|
1
|
Kondratskyi A, Kondratska K, Skryma R,
Klionsky DJ and Prevarskaya N: Ion channels in the regulation of
autophagy. Autophagy. 14:3–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Alexander SPH, Mathie A, Peters JA, Veale
EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD,
Pawson AJ, et al: The concise guide to pharmacology 2019/20: Ion
channels. Br J Pharmacol. 176 (Suppl 1):S142–S228. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roux B: Ion channels and ion selectivity.
Essays Biochem. 61:201–209. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cheng J, Wen J, Wang N, Wang C, Xu Q and
Yang Y: Ion channels and vascular diseases. Arterioscler Thromb
Vasc Biol. 39:e146–e156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Anderson KJ, Cormier RT and Scott PM: Role
of ion channels in gastrointestinal cancer. World J Gastroenterol.
25:5732–5772. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Armijo JA, Shushtarian M, Valdizan EM,
Cuadrado A, de las Cuevas I and Adín J: Ion channels and epilepsy.
Curr Pharm Des. 11:1975–2003. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moiseenkova-Bell V, Delemotte L and Minor
DL Jr: Ion channels: Intersection of structure, function, and
pharmacology. J Mol Biol. 433(167102)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang L and Yule DI: Differential
regulation of ion channels function by proteolysis. Biochim Biophys
Acta Mol Cell Res. 1865:1698–1706. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Catterall WA, Lenaeus MJ and Gamal El-Din
TM: Structure and pharmacology of voltage-gated sodium and calcium
channels. Annu Rev Pharmacol Toxicol. 60:133–154. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
De Logu F and Geppetti P: Ion channel
pharmacology for pain modulation. Handb Exp Pharmacol. 260:161–186.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pallet N, Bastard JP, Claeyssens S,
Fellahi S, Delanaye P, Piéroni L and Caussé E: groupe de travail
SFBC, SFNDT, SNP. Proteinuria typing: How, why and for whom? Ann
Biol Clin (Paris). 77:13–25. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barton M: Reversal of proteinuric renal
disease and the emerging role of endothelin. Nat Clin Pract
Nephrol. 4:490–501. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nowak A and Serra AL: Assessment of
proteinuria. Praxis (Bern 1994). 102:797–802. 2013.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
14
|
D'Amico G and Bazzi C: Pathophysiology of
proteinuria. Kidney Int. 63:809–825. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Menzel S and Moeller MJ: Role of the
podocyte in proteinuria. Pediatr Nephrol. 26:1775–1780.
2011.PubMed/NCBI View Article : Google Scholar
|
|
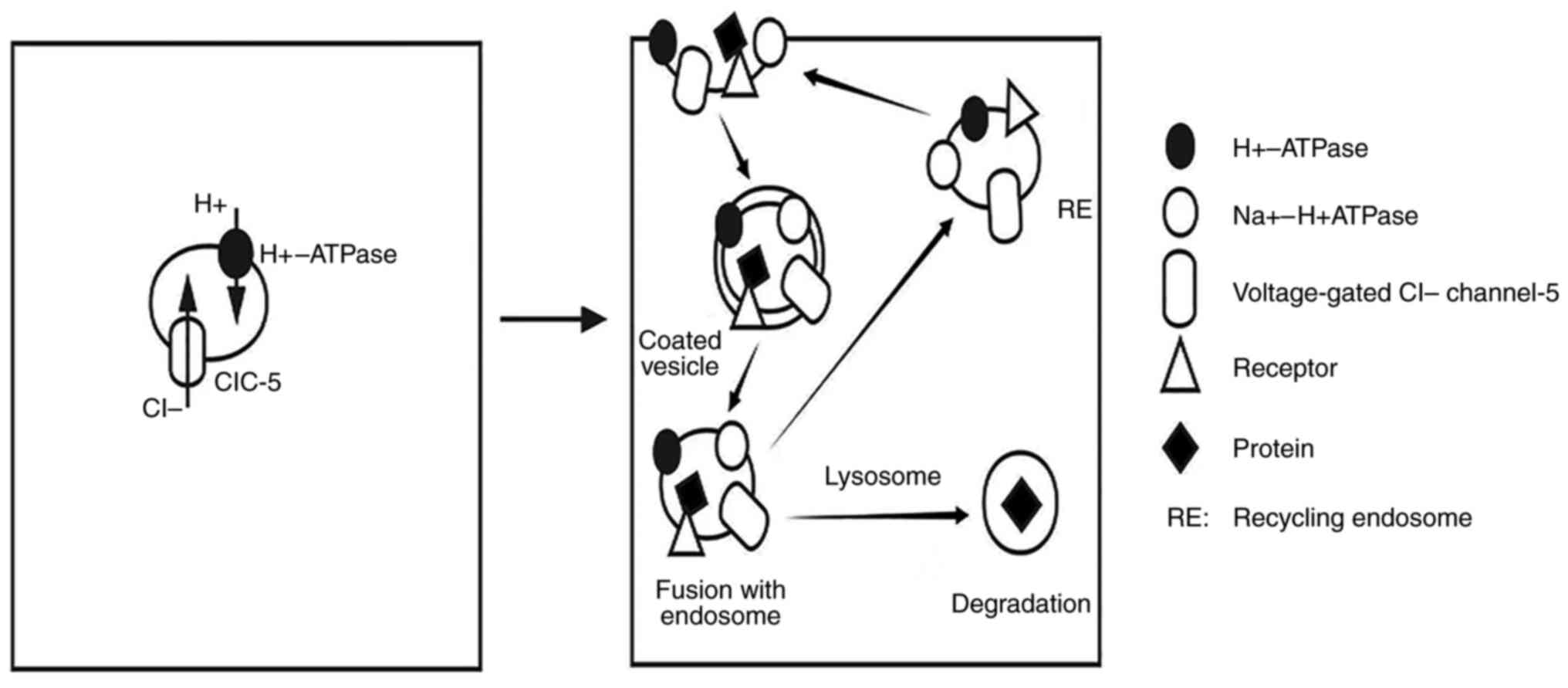
16
|
Miner JH: Glomerular basement membrane
composition and the filtration barrier. Pediatr Nephrol.
26:1413–1417. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kallen RG, Cohen SA and Barchi RL:
Structure, function and expression of voltage-dependent sodium
channels. Mol Neurobiol. 7:383–428. 1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee CH and Ruben PC: Interaction between
voltage-gated sodium channels and the neurotoxin, tetrodotoxin.
Channels (Austin). 2:407–412. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hanukoglu I and Hanukoglu A: Epithelial
sodium channel (ENaC) family: Phylogeny, structure-function, tissue
distribution, and associated inherited diseases. Gene. 579:95–132.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brunklaus A, Ellis R, Reavey E, Semsarian
C and Zuberi SM: Genotype phenotype associations across the
voltage-gated sodium channel family. J Med Genet. 51:650–658.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Soundararajan R, Pearce D, Hughey RP and
Kleyman TR: Role of epithelial sodium channels and their regulators
in hypertension. J Biol Chem. 285:30363–30369. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Büsst CJ: Blood pressure regulation via
the epithelial sodium channel: From gene to kidney and beyond. Clin
Exp Pharmacol Physiol. 40:495–503. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zachar RM, Skjødt K, Marcussen N, Walter
S, Toft A, Nielsen MR, Jensen BL and Svenningsen P: The epithelial
sodium channel γ-subunit is processed proteolytically in human
kidney. J Am Soc Nephrol. 26:95–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Svenningsen P, Friis UG, Bistrup C, Buhl
KB, Jensen BL and Skøtt O: Physiological regulation of epithelial
sodium channel by proteolysis. Curr Opin Nephrol Hypertens.
20:529–533. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ware AW, Rasulov SR, Cheung TT, Lott JS
and McDonald FJ: Membrane trafficking pathways regulating the
epithelial Na+ channel. Am J Physiol Renal Physiol.
318:F1–F13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bockenhauer D: Over- or underfill: Not all
nephrotic states are created equal. Pediatr Nephrol. 28:1153–1156.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Larionov A, Dahlke E, Kunke M, Zanon
Rodriguez L, Schiessl IM, Magnin JL, Kern U, Alli AA, Mollet G,
Schilling O, et al: Cathepsin B increases ENaC activity leading to
hypertension early in nephrotic syndrome. J Cell Mol Med.
23:6543–6553. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fila M, Sassi A, Brideau G, Cheval L,
Morla L, Houillier P, Walter C, Gennaoui M, Collignon L, Keck M, et
al: A variant of ASIC2 mediates sodium retention in nephrotic
syndrome. JCI Insight. 6(e148588)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Svenningsen P, Skøtt O and Jensen BL:
Proteinuric diseases with sodium retention: Is plasmin the link?
Clin Exp Pharmacol Physiol. 39:117–124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hinrichs GR, Jensen BL and Svenningsen P:
Mechanisms of sodium retention in nephrotic syndrome. Curr Opin
Nephrol Hypertens. 29:207–212. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Passero CJ, Hughey RP and Kleyman TR: New
role for plasmin in sodium homeostasis. Curr Opin Nephrol
Hypertens. 19:13–19. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Briet M and Schiffrin EL: Aldosterone:
Effects on the kidney and cardiovascular system. Nat Rev Nephrol.
6:261–273. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shapovalov G, Skryma R and Prevarskaya N:
Calcium channels and prostate cancer. Recent Pat Anticancer Drug
Discov. 8:18–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Prevarskaya N, Ouadid-Ahidouch H, Skryma R
and Shuba Y: Remodelling of Ca2+ transport in cancer: How it
contributes to cancer hallmarks? Philos Trans R Soc Lond B Biol
Sci. 369(20130097)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mignen O, Thompson JL and Shuttleworth TJ:
Ca2+ selectivity and fatty acid specificity of the noncapacitative,
arachidonate-regulated Ca2+ (ARC) channels. J Biol Chem.
278:10174–10181. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lewis RS: Store-operated calcium channels:
From function to structure and back again. Cold Spring Harb
Perspect Biol. 12(a035055)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou Y and Greka A: Calcium-permeable ion
channels in the kidney. Am J Physiol Renal Physiol.
310:F1157–F1167. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Patergnani S, Danese A, Bouhamida E,
Aguiari G, Previati M, Pinton P and Giorgi C: Various aspects of
calcium signaling in the regulation of apoptosis, autophagy, cell
proliferation, and cancer. Int J Mol Sci. 21(8323)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lou J, Yang X, Shan W, Jin Z, Ding J, Hu
Y, Liao Q, Du Q, Xie R and Xu J: Effects of calcium-permeable ion
channels on various digestive diseases in the regulation of
autophagy (review). Mol Med Rep. 24(680)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Perez-Reyes E: Molecular physiology of
low-voltage-activated t-type calcium channels. Physiol Rev.
83:117–161. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zamponi GW, Striessnig J, Koschak A and
Dolphin AC: The physiology, pathology, and pharmacology of
voltage-gated calcium channels and their future therapeutic
potential. Pharmacol Rev. 67:821–870. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Catterall WA: Voltage-gated calcium
channels. Cold Spring Harb Perspect Biol. 3(a003947)2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sairaman A, Cardoso FC, Bispat A, Lewis
RJ, Duggan PJ and Tuck KL: Synthesis and evaluation of
aminobenzothiazoles as blockers of N- and T-type calcium channels.
Bioorg Med Chem. 26:3046–3059. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tsunemi T, Saegusa H, Ishikawa K, Nagayama
S, Murakoshi T, Mizusawa H and Tanabe T: Novel Cav2.1 splice
variants isolated from Purkinje cells do not generate P-type Ca2+
current. J Biol Chem. 277:7214–7221. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yamaguchi S, Okamura Y, Nagao T and
Adachi-Akahane S: Serine residue in the IIIS5-S6 linker of the
L-type Ca2+ channel alpha 1C subunit is the critical determinant of
the action of dihydropyridine Ca2+ channel agonists. J Biol Chem.
275:41504–41511. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hansen PB: Functional and pharmacological
consequences of the distribution of voltage-gated calcium channels
in the renal blood vessels. Acta Physiol (Oxf). 207:690–699.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ohta M, Sugawara S, Sato N, Kuriyama C,
Hoshino C and Kikuchi A: Effects of benidipine, a long-acting
T-type calcium channel blocker, on home blood pressure and renal
function in patients with essential hypertension: A retrospective,
‘real-world’ comparison with amlodipine. Clin Drug Investig.
29:739–746. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mishima K, Maeshima A, Miya M, Sakurai N,
Ikeuchi H, Hiromura K and Nojima Y: Involvement of N-type Ca(2+)
channels in the fibrotic process of the kidney in rats. Am J
Physiol Renal Physiol. 304:F665–F673. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tamargo J and Ruilope LM: Investigational
calcium channel blockers for the treatment of hypertension. Expert
Opin Investig Drugs. 25:1295–1309. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Khanna R, Yu J, Yang X, Moutal A,
Chefdeville A, Gokhale V, Shuja Z, Chew LA, Bellampalli SS, Luo S,
et al: Targeting the CaVα-CaVβ interaction yields an antagonist of
the N-type CaV2.2 channel with broad antinociceptive efficacy.
Pain. 160:1644–1661. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ando K: L-/N-type calcium channel blockers
and proteinuria. Curr Hypertens Rev. 9:210–218. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lei B, Nakano D, Fujisawa Y, Liu Y, Hitomi
H, Kobori H, Mori H, Masaki T, Asanuma K, Tomino Y and Nishiyama A:
N-type calcium channel inhibition with cilnidipine elicits
glomerular podocyte protection independent of sympathetic nerve
inhibition. J Pharmacol Sci. 119:359–367. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fan YY, Kohno M, Nakano D, Ohsaki H,
Kobori H, Suwarni D, Ohashi N, Hitomi H, Asanuma K, Noma T, et al:
Cilnidipine suppresses podocyte injury and proteinuria in metabolic
syndrome rats: Possible involvement of N-type calcium channel in
podocyte. J Hypertens. 28:1034–1043. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tamargo J: New calcium channel blockers
for the treatment of hypertension. Hipertens Riesgo Vasc. 34 (Suppl
2):S5–S8. 2017.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
55
|
Hansen PB, Poulsen CB, Walter S, Marcussen
N, Cribbs LL, Skøtt O and Jensen BL: Functional importance of L-
and P/Q-type voltage-gated calcium channels in human renal
vasculature. Hypertension. 58:464–470. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Nilius B and Owsianik G: The transient
receptor potential family of ion channels. Genome Biol.
12(218)2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ramsey IS, Delling M and Clapham DE: An
introduction to TRP channels. Annu Rev Physiol. 68:619–647.
2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Montell C and Rubin GM: Molecular
characterization of the Drosophila trp locus: A putative integral
membrane protein required for phototransduction. Neuron.
2:1313–1323. 1989.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gees M, Owsianik G, Nilius B and Voets T:
TRP channels. Compr Physiol. 2:563–608. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Caterina MJ and Pang Z: TRP channels in
skin biology and pathophysiology. Pharmaceuticals (Basel).
9(77)2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Li H: TRP channel classification. Adv Exp
Med Biol. 976:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhao Y, McVeigh BM and Moiseenkova-Bell
VY: Structural pharmacology of TRP channels. J Mol Biol.
433(166914)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Nilius B: TRP channels in disease. Biochim
Biophys Acta. 1772:805–812. 2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Moran MM: TRP channels as potential drug
targets. Annu Rev Pharmacol Toxicol. 58:309–330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Koivisto AP, Belvisi MG, Gaudet R and
Szallasi A: Advances in TRP channel drug discovery: From target
validation to clinical studies. Nat Rev Drug Discov. 21:41–59.
2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Feng YL, Chen H, Chen DQ, Vaziri ND, Su W,
Ma SX, Shang YQ, Mao JR, Yu XY, Zhang L, et al: Activated
NF-κB/Nrf2 and Wnt/β-catenin pathways are associated with lipid
metabolism in CKD patients with microalbuminuria and
macroalbuminuria. Biochim Biophys Acta Mol Basis Dis.
1865:2317–2332. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Voets T, Vriens J and Vennekens R:
Targeting TRP channels-valuable alternatives to combat pain, lower
urinary tract disorders, and type 2 diabetes? Trends Pharmacol Sci.
40:669–683. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang Q, Tian X, Wang Y, Wang Y, Li J, Zhao
T and Li P: Role of transient receptor potential canonical channel
6 (TRPC6) in diabetic kidney disease by regulating podocyte actin
cytoskeleton rearrangement. J Diabetes Res.
2020(6897390)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Bacsa B, Tiapko O, Stockner T and
Groschner K: Mechanisms and significance of Ca2+ entry
through TRPC channels. Curr Opin Physiol. 17:25–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Möller CC, Flesche J and Reiser J:
Sensitizing the slit diaphragm with TRPC6 ion channels. J Am Soc
Nephrol. 20:950–953. 2009.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Möller CC, Wei C, Altintas MM, Li J, Greka
A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, et al:
Induction of TRPC6 channel in acquired forms of proteinuric kidney
disease. J Am Soc Nephrol. 18:29–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Schaldecker T, Kim S, Tarabanis C, Tian D,
Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR,
et al: Inhibition of the TRPC5 ion channel protects the kidney
filter. J Clin Invest. 123:5298–5309. 2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Tian D, Jacobo SM, Billing D, Rozkalne A,
Gage SD, Anagnostou T, Pavenstädt H, Hsu HH, Schlondorff J, Ramos A
and Greka A: Antagonistic regulation of actin dynamics and cell
motility by TRPC5 and TRPC6 channels. Sci Signal.
3(ra77)2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Shalygin A, Shuyskiy LS, Bohovyk R,
Palygin O, Staruschenko A and Kaznacheyeva E: Cytoskeleton
rearrangements modulate TRPC6 channel activity in podocytes. Int J
Mol Sci. 22(4396)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Schlondorff J: TRPC6 and kidney disease:
Sclerosing more than just glomeruli? Kidney Int. 91:773–775.
2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Schlöndorff JS and Pollak MR: TRPC6 in
glomerular health and disease: What we know and what we believe.
Semin Cell Dev Biol. 17:667–674. 2006.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kim EY, Yazdizadeh Shotorbani P and Dryer
SE: Trpc6 inactivation confers protection in a model of severe
nephrosis in rats. J Mol Med (Berl). 96:631–644. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hall G, Wang L and Spurney RF: TRPC
channels in proteinuric kidney diseases. Cells.
9(44)2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
van der Wijst J and Bindels RJM: Renal
physiology: TRPC5 inhibition to treat progressive kidney disease.
Nat Rev Nephrol. 14:145–146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Walsh L, Reilly JF, Cornwall C, Gaich GA,
Gipson DS, Heerspink HJL, Johnson L, Trachtman H, Tuttle KR, Farag
YMK, et al: Safety and efficacy of GFB-887, a TRPC5 channel
inhibitor, in patients with focal segmental glomerulosclerosis,
treatment-resistant minimal change disease, or diabetic
nephropathy: TRACTION-2 trial design. Kidney Int Rep. 6:2575–2584.
2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Reiser J, Polu KR, Möller CC, Kenlan P,
Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C,
et al: TRPC6 is a glomerular slit diaphragm-associated channel
required for normal renal function. Nat Genet. 37:739–744.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
82
|
Wiggins RC: The spectrum of
podocytopathies: A unifying view of glomerular diseases. Kidney
Int. 71:1205–1214. 2007.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zhou Y, Castonguay P, Sidhom EH, Clark AR,
Dvela-Levitt M, Kim S, Sieber J, Wieder N, Jung JY, Andreeva S, et
al: A small-molecule inhibitor of TRPC5 ion channels suppresses
progressive kidney disease in animal models. Science.
358:1332–1336. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Zhang H, Ding J, Fan Q and Liu S: TRPC6
up-regulation in Ang II-induced podocyte apoptosis might result
from ERK activation and NF-kappaB translocation. Exp Biol Med
(Maywood). 234:1029–1036. 2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang
S, Du P, Zhang X and Yi F: NADPH oxidase-derived ROS contributes to
upregulation of TRPC6 expression in puromycin
aminonucleoside-induced podocyte injury. Cell Physiol Biochem.
24:619–626. 2009.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kistler AD, Singh G, Altintas MM, Yu H,
Fernandez IC, Gu C, Wilson C, Srivastava SK, Dietrich A, Walz K, et
al: Transient receptor potential channel 6 (TRPC6) protects
podocytes during complement-mediated glomerular disease. J Biol
Chem. 288:36598–36609. 2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Zhou Y, Kim C, Pablo JLB, Zhang F, Jung
JY, Xiao L, Bazua-Valenti S, Emani M, Hopkins CR, Weins A and Greka
A: TRPC5 channel inhibition protects podocytes in
puromycin-aminonucleoside induced nephrosis models. Front Med
(Lausanne). 8(721865)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Randhawa PK and Jaggi AS: TRPV4 channels:
Physiological and pathological role in cardiovascular system. Basic
Res Cardiol. 110(54)2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Everaerts W, Nilius B and Owsianik G: The
vanilloid transient receptor potential channel TRPV4: From
structure to disease. Prog Biophys Mol Biol. 103:2–17.
2010.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kassmann M, Harteneck C, Zhu Z, Nürnberg
B, Tepel M and Gollasch M: Transient receptor potential vanilloid 1
(TRPV1), TRPV4, and the kidney. Acta Physiol (Oxf). 207:546–564.
2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Mannaa M, Markó L, Balogh A, Vigolo E,
N'diaye G, Kaßmann M, Michalick L, Weichelt U, Schmidt-Ott KM,
Liedtke WB, et al: Transient receptor potential vanilloid 4 channel
deficiency aggravates tubular damage after acute renal ischaemia
reperfusion. Sci Rep. 8(4878)2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Karasawa T, Wang Q, Fu Y, Cohen DM and
Steyger PS: TRPV4 enhances the cellular uptake of aminoglycoside
antibiotics. J Cell Sci. 121:2871–2879. 2008.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Gualdani R, Seghers F, Yerna X, Schakman
O, Tajeddine N, Achouri Y, Tissir F, Devuyst O and Gailly P:
Mechanical activation of TRPV4 channels controls albumin
reabsorption by proximal tubule cells. Sci Signal.
13(eabc6967)2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Duran C, Thompson CH, Xiao Q and Hartzell
HC: Chloride channels: Often enigmatic, rarely predictable. Annu
Rev Physiol. 72:95–121. 2010.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Xia J, Wang H, Li S, Wu Q, Sun L, Huang H
and Zeng M: Ion channels or aquaporins as novel molecular targets
in gastric cancer. Mol Cancer. 16(54)2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Gururaja Rao S, Ponnalagu D, Patel NJ and
Singh H: Three decades of chloride intracellular channel proteins:
From organelle to organ physiology. Curr Protoc Pharmacol.
80:11.21.1–11.21.17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Berend K, van Hulsteijn LH and Gans RO:
Chloride: The queen of electrolytes? Eur J Intern Med. 23:203–211.
2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Jentsch TJ, Stein V, Weinreich F and
Zdebik AA: Molecular structure and physiological function of
chloride channels. Physiol Rev. 82:503–568. 2002.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Suzuki M, Morita T and Iwamoto T:
Diversity of Cl(-) channels. Cell Mol Life Sci. 63:12–24.
2006.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Scheel O, Zdebik AA, Lourdel S and Jentsch
TJ: Voltage-dependent electrogenic chloride/proton exchange by
endosomal CLC proteins. Nature. 436:424–427. 2005.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Uchida S: In vivo role of CLC chloride
channels in the kidney. Am J Physiol Renal Physiol. 279:F802–F808.
2000.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Schriever AM, Friedrich T, Pusch M and
Jentsch TJ: CLC chloride channels in Caenorhabditis elegans. J Biol
Chem. 274:34238–34244. 1999.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Jentsch TJ and Pusch M: CLC chloride
channels and transporters: Structure, function, physiology, and
disease. Physiol Rev. 98:1493–1590. 2018.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Hryciw DH, Ekberg J, Pollock CA and
Poronnik P: ClC-5: A chloride channel with multiple roles in renal
tubular albumin uptake. Int J Biochem Cell Biol. 38:1036–1042.
2006.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Novarino G, Weinert S, Rickheit G and
Jentsch TJ: Endosomal chloride-proton exchange rather than chloride
conductance is crucial for renal endocytosis. Science.
328:1398–1401. 2010.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Devuyst O and Luciani A: Chloride
transporters and receptor-mediated endocytosis in the renal
proximal tubule. J Physiol. 593:4151–4164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Günther W, Piwon N and Jentsch TJ: The
ClC-5 chloride channel knock-out mouse-an animal model for Dent's
disease. Pflugers Arch. 445:456–462. 2003.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Faria D, Rock JR, Romao AM, Schweda F,
Bandulik S, Witzgall R, Schlatter E, Heitzmann D, Pavenstädt H,
Herrmann E, et al: The calcium-activated chloride channel Anoctamin
1 contributes to the regulation of renal function. Kidney Int.
85:1369–1381. 2014.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Yang YD, Cho H, Koo JY, Tak MH, Cho Y,
Shim WS, Park SP, Lee J, Lee B, Kim BM, et al: TMEM16A confers
receptor-activated calcium-dependent chloride conductance. Nature.
455:1210–1215. 2008.PubMed/NCBI View Article : Google Scholar
|
|
110
|
González C, Baez-Nieto D, Valencia I,
Oyarzún I, Rojas P, Naranjo D and Latorre R: K(+) channels:
Function-structural overview. Compr Physiol. 2:2087–2149.
2012.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Sigworth FJ: Potassium channel mechanics.
Neuron. 32:555–556. 2001.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Gulbis JM and Doyle DA: Potassium channel
structures: Do they conform? Curr Opin Struct Biol. 14:440–446.
2004.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Kuang Q, Purhonen P and Hebert H:
Structure of potassium channels. Cell Mol Life Sci. 72:3677–3693.
2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
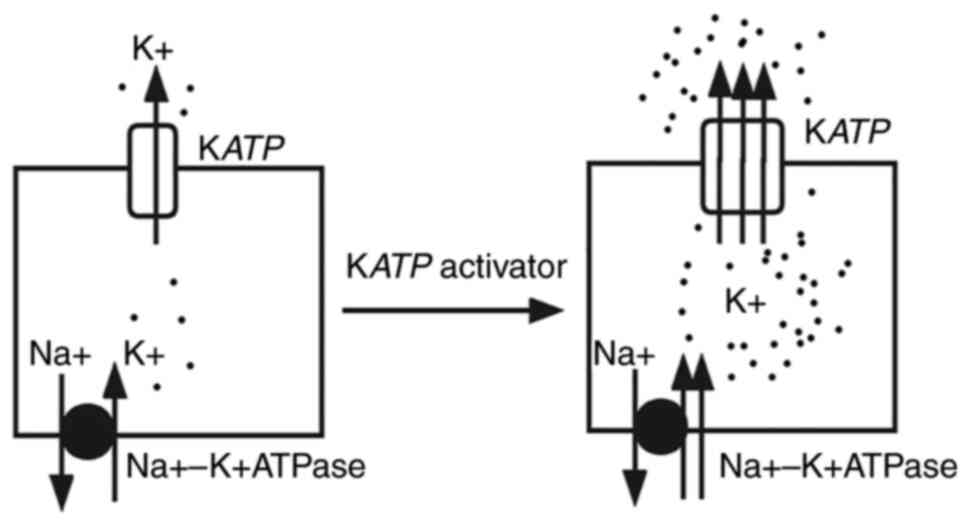
Noma A: ATP-regulated K+ channels in
cardiac muscle. Nature. 305:147–148. 1983.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Ashcroft FM and Rorsman P: K(ATP) channels
and islet hormone secretion: New insights and controversies. Nat
Rev Endocrinol. 9:660–669. 2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Rorsman P, Ramracheya R, Rorsman NJ and
Zhang Q: ATP-regulated potassium channels and voltage-gated calcium
channels in pancreatic alpha and beta cells: Similar functions but
reciprocal effects on secretion. Diabetologia. 57:1749–1761.
2014.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Tinker A, Aziz Q, Li Y and Specterman M:
ATP-sensitive potassium channels and their physiological and
pathophysiological roles. Compr Physiol. 8:1463–1511.
2018.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Cole WC, Chen TT and Clément-Chomienne O:
Myogenic regulation of arterial diameter: Role of potassium
channels with a focus on delayed rectifier potassium current. Can J
Physiol Pharmacol. 83:755–765. 2005.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Jackson MB, Konnerth A and Augustine GJ:
Action potential broadening and frequency-dependent facilitation of
calcium signals in pituitary nerve terminals. Proc Natl Acad Sci
USA. 88:380–384. 1991.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Guéguinou M, Chantôme A, Fromont G,
Bougnoux P, Vandier C and Potier-Cartereau M: KCa and Ca(2+)
channels: The complex thought. Biochim Biophys Acta.
1843:2322–2333. 2014.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Sforna L, Megaro A, Pessia M, Franciolini
F and Catacuzzeno L: Structure, gating and basic functions of the
Ca2+-activated K channel of intermediate conductance. Curr
Neuropharmacol. 16:608–617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Walewska A, Kulawiak B, Szewczyk A and
Koprowski P: Mechanosensitivity of mitochondrial large-conductance
calcium-activated potassium channels. Biochim Biophys Acta
Bioenerg. 1859:797–805. 2018.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Fezai M, Ahmed M, Hosseinzadeh Z and Lang
F: Up-regulation of the large-conductance Ca2+-activated K+ channel
by glycogen synthase kinase GSK3β. Cell Physiol Biochem.
39:1031–1039. 2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Giebisch G: Potassium channels and the
kidney. Nephrologie. 21:223–228. 2000.PubMed/NCBI(In French).
|
|
125
|
Sorensen CM, Braunstein TH,
Holstein-Rathlou NH and Salomonsson M: Role of vascular potassium
channels in the regulation of renal hemodynamics. Am J Physiol
Renal Physiol. 302:F505–F518. 2012.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Tamura Y, Tanabe K, Kitagawa W, Uchida S,
Schreiner GF, Johnson RJ and Nakagawa T: Nicorandil, a K(atp)
channel opener, alleviates chronic renal injury by targeting
podocytes and macrophages. Am J Physiol Renal Physiol.
303:F339–F349. 2012.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Snijder PM, Frenay AR, Koning AM, Bachtler
M, Pasch A, Kwakernaak AJ, van den Berg E, Bos EM, Hillebrands JL,
Navis G, et al: Sodium thiosulfate attenuates angiotensin
II-induced hypertension, proteinuria and renal damage. Nitric
Oxide. 42:87–98. 2014.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Hyodo T, Oda T, Kikuchi Y, Higashi K,
Kushiyama T, Yamamoto K, Yamada M, Suzuki S, Hokari R, Kinoshita M,
et al: Voltage-gated potassium channel Kv1.3 blocker as a potential
treatment for rat anti-glomerular basement membrane
glomerulonephritis. Am J Physiol Renal Physiol. 299:F1258–F1269.
2010.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Huang C, Zhang L, Shi Y, Yi H, Zhao Y,
Chen J, Pollock CA and Chen XM: The KCa3.1 blocker TRAM34 reverses
renal damage in a mouse model of established diabetic nephropathy.
PLoS One. 13(e0192800)2018.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Piwkowska A, Rogacka D, Audzeyenka I,
Kasztan M, Angielski S and Jankowski M: Insulin increases
glomerular filtration barrier permeability through PKGIα-dependent
mobilization of BKCa channels in cultured rat podocytes. Biochim
Biophys Acta. 1852:1599–1609. 2015.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Neverisky DL and Abbott GW: Ion
channel-transporter interactions. Crit Rev Biochem Mol Biol.
51:257–267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Veiras LC, McFarlin BE, Ralph DL, Buncha
V, Prescott J, Shirvani BS, McDonough JC, Ha D, Giani J, Gurley SB,
et al: Electrolyte and transporter responses to angiotensin II
induced hypertension in female and male rats and mice. Acta Physiol
(Oxf). 229(e13448)2020.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Orlov SN, Adragna NC, Adarichev VA and
Hamet P: Genetic and biochemical determinants of abnormal
monovalent ion transport in primary hypertension. Am J Physiol.
276:C511–C536. 1999.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Graves SW: Sodium regulation, sodium pump
function and sodium pump inhibitors in uncomplicated pregnancy and
preeclampsia. Front Biosci. 12:2438–2446. 2007.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Devuyst O, Jouret F, Auzanneau C and
Courtoy PJ: Chloride channels and endocytosis: New insights from
Dent's disease and ClC-5 knockout mice. Nephron Physiol.
99:p69–p73. 2005.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Shipman KE and Weisz OA: Making a dent in
dent disease. Function (Oxf). 1(zqaa017)2020.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Anglani F, Gianesello L, Beara-Lasic L and
Lieske J: Dent disease: A window into calcium and phosphate
transport. J Cell Mol Med. 23:7132–7142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Svenningsen P, Friis UG, Versland JB, Buhl
KB, Møller Frederiksen B, Andersen H, Zachar RM, Bistrup C, Skøtt
O, Jørgensen JS, et al: Mechanisms of renal NaCl retention in
proteinuric disease. Acta Physiol (Oxf). 207:536–545.
2013.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Gadau J, Peters H, Kastner C, Kühn H,
Nieminen-Kelhä M, Khadzhynov D, Krämer S, Castrop H, Bachmann S and
Theilig F: Mechanisms of tubular volume retention in
immune-mediated glomerulonephritis. Kidney Int. 75:699–710.
2009.PubMed/NCBI View Article : Google Scholar
|
|
140
|
de Seigneux S, Wilhelm-Bals A and
Courbebaisse M: On the relationship between proteinuria and plasma
phosphate. Swiss Med Wkly. 147(w14509)2017.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Shimizu MH, Volpini RA, de Bragança AC,
Campos R, Canale D, Sanches TR, Andrade L and Seguro AC:
N-acetylcysteine attenuates renal alterations induced by senescence
in the rat. Exp Gerontol. 48:298–303. 2013.PubMed/NCBI View Article : Google Scholar
|