1. Introduction
White fat cells or adipocytes are distributed
throughout the human body (1,2), and
damage to or necrotic changes in these cells are frequently
encountered in various diseases. In pathology, the term ‘fat
necrosis’ refers to intra-abdominal lesions composed of ghostly,
slightly basophilic, non-nucleated fat cells, with or without
calcification, associated with extravasated, activated pancreatic
juice (1,3). The intra-abdominal presence of this
fat necrosis, also called enzymic fat necrosis, suggests acute
pancreatitis (3-6).
Similar enzymic fat necrosis is occasionally observed in the
subcutaneous tissues of patients with pancreatic disorders,
indicating a possible diagnosis of pancreatic panniculitis
(7). Nonspecific necrosis of
generalized fat cells evokes an inflammatory reaction, accompanied
by epithelioid cells, multinucleated histiocytic giant cells, and
lymphocytes, a condition called fat granuloma (1,7).
Such necrotic fat cells or lipogranulomatous lesions can be
resolved during the relatively early stages of the disease.
However, another unique form of white fat necrosis, designated
lipomembranous fat necrosis (LFN) (7-15),
also called lipomembranous changes (8,15-21),
membranous fat necrosis (1,15,22-29),
membranocystic changes or fat necrosis (7,8,11,14-17,28-34),
membranous lipodystrophy-like changes (30,31),
and pseudomembranous fat necrosis (7,35),
is found in fibrotic tissues or later stages of various diseases
(9,12,14-16,18,19,21,35).
In this review, we describe clinicopathological features of this
interesting, but poorly characterized condition.
2. Morphological characteristics of LFN
LFN is microscopically characterized by eosinophilic
or hyaline, convoluted, crenulated, scalloped, and/or serpiginous
membrane formations on hematoxylin and eosin (H&E)-stained
sections (Fig. 1A) (7,8,11,15,17,19,22-28,30,31,35).
Some authors have designated LFN as membranes with an ‘arabesque’
(7-9,11,16,33)
or ‘frost on a windowpane’ (34)
appearance. LFN is scattered singly or in some clusters within
fatty and/or fibrous tissues and shows microcystic, macrocystic,
and/or crushed features (7-12,14-16,19,21-26,28).
Scattered LFN within fatty tissues represents early changes of LFN,
and LFN within fibrous tissues may represent chronic phase lesions
(9,12,14-16,18,19,21,35).
Microcystic LFN corresponds to necrotic changes in fat cells,
whereas macrocystic LFN may be composed of cohesive microcystic
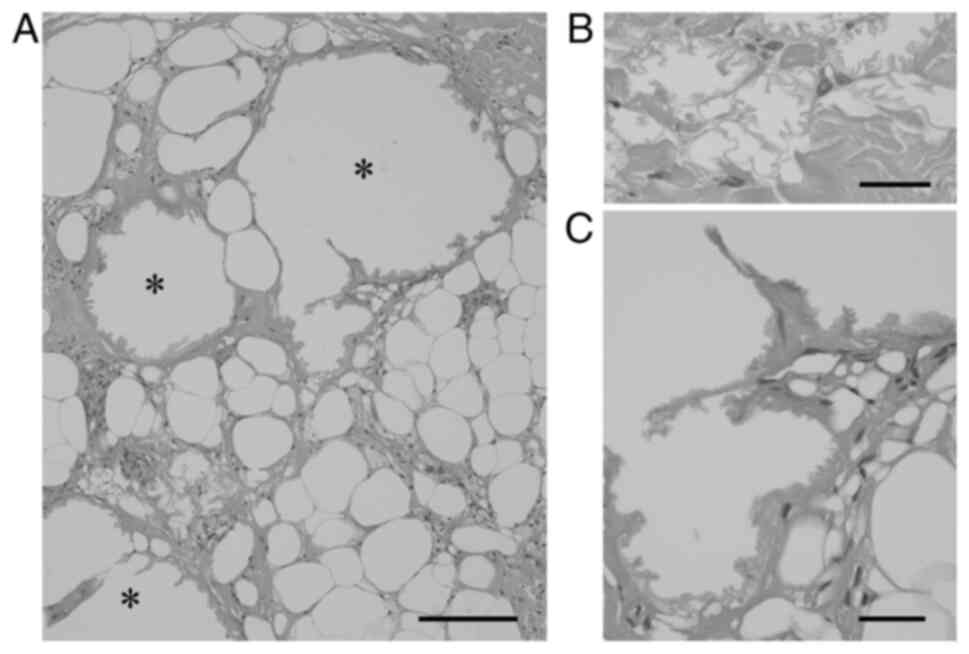
LFNs (Fig. 2A-C) (23). Macrocystic LFN can present as a
pea-sized or 2-cm cyst (8,22,23),
mimicking an epidermal cyst (22).
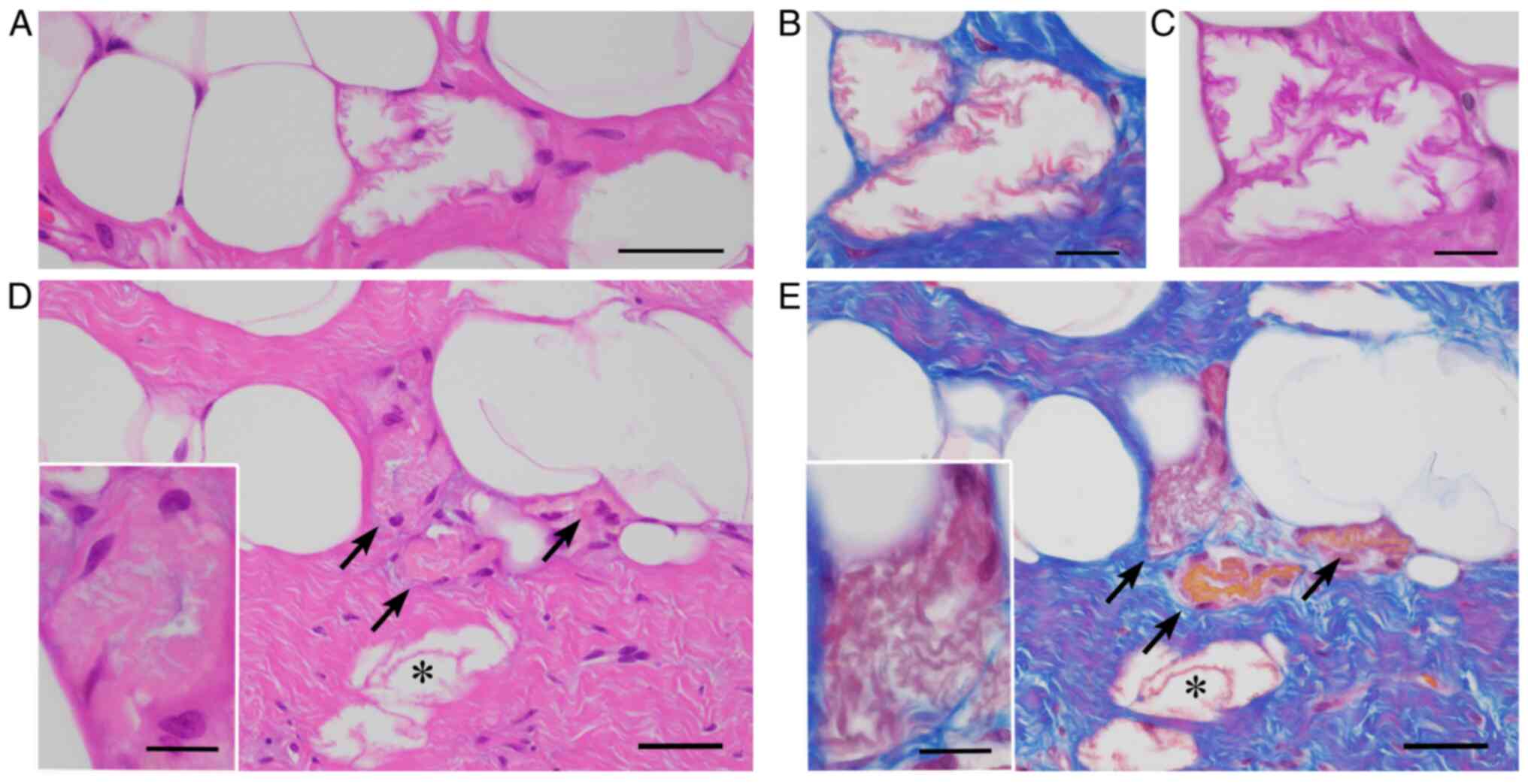 | Figure 1LFN. (A-C) Microcystic LFNs are
characterized by (A) eosinophilic, crenulated membranes on H&E
staining, stained (B) red by Masson trichrome stain, and (C)
enhanced by the periodic acid-Schiff reaction (A, bar=50 µm; B and
C, bar=10 µm). (D and E) Crushed LFNs (D, arrows) within fibrous
tissues adjacent to microcystic LFN (D, asterisk) on
H&E-stained sections are challenging to detect, but Masson
trichrome stain highlights LFNs in red (E, arrows and an asterisk)
on a bluish fibrous background (D and E, bar=50 µm). Insets in (D
and E) represent high-power views of crushed LFN (bar=10 µm). LFN,
lipomembranous fat necrosis; H&E, hematoxylin and eosin. |
LFN is stained red with Azan-Mallory or Masson
trichrome stain (Fig. 1B)
(11,28,30,31).
LFN is also highlighted by periodic acid-Schiff staining with or
without diastase digestion (Fig.
1C) (7-12,16,18,22-26,28-30,35),
Sudan black B staining (10,11,16,22,24-27,30,31),
oil red O staining (23), orcein
staining (10), long Ziehl-Neelsen
staining (16,23-27),
silver impregnation (30,31), phosphotungstic acid-hematoxylin
staining (11,16), and luxol fast blue staining
(11,16,22,23,27,30,31).
LFN occasionally shows noncystic, crushed features embedded within
fibrous tissues and may be difficult to recognize (Fig. 1D). Accordingly, LFN can be
discriminated from fibrosis using additional staining methods
(Fig. 1E). A recent report
(29) showed that LFN stains
maroon to purple when exposed to Russell-Movat pentachrome stain.
LFN is negative with alcian blue (11,22,24,30,31),
elastic stain (Weigert-van Gieson, elastica van Gieson stain, and
Verhoeff elastic stain) (22,28-31,35),
methenamine silver (11), and
Prussian blue (11,22). Immunohistochemically, LFN is
positive for CD68 (11,35) and lysozyme (11,35)
and negative for S-100 protein (28), CD34 (11,35),
muscle specific antigen (11,35),
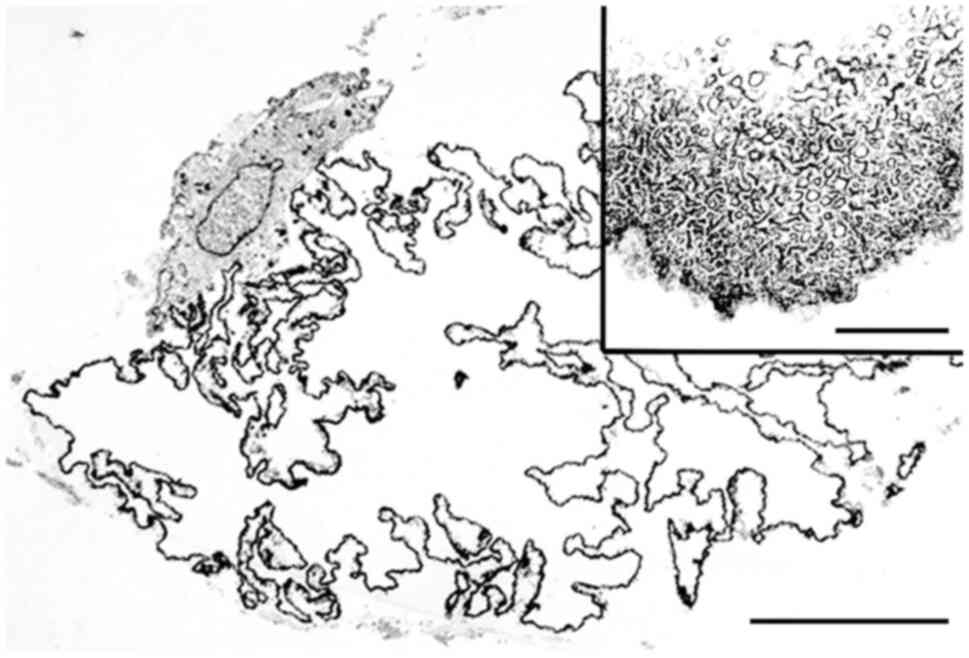
and factor XIIIa (11,35). On ultrastructural examination, LFN
is composed of poorly defined minute tubule-like structures
(Fig. 3) and/or tiny vesicle-like
structures (11,22,28,30,31).
Unstained LFN shows yellow-green autofluorescence on fluorescent
microscopy (10,11,23-27).
Older LFN sometimes exhibits weak red staining with Masson
trichrome stain, consistent with previous observations
demonstrating that LFN is weakly fuchsinophilic (29), or may show negative results with
Masson's trichrome stain (11,16).
In addition, older LFN may be positive for van Gieson elastic stain
(35) and may lack or exhibit weak
expression of CD68 and lysozyme (35). Older LFN can also show Kossa
stain-positive calcification (18,33,35).
3. Historical recognition of LFN
Approximately 60 years ago, the ‘arabesque’ or
‘membranocystose-like’ features of LFN were recognized in biopsy
specimens from osseous cystic lesions in a 28-year-old Japanese
male (36), as reported by
Terayama (37) at a Japanese
orthopedic meeting in 1961. From the detailed postmortem findings
of this case, Nasu et al (38) proposed the term ‘lipomembranous
dystrophy’ in 1973. Järvi et al (39) independently reported two cases
showing similar lipomembranous features as ‘membranous reticulin
dysplasia of bones’ in 1964. Hakola et al (40) and Hakola and Partanen (41) summarized cases of Finnish families
showing both progressive dementia and lipomembranous polycystic
osteodysplasia in 1970. Based on these historical aspects, this
autosomal recessive disorder has been designated ‘Nasu-Hakola
disease’, ‘Nasu-Hakola syndrome’, or ‘Järvi-Hakola-Nasu disease’
(9,11,28,35,42,43)
and is now known to be caused by loss-of-function variants in
TYROBP/DAP12 or TREM2 (44-46).
LFN was initially considered a specific morphology
of Nasu-Hakola disease (35,38,39).
However, subcutaneous LFN has occasionally been discovered in
patients without this hereditary disease (8-33,35,36,47,48).
Machinami (31) reported
subcutaneous LFN within necrotic legs caused by impaired arterial
blood supply, such as thromboangiitis obliterans, arteriosclerotic
obliterans, and progressive systemic sclerosis (LFN incidence
rates: 38, 75, and 50%, respectively). Poppiti et al
(22) observed LFN in the thoracic
subcutaneous tissues of a 66-year-old man without Nasu-Hakola
disease or other underlying diseases and identified LFN in 7 (21%)
of 33 consecutive cases of subcutaneous fat necrosis. Furthermore,
Coyne et al (23) found LFN
in 11 (44%) of 25 irradiated breast tissues and in 13 (31%) of 42
nonirradiated necrotic fat tissues of the breast. Therefore, LFN is
not rare and not specific to Nasu-Hakola disease. The most recent
version of the dermatopathology textbook Lever's Histopathology of
the Skin, 11th Edition (7) has
designated LFN as a distinct type of adipocyte necrosis in
panniculitis, although the previous versions (49,50)
had described LFN as a condition that was relatively specific to
lipodermatosclerosis.
4. LFN in various locations and
diseases
Non-neoplastic subcutaneous lesions
and breast tissues
Subcutaneous LFN has been reported in venous
insufficiency diseases (including hypodermatitis sclerodermiformis,
stasis dermatitis, deep venous thrombosis, thrombophlebitis,
varicose veins, and lipodermatosclerosis) (11,14-16,19,30-32,34,35,48),
erythema nodosum (9,11,14,35),
erythema induratum (11,35), traumatic panniculitis (11,35),
pancreatic panniculitis (11,35),
necrobiosis lipoidica (9,11,35),
nodular cystic fat necrosis (8,18),
sclerosing lipogranuloma (35),
morphea or scleroderma (9,11,18,35),
lupus panniculitis or discoid lupus erythematosus (9,11,17,20,21,33,35),
Behçet disease (11), Sjögren
syndrome (18), mixed connective
tissue disease (12),
polyarteritis nodosa or vasculitis (9,11,35),
lichen amyloidosis (47),
erysipelas (9), atypical
mycobacteria or miliary tuberculosis (11,13,35),
diabetes mellitus (11,16,35),
and subcutaneous sarcoidosis (35).
Abdominal lesions
Ramdial and Singh (25) reported that microcytic LFN was
found in 10 cases of appendix epiploica. Appendix epiploica is
characterized by calcified fibrous nodules protruding from the
colonic serosal surface or isolated as free bodies in the abdominal
cavity (25,51). Ramdial and Bagratee (26) found LFN in 9 (4%) of 217 ovarian
mature cystic teratomas. Nistal et al (10) identified LFN in 3 torn testes
accompanied by thrombosed veins.
Intra-articular loose bodies
Intra-articular loose bodies are caused by
osteochondral fracture, joint surface integration, torn meniscus,
fibrinous synovitis, and primary synovial chondromatosis (43,52-55).
LFN is found in necrotic bone marrow within intra-articular loose
bodies related to osteochondritis dissecans (43). Matsukuma et al (56) reported LFN in 7 (13%) of 55
intra-articular loose bodies; 4 were found in necrotic bone marrow
derived from osteochondral fracture, and the other 3 were
associated with viable fat cells without bone marrow
structures.
Cardiac valves and LFN
White fat cells can be found in cardiac valves,
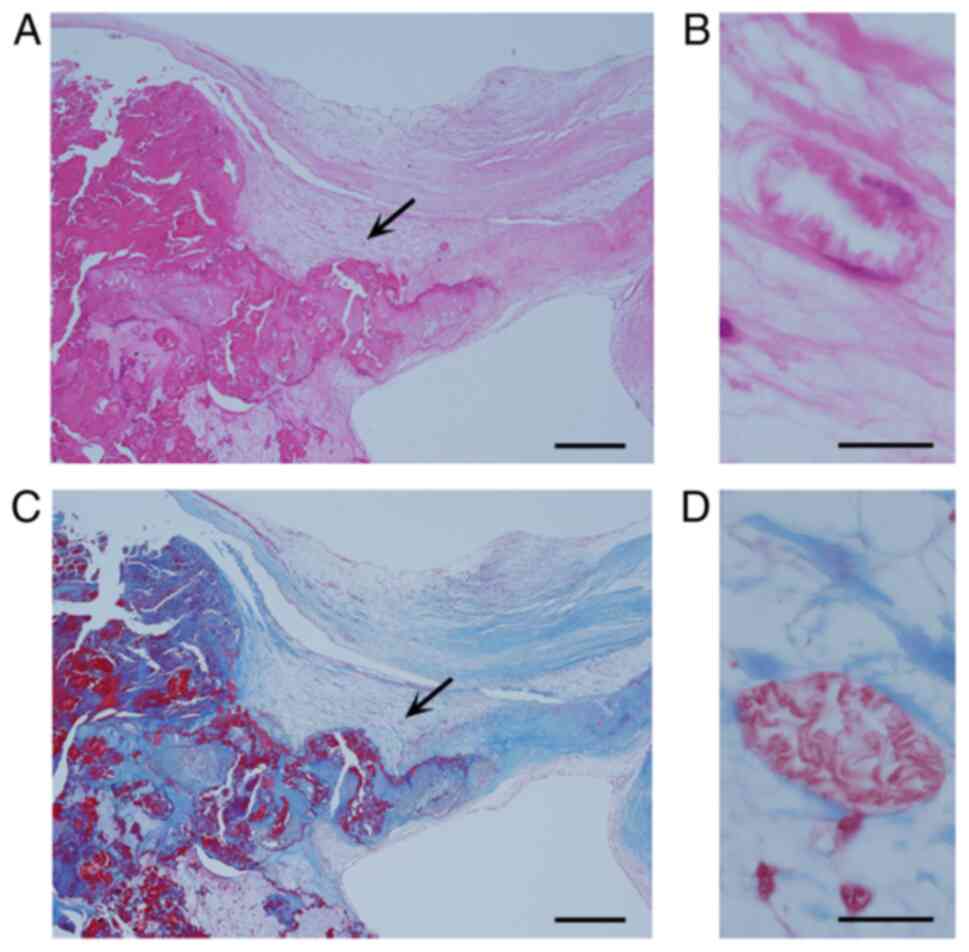
possibly representing fatty metaplasia (1). Matsukuma et al (28) reported concomitant age-dependent
fatty metaplasia of the aortic valves and LFN in 52 (63%) of 82
nondysfunctional aortic valves and in 58 (83%) of 70 dysfunctional
aortic valves (Fig. 4A-D). Sekulic
SP and Sekulic M (29) found LFN
with viable fat cells in 129 (18%) of 719 aortic valves, in 26 (9%)
of 284 mitral valves, but did not find LFN in 24 tricuspid valves
or 15 pulmonary valves.
LFN in other tumorous lesions
LFN was also reported in subcutaneous tissues of
patients with panniculitis-like T-cell lymphoma (11) and in 4 relatively large lipomas,
ranging in size from 9 to 22 cm (24).
5. Pathogenesis of LFN
LFN is relatively devoid of active inflammatory
reaction except for lipogranuloma or histiocytic reaction (8,9,11,16,22,24,33).
Some studies (32,57) have shown that patients with
subcutaneous LFN-related lesions recover after venous insufficiency
treatment. Subcutaneous LFN can also be caused by ischemia or
venous insufficiency, regardless of the underlying disease
(8,9,11,16,17,21,30-33).
Subcutaneous fatty tissues are highly vascularized (3) and would be resistant to ischemia,
thereby contributing to the uncommonness of subcutaneous LFN.
Appendix epiploica, torn testis, and twisted ovarian teratoma are
ischemic disorders, and LFN may be present in these lesions
(10,25,26).
Ramdial and Bagratee (26)
reported that LFN was found in only one (1.8%) of 56 mature cystic
teratomas removed from patients having a history, symptoms and/or
signs of teratoma torsion. In addition, they identified LFN in
another 8 (5%) of 161 teratomas removed from patients without a
history of teratoma torsion (26).
Thus, subclinical minor torsion of ovarian teratoma occurs in
approximately 5% of patients with ovarian teratoma. Coyne et
al (23) suggested that LFN
may be caused by a combination of factors, including prior surgery
and ischemia due to radiation-related vascular changes. Lipomas are
also well vascularized (3,58), but larger lipomas may also be
associated with ischemia or trauma (3,24).
Hence, the occurrence of LFN in larger lipomas is considered a
reasonable event (24).
By contrast, intra-articular loose bodies are in an
environment that is different from that of subcutaneous lesions.
Articular hyaline cartilage is exposed directly to joint fluid,
whereas bone and bone marrow fatty tissues are nourished by a
vessel-dependent blood supply (54,55).
Therefore, intra-articular loose bodies derived from articular
hyaline cartilage remain alive and still grow. However, detached
bone and bone marrow cannot survive and therefore may exhibit LFN
through ischemic necrosis of bone marrow fat cells (43,56).
Viable fat cells, which do not contain bone marrow structures,
within intra-articular loose bodies are considered uncommon fatty
metaplasia of detached cartilaginous cells (56). In a study by Matsukuma et al
(56), LFN was observed in 3 (43%)
of 7 loose bodies showing fatty metaplasia. Thus, intra-articular
loose bodies may be encountered under hypoxic or malnourished
conditions resembling ischemia (56). Furthermore, aortic valves are
similarly avascular (59,60) and receive nutritious permeation
directly from the blood flow. The presence of LFN in aortic valves
indicates a morbid condition that disrupts the circulation and
distribution of nutrients (28).
The close relationship between the occurrence of LFN and fibrously
thickened aortic valves supports that the impairment of nutrient
permeation may be related to valvular fibrous thickening (28). Sekulic SP and Sekulic M (29) showed that the higher incidence of
LFN in aortic valves than in mitral and tricuspid valves may be
related to differences in the rheological forces present in these
valves.
LFN is occasionally found in fatty tissues without
characteristic clinical and histological features of ischemia. Akay
et al (61) reported the
presence of LFN within abdominal and femoral subcutaneous tissues
without vascular changes in a patient with acute leukemia; they
concluded that this case represented chemotherapy-induced LFN.
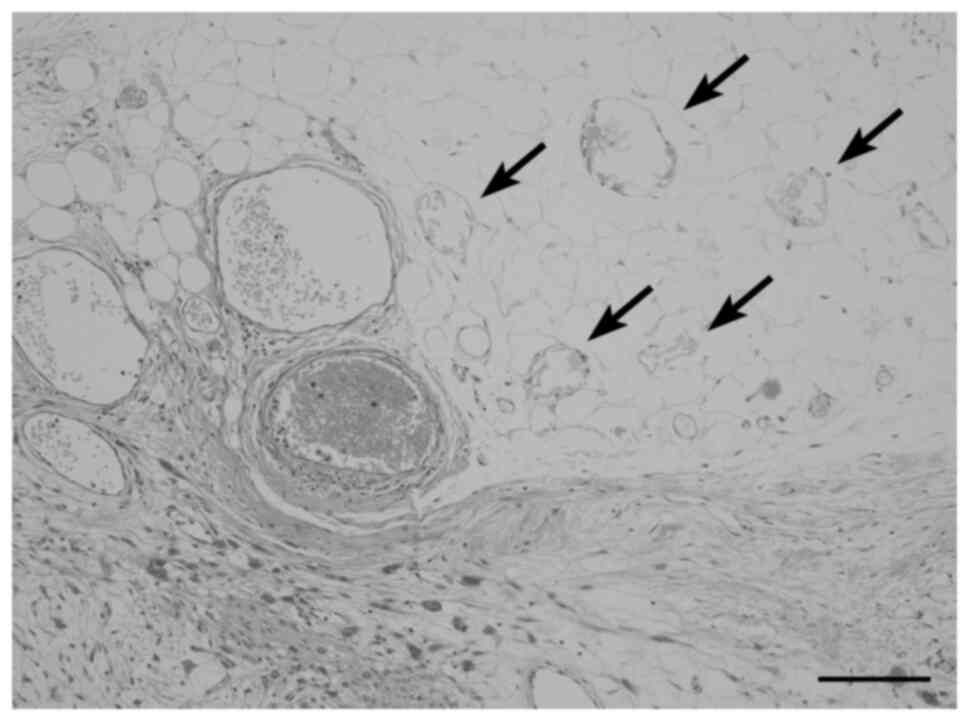
Fig. 5 shows scattered LFNs within
anconal subcutaneous fatty tissues adjacent to invading high-grade
sarcoma in a patient receiving no chemoradiotherapy. Small arteries
and veins in fatty tissues containing LFN are open and
well-preserved, but small fat granulomas are also multifocally
observed. We speculate that the presence of both LFN and fat
granulomas in this case may be related to the presence of hypoxic
or malnourished conditions.
Based on analysis of histochemical staining, LFN is
mainly composed of ceroids (22-24,26).
Some investigators have proposed several possible factors occurring
after ischemic/hypoxic injury that may contribute to the formation
of peculiar ceroid membranes, including anti-oxidants, reactive
oxygen intermediates, released cellular enzymes, and
lipoperoxidation (11,26). However, the specific mechanisms
causing LFN remain poorly understood.
6. Clinical and translational significance
of pathologically detected LFN
Fat necrosis is histologically divided into
lipogranuloma type fat necrosis, coagulation-like necrosis type fat
necrosis, enzymic fat necrosis, and LFN (1,3,7,8-35,47-49,56,57,61).
Table I summarizes these
clinicopathological features. Some types of fat necrosis are
occasionally intermingled and may not be specific to a disease or
condition. As described above, however, LFN is closely associated
with ischemia, hypoxia, or malnourishment. Therefore, when the
histopathological examination detects the presence of LFN in
inflamed or necrotic specimens of unknown etiology, clinicians
should rule out a possible circulatory disturbance. If a distinct
ischemic condition is not present clinically, a local hypoxic or
malnourishment-related condition can be considered. In addition,
clinicians should check the patient's history of radiation and
chemotherapy because previous reports have shown that LFN may be
possibly related to these modalities (23,61).
 | Table IClinicopathological features of
several types of fat necroses. |
Table I
Clinicopathological features of
several types of fat necroses.
| Type of fat
necrosis | Lipogranuloma type
fat necrosis | Coagulation-like
necrosis type fat necrosis | Enzymic fat
necrosis | Lipomembranous fat
necrosis |
|---|
| Favored
locations | Generalized fatty
tissues | Generalized fatty
tissues | Distal lower
extremities, buttock, abdomen, arm, elbow, scalp | Possible
generalized fatty tissues; breast, lower legs, cardiac valves,
abdominal cavities, testes, ovaries, intra-articular loose
bodies |
| Histological
features | Epithelioid and/or
foamy histiocytes, giant cells, with or without scattered lipid
vacuoles | Aggregated fat
cells losing nuclei, usually without inflammation | Basophilic or
eosinophilic liquefaction of fat cells with neutrophilia | Eosinophilic or
hyaline crenulated, arabesque-like membrane formation; crushed,
microcystic, and/or macrocystic |
| Pathogenesis | Nonspecific fat
cell damages due to various diseases/conditions | Due to
ischemia | Due to action of
pancreatic lipolytic enzymes | Due to ischemia,
hypoxia, or malnourishment-related conditions |
| Associated
lesions | Various
diseases/conditions, including inflammation, trauma, ischemia, and
lipoma | Erythema induratum,
calciphylaxis, appendix epiploicae, lipoma, other infarcted fatty
lesions | Acute
pancreatitis | Various
diseases/conditions causing local ischemia, hypoxia, or
malnourishment (including soft tissue tumors) |
7. Conclusions
LFN is characterized by a unique histopathology and
is detectable on routine H&E staining, although its occurrence
may be uncommon. We believe that LFN could be a hallmark of
unexpected, hidden ischemic or ischemia-like hypoxic/malnourished
conditions in various diseases. The exact pathogenesis of LFN,
however, remains unknown. In addition, other possible etiologies of
LFN, such as chemotherapy and radiotherapy, have not yet been
evaluated. Further large-scale studies are needed to assess these
factors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SM reviewed previous articles and drafted the
manuscript. AM collected and reviewed almost all of the reference
articles. OT and SO commented on the manuscript, and SO edited the
manuscript. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brooks JSJ: Adipose tissue. In: Histology
of Pathologists. Mills SE (ed). 5th edition. Wolters Kluwer,
Philadelphia, PA, pp133-165, 2020.
|
|
2
|
Goldblum JR, Folpe AL and Weiss SW (eds):
Enzinger & Weiss's soft tissue tumors. 7th edition. Elsevier
Inc., Philadelphia PA, pp225-231, 476-518, 2020.
|
|
3
|
Oakes SA: Cell injury, cell death, and
adaptations. In: Robbins and cotran pathologic basis of diseases.
Kumar V, Abbas AK and Aster JC (eds). 10th edition. Elsevier Inc.,
Philadelphia PA, pp33-69, 2021.
|
|
4
|
Klöppel G, von Gerkan R and Dreyer T:
Pathomorphology of acute pancreatitis-analysis of 357 autopsy cases
and 3 surgical specimens. In: Pancreatitis-concepts and
classification. Gyr KE, Singer MV and Sarles H (eds). Excerpta
Medica, Amsterdam, pp29-35, 1984.
|
|
5
|
Schmits-Moormann P: Comparative
radiological and morphological study of the human pancreas. IV.
Acute necrotizing pancreatitis in man. Pathol Res Pract.
171:325–335. 1981.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Maitra A: The pancreas. In: Robbins and
cotran pathologic basis of diseases. 10th edition. Kumar V, Abbas
AK and Aster JC (eds). Elsevier, Philadelphia, PA, pp881-894,
2021.
|
|
7
|
Fung MA and Requena L: Inflammatory
diseases of the subcutaneous fat. In: Lever's histopathology of the
skin. Elder DE, Elenitsas R, Rosenbach M, Murphy GF, Rubin AI and
Xu X (eds). 11th edition. Wolters Kluwer, Philadelphia, PA,
pp610-657, 2015.
|
|
8
|
Pujol RM, Wang CY, Gibson LE and Su WP:
Lipomembranous changes in nodular-cystic fat necrosis. J Cutan
Pathol. 22:551–555. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Snow JL and Su WP: Lipomembranous
(membranocystic) fat necrosis. Clinicopathologic correlation of 38
cases. Am J Dermatopathol. 18:151–155. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nistal M, González-Peramato P and Paniagua
R: Lipomembranous fat necrosis in three cases of testicular
torsion. Histopathology. 38:443–447. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Segura S and Pujol RM: Lipomembranous fat
necrosis of the subcutaneous tissue. Dermatol Clin. 26:509–517,
viii. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Halvorson CR, Kwon SY, Kao GF and Germanas
JP: Lipomembranous fat necrosis in a patient with mixed connective
tissue disease. J Am Acad Dermatol. 64:1010–1011. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yeh LJ, Shively NR, Isacke RN, Dowling CA
and Stogsdill PB: Miliary tuberculosis characterised by
lipomembranous fat necrosis. Lancet Infect Dis.
15(1497)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang TM and Lee JYY:
Lipodermatosclerosis: A clinicopathologic study of 17 cases and
differential diagnosis from erythema nodosum. J Cutan Pathol.
36:453–460. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Choonhakarn C, Chaowattanapanit S and
Julanon N: Lipodermatosclerosis: A clinicopathologic correlation.
Int J Dermatol. 55:303–308. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alegre VA, Winkelmann RK and Aliaga A:
Lipomembranous changes in chronic panniculitis. J Am Acad Dermatol.
19:39–46. 1988.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamamoto T, Furuhata Y and Tsuboi R:
Lipomembranous changes and calcification associated with systemic
lupus erythematosus. Clin Exp Dermatol. 32:278–280. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Toritsugi M, Yamamoto T and Nishioka K:
Nodular cystic fat necrosis with systemic sclerosis. Eur J
Dermatol. 14:353–355. 2004.PubMed/NCBI
|
|
19
|
Walsh SN and Santa Cruz DJ:
Lipodermatosclerosis: A clinicopathological study of 25 cases. J Am
Acad Dermatol. 62:1005–1012. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khoury T, Arayssi T, Kibbi AG and Ghosn S:
Extensive fat necrosis with lipomembranous changes and
calcification in lupus erythematosus panniculitis is not
necessarily associated with systemic lupus erythematosus. Am J
Dermatopathol. 32:742–743. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim JS, Kim HY, Kim YG, Paek JO and Yu HJ:
Lipomembranous changes associated with systemic lupus
erythematosus. Clin Exp Dermatol. 39:319–322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Poppiti RJ Jr, Margulies M, Cabello B and
Rywlin AM: Membranous fat necrosis. Am J Surg Pathol. 10:62–69.
1986.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Coyne JD, Parkinson D and Baildam AD:
Membranous fat necrosis of the breast. Histopathology. 28:61–64.
1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ramdial PK, Madaree A and Singh B:
Membranous fat necrosis in lipomas. Am J Surg Pathol. 21:841–846.
1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ramdial PK and Singh B: Membranous fat
necrosis in appendices epiploicae. A clinicopathological study.
Virchows Arch. 432:223–227. 1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ramdial PK and Bagratee JS: Membranous fat
necrosis in mature cystic teratomas of the ovary. Int J Gynecol
Pathol. 17:120–122. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ramdial PK and Chetty R:
Vasculitis-induced membranous fat necrosis. J Cutan Pathol.
26:405–410. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Matsukuma S, Takeo H, Kono T and Sato K:
Fat cells and membranous fat necrosis of aortic valves: A
clinicopathological study. Pathol Int. 63:345–352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pichler Sekulic S and Sekulic M:
Adipocytes and membranous fat necrosis within native cardiac
valves: Clinicopathologic characterization of histologic
constituents. Cardiovasc Pathol. 50(107276)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Machinami R: Membranous lipodystrophy-like
changes in ischemic necrosis of the legs. Virchows Arch A Pathol
Anat Histopathol. 399:191–205. 1983.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Machinami R: Incidence of membranous
lipodystrophy-like change among patients with limb necrosis caused
by chronic arterial obstruction. Arch Pathol Lab Med. 108:823–826.
1984.PubMed/NCBI
|
|
32
|
Demitsu T, Okada O, Yoneda K and Manabe M:
Lipodermatosclerosis-report of three cases and review of the
literature. Dermatology. 199:271–273. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Suda T, Hara H, Okada T and Suzuki H:
Coexistence of extensive calcification and membrano-cystic changes
in lupus erythematosus panniculitis associated with systemic lupus
erythematosus. Eur J Dermatol. 17:86–68. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Billings SD: Dermatosis. In: Rosai and
Ackerman's surgical pathology. 11th edition. Goldblum JR, Lamps LW,
McKenney JK and Myers JL (eds). Elsevier, Philadelphia, PA,
pp24-25, 2018.
|
|
35
|
Diaz-Cascajo C and Borghi S: Subcutaneous
pseudomembranous fat necrosis: New observations. J Cutan Pathol.
29:5–10. 2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fujiwara M: Histopathological and
histochemical studies of membranocystic lesion (Nasu). Shinshu Med
J. 27:78–100. 1979.(In Japanese).
|
|
37
|
Terayama K: Two cases of cystic bone
disease showing peculiar features. J Jap Orthop Ass.
35(626)1961.(In Japanese).
|
|
38
|
Nasu T, Tsukahara Y and Terayama K: A
lipid metabolic disease-‘membranous lipodystrophy’-an autopsy case
demonstrating numerous peculiar membrane-structures composed of
compound lipid in bone and bone marrow and various adipose tissues.
Acta Pathol Jpn. 23:539–558. 1973.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Järvi OH, Lauttamus LL and Solonen KA:
Membranous reticulin dysplasia of bone. Probably a new disease
entity. In: Proceedings of the 14th Scandinavian Congress of
Pathology and Microbiology. Universitetsforlaget, Oslo, p51,
1964.
|
|
40
|
Hakola HP, Järvi OH and Sourander P:
Osteodysplasia polycystica hereditaria combined with sclerosing
leucoencephalopathy, a new entity of the dementia praesenilis
group. Acta Psychiatr Scand. 46 (Suppl 43):S79–S80. 1970.PubMed/NCBI
|
|
41
|
Hakola HP and Partanen VS:
Neurophysiological findings in the hereditary presenile dementia
characterized by polycystic lipomembranous osteodysplasia and
sclerosing leukoencephalopathy. J Neurol Neurosurg Psychiatry.
46:515–520. 1983.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Verloes A, Maquet P, Sadzot B, Vivario M,
Thirty A and Franck G: Nasu-Hakola syndrome: Polycystic
lipomembranous osteodysplasia with sclerosing leucoencephalopathy
and presenile dementia. J Med Genet. 34:753–757. 1997.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Machinami R (ed): Atlas of bone and joint
disease. Bunkoudo, Tokyo, pp42-43, 64-65, 196-197, 1999 (In
Japanese).
|
|
44
|
Paloneva J, Kestilä M, Wu J, Salminen A,
Böhling T, Ruotsalainen V, Hakola P, Bakker ABH, Phillips JH,
Pekkarinen P, et al: Loss-of-function mutations in TYROBP (DAP12)
result in a presenile dementia with bone cysts. Nat Genet.
25:357–361. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
45
|
Paloneva J, Manninen T, Christman G,
Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R,
Salmaggi A, et al: Mutations in two genes encoding different
subunits of a receptor signaling complex result in an incidental
disease phenotype. Am J Hum Genet. 71:656–662. 2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Errichiello E, Dardiotis E, Mannino F,
Paloneva J, Mattina T and Zuffardi O: Phenotypic expansion in
Nasu-Hakola disease: immunological findings in three patients and
proposal of a unifying pathogenetic hypothesis. Front Immunol.
10(1685)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lee Y, Ahn SY, Ji JH, Hong SP, Bak H, Lee
SH and Ahn SK: A case of membranous lipodystrophy observed in
lichen amyloidosis. Ann Dermatol. 21:174–177. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ayele A, Tidman MJ and Biswas A:
Pseudomembranous changes in dermis: A novel observation and
potential clue for evolving lipodermatosclerosis? J Cutan Pathol.
44:1070–1074. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
McNutt NS, Moreno A and Contreras F:
Inflammatory diseases of the subcutaneous fat. In: Lever's
histopathology of the skin. Elder DE, Elenitsas R, Johnsons BL Jr,
Murphy GF, Rubin AI and Xu X (eds). 9th edition. Lippincott
Williams & Wilkins, Philadelphia, PA, pp519-549, 2005.
|
|
50
|
McNutt NS, Moreno A and Contreras F:
Inflammatory diseases of the subcutaneous fat. In: Lever's
histopathology of the skin. Elder DE, Elenitsas R, Johnsons BL Jr,
Murphy GF, Rubin AI and Xu X (eds). 10th edition. Lippincott
Williams & Wilkins, Philadelphia, PA, pp509-538, 2009.
|
|
51
|
Rosai J (ed): Rosai and Ackermans's
surgical pathology. 10th edition. Mosby/Elsevier, Philadelphia, PA,
pp2236, 2011.
|
|
52
|
Barrie HJ: Intra-articular loose bodies
regarded as organ cultures in vivo. J Pathol. 125:163–169.
1978.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Milgram JW (ed): Radiologic and histologic
pathology of nontumorous diseases of bones and joints. Northbook
Publishing Company, Brookfield, WI, pp281-334, 1990.
|
|
54
|
Ishida T and Imamura T (eds): Surgical
pathology of non-neoplastic bone and joint diseases. Bunkoudo,
Tokyo, pp48-61, 226-236, 2003 (In Japanese).
|
|
55
|
O'Connell JX: Pathology of the synovium.
Am J Clin Pathol. 114:773–784. 2000.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Matsukuma S, Takeo H, Okada K and Sato K:
Fatty lesions in intra-articular loose bodies: A histopathological
study of non-primary synovial chondromatosis cases. Virchows Arch.
460:103–108. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mullaaziz D, Kaptanoğlu A, Çalıkoğlu EE
and Özkayalar H: A case of lipomembranous panniculitis with a
dramatic response to the treatment of venous insufficiency.
Dermatol Reports. 10(7546)2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Fletcher CDM (ed): Diagnostic
histopathology of tumors. 5th edition. Elsevier, Philadelphia, PA,
pp1919-2001, 2021.
|
|
59
|
Schoen FJ and Sutton MS: Contemporary
issues in the pathology of valvular heart disease. Hum Pathol.
18:568–576. 1987.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Virmani R, Burke A, Farb A and Atkinson
JB: Cardiovascular pathology. In: Major problems in pathology.
LiVolsi VA (ed). Vol 40. 2nd edition. W.B. Saunders, Philadelphia,
PA, pp231-279, 2001.
|
|
61
|
Akay OM, Urer SM, Oner U and Gulbas Z:
Lipomembranous panniculitis in a patient with acute leukemia
induced by chemotherapy. Leuk Res. 32:669–671. 2008.PubMed/NCBI View Article : Google Scholar
|