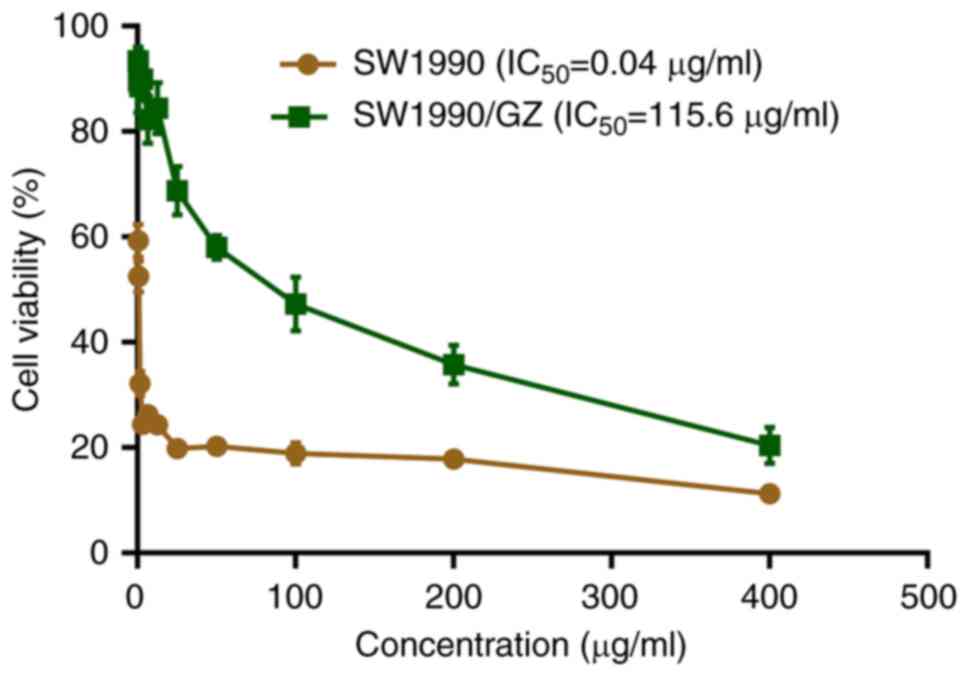
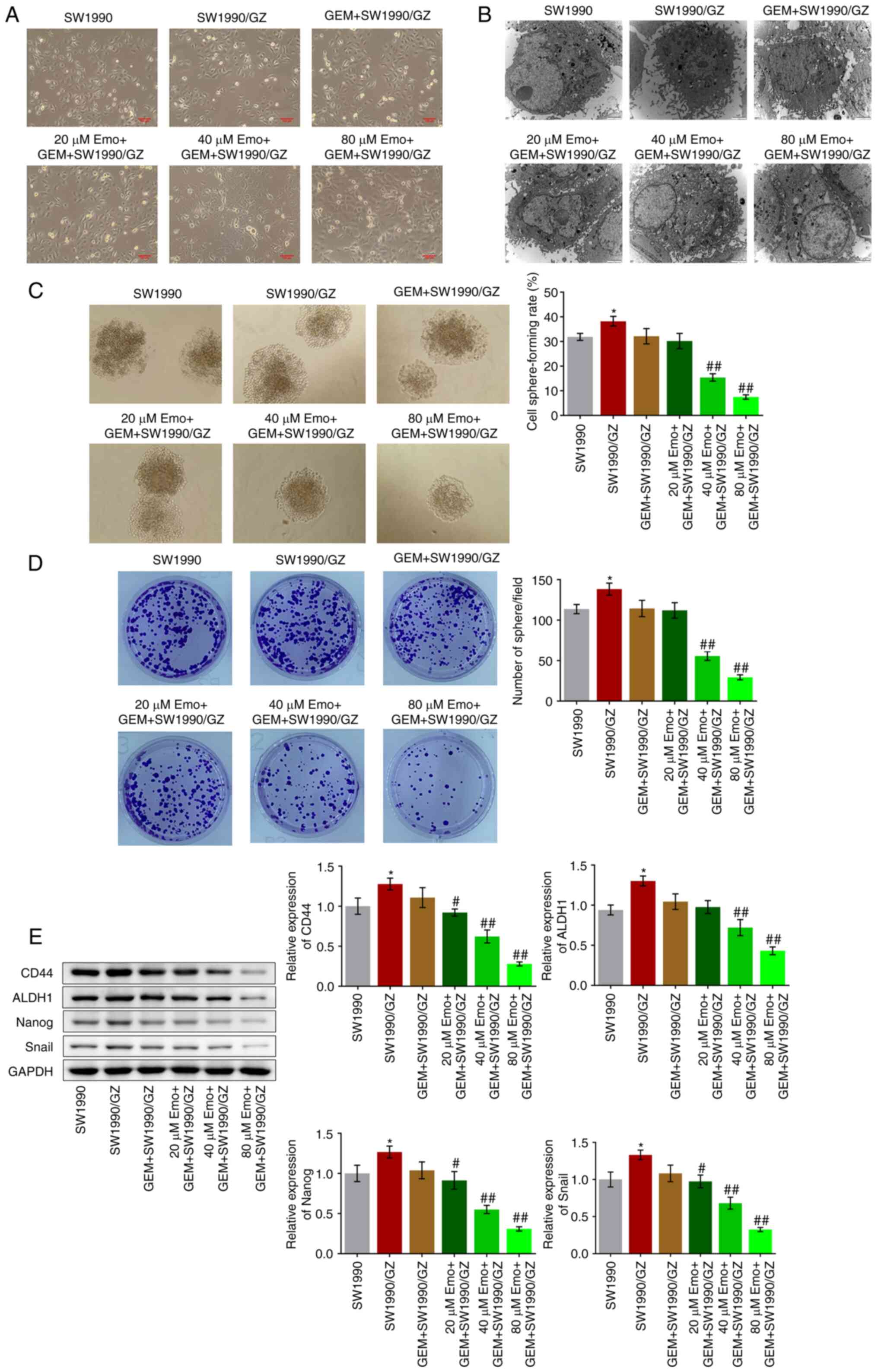
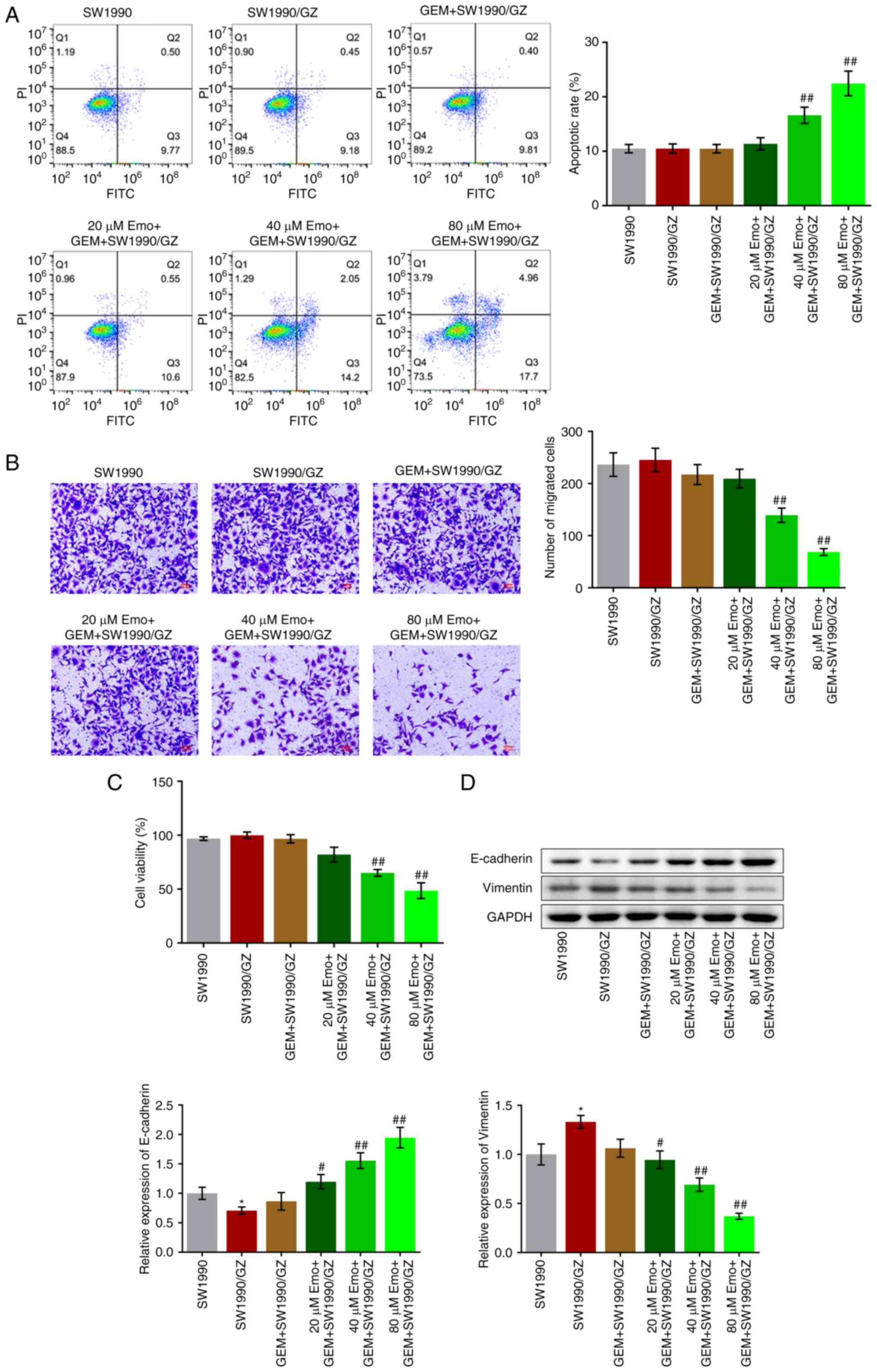
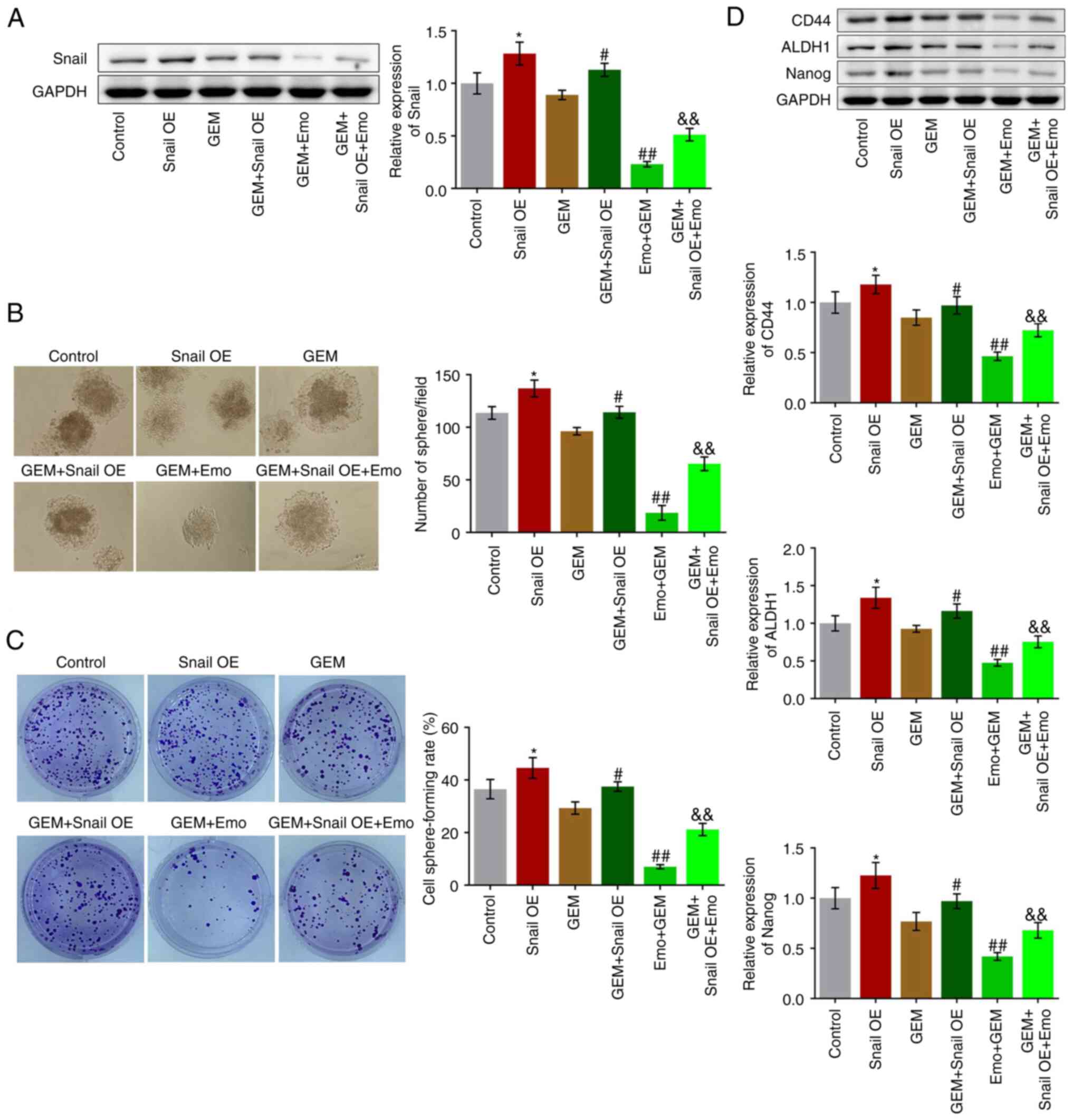
|
1
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sarvepalli D, Rashid MU, Rahman AU, Ullah
W, Hussain I, Hasan B, Jehanzeb S, Khan AK, Jain AG, Khetpal N and
Ahmad S: Gemcitabine: A review of chemoresistance in pancreatic
cancer. Crit Rev Oncog. 24:199–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang L, Dong P, Wang W, Huang M and Tian
B: Gemcitabine treatment causes resistance and malignancy of
pancreatic cancer stem-like cells via induction of lncRNA HOTAIR.
Exp Ther Med. 14:4773–4780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
de Sousa Cavalcante L and Monteiro G:
Gemcitabine: Metabolism and molecular mechanisms of action,
sensitivity and chemoresistance in pancreatic cancer. Eur J
Pharmacol. 741:8–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gangemi R, Paleari L, Orengo AM, Cesario
A, Chessa L, Ferrini S and Russo P: Cancer stem cells: A new
paradigm for understanding tumor growth and progression and drug
resistance. Curr Med Chem. 16:1688–1703. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Du Z, Qin R, Wei C, Wang M, Shi C, Tian R
and Peng C: Pancreatic cancer cells resistant to chemoradiotherapy
rich in ‘stem-cell-like’ tumor cells. Dig Dis Sci. 56:741–750.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Y, Kong D, Ahmad A, Bao B and Sarkar
FH: Pancreatic cancer stem cells: Emerging target for designing
novel therapy. Cancer Lett. 338:94–100. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abel EV and Simeone DM: Biology and
clinical applications of pancreatic cancer stem cells.
Gastroenterology. 144:1241–1248. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sancho P, Alcala S, Usachov V, Hermann PC
and Sainz B Jr: The ever-changing landscape of pancreatic cancer
stem cells. Pancreatology. 16:489–496. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Babaei G, Aziz SG and Jaghi NZZ: EMT,
cancer stem cells and autophagy; the three main axes of metastasis.
Biomed Pharmacother. 133(110909)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reiman JM, Knutson KL and Radisky DC:
Immune promotion of epithelial-mesenchymal transition and
generation of breast cancer stem cells. Cancer Res. 70:3005–3008.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tian K, Zhang H, Chen X and Hu Z:
Determination of five anthraquinones in medicinal plants by
capillary zone electrophoresis with beta-cyclodextrin addition. J
Chromatogr A. 1123:134–137. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li J, Liu P, Mao H, Wanga A and Zhang X:
Emodin sensitizes paclitaxel-resistant human ovarian cancer cells
to paclitaxel-induced apoptosis in vitro. Oncol Rep.
21:1605–1610. 2009.PubMed/NCBI
|
|
20
|
Way TD, Huang JT, Chou CH, Huang CH, Yang
MH and Ho CT: Emodin represses TWIST1-induced
epithelial-mesenchymal transitions in head and neck squamous cell
carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur J
Cancer. 50:366–378. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thacker PC and Karunagaran D: Curcumin and
emodin down-regulate TGF-β signaling pathway in human cervical
cancer cells. PLoS One. 10(e0120045)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pooja T and Karunagaran D: Emodin
suppresses Wnt signaling in human colorectal cancer cells SW480 and
SW620. Eur J Pharmacol. 742:55–64. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bai J, Wu J, Tang R, Sun C, Ji J, Yin Z,
Ma G and Yang W: Emodin, a natural anthraquinone, suppresses liver
cancer in vitro and in vivo by regulating VEGFR2 and
miR-34a. Invest New Drugs. 38:229–245. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zeng S, Pöttler M, Lan B, Grützmann R,
Pilarsky C and Yang H: Chemoresistance in pancreatic cancer. Int J
Mol Sci. 20(4504)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yin T, Wei H, Gou S, Shi P, Yang Z, Zhao G
and Wang C: Cancer stem-like cells enriched in Panc-1 spheres
possess increased migration ability and resistance to gemcitabine.
Int J Mol Sci. 12:1595–1604. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rao CV and Mohammed A: New insights into
pancreatic cancer stem cells. World J Stem Cells. 7:547–555.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Izumiya M, Kabashima A, Higuchi H,
Igarashi T, Sakai G, Iizuka H, Nakamura S, Adachi M, Hamamoto Y,
Funakoshi S, et al: Chemoresistance is associated with cancer stem
cell-like properties and epithelial-to-mesenchymal transition in
pancreatic cancer cells. Anticancer Res. 32:3847–3853.
2012.PubMed/NCBI
|
|
29
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Voon DC, Wang H, Koo JK, Chai JH, Hor YT,
Tan TZ, Chu YS, Mori S and Ito Y: EMT-induced stemness and
tumorigenicity are fueled by the EGFR/Ras pathway. PLoS One.
8(e70427)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Grau Y, Carteret C and Simpson P:
Mutations and chromosomal rearrangements affecting the expression
of snail, a gene involved in embryonic patterning in Drosophila
melanogaster. Genetics. 108:347–360. 1984.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xiong Y, Wang Y, Wang L, Huang Y, Xu Y, Xu
L, Guo Y, Lu J, Li X, Zhu M and Qian H: MicroRNA-30b targets Snail
to impede epithelial-mesenchymal transition in pancreatic cancer
stem cells. J Cancer. 9:2147–2159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Wang XF, Zhang G, Bu XZ, Cai SH and Du J: Epithelial-mesenchymal
transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated
stabilization of snail in colorectal cancer. PLoS One.
8(e56664)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hwang WL, Yang MH, Tsai ML, Lan HY, Su SH,
Chang SC, Teng HW, Yang SH, Lan YT, Chiou SH and Wang HW: SNAIL
regulates interleukin-8 expression, stem cell-like activity, and
tumorigenicity of human colorectal carcinoma cells.
Gastroenterology. 141:279–291, 291.e1-e5. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu W, Feng Q, Li Y, Ye L, Hu M and Liu Z:
Coupling of UDP-glucuronosyltransferases and multidrug
resistance-associated proteins is responsible for the intestinal
disposition and poor bioavailability of emodin. Toxicol Appl
Pharmacol. 265:316–324. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen J, Li S, Liu M, Lam CWK, Li Z, Xu X,
Chen Z, Zhang W and Yao M: Bioconcentration and metabolism of
emodin in zebrafish eleutheroembryos. Front Pharmacol.
8(453)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dong X, Fu J, Yin X, Cao S, Li X and Lin
L: Huyiligeqi and Ni J. Emodin: A review of its pharmacology,
toxicity and pharmacokinetics. Phytother Res. 30:1207–1218.
2016.PubMed/NCBI View Article : Google Scholar
|