Introduction
Systemic lupus erythematosus SLE is a complicated
inflammatory autoimmune disease. SLE frequently affects multiple
systems in the body, with the kidney being one of the most commonly
involved organs (1). Lupus
nephritis (LN) affects 40-70% of patients with SLE and has become a
major risk factor for end-stage renal disease and death (2). Previous studies have reported that
release of free radicals and inflammatory cytokines, as well as an
imbalance in T cell metabolism and subsets, are the primary causes
of autoimmune disease in patients with SLE (3,4).
Inflammatory signalling disorders, hyperactivation
of effector cell subtypes and autoantibody production have been
reported as causes of SLE (5-7).
Previous studies have reported that as an important element of the
effector and regulatory immune responses, T cell dysfunction is
widespread in patients with SLE (8,9).
Emerging evidence suggests that the imbalance of T helper 17 (Th17)
and regulatory T cells (Tregs) serves an important role in the
pathogenesis of SLE (8,10). Increased numbers of Th17 cells have
been reported to serve a key role in the pathogenesis of SLE and LN
(11,12). Moreover, blocking IL-17 is reported
to be a promising treatment strategy for SLE (13). However, based on the reduction and
dysfunction of Treg in patients with SLE, using Treg as a
therapeutic target is also expected to alleviate SLE (14). Therefore, it may be an effective
strategy to treat SLE and LN by correcting Treg/Th17 imbalance.
MicroRNAs (miRNAs or miRs), a class of
single-stranded small non-coding RNAs with lengths ranging from 19
to 23 nucleotides, typically regulate the expression of their
target gene by binding to the 3'-untranslated region (3'-UTR) of
mRNAs (15,16). Previous studies have reported that
miRNAs are important in the regulation of immune homeostasis and
abnormal miRNA expression has been reported in patients with
autoimmune disease (17,18). A recent study reported that
compared with healthy volunteers, miRNA in patients with SLE was
differentially expressed and may be a promising biological target
for SLE treatment (19).
miR-199a-5p alleviates SLE by promoting splenic CD4+ T
cell senescence (20). miR-654
treatment decreases macrophage migration inhibitory factor
expression and downstream inflammatory cytokine production, which
is reported to improve murine LN (21). Numerous studies have reported that
miR-146a underexpression contributes to changes in the type I
interferon (IFN) pathway in patients with SLE (22,23).
Furthermore, administration of miR-146a relieves renal injury in
mice with SLE (24); however, the
underlying mechanism is not clear. Previous studies have reported
that miRNA regulates the proportion of Treg/Th17 subsets in
vivo and in vitro and serves an immunotherapeutic role
in autoimmune diseases (25,26).
To the best of our knowledge, however, the immunotherapeutic action
of SLE by correction of the Treg/Th17 imbalance via miRNA
supplementation, has been rarely reported (27).
In the present study, the regulatory effect of
miR-146a-5p on differentiation of Treg/Th17 in MRL/lpr mice was
assessed to evaluate whether miR-146a-5p may be a potential
therapeutic target for treatment of SLE-induced renal lesions.
Materials and methods
Animals and groups
A total of 30 female MRL/lpr mice (weighing 37.9 ±
1.5 g age, 8 weeks) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. and housed in a specific-pathogen-free laboratory
under standard humidity (40-60%) and temperature (25˚C), with a
12/12-h light/dark cycle and with free access to standard diet and
water. The Ethics Board of Yantai Hospital of Traditional Chinese
Medicine approved all experimental procedures (approval no.
2021-09). The mice received humane care and all efforts were made
to alleviate suffering.
MRL/lpr mice were randomly divided into three groups
(n=10/group) as follows: i) Vehicle (VEH); ii) miR-146a-5p agomir
(M146AG) and iii) agomir negative control (NC) (Fig. 1A). At the age of 10-13 weeks, the
MRL/lpr mice in the M146AG group and NC group were administered 20
nmol M146AG or M146AG NC in 0.2 ml saline weekly via injection into
the tail vein. MRL/lpr mice in the VEH group were injected with
equal volumes of saline via the tail vein on a weekly basis. All
mice were sacrificed 1 week after the last injection. Guangzhou
RiboBio Co., Ltd. synthesized the M146AG and M146AG NC; sequences
used are presented in Table
SI.
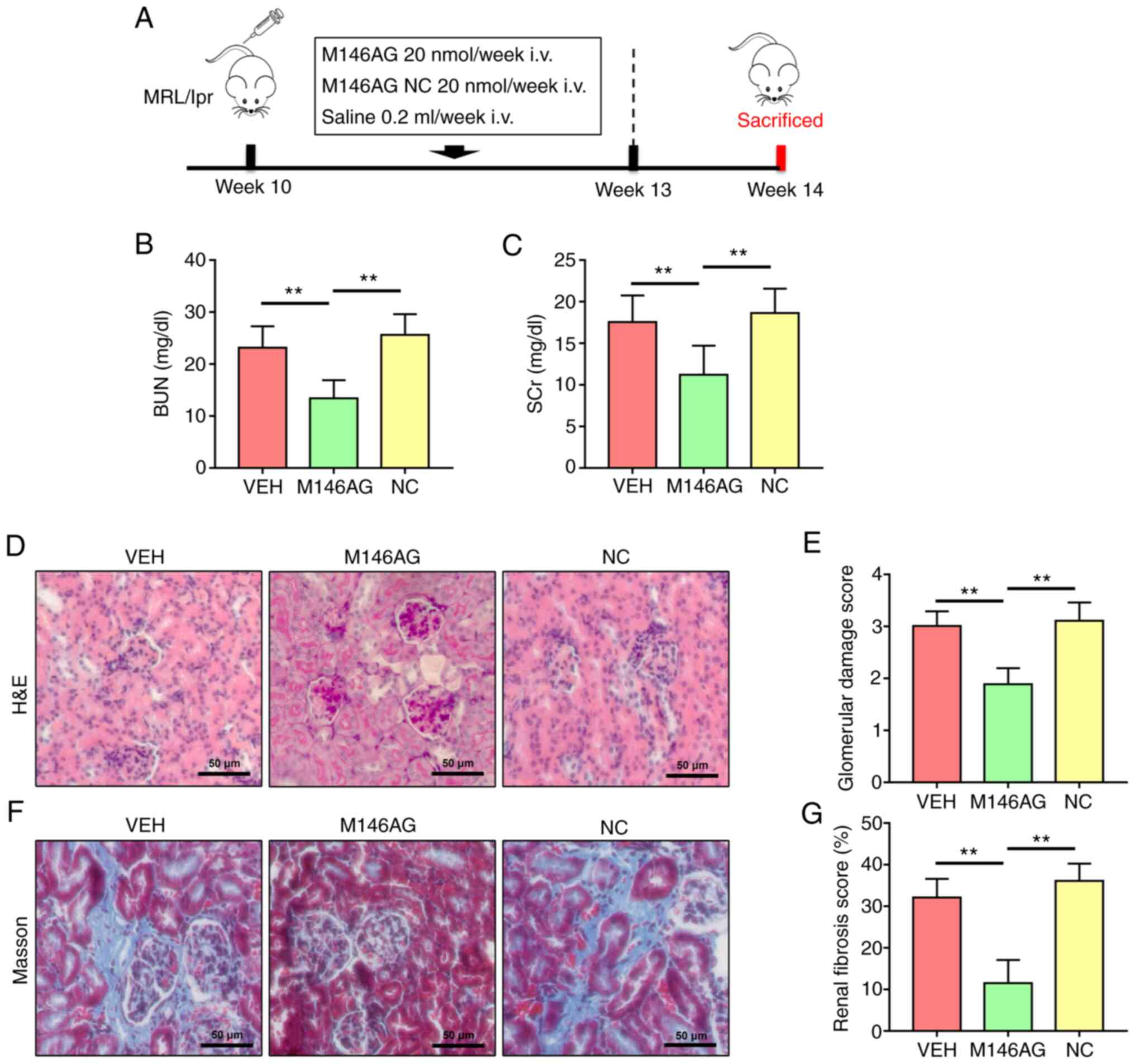 | Figure 1M146AG alleviates renal lesions in
MRL/lpr mice. (A) Schematic representation of the experimental
design. Renal function was assessed using (B) BUN and (C) SCr
levels. (D) Representative micrographs of H&E-stained renal
sections (scale bar, 50 µm). (E) Glomerular injury score. (F)
Representative micrographs of Masson's trichrome-stained renal
sections (scale bar, 50 µm). (G) Renal fibrosis score. All data are
presented as the mean ± standard deviation. **P<0.01.
i.v., intravenous; VEH, vehicle; NC, negative control; miR,
microRNA; M146AG, miR-146a-5p agomir; H&E, hematoxylin and
eosin; BUN, blood urea nitrogen; SCr, serum creatinine. |
Urine samples (500 µl) were collected once /week and
centrifuged at 3,000 x g for 15 min at 4˚C; supernatant was stored
at -80˚C until use for urinary protein assessment. At the end of
the experiment mice were anesthetized using ether and sacrificed
using exsanguination via the aorta, with blood samples (500 µl)
taken from the abdominal aorta. To collect serum, the blood was
centrifuged at 3,000 x g for 15 min at 4˚C. Mice were confirmed
dead when there was no autonomous respiration, no reflex activity
and no heart activity. The kidneys were dissected, with the left
kidney used for pathological analysis and the right kidney used for
western blotting. Separated serum and kidney were stored at -80˚C
until use in subsequent experiments.
Assessment of kidney function
Blood urea nitrogen (BUN) and serum creatinine (SCr)
concentrations were assessed using commercially available Urea
Nitrogen (BUN) Test (cat. no. C013-2-1; Nanjing Jiancheng
Bioengineering Institute) and Creatinine (Cr) Assay kit (cat. no.
C011-2-1; Nanjing Jiancheng Bioengineering Institute),
respectively. The manufacturer's instructions for the corresponding
assay kit were precisely followed.
Histological examination
Renal tissue was fixed using 10% formaldehyde for 24
h at 4˚C, embedded in paraffin, cut into 4 µm sections and mounted
on slides. The prepared slides were deparaffinized twice in xylene
at room temperature and rehydrated using an ethanol gradient before
being stained independently with Masson's trichrome (5 min, room
temperature) and hematoxylin and eosin (H&E) (5 min, room
temperature). Tissue injury was evaluated blindly and rated using a
glomerular damage score, as previously described (28). The severity of glomerulonephritis
was graded on a 0-4 scale as follows: 0, normal; 1, mild increase
in mesangial cellularity and matrix; 2, moderate increase in
mesangial cellularity and matrix, with thickening of the glomerular
basement membrane (GBM); 3, focal endocapillary hyper cellularity
with obliteration of capillary lumina and a substantial increase in
the thickness and irregularity of the GBM and 4, diffuse
endocapillary hyper cellularity, segmental necrosis, crescents and
hyalinized end-stage glomeruli.
Masson's trichrome staining was used to evaluate the
area of renal interstitial fibrosis according to the manufacturer's
protocol (cat. no. G1340, Beijing Solarbio Science & Technology
Co., Ltd.) (29). A DM4000 light
microscope was used to obtain images (Leica Microsystems GmbH).
ImageJ (version 1.53; National Institutes of Health) was used to
calculate fibrosis as the percentage of blue collagen-stained area
relative to total tissue in each field of view as follows: Renal
fibrosis score (%)=the area of Masson's trichrome-stained collagen
(blue)/total area.
Assessment of serum cytokines
The levels of cytokines IFN-γ, IL-6, TNF-α and
IL-17A were assessed in mouse serum using the Bio-Plex
Pro™ Mouse Cytokine TH17 Panel A 6-Plex kit (cat. no.
M6000007NY; Bio-Rad Laboratories, Inc.). The manufacturer's
instructions for the corresponding assay kit were followed.
Flow cytometry
A FACSCanto II flow cytometer (BD Biosciences) and
FlowJo software (version 10.7.1; Tree Star) were used to analyze
the percentage of Th17 and Treg cells in peripheral blood
mononuclear cells. Cells were incubated in the dark for 25 min at
4˚C with antibodies against surface markers. For intracellular
staining, cells were fixed, permeabilized using Cytofix/Cytoperm
kit (cat. no. 554714, BD Biosciences) for 15 min at 4˚C and stained
for 50 min at 4˚C in the dark with fluorescently labeled
antibodies. The following antibodies were used: Anti-mouse CD3 APC
(cat. no. MA1-81438; eBioscience; Thermo Fisher Scientific, Inc.),
CD4 FITC (cat. no. MHCD0401; eBioscience; Thermo Fisher Scientific,
Inc.), CD25 Percp-cy5.5 (cat. no. 45-0251-82; eBioscience; Thermo
Fisher Scientific, Inc.), Foxp3 PE (cat. no. 12-5773-82;
eBioscience; Thermo Fisher Scientific, Inc.) and IL-17 PE (cat. no.
12-7178-42; eBioscience; Thermo Fisher Scientific, Inc.).
Immunofluorescence
Immunofluorescence was performed as previously
described (30). Briefly,
CD4+ T cells were fixed using 4% paraformaldehyde at
room temperature for 30 min, washed with PBS and blocked using 0.3%
Triton X-100, 10% normal goat serum (cat. no. SL038, Beijing
Solarbio Science & Technology Co., Ltd) and 1% bovine serum
albumin (cat. no. S9020, Beijing Solarbio Science & Technology
Co., Ltd) at room temperature for 2 h. Cells were treated at 4˚C
overnight with primary antibodies against tumor necrosis factor
receptor-associated factor 6 (TRAF6; 1:400; cat. no. 8028, Cell
Signaling Technology, Inc.) and nuclear factor (NF)-κB (1:400; cat.
no. 6956, Cell Signaling Technology, Inc.). After adding
fluorescently labeled goat anti-mouse IgG (DyLight 549; 1:200; cat.
no. ab96881; Abcam) and goat anti-rabbit IgG (DyLight 488; 1:200;
cat. no. ab96899; Abcam) secondary antibodies, samples were
incubated at room temperature for 2 h. DAPI (cat. no. 4083, Cell
Signaling Technology, Inc) was used as a nuclear counterstain and
samples were incubated at room temperature for 2 min. The images
were obtained using a LSM880 fluorescent microscope with Airyscan
(Zeiss GmbH) and analyzed using ImageJ (version 1.53; National
Institutes of Health).
Western blotting
Western blot analysis was performed as described
previously (31,32). Briefly, CD4+ T cells
were lysed for 30 min on ice using RIPA buffer (cat. no. R0010;
Beijing Solarbio Science & Technology Co., Ltd.) containing 1%
phenylmethanesulfonyl fluoride (cat. no. P0100; Beijing Solarbio
Science & Technology Co., Ltd.). The protein content was
quantified using a BCA protein assay kit (cat. no. PC0020; Beijing
Solarbio Science & Technology Co., Ltd). SDS-PAGE was performed
on 5-15% linear acrylamide gradient gel (10 µg protein/lane). After
transferring proteins onto PVDF membranes, the membranes were
blocked for 2 h at room temperature using 5% skimmed milk powder.
The membranes were then incubated overnight at 4˚C with primary
antibodies as follows: TRAF6 (1:3,000; cat. no. 8028; Cell
Signaling Technology, Inc.), NF-κB (1:3,000; cat. no. 8242; Cell
Signaling Technology, Inc.), β-actin (1:5,000; cat. no. 4970; Cell
Signaling Technology, Inc.), FoxP3 (1:3,000; cat. no. 12632; Cell
Signaling Technology, Inc.) and IL-17A (1:3,000; cat. no. 13838;
Cell Signaling Technology, Inc.). The membranes were incubated with
an anti-rabbit IgG (H+L) (1:5,000; cat. no. 14708, Cell Signaling
Technology, Inc.) secondary antibody for 2 h at room temperature,
followed by washing 3 times using TBST (20% Tween 20). The protein
bands were visualized using developer a ECL kit (cat. no. P0018FS;
Beyotime Institute of Biotechnology), and semi-quantitative
densitometry was performed on the identified bands using Image
Quant 5.2 software (Molecular Dynamics, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the tissue samples using
TRIzon Reagent (cat. no. CW0580, CoWin Biosciences) according to
the manufacturer's protocol. A total of 1 µg of total RNA was
reverse transcribed into cDNA at 37˚C for 60 min and 4˚C for 5 min
using a First-strand cDNA Synthesis kit (cat. no. E6300L; New
England BioLabs, Inc.) according to the manufacturer's protocol.
qPCR assay was performed by using SYBR green dye on Step One
sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
initial predenaturation step at 95˚C for 3 min, followed by 39
cycles of 95˚C for 20 sec and 60˚C for 30 sec. The
2-∆∆Cq method was used to assess
the relative abundance (33). The
internal reference used for mRNA was GAPDH and the internal
reference used for miR-146a-5p was U6. Primers are presented in
Table SII.
Dual-luciferase reporter gene
assay
miR-146a-5p mimics/inhibitors and corresponding NC
were manufactured by Guangzhou RiboBio Co., Ltd. (Table SI). The 293T cell line was used
for the luciferase reporter assay. The 3'-UTR of TRAF6 which
contained the predicted binding targets of miR-146a-5p (targetscan.org/vert_72/) was cloned into the
c-myc luciferase reporter plasmid (Genomeditech) with either
original wild-type (WT) or altered mutant sequence (MUT) at the
binding sites. The 293T cells were co-transfected using 800 ng
TRAF6-WT 3'-UTR or TRAF6-MUT 3'-UTR dual luciferase reporter
plasmid vector and 20 µM miR-146a-5p mimic, inhibitor or NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Dual-Luciferase Reporter Assay System (E1910,
Promega Corporation) was used to assess Renilla and Firefly
luciferase activity of lysed 293T cells 24 h after
transfection.
Statistical analysis
Statistical analyses were performed using SPSS
(version 19.0; IBM, Corp.). Three independent experiments were
performed and data are presented as mean ± standard deviation.
One-way analysis of variance with Tukey's post hoc test and the
unpaired Student's t-test were used for comparisons between ≥3 and
2 groups, respectively. Spearman's rank correlation coefficient was
used to assess the correlation between two independent samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
M146AG alleviates renal lesions in
MRL/lpr mice
To evaluate the effect of M146AG on renal function
parameters of MRL/lpr mice, the concentration of BUN and SCr was
assessed. The levels of BUN and SCr were significantly decreased in
the M146AG group compared with both the VEH and NC groups (Fig. 1B and C).
H&E and Masson staining were used to evaluate
the effect of M146AG on renal histopathology of MRL/lpr mice. In
the VEH and NC groups, H&E staining demonstrated glomerular
swelling and extracellular matrix deposition, as well as a high
level of inflammatory cell infiltration (Fig. 1D). Furthermore, Masson staining
demonstrated that levels of renal interstitial fibrosis in the VEH
and NC group were markedly higher than those in the M146AG group
(Fig. 1F). However, M146AG
treatment significantly alleviated the glomerular damage and renal
interstitial fibrosis scores in MRL/lpr mice compared with both the
VEH and NC groups (Fig. 1D-G).
These results indicated that M146AG improved renal lesions in
MRL/lpr mice.
M146AG inhibits expression of
inflammatory factors in the serum and renal tissue of MRL/lpr
mice
The effect of M146AG on protein and mRNA expression
levels of inflammatory factors in serum and renal tissue of MRL/lpr
mice was assessed. Protein expression levels of serum inflammatory
factors IL-6, IL-17A, TNF-α and IFN-γ were significantly decreased
following M146AG intervention compared with the VEH and NC groups
(Fig. 2A-D). Similarly, mRNA
expression levels of inflammatory markers IL-6, IL-17A, TNF-α and
IFN-γ were also significantly decreased in the renal tissues of the
M146AG group compared with the VEH and NC groups (Fig. 2E-H). These results demonstrated
that M146AG alleviated the inflammatory response in MRL/lpr
mice.
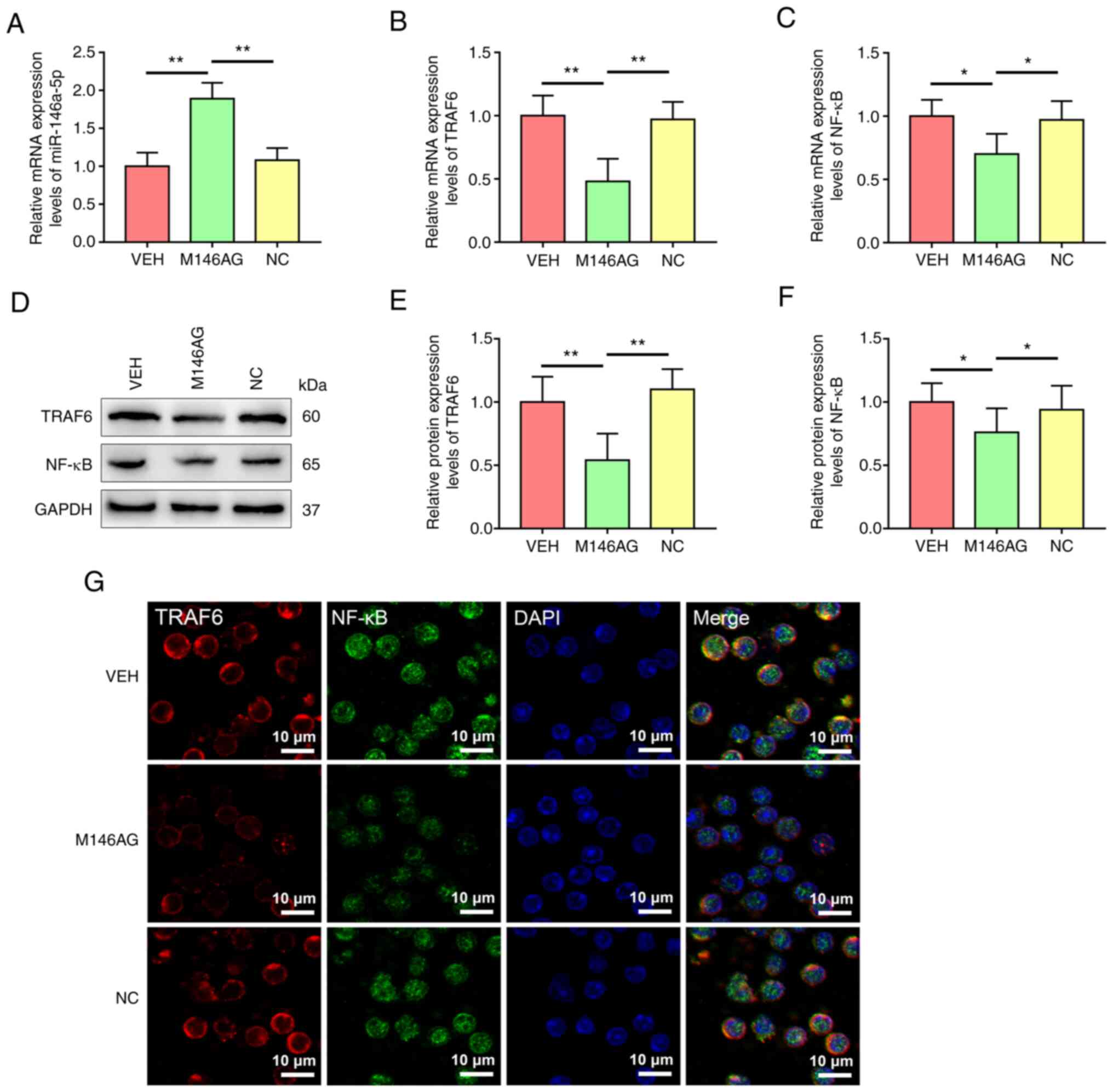
M146AG regulates mRNA expression
levels of miR-146a-5p and the TRAF6/NF-κB axis components in
CD4+ T cells of MRL/lpr mice
To evaluate the potential molecular mechanisms by
which M146AG alleviated renal lesions in MRL/lpr mice, expression
levels of miR-146a-5p and mRNA and protein expression levels of the
TRAF6/NF-κB axis components in CD4+ T cells were
assessed. The expression of miR-146a-5p was significantly increased
in CD4+ T cells in the M146AG treatment group compared
with the VEH and NC groups (Fig.
3A). Furthermore, compared with the VEH and NC groups, mRNA and
protein expression levels of TRAF6 and NF-κB were significantly
decreased in CD4+ T cells with M146AG treatment
(Fig. 3B-F). Subsequent
microscopic analysis of TRAF6 and NF-κB expression levels further
demonstrated the downregulate in protein expression levels in
CD4+ T cells of MRL/lpr mice after M146AG treatment
(Fig. 3G). These data indicated
that M146AG intervention inhibited mRNA and protein expression
levels of the TRAF6/NF-κB axis components by upregulating
expression of miR-146a-5p in the CD4+ T cells of MRL/lpr
mice.
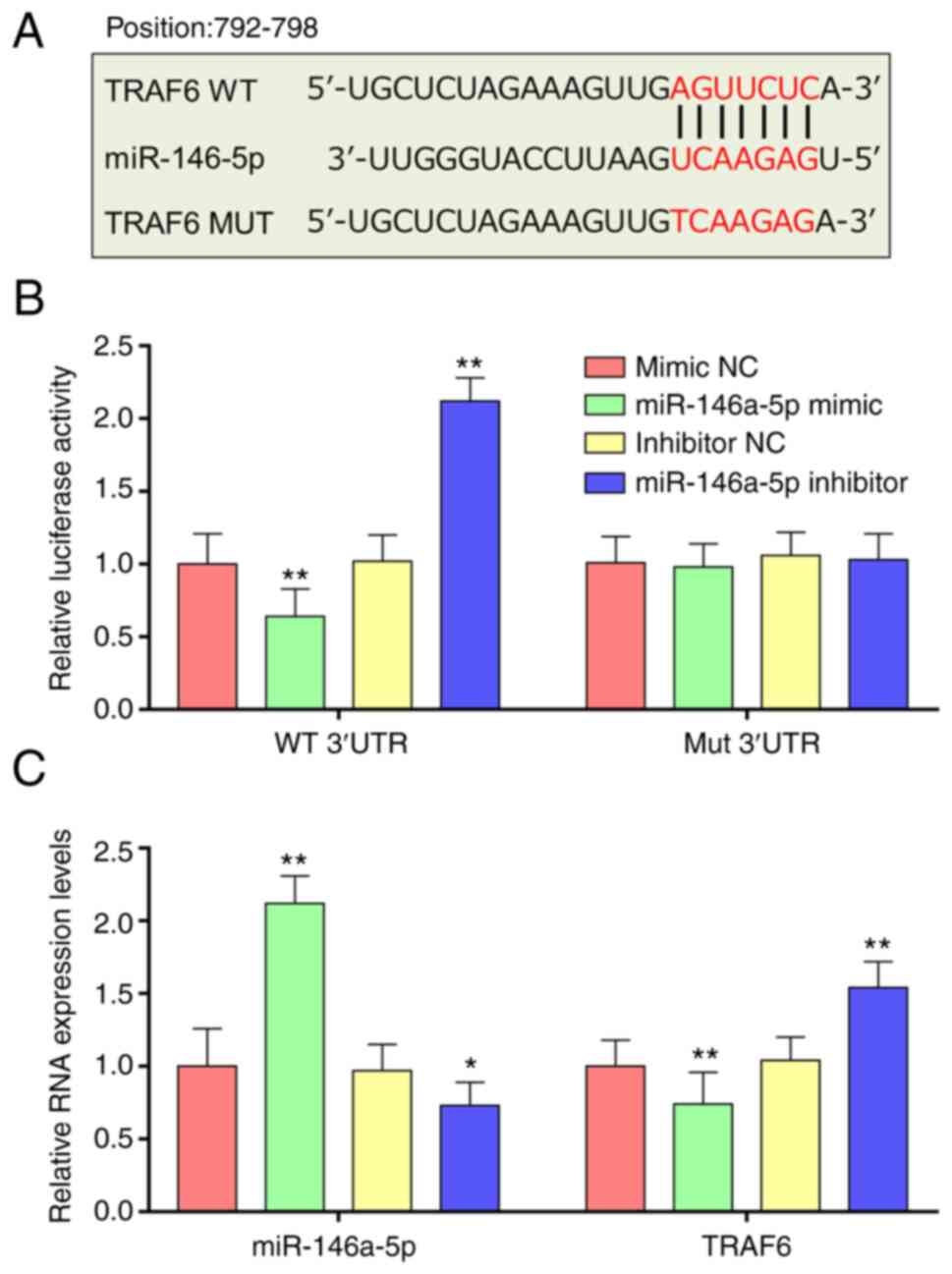
miR-146a-5p directly targets
TRAF6
Potential binding sites between miR-146a-5p and
TRAF6-3'-UTR sequences were assessed using Targetscan (Fig. 4A). To evaluate if miR-146a-5p
influenced TRAF6 expression levels by targeting its 3'-UTR, a
luciferase reporter system was constructed. Compared with the NC
group, miR-146a-5p mimic significantly decreased luciferase
activity, whereas miR-146a-5p inhibitor treatment significantly
increased relative luciferase activity compared with the NC group
(Fig. 4B). Furthermore, when the
seed sequence of the miR-146a-5p binding sites was altered, the
effect of miR-146a-5p on luciferase activity was not demonstrated
(Fig. 4B). Expression levels of
miR-146a-5p in 293T cells were significantly increased compared
with the NC. RT-qPCR demonstrated significantly lower TRAF6 mRNA
expression levels in cells treated with miR-146a-5p mimics compared
with NC; however, significantly increased TRAF6 mRNA expression
levels were demonstrated in cells treated with miR-146a-5p
inhibitor compared with NC (Fig.
4C). These results demonstrated that miR-146a-5p targeted and
negatively regulated TRAF6 expression.
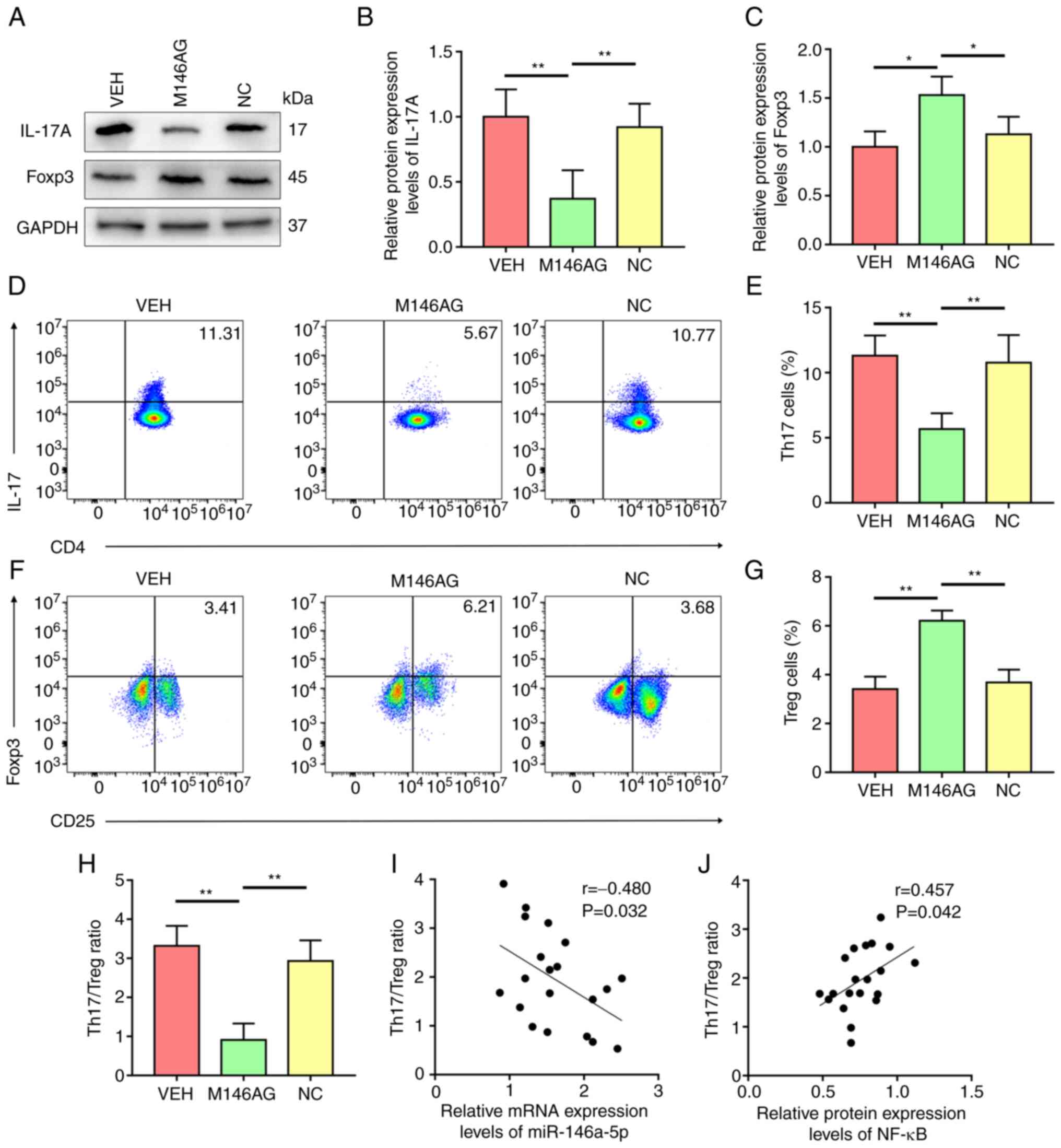
M146AG improves the Treg/Th17
imbalance in MRL/lpr mice
To elucidate the mechanism of M146AG-mediated
regulation of inflammatory responses, expression levels of IL-17A
and Foxp3 in CD4+ T cells of MRL/lpr mice were
evaluated. Compared with the VEH and NC group, the level of IL-17A
was considerably decreased and protein expression of Foxp3 was
significantly increased in CD4+ T cells following M146AG
treatment (Fig. 5A-C). Consistent
with this, the percentage of Th17 cells significantly decreased and
the percentage of Treg cells was significantly increased in the
M146AG group compared with the VEH and NC groups (Fig. 5D-G). Furthermore, M146AG treatment
significantly lowered the ratio of Th17 to Treg in MRL/lpr mice
compared with VEH and NC groups (Fig.
5H). Pearson's correlation analysis demonstrated a significant
negative association between Th17/Treg ratio and the mRNA
expression levels of miR-146a-5p in the CD4+ T cells of
MRL/lpr mice (Fig. 5I).
CD4+ T cells of MRL/lpr mice demonstrated a significant
positive correlation between Th17/Treg ratio and the relative
protein expression of NF-κB (Fig.
5J). These data indicated that M146AG may restore the Th17/Treg
balance in MRL/lpr mice by targeting the TRAF6/ NF-κB axis.
Discussion
The inflammatory response caused by T cell subset
differentiation and metabolic imbalance is a key cause of SLE.
miR-146a-5p in the peripheral blood of patients with SLE is
reported to be downregulated, but its direct targets and biological
functions are still unclear (22).
In the present study, M146AG intervention in vivo
demonstrated that M146AG treatment repaired the inflammatory
response and kidney pathological injury and improved the Treg/Th17
imbalance in peripheral blood of MRL/lpr mice. Furthermore, using
bioinformatics tools combined with cell experiments, it was
demonstrated that the TRAF6/NF-κB axis was the direct target of
miR-146a-5p for immunoregulation in CD4+ T cells.
The abnormal metabolism of miRNAs in patients with
SLE has received increased attention as the pathogenesis of lupus
has been studied in depth (18,34).
Therefore, it could be hypothesized that miRNA may be a potential
biomarker for the diagnosis of SLE and that a novel SLE therapies
with miRNAs as the intervention object could be developed. Thai
et al (35) reported that
elimination of miR-155 in MRL/lpr mice decreases renal inflammation
and autoantibody synthesis. Xia et al (36) reported that overexpression of
miR-326 in MRL/lpr mice results in B cell hyperactivity and serious
SLE. Previous studies reported that miR-146a is a significant
negative regulator in the immune response and its defect leads to
numerous immune disorders (24,37,38).
Fu et al (24) reported
that miR-146a protects the kidney from SLE-induced injury in
MRL/lpr mice by regulating both classical and non-classical NF-κB
signaling. In the present study, it was demonstrated that M146AG
alleviated abnormal renal function and repaired pathological damage
of renal tissue in MRL/lpr mice. According to previous reports, the
chronic inflammatory response induced by abnormal autoimmune
metabolism is the main inducing factor of LN, which primarily
manifests as significant upregulation of expression of various
pro-inflammatory cytokines, such as IL-6, IL-17A and IFN-γ
(39,40). In the present study, it was
demonstrated that M146AG intervention markedly downregulated levels
of the inflammatory factors IL-6, IFN-γ, TNF-α and IL-17A in both
peripheral blood and the renal tissue of MRL/lpr mice. These data
indicated that correcting the inflammatory reaction caused by
autoimmune disorder is a potential mechanism by which M146AG
alleviates LN.
Previous studies have reported that the imbalance of
pro-inflammatory and anti-inflammatory T cell subsets, especially
Treg and Th17, is a vital reason for the immune homeostasis
disorder in autoimmune diseases such as SLE (3,41).
Accumulated evidence indicates that the imbalance of Treg and Th17
is involved in the pathogenesis of SLE; however, the root causes of
Th17/Treg imbalance in SLE remain unelucidated (3,8). In
patients with SLE, the number of Th17 cells and Th17-related
inflammatory cytokines such as IL-6 and IL-17 are reported to be
increased (27). However, the
number of Treg cells and Treg-associated anti-inflammatory
cytokines such as TGF-β and IL-10 are reported to be decreased in
patients with SLE (42,43). The present study demonstrated that
M146AG intervention significantly reversed the imbalance of
Th17/Treg in MRL/lpr mice. These data demonstrated that reversing
the imbalance of T cell subsets may be a potential mechanism by
which M146AG repairs autoimmune disorders in SLE mice.
Previous studies have reported that the inflammatory
response mediated by the NF-κB signaling pathway is involved in LN
pathogenesis (44,45). Inhibition of the activation of the
NOD-like receptor protein 3-mediated inflammasome by inhibiting
NF-κB p65 nuclear migration has been reported to alleviate renal
inflammation (46). Increasing
studies have reported that the NF-κB pathway participates in
maintenance of Th17/Treg balance in numerous tissues and organs
(47,48). Furthermore, members of the TRAF
family are key signaling intermediaries in the NF-κB signaling
pathway upstream of the IκB kinase (IKK) complex. NF-κB activation
has been reported to be induced by TRAF6 in response to a range of
stimuli, such as cell surface or intracellular signals (49). Based on these reports, the
TRAF6/NF-κB axis as a target for treatment of SLE and other
autoimmune diseases has attracted increased attention (38,50).
It has been reported that the levels of miR-146a-5p in renal tissue
and peripheral blood are notably decreased in LN patients compared
with healthy controls, while the transcriptional activity of TRAF6
and NF-κB is enhanced (37,38).
In the present study, upregulation of expression levels of
miR-146a-5p by M146AG treatment suppressed activation of the
TRAF6/NF-κB axis and corrected the Th17/Treg imbalance in MRL/lpr
mice. Similarly, Meng et al (51) reported that miR-146a-5p inhibits
proliferation of pancreatic ductal adenocarcinoma cells by
targeting the 3'-UTR of TRAF6 and downregulation of the
TRAF6/NF-κB/p65/P-glycoprotein axis. Liu et al (52) also reported that miR-146a-5p
inhibits survival of non-small cell lung cancer cells by directly
binding to and suppressing TRAF6. In the present study, the ratio
of Th17/Treg in MRL/lpr mice was significantly correlated with
expression levels of miR-146a-5p and protein expression levels of
NF-κB in CD4+ T cells. This was consistent with a
previous report that miRNA contributes to the preservation of
immunological homeostasis in numerous physiological and
pathological situations by the regulation of Th17/Treg balance
(3). The aforementioned results
suggested that using miRNA as a therapeutic molecule to target
TRAF6 or NF-κB to inhibit NF-κB transcriptional activity and
inflammatory factor synthesis may be a promising therapeutic
strategy for SLE and LN. Nevertheless, the current study was
associated with a couple of limitations. For instance, there was a
lack of information on the time and dose dependence of M146AG
treatment.
In conclusion, the present study demonstrated a
potentially novel role for miR-146a-5p in mice with LN, which
involved alleviation of the inflammatory response via regulation of
the Th17/Treg balance. During this process, the TRAF6/NF-κB axis
may be a key target. Therefore, miR-146a-5p may be used as a
potential therapeutic target for treatment of LN.
Supplementary Material
Sequences of primers used for
constructs.
Sequences of primers used for reverse
transcription-quantitative PCR.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT and XL conceived and designed the experiments. JT
and FY performed the experiments. FY and XL analyzed the data. XL
wrote the paper. All authors have read and approved the final
manuscript. JT and XL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The Ethics Board of Yantai Hospital of Traditional
Chinese Medicine approved all experimental procedures (approval no.
2021-09).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mok CC and Lau CS: Pathogenesis of
systemic lupus erythematosus. J Clin Pathol. 56:481–490.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mohan C and Putterman C: Genetics and
pathogenesis of systemic lupus erythematosus and lupus nephritis.
Nat Rev Nephrol. 11:329–341. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheng T, Ding S, Liu S, Li X, Tang X and
Sun L: Resolvin D1 improves the Treg/Th17 imbalance in systemic
lupus erythematosus through miR-30e-5p. Front Immunol.
12(668760)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu S, Ji L, Fan X, Fang S, Bao J, Yuan X,
Fan Y and Xie G: Jieduquyuzishen prescription attenuates renal
fibrosis in MRL/lpr mice via inhibiting EMT and TGF-β1/Smad2/3
pathway. Evid Based Complement Alternat Med.
2022(4987323)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li M, Yu D, Ni B and Hao F: Interleukin-1
receptor associated kinase 1 is a potential therapeutic target of
anti-inflammatory therapy for systemic lupus erythematosus. Mol
Immunol. 87:94–101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Poissonnier A, Sanséau D, Le Gallo M,
Malleter M, Levoin N, Viel R, Morere L, Penna A, Blanco P, Dupuy A,
et al: CD95-mediated calcium signaling promotes T helper 17
trafficking to inflamed organs in lupus-prone mice. Immunity.
45:209–223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang N and Tian B: Brain-derived
neurotrophic factor in autoimmune inflammatory diseases (review).
Exp Ther Med. 22(1292)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shan J, Jin H and Xu Y: T cell metabolism:
A new perspective on Th17/Treg cell imbalance in systemic lupus
erythematosus. Front Immunol. 11(1027)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tenbrock K and Rauen T: T cell
dysregulation in SLE. Clin Immunol. 239(109031)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kubo S, Nakayamada S, Yoshikawa M,
Miyazaki Y, Sakata K, Nakano K, Hanami K, Iwata S, Miyagawa I,
Saito K and Tanaka Y: Peripheral immunophenotyping identifies three
subgroups based on T cell heterogeneity in lupus patients.
Arthritis Rheumatol. 69:2029–2037. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xiao JP, Wang DY, Wang XR, Yuan L, Hao L
and Wang DG: Increased ratio of Th17 cells to
SIGIRR+CD4+ T cells in peripheral blood of
patients with SLE is associated with disease activity. Biomed Rep.
9:339–344. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mesquita D Jr, Kirsztajn GM, Franco MF,
Reis LA, Perazzio SF, Mesquita FV, Ferreira VDS, Andrade LEC and de
Souza AWS: CD4+ T helper cells and regulatory T cells in
active lupus nephritis: an imbalance towards a predominant Th1
response? Clin Exp Immunol. 191:50–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rafael-Vidal C, Perez N, Altabas I, Garcia
S and Pego-Reigosa JM: Blocking IL-17: A promising strategy in the
treatment of systemic rheumatic diseases. Int J Mol Sci.
21(7100)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang X, Qiao Y, Yang L, Song S, Han Y,
Tian Y, Ding M, Jin H, Shao F and Liu A: Leptin levels in patients
with systemic lupus erythematosus inversely correlate with
regulatory T cell frequency. Lupus. 26:1401–1406. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu Q, Wu R, Zhao M, Garcia-Gomez A and
Ballestar E: miRNAs as therapeutic targets in inflammatory disease.
Trends Pharmacol Sci. 40:853–865. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schell SL and Rahman ZSM: miRNA-Mediated
control of B cell responses in immunity and SLE. Front Immunol.
12(683710)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang J, Liu Y and Shi G: The
circRNA-miRNA-mRNA regulatory network in systemic lupus
erythematosus. Clin Rheumatol. 40:331–339. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou S, Zhang J, Luan P, Ma Z, Dang J, Zhu
H, Ma Q, Wang Y and Huo Z: miR-183-5p is a potential molecular
marker of systemic lupus erythematosus. J Immunol Res.
2021(5547635)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cheng T, Ding S, Liu S, Li Y and Sun L:
Human umbilical cord-derived mesenchymal stem cell therapy
ameliorates lupus through increasing CD4+ T cell
senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics.
11:893–905. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tu Y, Guo R, Li J, Wang S, Leng L, Deng J,
Bucala R and Lu L: MiRNA regulation of MIF in SLE and attenuation
of murine lupus nephritis with miR-654. Front Immunol.
10(2229)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y,
Huang X, Zhou H, de Vries N, Tak PP, et al: MicroRNA-146A
contributes to abnormal activation of the type I interferon pathway
in human lupus by targeting the key signaling proteins. Arthritis
Rheum. 60:1065–1075. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo X, Yang W, Ye DQ, Cui H, Zhang Y,
Hirankarn N, Qian X, Tang Y, Lau YL, de Vries N, et al: A
functional variant in microRNA-146a promoter modulates its
expression and confers disease risk for systemic lupus
erythematosus. PLoS Genet. 7(e1002128)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fu HX, Fan XP, Li M, Liu MJ and Sun QL:
MiR-146a relieves kidney injury in mice with systemic lupus
erythematosus through regulating NF- κB pathway. Eur Rev Med
Pharmacol Sci. 23:7024–7032. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye X, Lu Q, Yang A, Rao J, Xie W, He C,
Wang W, Li H and Zhang Z: MiR-206 regulates the Th17/Treg ratio
during osteoarthritis. Mol Med. 27(64)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qu X, Han J, Zhang Y, Wang Y, Zhou J, Fan
H and Yao R: MiR-384 regulates the Th17/Treg ratio during
experimental autoimmune encephalomyelitis pathogenesis. Front Cell
Neurosci. 11(88)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang D, Huang S, Yuan X, Liang J, Xu R,
Yao G, Feng X and Sun L: The regulation of the Treg/Th17 balance by
mesenchymal stem cells in human systemic lupus erythematosus. Cell
Mol Immunol. 14:423–431. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Geng L, Tang X, Zhou K, Wang D, Wang S,
Yao G, Chen W, Gao X, Chen W, Shi S, et al: MicroRNA-663 induces
immune dysregulation by inhibiting TGF-β1 production in bone
marrow-derived mesenchymal stem cells in patients with systemic
lupus erythematosus. Cell Mol Immunol. 16:260–274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Remuzzi G, Zoja C, Gagliardini E, Corna D,
Abbate M and Benigni A: Combining an antiproteinuric approach with
mycophenolate mofetil fully suppresses progressive nephropathy of
experimental animals. J Am Soc Nephrol. 10:1542–1549.
1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xiong Y, Xiong Y, Zhang H, Zhao Y, Han K,
Zhang J, Zhao D, Yu Z, Geng Z, Wang L, et al: hPMSCs-derived
exosomal miRNA-21 protects against aging-related oxidative damage
of CD4+ T cells by targeting the PTEN/PI3K-Nrf2 axis.
Front Immunol. 12(780897)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Y and Xiong Y, Zhang A, Zhao N, Zhang
J, Zhao D, Yu Z, Xu N, Yin Y, Luan X and Xiong Y: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Y, Zhao N and Xiong Y, Zhang J, Zhao
D, Yin Y, Song L, Yin Y, Wang J, Luan X and Xiong Y: Downregulated
recycling process but not de novo synthesis of glutathione limits
antioxidant capacity of erythrocytes in hypoxia. Oxid Med Cell
Longev. 2020(7834252)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sui W, Liu F, Chen J, Ou M and Dai Y:
Microarray technology for analysis of microRNA expression in renal
biopsies of lupus nephritis patients. Methods Mol Biol.
1134:211–220. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Thai TH, Patterson HC, Pham DH, Kis-Toth
K, Kaminski DA and Tsokos GC: Deletion of microRNA-155 reduces
autoantibody responses and alleviates lupus-like disease in the
Fas(lpr) mouse. Proc Natl Acad Sci USA. 110:20194–20199.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xia Y, Tao JH, Fang X, Xiang N, Dai XJ,
Jin L, Li XM, Wang YP and Li XP: MicroRNA-326 upregulates B cell
activity and autoantibody production in lupus disease of MRL/lpr
mice. Mol Ther Nucleic Acids. 11:284–291. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu Y, Xue Z and Di L: Regulation of
MiR-146a and TRAF6 in the diagnose of lupus nephritis. Med Sci
Monit. 23:2550–2557. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zheng CZ, Shu YB, Luo YL and Luo J: The
role of miR-146a in modulating TRAF6-induced inflammation during
lupus nephritis. Eur Rev Med Pharmacol Sci. 21:1041–1048.
2017.PubMed/NCBI
|
|
39
|
Cai Z, Wong CK, Dong J, Jiao D, Chu M,
Leung PC, Lau CBS, Lau CP, Tam LS and Lam CWK: Anti-inflammatory
activities of Ganoderma lucidum (Lingzhi) and San-Miao-San
supplements in MRL/lpr mice for the treatment of systemic lupus
erythematosus. Chin Med. 11(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zickert A, Amoudruz P, Sundstrom Y,
Ronnelid J, Malmstrom V and Gunnarsson I: IL-17 and IL-23 in lupus
nephritis-association to histopathology and response to treatment.
BMC Immunol. 16(7)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Talaat RM, Mohamed SF, Bassyouni IH and
Raouf AA: Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus
erythematosus (SLE) patients: Correlation with disease activity.
Cytokine. 72:146–53. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ohl K and Tenbrock K: Regulatory T cells
in systemic lupus erythematosus. Eur J Immunol. 45:344–355.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Choi Y, Jung JH, Lee EG, Kim KM and Yoo
WH: 4-phenylbutyric acid mediates therapeutic effect in systemic
lupus erythematosus: Observations in an experimental murine lupus
model. Exp Ther Med. 21(460)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun F, Teng J, Yu P, Li W, Chang J and Xu
H: Involvement of TWEAK and the NF-κB signaling pathway in lupus
nephritis. Exp Ther Med. 15:2611–2619. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sun L, Zou LX, Han YC, Wu L, Chen T, Zhu
DD and Hu P: A20 overexpression exerts protective effects on
podocyte injury in lupus nephritis by downregulating UCH-L1. J Cell
Physiol: Feb 25, 2019 (Epub ahead of print).
|
|
46
|
Zhang H, Liu L and Li L:
Lentivirus-mediated knockdown of FcgammaRI (CD64) attenuated lupus
nephritis via inhibition of NF-κB regulating NLRP3 inflammasome
activation in MRL/lpr mice. J Pharmacol Sci. 137:342–349.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cui C, Zhang D, Sun K, Li H, Xu L, Lin G,
Guo Y, Hu J, Chen J, Nong L, et al: Propofol maintains Th17/Treg
cell balance and reduces inflammation in rats with traumatic brain
injury via the miR1453p/NFATc2/NF-κB axis. Int J Mol Med.
48(135)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen C, Hu N, Wang J, Xu L, Jia XL, Fan X,
Shi JX, Chen F, Tu Y, Wang YW and Li XH: Umbilical cord mesenchymal
stem cells promote neurological repair after traumatic brain injury
through regulating Treg/Th17 balance. Brain Res.
1775(147711)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dong C, Zhou Q, Fu T, Zhao R, Yang J, Kong
X, Zhang Z, Sun C, Bao Y, Ge X, et al: Circulating exosomes
derived-miR-146a from systemic lupus erythematosus patients
regulates senescence of mesenchymal stem cells. Biomed Res Int.
2019(6071308)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Meng Q, Liang C, Hua J, Zhang B, Liu J,
Zhang Y, Wei M, Yu X, Xu J and Shi S: A miR-146a-5p/TRAF6/NF-kB p65
axis regulates pancreatic cancer chemoresistance: Functional
validation and clinical significance. Theranostics. 10:3967–3979.
2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu X, Liu B, Li R, Wang F, Wang N, Zhang
M, Bai Y, Wu J, Liu L, Han D, et al: miR-146a-5p plays an oncogenic
role in NSCLC via suppression of TRAF6. Front Cell Dev Biol.
8(847)2020.PubMed/NCBI View Article : Google Scholar
|