|
1
|
Wyld L, Audisio RA and Poston GJ: The
evolution of cancer surgery and future perspectives. Nat Rev Clin
Oncol. 12:115–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hortobagyi GN and Buzdar AU: Current
status of adjuvant systemic therapy for primary breast cancer:
Progress and controversy. CA Cancer J Clin. 45:199–226.
1995.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pondé NF, Zardavas D and Piccart M:
Progress in adjuvant systemic therapy for breast cancer. Nat Rev
Clin Oncol. 16:27–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maehara Y, Baba H and Sugimachi K:
Adjuvant chemotherapy for gastric cancer: A comprehensive review.
Gastric Cancer. 4:175–184. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Minsky BD: Adjuvant radiation therapy for
colon cancer. Cancer Treat Rev. 21:407–414. 1995.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Osborne CK: Tamoxifen in the treatment of
breast cancer. N Engl J Med. 339:1609–1618. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Joensuu H, Eriksson M, Sundby Hall K,
Reichardt A, Hermes B, Schütte J, Cameron S, Hohenberger P, Jost
PJ, Al-Batran SE, et al: Survival outcomes associated with 3 years
vs 1 year of adjuvant imatinib for patients with high-risk
gastrointestinal stromal tumors: An analysis of a randomized
clinical trial after 10-year follow-up. JAMA Oncol. 6:1241–1246.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu YL, Tsuboi M, He J, John T, Grohe C,
Majem M, Goldman JW, Laktionov K, Kim SW, Kato T, et al:
Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N
Engl J Med. 383:1711–1723. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suhail Y, Cain MP, Vanaja K, Kurywchak PA,
Levchenko A, Kalluri R and Kshitiz : Systems biology of
cancer metastasis. Cell Syst. 9:109–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li T, Jia DD and Teng LS: Adjuvant
pembrolizumab versus high-dose interferon α-2b for Chinese patients
with resected stage III melanoma: A retrospective cohort study.
Invest New Drugs. 38:1334–1341. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ascierto PA, Del Vecchio M, Mandalá M,
Gogas H, Arance AM, Dalle S, Cowey CL, Schenker M, Grob JJ,
Chiarion-Sileni V, et al: Adjuvant nivolumab versus ipilimumab in
resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-Year
results from a multicentre, double-blind, randomised, controlled,
phase 3 trial. Lancet Oncol. 21:1465–1477. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eggermont AMM, Blank CU, Mandala M, Long
GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A,
Carlino MS, et al: Adjuvant pembrolizumab versus placebo in
resected stage III melanoma. N Engl J Med. 378:1789–1801.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choueiri TK, Tomczak P, Park SH, Venugopal
B, Ferguson T, Chang YH, Hajek J, Symeonides SN, Lee JL, Sarwar N,
et al: Adjuvant pembrolizumab after nephrectomy in renal-cell
carcinoma. N Engl J Med. 385:683–694. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bajorin DF, Witjes JA, Gschwend JE,
Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat
SF, Park SH, et al: Adjuvant nivolumab versus placebo in
muscle-invasive urothelial carcinoma. N Engl J Med. 384:2102–2114.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zimmer L, Livingstone E, Hassel JC, Fluck
M, Eigentler T, Loquai C, Haferkamp S, Gutzmer R, Meier F, Mohr P,
et al: Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy
versus placebo in patients with resected stage IV melanoma with no
evidence of disease (IMMUNED): A randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet. 395:1558–1568.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kelly RJ, Ajani JA, Kuzdzal J, Zander T,
Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre
A, et al: Adjuvant nivolumab in resected esophageal or
gastroesophageal junction cancer. N Engl J Med. 384:1191–1203.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bellmunt J, Hussain M, Gschwend JE, Albers
P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz
M, Degaonkar V, et al: Adjuvant atezolizumab versus observation in
muscle-invasive urothelial carcinoma (IMvigor010): A multicentre,
open-label, randomised, phase 3 trial. Lancet Oncol. 22:525–537.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
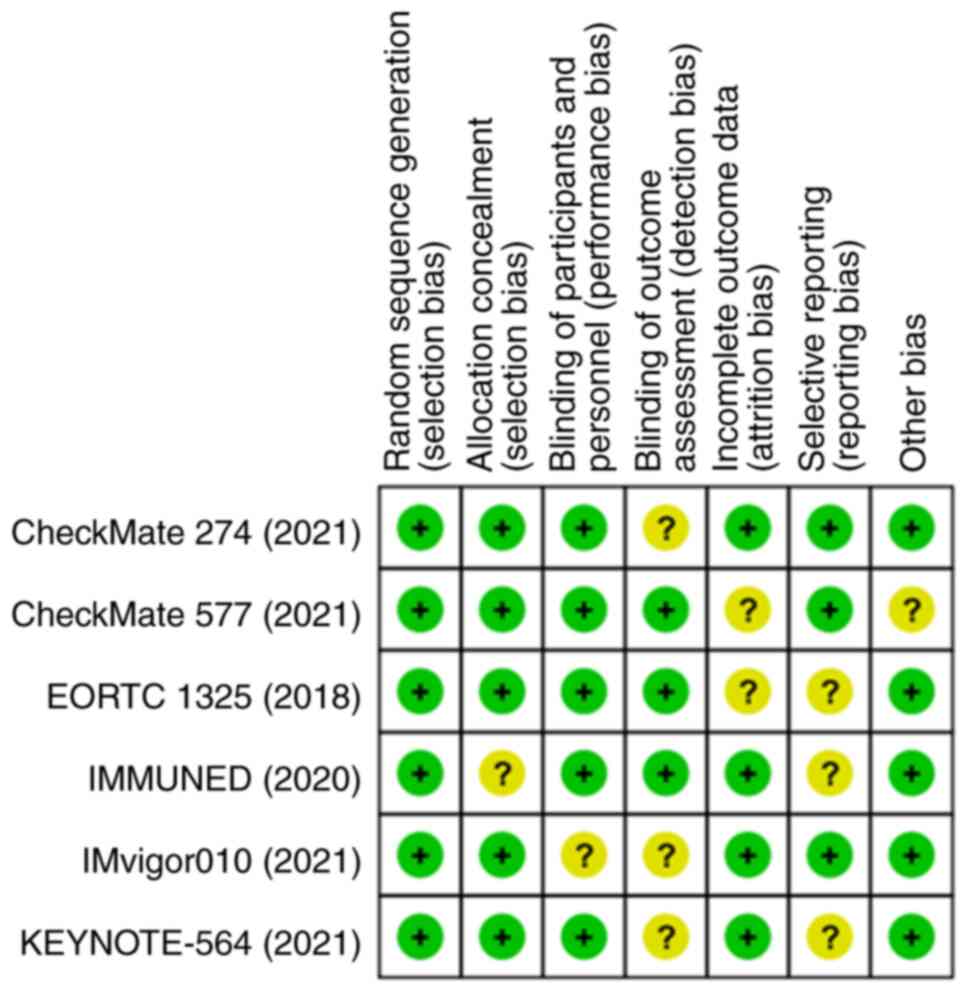
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Miguel M and Calvo E: Clinical
challenges of immune checkpoint inhibitors. Cancer Cell.
38:326–333. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao BY, Lin GH, Zhao YX and Wang BC: The
efficacy and safety of PD-1/PD-L1 inhibitors in breast cancer: A
systematic review and meta-analysis. Transl Cancer Res.
9:3804–3818. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eggermont AMM, Blank CU, Mandalà M, Long
GV, Atkinson VG, Dalle S, Haydon AM, Meshcheryakov A, Khattak A,
Carlino MS, et al: Adjuvant pembrolizumab versus placebo in
resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant
metastasis-free survival results from a double-blind, randomised,
controlled, phase 3 trial. Lancet Oncol. 22:643–654.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Adjuvant ipilimumab versus placebo after
complete resection of high-risk stage III melanoma (EORTC 18071): A
randomised, double-blind, phase 3 trial. Lancet Oncol. 16:522–530.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seya T, Takeda Y, Takashima K, Yoshida S,
Azuma M and Matsumoto M: Adjuvant immunotherapy for cancer: Both
dendritic cell-priming and check-point inhibitor blockade are
required for immunotherapy. Proc Jpn Acad Ser B Phys Biol Sci.
94:153–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mocellin S, Rossi CR, Lise M and Marincola
FM: Adjuvant immunotherapy for solid tumors: From promise to
clinical application. Cancer Immunol Immunother. 51:583–595.
2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Naidoo J, Page DB, Li BT, Connell LC,
Schindler K, Lacouture ME, Postow MA and Wolchok JD: Toxicities of
the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann
Oncol. 26:2375–2391. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6(8)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Galsky MD, Mortazavi A, Milowsky MI,
George S, Gupta S, Fleming MT, Dang LH, Geynisman DM, Walling R,
Alter RS, et al: Randomized double-blind phase II study of
maintenance pembrolizumab versus placebo after first-line
chemotherapy in patients with metastatic urothelial cancer. J Clin
Oncol. 38:1797–1806. 2020.PubMed/NCBI View Article : Google Scholar
|