1. Introduction
Osteoarthritis (OA) is a joint disease with a high
prevalence and serious consequences. It leads to pain and decline
in the quality of life of affected individuals (1,2). OA
is characterized by synovitis, osteophyte formation and
degeneration of joint-related tissues (such as articular
cartilage), which may eventually result in changes in bone
structure and disability of patients (3). Traditional treatments include
physical therapy, anti-inflammatory and analgesic drugs and
injecting glucocorticoid or hyaluronic acid into the joint
(4-6).
However, at present, most OA therapies have limited efficacy in
symptom relief. There are still no drugs that can reverse the
pathological process of OA (7).
Although there is continuing research to reveal the mechanism of
OA, more effective treatments are required. For the present review,
the following terms were searched using the PubMed database
(https://pubmed.ncbi.nlm.nih.gov/):
‘osteoarthritis’, ‘macrophage polarization’, ‘treatment’ and
‘cartilage damage’; only papers published in English were
assessed.
In OA, immune cells secrete inflammatory factors or
mediators affecting cartilage structure through intercellular
association to participate in the pathological process. Neutrophils
promote OA by secreting proinflammatory mediators and producing
enzymes that degrade cartilage. Dendritic cells activate T helper
(Th)1 and Th17 cells, which produce pro-inflammatory cytokines,
resulting in cartilage degradation, while Th2 cells secrete
anti-inflammatory factors to protect cartilage. In addition, Th2
cells can activate B cells to secrete proinflammatory factors and
antibodies to inhibit cartilage repair. Treg cells have beneficial
effects on OA. They do not directly participate in cartilage
repair, but act on neutrophils to secrete anti-inflammatory
factors. Natural killer cells are closely related to the
differentiation of mesenchymal stem cells (MSCs) and osteoclasts.
However, the largest number of immune cell types in OA synovium is
macrophages (8). Macrophages, as
an important part of the immune system, have long been considered
to be an crucial participant in OA (9).
Macrophages, derived from hematopoietic stem cells,
are immune cells that serve a key role in the immune response
(10). Macrophages are activated
by environmental factors such as TNF-α, IFN-γ and
lipopolysaccharide (LPS) (8). When
activated, they can absorb innate antigens or invading pathogens,
express costimulatory molecules and secrete more inflammatory
cytokines, which are essential for immune response and cartilage
reconstruction (11). Macrophages
are mainly polarized into M1 and M2 phenotypes (8). After being stimulated by IFN-γ, M0
macrophages are polarized into M1 macrophages, thereby expressing
pro-inflammatory cytokines and participating in the process of
pro-inflammation and degradation of extracellular matrix (ECM)
(9). When M0 macrophages are
stimulated by IL-4/IL-13, they polarized into M2 macrophages and
express anti-inflammatory cytokines and thus exert
anti-inflammatory and chondrogenic effects (12). Macrophage polarization participates
in a number of pathological processes, including metabolic changes,
virus infection, inflammatory environment and tumor immunity
(8,9,11).
Recent research has shown that it is beneficial to slow the
progress of OA by regulating the changes of macrophage phenotype
(12). This suggests that
macrophage polarization is an emerging target to treat OA. The
present study collected studies on the regulation of macrophage
polarization and OA progress in order to elaborate its mechanism
and provide clues for the subsequent development of treatment
methods based on the regulation of macrophage phenotype.
2. Development of OA
OA may be caused by factors such as heredity and
mechanical load (13-15).
OA can influence the entire joint, including cartilage, synovial
tissue, subchondral bone, joint capsule, ligaments and
periarticular muscles (16).
However, cartilage degeneration may be the most important
pathological process of OA and accumulating researches focus on
preventing its degeneration (3).
The inflammatory environment generated by cartilage injury will
cause chondrocyte apoptosis and hypertrophy, ECM decomposition,
eventually leading to cartilage injury and aggravating OA (17). Cartilage covers the end of bone and
is a type of hyaline cartilage without nerves and blood vessels
(18). Typically, it consists of
ECM and chondrocytes (19). The
ECM consists of matrix, collagen type 1A (col1A) and collagen type
2A1 (col12A1) (20). Proteoglycan
is the main component of matrix and participates in chondrocyte
synthesis and degradation (12).
The cascade effect is amplified by the production of MMP or a
disintegrin-like and metalloproteinase with thrombospondin motifs
(ADAMTS) by chondrocytes, synovium fibroblasts and macrophages when
mechanical loading on cartilage causes damage (8). The ECM degradation enzyme ADAMTS5 is
a proteoglycan enzyme of the ADAMTS family (21), whose main function is to clear
large proteoglycans such as aggrecan, leading to the destruction of
cartilage and OA (22). MMPs,
especially collagenase MMP1 and MMP13, are believed to cut collagen
type II, the main structural component of cartilage, leading to
irreversible loss of ECM structure and function (23). Therefore, the regulation of
cartilage homeostasis is particularly important for the development
of OA.
Existing studies have shown that the imbalance of
ECM composition synthesis and degradation is an important marker of
exacerbating OA (8,12,23,24).
However, the mechanism of imbalance between cartilage damage and
repair remains to be elucidated. Trauma can lead to bone and joint
friction or increased enzyme activity, followed by the formation of
‘wear’ particles that are then eaten by resident macrophages
(8). However, once the immune
system is unable to eliminate these ‘wear’ particles completely,
they act as mediators of inflammation, inducing chondrocytes to
release degrading enzymes (22).
Synovial macrophages phagocytose molecules produced by collagen or
proteoglycan breakdown and release pro-inflammatory cytokines such
as TNF-α, IL-1 and IL-6(23).
These cytokines can act on chondrocytes to further release
degradation enzymes and prevent the generation of type II collagen,
thus further increasing cartilage degradation (25). In conclusion, the mechanism of
cartilage destruction aggravating OA may be: i) The increased
production of degrading enzymes leads to decreased synthesis or
increased decomposition of collagen fibers or ii) the apoptosis and
senescence of chondrocytes. The morphologic change of articular
cartilage goes through several processes: At first, fibrotic areas
and cracks appear on the surface of the cartilage, leading to
cartilaginous softening and cartilage thickness reduction (24). When the articular cartilage is
completely destroyed, the underlying subchondral bone plate is
completely exposed (25). The
longer OA lasts, the more pronounced these changes become.
There are now a variety of induction methods to
damage joints in animals and build models to simulate OA (26-28).
One type of model is to simulate OA by surgical instability of
joint structure and joint injury. These include anterior cruciate
ligament (ACLT) transection or medial meniscus (DMM) instability,
which may result in changes in the mechanical load of the joint
(26). In addition, a closed
non-invasive injury model has been established, which can simulate
OA injury after mechanical injury regardless of whether there is
ACL fracture or not (27). Another
model triggers joint damage by injecting drugs into the joint that
disrupt cartilage homeostasis. Intra-articular injection of
monosodium iodoacetate (MIA) inhibits glycolysis of chondrocytes,
which leads to chondrocyte death and induces inflammation, thus
simulating OA (26). Occasionally
collagenase is injected into the joint, which will destroy the
stability of the joint and cause inflammation, leading to OA-like
joint injury (26).
3. Macrophages and OA
OA is characterized by the infiltration and invasion
of macrophages, the release of a great quantity of pro-inflammatory
and pro-catabolic mediators in the articular cavity and the
increase of synovial vascular distribution. These mediators are
recognized by synovial immune cells and activate the immune cells
to release more cytokines, resulting in chronic and repeated
inflammation. In short, these fragments released into the synovial
cavity stimulate macrophages to produce and release inflammatory
mediators (such as cytokines, chemokines, lipid mediators and
death-associated molecular patterns itself) into the synovial fluid
(29). In turn, these mediators
activate chondrocytes that produce metalloproteinases, leading to a
vicious cycle between cartilage and synovium (30). The pathological process is
generally described as mechanical stress directly damages cartilage
or activates chondrocytes, producing abnormal levels of degradation
enzymes and reactive oxygen species (ROS), leading to cartilage
destruction. Subsequently, microcrystals, osteochondral fragments
and ECM degradation products are released from the articular
cavity. These molecules and products trigger inflammatory
macrophages to secrete chemokines, cytokines, MMP, ROS and lipid
mediators. They can directly degrade ECM components or imbalance of
chondrocyte homeostasis, leading to imbalance of cartilage matrix
degradation and synthesis, thereby aggravating OA.
Macrophages can regulate the local microenvironment
under physiological and pathological conditions and respond quickly
to various stimuli (31). After
stimulation, macrophages are mainly activated into two phenotypes:
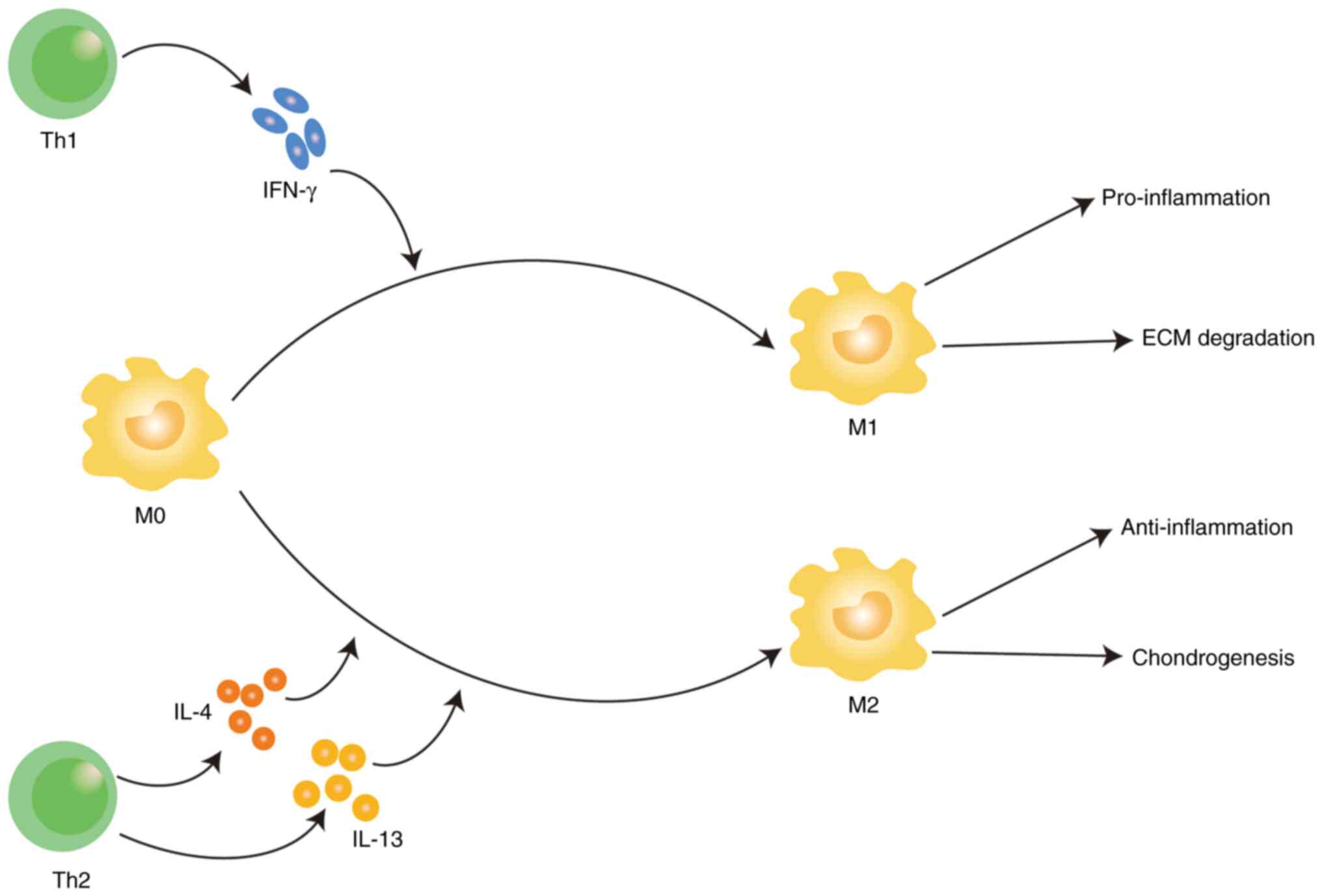
M1 and M2. Macrophages is polarized to M1 type by IFN-γ released by
Th1 cell and has high expression of proinflammatory cytokines,
including TNF-α, IL-1β, IL-6, IL-12, IL-23 and cyclooxygenase-2
(COX-2), therefore, have strong pro-inflammatory and pro-tumor
functions (32). M2 macrophages
are polarized by the STAT pathway activated by Th2 cytokines such
as IL4/IL-13 through interleukin receptor, which up regulates
arginine-1(Arg-1), CC motif chemokine ligand (CCL) 17 and CCL2, so
it has anti-inflammatory effect (33) (Fig.
1). Th1 cells release IFN-γ to polarize M0 macrophages into M1
macrophages, resulting in pro-inflammation and ECM degradation; Th2
cells release IL-4 and IL-13 polarizes M0 macrophages into M2
macrophages, resulting in anti-inflammation and chondrogenesis.
Therefore, macrophages are not only essential immune
cells, but also attractive therapeutic targets. In-depth
understanding of the molecular characteristics related to the
dynamic changes of macrophage polarization and their pathways is
crucial to elucidate the molecular basis of disease progression and
design of new macrophage therapy strategies (34). Identifying precise targets that
control the transition between given steady states is a huge
challenge.
4. Macrophages are emerging targets for OA
treatment
OA is an immune disease caused by dysfunction of
immune microenvironment. Reconstructing the balance of immune
microenvironment by regulating the polarization of macrophages is
an effective measure to treat OA. The factors affecting OA
development include chemical components and biomolecules.
Increasing studies have shed light on regulating inflammation by
reversing macrophage polarization and progress has been made. The
following is a review of the regulation of macrophages on OA,
providing clues for a deeper understanding of the regulation
mechanism of macrophages in OA (Table
I).
 | Table IOA treatment strategies based on
macrophage. |
Table I
OA treatment strategies based on
macrophage.
| Type | Name | OA sample;
cells | OA sample;
animal |
|---|
| Chemical
compound | | | |
| | Liraglutide | Mouse primary
chondrocytes, RAW 264.7 cells | MIA-induced OA
mouse model |
| |
Tert-butylhydroquinone | Mouse chondrocytes
and synovial macrophages | DMM-induced OA
mouse model |
| | Angelicin | Mouse bone marrow
macrophage | DMM-induced OA
mouse model |
| | Fargesin | Mouse primary
chondrocytes, RAW 264.7 cells | OA mouse model
induced by injection of collagenase |
| | Pseudolaric acid
B | RAW 264.7 cells,
bone marrow derived macrophages (BMDM) | DMM-induced OA
mouse model |
| | Eucommia
ulmoides Polysaccharides | RAW 264.7
cells | Anterior cruciate
ligament transection rabbit model |
| Cell | | | |
| | Human adipose stem
cells | Primary human
adipose stem cells, RAW264.7 cells | MIA-induced OA
mouse model |
| | Human umbilical
cord mesenchymal stem cells | 3rd-5th generation
human umbilical cord mesenchymal stem cells, CP-R092 cells, mouse
synovial macrophages | Anterior cruciate
ligament transection mouse model |
| | Human umbilical
cord mesenchymal stem cells | Primary human
umbilical cord mesenchymal stem cells, mouse derived macrophages
and rat derived chondrocytes | Anterior cruciate
ligament transection mouse model |
| | Transient receptor
potential vanillin 1 | RAW 264.7
cells | MIA-induced OA rat
model |
| Protein | | | |
| | Milk fat globule
epidermal growth factor 8 8 | Mouse primary
chondrocytes, RAW 264.7 cells | MIA-induced OA
mouse model |
| | E3 ubiquitin ligase
itch protein | Mouse bone marrow
macrophage | MIA-induced OA
mouse model |
Compounds. Liraglutide
Glucagon like peptide-1 (GLP-1) is secreted by
intestinal L cells and is a gastrointestinal hormone that processes
food intake. Insulin has a series of extrapancreatic functions
associated with anti-inflammatory properties (35). The GLP-1 analogue liraglutide is a
human GLP-1 applied in the patients with diabetes (36). New data suggest that liraglutide
inhibits ROS generation, pro-inflammatory cytokine secretion
(37).
In vitro, when liraglutide is added to
LPS-stimulated RAW264.7 cells, M1 macrophage phenotype reverts to
M2 phenotype (38). Liraglutide
significantly reduced the expression of MMP and ADAMTS and made
chondrocytes anti-catabolic. From a cellular perspective, the
decrease of proinflammatory factors in macrophages and chondrocytes
is dose-dependent (38). In
vivo results show that in the MIA-induced model, liraglutide
injection significantly reduced the secretion of IL-6, nitric oxide
and prostaglandin E2 and improved the synovitis severity score in
mice (38).
Liraglutide has been applied in clinical trials. A
randomized controlled trial applied liraglutide in patients with
knee arthritis, but it did not reduce knee pain compared with
placebo (39). More clinical
experiments are needed to verify the anti-inflammatory effect of
liraglutide. In conclusion, liraglutide may improve OA by changing
macrophage polarization and reducing cartilage degradation.
Tert-butylhydroquinone.
Tert-butylhydroquinone (tBHQ) is a compound with strong antioxidant
activity (40). There is
increasing evidence that tBHQ may be used to treat diseases related
to oxidative stress (41).
In vitro, tBHQ significantly reduces the
expression of phosphorylated (p-)STAT1 and p-STAT3 induced by LPS
and effectively inhibits the M1 polarization of synovial
macrophages. From a signaling pathway perspective, tBHQ inhibited
the production of ROS through MAPK and NF-κB signaling pathways,
which can alleviate OA (42). In
addition, IL-1β can induce chondrocyte apoptosis, promote
inflammation and inhibit differentiation. tBHQ treatment prevents
this effect of IL-1β (42). In
DMM-induced mouse model, tBHQ significantly reduces the expression
levels of IL-6, TNF-α and IL-1β and inhibits the development of OA
in mice (42). In brief, tBHQ may
be used for treating OA.
Angelicin. In China, Angelica sinensis
is a commonly used herbal medicine to treat a variety of ailments.
It is reported that Angelica sinensis extract has
antioxidant and anti-OA effects (43,44).
Angelicin (ANG) is an active ingredient isolated from Angelica
sinensis, which has the functions of anticoagulation, analgesia
and hemostasis. Therefore, ANG is considered to have a strong
immunomodulatory effect (45).
ANG significantly inhibits the transformation of
macrophages from M0 to M1 and the repolarization from M2 to M1 is
also significantly inhibited (46). From the perspective of signaling
pathway, ANG regulated macrophage polarization by activating the
STAT pathway to reduce secretion of pro-inflammatory factors. In
in vivo experiments, ANG effectively inhibits LPS-induced M1
polarization (46). The shape of
cartilage in the ANG treatment group was similar to that of healthy
cartilage, with smooth cartilage surface and complete cartilage
matrix. Although experimental data indicate that ANG is effective
in alleviating OA, more studies are needed to expand the
applicability of this study. Overall, these results suggest that
ANG has certain curative effects on OA.
Fargesin. Fargesin is a type of lignan with
pharmacological activity and is extracted from Flos
magnoliae (FM), which has long been used in the treatment of
emphysema, sinusitis and headaches. It is reported that FM extracts
have significant anti-allergic and anti-inflammatory effects
(47). Recent studies have found
that fargesin is critical for inflammatory response and glucose
metabolism (48).
In terms of signaling pathways, fargesin promotes
the polarization of macrophages into M2 through p38/ERK, P65/NF-κB
pathways. In addition, anti-inflammatory factors in synovium are
increased after fargesin treatment (49). Following fargesin treatment, the
markers of cartilage catabolism (MMP13, collagen type X and
Runt-related transcription factor 2) decrease and the markers of
cartilage formation (col2a1 and Sox9) increase. This is through the
mechanism of paracrine macrophages. In vivo, fargesin
treatment alleviates synovitis and cartilage damage in CIOA-induced
mice (49). Therefore, in-depth
study of the signaling pathways regulating macrophage polarization
and reprogramming or preventing macrophages from para-secreting
cytokines to chondrocytes may be one direction for alleviation of
OA.
Pseudolauric acid B. Pseudolaric acid B (PAB)
is extracted from the dried cortex of the roots of Pseudolarix
kaempferi Gord. It has a number of properties, such as
antifungal, anti-angiogenesis and anti-inflammation (50). PAB may inhibit angiogenesis by
promoting proteasome-mediated degradation (51).
In vitro experiments show that PAB inhibits
the degradation of IκBα by inhibiting the phosphorylation of P65.
At the same time, PAB inhibits NF-κB signaling by stabilizing
peroxisome proliferator activated receptor γ to prevent M1
polarization and angiogenesis, thereby further reducing synovitis
and OA progression (51). In
vivo, mice treated with PAB show significantly reduced
cartilage destruction and improved synovitis (52). These results expand the potential
clinical application of PAB and strengthen the possibility that
early targeting of synovitis or inhibiting angiogenesis may prevent
OA (52).
Eucommia ulmoides polysaccharide (EUP). EU is
a traditional herbal medicine that has been used in China for
thousands of years (53). In
recent years, a polysaccharide extracted from EU, as a drug
component, has been studied for its immunomodulatory effect
(53). It is reported that EUP has
anti-inflammatory and anti-hypertensive effects. In terms of
osteogenesis, it inhibits osteoclast and promotes osteogenesis
(54-56).
EUP inhibits the expression of cytokines IL-6, IL-1β
and IL-18, which inhibit chondrocyte apoptosis. In the
extracellular matrix, cytokines activate MMPs to cut collagen
fibers, so as to alleviate the progress of OA. In addition, the
expression of osteogenesis and cartilage regeneration related genes
such as BMP-6, TGF-β and Arg-1 increase under the treatment of EUP
(57). At the same time, EUP
treatment significantly decreases the expression of M1 macrophage
markers (F4/80 and inducible nitric oxide synthase), while a M2
macrophage marker (CD206) increases (57). Micro-CT of living rabbit model
showed that cartilage regeneration and subchondral bone
reconstruction were evident following EUP treatment.
A retrospective study combined meloxicam with EUP in
patients with OA showed significant improvement in pain, stiffness
and dysfunction (58). Therefore,
it can be considered that EUP alleviates the progress of OA and has
the potential to treat OA.
Cells. Human adipose stem cells
(hASCs)
hASCs are differentiated from mesenchymal stem cells
(MSCs), which can secrete growth factors, chemokines and cytokines
or other substances to improve the surrounding microenvironment and
promote the growth of surrounding cells (59). hASCs secrete extracellular vesicles
(EVs) to convey information between cells. EVs as carriers carry
protein and other signal molecules, serving a role in targeting
cells, such as chondrocytes and monocytes (60). EVs can improve OA by protecting
chondrocytes from apoptosis and stimulating macrophages to polarize
into the anti-inflammatory phenotype. Therefore, hASCs have been
applied in regulating cell growth, regulating inflammatory response
and repairing tissue defects (60,61).
In vitro, LPS was added to RAW264.7 cells to
induce inflammation. The expression of proinflammatory cytokines
(COX-2, IL-1, IL-6 and TNF-α) decreased significantly following
hASCs-EVs treatment. In addition, hASCs-EVs promoted the
transformation of macrophage to M2 subtype (62). In a mouse OA model, the number of
M1 macrophages in OA synovium decrease and the expression of
proinflammatory IL-1β in synovium and cartilage is also inhibited
following intra-articular injection of EVs (62). In addition, hASCs-EVs treatment can
regulate immune reactivity and effectively prevent proteoglycan
degradation. It also reduces cartilage damage and slowed the
progression of OA (62). In the
DMM model, hASCs-EVs reduces the number of MMP-13 positive cells
and reduces cartilage damage (62). Although this previous article shows
that hASCs-EVs are helpful in reducing cartilage damage and OA, it
is necessary to optimize the injection dose and study the mechanism
in the future. Chen et al (63) studied the injection of ADSCs into
the joint to treat knee OA and it effectively reduced pain scores
and improved functional scores. In conclusion, hASCs-EVs should be
considered as a potential treatment for OA.
Human umbilical cord mesenchymal stem cells
(hUC-MSCs). There are two studies that reveal the effect of EVs
secreted by hUC-MSCs on OA. The first study shows that hUC-MSC-EVs
regulated OA in vitro mainly through the following: i)
Releasing EVs to inhibit the infiltration of synovial M1
macrophages; ii) reducing the expression of CD14 and IL-1β, and
iii) increasing the expression of CD206 and IL-10. In vivo
experimental data show that in a rat model, hUC-MSC-EVs treatment
can reverse the cartilage rupture and subchondral bone thickening
(64).
The second study shows that hUC-MSC-EVs may transmit
target proteins and microRNA through PI3K/Akt signaling pathway, so
as to achieve the repolarization of M2 macrophages. In addition,
the paracrine secretion of polarized M2 macrophages induced by
hUC-MSC-EVs decreases the expression of MMP and apoptosis of
chondrocytes. An in vivo experimental study reported that
hUC-MSC-EVs can improve the progression of OA caused by ACLT
(65).
Although hUC-MSCs have been proved to be effective
in alleviating OA, there are still a number of problems with sEVs.
The pain caused by repeated injections and the optimal dosage of
injections are all difficult issues (64). However, hUC-MSC and their
derivatives (such as EVs) have made remarkable achievements in
promoting OA repair. These two studies further elucidated the
molecular mechanism of hUC-MSC-EVs in the remission of OA and
greatly improved the possibility of clinical application.
Proteins. Transient receptor potential
vanilloid 1 (TRPV1)
TRPV1 is highly expressed on primary afferent
neurons and participates in the conduction of pain (66). Activation of TRPV1 is closely
associated with inflammation. However, TRPV1 exhibits both
pro-inflammatory and anti-inflammatory effects, suggesting that the
mechanism by which TRPV1 regulates inflammation may be complex
(67).
In vitro experimental studies show that at
the level of signaling pathways, TRPV1 inhibits the polarization of
M1 macrophages in synovium through Ca2+/CaMKII/Nrf2
axis, thereby reversing the progress of OA (68). A rat OA model showed that
activation of TRPV1 could reduce knee swelling, improve synovitis
score and reduce M1 macrophage level, so as to reduce cartilage
destruction and osteophyte formation (68).
In a study in which the target disease was knee OA,
the pain of patients was significantly reduced after the
application of TRPV1 antagonist (69). In conclusion, this previous paper
clarifies that TRPV1 is an attractive research direction and
reveals a new mechanism of local capsaicin in the treatment of
OA.
Milk fat globule epidermal growth
factor 8 (MFG-E8)
MFG-E8, also known as lactomyxin, is a peripheral
secreted glycoprotein that can phagocytize apoptotic cells
(70). In addition to being used
to clear apoptotic cells, accumulating evidence shows that MFG-E8
serves a critical role in inhibiting inflammation, repairing
injury, arterial remodeling and improving prognosis (70).
At the signaling pathway level, MFG-E8 targets
chondrocyte growth and macrophage repolarization through the NF-κB
pathway to prevent OA (71). In
addition, lack of MFG-E8 leads to dysregulation of articular
cartilage synthesis and decomposition homeostasis. Exogenous
administration of MFG-E8 can promote the transformation of
macrophages into M2 and reduce the release of inflammatory
cytokines during the development of OA (72). In a DMM-mediated mouse model,
MFG-E8 can prevent OA from developing from cartilage injury to
osteophyte formation (72).
In conclusion, these results broaden the field of
clinical application of MFG-E8. Targeting MFG-E8 through
intra-articular supplements represents a method to delay the
development of OA.
E3 ubiquitin ligase ITCH protein
Ubiquitin E1, E2 and E3 ligases can mediate
ubiquitination. This modification occurs at the level of protein
translation. Proteins usually degrade and lose their biological
functions after ubiquitination. ITCH is a monomeric protein that
serves a key role in different cellular environments, including DNA
damage, immune response, T cell differentiation and cell death
(73).
In vitro data show that ITCH-deficient
macrophageswill promote the pro-inflammatory polarization of
macrophages and aggravate the progress of post-traumatic OA (PTOA).
In vivo studies show that in the mouse OA model, the
decrease of ITCH protein level is related to the severity of PTOA,
indicating that the decrease of ITCH protein level may aggravate
human OA (74).
In conclusion, ubiquitination and degradation of
ITCH protein may be involved in PTOA process and studying the
mechanism of its degradation may be an exciting direction for
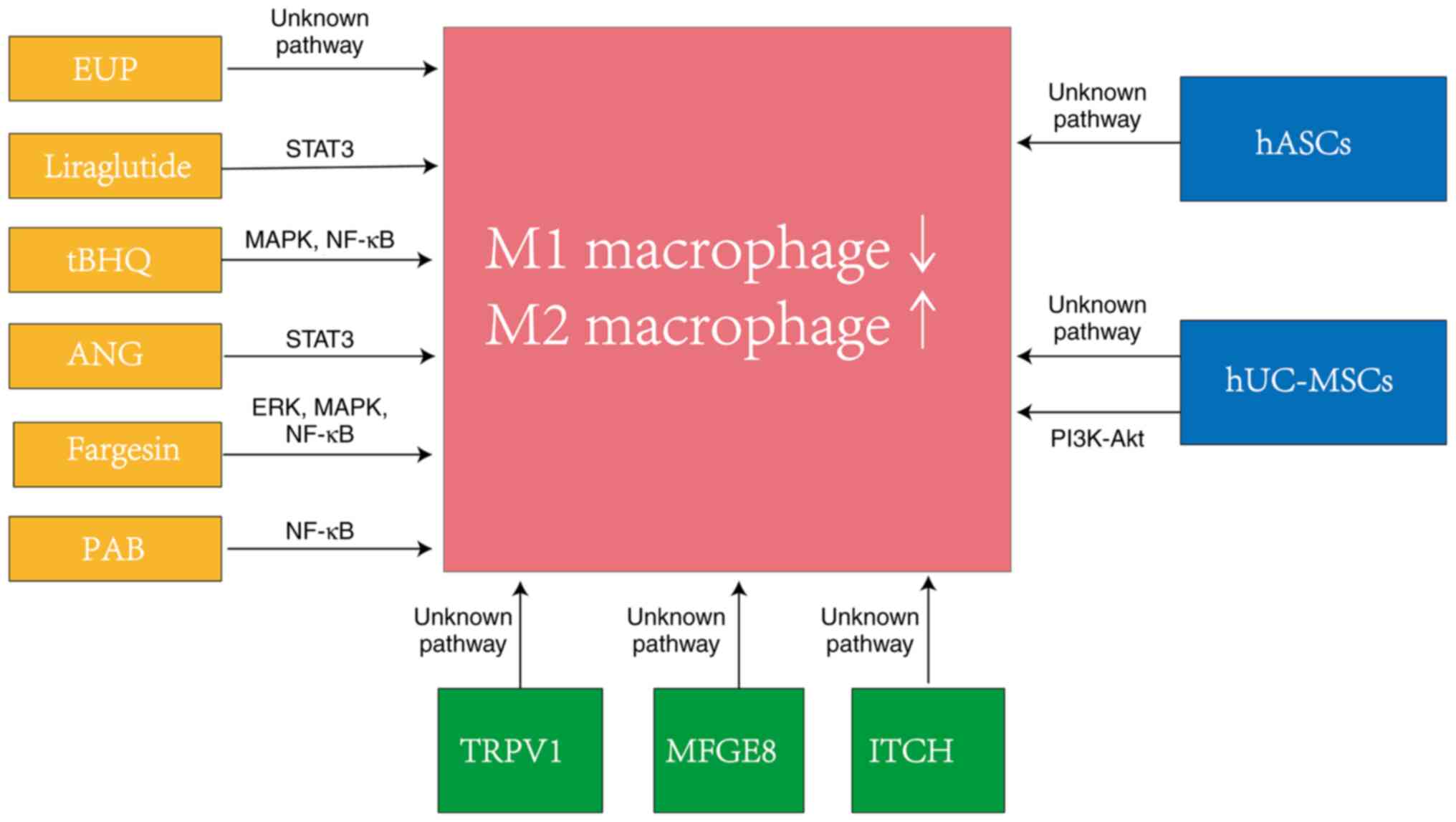
preventing the progression of OA (Fig.
2).
Fig. 2 shows the
relevant pathways of substances involved in macrophage polarization
regulation in different studies. A previous study reported that the
pathway underlying how hUC-MSCs regulate macrophage polarization is
unknown (62), whereas another
previous study reported that hUC-MSCs act through the PI3K/Akt
pathway (64).
5. Conclusion
OA is a chronic disease that causes physical
disability. By 2030, the number of OA patients in the United States
is expected to increase to ~67 million (75). Traditional treatments include
physical therapy, anti-inflammatory and analgesic drugs and
injections of glucocorticoids or hyaluronic acid into the joints.
However, these therapies are ineffective and have side effects. For
example, non-steroidal anti-inflammatory drugs are associated with
cardiovascular risk and gastrointestinal bleeding problems
(76,77). The optimal dose and time of
intra-articular hormone injection remain controversial (6). Gene editing, including RNAi and
CRISPR/Cas9 is an emerging therapy for OA. RNAi is regulated by
small interfering RNA (siRNA) at the level of gene transcription
(78). In OA, a number of animal
studies confirm that RNAi can reduce cartilage degradation and
alleviate OA (79-81).
However, there are currently no clinical trials using siRNA for OA
treatment. CRISPR/Ccas9 has great potential in the treatment of OA.
One study summarizes the potential targets of CRISPR/Cas9 in the
treatment of OA (82). However,
CRISPR/Cas9 has ethical and effective targeting range problems.
Notably, gene editing can be easily completed using CRISPR/Cas9,
which raises concerns regarding the application of this technology
to embryos (83). Single guide RNA
(sgRNA) can only bind to a specific region near the PAM sequence on
DNA (82). The PAM sequence for
Cas9 is 5'-NGG-3', where ‘N’ can be any nucleotide base, but the
third base must be G. This results in a significant reduction in
the potential target locations for DNA editing (83).
However, these OA treatment methods can only improve
the symptoms of OA but cannot reverse its process. Immune
microenvironment serves an important role in OA (18). Osteoarthritis is characterized by
the invasion and activation of macrophages and lymphocytes.
Macrophages will polarize into M1 macrophages or M2 macrophages
following stimulation. Regulating the phenotype of macrophages is
important in the treatment of OA and may be the most promising
treatment.
The novelty of the present study is from the
perspective of immunology; it collated the latest studies of the
enriched signal pathways and target molecules of macrophage
polarization in OA, so as to provide insights into the treatment of
OA. The pathways involved are ERK, PI3K/Akt, MAPK, STAT and NF-κB
pathways, different substances including compounds, cells and
proteins can alleviate OA by regulating the balance of M1 and M2
macrophages polarization. This suggests that maintaining the
homeostasis of M1 macrophages and M2 macrophages is important for
the treatment of OA. In addition, in the in vivo models of
these studies, cartilage destruction has been improved, which again
shows that cartilage homeostasis is very important for the
development of OA.
Unfortunately, most of these studies are only in the
preclinical stage and further experiments are needed to verify the
efficacy of their clinical use. It may bring more benefits to the
future research on macrophage regulation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Project of Nantong City (grant no. JC2020013) and
Postgraduate Research and Practice Innovation Program of Jiangsu
Province (grant no. KYCX21_3107).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BSC, HXH and ZMC contributed to the study design,
participated in the review process and prepared the first draft.
YYS, CC and GFB contributed to collecting the relevant literature
and important information, generating figures and modified the
manuscript. BSC, ZMC, CSW and GHX conceived the review and approved
the final version of the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y and Jordan JM: Epidemiology of
osteoarthritis. Clin Geriatr Med. 26:355–369. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wallace IJ, Worthington S, Felson DT,
Jurmain RD, Wren KT, Maijanen H, Woods RJ and Lieberman DE: Knee
osteoarthritis has doubled in prevalence since the mid-20th
century. Proc Natl Acad Sci USA. 114:9332–9336. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Young DA, Barter MJ and Wilkinson DJ:
Recent advances in understanding the regulation of
metalloproteinases. F1000Res. 8(F1000 Faculty
Rev-195)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Glazier RH, Dalby DM, Badley EM, Hawker
GA, Bell MJ, Buchbinder R and Lineker SC: Management of common
musculoskeletal problems: A survey of Ontario primary care
physicians. CMAJ. 158:1037–1040. 1998.PubMed/NCBI
|
|
5
|
Gnylorybov AM, Ter-Vartanian SK, Golovach
IY, Vyrva OE, Burianov OA, Yesirkepova GS, Irismetov ME,
Rizamuhamedova MZ, Vardanyan VS and Ginosyan KV: Expert opinion on
the extensive use of prescription crystalline glucosamine sulfate
in the multimodal treatment of osteoarthritis in Ukraine,
Kazakhstan, Uzbekistan, and Armenia. Clin Med Insights Arthritis
Musculoskelet Disord. 13(1179544120946743)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nowaczyk A, Szwedowski D, Dallo I and
Nowaczyk J: Overview of first-line and second-line
pharmacotherapies for osteoarthritis with special focus on
intra-articular treatment. Int J Mol Sci. 23(1566)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Le Graverand-Gastineau MP: Disease
modifying osteoarthritis drugs: Facing development challenges and
choosing molecular targets. Curr Drug Targets. 11:528–535.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Thomson A and Hilkens CMU: Synovial
macrophages in osteoarthritis: The key to understanding
pathogenesis? Front Immunol. 12(678757)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu X, Lee CW, Xu H, Wang YF, Yung PSH,
Jiang Y and Lee OK: Phenotypic alteration of macrophages during
osteoarthritis: A systematic review. Arthritis Res Ther.
23(110)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rao KN and Brown MA: Mast cells:
Multifaceted immune cells with diverse roles in health and disease.
Ann N Y Acad Sci. 1143:83–104. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xie J, Huang Z, Yu X, Zhou L and Pei F:
Clinical implications of macrophage dysfunction in the development
of osteoarthritis of the knee. Cytokine Growth Factor Rev.
46:36–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang H, Cai D and Bai X: Macrophages
regulate the progression of osteoarthritis. Osteoarthritis
Cartilage. 28:555–561. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Felson DT, Lawrence RC, Dieppe PA, Hirsch
R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y,
et al: Osteoarthritis: New insights. Part 1: The disease and its
risk factors. Ann Intern Med. 133:635–646. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jeffries MA: Osteoarthritis year in review
2018: Genetics and epigenetics. Osteoarthritis Cartilage.
27:371–377. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
DeFrate LE, Kim-Wang SY, Englander ZA and
McNulty AL: Osteoarthritis year in review 2018: Mechanics.
Osteoarthritis Cartilage. 27:392–400. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nunez-Carro C, Blanco-Blanco M, Montoya T,
Villagran-Andrade KM, Hermida-Gomez T, Blanco FJ and de Andres MC:
Histone extraction from human articular cartilage for the study of
epigenetic regulation in osteoarthritis. Int J Mol Sci.
23(3355)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li M, Yin H, Yan Z, Li H, Wu J, Wang Y,
Wei F, Tian G, Ning C, Li H, et al: The immune microenvironment in
cartilage injury and repair. Acta Biomater. 140:23–42.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bhat S, Tripathi A and Kumar A:
Supermacroprous chitosan-agarose-gelatin cryogels: In vitro
characterization and in vivo assessment for cartilage tissue
engineering. J R Soc Interface. 8:540–554. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sandell LJ, Morris N, Robbins JR and
Goldring MB: Alternatively spliced type II procollagen mRNAs define
distinct populations of cells during vertebral development:
Differential expression of the amino-propeptide. J Cell Biol.
114:1307–1319. 1991.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stocker W and Bode W: Structural features
of a superfamily of zinc-endopeptidases: The metzincins. Curr Opin
Struct Biol. 5:383–390. 1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chan CM, Macdonald CD, Litherland GJ,
Wilkinson DJ, Skelton A, Europe-Finner GN and Rowan AD:
Cytokine-induced MMP13 expression in human chondrocytes is
dependent on activating transcription factor 3 (ATF3) Regulation. J
Biol Chem. 292:1625–1636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Heijink A, Vanhees M, van den Ende K, van
den Bekerom MP, van Riet RP, Van Dijk CN and Eygendaal D:
Biomechanical considerations in the pathogenesis of osteoarthritis
of the elbow. Knee Surg Sports Traumatol Arthrosc. 24:2313–2318.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stannus O, Jones G, Cicuttini F,
Parameswaran V, Quinn S, Burgess J and Ding C: Circulating levels
of IL-6 and TNF-α are associated with knee radiographic
osteoarthritis and knee cartilage loss in older adults.
Osteoarthritis Cartilage. 18:1441–1447. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kuyinu EL, Narayanan G, Nair LS and
Laurencin CT: Animal models of osteoarthritis: Classification,
update, and measurement of outcomes. J Orthop Surg Res.
11(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Blaker CL, Clarke EC and Little CB: Using
mouse models to investigate the pathophysiology, treatment, and
prevention of post-traumatic osteoarthritis. J Orthop Res.
35:424–439. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Man GS and Mologhianu G: Osteoarthritis
pathogenesis-a complex process that involves the entire joint. J
Med Life. 7:37–41. 2014.PubMed/NCBI
|
|
29
|
Gordon S: Pattern recognition receptors:
Doubling up for the innate immune response. Cell. 111:927–930.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nefla M, Holzinger D, Berenbaum F and
Jacques C: The danger from within: Alarmins in arthritis. Nat Rev
Rheumatol. 12:669–683. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ito T, Kurata N and Fukunaga Y:
Tissue-Resident Macrophages in the Stria Vascularis. Front Neurol.
13(818395)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Davies LC and Taylor PR: Tissue-resident
macrophages: Then and now. Immunology. 144:541–548. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Geiß C, Salas E, Guevara-Coto J,
Regnier-Vigouroux A and Mora-Rodriguez RA: Multistability in
macrophage activation pathways and metabolic implications. Cells.
11(404)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Andersen A, Lund A, Knop FK and Vilsboll
T: Glucagon-like peptide 1 in health and disease. Nat Rev
Endocrinol. 14:390–403. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schisano B, Harte AL, Lois K, Saravanan P,
Al-Daghri N, Al-Attas O, Knudsen LB, McTernan PG, Ceriello A and
Tripathi G: GLP-1 analogue, Liraglutide protects human umbilical
vein endothelial cells against high glucose induced endoplasmic
reticulum stress. Regul Pept. 174:46–52. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mehan S, Bhalla S, Siddiqui EM, Sharma N,
Shandilya A and Khan A: Potential roles of glucagon-like peptide-1
and its analogues in dementia targeting impaired insulin secretion
and neurodegeneration. Degener Neurol Neuromuscul Dis. 12:31–59.
2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Meurot C, Martin C, Sudre L, Breton J,
Bougault C, Rattenbach R, Bismuth K, Jacques C and Berenbaum F:
Liraglutide, a glucagon-like peptide 1 receptor agonist, exerts
analgesic, anti-inflammatory and anti-degradative actions in
osteoarthritis. Sci Rep. 12(1567)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gudbergsen H, Overgaard A, Henriksen M,
Waehrens EE, Bliddal H, Christensen R, Nielsen SM, Boesen M, Knop
FK, Astrup A, et al: Liraglutide after diet-induced weight loss for
pain and weight control in knee osteoarthritis: A randomized
controlled trial. Am J Clin Nutr. 113:314–323. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li J, Bi Y, Yang H and Wang D:
Antioxidative properties and interconversion of
tert-Butylhydroquinone and tert-Butylquinone in Soybean Oils. J
Agric Food Chem. 65:10598–10603. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Song H, Xu Y, Yang X, Rong X, Wang Y and
Wei N: Tertiary butylhydroquinone alleviates gestational diabetes
mellitus in C57BL/KsJ-Lep db/+ mice by suppression of oxidative
stress. J Cell Biochem. 120:15310–15319. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang H, Li J, Xiang X, Zhou B, Zhao C,
Wei Q, Sun Y, Chen J, Lai B, Luo Z and Li A: Tert-butylhydroquinone
attenuates osteoarthritis by protecting chondrocytes and inhibiting
macrophage polarization. Bone Joint Res. 10:704–713.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wu SJ, Ng LT and Lin CC: Antioxidant
activities of some common ingredients of traditional chinese
medicine, Angelica sinensis, Lycium barbarum and Poria cocos.
Phytother Res. 18:1008–1012. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Qin J, Liu YS, Liu J, Li J, Tan Y, Li XJ,
Magdalou J, Mei QB, Wang H and Chen LB: Effect of angelica sinensis
polysaccharides on osteoarthritis in vivo and in vitro: A possible
mechanism to promote proteoglycans synthesis. Evid Based Complement
Alternat Med. 2013(794761)2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lampronti I, Manzione MG, Sacchetti G,
Ferrari D, Spisani S, Bezzerri V, Finotti A, Borgatti M, Dechecchi
MC, Miolo G, et al: Differential effects of angelicin analogues on
NF-κB Activity and IL-8 gene expression in cystic fibrosis IB3-1
cells. Mediators Inflamm. 2017(2389487)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tian Z, Zeng F, Zhao C and Dong S:
Angelicin alleviates post-trauma osteoarthritis progression by
regulating macrophage polarization via STAT3 signaling pathway.
Front Pharmacol. 12(669213)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hong PTL, Kim HJ, Kim WK and Nam JH: Flos
magnoliae constituent fargesin has an anti-allergic effect via
ORAI1 channel inhibition. Korean J Physiol Pharmacol. 25:251–258.
2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lee GE, Lee CJ, An HJ, Kang HC, Lee HS,
Lee JY, Oh SR, Cho SJ, Kim DJ and Cho YY: Fargesin Inhibits
EGF-Induced cell transformation and colon cancer cell growth by
suppression of CDK2/Cyclin E signaling pathway. Int J Mol Sci.
22(2073)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lu J and Zhang H, Pan J, Hu Z, Liu L, Liu
Y, Yu X, Bai X, Cai D and Zhang H: Fargesin ameliorates
osteoarthritis via macrophage reprogramming by downregulating MAPK
and NF-κB pathways. Arthritis Res Ther. 23(142)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Guan D, Li C, Lv X and Yang Y: Pseudolaric
acid B inhibits PAX2 expression through Wnt signaling and induces
BAX expression, therefore promoting apoptosis in HeLa cervical
cancer cells. J Gynecol Oncol. 30(e77)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li MH, Miao ZH, Tan WF, Yue JM, Zhang C,
Lin LP, Zhang XW and Ding J: Pseudolaric acid B inhibits
angiogenesis and reduces hypoxia-inducible factor 1alpha by
promoting proteasome-mediated degradation. Clin Cancer Res.
10:8266–8274. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lu J, Guan H, Wu D, Hu Z, Zhang H, Jiang
H, Yu J, Zeng K, Li H, Zhang H, et al: Pseudolaric acid B
ameliorates synovial inflammation and vessel formation by
stabilizing PPARγ to inhibit NF-κB signalling pathway. J Cell Mol
Med. 25:6664–6678. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
He X, Wang J, Li M, Hao D, Yang Y, Zhang
C, He R and Tao R: Eucommia ulmoides Oliv: Ethnopharmacology,
phytochemistry and pharmacology of an important traditional Chinese
medicine. J Ethnopharmacol. 151:78–92. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hong YK, Liu WJ, Li T and She SY:
Optimization of extraction of Eucommia ulmoides polysaccharides by
response surface methodology. Carbohydr Polym. 92:1761–1766.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Deng Y, Ma F, Ruiz-Ortega LI, Peng Y, Tian
Y, He W and Tang B: Fabrication of strontium Eucommia ulmoides
polysaccharides and in vitro evaluation of their
osteoimmunomodulatory property. Int J Biol Macromol. 140:727–735.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gao W, Feng Z, Zhang S, Wu B, Geng X, Fan
G, Duan Y, Li K, Liu K and Peng C: Anti-Inflammatory and
Antioxidant Effect of Eucommia ulmoides Polysaccharide in Hepatic
Ischemia-Reperfusion Injury by Regulating ROS and the TLR-4-NF-κB
Pathway. Biomed Res Int. 2020(1860637)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sun Y, Huang K, Mo L, Ahmad A, Wang D,
Rong Z, Peng H, Cai H and Liu G: Eucommia ulmoides polysaccharides
attenuate rabbit osteoarthritis by regulating the function of
macrophages. Front Pharmacol. 12(730557)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hu CX, Hu KY and Wang JF: Potential role
of the compound Eucommia bone tonic granules in patients with
osteoarthritis and osteonecrosis: A retrospective study. World J
Clin Cases. 8:46–53. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Mazini L, Rochette L, Admou B, Amal S and
Malka G: Hopes and limits of adipose-derived stem cells (ADSCs) and
mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci.
21(1306)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cosenza S, Ruiz M, Toupet K, Jorgensen C
and Noel D: Mesenchymal stem cells derived exosomes and
microparticles protect cartilage and bone from degradation in
osteoarthritis. Sci Rep. 7(16214)2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Furuta T, Miyaki S, Ishitobi H, Ogura T,
Kato Y, Kamei N, Miyado K, Higashi Y and Ochi M: Mesenchymal stem
cell-derived exosomes promote fracture Healing in a mouse model.
Stem Cells Transl Med. 5:1620–1630. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS,
Yun YE, Han J, Lee J, Kim WS, Choi JS, et al: Small extracellular
vesicles from human adipose-derived stem cells attenuate cartilage
degeneration. J Extracell Vesicles. 9(1735249)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chen CF, Hu CC, Wu CT, Wu HH, Chang CS,
Hung YP, Tsai CC and Chang Y: Treatment of knee osteoarthritis with
intra-articular injection of allogeneic adipose-derived stem cells
(ADSCs) ELIXCYTE®: a phase I/II, randomized,
active-control, single-blind, multiple-center clinical trial. Stem
Cell Res Ther. 12(562)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Tang S, Chen P, Zhang H, Weng H, Fang Z,
Chen C, Peng G, Gao H, Hu K, Chen J, et al: Comparison of curative
effect of human umbilical cord-derived mesenchymal stem cells and
their small extracellular vesicles in treating osteoarthritis. Int
J Nanomedicine. 16:8185–8202. 2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Li K, Yan G, Huang H, Zheng M, Ma K, Cui
X, Lu D, Zheng L, Zhu B, Cheng J and Zhao J: Anti-inflammatory and
immunomodulatory effects of the extracellular vesicles derived from
human umbilical cord mesenchymal stem cells on osteoarthritis via
M2 macrophages. J Nanobiotechnology. 20(38)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mickle AD, Shepherd AJ and Mohapatra DP:
Sensory TRP channels: The key transducers of nociception and pain.
Prog Mol Biol Transl Sci. 131:73–118. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zhang X, Ye L, Huang Y, Ding X and Wang L:
The potential role of TRPV1 in pulmonary hypertension: Angel or
demon? Channels (Austin). 13:235–246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lv Z, Xu X, Sun Z, Yang YX, Guo H, Li J,
Sun K, Wu R, Xu J, Jiang Q, et al: TRPV1 alleviates osteoarthritis
by inhibiting M1 macrophage polarization via
Ca2+/CaMKII/Nrf2 signaling pathway. Cell Death Dis.
12(504)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mayorga AJ, Flores CM, Trudeau JJ, Moyer
JA, Shalayda K, Dale M, Frustaci ME, Katz N, Manitpisitkul P,
Treister R, et al: A randomized study to evaluate the analgesic
efficacy of a single dose of the TRPV1 antagonist mavatrep in
patients with osteoarthritis. Scand J Pain. 17:134–143.
2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yi YS: Functional Role of Milk fat
globule-epidermal growth factor VIII in macrophage-mediated
inflammatory responses and inflammatory/autoimmune diseases.
Mediators Inflamm. 2016(5628486)2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Uchiyama A, Motegi SI, Sekiguchi A,
Fujiwara C, Perera B, Ogino S, Yokoyama Y and Ishikawa O:
Mesenchymal stem cells-derived MFG-E8 accelerates diabetic
cutaneous wound healing. J Dermatol Sci. 86:187–197.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lu Y, Liu L, Pan J, Luo B, Zeng H, Shao Y,
Zhang H, Guan H, Guo D, Zeng C, et al: MFG-E8 regulated by
miR-99b-5p protects against osteoarthritis by targeting chondrocyte
senescence and macrophage reprogramming via the NF-κB pathway. Cell
Death Dis. 12(533)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Han S, Zhang Y, Guo C and Chang C: The E3
protein ubiquitin ligase Itch is a potential target in myeloid
malignancies with marrow fibrosis. Transl Cancer Res. 10:2368–2378.
2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lin X, Wang W, McDavid A, Xu H, Boyce BF
and Xing L: The E3 ubiquitin ligase Itch limits the progression of
post-traumatic osteoarthritis in mice by inhibiting macrophage
polarization. Osteoarthritis Cartilage. 29:1225–1236.
2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Hootman JM and Helmick CG: Projections of
US prevalence of arthritis and associated activity limitations.
Arthritis Rheum. 54:226–229. 2006.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cutolo M, Berenbaum F, Hochberg M, Punzi L
and Reginster JY: Commentary on recent therapeutic guidelines for
osteoarthritis. Semin Arthritis Rheum. 44:611–617. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhang W, Nuki G, Moskowitz RW, Abramson S,
Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty
M, et al: OARSI recommendations for the management of hip and knee
osteoarthritis: Part III: Changes in evidence following systematic
cumulative update of research published through January 2009.
Osteoarthritis Cartilage. 18:476–499. 2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Rai MF, Pan H, Yan H, Sandell LJ, Pham CTN
and Wickline SA: Applications of RNA interference in the treatment
of arthritis. Transl Res. 214:1–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ye C, Bhan AK, Deshpande V, Shankar P and
Manjunath N: Silencing TNF-α in macrophages and dendritic cells for
arthritis treatment. Scand J Rheumatol. 42:266–269. 2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Shi Q, Rondon-Cavanzo EP, Dalla Picola IP,
Tiera MJ, Zhang X, Dai K, Benabdoune HA, Benderdour M and Fernandes
JC: In vivo therapeutic efficacy of TNFα silencing by
folate-PEG-chitosan-DEAE/siRNA nanoparticles in arthritic mice. Int
J Nanomedicine. 13:387–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lee SJ, Lee A, Hwang SR, Park JS, Jang J,
Huh MS, Jo DG, Yoon SY, Byun Y, Kim SH, et al: TNF-α gene silencing
using polymerized siRNA/thiolated glycol chitosan nanoparticles for
rheumatoid arthritis. Mol Ther. 22:397–408. 2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Tanikella AS, Hardy MJ, Frahs SM, Cormier
AG, Gibbons KD, Fitzpatrick CK and Oxford JT: Emerging gene-editing
modalities for osteoarthritis. Int J Mol Sci.
21(6046)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Nishimasu H, Shi X, Ishiguro S, Gao L,
Hirano S, Okazaki S, Noda T, Abudayyeh OO, Gootenberg JS, Mori H,
et al: Engineered CRISPR-Cas9 nuclease with expanded targeting
space. Science. 361:1259–1262. 2018.PubMed/NCBI View Article : Google Scholar
|