Introduction
Follicular atresia is an inevitable degenerative
process that occurs at all stages of follicular development in
mammals (1,2). Among domesticated animals, sheep and
cows, >99% of these follicles undergo atresia (3-5),
however the regulatory mechanism of follicular atresia remains
largely unclear. Granulosa cells (GCs) are shown to play a key role
in the processes of follicular development and atresia (5). GC proliferation and estradiol
synthesis is closely associated with follicular atresia (6,7).
Thus, elucidating regulatory mechanisms such as follicular GC
proliferation and estradiol synthesis is important to decrease the
occurrence of these atresia processes.
The growth, differentiation and apoptosis of GCs are
modulated by a large number of molecules including non-coding RNAs
(ncRNAs) genes (8,9). Among ncRNAs, long ncRNAs (lncRNAs),
which are defined as transcripts >200 nucleotides without
protein-coding ability, have gained widespread attention in disease
diagnosis and treatment (10,11).
In the field of reproduction, dysregulation of lncRNAs has been
shown to play important roles in ovarian function, follicle
development and luteal formation in humans, mice, bovines and pigs
(12,13).
LncRNA nuclear-enriched abundant transcript 1
(NEAT1) has been reported to be highly expressed in multiple types
of cancer, such as breast, colorectal and thyroid cancer, and plays
an oncogenic role in tumorigenesis by regulating cell
proliferation, apoptosis, invasion and metastasis (14,15).
NEAT1 has been reported to be involved in polycystic ovary syndrome
by regulating GC cell proliferation and apoptosis via the microRNA
(miR)-381/insulin-like growth factor 1 (IGF1) axis (16). However, its functional roles and
regulatory mechanisms in GC remain elusive. Therefore, the present
study aimed to investigate the biological effects of NEAT1 on mouse
GC proliferation, apoptosis and estradiol synthesis. Additionally,
the regulatory mechanisms of NEAT1 in GCs were further explored
through a series of experiments.
Materials and methods
Animals and follicles isolation
A total of 10 female C57BL/6j mice (age, 4-5 weeks;
weight, 18-25 g) were obtained from the Changchun Institute of
Biological Products Co., Ltd., and housed at 21-25˚C, humidity
52-60% and a 12-h light/dark cycle under specific pathogen-free
conditions. All of the mice were provided a standard diet and
sterile water ad libitum for one week. Animal care and
experiments were done in accordance with the ‘Principles for the
Utilization and Care of Vertebrate Animals’ of the National
Institutes of Health (17).
The behavior and health status of the mice were
observed every day, and the mice were weighed every 2 days. The
mice were sacrificed by inhalation of CO2 (50% of the
chamber volume/min) before dissection for excision of ovarian
tissue. Confirmation of mice mortality was verified lack of
heartbeat and dilation of pupils. Follicles were isolated from
ovarian tissue according to a previous study (18). Follicles were divided into healthy
follicles (HFs), early atretic follicles (EAFs) and progressively
atretic follicles (PAFs) according to follicle morphological
characteristics as previously described by Miller et al
(19).
Mouse granulosa cells (mGCs) isolation
and culture
Primary mGCs were isolated from follicles and
cultured as described previously (20). Dulbecco's Modified Eagle
medium/nutrient mixture F-12 (DMEM/F12; MilliporeSigma) was changed
every 48 h. The two or three passage mGCs were selected for
subsequent experiments. All experiments using laboratory animals
were approved by the Animal Ethics Committee of Jilin Academy of
Agricultural Sciences (approval no. JNK20210719-2; Changchun,
China).
mGCs transfection
A siRNA that directly targeted mouse NEAT1
(si-NEAT1; 5'-TGGTAATGGTGGAGGAAGA-3') and appropriate non-targeting
siRNA (si-NC; 5'-GGCUCCGAACGUGUCACGUU-3') were bought from Takara
Biotechnology Co., Ltd. In addition, Shanghai GenePharma Co., Ltd.
provided miR-874-3p mimics (miR-874-3p;
5'-UGAGCUGUAAUCAGGUCCCGUC-3'), scrambled negative control (miR-NC;
5'-UUGUACUACACAAAAGUACUG-3'), miR-874-3p inhibitor
(anti-miR-874-3p; 5'-GACGGGACCUGAUUACAGCUCA-3') as well as its
scrambled negative control (anti-miR-NC;
5'-CAGUACUUUUGUGUAGUACAA-3'). mGCs (5x103 cells/well)
were cultured in six-well plates until they reached 80% confluence.
After 4 h of cell starvation in serum-free medium, mGCs were
treated with a mixture containing 100 nM mimics, inhibitors or
siRNAs and Lipofectamine® 3000 reagent (Thermo Fisher
Scientific, Inc.). After 24 h of transfection at 37˚C, the cells
were used for follow-up experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from mGCs using a
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Subsequently, RNA was
reverse transcribed into complementary DNA using TaqMan Reverse
Transcription reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The mRNA expression levels were
examined using the Power SYBR® Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) under the 7500
Fast Real Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers used in the present study have been
previously reported (21-23)
and listed in Table I. The PCR
amplification conditions were as follows: Pre-denaturation at 95˚C
for 30 sec, denaturation at 95˚C for 10 sec, and annealing and
extension at 60˚C for 30 sec. These steps were repeated for 40
cycles. GAPDH and U6 served as endogenous controls for
NEAT1/cytochrome P450 family 19 subfamily A member 1
(CYP19A1)/cytochrome P450 family 1 subfamily B member 1
(CYP1B1) and miR-874-3p expression, respectively. The
relative expression levels were calculated using the
2-ΔΔCq method (24).
 | Table IPrimers for reverse
transcription-quantitative PCR analysis. |
Table I
Primers for reverse
transcription-quantitative PCR analysis.
| Target gene | Sequence
(5'-3') |
|---|
| NEAT1-F |
TGAGTAGTGGAAGCAGGAGGAT |
| NEAT1-R |
GGAGGCAAGGACGAGACAGA |
| CYP19A1-F |
GACACATCATGCTGGACACC |
| CYP19A1-R |
CAAGTCCTTGACGGATCGTT |
| CYP1B1-F |
CACTATTACGGACATCTTCGG |
| CYP1B1-R |
AGGTTGGGCTGGTCACTC |
| GAPDH-F |
GAGTCCACTGGCGTCTTCAC |
| GAPDH-R |
ATCTTGAGGCTGTTGTCATACTTCT |
| miR-874-3p-F |
GAACTCCACTGTAGCAGAGATGGT |
| miR-874-3p-R |
CATTTTTTCCACTCCTCTTCTCTC |
| U6-F |
CTCGCTTCGGCAGCACATATACT |
| U6-R |
ACGCTTCACGAATTTGCGTGTC |
Cell proliferation assay
The CellTiter 96 Aqueous One Solution Cell
Proliferation kit (MTS; Promega Corporation) was used to examine
cell proliferation. Briefly, at 24 h post-transfection, a total of
5x103 mGCs/well were seeded into 96-well plates and
cultured for an additional 24-72 h. At 24, 48 and 72 h, 20 µl MTS
was added into each well for an additional 2 h. The absorbance at
490 nm was measured with a spectrophotometric plate reader
(Synergy2; BioTek Instruments, Inc.).
Apoptosis detection
Following transfection for 48 h, the apoptosis
(early + late phases) of the mGCs was detected using the Apoptosis
Detection Kit Annexin V-FITC (Becton, Dickinson and Company) under
a flow cytometer (BD Biosciences) as per manufacturer's
instructions. The apoptosis ratio was analyzed using the CellQuest
3.0 software (BD Biosciences).
Steroid hormone detection
Transfected cells were grown in serum-free medium
for 48 h. The production of 17β-Estradiol (E2) and progesterone
(P4) were measured using enzyme-linked immunosorbent assay (ELISA)
kits (cat nos. E03E0023 and E03P0200; BlueGene) based on the
manufacturer's instructions, respectively. The minimum detectable
concentrations were 5 pg/ml for E2 and 0.2 ng/ml for P4.
Isolation of cytoplasmic and nuclear
RNA
The Cytoplasmic and Nuclear RNA Purification kit
(Norgen Biotek Corp.,) was used to isolate cytoplasmic and nuclear
RNA from mGCs following the manufacturer's instruction. The
expression of NEAT1 in cytoplasmic and nuclear RNA of mGCs was
measured using RT-qPCR as mentioned above.
Luciferase reporter assay
The ENCORI database (https://starbase.sysu.edu.cn/) was used to decode the
miR-874-3p-NEAT1 binding interaction. The wild-type sequence NEAT1
(WT-NEAT1) containing a binding target site of miR-874-3p
(5'-UGAGCUGUAAUCACCAGGGCAC-3') was synthesized and inserted into a
dual-luciferase miRNA target expression vector (pmirGLO; Promega
Corporation). In addition, another reporter plasmid was conducted
by inserting the mutated target sequence of miR-874-3p
(5'-UGAGCUGUAAUCAGGUCCCGUC-3') into a pmirGLO vector to form a
negative control plasmid, namely NEAT1-mutant (MUT-NEAT1).
Subsequently, both reporter plasmids and miR-874-3p mimics
(5'-UGAGCUGUAAUCAGGUCCCGUC-3') or miR-NC mimics
(5'-UUGUACUACACAAAAGUACUG-3') were co-transfected into mGCs cells
using Lipofectamine® 3000 reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Luciferase activity assay was performed at 48 h post-transfection
via a Dual Luciferase Reporter Assay System (Promega Corporation)
according to the kit specification sheet. The firefly luciferase
activity of each sample was normalized to Renilla luciferase
activity.
RNA immunoprecipitation (RIP)
The EZ-Magna RIP RNA-binding protein
immunoprecipitation kit (cat. no. 17-701; Merck KGaA) was applied
to analyze the association between miR-874-3p and NEAT1 in mGCs
according to the manufacturer's instructions. Briefly, following
washing of the cells in the flasks or plates twice with 10 ml
ice-cold PBS, 2.0x107 mGCs were lysed using 100 µl RIP
lysis buffer (cat no. CS203176, MilliporeSigma) containing 0.5 µl
protease inhibitor cocktail and 0.25 µl RNase inhibitor. Cells were
collected by centrifugation at 1,000 x g for 5 min at 4˚C and the
supernatant was discarded, then 100 µl RIP lysis buffer was added
for mGCs RIP lysis. Magnetic beads (protein A/G; cat no. CS203178;
MilliporeSigma) were completely dispersed and re-suspended by
pipetting according to the manufacturer's protocol, then 50 µl
magnetic bead suspension was transferred to each tube, and 0.5 ml
RIP Wash Buffer (cat. no. CS203177; MilliporeSigma) was added to
each tube and vortexed briefly. Subsequently, ~5 µg human
anti-argonaute-2 (Ago2; cat. no. MABE56; MilliporeSigma) or normal
mouse immunoglobulin G (IgG; cat. no. 12-370; MilliporeSigma) as
control antibodies was added for 4 h at 4˚C according to the
manufacturer's protocol. A total of 100 µl mGCs RIP lysate was
centrifuged at 3,000 x g for 10 min at 4˚C. Subsequently, 100 µl of
the supernatant was removed and added to each beads-antibody
complex in 900 µl RIP Immunoprecipitation Buffer (860 µl RIP Wash
Buffer, 35 µl 0.5 M EDTA and 5 µl RNase Inhibitor), and gently
rotated using a rotary mixer (cat. no. CC8039-01; As One Shanghai
Corporation) for 3 h at 4˚C. Immunoprecipitation tubes were
centrifuged at 3,000 x g for 10 min at 4˚C and the supernatant was
discarded. The beads were washed six times with 500 µl cold RIP
Wash Buffer (cat. no. CS203177; MilliporeSigma). Ago2 or IgG
complex (100 µl) isolation from beads was performed using 150 µl
proteinase K buffer containing 117 µl RIP Wash Buffer, 15 µl 10%
SDS and 18 µl 10 mg/ml proteinase K at 55˚C for 30 min. RNA was
isolated and purified from the precipitate using TRIzol®
reagent according to the manufacturer's protocol. RT-qPCR was
performed to measure the expression levels of NEAT1 and
miR-874-3p.
Statistical analysis
All statistical analyses were performed using the
SPSS 20.0 software (IBM Corp., SPSS). All results are expressed as
the mean ± standard deviation (SD) from at least three independent
experiments. Unpaired Student's t-test and one-way ANOVA followed
by the Tukey's post hoc test or Pearson's correlation analysis were
applied to analyze the significant differences, as appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of NEAT1 and miR-874-3p in
EAFs and PAFs
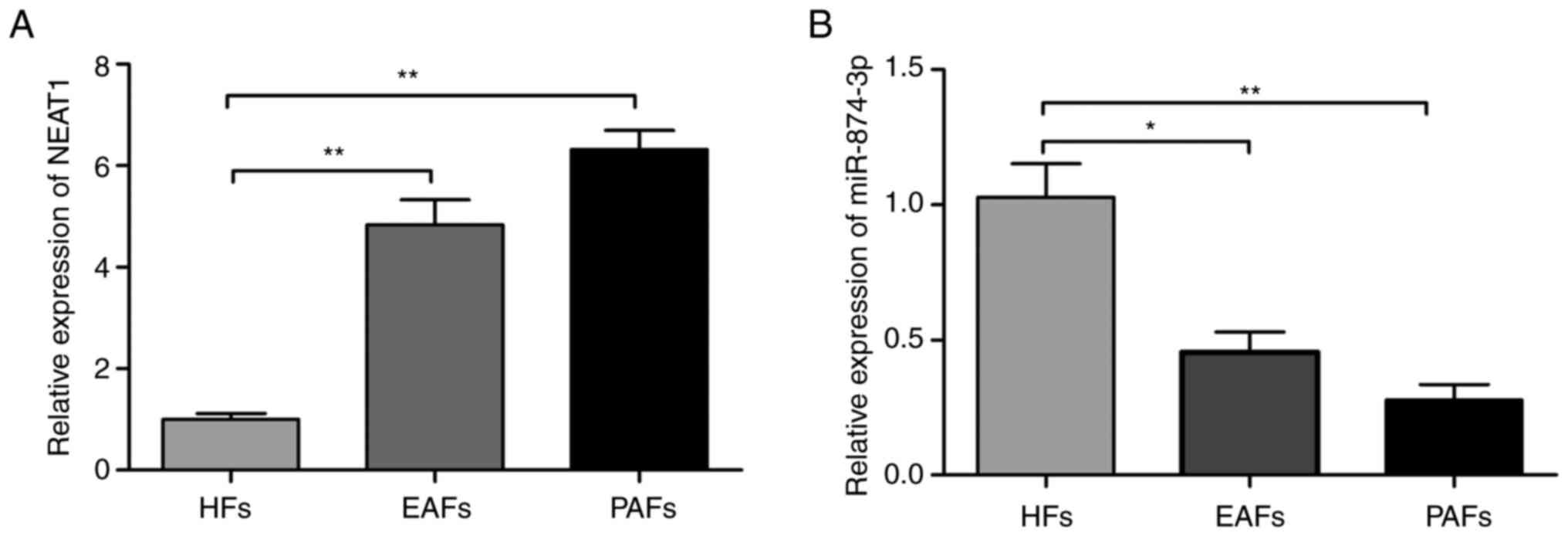
The expression levels of NEAT1 and miR-874-3p in
HFs, EAFs and PAFs were detected using RT-qPCR. The results
displayed that the expression level of NEAT1 was significantly
increased in EAFs and PAFs compared with HFs (Fig. 1A). However, the miR-874-3p
expression level was significantly downregulated in EAFs and PAFs
tissues (Fig. 1B). These results
suggested that NEAT1 and miR-874-3p may be involved in follicular
atresia progression.
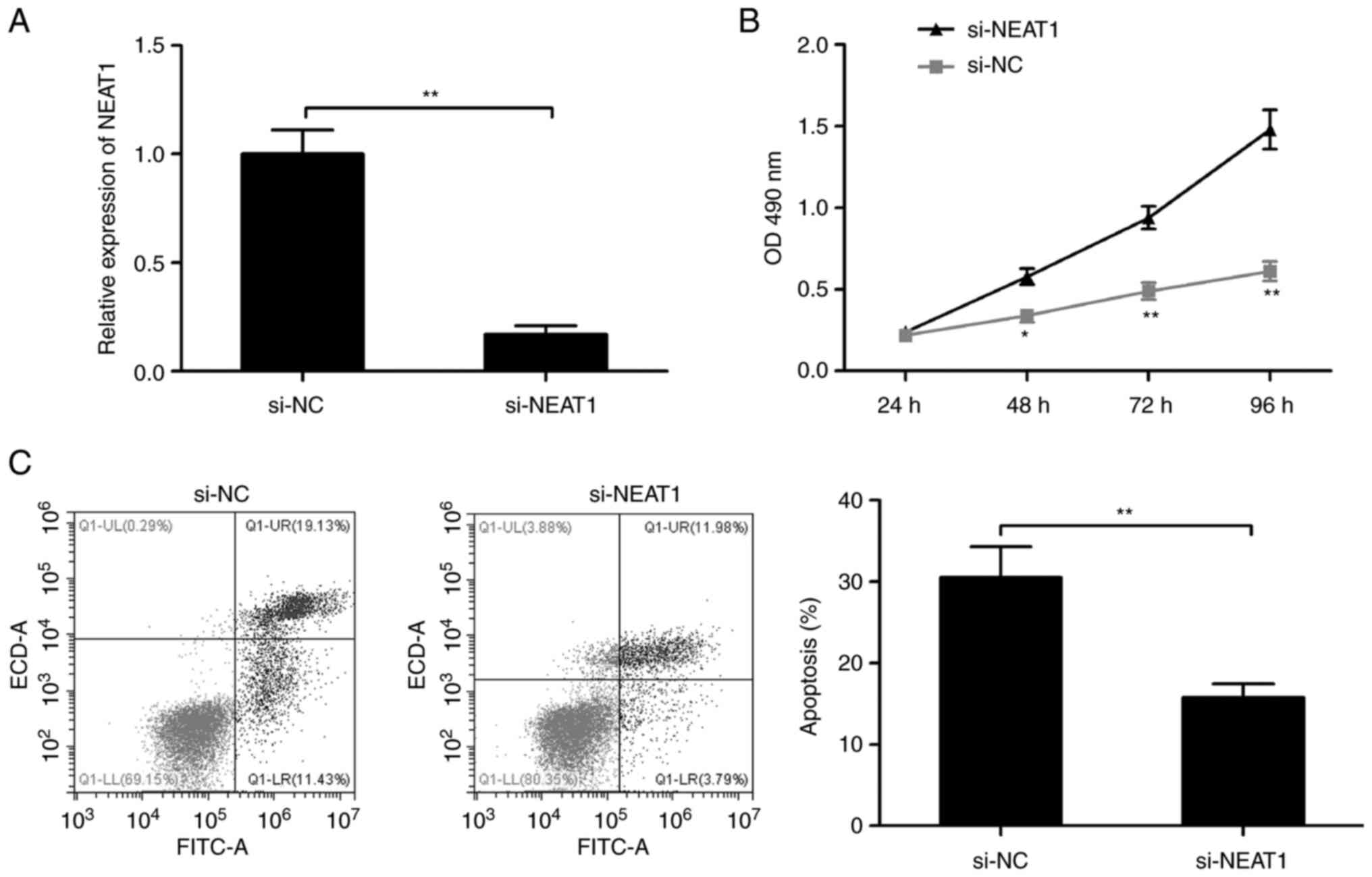
Effects of NEAT1-knockdown on mGC
proliferation and apoptosis
To investigate the potential role of NEAT1 in
follicular atresia, mGCs were transfected with siRNA against NEAT1
(si-NEAT1) to knockdown its expression. The results revealed that
knockdown of NEAT1 significantly decreased the NEAT1 expression of
mGCs (Fig. 2A). Furthermore, the
MTS assay demonstrated that NEAT1 depletion significantly increased
the proliferation of mGCs cells after 48 h compared with the si-NC
group (Fig. 2B). Finally, the flow
cytometry results showed that the knockdown of NEAT1 significantly
decreased the apoptosis ratio in mGCs (Fig. 2C).
Effects of NEAT1 knockdown on
estradiol synthesis
The effect of NEAT1-knockdown on estradiol synthesis
using ELISA assay was investigated. The results demonstrated that
suppression of NEAT1 significantly increased the production of E2
and P4 in mGCs compared with the si-NC group (Fig. 3A and B). The expression of two genes encoding
steroidogenic enzymes, CYP1B1 and CYP19A1, two key genes in
estradiol synthesis (25) were
also analyzed in mGCs transfected with si-NEAT1 or si-NC by
RT-qPCR. The results revealed that knockdown of NEAT1 significantly
increased the CYP1B1 and CYP19A1 expression levels in
mGCs compared with the si-NC group (Fig. 3C and D). These results suggested that NEAT1 may
play an important role in the regulation of steroidogenesis in
mGCs.
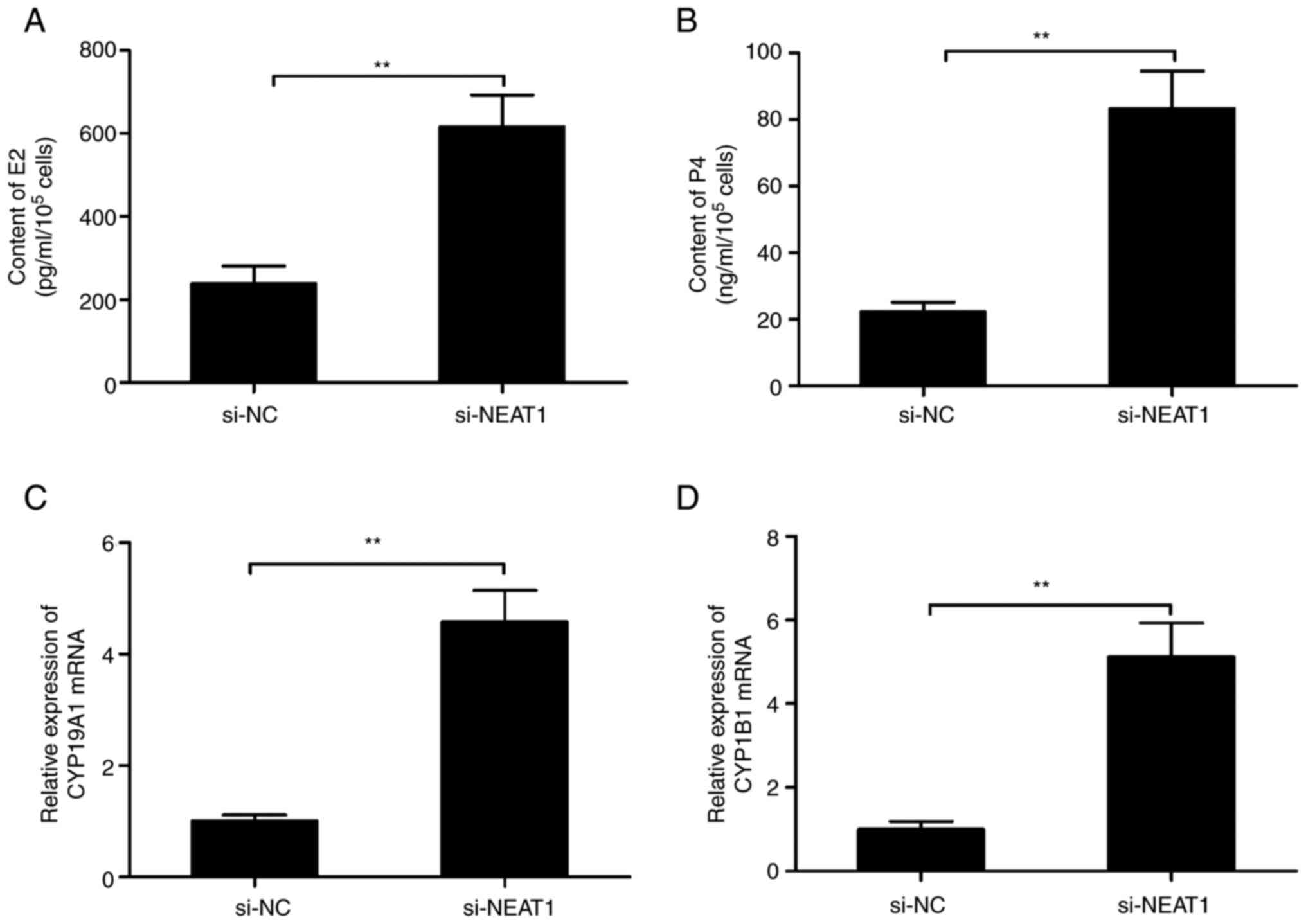 | Figure 3Knockdown of NEAT1 affects the
concentrations of estradiol and progesterone in mGCs. The (A) E2
and (B) P4 concentrations were measured in mGCs transfected with
the si-NC or si-NEAT1 using ELISA. The (C) CYP1B1 and (D)
CYP19A1 mRNA expression levels were analyzed in mGCs
transfected with si-NC or si-NEAT1 using reverse
transcription-quantitative PCR. **P<0.01. mGCs, mouse
granulosa cells; NEAT1, nuclear-enriched abundant transcript;
CYP1B1, cytochrome P450 1B1; CYP19A1, cytochrome
P450, family 19, subfamily a, polypeptide 1; E2, estradiol; P4,
progesterone; si-, short interfering-; NC, negative control. |
MiR-874-3p is negatively regulated by
NEAT1 in mGCs
Accumulating evidence has suggested that cytoplasmic
lncRNAs exert their biological functions by acting as sponges for
miRNAs to negatively regulate miRNAs expression (26). The present study revealed that
NEAT1 was mainly located in the cytoplasm of mGCs (Fig. 4A). The online software ENCORI was
used to identify the potential miRNA targets of NEAT1. The
screening results revealed that miR-874-3p could bind to
complementary sequences in NEAT1 (Fig.
4B). Subsequently, luciferase reporter assay was performed to
investigate whether miR-874-3p can bind with NEAT1 via direct
binding effects. As presented in Fig.
4C, overexpression of miR-874-3p significantly decreased the
luciferase activity of WT-NEAT1, but not that of MUT-NEAT1.
Furthermore, RIP assay was applied on mGC extracts to verify the
direct association between NEAT1 and miR-874-3p using antibodies
against Ago2. The results demonstrated that NEAT1 and miR-874-3p
were significantly enriched in Ago2 pellets compared with control
IgG (Fig. 4D). Additionally, NEAT1
downregulation significantly increased the expression levels of
miR-874-3p in mGCs (Fig. 4E),
while overexpression of miR-874-3p significantly decreased the
expression levels of NEAT1 in mGCs (Fig. 4F). Overall, these results suggested
that NEAT1 acted as a sponge for decreasing miR-874-3p
expression.
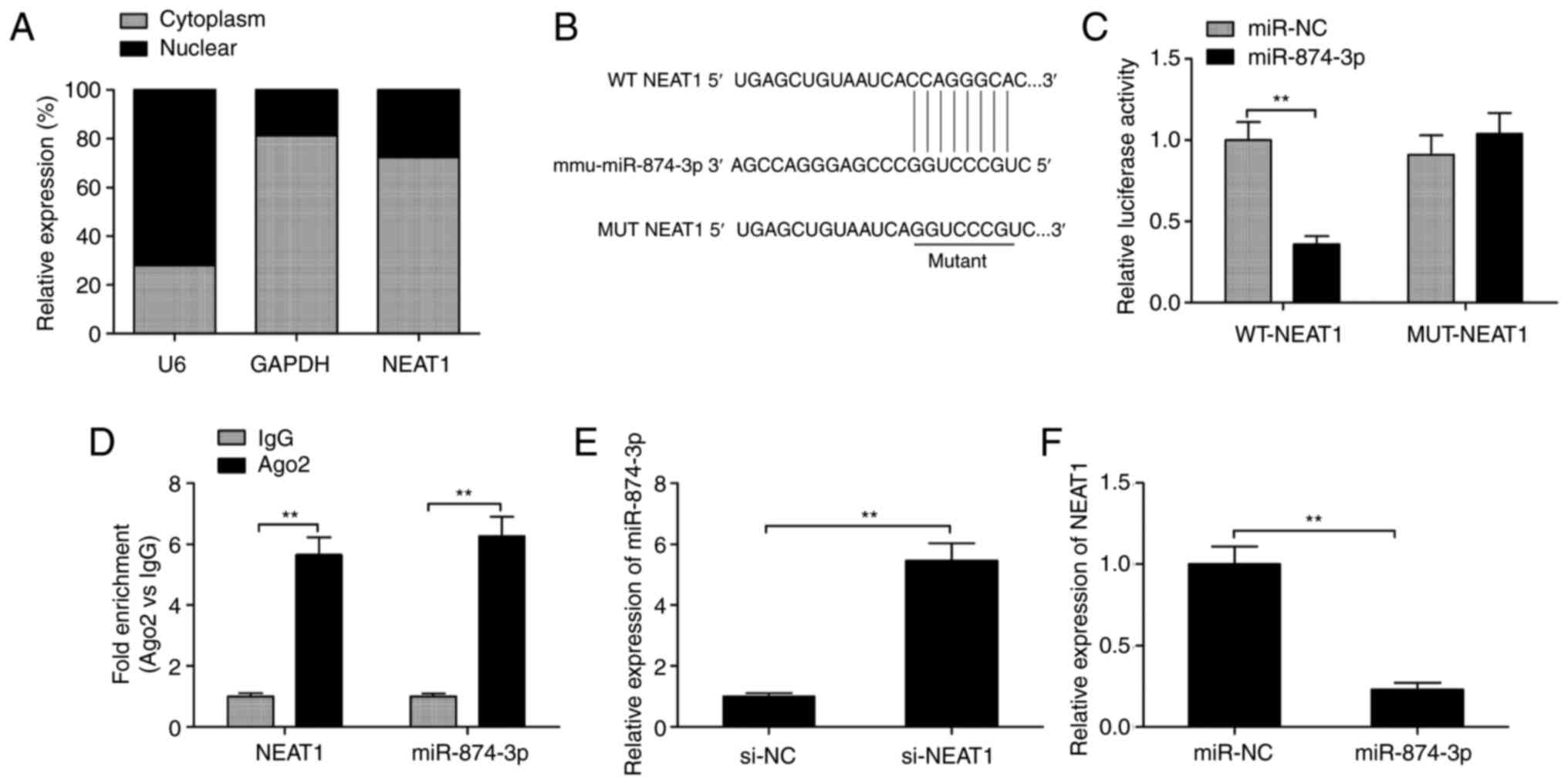 | Figure 4miR-874-3p is negatively regulated by
NEAT1 in mGCs. (A) Expression of NEAT1 was examined in cytoplasm
and nuclear of mGCs by RT-qPCR. (B) WT and MUT binding sites of
miR-874-3p on NEAT1 are shown. (C) Luciferase activity was
determined in mGCs following co-transfection with miR-874-3p/miR-NC
mimics and WT-NEAT1 or MUT-NEAT1 reporter plasmid. (D) Association
between NEAT1 and miR-874-3p using RIP assay. (E) Relative
expression levels of miR-874-3p were examined by RT-qPCR in mGCs
transfected with si-NEAT1 or si-NC. (F) Relative expression level
of NEAT1 was examined using RT-qPCR in mGCs cells transfected with
miR-874-3p mimics or miR-NC. **P<0.01. mGCs, mouse
granulosa cells; NEAT1, nuclear-enriched abundant transcript;
RT-qPCR, reverse transcription-quantitative PCR; NEAT1,
nuclear-enriched abundant transcript 1; MUT, mutant; WT, wild-type;
IgG, immunoglobulin G; Ago2, argonaute RISC catalytic component 2,
miR, microRNA; NC, negative control; si-, short interfering-. |
NEAT1-knockdown affects mGCs
proliferation and estradiol synthesis by regulating miR-874-3p
RT-qPCR analysis demonstrated notable miR-874-3p
upregulation following transfection with miR-874-3p mimics and
downregulation after transfection with miR-874-3p inhibitors
(Fig. 5A and B). To verify whether NEAT1 regulated mGCs
proliferation and estradiol synthesis by targeting miR-874-3p, a
rescue experiment was subsequently conducted. It was revealed that
the inhibition of miR-874-3p significantly reversed the NEAT1
knockdown-induced increase of miR-874-3p expression levels in mGCs
(Fig. 5C). In addition, miR-874-3p
inhibition significantly rescued the effects of NEAT1 depletion on
mGCs apoptosis and proliferation, as well as the production of E2
and P4 (Fig. 5D-G). The
aforementioned data suggested that NEAT1 regulated mGCs
proliferation and estradiol synthesis by sponging miR-874-3p.
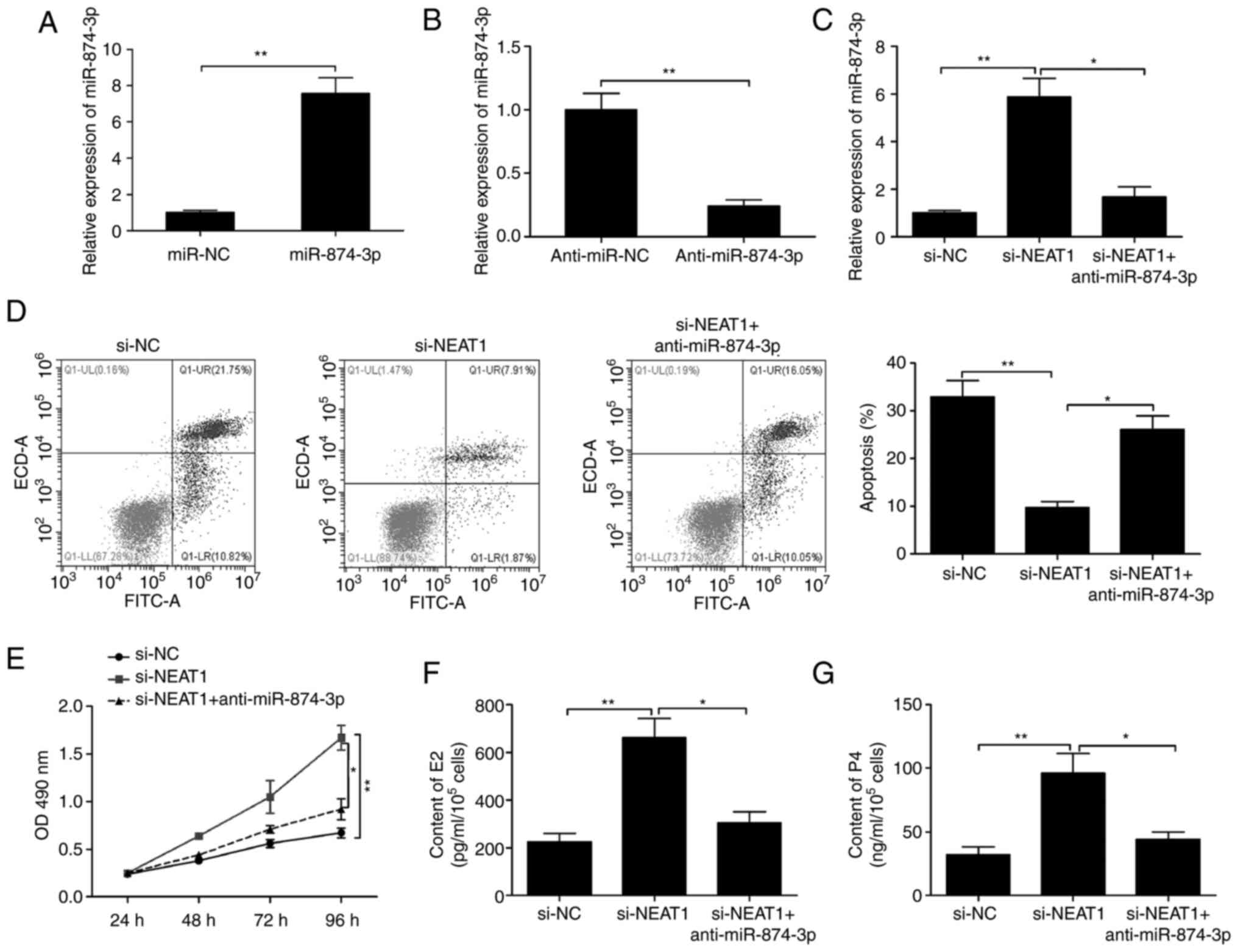 | Figure 5NEAT1 exerts a biological role in
mGCs by sponging miR-874-3p. (A) Expression of miR-874-3p was
examined in mGCs transfected with miR-874-3p mimics and miR-NC by
RT-qPCR. (B) Expression of miR-874-3p was examined in mGCs
transfected with miR-874-3p inhibitor (anti-miR-874-3p) and
anti-miR-NC by RT-qPCR. (C) Expression of miR-874-3p was examined
in mGCs transfected with si-NC, si-NEAT1 and si-NEAT1 +
anti-miR-874-3p using reverse transcription-quantitative PCR. The
(D) apoptosis, (E) cell proliferation, (F) E2 and (G) P4 production
were determined in mGCs transfected with si-NC, si-NEAT1 and
si-NEAT1 + anti-miR-874-3p. *P<0.05 and
**P<0.01. mGCs, mouse granulosa cells; NEAT1,
nuclear-enriched abundant transcript 1; si-, short interfering;
miR, microRNA; NC, negative control; E2, estradiol; P4,
progesterone; OD, optical density; RT-qPCR, reverse
transcription-quantitative PCR. |
Discussion
Several lncRNAs have been identified to be key
regulators of the normal development of GCs and are thereby
involved in both physiological conditions and pathological
processes, such as human oocyte maturation, fertilization, embryo
development and ovarian failure (12,13).
For example, lncRNA NORHA significantly induces GC apoptosis by
influencing the activities of the miR-183-96-182 cluster and the
forkhead box protein O1 axis (27). The LINC00477/miR-128 axis
contributes to the progression of polycystic ovary syndrome by
regulating ovarian granulosa cell proliferation and apoptosis
(28). LncRNA steroid receptor RNA
activator increases cell growth, inhibited apoptosis and induced
secretion of E2 and P4 in mGCs (29). Metastasis-associated lung
adenocarcinoma transcript 1 regulates mGC apoptosis and the
secretion of E2 and P4 by regulating the miR-205/CREB1 axis
(30). The present study revealed
that knockdown of NEAT1 promoted cell proliferation, inhibited
apoptosis and induced secretion of E2 and P4 by sponging
miR-874-3p, thus suggesting that NEAT1 might be involved in
follicular atresia.
GC proliferation and gonadal steroid hormones play
key roles in both normal reproductive processes and some
reproductive and nonreproductive pathology, including follicular
atresia (6,7). GC apoptosis has been confirmed to be
a main reason for follicular atresia (2). Thus, understanding of the underlying
molecular mechanisms controlling steroid production, cell
proliferation and apoptosis within GCs is needed for controlling
follicular atresia. In the present study, the results demonstrated
that the basal expression levels of NEAT1 in EAFs and PAFs were
significantly upregulated compared with HFs. The results also
demonstrated that NEAT1 depletion significantly increased cell
proliferation, inhibited apoptosis and promoted the production of
E2 and P4 in mGCs, thus implying that NEAT1 could participate in
follicular atresia progression.
LncRNA NEAT1 has been revealed to function as an
oncogene in ovarian carcinogenesis and serves as a potential
biomarker for this disease (14,15).
Moreover, NEAT1 depletion is able to affect human ovarian GC
proliferation and apoptosis through regulation of the miR-381/IGF1
axis (16). However, its
functional roles and regulatory mechanisms in GC remain largely
unclear. The present study discovered that knockdown of NEAT1
significantly increased cell proliferation and inhibited apoptosis
in mGCs, which was consistent with previous results (16). Notably, the present study's results
also revealed that NEAT1 downregulation significantly promoted the
production of E2 and P4 in mGCs.
Accumulating evidence has suggested that lncRNAs
exerts diverse biological outcomes by sponging miRNAs involved in
normal development and pathological responses (25,26,31).
Based on the basic principles of interactions between miRNAs and
lncRNAs, the underlying mechanism of NEAT1 affecting GC
proliferation and apoptosis was subsequently investigated in the
present study. The bioinformatical analysis indicated that NEAT1
could bind with multiple miRNAs. MiR-874-3p has been shown to be
downregulated and serves as a tumor suppressor in several types of
cancer, including ovarian cancer (32). Notably, miR-874-3p has been
reported to promote testosterone-induced GC apoptosis by
suppressing histone deacetylase 1-mediated p53 deacetylation
(33). Furthermore, the luciferase
reporter activity and RIP assays in the present study confirmed
this interaction. NEAT1 knockdown increased the expression level of
miR-874-3p in mGCs, while overexpression of miR-874-3p decreased
the expression level of NEAT1. The effects of NEAT1 depletion on
MGCs proliferation, apoptosis and the production of E2 and P4 were
partially reserved following miR-874-3p inhibition. Taken together,
the aforementioned results indicated that NEAT1 regulated mGC
proliferation and estradiol synthesis by sponging miR-874-3p.
In summary, the present study demonstrated that
NEAT1 functioned as an important regulator of E2 release, cell
proliferation and apoptosis in mGCs by sponging miR-874-3p.
Although further studies are needed to study other miRNAs that bind
with NEAT1 to clarify the regulatory mechanism of NEAT1, the
present study demonstrated that NEAT1 can regulate GC cell
proliferation and steroidogenesis, suggesting that NEAT1 has
therapeutic implications for the control of follicular atresia and
fertility.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Natural Science
Foundation of Basic Research Fund (grant no. KYJF2021ZR003) and the
Program of Science and Technology Development Plan of Jilin
Province (grant no. 20191001003XH).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and XLi conceived the study. PZ, JG, SL and GL
performed the experiments and wrote the manuscript. WW and CT
analyzed the data. XLiu interpreted data. PZ and GL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Jilin Academy of Agricultural Sciences (approval no.
JNK20210719-2; Changchun, China) and was in accordance with the
Declaration of Helsinki (2000).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moley KH and Schreiber JR: Ovarian
follicular growth, ovulation and atresia. Endocrine, paracrine and
autocrine regulation. Adv Exp Med Biol. 377:103–119.
1995.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Manabe N, Goto Y, Matsuda-Minehata F,
Inoue N, Maeda A, Sakamaki K and Miyano T: Regulation mechanism of
selective atresia in porcine follicles: Regulation of granulosa
cell apoptosis during atresia. J Reprod Dev. 50:493–514.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ma L, Tang X, Guo S, Liang M, Zhang B and
Jiang Z: miRNA-21-3p targeting of FGF2 suppresses autophagy of
bovine ovarian granulosa cells through AKT/mTOR pathway.
Theriogenology. 157:226–237. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Driancourt MA, Gibson WR and Cahill LP:
Follicular dynamics throughout the oestrous cycle in sheep. A
review. Reprod Nutr Dev (1980). 25:1–15. 1985.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Matsuda F, Inoue N, Manabe N and Ohkura S:
Follicular growth and atresia in mammalian ovaries: Regulation by
survival and death of granulosa cells. J Reprod Dev. 58:44–50.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Webb R, Nicholas B, Gong JG, Campbell BK,
Gutierrez CG, Garverick HA and Armstrong DG: Mechanisms regulating
follicular development and selection of the dominant follicle.
Reproduction. 61:71–90. 2003.PubMed/NCBI
|
|
7
|
Quirk SM, Cowan RG, Harman RM, Hu CL and
Porter DA: Ovarian follicular growth and atresia: The relationship
between cell proliferation and survival. J Anim Sci. 82
(E-Suppl):E40–E52. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang J, Xu Y, Liu H and Pan Z: MicroRNAs
in ovarian follicular atresia and granulosa cell apoptosis. Reprod
Biol Endocrinol. 17(9)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Maalouf SW, Liu WS and Pate JL: MicroRNA
in ovarian function. Cell Tissue Res. 363:7–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jin L, Yang Q, Zhou C, Liu L, Wang H, Hou
M, Wu H, Shi F, Sheng J and Huang H: Profiles for long non-coding
RNAs in ovarian granulosa cells from women with PCOS with or
without hyperandrogenism. Reprod Biomed Online. 37:613–623.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tu J, Chen Y, Li Z, Yang H, Chen H and Yu
Z: Long non-coding RNAs in ovarian granulosa cells. J Ovarian Res.
13(63)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu X, Li Z, Zheng H, Chan MT and Wu WK:
NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif.
50(e12329)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou H, Wang Y, Liu Z, Zhang Z, Xiong L
and Wen Y: Recent advances of NEAT1-miRNA interactions in cancer.
Acta Biochim Biophys Sin (Shanghai). 54:153–162. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhen J, Li J, Li X, Wang X, Xiao Y, Sun Z
and Yu Q: Downregulating lncRNA NEAT1 induces proliferation and
represses apoptosis of ovarian granulosa cells in polycystic ovary
syndrome via microRNA-381/IGF1 axis. J Biomed Sci.
28(53)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
U.S. Office of Science and Technology
Policy. Laboratory animal welfare; U.S. government principles for
the utilization and care of vertebrate animals used in testing,
research and training; notice. Fed Regist. 50:20864–20865.
1985.PubMed/NCBI
|
|
18
|
Kim EJ, Lee J, Youm HW, Kim SK, Lee JR,
Suh CS and Kim SH: Comparison of follicle isolation methods for
mouse ovarian follicle culture in vitro. Reprod Sci. 25:1270–1278.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miller KP, Gupta RK, Greenfeld CR, Babus
JK and Flaws JA: Methoxychlor directly affects ovarian antral
follicle growth and atresia through Bcl-2- and Bax-mediated
pathways. Toxicol Sci. 88:213–221. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kipp JL, Kilen SM, Woodruff TK and Mayo
KE: Activin regulates estrogen receptor gene expression in the
mouse ovary. J Biol Chem. 282:36755–36765. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xie K, Cai Y, Yang P, Du F and Wu K:
Upregulating microRNA-874-3p inhibits CXCL12 expression to promote
angiogenesis and suppress inflammatory response in ischemic stroke.
Am J Physiol Cell Physiol. 319:C579–C588. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma J, Zhao N, Du L and Wang Y:
Downregulation of lncRNA NEAT1 inhibits mouse mesangial cell
proliferation, fibrosis, and inflammation but promotes apoptosis in
diabetic nephropathy. Int J Clin Exp Pathol. 12:1174–1183.
2019.PubMed/NCBI
|
|
23
|
Zhao F, Wang N, Yi Y, Lin P, Tang K, Wang
A and Jin Y: Knockdown of CREB3/Luman by shRNA in mouse granulosa
cells results in decreased estradiol and progesterone synthesis and
promotes cell proliferation. PLoS One. 11(e0168246)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yao W, Pan Z, Du X, Zhang J, Liu H and Li
Q: NORHA, a novel follicular atresia-related lncRNA, promotes
porcine granulosa cell apoptosis via the miR-183-96-182 cluster and
FoxO1 axis. J Anim Sci Biotechnol. 12(103)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gao H, Jiang J, Shi Y, Chen J, Zhao L and
Wang C: The LINC00477/miR-128 axis promotes the progression of
polycystic ovary syndrome by regulating ovarian granulosa cell
proliferation and apoptosis. Reprod Biol Endocrinol.
19(29)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li Y, Wang H, Zhou D, Shuang T, Zhao H and
Chen B: Up-Regulation of long noncoding RNA SRA promotes cell
growth, inhibits cell apoptosis, and induces secretion of estradiol
and progesterone in ovarian granular cells of mice. Med Sci Monit.
24:2384–2390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun L, Zhang P and Lu W: lncRNA MALAT1
regulates mouse granulosa cell apoptosis and 17beta-estradiol
synthesis via regulating miR-205/CREB1 Axis. Biomed Res Int.
2021(6671814)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chan JJ and Tay Y: Noncoding RNA: RNA
regulatory networks in cancer. Int J Mol Sci.
19(1310)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xia B, Lin M, Dong W, Chen H, Li B, Zhang
X, Hou Y and Lou G: Upregulation of miR-874-3p and miR-874-5p
inhibits epithelial ovarian cancer malignancy via SIK2. J Biochem
Mol Toxicol. 32(e22168)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wei Y, Wang Z, Wei L, Li S, Qiu X and Liu
C: MicroRNA-874-3p promotes testosterone-induced granulosa cell
apoptosis by suppressing HDAC1-mediated p53 deacetylation. Exp Ther
Med. 21(359)2021.PubMed/NCBI View Article : Google Scholar
|