3 years
vs 1 year of adjuvant imatinib for patients with high-risk
gastrointestinal stromal tumors: An analysis of a randomized
clinical trial after 10-year follow-up. JAMA Oncol. 6:1241–1246.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu YL, Tsuboi M, He J, John T, Grohe C,
Majem M, Goldman JW, Laktionov K, Kim SW, Kato T, et al:
Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N
Engl J Med. 383:1711–1723. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suhail Y, Cain MP, Vanaja K, Kurywchak PA,
Levchenko A, Kalluri R and Kshitiz : Systems biology of
cancer metastasis. Cell Syst. 9:109–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li T, Jia DD and Teng LS: Adjuvant
pembrolizumab versus high-dose interferon α-2b for Chinese patients
with resected stage III melanoma: A retrospective cohort study.
Invest New Drugs. 38:1334–1341. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ascierto PA, Del Vecchio M, Mandalá M,
Gogas H, Arance AM, Dalle S, Cowey CL, Schenker M, Grob JJ,
Chiarion-Sileni V, et al: Adjuvant nivolumab versus ipilimumab in
resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-Year
results from a multicentre, double-blind, randomised, controlled,
phase 3 trial. Lancet Oncol. 21:1465–1477. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eggermont AMM, Blank CU, Mandala M, Long
GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A,
Carlino MS, et al: Adjuvant pembrolizumab versus placebo in
resected stage III melanoma. N Engl J Med. 378:1789–1801.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choueiri TK, Tomczak P, Park SH, Venugopal
B, Ferguson T, Chang YH, Hajek J, Symeonides SN, Lee JL, Sarwar N,
et al: Adjuvant pembrolizumab after nephrectomy in renal-cell
carcinoma. N Engl J Med. 385:683–694. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bajorin DF, Witjes JA, Gschwend JE,
Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat
SF, Park SH, et al: Adjuvant nivolumab versus placebo in
muscle-invasive urothelial carcinoma. N Engl J Med. 384:2102–2114.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zimmer L, Livingstone E, Hassel JC, Fluck
M, Eigentler T, Loquai C, Haferkamp S, Gutzmer R, Meier F, Mohr P,
et al: Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy
versus placebo in patients with resected stage IV melanoma with no
evidence of disease (IMMUNED): A randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet. 395:1558–1568.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kelly RJ, Ajani JA, Kuzdzal J, Zander T,
Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre
A, et al: Adjuvant nivolumab in resected esophageal or
gastroesophageal junction cancer. N Engl J Med. 384:1191–1203.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bellmunt J, Hussain M, Gschwend JE, Albers
P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz
M, Degaonkar V, et al: Adjuvant atezolizumab versus observation in
muscle-invasive urothelial carcinoma (IMvigor010): A multicentre,
open-label, randomised, phase 3 trial. Lancet Oncol. 22:525–537.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Miguel M and Calvo E: Clinical
challenges of immune checkpoint inhibitors. Cancer Cell.
38:326–333. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao BY, Lin GH, Zhao YX and Wang BC: The
efficacy and safety of PD-1/PD-L1 inhibitors in breast cancer: A
systematic review and meta-analysis. Transl Cancer Res.
9:3804–3818. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eggermont AMM, Blank CU, Mandalà M, Long
GV, Atkinson VG, Dalle S, Haydon AM, Meshcheryakov A, Khattak A,
Carlino MS, et al: Adjuvant pembrolizumab versus placebo in
resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant
metastasis-free survival results from a double-blind, randomised,
controlled, phase 3 trial. Lancet Oncol. 22:643–654.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Adjuvant ipilimumab versus placebo after
complete resection of high-risk stage III melanoma (EORTC 18071): A
randomised, double-blind, phase 3 trial. Lancet Oncol. 16:522–530.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seya T, Takeda Y, Takashima K, Yoshida S,
Azuma M and Matsumoto M: Adjuvant immunotherapy for cancer: Both
dendritic cell-priming and check-point inhibitor blockade are
required for immunotherapy. Proc Jpn Acad Ser B Phys Biol Sci.
94:153–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mocellin S, Rossi CR, Lise M and Marincola
FM: Adjuvant immunotherapy for solid tumors: From promise to
clinical application. Cancer Immunol Immunother. 51:583–595.
2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Naidoo J, Page DB, Li BT, Connell LC,
Schindler K, Lacouture ME, Postow MA and Wolchok JD: Toxicities of
the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann
Oncol. 26:2375–2391. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6(8)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Galsky MD, Mortazavi A, Milowsky MI,
George S, Gupta S, Fleming MT, Dang LH, Geynisman DM, Walling R,
Alter RS, et al: Randomized double-blind phase II study of
maintenance pembrolizumab versus placebo after first-line
chemotherapy in patients with metastatic urothelial cancer. J Clin
Oncol. 38:1797–1806. 2020.PubMed/NCBI View Article : Google Scholar
|