1. Introduction
Gastrointestinal diseases are the most frequent
cause for medical consultation and one of the leading causes of
death worldwide (1,2). In America, 77 million individuals get
sick annually due to food poisoning and, according to the World
Health Organization (WHO), one in ten individuals get sick each
year for the same reason worldwide. As a result, ~420,000
individuals die on a yearly basis, of which ~30% are children under
five years of age. It must be noted that diarrheal diseases
correspond to more than half of the cases of gastrointestinal
illnesses, for which ~95% of cases can be associated with
Campylobacter spp., Escherichia coli and non-typhoidal
Salmonella spp. (3).
In 2017, the WHO published a list of drug resistant
bacteria for which there is a growing need to develop new
antibiotics as even the current most effective of them, such as
carbapenems and cephalosporins, are now ineffective. This list is
divided into three categories (critical, high, and medium priority)
based on how urgently these antibiotics are needed. The critical
priority group includes multidrug resistant (MDR) bacteria that are
especially dangerous for vulnerable individuals, or individuals
under specialized care, due to the high risk of infection,
complications, disease severity and mortality (4). Some of the bacteria included in this
group are: Acinetobacter spp., Pseudomonas spp., Klebsiella
spp., Escherichia coli, Serratia spp. and Proteus spp.
(4), all of which have different
infection pathways in the host (5).
Different molecular mechanisms of bacterial
resistance to antibiotics have been described so far. Due to the
importance of this phenomenon in public health, the present review
gathers engaging and relevant information concerning the most
common enteropathogenic bacteria in clinical practice and describes
the molecular mechanisms for the acquisition or de novo
development of antibiotic resistance, thus seeking to enlighten the
reader in this regard and provide a greater understanding of this
process.
Thorough research was conducted in the writing of
the present manuscript, primarily employing informatic tools such
as PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/home.uri), Scielo
(https://scielo.org/), Medigraphic (https://www.medigraphic.com/newMedi/)
and Science Direct (https://www.sciencedirect.com/). The terms used in
this search included: Enterobacteria, multidrug resistance,
enteropathogenic bacteria, multidrug-resistant bacteria, bacterial
drug resistance, horizontal gene transfer and gastrointestinal
diseases. Inclusion criteria included English language and
full-length articles. Exclusion criteria: Publications from 2012 to
2022 were prioritized. Older publications were also reviewed and
introduced in the present study if deemed relevant. A total of 99
research and review articles were used in the present study
(Fig. 1).
2. Multidrug resistant enteropathogenic
bacteria
The family Enterobacteriaceae includes several genus
and species of both gram-negative and -positive bacilli (for
example Enterococcus spp.), a number of which are present in
water, soil, plants and the intestinal microbiota of humans and
animals; however, their diversity is often dictated by geographical
area (6) and often develop as
opportunistic pathogens causing severe infections in humans
(Table I) (7).
 | Table ICommon enteropathogenic bacteria in
clinical practice and their symptoms. |
Table I
Common enteropathogenic bacteria in
clinical practice and their symptoms.
| Bacteria | Antibiotic
resistance | Pathology | Mechanism of
action | (Refs.) |
|---|
| Klebsiella
pneumoniae | Carbapenems,
β-lactams, aminoglycosides, quinolones, tigecycline,
polymyxin. | Acute Diarrheic
Syndrome, urinary tract infections, cystitis, pneumonia,
endocarditis, septicemia. | Adherence and
biofilm formation by type 1 and type 3 pili. | (8,85,86) |
| Escherichia
coli | Cephalosporins,
fluoroquinolones. | Acute watery
diarrhea, bloody diarrhea. | A/E and changes of
the host apical enterocyte membrane. Activation of T3SS and
formation of A/E. | (2,5,8,87) |
| Shigella
spp. | Cephalosporins,
ampicillin, co-trimoxazole, nalidixic acid. | Bloody
diarrhea. | T3SS encoded on a
large plasmid and transport of effector proteins. | (5,10,88,89) |
| Salmonella
spp. | Quinolones,
nalidixic acid. | Acute watery
diarrhea, bloody diarrhea, enteric fever. | Activation of T3SS
and transport of effector proteins. | (5,10,90) |
| Campylobacter
spp. | Quinolones,
tetracycline. | Enteric fever,
acute watery diarrhea, bloody diarrhea. | Presence of
flagella, high molecular weight plasmids, surface adhesins and
chemotactic factors. | (10,90,91) |
| Vibrio
cholerae | Ampicillin,
nalidixic acid, co-trimoxazole. | Acute liquid
diarrhea. | Biofilms formation
and production of extended-spectrum β-lactamases. | (90,92,93) |
| Aeromonas
spp. | Beta-lactams,
tetracyclines, glycylcyclines, fluoroquinolones, aminoglycosides,
sulfamethoxazole-trimethoprim. | Acute watery
diarrhea, bloody diarrhea. | Travel by the blood
to the first organ it finds where it produces the toxic enterotoxin
aerolysin. | (2,94,95) |
| Yersinia
enterocolitica | Nalidixic
acid. | Enteric fever,
bloody diarrhea. | Activation of T3SS
and transport of effector proteins and/or apoptosis. Yersinia forms
microcolonies and starts replication. | (5,10,90,96) |
| Staphylococcus
aureus | Penicillin,
methicillin. | Acute liquid
diarrhea. | Inoculation into an
open wound. adhesion and invasion of host epithelial cells by
microbial surface components recognizing adhesive matrix
molecules. | (2,90,97) |
| Enterococcus
spp. | Vancomycin,
Beta-lactams, glycopeptides, aminoglycosides, tetracyclines,
quinolones, macrolides. | Sepsis,
endocarditis, urinary tract infections. | When pathologic
alterations are caused by either direct toxin activity or
indirectly by bystander damage from the inflammatory response,
enterococci are able to outpace host defenses, multiply at rates
that are faster than clearance, and overwhelm the host. | (18,98,99) |
These bacteria are associated with 10-20% cases of
infectious diarrhea in children worldwide (8,9). The
majority of patients affected by these bacteria only require an
hydroelectrolytic imbalance intervention, caused by dehydration or
antibiotics treatment, the latter of which diminishes the duration
of the disease, reduces its transmission and prevents complications
(10). In some cases, it is
possible that severe infections can be caused by
multidrug-resistant enterobacteria or by enterotoxin producing
bacteria, and for this reason special epidemiological surveillance
is necessary (10,11). A report made in 2017 revealed that
antibiotic resistance in Latin America was as high as 45%, followed
by Europe with 39%, the US with 8%, and Canada with 5% (12).
In 2019 Levin-Reisman et al (13) described the different phenotypic
traits enabling bacteria to acquire resistance to antibacterial
agents, such as tolerance, persistence and resistance. Tolerance is
the ability of a bacterial population to survive and grow under
toxic conditions, such as high concentrations of antibiotics, thus
prolonging treatment duration; notably, this acquired resistance
may or may not be inherited to daughter cells (13-15).
Persistence is the ability of bacteria to survive a specific drug
concentration, prolonging the duration of treatment unless
corrected (13). These persistent
bacteria can withstand antibiotic treatment without affecting the
drugs' minimum inhibitory concentration (MIC), presenting a
biphasic death curve because the majority of the bacterial
population dies, with only a small subpopulation persisting for a
longer time (13-15).
Resistance is the ability to grow in the presence of environmental
stress or high concentrations of antibiotics, regardless of the
treatment's duration, due to the increased MIC required to
effectively destroy the microorganism (13-16).
The acquisition of antibiotic or antimicrobial
resistance is a natural selection process of bacteria and thus
considered as part of their evolutionary path. In this regard, the
indiscriminate use of antibiotics exerts a high selective pressure
on them, which results in genomic changes that translate into
multidrug resistance, as seen with greater frequency in developing
countries (15,17).
Depending on the number or type of antimicrobials,
resistant bacteria can be classified as MDR, which occurs when
clinically relevant microorganisms have developed resistance to
three or more classes of commonly used antibiotics and/or
antimicrobials (18,19); extensively drug-resistant (XDR),
microorganisms resistant to at least one agent of all antimicrobial
classes; and pandrug-resistant, which includes microorganisms
resistant to all agents in all antimicrobial classes (20). Most of these multidrug-resistant
bacteria are typically gram-negative enterobacteria representing an
important therapeutic challenge in the treatment of
life-threatening infections (12,15).
3. Molecular mechanisms of multidrug
resistance
Antibiotic resistance can be permanently maintained
once it has been fixed in the genome or it can be just temporary if
the selective pressure is absent causing non-resistant bacteria to
proliferate instead. Drug resistance often appears due to the
acquisition of exogenous DNA or through genomic DNA mutations
(21).
From an evolutionary perspective, bacteria have
several advantages over other organisms because they have short
replication time, large populations and capacity for horizontal
gene transfer, which enables bacteria to adopt, use, propagate and
fix advantageous genetic information between strains and species,
such as antibiotic resistance (22).
The limitation of both resources and nutrients
within the environment is a decisive factor exerting great
selective pressure on bacteria, forcing the stressed populations to
adapt or die. As a result, the genetic variations providing a
survival advantage become fixed in the bacterial population, thus
taking another step in their evolution as a species (23,24).
The genetic evolution of bacteria mostly occurs due
to recombination events allowing gene acquisition, segment
duplication, fusion of homologous regions, functional domain
exchange and gene deletion (24,25).
Acquisition of exogenous genomic material occurs via horizontal
gene transfer (HGT), which enables bacteria to absorb and
incorporate genetic material of diverse origin, thus giving rise to
different genotypes between populations of the same species.
Further, HGT events can also confer pathogenicity factors related
to virulence, symbiosis, resistance and metabolism, among others
(24,25).
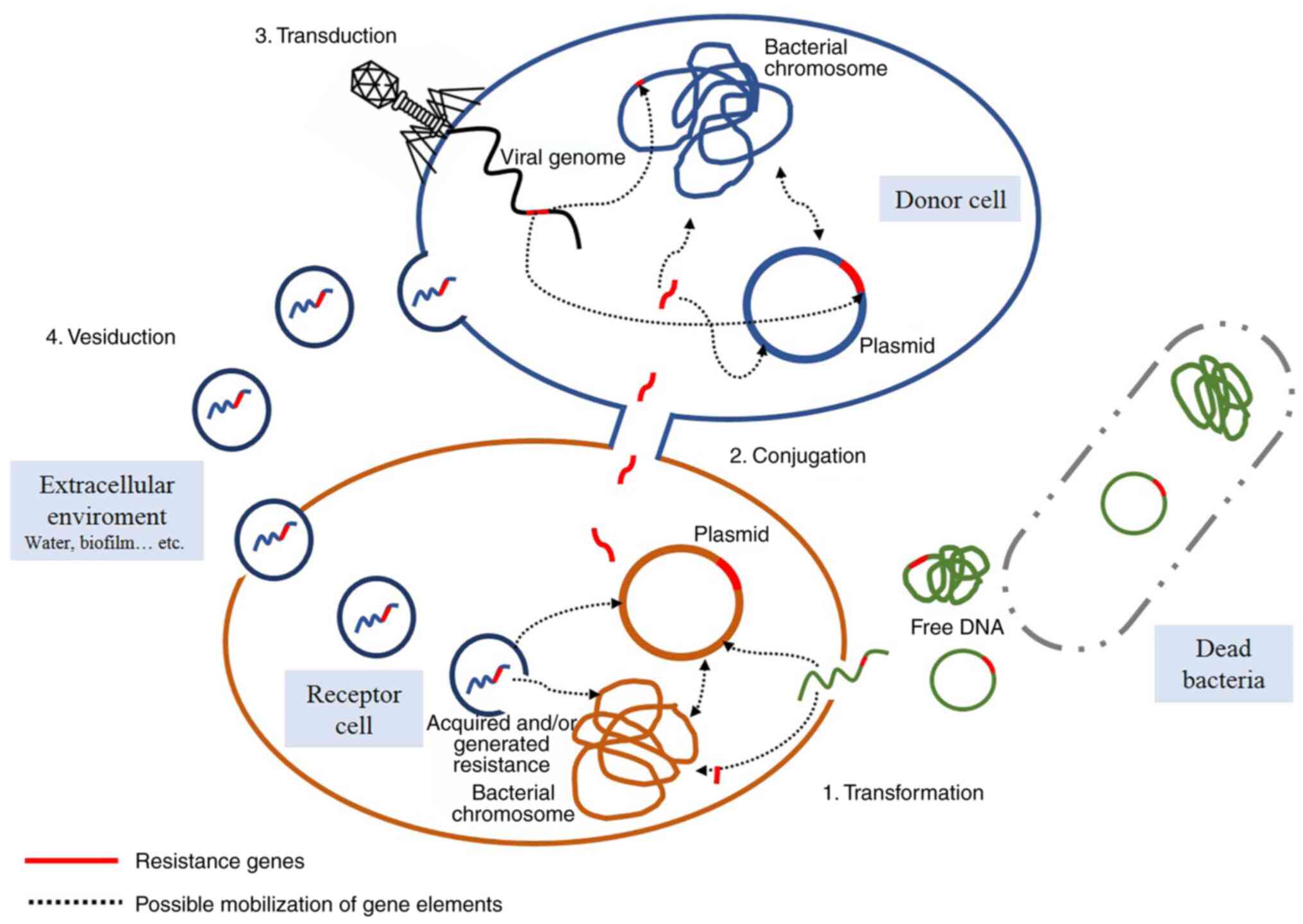
Three major mechanisms of HGT have been described
until 2019: i) Natural transformation (26); ii) conjugation (27); and iii) transduction (28). However, Soler and Forterre
(25) proposed a fourth mechanism
called vesiduction in 2020 (Fig.
2).
Natural transformation
This phenomenon represents the active transfer of
genes from extracellular free DNA from lysed bacteria to a living,
competent bacterium that captures and incorporates it into its
genome through DNA recombination. This process contributes to
genetic variability, shapes evolution and, in the case of
pathogenic bacteria, it is responsible for their adaptation to host
cells, fosters the spread of antibiotics resistance, promotes
antigenic variation and leads to the acquisition of new virulence
factors (29-31).
In addition, natural transformation also promotes DNA exchange
between taxonomically distant bacteria (29-31).
In 2018, Ellison et al (31) demonstrated the ability of bacteria
to capture and introduce free DNA molecules through surface
appendages known as competing pili. Using Vibrio cholerae as
a model, type IV competing pili were demonstrated to be able to
capture and bind double-stranded DNA from the extracellular space.
Once bound to DNA, the pili retracts and mobilizes the captured DNA
molecule towards the cell surface, where it is finally
internalized.
Conjugation
This process explains the exchange of genetic
material from donor bacteria with an adjacent recipient through a
sexual pilus or physical contact, requiring the formation of a pore
between both bacteria while connected as a mating pair. The
exchanged genetic elements can usually provide resistance to drugs,
antiseptics and/or disinfectants (30,32).
The occurrence of a conjugation event, and thus of
an effective DNA transfer, requires cell-cell contact between a
donor and a recipient cell. There are two types of genetic elements
than can be exchanged during this conjugation: i) Conjugative
plasmids, found in free form within the intracellular space and
with autonomous replication capacity; and ii)
integrated-conjugative elements, or conjugative transposons, that
can integrate into the genome of the recipient cell. Since these
plasmids were part of the donor's genome prior to the exchange, the
latter are rarely, if ever, found free in the cytoplasm and do not
replicate autonomously (33,34).
In the majority of bacteria, conjugation occurs by transferring
single-stranded DNA molecules through the type IV secretion system
contained in the conjugative element, which can also transfer DNA
from bacteria to eukaryotes (33,34).
Transduction
Transduction is mediated by bacterial viruses called
bacteriophages. When a phage infection culminates in bacterial
lysis, some viral particles can encapsulate bacterial DNA
fragments, thus producing transducer particles. Upon subsequent
infection, the transducer particles inject bacterial DNA into the
next bacterium host, which may acquire new genetic traits after
adopting the exogenous DNA (35).
Generalized transduction occurs when any of the bacterial genes
maintains the same probability of being encapsulated in a
transducing particle and transferred into a recipient. On the other
hand, specialized transduction defines the transference of specific
genes, such as those located next to the bacteriophage's DNA
(30,36,37).
Vesiduction
Vesiduction was proposed in 2020 by Soler and
Forterre (25). It involves the
donation and/or acquisition of exogenous material from
extracellular vesicles, a phenomenon that has been observed in all
three domains of life. Vesicles are secreted through the cell
membrane of Gram-positive bacteria and the outer cell membrane of
Gram-negative bacteria. The precise mechanism is not yet fully
understood, and it may be possible that it differs according to the
composition of the cell wall and the proteins used in the
construction of the vesicles (38,39).
These vesicles can fulfill different physiological roles that are
not mutually exclusive with genetic material transference, such as
the transport of peptidoglycan hydrolases or toxins, and other
effector proteins that may be involved in the elimination of
concurrent microorganisms through competition or pathogenicity.
Some vesicles can also transport intercellular communication
molecules (39).
Regardless of their inherent differences, all of the
previously mentioned mechanisms for genetic acquisition can be
driven by RecA-dependent recombination, illegitimate recombination,
transposition or integration (25).
4. Molecular mechanisms of multidrug
resistance generation
There are different mechanisms of natural resistance
that can appear through other pathways; however, these are usually
induced by the presence or prolonged exposure of hazardous
molecules (such as antibiotics) resulting in the proliferation of
those populations with advantageous biological changes (40,41).
The majority of these mechanisms are specifically developed by
bacteria to generate resistance to antibiotics or antimicrobials
and may involve the modification of existing genomic material
through spontaneous mutations that might be punctual or massive.
These resistant populations thrive thanks to the action of
bactericidal molecules eliminating the cells lacking tolerance or
resistance; in other words, the microorganisms are forced to evolve
in order to survive (21). This
selective pressure has become the standard in areas such as
hospitals, biohazard waste disposal areas, pharmaceutical industry
effluents, wastewater, manure treated soils, animal breeding and
aquaculture areas (21).
Inherent (natural) resistance
Natural resistance to drugs, antibiotics or
antimicrobials is a trait often shared between different species of
microorganisms, which may be due to the same physiology or
spontaneous genetic mutations regardless of previous exposure to
these molecules (42,43). An example of intrinsic antibiotics
resistance conferred by physiology can be seen in bacteria of the
Mycoplasma genus, whose members are highly resistant to
drugs targeting the cell wall, such as β-lactams and glycopeptides
(44,45); although some antibiotics normally
have difficulty crossing the outer membrane of Gram-negative
bacteria. For example, vancomycin inhibits cell wall synthesis by
targeting d-Ala-d-Ala peptide precursor units of Gram-positive
bacteria, thus preventing the assembly of peptidoglycan layers and
transpeptidation (46). By
contrast, this antibiotic cannot affect Gram-negative bacteria
since it is unable to cross the outer membrane, and thus kept from
accessing the cell wall (46).
Even though these events occur naturally in the environment,
(47) it must be mentioned that
the intrinsic resistance to antibiotics is not considered as a
clinical problem because previously developed antibiotics do not
target these bacteria.
Spontaneous mutations
Spontaneous mutations occur by random nucleotide
changes that induce different effects; for example, amino acid
sequence variations that may lead to altered phenotypes. These
mutations can be caused by DNA replication errors or through the
action of mutagenic agents. It must be noted that acquired
mutations are often detrimental, so these are usually not
inherited, are rarely widespread and often are just isolated events
(21,48). However, when a mutation provides a
biological advantage, this change can become fixed in the
population through vertical gene transfer and become a dominant
trait (21,48). The frequency of spontaneous
mutations related to antibiotic resistance occur at a rate of
1x10-5 to 2x10-8 in members of the
Chlamydiaceae and Helicobacter pylori (49,50).
Though this would appear to be a rare event, in reality antibiotic
resistance appears in bacterial populations within a relatively
short period of time, accelerating further when exposed to a
selective agent due to exponential growth rate and the number of
cells generated per replication cycle (51). For example, the gastric pathogen
Helicobacter pylori can have different mutations in the 23S
rRNA, gyrA and rpoB genes, which are responsible for
resistance to clarithromycin, ciprofloxacin and rifampicin,
respectively (50). The capacity
of Chlamydia trachomatis to resist antibiotics such as
azithromycin, tetracycline and fluoroquinolone has also been
attributed to spontaneous mutations (52). Although this mutation rate is not
even across the board, there are bacterial subpopulations with a
significant tendency to acquire and accumulate spontaneous
mutations, which is why they often present a greater number of
mutation events compared with what is commonly observed (21). These subpopulations are known as
hypermutable and, although not all spontaneous mutations confer
antibiotics resistance, this hypermutability is directly
proportional with the increased resistance capacity (21).
Duplications
Gene duplications are often overlooked as the
primary source of functional genomic diversity, originating new
functions from a pre-existing gene. In addition, the generation of
genetic copies derives into elements that can evolve independently
due to inexistent selective pressure, further diversifying their
functions (53). For example, the
Plasmodium falciparum multidrug resistance protein
transporter 1 gene plays an important role in the parasite's
resistance to drugs due to the strong correlation between the
number of copies and the resistance to artemisinin-based therapies,
an anti-malaria drug used to reduce its mortality rate since the
year 2000. By 2020, Calçada et al (54) reported the threat of resistance
against this drug due to the appearance of new duplication events
and the presence of single nucleotide polymorphisms in current
strains.
5. Drugs and bacterial response
As aforementioned, the genetic elements leading to
drug resistance can be spread between different microorganisms in
different manner, from the horizontal (transformation,
transduction, conjugation or vesiduction) to vertical gene transfer
(from mother to daughter cells) of either intrinsic or
extrinsically acquired genomic modifications, such as spontaneous
mutations, duplications, insertions, deletions or transpositions.
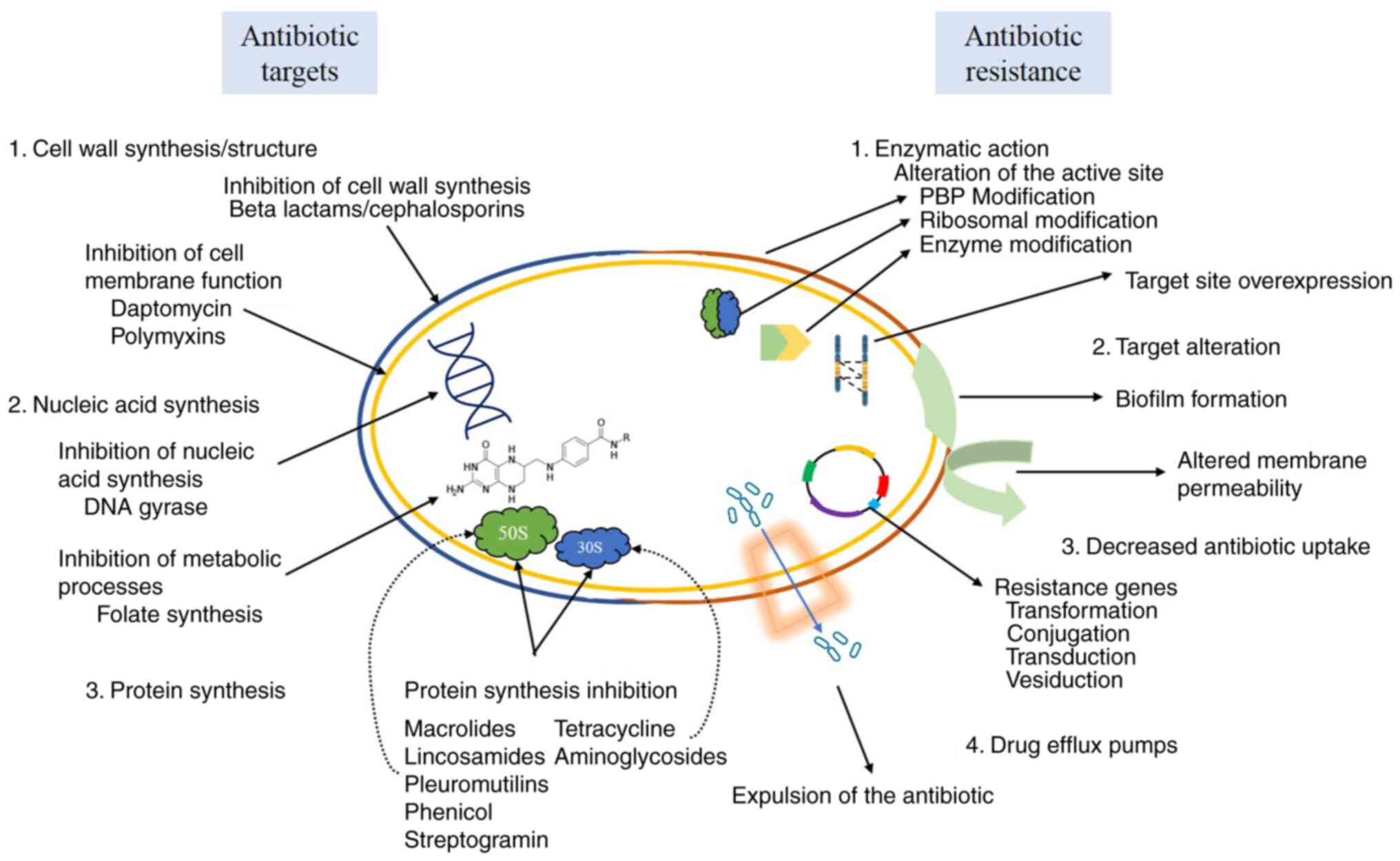
The mechanism of antibiotics resistance is highly dependent on the
way the drug itself works against the bacterium, regardless of how
this resistance was acquired, thus deriving in different survival
pathways that may limit the absorption of drugs, modify the target
molecules, directly inactivate the drug and/or secrete it into the
microenvironment (Fig. 3)
(55).
6. Mechanisms of antibiotics
Antibiotics with the capacity to inhibit or kill a
wide range of bacteria are known as broad-spectrum antibiotics,
whereas that those that only affect certain types of bacteria are
known as narrow-spectrum antibiotics. Antibiotics typically target
the structure or metabolic processes of bacteria, preventing their
replication (56-58).
In this regard, the most common mechanisms consist in the
inhibition of cell wall synthesis, DNA replication or
transcription, protein synthesis, metabolic pathways or directly
through cell membrane degradation (41,57).
Cell wall synthesis inhibition
The majority of bacterial cells are surrounded by a
rigid peptidoglycan layer consisting of long sugar polymers linked
through peptide bonds. This structure is needed for survival as it
protects the bacteria from osmotic pressure and other hostile
conditions from the environment (56,57,59).
Drugs such as penicillin and cephalosporins inhibit the formation
of peptide bonds in the bacterial cell wall, thus effectively
killing the microorganism (56).
By contrast, glycopeptides inhibit bacterial growth by inhibiting
peptidoglycan synthesis (56,57).
Cell membrane function inhibition
In comparison with gram-positive bacteria,
gram-negatives have a greater resistance to antimicrobials due to
the existence of an external cell membrane regulating both
intracellular and extracellular substance flow (56,60).
The drugs targeting this external cell membrane are specific for
each microbial group because their function depends on the lipid
content of such membrane; however, these drugs can sometimes be
toxic, thus limiting their use (56,57).
For example, Daptomycin can rupture the cell membrane due to
depolarization, whereas that polymyxins bind to the lipid fraction
of the membrane's lipopolysaccharide layer, thus causing its
disintegration (57).
Nucleic acid synthesis inhibition
Nucleic acid synthesis is important for the survival
of living beings, including bacteria. The cellular processes
responsible for cell replication and bacterial conservation can be
negatively affected due to the interruption of this process by
drugs that block DNA replication or transcription (56,57).
In this regard, antibiotics such as quinolones interfere with the
functionality of the helicase enzyme preventing the function of
unwinding DNA, effecting the process of DNA replication and repair.
On the other hand, they can exert their action by inhibiting
topoisomerase II and IV of bacteria, preventing the synthesis of
RNA (61).
Metabolic inhibitors
Some drugs act against important metabolic processes
for survival, such as the folic acid pathway, which is necessary to
produce important precursors in DNA synthesis (57). In this case, sulphonamides and
trimethoprim release similar substrates to those produced and used
by the bacteria in its normal metabolism (56,57).
Each of these drugs is responsible for inhibiting different stages
of folic acid metabolism. For example, the sulphonamides
competitively inhibit dihydropteroate synthase, binding to it with
greater affinity compared with the substrate produced by the
bacteria; while trimethoprim is responsible for inhibiting
dihydrofolate reductase at a later stage of folic acid synthesis
(56,57,59).
Protein synthesis inhibition
Proteins play a role in various cellular structures
and physiological processes; therefore, their synthesis is
fundamental for survival (56,57).
For this reason, drugs that inhibit protein biosynthesis by
targeting the 70S prokaryotic ribosome (30S and 50S ribosomal
subunits) constitute the largest class of antibiotics (56,57,59).
30S subunit inhibitors
Antibiotics such as tetracycline, aminoglycosides
and streptomycin target and inhibit the 30S ribosome, blocking the
passage of aminoacyl-tRNA towards the ribosome (57,59).
50S subunit inhibitors
Antibiotics targeting the 50S ribosomal subunit can
act in two different ways, by blocking protein translation
(oxazolidinones) or by blocking the elongation phase of protein
synthesis (for example, macrolides). However, the latter may be
ineffective when the elongation phase has advanced significantly
(57,59). Natural antibiotics such as
aminoglycosides are considered as bactericidal, whereas macrolides,
tetracyclines, chloramphenicol, streptogramins and spectinomycin
are considered as bacteriostatic (57).
7. Mechanisms of drug resistance
Antibiotics outlet, secretion or
efflux pumps
Some bacteria have exporter proteins on their cell
membrane that can rapidly transport the antibiotics from the
cytoplasmic membrane in gram-positive bacteria and from the
intermembrane space in gram-negative bacteria to the exterior of
the cell without the help of energy-dependent efflux pumps, thus
preventing the antibiotics from reaching their target (30,62,63).
There are two groups of efflux pumps, some of them are specific
whereas others can secrete diverse substances. These pumps are
classified according to energy source and function; in this regard,
the first group uses ATP as an energy source and functions through
hydrolysis (63,64). By contrast, the second group uses
the mobile force of protons as an energy source, enhancing
secretion through the electrochemical potential of the membrane
(63,64). A total of five families of efflux
pumps have been described within the second group: i) The multidrug
and toxic extrusion family; ii) the major facilitator super family;
iii) the resistance nodulations cell division (RND) family; iv) the
small MDR family; and v) the multidrug endosomal transporter family
(62-64).
These efflux pumps are widely distributed among gram-positive and
-negative bacteria, except for the RND poly-selective superfamily,
which is found gram-negative bacteria with very high frequency
(62,63). These efflux pumps play a notable
role in multidrug resistance due to their capacity to secrete a
wide range of structurally unrelated drugs and molecules (62-64).
Permeability alterations in the outer
cell membrane
The majority of antibiotics penetrate the bacterial
membrane and target diverse intracellular processes; therefore, the
concentration of antibiotics within the cell can be affected by
alterations in the lipid bilayer of the membrane, modifying either
the cell's diameter or number of porins (30,65).
The bacterial cell envelope provides a selective barrier allowing
the exchange of nutrients and signaling molecules with the
microenvironment. This envelope is formed by the cell wall and the
plasma membrane, and, in Gram-negatives, it provides an additional
function as a physical barrier that reduces the permeability of a
number of drugs (53,65). Notably, this envelope can also be
targeted by antibiotics (53,65).
The outer membrane of gram-negative bacteria is populated by
proteins called porins, which determine its permeability and allow
the entry of hydrophilic compounds into the cell. The absence or
low number of porins can also prevent the entry of antimicrobial
molecules, thus hindering their action in the cytoplasm and/or cell
envelope (62,63).
Active site alterations
Bacteria have the ability to form metabolic
substances that compete with antimicrobial drugs for the active
site, preventing it from binding due to loss of affinity (30,63).
There are two types of modifications in this regard as follows.
Penicillin-Binding-Protein (PBP)
modification. Observed in Gram-positive bacteria, this effect
is caused by variations in the peptidoglycan gene, which modify the
antimicrobial binding site in the cell wall (30,62).
Ribosomal modification. The ermA and
ermB genes can modify the ribosome's active site through
methylation. These modifications occur in the 30S and 50S subunits
of the 70S ribosome, affecting the target site of drugs such as
aminoglycosides, macrolides, tetracyclines and lincosamides
(30,66).
Enzymatic modification or inactivation
of antibiotics
This is the most common mechanism of resistance
observed in bacteria. It is achieved through the expression of
enzymes with the capacity to modify the active component of the
antibiotics, thus reducing their effectiveness. Three mechanisms
have been reported so far: i) Redox reactions; ii) group transfer;
and iii) enzymatic hydrolysis. The latter is the primary mechanism
of resistance, with the clearest example being the hydrolysis of
the beta-lactam ring of antibiotics. The enzymes of gram-negative
bacteria typically originate from a plasmid or have a transposon
origin with constitutive and periplasmic expression. By contrast,
this resistance is solely provided by a plasmid in gram-positive
bacteria, which can be inducible and/or extracellular (40,65).
Biofilm formation
Biofilms are structured aggregations of bacterial
cells enclosed in a self-synthesized extracellular matrix composed
of different macromolecules such as proteins, nucleic acids and
polysaccharides (63,67). Biofilms bacterial production
protects them from ultraviolet light, dehydration, immune system or
certain antibiotics. There are three important steps in biofilm
formation: i) Adhesion, in this phase bacteria can attach to any
give surface; ii) growth and maturation, occurs when bacteria
secrete an exopolysaccharides matrix and mature from microcolonies
to multi-layered cell clusters; and iii) shedding, which can be
either active (initiated by the bacteria) or passive (caused by
external factors) (30,62). Amongst the most common pathogens
that develop biofilms are S. aureus, P. aeruginosa, A. baumannii
and K. pneumonia (30,62).
Target site overexpression
This mechanism has been described in clinical
isolates of mycobacteria with promoter duplications. This often
results in the overexpression of genes that may include mutations
affecting the target site of antibiotics or antimicrobials
(30). In this regard, Martinez
et al (68) describe the
presence of plasmids in E. coli that provide resistance to
amoxicillin-clavulanate as a result of the hyperproduction of
plasmid-determined TEM-1 P-lactamase. TEM-1 β-lactamase is a known
determinant of resistance to antibiotics, such as penicillin,
cephalosporins and their derivatives, including second, third and
fourth generation cephalosporins, monobactams and β-lactamase
inhibitors. This enzyme inactivates the aforementioned compounds by
hydrolyzing their lactam rings (69,70).
8. Treatment against multidrug-resistant
bacteria
Some of the first-line drugs used in the treatment
of serious infections caused by Enterobacteriaceae include
penicillin, cephalosporins, carbapenems, fluoroquinolones,
monobactams and, occasionally, aminoglycosides. However, bacterial
resistance against these drugs is rapidly becoming widespread, thus
making difficult these treatment (20,71).
In some cases, second-line drugs are more effective against
enterobacteria, as would be the case with polymyxins, tigecycline,
aminoglycosides and fosfomycin (72). Pathogenic bacteria have evolved
different strategies to overcome the host's response by avoiding
highly competitive environments. For example, the mucosal barrier
can be breached by mucinases, such as the Pic enzyme from
Shigella and enteroaggregative from Escherichia coli
(EAEC). Notably, the Pic gene can be found in a
‘pathogenicity island’ flanked by insert-like EAEC elements that
have been acquired through horizontal gene transfer (24).
Empirical treatment with
antibiotics
As a first line decision, empirical therapy becomes
essential in the treatment of serious infections caused by
bacteria. However, the emergence of bacterial resistance
complicates its implementation, thus causing a serious dilemma
between the selection of a broad-spectrum drug, which could induce
greater drug resistance, or a narrow-spectrum drug, which could be
completely ineffective (71,73).
Regardless of its potential shortcomings, the latter could supply
important information on the pathogen's susceptibility to certain
antimicrobials (71,73). Several factors must be evaluated
during the selection of antibiotics treatment, including
susceptibility, risk of developing resistance, potential side
effects, comorbidities, local epidemiology and clinical severity
(10,71,74).
Combination antibiotic therapy, The combined
therapy of antibiotics enables the synergistic effect of one or
more drugs, potentially increasing the probability of an effective
treatment and lowering the risk of bacterial resistance. However,
the results of drug synergy tests observed in vitro do not
always translate well into a clinical setting (71,75).
Alternative treatments
Alternative treatments can also be implemented in
addition to antibiotics therapy if their contribution proves safe
for the patient and does not enable the development of bacterial
resistance, for example, phage therapy or competing microorganisms
(76). There are some reports
demonstrating the benefits of these treatments against
multidrug-resistant pathogens, even suggesting they could be used
as replacements for common drugs (76).
Phage therapy
Bacteriophages are bacteria-specific viruses that
can infect bacteria through the binding of specific receptors on
the cell's surface and injecting their genetic material. Once
infected, the phage can go through a lysogenic cycle, where the
phage's genome is integrated in the bacterial chromosome as an
endogenous prophage, spreading horizontally during cell division.
The virus can remain latent for prolonged periods of time during
this cycle; however, environmental or cellular stress factors can
re-activate the phage and induce its lytic cycle, in which the
viral genome is no longer integrated in the bacterial chromosome
and goes into a massive replication event, finally causing cell
death after the phage's lytic proteins hydrolyze the cell wall.
These liberated phage particles can then infect other bacteria and
start the lytic cycle again (76-78).
It must be mentioned that the infective capacity of bacteriophages
is constrained to particular bacterial species, thus resulting in a
reduced spectrum. Although this could be considered a shortcoming,
it could also be considered as a positive aspect since they are
unable to affect the intestinal microbiota or the host (76,78).
Probiotics, prebiotics and
synbiotics
Probiotics are live microorganisms that play a
beneficial role if administered in adequate amounts, regardless of
those present in the essential diet or naturally in the intestinal
microbiota of the host (79-81).
On the other hand, prebiotics are non-digestible compounds
(non-starch polysaccharides and non-digestible oligosaccharides)
present in the daily diet and which help stimulate the growth or
activity of the intestinal microbiota, favoring the development of
beneficial microorganisms (79,80,82).
Finally, synbiotics are a composition of the previous two and which
are often found in the form of pharmaceutical or food preparations
containing one or more probiotic organisms and prebiotic compounds
in order to provide a synergistic effect on the prebiotics,
enhancing the development, activity and nutritional properties of
the probiotics. The inclusion of synbiotics increases the density
of probiotics and their health benefits (80,82).
The probiotics used in clinical treatments are mainly composed of
Gram-positive strains such as Lactobacillus that are
resistant to the human digestive process. The administration of
these microorganisms improves the epithelial barrier function,
promotes the growth of beneficial bacteria, the proliferation of
epithelial cells in the host (by upregulation of cell growth and
downregulation of apoptosis), prevents the adhesion and
colonization of pathogenic microorganisms and toxins, improves
lactose digestion, produces antimicrobial peptides, regulates the
immune response and improves the ability to regulate pH (76,83).
Regarding the functional foods that seem to exert the best
prebiotic effect, fructooligosaccharides, galacto-oligosaccharides
and xyloseoligosaccharide, inulin and lactulose, can be mentioned.
Some extracted from sources such as chicory and yacon roots, are
reported (84). These can be used
in symbiotic formulations with Lactobacilli, Bifidobacteria spp,
S. boulardii and B. coagulans, among other probiotic
agents (84).
9. Conclusions
Several clinically relevant bacterial strains are
now resistant to multiple drugs. To counteract this phenomenon,
novel compounds with the capacity to kill and/or prevent their
proliferation are constantly being developed. However, the
epidemiology and resistance of these strains varies widely
according to geographical region. Therefore, alternative treatments
are also being sought to enhance the effectiveness of antibiotics
to reduce bacterial proliferation and prevent further spread of
antibiotics resistance.
Acknowledgements
Thanks to Dr Daniel Díaz (Department of
Pharmacology, Faculty of Medicine in Hradec Králové; Charles
University, Prague, Czech Republic) for his kind assistance in
proofreading and editing this manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JAGV, KJGL and MASS contributed to the study
design, and performed the literature review and data collection.
JAGV, KJGL, ETAC and MASS contributed to the selection the relevant
literature and critical interpretation. JAGV, KJGL, ETAC, MASS,
JAMC, MPLE and NB drafted and improved the manuscript. JAGV, KJGL,
ETAC, MASS, JAMC, MPLE and NB critically read and modified the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sell J and Dolan B: Common
gastrointestinal infections. Prim Care. 45:519–532. 2018.
|
|
2
|
Hernández CC, Aguilera AMG and Castro EG:
Gastrointestinal diseases, situation in Mexico. Enf Infec
Microbiol. 31:137–151. 2011.
|
|
3
|
World Health Organization: WHO's first
ever global estimates of foodborne diseases find children under 5
account for almost one third of deaths. Geneva, Switzerland, 2015.
Available from: https://www.who.int.
|
|
4
|
World Health Organization: WHO publishes
list of bacteria for which new antibiotics are urgently needed.
Geneva, Switzerland, 2017. Available from: https://www.who.int.
|
|
5
|
Reis RS and Horn F: Enteropathogenic
Escherichia coli, Samonella, Shigella and Yersinia: Cellular
aspects of host-bacteria interactions in enteric diseases. Gut
Pathog. 2(8)2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zaheer R, Cook SR, Barbieri R, Goji N,
Cameron A, Petkau A, Polo RO, Tymensen L, Stamm C, Song J, et al:
Surveillance of Enterococcus spp reveals distinct species
and antimicrobial resistance diversity across a one-health
continuum. Sci Rep. 10(3937)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fariñas MC and Martínez-Martínez L:
Multiresistant gram-negative bacterial infections: Enterobacteria,
pseudomonas aeruginosa, Acinetobacter baumannii and other
non-fermenting gram-negative bacilli. Enferm Infecc Microbiol Clin.
31:402–409. 2013.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
8
|
Silva-Díaz H, Bustamante-Canelo O,
Aguilar-Gamboa F, Mera-Villasis K, Ipanaque-Chozo J, Seclen-Bernabe
E and Vergara-Espinosa M: Predominant enteropathogens in acute
diarrhea and associated variables in children at the lambayeque
regional hospital, Peru. Horiz Med. 17:38–44. 2017.
|
|
9
|
Oliva-Menacho J, Oliva-Candela J and
Garcia-Hjarles M: Multi-drug resistant bacteria isolated from
medical stethoscopes in a level III hospital. Rev Med Hered.
28:242–246. 2017.
|
|
10
|
Hernandez del Sol CR and Vazquez Hernandez
G, Mesa Delgado Z, Bermudez Aleman RI, Sotolongo Rodriguez Y and
Vazquez Hernandez G: Enteropathogenic bacteria associated with
acute diarrheal disease in children. Acta Médica del Centro.
11:28–34. 2017.
|
|
11
|
López-Pueyo MJ, Barcenilla-Gaite F,
Amaya-Villar R and Garnacho-Montero J: Antibiotic multiresistance
in critical care units. Med Intensiva. 35:41–53. 2011.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
12
|
Torres C, Alonso CA, Ruiz-Ripa L,
León-Sampedro R, del Campo R and Coque TM: Antimicrobial Resistance
in Enterococcus spp. of animal origin. In: Antimicrobial
Resistance in Bacteria from Livestock and Companion Animals.
Schwarz S, Cavaco LM and Shen J (eds). Wiley Online Library, USA,
pp185-227, 2018.
|
|
13
|
Levin-Reisman I, Brauner A, Ronin I and
Balaban NQ: Epistasis between antibiotic tolerance, persistence,
and resistance mutations. Proc Natl Acad Sci USa. 116:14734–14739.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fernández-García L, Fernandez-Cuenca F,
Blasco L, López-Rojas R, Ambroa A, Lopez M, Pascual Á, Bou G and
Tomás M: Relationship between tolerance and persistence mechanisms
in Acinetobacter baumannii strains with AbkAB
toxin-antitoxin system. Antimicrob Agents Chemother. 62:e00250–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pacios O, Blasco L, Bleriot I,
Fernandez-Garcia L, González Bardanca M, Ambroa A, López M, Bou G
and Tomás M: Strategies to combat multidrug-resistant and
persistent infectious diseases. Antibiotics (Basel).
9(65)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brauner A, Fridman O, Gefen O and Balaban
NQ: . Distinguishing between resistance, tolerance and persistence
to antibiotic treatment. Nat Rev Microbiol. 14:320–330.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Remes Troche JM: Reflections on antibiotic
resistance and what to do about it. Rev Gastroenterol Mex. 81:1–2.
2016.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
18
|
Thapa Shrestha U, Adhikari N, Maharjan R,
Banjara MR, Rijal KR, Basnyat SR and Agrawal VP: Mutildrug
resistant Vibrio cholerae O1 from clinical and environmental
samples in Kathmandu city. BMC Infect Dis. 15(104)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hawkey PM, Warren RE, Livermore DM,
McNulty CAM, Enoch DA, Otter JA and Wilson A: Treatment of
infections caused by multidrug-resistant gram-negative bacteria:
Report of the British society for antimicrobial
chemotherapy/healthcare infection society/British infection
association joint working party. J Antimicrob Chemother. 73 (Suppl
3):iii2–iii78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alkofide H, Alhammad AM, Alruwaili A,
Aldemerdash A, Almangour TA, Alsuwayegh A, Almoqbel D, Albati A,
Alsaud A and Enani M: Multidrug-resistant and extensively
drug-resistant Enterobacteriaceae: Prevalence, treatments, and
outcomes-a retrospective cohort study. Infect Drug Resist.
13:4653–4662. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nadeem SF, Gohar UF, Tahir SF, Mukhtar H,
Pornpukdeewattana S, Nukthamna P, Moula Ali AM, Bavisetty S and
Masa S: Antimicrobial resistance: More than 70 years of war between
humans and bacteria. Crit Rev Microbiol. 46:578–599.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wiedenbeck J and Cohan FM: Origins of
bacterial diversity through horizontal genetic transfer and
adaptation to new ecological niches. FEMS Microbiol Rev.
35:957–976. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Toft C and Andersson SGE: Evolutionary
microbial genomics: Insights into bacterial host adaptation. Nat
Rev Genet. 11:465–475. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bliven KA and Maurelli AT: Evolution of
bacterial pathogens within the human host. Microbiol Spectr 4:
10.1128/microbiolspec.VMBF-0017-2015, 2016.
|
|
25
|
Soler N and Forterre P: Vesiduction: The
fourth way of HGT. Environ Microbiol. 22:2457–2460. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Griffith F: The significance of
pneumococcal types. J Hyg (Lond). 27:113–159. 1928.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lederberg J and Tatum EL: Gene
recombination in Escherichia coli. Nature.
158(558)1946.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zinder ND and Lederberg J: Genetic
exchange in Salmonella. J Bacteriol. 64:679–699. 1952.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Domingues S, Nielsen KM and da Silva GJ:
Various pathways leading to the acquisition of antibiotic
resistance by natural transformation. Mob Genet Elements.
2:257–260. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Calderón G and Aguilar L: Antimicrobial
resistance: More resistant microorganisms and antibiotics. Rev Méd
Costa Rica Centroam. 73:757–763. 2016.
|
|
31
|
Ellison CK, Dalia TN, Vidal Ceballos A,
Wang JC, Biais N, Brun YV and Dalia AB: Retraction of DNA-bound
type IV competence pili initiates DNA uptake during natural
transformation in Vibrio cholerae. Nat Microbiol. 3:773–780.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Graf FE, Palm M, Warringer J and Farewell
A: Inhibiting conjugation as a tool in the fight against antibiotic
resistance. Drug Dev Res. 80:19–23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Krenek P, Samajova O, Luptovciak I,
Doskocilova A, Komis G and Samaj J: Transient plant transformation
mediated by Agrobacterium tumefaciens: Principles, methods and
applications. Biotechnol Adv. 33:1024–1042. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bitto NJ, Chapman R, Pidot S, Costin A, Lo
C, Choi J, D'Cruze T, Reynolds EC, Dashper SG, Turnbull L, et al:
Bacterial membrane vesicles transport their DNA cargo into host
cells. Sci Rep. 7(7072)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Parkinson JS: Classic spotlight: The
discovery of bacterial transduction. J Bacteriol. 198:2899–2900.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Abebe E, Tegegne B and Tibebu S: A review
on molecular mechanisms of bacterial resistance to antibiotics. Eur
J Appl Sci. 8:301–310. 2016.
|
|
37
|
Balcázar JL: Implications of
bacteriophages on the acquisition and spread of antibiotic
resistance in the environment. Int Microbiol. 23:475–479.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Brown L, Wolf JM, Prados-Rosales R and
Casadevall A: Through the wall: Extracellular vesicles in
gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol.
13:620–630. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schwechheimer C and Kuehn MJ:
Outer-membrane vesicles from gram-negative bacteria: Biogenesis and
functions. Nat Rev Microbiol. 13:605–619. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Acosta RG and Vargas CM: Bacterial
resistance mechanism. Diagnóstico. 57:82–86. 2018.
|
|
41
|
Reygaert WC: An overview of the
antimicrobial resistance mechanisms of bacteria. AIMS Microbiol.
4:482–501. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cox G and Wright GD: Intrinsic antibiotic
resistance: Mechanisms, origins, challenges and solutions. Int J
Med Microbiol. 303:287–292. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Martinez JL: General principles of
antibiotic resistance in bacteria. Drug Discov Today Technol.
11:33–39. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bébéar CM and Pereyre S: Mechanisms of
drug resistance in Mycoplasma pneumoniae. Curr Drug Targets
Infect Disord. 5:263–271. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao F, Liu J, Shi W, Huang F, Liu L, Zhao
S and Zhang J: Antimicrobial susceptibility and genotyping of
Mycoplasma pneumoniae isolates in Beijing, China, from 2014
to 2016. Antimicrob Resist Infect Control. 8(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
O'Shea R and Moser HE: Physicochemical
properties of antibacterial compounds: Implications for drug
discovery. J Med Chem. 51:2871–2878. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Davies J and Davies D: Origins and
evolution of antibiotic resistance. Microbiol Mol Biol Rev.
74:417–433. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Martinez JL and Baquero F: Mutation
frequencies and antibiotic resistance. Antimicrob Agents Chemother.
44:1771–1777. 2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Binet R and Maurelli AT: Frequency of
spontaneous mutations that confer antibiotic resistance in
Chlamydia spp. Antimicrob Agents Chemother. 49:2865–2873.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang G, Wilson TJM, Jiang Q and Taylor DE:
Spontaneous mutations that confer antibiotic resistance in
Helicobacter pylori. Antimicrob Agents Chemother.
45:727–733. 2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Coculescu BI: Antimicrobial resistance
induced by genetic changes. J Med Life. 2:114–123. 2009.PubMed/NCBI
|
|
52
|
Meštrović T, Virok DP, Ljubin-Sternak S,
Raffai T, Burián K and Vraneš J: Antimicrobial resistance screening
in Chlamydia trachomatis by optimized McCoy cell culture
system and direct qPCR-based monitoring of chlamydial growth.
Methods Mol Biol. 2042:33–43. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kondrashov FA: Gene duplication as a
mechanism of genomic adaptation to a changing environment. Proc
Biol Sci. 279:5048–5057. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Calçada C, Silva M, Baptista V, Thathy V,
Silva-Pedrosa R, Granja D, Ferreira PE, Gil JP, Fidock DA and Veiga
MI: Expansion of a specific Plasmodium falciparum PfMDR1
haplotype in southeast Asia with increased substrate transport.
mBio. 11:e02093–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Munita JM and Arias CA: Mechanisms of
antibiotic resistance. Microbiol Spectr 4: 10.1128/microbiolspec.
VMBF-0016-2015, 2016.
|
|
56
|
Begum S, Begum T, Rahman N and Khan RA: A
review on antibiotic resistance and way of combating antimicrobial
resistance. GSC Biol Pharm Sci. 14:87–97. 2021.
|
|
57
|
Etebu E and Arikekpar I: Antibiotics:
Classification and mechanisms of action with emphasis on molecular
perspectives. Int J Appl Microbiol Biotechnol Res. 4:90–101.
2016.
|
|
58
|
Poulikakos P, Tansarli GS and Falagas ME:
Combination antibiotic treatment versus monotherapy for
multidrug-resistant, extensively drug-resistant, and
pandrug-resistant Acinetobacter infections: A systematic
review. Eur J Clin Microbiol Infect Dis. 33:1675–1685.
2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kapoor G, Saigal S and Elongavan A: Action
and resistance mechanisms of antibiotics: A guide for clinicians. J
Anaesthesiol Clin Pharmacol. 33:300–305. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Epand RM, Walker C, Epand RF and Magarvey
NA: Molecular mechanisms of membrane targeting antibiotics. Biochim
Biophys Acta. 1858:980–987. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hooper DC and Jacoby GA: Topoisomerase
inhibitors: Fluoroquinolone mechanisms of action and resistance.
Cold Spring Harb Perspect Med. 6(a025320)2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Santajit S and Indrawattana N: Mechanisms
of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int.
2016(2475067)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Troncoso C, Pavez M, Santos A, Salazar R
and Barrientos Díaz L: Structural and physiological implications of
bacterial cell in antibiotic resistance mechanisms. Int J Morphol.
35:1214–1223. 2017.
|
|
64
|
Zhou G, Shi QS, Huang XM and Xie XB: The
three bacterial lines of defense against antimicrobial agents. Int
J Mol Sci. 16:21711–21733. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Borges A, Abreu AC, Dias C, Saavedra MJ,
Borges F and Simões M: New perspectives on the use of
phytochemicals as an emergent strategy to control bacterial
infections including biofilms. Molecules. 21(877)2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Pérez-Cano H and Robles-Contreras A: Basic
aspects of the mechanisms of bacterial resistance. Rev Med MD.
4:186–191. 2013.
|
|
67
|
Sager M, Benten WP, Engelhardt E, Gougoula
C and Benga L: Characterization of biofilm formation in
[Pasteurella] pneumotropica and [Actinobacillus] muris isolates of
mouse origin. PLoS One. 10(e0138778)2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Martinez JL, Vicente MF, Delgado-Iribarren
A, Perez-Diaz JC and Baquero F: Small plasmids are involved in
amoxicillin-clavulanate resistance in Escherichia coli.
Antimicrob Agents Chemother. 33(595)1989.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sideraki V, Huang W, Palzkill T and
Gilbert HF: A secondary drug resistance mutation of TEM-1
beta-lactamase that suppresses misfolding and aggregation. Proc
Natl Acad Sci USA. 98:283–288. 2001.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Salverda ML, De Visser JA and Barlow M:
Natural evolution of TEM-1 β-lactamase: experimental reconstruction
and clinical relevance. FEMS Microbiol Rev. 34:1015–1036.
2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Delgado-Valverde M, Sojo-Dorado J, Pascual
A and Rodríguez-Baño J: Clinical management of infections caused by
multidrug-resistant Enterobacteriaceae. Ther Adv Infect Dis.
1:49–69. 2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rodríguez-Baño J, Gutiérrez-Gutiérrez B,
Machuca I and Pascual A: Treatment of infections caused by
extended-spectrum-beta-lactamase-, AmpC-, and
carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev.
31:e00079–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Retamar P, Portillo MM, López-Prieto MD,
Rodríguez-López F, de Cueto M, García MV, Gómez MJ, Del Arco A,
Muñoz A, Sánchez-Porto A, et al: Impact of inadequate empirical
therapy on the mortality of patients with bloodstream infections: A
propensity score-based analysis. Antimicrob Agents Chemother.
56:472–478. 2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Nørgaard SM, Jensen CS, Aalestrup J,
Vandenbroucke-Grauls CM, de Boer MG and Pedersen AB: Choice of
therapeutic interventions and outcomes for the treatment of
infections caused by multidrug-resistant gram-negative pathogens: A
systematic review. Antimicrob Resist Infect Control.
8(170)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Falagas ME, Karageorgopoulos DE and
Nordmann P: Therapeutic options for infections with
Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future
Microbiol. 6:653–666. 2011.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gill EE, Franco OL and Hancock REW:
Antibiotic adjuvants: Diverse strategies for controlling
drug-resistant pathogens. Chem Biol Drug Des. 85:56–78.
2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lin DM, Koskella B and Lin HC: Phage
therapy: An alternative to antibiotics in the age of multi-drug
resistance. World J Gastrointest Pharmacol Ther. 8:162–173.
2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Reina J and Reina N: Phage theraphy, an
alternative to antibiotic therapy? Rev Esp Quimioter. 31:101–104.
2018.PubMed/NCBI(In Spanish).
|
|
79
|
Castañeda GCD: Intestinal microbota,
probiotics and prebiotics. Enferm Inv (Ambato). 2:156–160.
2017.
|
|
80
|
Játiva-Mariño E, Manterola C, Macias R and
Narváez D: Probiotics and Prebiotics: Its role in childhood acute
diarrheal disease therapy. Int J Morphol. 39:294–301. 2021.
|
|
81
|
Olveira G and González-Molero I: An update
on probiotics, prebiotics and symbiotics in clinical nutrition.
Endocrinol Nutr. 63:482–494. 2016.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
82
|
Suárez JE: Autochthonous microbiota,
probiotics and prebiotics. Nutr Hosp. 31 (Suppl 1):S3–S9.
2015.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
83
|
Feria MG, Taborda NA, Hernandez JC and
Rugeles MT: Effects of prebiotics and probiotics on
gastrointestinal tract lymphoid tissue in hiv infected patients.
Rev Med Chil. 145:219–229. 2017.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
84
|
Pandey KR, Naik SR and Vakil BV:
Probiotics, prebiotics and synbiotics-a review. J Food Sci Technol.
52:7577–7587. 2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
González-Torralba A, García-Esteban C and
Alós JI: Enteropathogens and antibiotics. Enferm Infecc Microbiol
Clin (Engl Ed). 36:47–54. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
86
|
Alcántar-Curiel MD, Blackburn D, Saldaña
Z, Gayosso-Vázquez C, Iovine NM, De la Cruz MA and Girón JA:
Multi-functional analysis of Klebsiella pneumoniae fimbrial types
in adherence and biofilm formation. Virulence. 4:129–138.
2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Clarke SC, Haigh RD, Freestone PP and
Williams PH: Virulence of enteropathogenic Escherichia coli,
a global pathogen. Clin Microbiol Rev. 16:365–378. 2003.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Gallego-Maldonado G, Otálora-Díaz AS,
Urbano-Cáceres EX and Morales-Súarez C: Bacterial multiresistance:
Therapeutic challenge in renal transplantation. Univ Salud.
21:72–87. 2019.
|
|
89
|
Schroeder GN and Hilbi H: Molecular
pathogenesis of Shigella spp.: Controlling host cell
signaling, invasion, and death by type III secretion. Clin
Microbiol Rev. 21:134–156. 2008.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Tanwar J, Das S, Fatima Z and Hameed S:
Multidrug resistance: An emerging crisis. Interdiscip Perspect
Infect Dis. 2014(541340)2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Alonso-Pérez C, Alcántara-Salinas A,
Escobar-Rojas V, Ramírez-Sandoval MP, Reyes-Hernández MU,
Guerrero-Becerra M, Vargas-Mosso ME, Hernández-Magaña R,
Anzures-Gutiérrez SA, Cuevas-López LL, et al: Gastroenteritis by
Campylobacter in children. Current concepts. Bol Clin Hosp
Infant Edo Son. 36:88–101. 2019.
|
|
92
|
Navon-Venezia S, Kondratyeva K and
Carattoli A: Klebsiella pneumoniae: A major worldwide source and
shuttle for antibiotic resistance. FEMS Microbiol Rev. 41:252–275.
2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Fernández-Abreu A, Bravo-Fariñas LC,
Rivero-Navea G, Nuñez-Fernández FA, Cruz-Infante Y, Águila-Sánchez
A and Hernández-Martínez JL: Determination of biofilms and
extended-spectrum beta-lactamases in Vibrio cholerae non-O1,
non-O139 isolates from patients with diarrhea in Cuba. Rev Cubana
Med Trop. 71:1–7. 2019.
|
|
94
|
Riveros M and Ochoa TJ: Relevant public
health enteropathogens. Rev Peru Med Exp Salud Publica. 32:157–164.
2015.PubMed/NCBI(In Spanish).
|
|
95
|
Macero-Gualpa LJ, Vásquez-Véliz RM and
Reyes-Sánchez RR: Wound infection by aeromona hydrophila, a case
report in ecuador. Rev Med FCM-UCSG. 23:95–99. 2019.
|
|
96
|
Fàbrega A and Vila J: Yersinia
enterocolitica: Pathogenesis, virulence and antimicrobial
resistance. Enferm Infecc Microbiol Clin. 30:24–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Liu GY: Molecular pathogenesis of
Staphylococcus aureus infection. Pediatr Res. 65:71R–77R.
2009.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Harnisz M and Korzeniewska E: The
prevalence of multidrug-resistant Aeromonas spp in the
municipal wastewater system and their dissemination in the
environment. Sci Total Environ. 626:377–383. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Fiore E, Van Tyne D and Gilmore MS:
Pathogenicity of enterococci. Microbiol Spectr.
7:10.1128/microbiolspec.GPP3-0053-2018. 2019.
|