1. Introduction
Pre-eclampsia (PE) is a complicated
pregnancy-specific disease that is characterized by hypertension
(≥140/90 mmHg on at least two occasions ~4 h apart) and impaired
function of one or more organs or systems, with proteinuria no
longer considered as a symptom of the disease (1). Affecting 3-8% of pregnant people
worldwide, PE is considered a leading cause of maternal and foetal
mortality and morbidity (2). It
can lead to severe multisystem complications such as eclampsia,
liver and kidney failure, as well as cerebral haemorrhage. In
addition, foetuses can be affected by PE, such as prematurity and
intrauterine growth restriction (IUGR) (3). PE is classified as early- (<34
weeks of gestation) and late-onset PE (>34 weeks of gestation)
(4). Early-onset PE, accounting
for 20% of PE cases, tends to have more serious consequences for
the mother and foetus, including IUGR, intrauterine death,
premature delivery and placental abruption, while late-onset PE,
accounting for 80% of PE cases, is associated with risk factors
such as insulin resistance, obesity, chronic hypertension,
dyslipidaemia and thrombophilia, but not IUGR (4,5).
Women with PE have a 4-fold increased risk of heart failure in
later life, a 2-fold increased risk of coronary artery disease and
stroke and an increased risk of cardiovascular disease in the
offspring (2).
The pathogenesis of PE is attributed to
multifactorial causes involving multiple risk factors and
mechanisms (1). Nonetheless, the
pathogenesis of PE remains unclear and it has been shown that it
may be associated with placental dysfunction, an insufficient
recast of uterine spiral arterioles, excessive activation of the
immune system, damage to vascular endothelial cells, abnormal
balance of angiogenic and antiangiogenic factors, as well as
genetic factors (6,7). At present, control of gestational
hypertension and termination of pregnancy is considered the best
available treatment option for PE (1,8).
Several interventions, such as calcium and low-dose aspirin, have
been shown to decrease mortality and morbidity in pregnant people
at high risk for PE (8). To the
best of our knowledge, no single test can reliably predict the risk
of PE, although tests of maternal angiogenic factors have some
predictive value, while risk prediction models and biomarkers may
be different among different populations (9).
In the human genome, non-coding RNAs (ncRNAs)
constitute 98% of the human genome and include microRNAs (miRNAs or
miRs), long ncRNAs (lncRNAs) and circular RNAs (circRNAs) (10). ncRNAs are involved in several
pathological and physiological processes in the human body such as
cell proliferation and adhesion as well as angiogenesis (10). The regulation of ncRNAs alters gene
activity, thereby regulating gene expression and transcription, as
well as chromatin structure, epigenetic memory and protein
translation (11). Epigenetic
changes, in particular altered expression of certain miRs, may play
key roles in placental-related disease such as PE and IUGR, which
cause changes in placental gene expression, mediate downstream
effects and promote development of placental dysfunction (12). Some lncRNAs and circRNAs inhibit
the function of miRs by binding to other miRs, thereby acting as
miR sponges (13). As a large
family of ncRNAs, the association between miRs and PE has been
studied extensively in the field of perinatal medicine. Several
miRs are differentially expressed in PE (14-16).
Dysregulation of miRs is also found in endometriosis, where they
cause differential expression of endometrial stem/progenitor cells
(17). As a key component of the
placenta, dysfunction of trophoblasts is crucial to the development
of PE (5). The present review
summarizes the function of miRs regulating trophoblast and
discusses their application in the treatment of PE.
2. Biological characteristics and production
of miRs
miRs are short-sequence and single-stranded RNAs
with a stem-loop structure that do not encode proteins. They are
19-25 nucleotides in length and bind to the 3'-untranslated regions
of mRNAs to regulate mRNA translation or direct shear of mRNAs at
the post-transcriptional level (18). The regulation of miRs is complex
because a miR can target multiple genes, while one gene can be the
target of several miRs (19). miRs
are involved in almost all life processes. For example, miRs
participate in cell function regulation and serve critical roles in
the occurrence and development of disease (19). In addition, miRs exhibit high
stability in extracellular fluids, which provides a foundation for
identifying miRs as biomarkers (20).
Under the action of polymerase II, DNA is
transcribed into a primary miR with a hairpin structure and a
polyadenylic acid tail composed of several thousand nucleotides
(21). Following cutting by the
RNase III Drosha and its cofactor DiGeorge syndrome critical region
8, a stem-loop-like miR pre-cursor miR (pre-miR) is formed, which
is ~70 nucleotides in length (18). Ras-related nuclear protein GTPase
and exportin-5 transport pre-miR from the nucleus to the cytoplasm
(22). Under the shearing action
of the nuclease Dicer and transactivation response element
RNA-binding protein or the protein activator of the
interferon-induced protein kinase, pre-miR is processed to form a
double-stranded RNA (dsRNA) with a length of ~22 nucleotides
(23). Under the action of
argonaute protein, one strand of dsRNA can be guided into the
RNA-induced silencing complex to become a mature single-stranded
miR that regulates genes, while the other strand is degraded
(24). The biosynthesis of miRs is
shown in Fig. 1. The absence of
Dicer protein in the miRNA biogenesis pathway can lead to severe
defects in reproductive function. For example, defects in the Dicer
protein damage the corpus luteum and lead to female rat
infertility, while defects of embryonic Dicer lead to early
embryonic death associated with the loss of mouse stem cells
(25). In addition, mutations in
mouse argonaute-2 protein cause placental development defects or
foetal death during the second trimester of pregnancy (26).
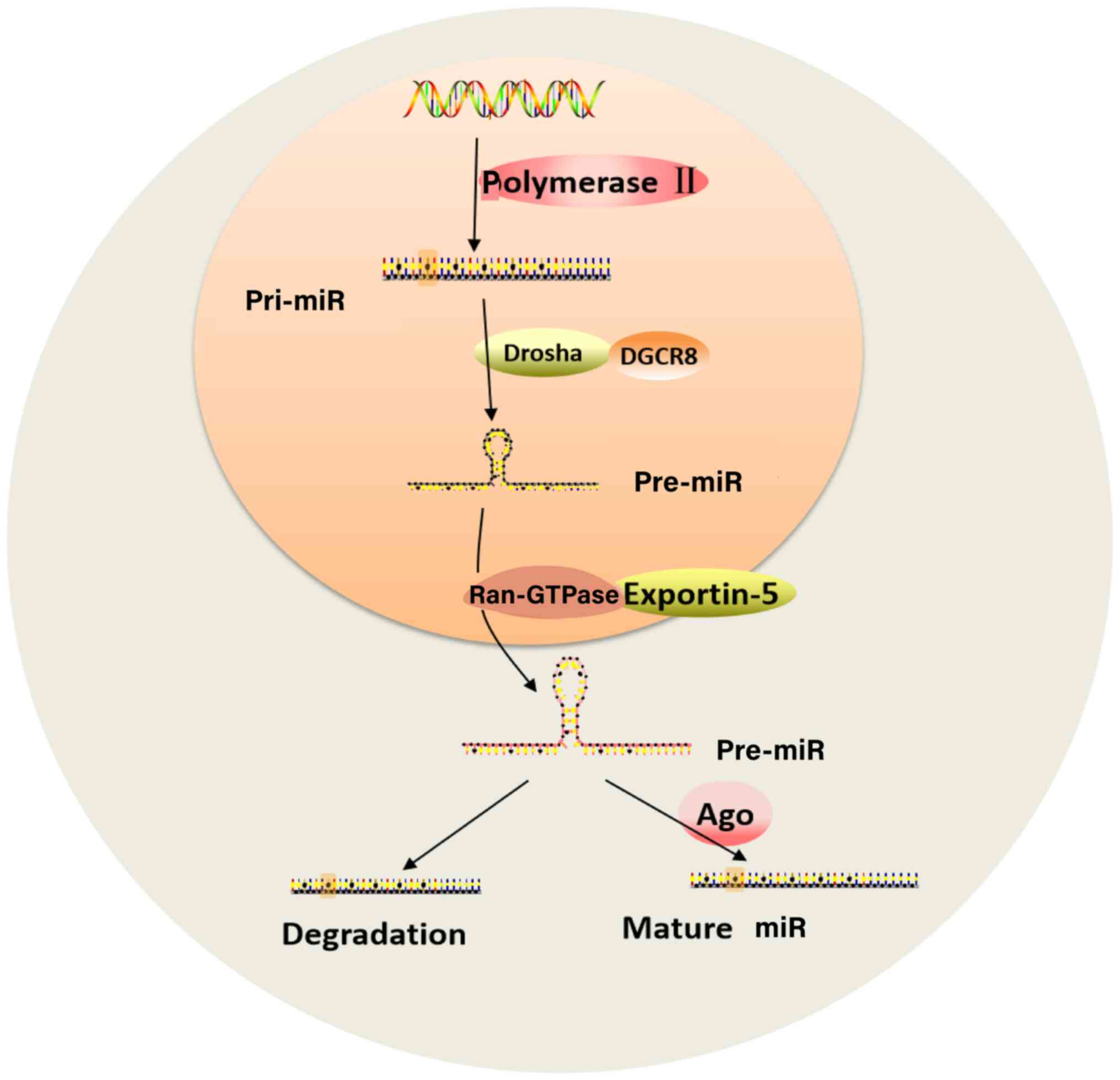 | Figure 1Biosynthesis of miRs. Under the
action of Ppolymerase II, DNA is transcribed into a pri-miR.
Following cutting by the RNase III Drosha and its cofactor DGCR8,
pre-miR is formed. Ran-GTPase and exportin-5 transport pre-miR from
the nucleus to the cytoplasm where it is processed to form dsRNA.
Then, one strand of the dsRNA becomes a mature single-stranded miR
that regulates genes, while the other strand is degraded. miR,
microRNA; DGCR8, DiGeorge syndrome critical region 8; Ran-GTPase,
Ras-related nuclear protein GTPase; dsRNA, double strand RNA; pri-,
primary; Ago, argonaute protein. |
3. miRs are expressed in PE
It has been estimated that ~400 miRs exist in
trophoblasts of the normal human placenta (27). miRs are expressed in specific
chromosomal regions and are regulated by the same promoter such as
placenta-derived chromosome 19 miR cluster (C19MC; containing 54
different miRs with functions in the placenta and reproductive
system during the third trimester of pregnancy) and chromosome 14
miR cluster (C14MC; containing 34 miRs with high expression in
placental tissue and embryos during early pregnancy) (27). Decreased expression of C19MC miRs
(miR-515-5p, miR-518b, miR-518f, miR-519d and miR-520h) has been
reported in the placenta of patients with PE, with these miRs
regulating the immune system and inflammatory response (28). However, there is no significant
difference in C19MC miRs in PE complicated by IUGR compared with PE
alone (29). miR-378a-5p, which is
located on C14MC, is downregulated in the placenta of patients with
PE and it regulates expression of Nodal (a transforming growth
factor) to inhibit trophoblast proliferation and migration and
induce trophoblast apoptosis (30). In mouse models, several miRs
identified as key immunomodulators in other tissue are
differentially expressed in early pregnancy, such as miR-146a,
miR-155 and miR-223, suggesting that they are involved in the
maternal adaptation to pregnancy (25,31).
Furthermore, miRs that exert angiogenic and antiapoptotic
properties are primarily expressed in the placenta during early
pregnancy, while miRs that promote cell differentiation are
strongly expressed in the placenta during late pregnancy (32). Therefore, miRs promote successful
pregnancy and regulate differentiation and development of the
placenta and foetus during pregnancy. An imbalance of miRs leads to
pregnancy-related disorders such as gestational hypertension and
diabetes, IUGR and prematurity (33).
The expression of miRs in the placenta is influenced
by changes in pregnancy, which includes genetic, environmental and
immunological factors; thus, miR expression profiles change
dynamically (34). For example,
let-7d is downregulated in the circulation of pregnant patients
with PE, but there is no difference compared with patients with
normal pregnancy, while let-7d expression is upregulated in the
placenta, indicating that apoptosis is critical in the control of
trophoblasts (33,35). Furthermore, expression miR-584 and
miR-17 (both play important roles in malignant tumour progression)
is inconsistent in different studies (36,37).
The levels of six miRs (miR-1, miR-328, miR-363, miR-377, miR-500
and miR-584), which are downregulated in the placenta of patients
with PE, are not statistically different in sera from patients with
PE and healthy pregnancy (38). PE
may have multiple predisposing factors and placental pathology,,
which may be the reason for the inconsistent regulation of some
miRs in the pathogenesis of PE (36). For example, miR expression in the
placenta is influenced by maternal nutritional status, obesity and
pregnancy body mass index (39).
4. miRs regulate the activity of
trophoblasts
Placental development includes differentiation,
invasion and migration of trophoblasts as well as the remodelling
of uterine spiral arteries. During the early stages of placental
development, dynamic replacement of trophoblasts is strictly
regulated by several molecular pathways; however, an imbalance of
these molecular pathways leads to severe placental lesions and
pregnancy complications (40). The
upregulation of miR-370-3p can inhibit trophoblast proliferation,
migration and invasion while promoting apoptosis (41). miR-326 targets paired box 8 via the
Hippo pathway to inhibit trophoblast proliferation migration and
invasion (42). In addition,
expression of let-7b is significantly decreased in the placenta of
patients with PE. Let-7b adversely affects the function of
trophoblasts via the ERK1/2 signalling pathway and it inhibits cell
proliferation and invasion, promotes cell apoptosis and autophagy,
as well as increasing TNF-α expression in trophoblasts of patients
with PE (43). On the other hand,
miR-134 inhibits infiltration of trophoblasts by targeting
ITGB1(44). Migration and invasion
of trophoblasts are similar to metastatic behaviour of tumour
cells. Certain miRs that are abnormally expressed in malignant
tumours are also differentially expressed in PE. For example,
miR-21, which affects tumour cell proliferation and viability,
inhibits invasion and promotes apoptosis of trophoblasts (45). miRs can also combine with other
ncRNAs to play a role in regulating downstream target molecules.
The lncRNA SNHG5 affects expression of miRs by adsorbing miRs and
upregulating the transcription of the secreted protein acidic and
cysteine-rich gene, thereby inhibiting autophagy in trophoblasts of
patients with PE (46). miRs that
have been reported to regulate trophoblasts are listed in Table I (47-58).
 | Table IDifferentially expressed miRs
associated with pre-eclampsia trophoblast. |
Table I
Differentially expressed miRs
associated with pre-eclampsia trophoblast.
| First author/s,
year | miR | Source | Expression | Target | Effect | (Refs.) |
|---|
| Yuan et al,
2020 | miR-16 | Placenta | Up | Notch2 | Inhibits
proliferation, migration, invasion and promotes apoptosis of
trophoblast | (47) |
| Wang et al,
2019; Zhang et al, 2012 | miR-210 | Placenta | Up | Notch1, Ephrin-A3,
homeobox -A9 | Inhibits
proliferation, migration, invasion and promotes apoptosis of
trophoblast | (48,49) |
| Xiaobo et
al, 2019 | miR-149-5p | Placenta | Up | Endoglin | Inhibits
proliferation, migration, invasion and promotes apoptosis of
trophoblast | (50) |
| Liu et al,
2021 | miR-126 | Placenta | Up | VCAM-1 | Inhibits invasion
of trophoblast | (51) |
| Ali et al,
2021 | miR-16 | Maternal serum | Up | TP53 | Promotes apoptosis
of trophoblast | (52) |
| Shi et al,
2019 | miR-454 | Placenta | Down | Activin
receptor-like kinase 7 | Proliferation,
invasion of trophoblast | (53) |
| Liu et al,
2021 | miR-126 | Placenta | Up | VCAM-1 | Inhibits invasion
of trophoblast | (51) |
| Lai and Yu,
2020 | miR-183 | Placenta | Up | FOXP1, G protein
subunit γ7 | Inhibits
proliferation, invasion, and angiogenesis of trophoblast | (54) |
| Wang et al,
2020 | miR-132 | Placenta | Up | Death associated
protein kinase 1 | Proliferation,
migration, invasion and apoptosis | (55) |
| Wang et al,
2019 | miR-141,
miR-200a | Placenta, maternal
serum | Up | Endocrine
gland-derived-VEGF | Inhibits
proliferation, migration, invasion and promotes apoptosis of
trophoblast | (56) |
| Yang and Meng,
2020 | miR-215-5p | Placenta | Up | CDC6 | Inhibits migration
and invasion of trophoblast | (57) |
| Ni et al,
2021 | miR-95-5p | Placenta | Up | Low-density
lipoprotein receptor-related protein 6 | Inhibits migration
and invasion of trophoblast | (58) |
The differentiation and proliferation of
trophoblasts in the placenta are inseparable from the blood supply.
The development of blood vessels in the placenta requires vascular
factors such as VEGF, placenta growth factor, angiopoietin and
soluble endothelin. The current theory is that an imbalance between
angiogenic factors and antiangiogenic factors may be the cause of
PE (59). Studies have reported
that these angiogenic genes are targeted by multiple miRs, which
are associated with pathogenesis of PE (33,36);
among them, 127 miRs have been demonstrated to be associated with
PE and VGEF-A can be targeted by ten different miRs, while VEGF-B
is targeted by nine different miRs (36), suggesting that miRs may promote the
development of PE by influencing synthesis and secretion of
vascular factors.
5. miRs regulate the stress response of
trophoblasts
The failure of uterine spiral artery remodelling
leads to placental ischemia and hypoxia, which stimulates maternal
immune cells. Under these conditions, active T lymphocytes increase
production of inflammatory cytokines such as TNF-α and
interleukin-6(60). TNF-α
decreases transcription of nitric oxide synthase and increases
production of endothelin-1, a potent vasoconstrictor (60). In addition, placental ischemia
triggers oxidative stress characterized by production of excessive
reactive oxygen species in the endoplasmic reticulum and cell
chambers, leading to protein and DNA damage (61). In response to chronic inflammatory
stimulation, miR-195 expression is downregulated, which inhibits
mitochondrial energy production by targeting flavin adenine
dinucleotide-dependent oxidoreductase domain-containing protein 1
and pyruvate dehydrogenase phosphatase regulatory subunit coding
genes, leading to apoptosis of trophoblasts under oxidative stress
(62). Furthermore, plasma miR-210
levels in patients with mild or severe PE are ~4- and 10-fold
higher than those in healthy individuals, respectively (63). In patients with PE and trophoblasts
cultured under hypoxic conditions, upregulated miR-210 expression
in the placenta and plasma is associated with cell migration and
vascular remodelling (64). In
addition, the upregulated miR-210 expression in patients with PE
may also mediate mitochondrial damage via iron-sulphur cluster
scaffold homolog, thereby promoting pathogenesis of PE (65). Collectively, these mechanisms
induce chronic inflammation in the placenta. Inflammation promotes
apoptosis and disrupts migration of trophoblasts, affects formation
of placental blood vessels and aggravates the immune response. The
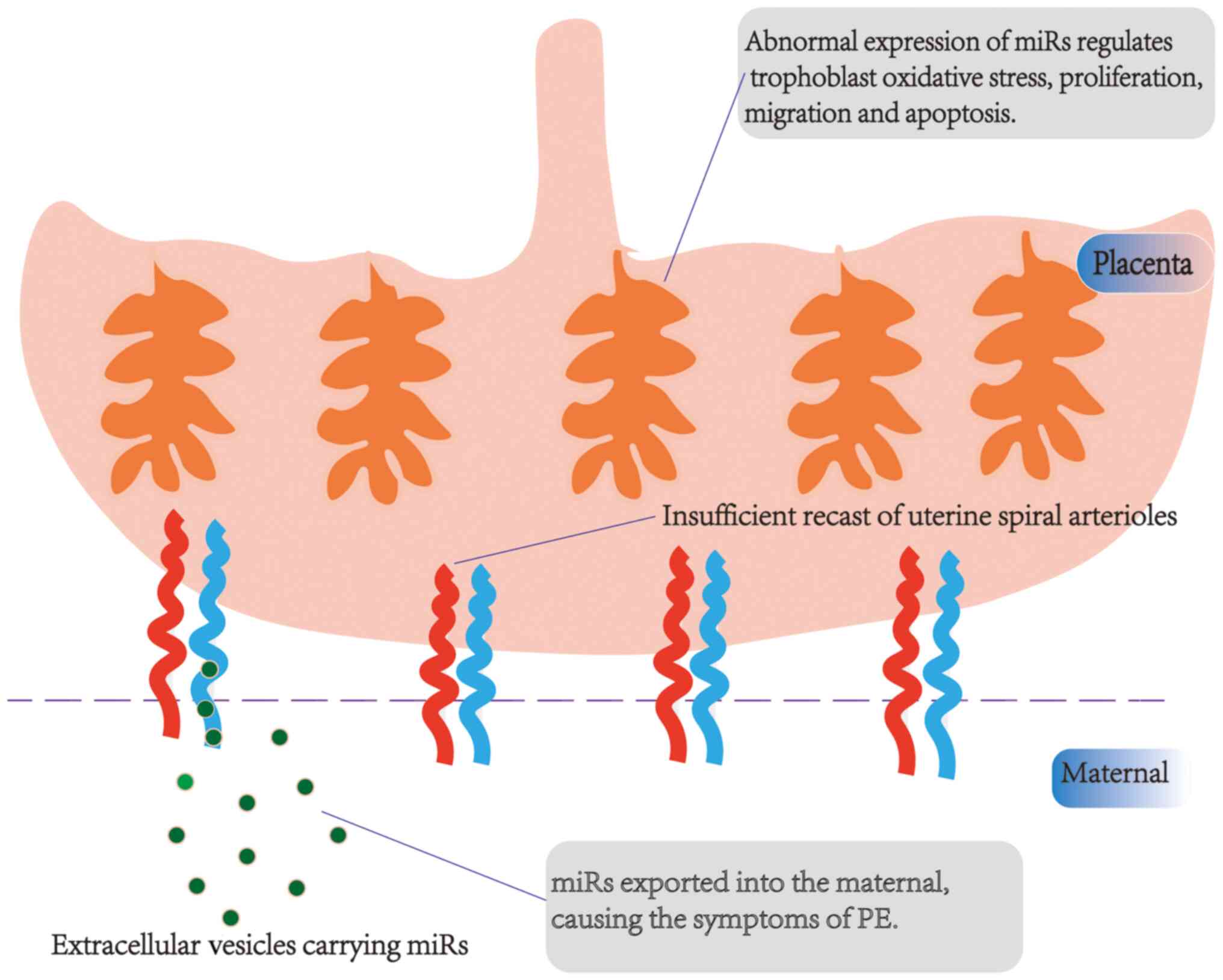
role of miRs in the pathogenesis of PE is shown in Fig. 2.
6. Applications of miRs in PE
PE cause great harm to the foetus (IUGR,
intrauterine death, prematurity and placental abruption) (36). In addition, symptoms of PE are
usually detected after 20 weeks of gestation; thus, it is difficult
to diagnose PE before this time (1). Therefore, PE prediction in the first
trimester may help to treat the complications associated with PE.
In this regard, it is essential to explore molecular mechanisms of
PE and to identify the early biomarkers of PE. The dysregulation of
miRs in the placenta not only affects the function of the placenta,
these miRs may also affect maternal physiology and foetal growth
and development (2).
A recent study reported that miR-363 is associated
with early-onset PE and can be used as a potential biomarker to
diagnose early- and late-onset PE (66). Gan et al (67) demonstrated that the areas under the
curves (AUCs) of miR-210 and miR-155, which are upregulated in the
serum of patients with PE, are 0.750 and 0.703, respectively.
Furthermore, miR-152 (AUC=0.94), miR-183 (AUC=0.97) and miR-210
(AUC=0.93) in maternal serum at 20-24 weeks of gestation predict PE
(33). In maternal serum of
patients with early stage of pregnancy, Hromadnikova et al
(29) reported that miR-517-5p, a
placenta-specific C19MC miR, has the best predictive performance
for PE, with a sensitivity of 42.9% and a specificity of 86.2%, but
that circulating C19MC miR has no predictive value for IUGR. A
clinical trial demonstrated that serum miRs in early pregnancy do
not have a predictive value for early PE (68). By using miR microarray and
quantitative polymerase chain reaction analysis, Luque et al
(68) reported no differences in
754 miRs in the serum of 31 early-onset patients with PE with early
pregnancy compared with the serum of 44 patients with normal early
pregnancies. A previous study found that miRs in maternal serum in
early pregnancy predict late-onset PE and miscarriage (AUC=0.90),
but the authors used a miR panel of 30 miRs, which increases the
cost of screening (69). It has
been established that miRs form a huge network of ncRNAs. For
example, miR-210 is differentially expressed in a variety of
cancers and cardiovascular and inflammatory diseases, which renders
miRs non-specific biomarkers of PE (2,70,71).
Furthermore, PE is a disease involving multiple factors and
mechanisms, which makes it difficult to identify highly specific
miRs in PE (33). At the same
time, due to the complex regulatory characteristics of miRs,
similar situations may exist for other diseases (33). Therefore, further studies are
required in the future.
miRs are present in extracellular vesicles (EVs),
indicating that they can be exported into the maternal blood and
in vitro experiments have reported that EVs in pregnant
patients with severe PE impair endothelial cell function (72,73).
Development of EV inhibitors based on characteristics of EVs and
the pathway targets of miRs may serve an important role in
prevention and treatment of PE. Thus, miRs have potential for
disease diagnosis and treatment and it may be possible to prevent
and treat PE at the miR level. At the same time, artificial
intelligence and machine learning techniques, which can find
important connections between data items in diverse data sets, can
be used to identify miRs as improved biomarkers of diseases with
potential in the diagnosis and treatment of other disease (74).
7. Summary
An increasing number of miRs have been reported to
be involved in the pathogenesis of PE (2). miRs play important roles in the
regulation of genes in the pathogenesis of PE, thereby
participating in oxidative stress of trophoblasts while inhibiting
proliferation and invasion and promoting apoptosis (75). The identification of biomarkers of
PE has been actively researched in perinatal medicine (76). However, to the best of our
knowledge, current research on miRs lacks detail and the roles of
miRs in the pathogenesis of PE remain unclear. Highly stable miRs
are potential biomarkers and therapeutic targets for PE. However,
the pathogenesis of PE, as well as the roles of miRs, are complex
and diverse. PE is regulated by different mechanisms and patients
exhibit different miR expression profiles (68,76).
Therefore, the challenge in molecular medicine is to find a
biomarker, or panel of biomarkers, for early diagnosis of PE, as
well as individualized prediction and treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Scientific
Research Fund of Zhejiang Provincial Education Department (grant
no. Y202145947).
Availability of data and materials
Not applicable.
Authors' contributions
YimC designed the study. WN and BW wrote the
manuscript. YijC and JL wrote, reviewed and edited the manuscript.
Data authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
YimC, ORCID no. 0000-0003-1532-6049.
References
|
1
|
Lowe SA, Bowyer L, Lust K, McMahon LP,
Morton M, North RA, Paech M and Said JM: SOMANZ guidelines for the
management of hypertensive disorders of pregnancy 2014. Aust N Z J
Obstet Gynaecol. 55:e1–e29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pankiewicz K, Fijałkowska A, Issat T and
Maciejewski TM: Insight into the key points of preeclampsia
pathophysiology: Uterine artery remodeling and the role of
MicroRNAs. Int J Mol Sci. 22(3132)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wojczakowski W, Kimber-Trojnar Ż, Dziwisz
F, Słodzińska M, Słodziński H and Leszczyńska-Gorzelak B:
Preeclampsia and cardiovascular risk for offspring. J Clin Med.
10(3154)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Parada-Niño L, Castillo-León LF and Morel
A: Preeclampsia, natural history, genes and miRs associated with
the syndrome. J Pregnancy. 2022(3851225)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huppertz B: Placental origins of
preeclampsia: Challenging the current hypothesis. Hypertension.
51:970–975. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ives CW, Sinkey R, Rajapreyar I, Tita ATN
and Oparil S: Preeclampsia-Pathophysiology and clinical
presentations: JACC State-of-the-Art Review. J Am Coll Cardiol.
76:1690–1702. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Henderson JT, O'Connor E and Whitlock EP:
Low-dose aspirin for prevention of morbidity and mortality from
preeclampsia. Ann Intern Med. 161:613–614. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang Y, Le Ray I, Zhu J, Zhang J, Hua J
and Reilly M: Preeclampsia prevalence, risk factors and pregnancy
outcomes in Sweden and China. JAMA Netw Open.
4(e218401)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun N, Qin S, Zhang L and Liu S: Roles of
noncoding RNAs in preeclampsia. Reprod Biol Endocrinol.
19(100)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ashraf UM, Hall DL, Rawls AZ and Alexander
BT: Epigenetic processes during preeclampsia and effects on fetal
development and chronic health. Clin Sci (Lond). 135:2307–2327.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kulcheski FR, Christoff AP and Margis R:
Circular RNAs are miRNA sponges and can be used as a new class of
biomarker. J Biotechnol. 238:42–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Munjas J, Sopić M, Stefanović A, Košir R,
Ninić A, Joksić I, Antonić T, Spasojević-Kalimanovska V and Prosenc
Zmrzljak U: Non-Coding RNAs in preeclampsia-molecular mechanisms
and diagnostic potential. Int J Mol Sci. 22(10652)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brodowski L, Schröder-Heurich B, von
Hardenberg S, Richter K, von Kaisenberg CS, Dittrich-Breiholz O,
Meyer N, Dörk T and von Versen-Höynck F: MicroRNA Profiles of
Maternal and Neonatal Endothelial Progenitor Cells in Preeclampsia.
Int J Mol Sci. 22(5320)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bao S, Zhou T, Yan C, Bao J, Yang F, Chao
S, Zhou M and Xu Z: A blood-based miRNA signature for early
non-invasive diagnosis of preeclampsia. BMC Med.
20(303)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Laganà AS and Naem A: The Pathogenesis of
Endometriosis: Are Endometrial Stem/Progenitor Cells Involved? Stem
Cells in Reproductive Tissues and Organs. Virant-Klun I (ed). Stem
Cell Biology and Regenerative Medicine, Humana. 70:193–216.
2022.
|
|
18
|
Lv Y, Lu C, Ji X, Miao Z, Long W, Ding H
and Lv M: Roles of microRNAs in preeclampsia. J Cell Physiol.
234:1052–1061. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gantier MP, McCoy CE, Rusinova I, Saulep
D, Wang D, Xu D, Irving AT, Behlke MA, Hertzog PJ, Mackay F and
Williams BR: Analysis of microRNA turnover in mammalian cells
following Dicer1 ablation. Nucleic Acids Res. 39:5692–5703.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Borchert GM, Lanier W and Davidson BL: RNA
polymerase III transcribes human microRNAs. Nat Struct Mol Biol.
13:1097–1101. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Bohnsack MT, Czaplinski K and Gorlich D:
Exportin 5 is a RanGTP-dependent dsRNA-binding protein that
mediates nuclear export of pre-miRs. RNA. 10:185–191.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bernstein E, Caudy AA, Hannon GJ and
Hammond SM: Role for a bidentate ribonuclease in the initiation
step of RNA interference. Nature. 409:363–366. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Golden RJ, Chen B, Li T, Braun J,
Manjunath H, Chen X, Wu J, Schmid V, Chang TC, Kopp F, et al: An
Argonaute phosphorylation cycle promotes microRNA-mediated
silencing. Nature. 542:197–202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Robertson SA, Zhang B, Chan H, Sharkey DJ,
Barry SC, Fullston T and Schjenken JE: MicroRNA regulation of
immune events at conception. Mol Reprod Dev. 84:914–925.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lykke-Andersen K, Gilchrist MJ, Grabarek
JB, Das P, Miska E and Zernicka-Goetz M: Maternal Argonaute 2 is
essential for early mouse development at the maternal-zygotic
transition. Mol Biol Cell. 19:4383–4392. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Morales-Prieto DM, Chaiwangyen W,
Ospina-Prieto S, Schneider U, Herrmann J, Gruhn B and Markert UR:
MicroRNA expression profiles of trophoblastic cells. Placenta.
33:725–734. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hromadnikova I, Kotlabova K, Ondrackova M,
Pirkova P, Kestlerova A, Novotna V, Hympanova L and Krofta L:
Expression profile of C19MC microRNAs in placental tissue in
pregnancy-related complications. DNA Cell Biol. 34:437–457.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hromadnikova I, Kotlabova K, Ivankova K
and Krofta L: First trimester screening of circulating C19MC
microRNAs and the evaluation of their potential to predict the
onset of preeclampsia and IUGR. PLoS One.
12(e0171756)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo L, Ye G, Nadeem L, Fu G, Yang BB,
Honarparvar E, Dunk C, Lye S and Peng C: MicroRNA-378a-5p promotes
trophoblast cell survival, migration and invasion by targeting
Nodal. J Cell Sci. 125(Pt 13):3124–3132. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hassan SS, Romero R, Pineles B, Tarca AL,
Montenegro D, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S,
Espinoza J, et al: MicroRNA expression profiling of the human
uterine cervix after term labor and delivery. Am J Obstet Gynecol.
202:80.e1–e8. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gu Y, Sun J, Groome LJ and Wang Y:
Differential miRNA expression profiles between the first and third
trimester human placentas. Am J Physiol Endocrinol Metab.
304:E836–E843. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Skalis G, Katsi V, Miliou A, Georgiopoulos
G, Papazachou O, Vamvakou G, Nihoyannopoulos P, Tousoulis D and
Makris T: MicroRNAs in Preeclampsia. Microrna. 8:28–35.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Qiu C, Chen G and Cui Q: Towards the
understanding of microRNA and environmental factor interactions and
their relationships to human diseases. Sci Rep.
2(318)2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hromadnikova I, Kotlabova K, Doucha J,
Dlouha K and Krofta L: Absolute and relative quantification of
placenta-specific micrornas in maternal circulation with placental
insufficiency-related complications. J Mol Diagn. 14:160–167.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ali A, Hadlich F, Abbas MW, Iqbal MA,
Tesfaye D, Bouma GJ, Winger QA and Ponsuksili S: MicroRNA-mRNA
networks in pregnancy complications: A comprehensive downstream
analysis of potential biomarkers. Int J Mol Sci.
22(2313)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang W, Feng L, Zhang H, Hachy S, Satohisa
S, Laurent LC, Parast M, Zheng J and Chen DB: Preeclampsia
up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a,
and -20b) that target ephrin-B2 and EPHB4 in human placenta. J Clin
Endocrinol Metab. 97:E1051–E1059. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Q, Long A, Jiang L, Cai L, Xie LI, Gu
J, Chen X and Tan L: Quantification of preeclampsia-related
microRNAs in maternal serum. Biomed Rep. 3:792–796. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jing J, Wang Y, Quan Y, Wang Z, Liu Y and
Ding Z: Maternal obesity alters C19MC microRNAs expression profile
in fetal umbilical cord blood. Nutr Metab (Lond).
17(52)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ali A, Bouma GJ, Anthony RV and Winger QA:
The Role of LIN28-let-7-ARID3B pathway in placental development.
Int J Mol Sci. 21(3637)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu J, Sun Y, Cao Y and Zhang Y: Small RNA
sequencing reveals placenta-derived exosomal microRNAs associated
with preeclampsia. J Hypertens. 40:1030–1041. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zang J, Yan M, Zhang Y, Peng W, Zuo J,
Zhou H, Gao G, Li M, Chu Y and Ye Y: MiR-326 inhibits trophoblast
growth, migration and invasion by targeting PAX8 via Hippo pathway.
Reprod Biol Endocrinol. 20(38)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gao Y, Zhang X and Meng T: Overexpression
of let-7b exerts beneficial effects on the functions of human
placental trophoblasts by activating the ERK1/2 signaling pathway.
Mol Reprod Dev. 89:39–53. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zou AX, Chen B, Li QX and Liang YC:
MiR-134 inhibits infiltration of trophoblast cells in placenta of
patients with preeclampsia by decreasing ITGB1 expression. Eur Rev
Med Pharmacol Sci. 22:2199–2206. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ojeda-Casares H and Paradisi I: The
regulatory network played by miRANs during normal pregnancy and
preeclampsia: A comparative study. Microrna. 10:263–275.
2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yang L, Liu C, Zhang C, Shang R, Zhang Y,
Wu S and Long Y: LncRNA small nucleolar RNA host gene 5 inhibits
trophoblast autophagy in preeclampsia by targeting microRNA-31-5p
and promoting the transcription of secreted protein acidic and rich
in cysteine. Bioengineered. 13:7221–7237. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yuan Y, Wang X, Sun Q, Dai X and Cai Y:
MicroRNA-16 is involved in the pathogenesis of pre-eclampsia via
regulation of Notch2. J Cell Physiol. 235:4530–4544.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang R, Liu W, Liu X, Liu X, Tao H, Wu D,
Zhao Y and Zou L: MicroRNA-210 regulates human trophoblast cell
line HTR-8/SVneo function by attenuating Notch1 expression:
Implications for the role of microRNA-210 in pre-eclampsia. Mol
Reprod Dev. 86:896–907. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li
L, Xin H and Sun S: Elevated levels of hypoxia-inducible
microRNA-210 in pre-eclampsia: New insights into molecular
mechanisms for the disease. J Cell Mol Med. 16:249–259.
2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Xiaobo Z, Qizhi H, Zhiping W and Tao D:
Down-regulated miR-149-5p contributes to preeclampsia via
modulating endoglin expression. Pregnancy Hypertens. 15:201–208.
2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu B, Liu L, Cui S, Qi Y and Wang T:
Expression and significance of microRNA-126 and VCAM-1 in placental
tissues of women with early-onset preeclampsia. J Obstet Gynaecol
Res. 47:2042–2050. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ali Z, Zafar U, Zaki S, Ahmad S, Khaliq S
and Lone KP: Expression levels of MiRNA-16, SURVIVIN and TP53 in
Preeclamptic and Normotensive women. J Pak Med Assoc. 71:2208–2213.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shi Z, She K, Li H, Yuan X, Han X and Wang
Y: MicroRNA-454 contributes to sustaining the proliferation and
invasion of trophoblast cells through inhibiting Nodal/ALK7
signaling in pre-eclampsia. Chem Biol Interact. 298:8–14.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lai W and Yu L: Elevated MicroRNA 183
impairs trophoblast migration and invasiveness by downregulating
FOXP1 expression and elevating GNG7 Expression during Preeclampsia.
Mol Cell Biol. 41(e00236)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang YP, Zhao P, Liu JY, Liu SM and Wang
YX: MicroRNA-132 stimulates the growth and invasiveness of
trophoblasts by targeting DAPK-1. Eur Rev Med Pharmacol Sci.
24:9837–9843. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang CY, Tsai PY, Chen TY, Tsai HL, Kuo PL
and Su MT: Elevated miR-200a and miR-141 inhibit endocrine
gland-derived vascular endothelial growth factor expression and
ciliogenesis in preeclampsia. J Physiol. 597:3069–3083.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yang X and Meng T: miR-215-5p decreases
migration and invasion of trophoblast cells through regulating CDC6
in preeclampsia. Cell Biochem Funct. 38:472–479. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ni H, Wang X, Qu H, Gao X and Yu X:
MiR-95-5p involves in the migration and invasion of trophoblast
cells by targeting low density lipoprotein receptor-related protein
6. J Obstet Gynaecol Res. 47:184–197. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Umapathy A, Chamley LW and James JL:
Reconciling the distinct roles of angiogenic/anti-angiogenic
factors in the placenta and maternal circulation of normal and
pathological pregnancies. Angiogenesis. 23:105–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cornelius DC: Preeclampsia: From
inflammation to immunoregulation. Clin Med Insights Blood Disord.
11(1179545X17752325)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Schoots MH, Gordijn SJ, Scherjon SA, van
Goor H and Hillebrands JL: Oxidative stress in placental pathology.
Placenta. 69:153–161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang H, Zhang L, Guo X, Bai Y, Li YX, Sha
J, Peng C, Wang YL and Liu M: MiR-195 modulates oxidative
stress-induced apoptosis and mitochondrial energy production in
human trophoblasts via flavin adenine dinucleotide-dependent
oxidoreductase domain-containing protein 1 and pyruvate
dehydrogenase phosphatase regulatory subunit. J Hypertens.
36:306–318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wu P, van Den Berg C, Alfirevic Z, O'Brien
S, Röthlisberger M, Baker PN, Kenny LC, Kublickiene K and Duvekot
JJ: Early pregnancy biomarkers in pre-eclampsia: A systematic
review and meta-analysis. Int J Mol Sci. 16:23035–23056.
2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhao G, Miao H, Li X, Chen S, Hu Y, Wang Z
and Hou Y: TGF-β3-induced miR-494 inhibits macrophage polarization
via suppressing PGE2 secretion in mesenchymal stem cells. FEBS
Lett. 590:1602–1613. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Muralimanoharan S, Maloyan A, Mele J, Guo
C, Myatt LG and Myatt L: MIR-210 modulates mitochondrial
respiration in placenta with preeclampsia. Placenta. 33:816–823.
2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Abdelazim SA, Shaker OG, Aly YAH and
Senousy MA: Uncovering serum placental-related non-coding RNAs as
possible biomarkers of preeclampsia risk, onset and severity
revealed MALAT-1, miR-363 and miR-17. Sci Rep.
12(1249)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gan L, Liu Z, Wei M, Chen Y, Yang X, Chen
L and Xiao X: MiR-210 and miR-155 as potential diagnostic markers
for pre-eclampsia pregnancies. Medicine (Baltimore).
96(e7515)2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Luque A, Farwati A, Crovetto F, Crispi F,
Figueras F, Gratacos E and Aran JM: Usefulness of circulating
microRNAs for the prediction of early preeclampsia at
first-trimester of pregnancy. Sci Rep. 4(4882)2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Winger EE, Reed JL and Ji X: First
trimester PBMC microRNA predicts adverse pregnancy outcome. Am J
Reprod Immunol. 72:515–526. 2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yu P, Fan S, Huang L, Yang L and Du Y:
MIR210 as a potential molecular target to block invasion and
metastasis of gastric cancer. Med Hypotheses. 84:209–212.
2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Jaszczuk I, Koczkodaj D, Kondracka A,
Kwaśniewska A, Winkler I and Filip A: The role of miRNA-210 in
pre-eclampsia development. Ann Med. 54:1350–1356. 2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang Z, Zhao G, Zeng M, Feng W and Liu J:
Overview of extracellular vesicles in the pathogenesis of
preeclampsia†. Biol Reprod. 105:32–39. 2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Cui J, Chen X, Lin S, Li L, Fan J, Hou H
and Li P: MiR-101-containing extracellular vesicles bind to BRD4
and enhance proliferation and migration of trophoblasts in
preeclampsia. Stem Cell Res Ther. 11(231)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Bendifallah S, Dabi Y, Suisse S, Jornea L,
Bouteiller D, Touboul C, Puchar A and Daraï E: MicroRNome analysis
generates a blood-based signature for endometriosis. Sci Rep.
12(4051)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Hemmatzadeh M, Shomali N, Yousefzadeh Y,
Mohammadi H, Ghasemzadeh A and Yousefi M: MicroRNAs: Small
molecules with a large impact on pre-eclampsia. J Cell Physiol.
235:3235–3248. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Chaemsaithong P, Sahota DS and Poon LC:
First trimester preeclampsia screening and prediction. Am J Obstet
Gynecol. 226 (2S):S1071–S1097.e2. 2022.PubMed/NCBI View Article : Google Scholar
|