Introduction
The deterioration of neurological deficits is a
frequent complication in acute stroke (1). Early progressive ischemic stroke
(PIS) tightly related to poor prognosis (2,3). The
existence of this gradual deterioration implies that it should
theoretically be possible to at least arrest or delay this process.
To the best of our knowledge, there is as yet no unified
international definition or diagnostic criteria for PIS. The main
differences in the types of PIS lie in the definition of the time
window of progression and the severity of disease progression
(4-6).
PIS is generally defined as an increase of ≥2 points according to
the National Institutes of Health Stroke Scale (NIHSS) within 48 or
72 h of stroke onset (4,5). A small NIHSS change (e.g., ∆NIHSS ≥2)
is particularly applicable to the definition of PIS in minor
strokes (defined as admission NIHSS ≤5) (6). The mechanism underlying PIS remains
unclear, for which there is no effective treatment methods
(7). As a result, PIS represents a
clinical challenge.
Patients with PIS have been extensively treated
using high-dose heparin (1).
However, this is discouraged by the current stroke guidelines,
since scientific support for its efficacy is poor (1). Therefore, a clinical trial with
another therapeutic drug operating through a different mechanism is
desired. The final common pathway of platelet aggregation and
subsequent thrombus formation is the activation of glycoprotein
(GP) IIb/IIIa receptors (8).
Currently available drugs for acute ischemic stroke and acute
coronary syndrome are abciximab, tirofiban and eptifibatide, all
three of which are administered by intravascular and exert potent,
rapid anti-platelet aggregation effects (9). Abciximab binds irreversibly to
receptors and the platelet recovery time is 24-48 h after the
treatment, but results in a rather higher risk of bleeding
(10). By contrast, tirofiban and
eptifibatide bind to receptors in a reversible manner, with
platelet recovery times of 4-8 and 2-4 h, respectively, and might
be relatively safer. A previous study showed that eptifibatide had
a higher GP IIb/IIIa receptor occupancy ratio compared with
tirofiban, where the incidence of 30-day adverse cardiovascular
events was also lower compared with that of tirofiban (11). A meta-analysis also previously
showed that the safety of eptifibatide was slightly superior to
tirofiban in patients with acute coronary syndrome (12).
It has been previously shown that platelet GP
IIb/IIIa receptor inhibitors could be used for treating thrombosis
mediated by activated platelets (9,13).
Previous literature reviews summarized that a number of exploratory
studies have tested them either alone or in combination with
intravenous thrombolysis or endovascular therapy as an alternative
strategy for the management of acute ischemic stroke (9,13).
In a single-center retrospective study, 25 patients with
subcortical progressive stroke were found to be safely treated with
eptifibatide (14). However,
additional research on the efficacy of eptifibatide is required
(14). For the study of tirofiban,
another (GP) IIb/IIIa receptor inhibitor, Philipps et al
(15) previously found that during
the early stages of PIS, patients with small-vessel occlusions
tended to exhibit superior responses to tirofiban compared with
those by patients with large-vessel occlusions. Therefore, outcomes
in patients with progressive stroke may be associated with the
blood vessels that are involved.
The present report aimed to provide evidence
regarding the safety and validity of a specific low-dose
eptifibatide antiplatelet strategy for the treatment of PIS. In
addition, another aim of the present study was to test whether the
efficacy of eptifibatide in patients with PIS is related to
vascular classification.
Patients and methods
Patients
A total of 74 patients [mean age, 64.3±11.4 years;
53 males (71.6%)] with clinically confirmed PIS were enrolled in
Xiangtan Central Hospital (Xiangtan, China) between March 2020 and
March 2021. PIS was diagnosed as the condition worsening by ≥2
points according to the NIHSS within 72 h after stroke onset
(2). Inclusion criteria were the
following: i) Diagnosis of stroke as per the criteria published in
2018 in the Chinese Guidelines on the Diagnosis and Treatment of
Acute Ischemic Stroke (16); ii)
aged 18-80 years; iii) first onset, new infarction on head MRI; and
iv) stroke due to large artery atherosclerosis (LAA) or small
artery occlusion (SAO). Exclusion criteria were as follows: i)
Haemorrhagic infarction; ii) transient ischemic attack; iii)
cardiac cerebral infarction; iv) cerebral infarction with unknown
pathogenesis or other less common causes; v) received intravenous
thrombolysis or endovascular treatment in the acute phase; vi)
disturbance of blood coagulation, aberrant thrombocytopenia or
neutropenia; vii) history of previous diagnosis of cancer, brain
injury or surgery; viii) history of neurodegenerative disease or
other central nervous system diseases; and ix) history of liver
disease or another end-stage disease.
Patients were divided into the eptifibatide group
(n=32) and the control group (n=42) according to the different
treatment regimens they received. For the eptifibatide group,
eptifibatide was given to patients as a bolus injection at a
loading dose of 135 µg/kg at the time of stroke progression,
followed by a maintenance dose of 0.75 µg/kg/min for 48 h. In
addition, oral antiplatelet agents were also given 4 h before
eptifibatide administration was stopped. Patients in the control
group received oral antiplatelet medication. For the specific oral
antiplatelet drug regimens, patients with mild ischemic stroke
(NIHSS score=3) were given dual-antiplatelet medication (aspirin
100 mg/day + clopidogrel hydrochloride 75 mg/day) for 21 days
(17), followed by aspirin 100
mg/day. By contrast, patients with symptomatic intracranial artery
stenosis (stenosis rate, 70-99%) were treated with
dual-antiplatelet therapy for 90 days (18,19),
followed by aspirin 100 mg/day. Other patients were only given
aspirin 100 mg/day. After discharge, patients were recommended to
use this plan for a long time and regularly visit the clinic.
According to the blood vessel involved, patients were then divided
into the following two subgroups: The LAA subgroup and the SAO
subgroup.
The present study was approved by the Xiangtan
Central Hospital ethics committee (Xiangtan, China). All patients
signed informed consent and all data were anonymized.
Data acquisition and outcomes
The patients' demographic data (age and sex), lesion
location, hypertension, diabetes, hypercholesterinaemia, current
smoking habit, drinking history, coronary heart disease,
hyperhomocysteinemia, classification for ischemic stroke based on
the Trial of ORG 10172(20) in
Acute Stroke Treatment and other complications were obtained. In
addition, intracranial haemorrhage was evaluated by CT and MRI (the
head CT or MR of the patients in the eptifibatide group was checked
within 48 h after eptifibatide administration, and the head CT or
MR of the patients in the control group was checked after treatment
during hospitalization), whilst other related bleeding events were
also recorded (if the patient's gastric juice occult blood or fecal
occult blood was positive during hospitalization, it was judged as
gastrointestinal bleeding; if the patient's electronic system
recorded skin and mucous membrane bleeding during hospitalization,
it was judged as ecchymosis). The NIHSS scores were used to
evaluate the degree of neurological deficit. The NIHSS score was
evaluated on admission, at the time of progression (the disease is
the most serious within 72 h) and 7 days after treatment. The
samples used in the present study were collected when the patients
were hospitalized. All laboratory test results were obtained from
the Laboratory Department and Radiology Department of Xiangtan
Central Hospital. The basic information and condition assessment
data were obtained from the electronic medical record system of the
Neurology Department of Xiangtan Central Hospital.
Statistical analysis
Categorical data are shown as numbers and
percentages (%), whereas continuous data are shown as the means ±
standard deviation or median with interquartile range (IQR). The
continuous and categorical variables were compared using a
two-tailed independent samples t-test or Mann-Whitney U test and
the χ2 test or Fisher's exact test, respectively. SPSS
version 26.0 software (IBM Corp.) was used for analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
Baseline characteristics and clinical
course of eptifibatide and control group
The present retrospective study enrolled 74
participants, all patients received routine basic treatment,
including routine nursing, oxygen inhalation, management of blood
pressure and blood sugar, intensive lipid lowering, maintenance of
water electrolyte balance and rehabilitation treatment. Among them,
32 patients received eptifibatide whereas 42 patients did not. At
baseline, the mean age in the eptifibatide group was 64.0±2.2
years, where 23 (71.9%) of the patients were male. The mean age and
number of male patients in the control group was 65.7±1.9 years and
30 (71.4%), respectively (Table
I). The proportion of the vessels involved [LAA, 21 (65.6%) vs.
23 (54.8%) for the eptifibatide and control group, respectively]
were generally similar (Table I).
The median NIHSS score at progression was 7.5 (IQR, 4.0-11.8) in
the eptifibatide group and 6.0 (IQR, 5.0-9.0) in the control group
(Table I). Overall, baseline
demographic and clinical features of the two groups were largely
comparable (Table I). Changes in
NIHSS scores (∆NIHSS) between progression and 7 days after
treatment were used to evaluate the degree of early neurological
recovery. The median ∆NIHSS was 2.0 (IQR, 0.3-4.0) in the
eptifibatide group and 1.5 (IQR, 0.0-2.0) in the control group.
During hospitalization, the incidence rates of subcutaneous
ecchymosis in the eptifibatide group and control group were 6.3 and
4.8%, respectively. The rates of asymptomatic cerebral haemorrhage
were 3.1 and 2.4%, respectively. The rates of gastrointestinal
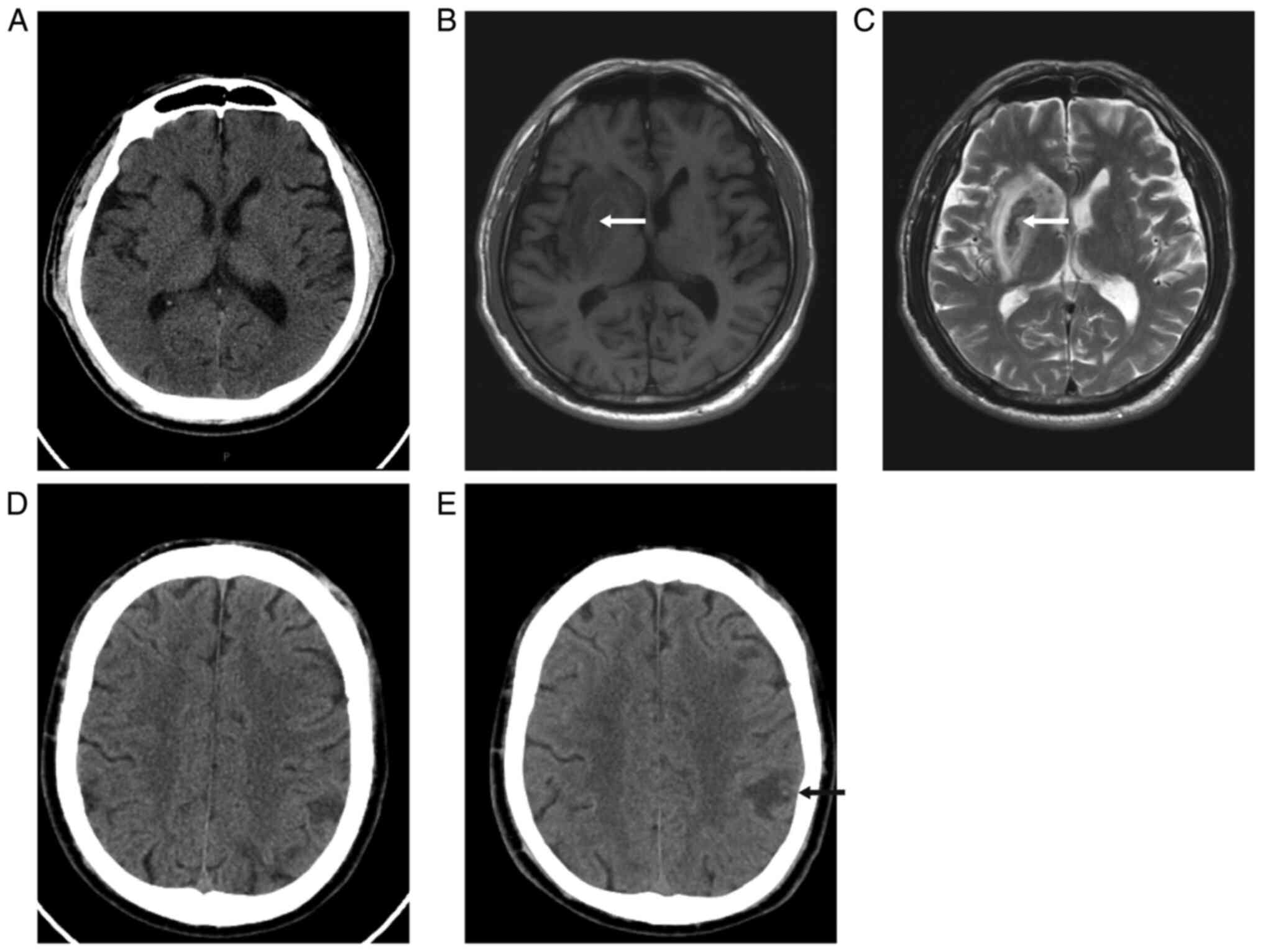
bleeding were 6.3 and 7.0%, respectively (Table I). Images of patients with
asymptomatic intracerebral haemorrhage are shown in Fig. 1.
 | Table IBaseline clinical characteristics and
clinical course of patients. |
Table I
Baseline clinical characteristics and
clinical course of patients.
| Characteristic | Eptifibatide group
(n=32) | Control group
(n=42) | P-value |
|---|
| Age,
yearsa | 64.0±2.2 | 65.7±1.9 | 0.549 |
| Sex, n (%) | | | |
|
Male | 23 (71.9) | 30 (71.4) | 0.996 |
|
Female | 9 (28.1) | 12 (28.6) | |
| Lesion location, n
(%) | | | 0.877 |
|
Brainstem/cerebellum | 13 (40.6) | 13 (31.0) | |
|
Basal
ganglia | 6 (18.8) | 10 (23.8) | |
|
Cortical and
subcortical of MCA | 9 (28.1) | 11 (26.2) | |
|
Cortical and
subcortical of ACA | 1 (3.1) | 3 (7.1) | |
|
Cortical and
subcortical of PCA | 3 (9.4) | 5 (11.9) | |
| Involved vessel, n
(%) | | | |
|
Small artery
occlusion | 11 (34.4) | 19 (45.2) | 0.346 |
|
Large artery
atherosclerosis | 21 (65.6) | 23 (54.8) | |
| Risk factors, n
(%) | | | |
|
Hypertension | 26 (81.3) | 33 (78.6) | 0.776 |
|
Diabetes
mellitus | 8 (25.0) | 16 (38.1) | 0.233 |
|
Hypercholesterinemia | 13 (40.6) | 22 (52.4) | 0.316 |
|
Current
smoking | 9 (28.1) | 10 (23.8) | 0.674 |
|
Drinking | 9 (28.1) | 6 (14.3) | 0.142 |
|
Coronary
heart disease | 8 (25.0) | 12 (28.6) | 0.732 |
|
Hyperhomocysteinemia | 3 (9.4) | 7 (16.7) | 0.572 |
|
Family
history of stroke | 2 (6.3) | 4 (9.5) | 0.693 |
| Antiplatelet regimen,
n (%) | | | 0.715 |
|
Dual
antiplatelet 21 days | 5 (15.6) | 4 (9.5) | |
|
Dual
antiplatelet 90 days | 10 (31.3) | 15 (35.7) | |
|
Monoclonal
antiplatelet | 17 (53.1) | 23 (54.8) | |
| Clinical сouгse,
pointsb | | | |
|
NIHSS on
admission | 3.0 (1.0-5.8) | 3.0 (1.8-5.0) | 0.821 |
|
NIHSS at
progression | 7.5 (4.0-11.8) | 6.0 (5.0-9.0) | 0.588 |
|
∆NIHSS
improvement | 2.0 (0.3-4.0) | 1.5 (0.0-2.0) | 0.053 |
|
NIHSS 7 days
after treatment | 4 (2.0-10.0) | 5.0 (3.0-7.5) | 0.425 |
| Complications, n
(%) | | | |
|
Ecchymosis | 2 (6.3) | 2 (4.8) | 1.000 |
|
Asymptomatic
cerebral haemorrhage | 1 (3.1) | 1 (2.4) | 1.000 |
|
Gastrointestinal
haemorrhage | 2 (6.3) | 3 (7.1) | 1.000 |
SAO subgroup analysis
There were 30 patients in the SAO subgroup, 11
patients were treated with eptifibatide whereas 19 patients were
not. At baseline, in the eptifibatide group, the mean age was
64.1±15.7 years and 9 (81.8%) of the patients were male, whereas in
the control group the mean age was 63.0±12.8 with 12 (63.2%) male
patients (Table II). The median
NIHSS score at progression was 7.0 (IQR, 5.0-10.0) in the
eptifibatide group and 6.0 (IQR, 5.0-7.0) in the control group
(Table II). Overall, the baseline
demographic and clinical characteristics of the two groups were
comparable (Table II). The median
∆NIHSS was 4.0 (IQR, 2.0-5.0) in the eptifibatide group,
significantly higher than 2.0 (IQR, 1.0-3.0) in the control group
(Table II).
 | Table IISubgroup analysis of patients with
small artery occlusion. |
Table II
Subgroup analysis of patients with
small artery occlusion.
| Characteristic | Eptifibatide group
(n=11) | Control group
(n=19) | P-value |
|---|
| Age,
yearsa | 64.1±15.7 | 63.0±12.8 | 0.830 |
| Sex, n (%) | | | 0.508 |
|
Male | 9 (81.8) | 12 (63.2) | |
|
Female | 2 (18.2) | 7 (36.8) | |
| Lesion location, n
(%) | | | 0.444 |
|
Brainstem/cerebellum | 4 (36.4) | 8 (42.1) | |
|
Basal
ganglia | 4 (36.4) | 6 (31.6) | |
|
Cortical and
Subcortical of MCA | 3 (27.3) | 2 (10.5) | |
|
Cortical and
Subcortical of ACA | 0 (0.0) | 3 (15.8) | |
|
Cortical and
Subcortical of PCA | 0 (0.0) | 0 (0.0) | |
| Risk factors, n
(%) | | | |
|
Hypertension | 9 (81.8) | 15 (78.9) | 1.000 |
|
Diabetes
mellitus | 2 (18.2) | 7 (36.8) | 0.508 |
|
Hypercholesterinaemia | 4 (36.4) | 12 (63.2) | 0.156 |
|
Current
smoking | 2 (18.2) | 5 (26.3) | 0.952 |
|
Drinking | 4 (36.4) | 3 (15.8) | 0.403 |
|
Coronary
heart disease | 2 (18.2) | 4 (21.1) | 1.000 |
|
Hyperhomocysteinemia | 0 (0.0) | 2 (10.5) | 0.520 |
|
Family
history of stroke | 1 (9.1) | 0 (0.0) | 0.367 |
| Antiplatelet
regimen, n (%) | | | 0.520 |
|
Dual
antiplatelet 21 days | 0 (0.0) | 2 (8.7) | |
|
Dual
antiplatelet 90 days | 0 (0.0) | 0 (0.0) | |
|
Monoclonal
antiplatelet | 11 (100.0) | 17 (89.5) | |
| Clinical сouгse,
pointsb | | | |
|
NIHSS on
admission | 3.0 (2.0-7.0) | 2.0 (1.0-3.0) | 0.094 |
|
NIHSS at
progression | 7.0 (5.0-10.0) | 6.0 (5.0-7.0) | 0.171 |
|
∆NIHSS
improvement | 4.0 (2.0-5.0) | 2.0 (1.0-3.0) | 0.008 |
|
NIHSS at 7
days after treatment | 2.0 (2.0-5.0) | 3.0 (2.0-5.0) | 0.641 |
LAA subgroup analysis
There were 44 patients in the LAA subgroup. In
total, 21 patients were treated with eptifibatide whereas 23
patients were not. At baseline, in the eptifibatide group, the mean
age was 63.9±10.9 years and 14 (66.7%) of the patients were male,
whereas in the control group the mean age was 68.0±11.4 years with
18 (78.3%) male patients (Table
III). The median NIHSS score at progression was 8.0 (IQR,
3.5-14.0) in the eptifibatide group and 7.0 (IQR, 5.0-12.0) in the
control group (Table III).
Overall, the baseline demographic and clinical characteristics of
the two groups were also comparable with no significant differences
(Table III). The median ∆NIHSS
was 2.0 (IQR, 0.0-2.5) in the eptifibatide group and 1.0 (IQR,
0.0-2.0) in the control group (Table
III).
 | Table IIISubgroup analysis of patients with
large arteries atherosclerosis. |
Table III
Subgroup analysis of patients with
large arteries atherosclerosis.
|
Characteristics | Eptifibatide group
(n=21) | Control group
(n=23) | P-value |
|---|
| Age,
yearsa | 63.9±10.9 | 68.0±11.4 | 0.231 |
| Sex, n (%) | | | 0.388 |
|
Male | 14 (66.7) | 18 (78.3) | |
|
Female | 7 (33.3) | 5 (21.7) | |
| Lesion location, n
(%) | | | 0.481 |
|
Brainstem/cerebellum | 9 (42.9) | 5 (21.7) | |
|
Basal
ganglia | 2 (9.5) | 4 (17.4) | |
|
Cortical and
Subcortical of MCA | 6 (28.6) | 9 (39.1) | |
|
Cortical and
Subcortical of ACA | 1 (4.8) | 0 (0.0) | |
|
Cortical and
Subcortical of PCA | 3 (14.3) | 5 (21.7) | |
| Risk factors, n
(%) | | | |
|
Hypertension | 17 (81.0) | 18 (78.3) | 1.000 |
|
Diabetes
mellitus | 6 (28.6) | 9 (39.1) | 0.460 |
|
Hypercholesterinaemia | 9 (42.9) | 10 (43.5) | 0.967 |
|
Current
Smoking | 7 (33.3) | 5 (21.7) | 0.388 |
|
Drinking | 5 (23.8) | 3 (13.0) | 0.594 |
|
Coronary
heart disease | 6 (28.6) | 8 (34.8) | 0.659 |
|
Hyperhomocysteinemia | 3 (14.3) | 5 (21.7) | 0.803 |
|
Family
history of stroke | 1 (4.8) | 4 (17.4) | 0.348 |
| Antiplatelet
regimen, n (%) | | | 0.342 |
|
Dual
antiplatelet 21 days | 5 (23.8) | 2 (8.7) | |
|
Dual
antiplatelet 90 days | 10 (47.6) | 15 (65.2) | |
|
Monoclonal
antiplatelets | 6 (28.6) | 6 (26.1) | |
| Clinical сouгse,
pointsb | | | |
|
NIHSS on
admission | 2.0 (1.0-5.0) | 4.0 (2.0-8.0) | 0.155 |
|
NIHSS at
progression | 8.0 (3.5-14.0) | 7.0 (5.0-12.0) | 0.934 |
|
∆NIHSS
improvement | 2.0 (0.0-2.5) | 1.0 (0.0-2.0) | 0.334 |
|
NIHSS at 7
days after treatment | 5.0 (2.0-12.5) | 6.0 (3.0-11.0) | 0.663 |
Discussion
The selection of the eptifibatide dosage for the
present study was based on limited evidence (14,21).
To the best of our knowledge, there is currently no guideline or
consensus for the treatment of PIS with eptifibatide. In a large
Chinese clinical trial (ClinicalTrials.gov Identifier: NCT03844594) to
determine the efficacy and safety of eptifibatide [Eptifibatide in
Endovascular Treatment of Acute Ischemic Stroke (EPOCH) trial],
intravenous or combined intra-catheter bolus treatment at a dose of
135-180 µg/kg administered over <5 min, followed by a
maintenance dose of 0.75 to 2 µg/kg/min for 24 h (22). The results from this previous trial
confirmed that this regimen was effective and safe (22). Considering that the process of
platelet activation is consistent in patients with PIS and in
patients treated with endovascular therapy, in addition to a
previous report stating that eptifibatide has a high safety profile
in PIS (14), the treatment
protocol (135 µg/kg bolus followed by 0.75 µg/kg/min for 48 h) was
used for the 32 patients in the present study.
In the present study, ∆NIHSS in the eptifibatide
group was not significantly different compared with that in the
control group. Further subgroup analysis revealed that in the
eptifibatide group, the ∆NIHSS was significantly higher compared
with that in the control group in patients with SAO, but not in
patients with LAA. This suggests that treatment with eptifibatide
could promote the early recovery of patients with PIS, specifically
with SAO. Compared with previous studies using eptifibatide in
patients with PIS, the present study established the control group
as a reference and subgroup analyses were conducted according to
different blood vessel types involved. The establishment of a
control group can exclude other factors except the anti-platelet
aggregation program. Subgroup analyses based on vascular type
further helped to identify the population that can benefit the most
from this treatment. The present study showed that eptifibatide may
exert different effects on acute PIS caused by different types of
vessels, where it appeared to significantly improve the early
neurological deficits in patients with SAO.
Unlike SAO, early efficacy of eptifibatide in LAA
could not be found in the present study and the reason for this
remains unclear. SAO-associated stroke is known to be caused by
small arterial hyalinosis, endothelial dysfunction and/or
intracranial perforator atherosclerosis (23,24).
SAO is a common cause of capsular warning syndrome (CWS), since CWS
is most often due to small penetrating vessel hemodynamic disorder
(25,26). A previous study on tirofiban showed
that it could stop early symptomatic fluctuations and decrease the
duration of functional impairments in patients with CWS compared
with oral antiplatelet drugs (27). In addition, there is evidence that
tirofiban is safe and efficacious in patients with ischemic stroke
outside the thrombolytic therapy window compared with oral
antiplatelet medications (28).
This supports the likelihood of beneficial effects conferred by GP
IIb/IIIa receptor antagonists for treatment during the early stages
of ischemia episodes.
In the present retrospective study of 74 patients
with PIS, the rates of complications, including ecchymosis,
gastrointestinal bleeding and asymptomatic intracranial
haemorrhage, did not differ between the eptifibatide and control
group. In addition, no symptomatic intracranial haemorrhage
occurred in either group. This suggests that the patients who
received eptifibatide tolerated this drug well, consistent with a
previous study (14).
There are several limitations in the present study.
The possibility of selection bias cannot be excluded, since
eptifibatide therapy was provided to the patients based on their
preferences. Additionally, the sample size is relatively small, and
the present study was a single-centre study in China. Due to the
relatively high cost of eptifibatide, only a small proportion of
individuals with PIS could afford this medication. With the reform
of the national medical insurance policy, non-cerebral vascular
interventional treatment using eptifibatide has become increasingly
difficult. This has prevented the increase in sample size. In
addition, methodological biases, such as the heterogeneity of
individuals and potential drug interactions, may influence the
efficacy of eptifibatide. Due to the difficulty in obtaining valid
post-discharge assessments, long-term follow-up analysis was not
performed in the present study.
In conclusion, the present study showed that
eptifibatide treatment was well tolerated in patients with PIS.
Furthermore, compared with oral antiplatelet regimen alone, adding
eptifibatide promoted early recovery in patients with SAO but not
in patients with LAA. Due to the limitations of the retrospective
study design itself, more multi-center, randomized controlled
trials are needed in the future to study the effect of eptifibatide
on progressive stroke.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Xiangtan Medical
Association (grant no. 2021-xtyx-7).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and JL designed the study. YD, ZL, YY and WZ
collected the data. LL and JL confirm the authenticity of all the
raw data. LL and YD analysed the data, and ZL, JL, YY and WZ helped
perform the analysis, with constructive discussions. LL and JL
wrote the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The studies involving human participants were
reviewed and approved by the Ethics Committee of Xiangtan Central
Hospital (Xiangtan, China). All participants in this study provided
written informed consent for the use of their samples and
publication of their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eriksson M, Stecksén A, Glader EL,
Norrving B, Appelros P, Hulter Åsberg K, Stegmayr B, Terént A and
Asplund K: Riks-Stroke Collaboration. Discarding heparins as
treatment for progressive stroke in Sweden 2001 to 2008. Stroke.
41:2552–2558. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Birschel P, Ellul J and Barer D:
Progressing stroke: Towards an internationally agreed definition.
Cerebrovasc Dis. 17:242–252. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Helleberg BH, Ellekjaer H and Indredavik
B: Outcomes after early neurological deterioration and transitory
deterioration in acute ischemic stroke patients. Cerebrovasc Dis.
42:378–386. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Weimar C, Mieck T, Buchthal J, Ehrenfeld
CE, Schmid E and Diener HC: German Stroke Study Collaboration.
Neurologic worsening during the acute phase of ischemic stroke.
Arch Neurol. 62:393–397. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kwan J and Hand P: Early neurological
deterioration in acute stroke: Clinical characteristics and impact
on outcome. QJM. 99:625–633. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Seners P and Baron JC: Revisiting
‹progressive stroke›: Incidence, predictors, pathophysiology, and
management of unexplained early neurological deterioration
following acute ischemic stroke. J Neurol. 265:216–225.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Caplan LR: Treatment of ‘progressive’
stroke. Stroke. 22:694–695. 1991.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schwarz M, Meade G, Stoll P, Ylanne J,
Bassler N, Chen YC, Hagemeyer CE, Ahrens I, Moran N, Kenny D, et
al: Conformation-specific blockade of the integrin GPIIb/IIIa: A
novel antiplatelet strategy that selectively targets activated
platelets. Circ Res. 99:25–33. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Casserly IP and Topol EJ: Glycoprotein
IIb/IIIa antagonists-from bench to practice. Cell Mol Life Sci.
59:478–500. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Adams HP Jr, Effron MB, Torner J, Dávalos
A, Frayne J, Teal P, Leclerc J, Oemar B, Padgett L, Barnathan ES,
et al: Emergency administration of abciximab for treatment of
patients with acute ischemic stroke: Results of an international
phase III trial: Abciximab in Emergency Treatment of Stroke Trial
(AbESTT-II). Stroke. 39:87–99. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Puri A, Bansal A, Narain VS, Sethi R,
Dwivedi SK, Puri VK and Saran RK: Comparative assessment of
platelet GpIIb/IIIa receptor occupancy ratio with
Eptifibatide/Tirofiban in patients presenting with ACS and
undergoing PCI. Indian Heart J. 65:152–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou X, Wu X, Sun H and Li J: Efficacy and
safety of eptifibatide versus tirofiban in acute coronary syndrome
patients: A systematic review and meta-analysis. J Evid Based Med.
10:136–144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang M, Huo X, Miao Z and Wang Y: Platelet
glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute
ischemic stroke. Drugs. 79:515–529. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martin-Schild S, Shaltoni H, Abraham AT,
Barreto AD, Hallevi H, Gonzales NR, Grotta JC and Savitz SI: Safety
of eptifibatide for subcortical stroke progression. Cerebrovasc
Dis. 28:595–600. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Philipps J, Thomalla G, Glahn J, Schwarze
M and Rother J: Treatment of progressive stroke with
tirofiban-experience in 35 patients. Cerebrovasc Dis. 28:435–438.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Peng B and Wu B: Guidelines for the
diagnosis and treatment of acute ischemic stroke in China 2018.
Chin J Neurol. 51:666–682. 2018.
|
|
17
|
Wang Y, Wang Y, Zhao X, Liu L, Wang D,
Wang C, Wang C, Li H, Meng X, Cui L, et al: Clopidogrel with
aspirin in acute minor stroke or transient ischemic attack. N Engl
J Med. 369:11–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Derdeyn CP, Chimowitz MI, Lynn MJ,
Fiorella D, Turan TN, Janis LS, Montgomery J, Nizam A, Lane BF,
Lutsep HL, et al: Aggressive medical treatment with or without
stenting in high-risk patients with intracranial artery stenosis
(SAMMPRIS): The final results of a randomised trial. Lancet.
383:333–341. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chimowitz MI, Lynn MJ, Derdeyn CP, Turan
TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters
MF, et al: Stenting versus aggressive medical therapy for
intracranial arterial stenosis. N Engl J Med. 365:993–1003.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Adams HP Jr, Bendixen BH, Kappelle LJ,
Biller J, Love BB, Gordon DL and Marsh EE III: Classification of
subtype of acute ischemic stroke. Definitions for use in a
multicenter clinical trial. TOAST. Trial of Org 10172 in acute
stroke treatment. Stroke. 24:35–41. 1993.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kirmani JF, Korya D, Choi G, Brar J,
Chahal H, Hussain M, Mehta S, Panezai S and Moussavi M: Abstract T
P3: Safety of eptifibatide after full dose IV tPA and endovascular
treatment for ischemic stroke. Stroke. 46 (suppl_1)(ATP3)2015.
|
|
22
|
Ma G, Sun X, Cheng H, Burgin WS, Luo W,
Jia W, Liu Y, He W, Geng X, Zhu L, et al: Combined approach to
eptifibatide and thrombectomy in acute ischemic stroke because of
large vessel occlusion: A matched-control analysis. Stroke.
53:1580–1588. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim SK, Song P, Hong JM, Pak CY, Chung CS,
Lee KH and Kim GM: Prediction of progressive motor deficits in
patients with deep subcortical infarction. Cerebrovasc Dis.
25:297–303. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Steinke W and Ley SC: Lacunar stroke is
the major cause of progressive motor deficits. Stroke.
33:1510–1516. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Donnan GA, O'Malley HM, Quang L, Hurley S
and Bladin PF: The capsular warning syndrome: Pathogenesis and
clinical features. Neurology. 43:957–962. 1993.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Foschi M, Pavolucci L, Rondelli F, Barone
V, Rinaldi R, Spinardi L, Favaretto E, Rucci P, Giostra F, Cosmi B,
et al: Capsular warning syndrome: Features, risk profile, and
prognosis in a large prospective TIA cohort. Cerebrovasc Dis: Sep
9, 2022 (Epub ahead of print).
|
|
27
|
Li W, Wu Y, Li XS, Liu CC, Huang SH, Liang
CR, Wang H, Zhang LL, Xu ZQ, Wang YJ and Zhang M: Intravenous
tirofiban therapy for patients with capsular warning syndrome.
Stroke Vasc Neurol. 4:22–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin L, Li W, Liu CC, Wu Y, Huang SH, Li
XS, Liang CR, Wang H, Zhang LL, Xu ZQ, et al: Safety and
preliminary efficacy of intravenous tirofiban in acute ischemic
stroke patient without arterial occlusion on neurovascular imaging
studies. J Neurol Sci. 383:175–179. 2017.PubMed/NCBI View Article : Google Scholar
|