Introduction
Glaucoma is one of the leading causes of
irreversible blindness worldwide, the prevalence of which is
projected to reach 111.8 million by 2040, with ~10% of patients
succumbing to blindness (1).
Glaucoma is a chronic neurodegenerative disease that is
characterized by the progressive loss of retinal ganglion cells
(RGCs), including the neurons and their axons, resulting in
structural and functional defects in the visual field (2). Intraocular pressure (IOP) reduction
is considered to be the most promising intervention strategy to
protect the optic nerve from glaucomatous damage (3,4).
However, the deterioration of glaucomatous neuropathy cannot be
prevented in some patients, for whom lowering the IOP is either
insufficient or difficult to achieve (5). Furthermore, accumulating evidence
suggests that optic nerve damage can continue despite effective IOP
reduction (3-6).
Therefore, the possible use of neuroprotective strategies to
prevent visual loss in glaucoma is garnering the attention of this
research field (7,8).
A number of causes have been reported to be
responsible for RGC damage and death in glaucoma, including IOP
elevation, ischemia/reperfusion (I/R) damage of the retina,
oxidative stress, glutamate neurotoxicity, neurotrophic growth
factor deprivation and immune disturbance (9). Acute ocular hypertension (AOH) mimics
the pathophysiological process of acute glaucomatous damage
(10), as well as I/R injury to
the retina (11,12). Therefore, animal models of AOH are
frequently used for glaucoma research. Aberrant IOP increase
induces stress and strain to the eye, resulting in the compression,
deformation and remodeling of the lamina cribrosa to induce
mechanical axonal damage and disruption in axonal transport
(13,14). Therefore, retrograde delivery of
essential neurotrophic factors, such as brain-derived neurotrophic
factor and its receptor, interleukin-6 and neural growth factor, to
RGCs from the central nervous system is interrupted (15). I/R damage is also reported to be
involved in retinal damage induced by AOH (16). During ischemia, glutamate is
released in the retina and induces the death of neurons expressing
ionotropic glutamate (N-methyl-D-aspartate) receptors (17). RGC apoptosis has been reported to
be caused by the activation of caspase signaling induced by the
abnormally high concentration of glutamate (18).
Several cytokines have been previously found to be
involved in the pathophysiology of glaucoma, such as tumor necrosis
factor-α, interleukin-1β, interleukin-6 and interleukin-18(19). In particular, leukemia inhibitory
factor (LIF) is a member of the IL-6 cytokine family and has been
reported to be present in the retina (20,21).
The LIF signaling pathway is considered to be one of the major
endogenous factors mediating neuroprotection in the retina
(20,21). Mechanistically, LIF activates the
Janus kinase (JAK)/STAT3, PI3K/AKT and ERK1/2 signaling pathways to
facilitate the neuroprotection of retina from injury (22-27).
Photoreceptor injury or degeneration activates a subset of Müller
glial cells into expressing LIF, which initiates a neuroprotective
signaling cascade between photoreceptors and Müller cells (20,28).
These signaling events include activation of the JAK/STAT3 pathway
(29-31),
which result in the upregulation of the expression of several
genes, including STAT3(18). In
addition, LIF was shown to protect the degenerative retina from
apoptosis (32). Activation of the
mTOR/70-kDa ribosomal protein S6 kinase (p70S6K) signaling pathway
has also been reported to be activated by exogenous LIF (33), which may be responsible for
neuroprotection against ischemic brain injury (34).
Our previous study on a rat AOH model has revealed
that LIF and LIF receptor protein expression is upregulated
associated with activation of the STAT3 and AKT signaling pathways
(35). Therefore, in the present
study the potential effects of exogenous LIF treatment on retinal
damage induced by AOH in rats were investigated.
Materials and methods
Animals
The protocol of the present study was approved by
the Experimental Animal Ethics Committee of Xiamen University,
School of Medicine (Xiamen, China; approval no. 20150306155209) and
followed the Association for Research in Vision and Ophthalmology
Statement for the Use of Animals in Ophthalmic and Vision Research
(https://www.arvo.org/About/policies/arvo-statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/).
Adult male Sprague-Dawley rats (aged 8-12 weeks old; weight 250±30
g; n=140) were obtained from the Shanghai SLAC Laboratory Animal
Co., Ltd. The rats were maintained on a 12-h light-dark cycle
(~20˚C; humidity ~50%) and were dark-adapted for ≥2 h before any
experiments. All animals had access to food (standard lab chow) and
water ad libitum. All efforts were made to minimize the
number of animals used and their suffering. Prior to AOH induction
and drug injection procedures, deep anesthesia was induced by an
intraperitoneal injection of pentobarbital sodium (30 mg/kg;
Sinopharm Chemical Reagent Co., Ltd.).
Induction of AOH
The experimental procedure has previously been
described (35). Briefly,
following the topical administration of 0.5% proparacaine (Alcon),
the eye pupil was dilated with 0.5% tropicamide (Alcon). Under a
Spot OPMI 11 operation microscope (Carl Zeiss AG), the anterior
chamber of the eye was cannulated by 7-scalp infusion acupuncture
needle connected to a 500-ml container of sterile saline. Only the
right eyes were chosen for the experiment; the left eyes remained
untouched and served as control. The IOP was then elevated to ~110
mmHg by raising the height of the saline container to 150 cm above
the eye for 60 min. The infusion needle was then removed before
further experiments on the rats were performed.
LIF injection
After the needle was removed and AOH was ceased,
intravitreal injection of 1 µg/µl LIF was conducted immediately.
Topical anesthesia by 0.5% proparacaine eyedrops (Alcon Inc.) was
performed as needed. A total volume of 5 µl LIF was injected into
the vitreous through the par plana using a 33G microsyringe
(Hamilton Co.). For the untreated AOH group, a dose of 5 µl PBS was
injected instead of LIF solution.
In another set of experiments, to test the
involvement of STAT3 and the PI3K/AKT/mTOR signaling pathway in the
effect induced by LIF injection, the pretreatment of intravitreal
injection through the par plana of either the STAT3 inhibitor
C188-9 (10 mM; Selleck Chemicals) or the PI3K/AKT/mTOR inhibitor
LY3023414 (50 nM; Selleck Chemicals), was conducted 3 h prior to
LIF injection (~2 h before AOH induction). A dose of 5 µl PBS was
used as a control. C188-9, LY3023414 and PBS injections were all
performed under topical anesthesia by 0.5% proparacaine and general
anesthesia by pentobarbital sodium (30 mg/kg).
Histological assessment of the ocular
tissue
One day or 3 days after AOH induction, rats were
sacrificed by anesthetic overdose with an intraperitoneal injection
of pentobarbital sodium (150 mg/kg), and the eyeball was
immediately enucleated and frozen (-20˚C) in the optimal cutting
temperature compound (Sakura Finetek Japan Co., Ltd.). The eyeball
was sectioned along the meridian to a thickness of 10 mm to assess
the histological changes in the anterior part of the eyes. Tissue
preparations were stained with hematoxylin (3 min) and eosin (1
min) at room temperature and viewed under a light optical
microscope (Nikon Co., cTokyo, Japan). The retinal thickness was
measured using Image-Pro Plus 6.0 (Media Cybernetics) and the data
were proceeded for statistical analysis.
Fluoro-Gold (FG) retrograde labeling
and cell counting of the RGCs
RGCs were retrogradely labeled with Fluoro-Gold™
(Fluorochrome, LLC) 7 days before the induction of AOH. The
procedures were described in our previous reports (9,31).
Briefly, the rats were deeply anesthetized with 30 mg/kg
pentobarbital sodium (i.p.) and placed in a prone position on the
stereotaxic apparatus (RWD Life Science Co. Ltd.). RGC labeling of
both eyes was conducted by injecting 4% FG into the superior
colliculus at 6.0 mm caudal to the bregma and 1.0 mm lateral to the
midline on both sides (3 µl each), to a depth of 5.0 mm from the
surface of the skull. A total of 3 days after LIF or PBS injection,
the rats were sacrificed by overdose of pentobarbital sodium before
the eyes were enucleated and fixed in 4% paraformaldehyde solution
for 40 min at room temperature. The retinas were dissected free and
flatly mounted onto a glass slide. The FG-labeled RGCs were then
identified under a fluorescence microscope (Leica DM2500; Leica
Microsystems GmbH) with a wide band ultraviolet filter (0.1%
fluorogold solution in distilled water with a pH of 4.5, Ex 414 nm,
Em 541 nm). RGCs were manually counted by another investigator (CH)
blinded to the experiment protocols. For each quadrant of the
retina, three images were captured at 1, 2 and 3 mm radially from
the optic disc in the identical retinal preparation (magnification,
x10); the total number of RGCs was counted in all four quadrants
using the image analysis program Image J (version 1.52; National
Institutes of Health).
TUNEL staining
According to our previous report (35), RGC apoptosis, as well as the
protein expression of apoptosis-related cytokines, was detected
mostly on day 1 post AOH. One day after AOH induction, rats were
deeply anesthetized with 30 mg/kg pentobarbital sodium prior to
cardiac perfusion with 4% paraformaldehyde (Sinopharm Chemical
Reagent Co., Ltd.) for 24 h. The enucleated eyes were embedded in
paraffin (65˚C) and sectioned at a thickness of 7 µm using a Leica
DM2500 microtome (Leica Microsystems GmbH). TUNEL staining (37˚C; 1
h) of the retina was performed using a TUNEL assay kit (Promega
Corporation), whereas the cell nuclei were stained with 50 mM DAPI
(37˚C; 15 min, Vector Laboratories, Inc.). As a positive control,
sections were incubated (room temperature; 10 min) with 0.5 µg/ml
DNase I (Promega Corporation) before adding the equilibration
buffer of the TUNEL assay kit. TUNEL-positive cells were observed
and counted under fluorescence microscopy. The TUNEL-positive cells
in each section were counted and quantified as per mm2
of the retina by using the image analysis program Image J. A total
of six images on x20 magnification were used from two sections per
animal.
Western blotting
The rat retinas were dissected free after the global
enucleation and homogenized with lysis buffer (Solarbio Science
& Technology, China), and the protein concentration was
determined using the Pierce™ BCA Protein Assay Kit (Thermo Fisher
Scientific, Inc.). SDS-PAGE (12%) of the protein (20 mg per lane)
was performed for 1-2 h and then transferred onto a PVDF membrane
(MilliporeSigma). After blocking with 2% bovine serum albumin
(Ameresco, Inc.) for 2 h at room temperature, the PVDF membranes
were incubated with primary polyclonal antibodies against
cleaved-caspase-3 (1:1,000), poly (ADP-ribose) polymerase (PARP;
1:1,000), STAT3 (1:500), phosphorylated (p-)-STAT3 (STAT3; 1:500),
AKT (1:1,000), p-AKT (1:2,000), mTOR (1:1,000), p-mTOR (1:1,000),
p70S6K (1:1,000), p-p70S6K (1:1,000) or β-actin (1:10,000; cat. no.
A5441, Sigma-Aldrich; Merck KGaA) overnight at 4˚C. The antibodies
of cleaved-caspase-3 (cat. no. 9579), PARP (cat. no. 9542), AKT
(cat. no. 9272), p-AKT (cat. no. 31957), mTOR (cat. no. 2972),
p-mTOR (cat. no. 5536), p70S6K (cat. no. 9202) and p-p70S6K (cat.
no. 9208) were purchased from Cell Signaling Technology, Inc.,
whilst the antibodies of STAT3 (cat. no. APR13562G) and p-STAT3
(cat. no. APR11162G) were bought from Santa Cruz Biotechnology,
Inc. After washing with TBS + 1% Tween 20 three times, the
membranes were incubated with an HRP-conjugated goat anti-rabbit
IgG secondary antibody (1:10,000; cat. no. 172-1050; Bio-Rad
Laboratories, Inc.) for 2 h at room temperature. The protein bands
were visualized with Enhanced Chemiluminescence reagents
(SuperSignal; cat. no. 46641; Thermo Fisher Scientific, Inc.) and
images captured using a transilluminator (ChemiDoc XRS; Bio-Rad
Laboratories). Image Lab software (version 6.1; Bio-Rad
Laboratories, Inc.) was used for the densitometry of the bands.
Statistical analysis
All data are presented as the mean ± standard
deviation. One-way ANOVA followed by Tukey's multiple comparisons
were performed using SPSS (version 17.0; SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
LIF alleviates retinal damage by
AOH
H&E staining was used to assess the effect of
LIF on retinal histopathology induced by AOH. As shown in Fig. 1A (n=3), on day 1 after AOH, the
thickness of the inner nuclear layer (INL) was decreased, whereas
that of the inner plexiform layer (IPL) was markedly increased. The
nuclei in the RGC layer also appeared larger compared with those in
the normal control retina. On day 3 after AOH, a marked decrease in
the IPL was observed After LIF treatment, the change in the
thickness of the IPL was reversed compared with AOH group without
LIF treatment. The change in the total retinal thickness (TRT) is
presented in Fig. 1B and C (n=3/group). At 1 day after AOH, the TRT
increase in both AOH and LIF treatment groups was statistically
significant compared with the control (both P<0.05). At 3 days
after AOH, significant decrease in the TRT was noticed in AOH rats
compared with the control (P<0.001), while the TRT in AOH rats
treated with LIF was significantly reversed compared with the AOH
group (P<0.001).
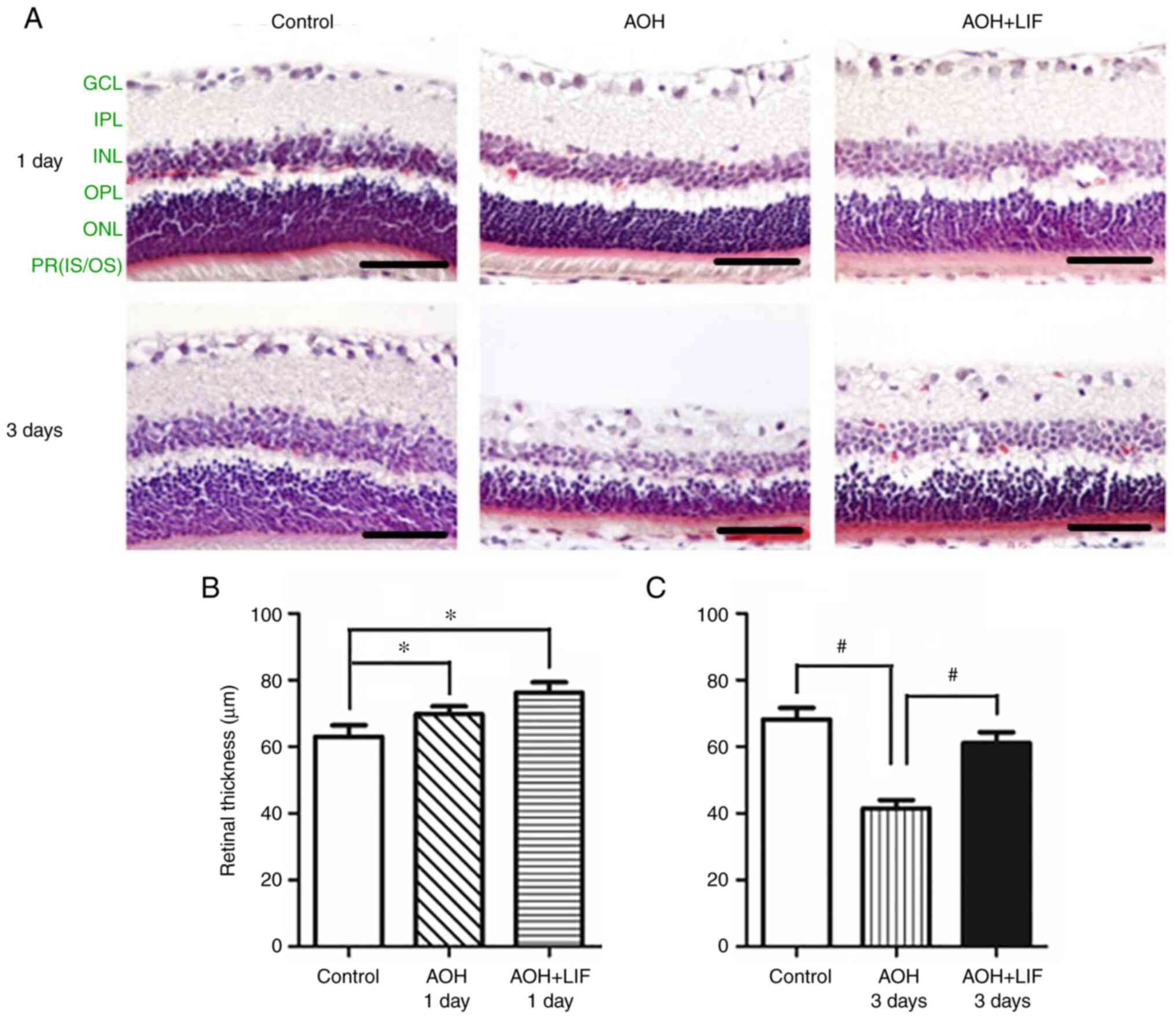 | Figure 1Protective effect of LIF on the rat
retina 1 and 3 days after AOH. (A) Morphological changes in the rat
retina. On day 1 after AOH, the thickness of the INL was decreased,
whereas that of the IPL was increased. On day 3 after AOH, the IPL
decreased markedly. After LIF treatment, the change in the
thickness of the IPL was reversed compared with AOH group without
LIF treatment. Scale bar, 100 µm. Changes in retinal thickness (B)
1 day and (C) 3 days after AOH. n=3/group. *P<0.05
and #P<0.001. AOH, acute ocular hypertension; LIF,
leukemia inhibitory factor; INL, inner nuclear layer; IPL, inner
plexiform layer; GCL, ganglion cell layer; OPL, outer plexiform
layer; ONL, outer nuclear layer; PR (IS/OS), photoreceptor (inner
segment/outer segment). |
LIF improves RGC survival after
AOH
An FG tracer was used to label RGCs to assess the
effect of LIF on RGC survival after AOH (Fig. 2A). Quantitative analyses indicated
that the FG-labeled RGC density in the AOH model retina
(1,572.6±21.3/mm2) was significantly less compared with
that in the normal control retina (2,390.4±68.8/mm2;
P<0.001) (Fig. 2B). In retinae
that received intravitreal LIF treatment, the RGC density was
significantly higher compared with those in the AOH group without
treatment (2,131.2±85.6/mm2; P<0.001; Fig. 2).
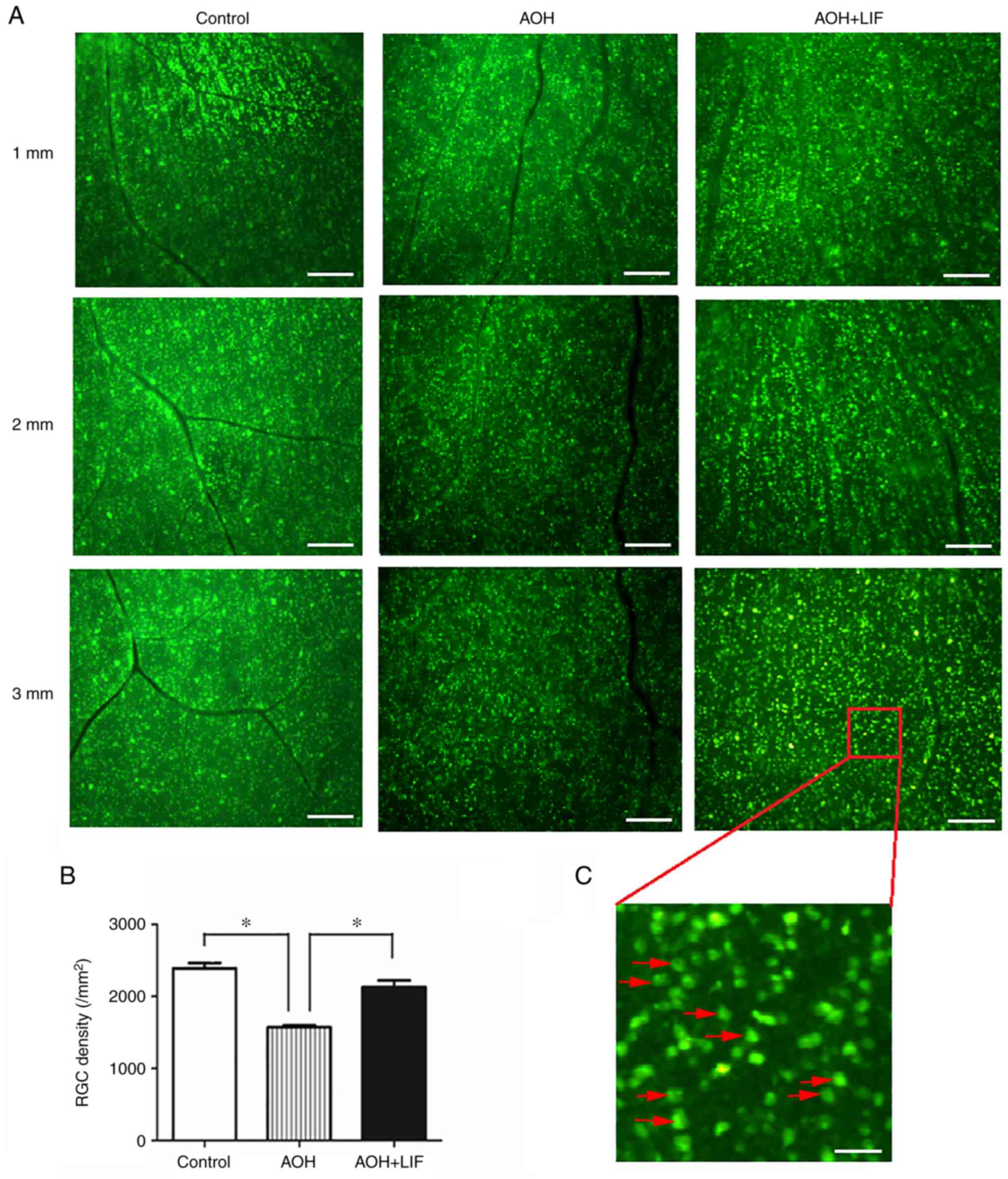 | Figure 2FG retrograde labeling to determine
the effects of LIF on RGC survival after AOH. (A) FG retrograde
labeling of RGCs; 1, 2 and 3 mm indicate the radial distances to
the optic disc in the identical retinal preparation for each
treatment condition. Scale bar, 200 µm. (B) The results of RGC
counting under the fluorescence microscope (Leica DM2500). For each
quadrant of the retina, three images were captured at 1, 2 and 3 mm
radially from the optic disc in the identical retinal preparation
(magnification, x10). RGCs were manually counted by an investigator
blinded to the experiment protocols. *P<0.001,
n=4/group. (C) Partial enlargement (magnification, x10) of an
FG-labeled image. The red arrows refer to examples of RGCs. Scale
bar, 40 µm. AOH, acute ocular hypertension; FG, Fluoro-Gold; LIF,
leukemia inhibitory factor; RGC, retinal ganglion cell. |
LIF inhibits apoptosis in the retina
after AOH
TUNEL staining was used to measure the degree of
apoptosis in the retina 1 day after AOH, particularly in the RGC
and inner nuclear layers (Fig. 3A,
n=3). As shown in Fig. 3B, the
number of TUNEL-positive cells was increased significantly in the
AOH group (127.6±30.0/mm2, n=3) compared with that in
the normal control group (1.8±3.3/mm2, n=3)
(P<0.001). Accordingly, 1 day after AOH, the protein expression
levels of cleaved-caspase-3 and PARP, indicators of apoptotic
activity in the tissue, were significantly increased (Fig. 3C-E). After treatment with
intravitreal LIF injection, the number of apoptotic cells
(26.9±13.3/mm2, n=3) was reduced significantly compared
with that in the AOH group (P<0.001; Fig. 3B). In addition, the expression of
cleaved-caspase-3 (P<0.001) and PARP (P<0.01) were
significantly reduced compared with that in the AOH group (Fig. 3C-E).
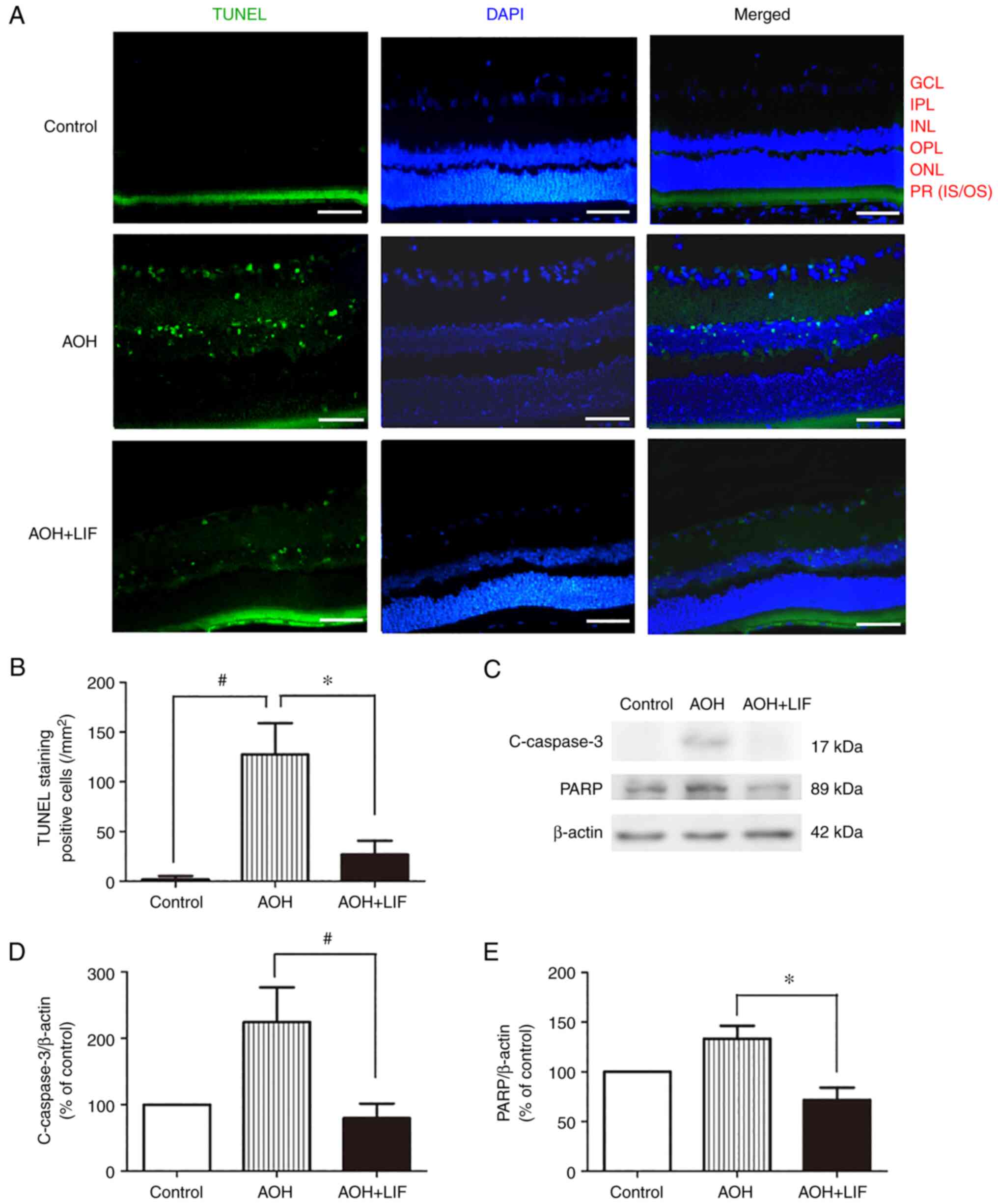 | Figure 3Effects of LIF on the apoptosis of
retinal cells in AOH model rats. (A) TUNEL staining of the retinae.
Green, TUNEL staining; blue, DAPI nuclear staining. Scale bar, 100
µm. (B) Quantitative analysis of the number of TUNEL-stained cells
in the retinae. (C) Representative western blotting images and
semi-quantitative analysis of (D) c-caspase-3 and (E) PARP protein
expression in the retina 1 day after AOH. *P<0.01 and
#P<0.001, n=3/group. AOH, acute ocular hypertension;
c-caspase-3, cleaved-caspase-3; LIF, leukemia inhibitory factor;
PARP, poly (ADP-ribose) polymerase; GCL, ganglion cell layer; IPL,
inner plexiform layer; INL, inner nuclear layer; OPL, outer
plexiform layer; ONL, outer nuclear layer; PR (IS/OS),
photoreceptor (inner segment/outer segment). |
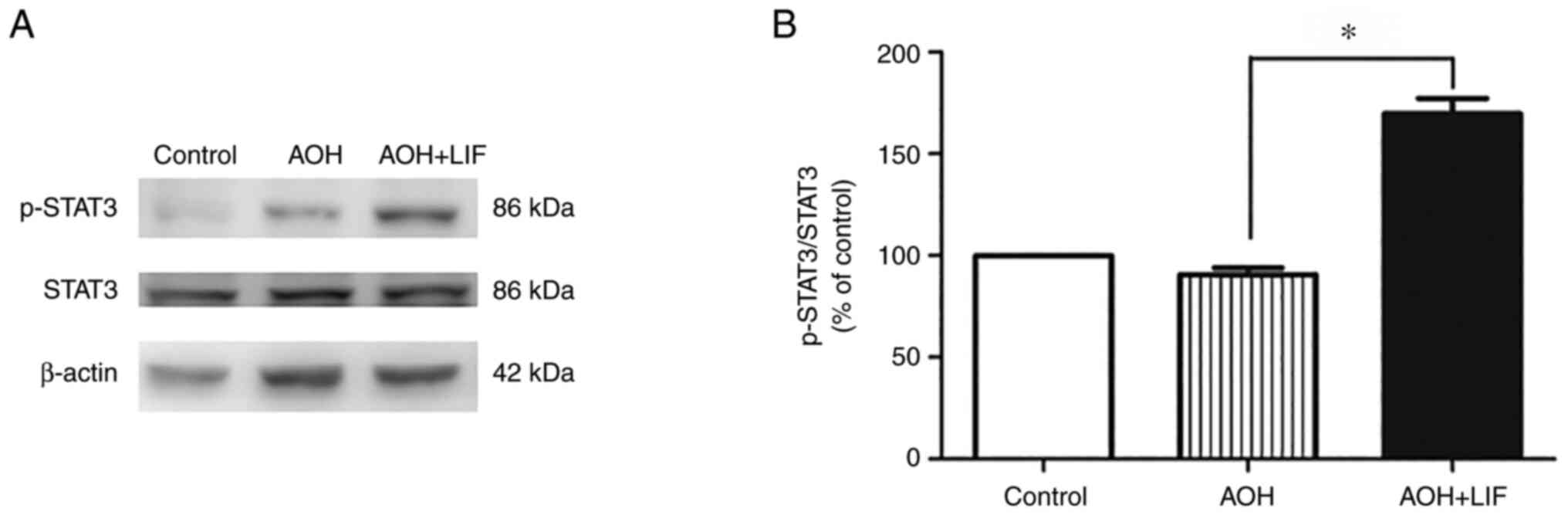
LIF upregulates the JAK/STAT signaling
pathway after AOH
As reported previously, the expression of STAT3,
AKT/mTOR/p70S6K signaling pathways components peaked at around day
3 post AOH (35). The level of
STAT3 phosphorylation in the rat retina was tested 3 days after
AOH. A significant increase in the phosphorylation of STAT3 was
observed only in the LIF treatment group compared with the AOH
group (P<0.001, n=3/group; Fig.
4).
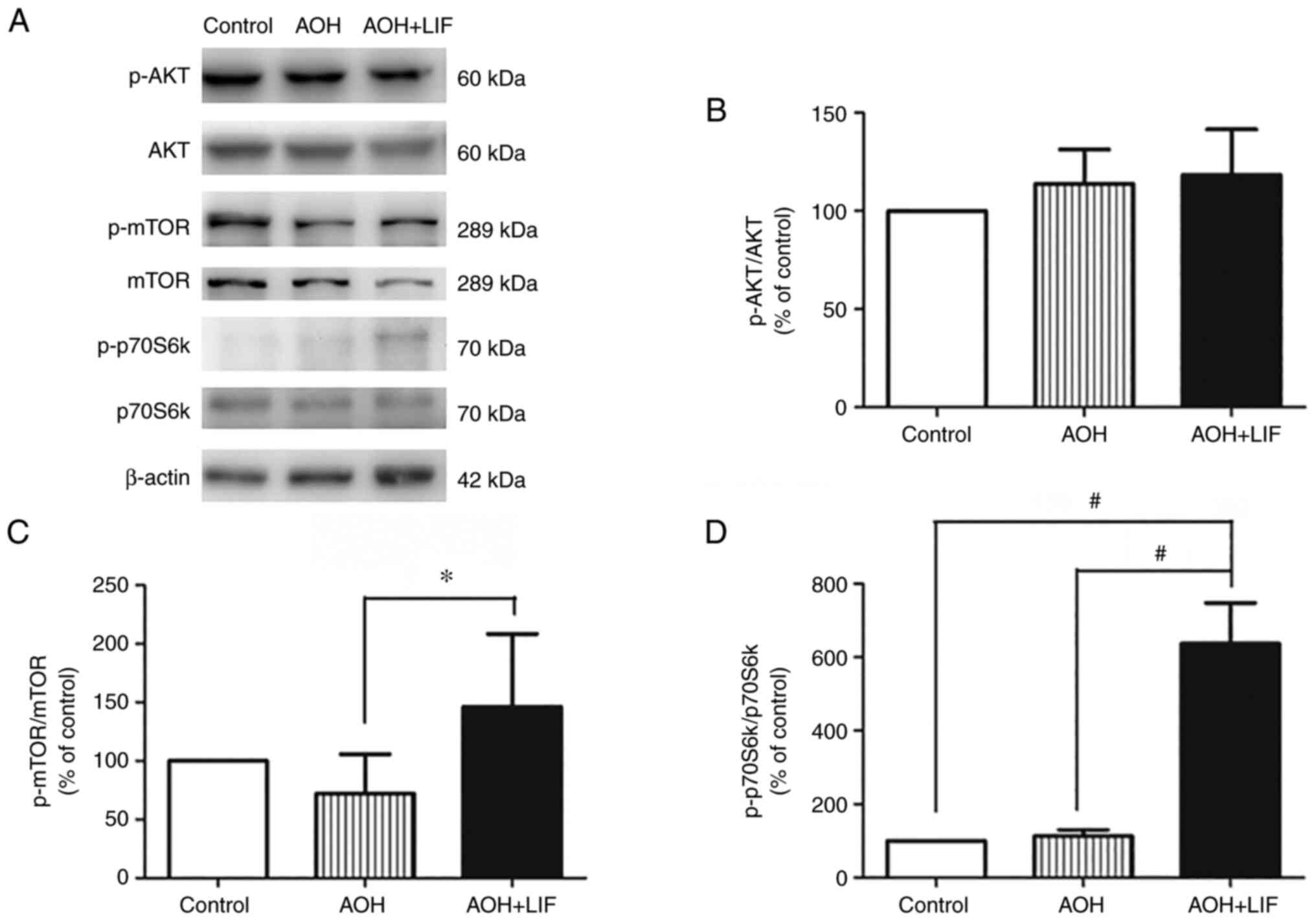
LIF upregulates the AKT/mTOR/p70S6K
signaling pathway after AOH
The expression of the AKT/mTOR/p70S6k signaling
pathway components in the retina after AOH was assessed by western
blotting (Fig. 5A). AOH didn't
induce any significant change in the expression of any signaling
pathway components compared with the control. After LIF treatment,
a slight but insignificant increase in the phosphorylation of AKT
was observed (Fig. 5B, n=3). By
contrast, the phosphorylation levels of mTOR (P<0.05; Fig. 5C, n=3) and p70S6K (P<0.001;
Fig. 5D, n=3) were significantly
upregulated in the LIF treatment group compared with the untreated
AOH group.
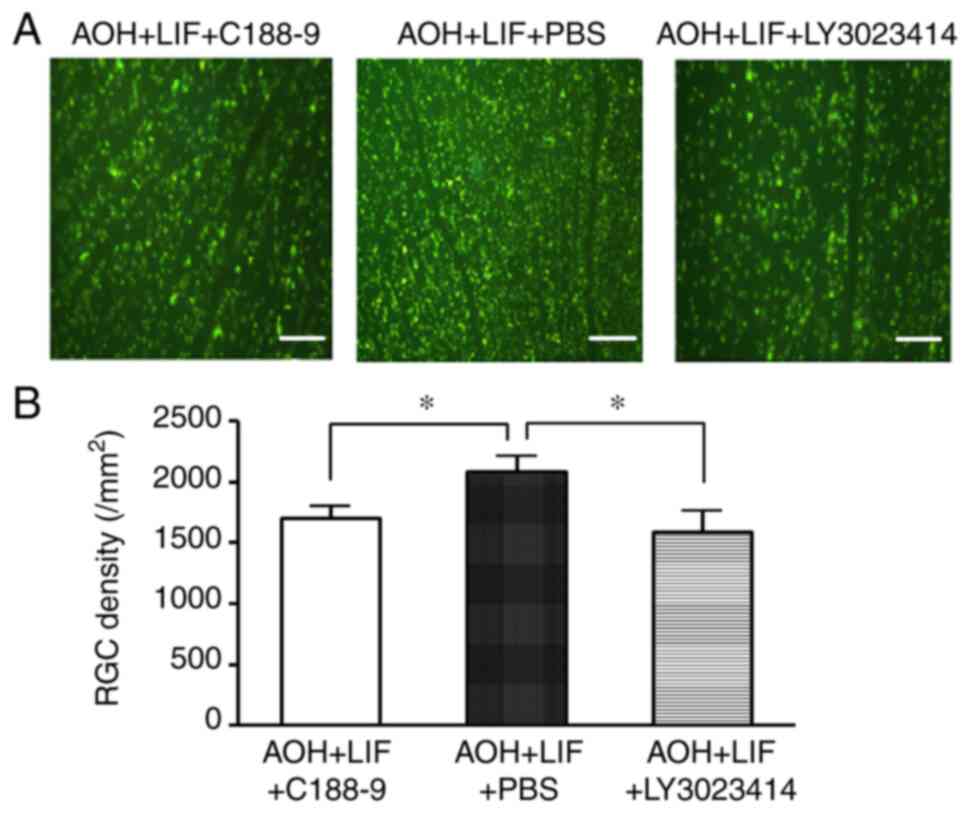
Inhibition of JAK/STAT3 or
PI3K/AKT/mTOR pathways reverses LIF-induced RGC protection after
AOH
Compared with that in the AOH retina treated with
LIF and PBS intravitreal injection, the RGC density was
significantly lower in AOH rats receiving intravitreal injection of
the JAK/STAT3 inhibitor C188-9 or with the PI3K/AKT/mTOR inhibitor
LY3023414 (both P<0.01; Fig. 6,
n=4/group).
Discussion
In the present study, the effects of exogenous LIF
on the survival of RGCs was investigated in AOH model rats. When
injected into the vitreous, LIF significantly inhibited the retinal
atrophy and RGC loss induced by AOH. Furthermore, apoptosis was
reduced after LIF injection. Activation of the AKT/mTOR/p70S6K and
JAK/STAT signaling pathways may be associated with these
neuroprotective effects of LIF.
Several mechanisms are involved in the pathological
changes in the retina after AOH. In the early stages, direct stress
on the inner retina leads to the death of RGCs and axonal damage
(13,14). IOP elevation may also directly
obstruct retinal blood vessels and decrease retinal blood flow
(36). I/R damage of the retina
also serves a key role in neuronal cell death in the latter
pathological stages (37,38). In the present study, tissue edema
and disorder in cell arrangement were observed 1 day after AOH,
followed by atrophy of the retina and cell loss 2 days later. It
has been observed that the degree of neuronal loss is associated
with the duration of AOH (I/R) imposed on the eye (11,12).
Retinal thinning and RGC loss are more evident with longer
reperfusion times (11,12). Treatment with intravitreal LIF
injection prevented this RGC loss and atrophy of the retina after
AOH. However, the underlying mechanism of this type cell death
after I/R induced by AOH, as well as the protective effects of LIF,
remain largely unknown and warrant further study.
Apoptosis inhibition is reported to be associated
with the neuroprotective effect of LIF in the retina. In a previous
model of light-mediated retinal injury, apoptosis of photoreceptor
cells triggers the expression of LIF from Müller cells (20). LIF accumulation then promotes the
expression of fibroblast growth factor-2(18) and activates the STAT3 signaling
pathway (39) to protect
photoreceptors from apoptosis. In cultured human neural progenitor
cells, exogenous LIF has also been reported to inhibit
caspase-mediated apoptosis (40).
In the present study on a rat AOH model, TUNEL-stained cells were
noticed in the RGC, INL and ONL layers 24 h after AOH After
treatment with exogenous LIF, the apoptotic cells were
significantly reduced compared with those in the untreated AOH
group, suggesting that inhibition of apoptosis is associated with
the protective effects of LIF on the RGC loss induced by AOH. The
protein expression of cleaved caspase-3 and PARP was also reduced
in the LIF treatment group, which is in accordance with the
aforementioned observation, since both cleaved caspase-3 and PARP
are indicators of apoptosis activation (41,42).
Activation of caspase family members, especially caspase-3, by the
accumulation of neurotoxic glutamate, is considered to be one of
the major mechanisms responsible for RGC death observed in glaucoma
(43). Nevertheless, other
mechanisms aside from apoptosis may be responsible for the
neuroprotective effects of LIF against RGC damage and death.
Indeed, it has been previously shown that LIF can block amyloid
β-mediated induction of autophagy-related activity in HT-22 mouse
hippocampal cells. In addition, suppression of the autophagy
marker, light chain 3II, by LIF has also been observed in a
Drosophila model of Alzheimer's disease (44). However, whether autophagy-related
mechanisms are involved in the neuroprotective effects of LIF
against AOH-induced retinal damage requires further
investigation.
Our previous study showed that AOH activated the
expression of intrinsic LIF and LIF receptors in the retina, which
is accompanied by the upregulation of STAT3 activity and AKT
protein expression (35). It was
therefore hypothesized that this LIF activation can exert a
protective effect against retinal damage induced by AOH through the
JAK/STAT and AKT signaling pathways. In the present study,
exogenous LIF treatment in AOH prevented RGC damage/death whilst
also upregulating the STAT3 pathway. In contrast to what occurred
in AOH without treatment, when treated with exogenous LIF the ratio
of phosphorylated/non-phosphorylated JAK/STAT and AKT signaling
pathway components increased markedly, indicating the enhancement
of these pathways' activation. It has also been reported that LIF
is involved in protecting photoreceptors against light-induced
injury, predominantly through activation of the JAK/STAT3 pathway
(20,45). In addition, increased, although not
statistically significant, phosphorylation of AKT in the retina
after LIF injection was also observed in the present study, which
is accompanied by the increased phosphorylation of mTOR and p70S6K.
It has been shown that the mTOR/p70S6K signaling pathway has a
crucial effect in modulating axonal protein synthesis and neurite
growth during the development or recovery of the nervous system in
response to injury (46). RGC
counting using FG labeling revealed the inhibition of LIF-induced
neuroprotection against RGC loss by potent STAT3 or PI3K/AKT/mTOR
pathway inhibitors. Results of the present study therefore
suggested that the neuroprotective effects of LIF against
AOH-induced retinal damage may be associated with the activation of
both the STAT3 and the mTOR/p70S6K signaling pathways.
The present study has several limitations. First of
all, the effect of LIF on RGC survival after AOH needs to be
monitored for a longer time, and a combination of any functional
assessment would be valuable. Additionally, the mechanism
underlying the neuroprotection of LIF injection needs further
investigation and the involvement of the signaling pathways need to
be clarified. Nevertheless, the present study demonstrated the
neuroprotective effects of exogenous LIF treatment against retinal
damage observed in AOH, which suggests that LIF may serve a role in
the neuroprotective treatment for glaucoma. Further studies are
needed to confirm this, as well as the neuroprotective effects of
LIF treatment in patients with glaucoma.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by The National Natural
Science Foundation of China (grant no. 81570844), The Natural
Science Foundation of Fujian Province (grant no. 2011D001), The
Medical Innovation Program of Fujian Province (grant no.
2011-CXB-47), The Huaxia Translational Medicine Youth Foundation
(grant no. 2017-A-00301) and The Xiamen Science and Technology
Program Guiding Project (grant no. 3502Z20189033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, CH and RW designed the study. JL and RW were
responsible for the data collection. JL, RG, CH and YW conducted
the experiments. JL, RG and RW were responsible for data analysis.
JL and CH confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of Xiamen University (Xiamen, China; approval no.
20150306155209).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: A systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heijl A, Leske MC, Bengtsson B, Hyman L,
Bengtsson B and Hussein M: Reduction of intraocular pressure and
glaucoma progression: Results from the early manifest glaucoma
trial. Arch Ophthalmol. 120:1268–1279. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lichter PR, Musch DC, Gillespie BW, Guire
KE, Janz NK, Wren PA and Mills RP: CIGTS Study Group. Interim
clinical outcomes in the Collaborative Initial Glaucoma Treatment
Study comparing initial treatment randomized to medications or
surgery. Ophthalmology. 108:1943–1953. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Leske MC, Heijl A, Hyman L, Bengtsson B
and Komaroff E: Factors for progression and glaucoma treatment: The
Early Manifest Glaucoma Trial. Curr Opin Ophthalmol. 15:102–106.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Comparison of glaucomatous progression
between untreated patients with normal-tension glaucoma and
patients with therapeutically reduced intraocular pressures.
Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol.
126:487–497. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Diekmann H and Fischer D: Glaucoma and
optic nerve repair. Cell Tissue Res. 353:327–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gauthier AC and Liu J: Neurodegeneration
and neuroprotection in glaucoma. Yale J Biol Med. 89:73–79.
2016.PubMed/NCBI
|
|
9
|
He S, Stankowska DL, Ellis DZ,
Krishnamoorthy RR and Yorio T: Targets of neuroprotection in
glaucoma. J Ocul Pharmacol Ther. 34:85–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Lv J, Huang C, Li X, Chen Y, Wu W
and Wu R: Human Umbilical Cord-mesenchymal stem cells survive and
migrate within the vitreous cavity and ameliorate retinal damage in
a novel rat model of chronic glaucoma. Stem Cells Int.
2021(8852517)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Büchi ER: Cell death in rat retina after
pressure-induced ischaemia-reperfusion insult: Electron microscopic
study. II. Outer nuclear layer. Jpn J Ophthalmol. 36:62–68.
1992.PubMed/NCBI
|
|
12
|
Büchi ER: Cell death in the rat retina
after a pressure-induced ischaemia-reperfusion insult: An electron
microscopic study. I. Ganglion cell layer and inner nuclear layer.
Exp Eye Res. 55:605–613. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Burgoyne CF, Downs JC, Bellezza AJ, Suh JK
and Hart RT: The optic nerve head as a biomechanical structure: A
new paradigm for understanding the role of IOP-related stress and
strain in the pathophysiology of glaucomatous optic nerve head
damage. Prog Retin Eye Res. 24:39–73. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fechtner RD and Weinreb RN: Mechanisms of
optic nerve damage in primary open angle glaucoma. Surv Ophthalmol.
39:23–42. 1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fahy ET, Chrysostomou V and Crowston JG:
Mini-review: Impaired axonal transport and glaucoma. Curr Eye Res.
41:273–283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hartsock MJ, Cho H, Wu L, Chen WJ, Gong J
and Duh EJ: A Mouse model of retinal ischemia-reperfusion injury
through elevation of intraocular pressure. J Vis Exp.
54065:2016.PubMed/NCBI View
Article : Google Scholar : doi:
10.3791/54065.
|
|
17
|
Osborne NN, Ugarte M, Chao M, Chidlow G,
Bae JH, Wood JP and Nash MS: Neuroprotection in relation to retinal
ischemia and relevance to glaucoma. Surv Ophthalmol. 43 (Suppl
1):S102–S128. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Faiq MA, Wollstein G, Schuman JS and Chan
KC: Cholinergic nervous system and glaucoma: From basic science to
clinical applications. Prog Retin Eye Res.
72(100767)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adornetto A, Russo R and Parisi V:
Neuroinflammation as a target for glaucoma therapy. Neural Regen
Res. 14:391–394. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Joly S, Lange C, Thiersch M, Samardzija M
and Grimm C: Leukemia inhibitory factor extends the lifespan of
injured photoreceptors in vivo. J Neurosci. 28:13765–13774.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Leibinger M, Müller A, Andreadaki A, Hauk
TG, Kirsch M and Fischer D: Neuroprotective and axon
growth-promoting effects following inflammatory stimulation on
mature retinal ganglion cells in mice depend on ciliary
neurotrophic factor and leukemia inhibitory factor. J Neurosci.
29:14334–14341. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Burdon T, Smith A and Savatier P:
Signalling, cell cycle and pluripotency in embryonic stem cells.
Trends Cell Biol. 12:432–438. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mathieu ME, Saucourt C, Mournetas V,
Gauthereau X, Thézé N, Praloran V, Thiébaud P and Bœuf H:
LIF-dependent signaling: New pieces in the Lego. Stem Cell Rev Rep.
8:1–15. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Peñuelas S, Anido J, Prieto-Sánchez RM,
Folch G, Barba I, Cuartas I, García-Dorado D, Poca MA, Sahuquillo
J, Baselga J and Seoane J: TGF-beta increases glioma-initiating
cell self-renewal through the induction of LIF in human
glioblastoma. Cancer Cell. 15:315–327. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pera MF and Tam PP: Extrinsic regulation
of pluripotent stem cells. Nature. 465:713–720. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zeng X, Huang Z, Mao X, Wang J, Wu G and
Qiao S: N-carbamylglutamate enhances pregnancy outcome in rats
through activation of the PI3K/PKB/mTOR signaling pathway. PLoS
One. 7(e41192)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zouein FA, Kurdi M and Booz GW: LIF and
the heart: Just another brick in the wall? Eur Cytokine Netw.
24:11–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rattner A and Nathans J: The genomic
response to retinal disease and injury: Evidence for endothelin
signaling from photoreceptors to glia. J Neurosci. 25:4540–4549.
2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Samardzija M, Wariwoda H, Imsand C, Huber
P, Heynen SR, Gubler A and Grimm C: Activation of survival pathways
in the degenerating retina of rd10 mice. Exp Eye Res. 99:17–26.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Samardzija M, Wenzel A, Aufenberg S,
Thiersch M, Remé C and Grimm C: Differential role of Jak-STAT
signaling in retinal degenerations. Faseb J. 20:2411–2413.
2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schaeferhoff K, Michalakis S, Tanimoto N,
Fischer MD, Becirovic E, Beck SC, Huber G, Rieger N, Riess O,
Wissinger B, et al: Induction of STAT3-related genes in fast
degenerating cone photoreceptors of cpfl1 mice. Cell Mol Life Sci.
67:3173–3186. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Agca C and Grimm C: Leukemia inhibitory
factor signaling in degenerating retinas. Adv Exp Med Biol.
801:389–394. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu SC, Tsang NM, Chiang WC, Chang KP,
Hsueh C, Liang Y, Juang JL, Chow KP and Chang YS: Leukemia
inhibitory factor promotes nasopharyngeal carcinoma progression and
radioresistance. J Clin Invest. 123:5269–5283. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Chen H, Qu Y, Tang B, Xiong T and Mu D:
Role of mammalian target of rapamycin in hypoxic or ischemic brain
injury: Potential neuroprotection and limitations. Rev Neurosci.
23:279–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hu Q, Huang C, Wang Y and Wu R: Expression
of leukemia inhibitory factor in the rat retina following acute
ocular hypertension. Mol Med Rep. 12:6577–6583. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu J, Li Y, Song S, Cepurna W, Morrison J
and Wang RK: Evaluating changes of blood flow in retina, choroid,
and outer choroid in rats in response to elevated intraocular
pressure by 1300 nm swept-source OCT. Microvasc Res. 121:37–45.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lafuente MP, Villegas-Pérez MP,
Sellés-Navarro I, Mayor-Torroglosa S, Miralles de Imperial J and
Vidal-Sanz M: Retinal ganglion cell death after acute retinal
ischemia is an ongoing process whose severity and duration depends
on the duration of the insult. Neuroscience. 109:157–168.
2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liao XX, Chen D, Shi J, Sun YQ, Sun SJ, So
KF and Fu QL: The expression patterns of Nogo-A, myelin associated
glycoprotein and oligodendrocyte myelin glycoprotein in the retina
after ocular hypertension: The expression of myelin proteins in the
retina in glaucoma. Neurochem Res. 36:1955–1961. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ueki Y, Wang J, Chollangi S and Ash JD:
STAT3 activation in photoreceptors by leukemia inhibitory factor is
associated with protection from light damage. J Neurochem.
105:784–796. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Majumder A, Banerjee S, Harrill JA,
Machacek DW, Mohamad O, Bacanamwo M, Mundy WR, Wei L, Dhara SK and
Stice SL: Neurotrophic effects of leukemia inhibitory factor on
neural cells derived from human embryonic stem cells. Stem Cells.
30:2387–2399. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Crowley LC and Waterhouse NJ: Detecting
cleaved Caspase-3 in apoptotic cells by flow cytometry. Cold Spring
Harb Protoc. (2016)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Soldani C and Scovassi AI:
Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update.
Apoptosis. 7:321–328. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Seki M and Lipton SA: Targeting
excitotoxic/free radical signaling pathways for therapeutic
intervention in glaucoma. Prog Brain Res. 173:495–510.
2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lee HJ, Lee JO, Lee YW, Kim SA, Seo IH,
Han JA, Kang MJ, Kim SJ, Cho YH, Park JJ, et al: LIF, a novel
myokine, protects against amyloid-beta-induced neurotoxicity via
akt-mediated autophagy signaling in hippocampal cells. Int J
Neuropsychopharmacol. 22:402–414. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bürgi S, Samardzija M and Grimm C:
Endogenous leukemia inhibitory factor protects photoreceptor cells
against light-induced degeneration. Mol Vis. 15:1631–1637.
2009.PubMed/NCBI
|
|
46
|
Morgan-Warren PJ, Berry M, Ahmed Z, Scott
RA and Logan A: Exploiting mTOR signaling: A novel translatable
treatment strategy for traumatic optic neuropathy? Invest
Ophthalmol Vis Sci. 54:6903–6916. 2013.PubMed/NCBI View Article : Google Scholar
|