Introduction
Autoimmune thyroid diseases (AITDs) involve multiple
factors such as heritage, environment and infection and include
Graves' disease (GD) and Hashimoto's thyroiditis (HT) as the most
common clinical diseases (1).
Autoimmune thyroid disease patients are prone to fat metabolism
disorder, and the serum thyroid hormone level has a close
correlation with blood lipid metabolism, insulin metabolism and
inflammatory factors (2).
Autoimmune thyroid illnesses are a category of disorders
characterized by aberrant lymphocyte activity directed against
self-tissues, which affect 2-3% of the population, with a female
predominance (3). NF-κB is a key
nuclear transcription factor expressed in nearly all cell and
tissue types. The transcription factor NF-κB, regulates multiple
aspects of innate and adaptive immune functions and serves as a
pivotal mediator of the inflammatory response (4). NF-κB is a central mediator of
inflammation in response to DNA damage and oxidative stress.
Because of its central role in numerous cellular processes, NF-κB
dysregulation is implicated in the pathology of numerous important
human diseases (5). NF-κB
activation causes inappropriate inflammatory responses in diseases
including rheumatoid arthritis, multiple sclerosis, coronavirus
disease-2019 and severe acute respiratory syndrome-coronavirus-2
cases of pneumonia (6).
The present study investigated the differences in
the expression levels of IL-17, NF-κB, IFN-γ, TNF-α, IL-2, IL-4,
IL-6 and IL-10 in GD and HT, and the differences in the changes of
NF-κB signaling pathway and the pathogenesis induced by different
inflammatory stimuli. To investigate this and further clarify the
pathogenesis of AITDs, the present study analyzed the relationships
between IL-17, NF-κB, IL-6, IL-10 proteins and their mRNAs,
thyroid-stimulating hormone (TSH), free triiodothyronine (FT3),
free thyroxine (FT4), thyroid peroxidase antibody (ATPO), thyroid
gland globulin (TG), thyroglobulin antibody (TGAb) and
thyroid-stimulating receptor antibody (TRAb).
Materials and methods
Patients
The present study selected 82 patients with AITDs
(41 with GD and 41 with HT) and 53 age-matched healthy controls who
were outpatients and inpatients of Hebei General Hospital
(Shijiazhuang, China) from June 2020 to November 2020. The GD group
was 19-63 years old (mean, 38.49±8.81 years). The HT group was
17-66 years old (mean, 40.71±13.08 years). The controls were 15-67
years old (mean, 43.06±12.57 years). Sex and age did not
significantly differ among the three groups. Basic parameters for
all patients are presented in Table
I.
 | Table IPatient demographics. |
Table I
Patient demographics.
| | Autoimmune thyroid
diseases | |
|---|
| Characteristics | Graves' disease
patients | Hashimoto's
thyroiditis patients | Control |
|---|
| Numbers | 41 | 41 | 53 |
| Age, years | 38.49±8.81 | 40.71±13.08 | 43.06±12.57 |
| Sex, W:M | 34:7 | 36:5 | 41:12 |
Diagnostic criteria for HT. HT was diagnosed
per the following: i) Thyroid peroxidase antibody (TPOAb)-positive
or thyroglobulin antibody (TGAb)-positive in serum autothyroid
antibodies; ii) thyroid gland exhibited toughness or diffuse
enlargement or non-obvious enlargement; iii) transient
hyperthyroidism or permanent hypothyroidism; iv) ultrasound showed
goiter or no obvious enlargement, diffuse changes in the thyroid
gland, decreased echo, or nodules; v) cytological examination by
fine needle puncture of the thyroid gland indicated lymphocyte
infiltration and eosinophilic changes of follicular cells; and vi)
normal or low thyroid uptake (7).
Diagnostic criteria for GD. GD was diagnosed
per the following: i) Hypermetabolic symptoms and signs caused by
thyrotoxicosis, which were confirmed by clinical manifestations;
ii) diffuse thyroid enlargement confirmed by physical examination
and imaging; iii) serum thyroid-stimulating hormone (TSH) level
decreased and thyroid hormone level increased; iv) no obvious
exophthalmia or other invasive eye signs; v) anterior tibial
myxedema; vi) both TRAb-positive (>1.75 IU/l); vii) increased
thyroid iodine uptake or enhanced thyroid uptake on thyroid nuclide
imaging; and viii) pathological examination showed hyperplasia of
thyroid cells, cells in cubic or columnar shape and visible
papilla. Subclinical hyperthyroidism with reduced serum TSH levels
and normal thyroid hormone levels may be caused by GD, but patients
with this condition may be asymptomatic (7).
Exclusion criteria. Exclusion criteria were
other acute and chronic inflammatory diseases, liver and kidney
dysfunction, severe respiratory diseases, tumor radiotherapy and
chemotherapy, severe cardiovascular diseases, pregnancy or
lactation, severe mental diseases and infectious diseases. The
Ethics Committee of Hebei General Hospital approved informed
consent and voluntary participation [approval no. 2020(204)].
Electrochemiluminescence analyzer. TSH, FT3,
FT4, ATPO, TG, TGAb, TRAb were detected using an
electrochemiluminescence analyzer (Cobas e602; Roche
Diagnostics).
Flow cytometry. There were seven types of
capture microspheres with different fluorescence intensity in the
capture microsphere mixture in the Human Th1/Th2/Th17 subpopulation
detection kit (cat. no. P010002; Jiangxi Saiji Biological Co.,
Ltd.). The surface of the capture beads provided by this
aforementioned kit were coated with IL-2, IL-4, IL-6, IL-10, IL-17,
TNF-α and IFN-γ specific antibodies, and diluted with twice the
capture bead buffer and incubate at 37˚C away from light for 30
min. The capture beads were specifically bound to IL-2, IL-4, IL-6,
IL-10, IL-17, TNF-α, IFN-γ in the samples to be tested, and then
bound to the provided fluorescent detection antibody labeled by PE
to form a double-antibody sandwich complex (capture bead + sample
to be tested + antibody to be detected). After incubation for 2.5 h
at room temperature and away from light, the fluorescence intensity
of the double-antibody sandwich complex was analyzed. The contents
of IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α and IFN-γ in the samples
to be tested were obtained. Multiprotein quantitative assay (CBA)
was performed (FACS Canto, BD Biosciences), according to the kit
Instruction Manual Software (8).
FCAP Array v3 was used to analyze the results (Cellgene Biotech Co.
Ltd).
ELISA. Serum NF-κB was detected using ELISA
(cat. no. NBP2-29661; Novus Biologicals, LLC).
Cell collection. In the morning, 5 ml of
venous blood was drawn from all participants on an empty stomach
and treated as below.
Preparation of monocytes
Peripheral venous blood (1-2 ml) was drawn from all
subjects on an empty stomach in the morning, the samples were
treated with EDTA anticoagulant and added with 1.5X
phosphate-buffered saline (PBS), and the treatment was completed
within 4 h. Lymphocyte isolation solution was added within 1 h
(Tiangen Biotech Co., Ltd.). The diluted blood cells were evenly
vortexed, then the vortexed blood was added to the 1.5X lymphocyte
separation solution (Tiangen Biotech Co., Ltd.) to ensure that the
blood was in the upper layer and centrifuged at 1,220 x g for 20
min at 4˚C. The supernatant was removed and added to another glass
test tube with 1.5x PBS. The mixture was centrifuged at 1,220 x g
for 20 min at 4˚C, then the precipitate was removed from the
discarded solution. Next, the 1.5x PBS was added, mixed and
centrifuged at 1,220 x g for 20 min at 4˚C. The precipitate was
removed from the discarded solution, and 1 ml of PBS was added,
homogenized and transferred to a 1.5-ml centrifuge tube and
centrifuged at 12 x g for 5 min at 4˚C. The precipitate was removed
from the discarded solution to form mononuclear cells. The SYBR
Green I (Tiangen Biotech Co., Ltd.) chimeric fluorescence method
was used for quantitative detection (7500 Real-Time PCR System;
Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR). IL-6, IL-10, IL-17 and NF-κB mRNA expression levels
were detected using RT-qPCR. Total RNA was extracted from the
mononuclear cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), then amplified using a genomic DNA
removal reaction. RT was performed using FastKing RT Kit (with
gDNase) (Tiangen Biotech Co., Ltd.) according to the manufacturer's
instructions. qPCR was performed as per the instructions of the
SuperReal PreMix Plus (SYBR Green) kit (Tiangen Biotech Co., Ltd.).
The amplification procedure was pre-denaturation at 95˚C for 10 min
for one cycle and amplification at 95˚C for 15 sec, 60˚C for 15 sec
and 72˚C for 30 sec for 40 cycles. The ΔCq was calculated by
subtracting the Cq value of the reference β-actin gene from the Cq
value of the target gene, and the relative mRNA expression of the
target gene was calculated via 2-ΔΔCq (9). The reference method comes from the
specification of Tiangen Biotech Co., Ltd. Primer sequences are
presented in Table II.
 | Table IIPrimer sequences used for reverse
transcription-quantitative PCR. |
Table II
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene name | Upstream primer
(5'-3') | Downstream primer
(5'-3') |
|---|
| β-actin |
ACGAGGCCCAGAGCAAGAGA |
GGTCTTTGCGGATGTCCACG |
| IL-6 |
ACTCACCTCTTCAGAACGAATTG |
CCATCTTTGGAAGGTTCAGGTTG |
| IL-10 |
TGAATTCCCTGGGTGAGAAG |
CTCTTCACCTCCTCCACTGC |
| IL-17 |
ACGATGACTCCTGGGAAGACC |
GGGATTGTGATTCCTGCCTTC |
| NF-κB |
TGGCGCAGAAATTAGGTCTGG |
GATCACTTCAATTGCTTCGGTGTA |
Statistical analysis
IBM SPSS 21.0 software (IBM Corp.) was used for
statistical analysis. Measurement data are expressed as the mean ±
standard deviation (X ± S) for normally distributed variables in
each group, Homogeneity of variance test and one-way analysis of
variance were performed among multiple groups, after which multiple
comparisons of LSD were performed. Non-normally distributed
variables are presented as the median and interquartile range (M
[Q1-Q3]). Pearson correlation analysis was used for correlation
analysis between variables and Spearman correlation analysis was
used for non-parametric variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of the thyroid hormone,
antibodies and NF-κB
Index levels differed significantly between the GD
and HT groups and the controls (P<0.05 or P<0.01). The levels
of TSH, TGAb and NF-κB were significantly higher in the HT group
compared with the GD group and controls; while the levels FT3, FT4
and TRAb were significantly lower in the HT group compared with the
GD group (P<0.05 or P<0.01; Table III).
 | Table IIIComparison of indexes between the
patients and controls. |
Table III
Comparison of indexes between the
patients and controls.
| Groups | NC (n=53) | GD (n=41) | HT (n=41) |
|---|
| TSH (uIU/ml) | 1.5 (1.16-2.57) | 0.015
(0.005-0.145)a | 3.72
(2.24-5.16)a,b |
| FT3 (pmol/l) | 4.69±0.77 |
12.96±7.37a | 4.5±1.2b |
| FT4 (pmol/l) | 16.19±1.80 |
33.64±19.81a |
15.87±5.15b |
| ATPO (IU/ml) | 13.09±6.39 |
148.15±108.41a |
189.28±111.32a |
| TG (ng/ml) | 17.81±2.45 |
44.63±34.73a |
56.67±42.02a |
| TGAb (IU/ml) |
13.66(10.53-26.25) |
116.7(48.20-232.60)a |
285.8(171.89-476.90)a,c |
| TRAb (IU/l) | 0.64±0.26 |
14.83±11.91a |
0.94±0.39a,b |
| NF-κB (ng/ml) | 1.45±0.59 |
2.81±1.28a |
3.62±1.67a,c |
Comparison of cytokine levels
Cytokine levels differed significantly between the
GD and HT groups and the controls (P<0.05 or P<0.01). TNF-α,
IL-6 and IL-17A were significantly higher in the HT group compared
with the GD group and controls. IFN-γ and IL-10 were significantly
lower in the HT group compared with the controls (P<0.05). These
results suggest an immunoinflammatory mechanism, especially in the
HT group, which might be related to severer inflammatory lymphocyte
infiltration (Table IV).
Representative flow cytometric view of each cytokine analyzed by
flow cytometry is presented in (Fig.
S1).
 | Table IVComparison of cytokine levels between
patients and controls. |
Table IV
Comparison of cytokine levels between
patients and controls.
| Cytokines | NC (n=53) | GD (n=41) | HT (n=41) |
|---|
| IFN-γ (pg/ml) | 4.43±2.78 |
3.66±2.29a |
3.21±1.86b |
| TNF-α (pg/ml) | 2.24±1.03 |
3.42±1.54b |
4.33±1.88b,c |
| IL-2 (pg/ml) | 3.45±1.99 |
4.14±2.28a | 3.91±1.89 |
| IL-4 (pg/ml) | 1.95±1.02 | 1.72±0.98 | 1.69±0.94 |
| IL-6 (pg/ml) | 2.76±1.55 |
3.61±1.82b |
4.94±2.04b,d |
| IL-10 (pg/ml) | 2.49±1.43 |
1.89±0.94a |
1.79±0.95a |
| IL-17A (pg/ml) | 4.76±1.71 |
7.79±1.12b |
8.59±1.74b,c |
mRNA comparisons among groups
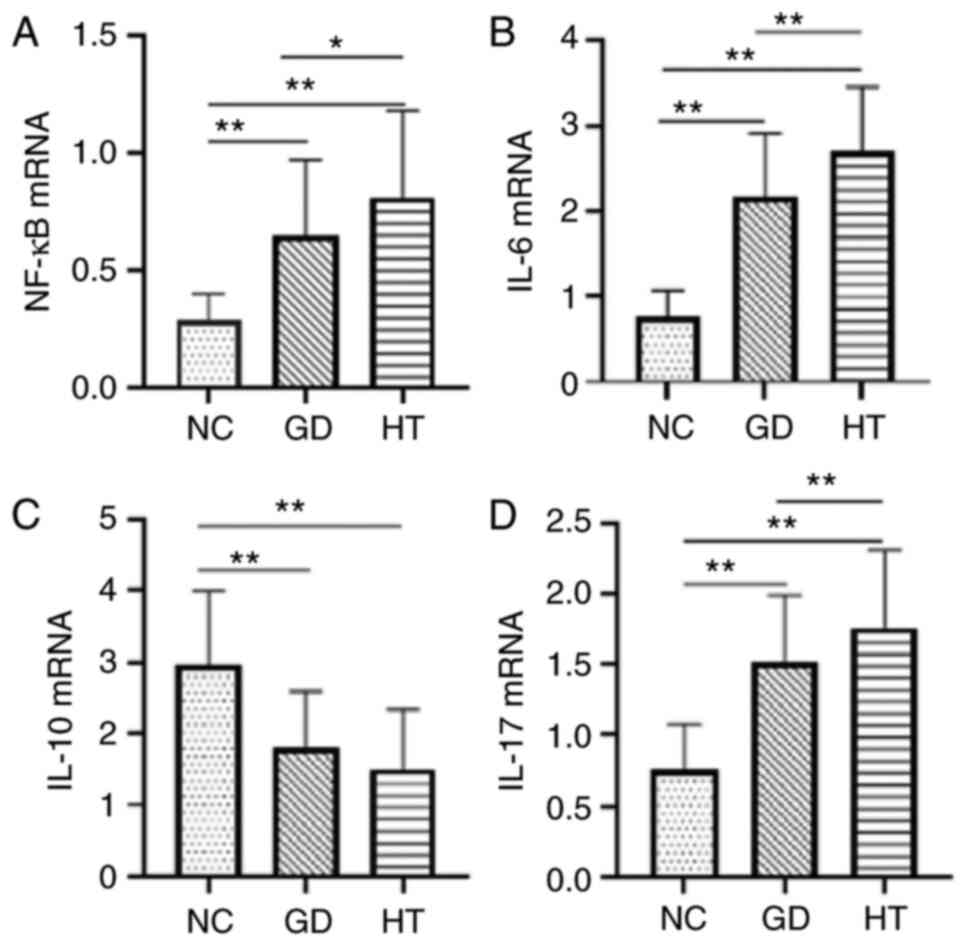
RT-qPCR was used to analyze the mRNA expression
levels of NF-κB (Fig. 1A), IL-6
(Fig. 1B), IL-17 (Fig. 1C) and IL-10 (Fig. 1D). mRNA levels in the GD and HT
groups differed significantly from those of the controls
(P<0.01). The mRNA expression levels of NF-κB, IL-6 and IL-17
were significantly higher in the HT group compared with the GD
group and controls (P<0.05 or P<0.01). While IL-10 mRNA was
lower in the HT and GD groups compared with the controls
(P<0.01) (Fig. 1).
Correlation analysis
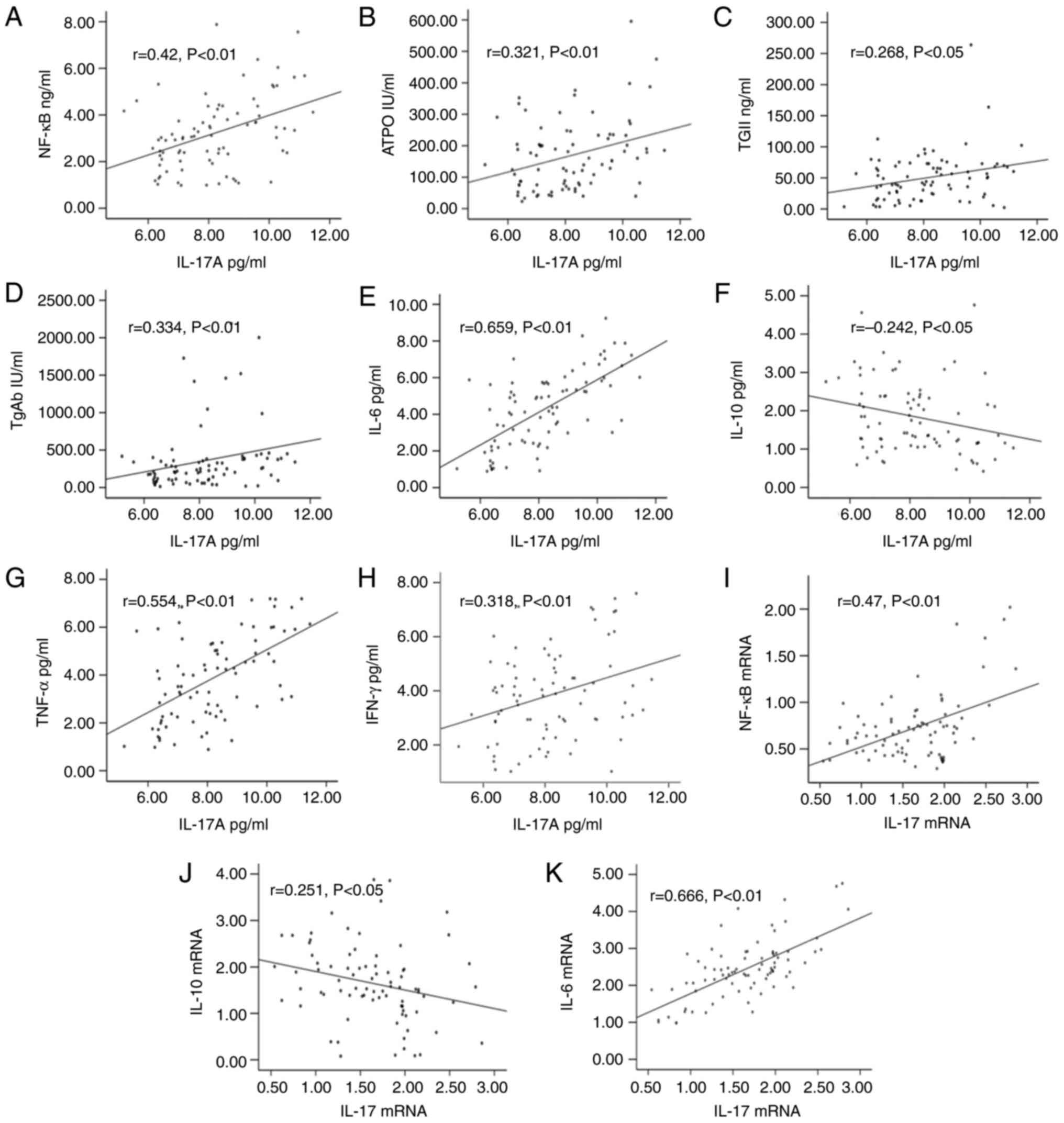
Pearson and Spearman correlation analyses revealed
that IL-17A was significantly positively correlated with ATPO,
NF-κB, TGAb, TNF-α, IL-6 and IFN-γ (r=0.321, 0.42, 0.334, 0.554,
0.659 and 0.318, respectively; P<0.05 or P<0.01) and
significant correlation with IL-10, TG (r=-0.242, 0.268;
P<0.05). IL-17 mRNA was positively correlated with NF-κB and
IL-6 mRNA (r=0.47 and 0.666, respectively; P<0.01) and
significant correlation with IL-10 mRNA (r=-0.251; P<0.05).
These data suggested that IL-17 activated the NF-κB signaling
pathway to produce inflammatory factors, and the high expression
difference may be caused by abnormal NF-κB expression (Fig. 2).
Discussion
IL-17, also known as IL-17A (10), is an important inflammatory factor
that promotes recruitment, chemotaxis and amplification of
neutrophils. It also recruits monocytes and neutrophils to gather
at inflammatory sites by increasing production of C-X-C motif
ligand (CXCL)1 and CXCL2 chemokines in tissues, thus causing
chronic inflammation (11).
Studies have revealed that IL-17 levels in thyroid
tissues are highly expressed in patients with both HT and GD
(12,13) and can be used as a novel marker and
potential prognostic indicator for diagnosing HT deterioration.
Such high expression stimulates Th17 cells under the synergistic
effect of IL-23 and IL-6 and activates relevant signaling pathways
to induce pathogenic phenotypes to induce AITDs (14). IL-17 further aggravates the local
inflammatory response of HT thyroid tissue by promoting
interstitial fibrosis, which leads to local fibrosis of thyroid
tissue and accelerates disease progression (15). Studies have shown that IL-17 is
closely associated with thyroid hormone levels and has different
correlations with various thyroid disease inflammatory factors,
such as IL-6, IL-23 and IL-10, and thyroid antibody titers,
indicating that Th17 cells have an independent stimulating effect
on thyroid cells (16).
In the present study, the thyroid hormone levels
were much higher in the GD group compared with the HT group, but
the ATPO, TGAb and TRAb levels were significantly higher in the HT
group, indicating that NF-κB levels also differ significantly under
different stimuli. IL-17A, TNF-α and IL-6 levels were significantly
higher in the patients compared with the controls, whereas IFN-γ
and IL-4 levels were lower, indicating the existence of an
immunoinflammatory mechanism. The HT group demonstrated the most
significant inflammation, possibly owing to the more severe degree
of inflammatory lymphocyte infiltration.
Inflammation destroys thyroid follicular cells and
lymphocytes and activates lymphocyte chemokine expression (17), resulting in disorder of the thyroid
hormones, increased antibody levels and toxic effects.
Additionally, IL-17 significantly inhibits the anti-inflammatory
and antitumor effects of IFN-γ and further upregulates the
expression of protein inhibitor of activated STAT1, a negative
feedback regulator of the JAK/STAT1 pathway, by enhancing NF-κB
activation, thereby accelerating tumor development (18). Autoantigen TG induces B cells and
CD4 T cells to secrete IL-10 and IL-6 and induce Th17
differentiation biased by HT to further produce IL-17, thus
resulting in a vicious cycle (19). Long-term inflammation destroys the
immune microenvironment of the thyroid tissue, rendering the immune
self-stabilizing mechanism ineffective (20), further causing carcinogenesis and
significantly increasing TG and cytokines Bcl-2, IL-8 and
TNF-α.
In the present study, IL-10 was lower in patients
compared with the controls, suggesting that the effects of IL-17,
IL-6 and TNF-α significantly weakened the anti-inflammatory effect
and major histocompatibility complex-II expression on the
downregulated antigen-presenting cells, and the T-cell response was
not effectively inhibited, thus promoting disease progression.
A number of cells express NF-κB, a member of the
nuclear hormone receptor family and protein molecule with
multidirectional regulatory effects, which can be activated by
inflammatory factors, growth factors and chemokines to activate a
cytokine cascade and produce proinflammatory mediators to regulate
the inflammatory response (18).
For example, activation of the NF-κB signaling pathway and an
imbalance of Treg/Th17 may be involved in the GD pathogenesis
(21). IL-17 directly activates
NF-κB through the classic pathway by promoting NF-κB p65 and other
nuclear ectopic genes. Stimulation of IL-17-expressing cells
induces MCP-1 expression dose- and time-dependently, resulting in
the persistence and aggravation of chronic inflammatory processes
(22). IL-17A signals
intracellular responses by activating IL-17R and synergistically
induces interstitial transformation, proliferation and inflammation
of bronchial epithelial cells via IL17R/NF-κB signal transduction
(23). IL-17 upregulates IL-6
expression by activating the NF-κB pathway, upregulates the
expression of programmed cell death ligand 1 in thyroid cells,
accelerates inflammatory infiltration and promotes tumor
development (24).
TNF-α activates the NF-κB signaling pathway, and its
expression is regulated by NF-κB (25). TNF-α contains specific NF-κB
binding sites; thus, a positive feedback loop can be formed between
TNF-α and the NF-κB signaling pathway, resulting in continuous
activation of the NF-κB pathway and Bcl-2 and intercellular
adhesion molecule-1 genes. This promotes proliferation and
infiltration of inflammatory cells into thyroid tissues and
occurrence and development of AITDs (26). Inhibiting the NF-κB pathway can
regulate the release of inflammatory factors such as IL-6, IL-10,
IL-12 and TNF-α (27) and improve
autoimmune thyroid function. The present study demonstrated that
IL-6, TNF-α and NF-κB increased successively in the three groups
and were positively correlated with IL-17A. IL-17 mRNA was also
positively correlated with IL-6 mRNA and NF-κB mRNA. These results
suggested that IL-17 activated the NF-κB signaling pathway to
produce a number of inflammatory factors such as IL-6 and TNF-α,
forming a positive feedback effect that leads to the occurrence and
development of AITDs.
In the present study, IFN-γ, IL-4, IL-2, IL-10 and
IL-10 mRNA did not significantly differ between the HT and GD
groups, but they showed a decreasing trend. IL-17A was negatively
correlated with IL-10, and IL-17 mRNA was negatively correlated
with IL-10 mRNA. The proinflammatory effects of IL-6 and TNF-α
formation are likely much greater compared with their
anti-inflammatory effects, and abnormal NF-κB expression may cause
the large differences in their expressions. A previous study has
shown that although IL-17 is highly expressed in thyroid cells in
patients with HT and GD, the levels of IL-23 that induce Th17
differentiation differ, and the correlation between IL-17 and IL-23
also differs (28). IL-23 induces
peripheral blood mononuclear cells to secrete prostaglandin E2,
which further increases the proportion of
IL-23R+CD4+T cells, promotes IL-17A
secretion, reduces secretion of anti-inflammatory factor IL-38 and
increases inflammatory cell release (29). These factors induce the different
pathogenic phenotypes among AITDs, including GD and HT, which
requires further research.
Supplementary Material
Flow cytometry was used to analyze the
representative views of IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10 and
IL-17A in the HT, GD and NC groups. The figure on the left is a
quantitative measure of cytokine representativeness and on the
right is a histogram of representational peaks. The y-axis is
pg/ml. HT, Hashimoto's thyroiditis; GD, Graves' disease; NC,
negative control.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the foundation Item:
Mandatory Research Project of Medical Science in Hebei Province
(grant no. 20210089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, CX and DZ designed the study, collected data,
analyzed relevant information, wrote the manuscript and approved
the final submission. GL, FC, ZH and CZ performed the formal
analysis, analyzed data, organized articles and checked papers YL
and XL designed the investigation and wrote the original draft. CX
and YL designed the methodology. YL, CX and XL were project
administrators. YL and CX confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Hebei General Hospital [approval no. 2020(204)], and all subjects
signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rayman MP: Multiple nutritional factors
and thyroid disease, with particular reference to autoimmune
thyroid disease. Proc Nutr Soc. 78:34–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lei Y, Yang J, Li H, Zhong H and Wan Q:
Changes in glucose-lipid metabolism, insulin resistance, and
inflammatory factors in patients with autoimmune thyroid disease. J
Clin Lab Anal. 33(e22929)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dashdamirova G, Rahimova R, Baghirova S
and Azizova U: Pathogenic mechanisms of autoimmune thyroid disease.
Int J Med Sci Health Res. 06:26–33. 2022.
|
|
4
|
Barnabei L, Laplantine E, Mbongo W,
Rieux-Laucat F and Weil R: NF-κB: At the borders of autoimmunity
and inflammation. Front Immunol. 12(716469)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Capece D, Verzella D, Flati I, Arboretto
P, Cornice J and Franzoso G: NF-κB: Blending metabolism, immunity,
and inflammation. Trends Immunol. 43:757–775. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roberti A, Chaffey LE and Greaves DR:
NF-κB Signaling and inflammation-drug repurposing to treat
inflammatory disorders? Biology (Basel). 11(372)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Endocrine Society of Chinese Medical
Association, Compilation group of《Guidelines for diagnosis and
treatment of thyroid diseases in China》. Diagnosis and treatment of
thyroid diseases in China-Laboratory and auxiliary examination of
thyroid diseases. Chinese Journal of Internal Medicine,. 2007.
4:697–702. 2007.
|
|
8
|
Quintanal-Villalonga Á, Chan JM,
Masilionis I, Gao VR, Xie Y, Allaj V, Chow A, Poirier JT, Pe'er D,
Rudin CM and Mazutis L: Protocol to dissociate, process, and
analyze the human lung tissue using single-cell RNA-seq. STAR
Protoc. 3(101776)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chimenz R, Tropeano A, Chirico V, Ceravolo
G, Salpietro C and Cuppari C: IL-17 serum level in patients with
chronic mucocutaneous candidiasis disease. Pediatr Allergy Immunol.
33 (Suppl 27):S77–S79. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang C, Chen J, Wang H, Chen J, Zheng MJ,
Chen XG, Zhang L, Liang CZ and Zhan CS: IL-17 exacerbates
experimental autoimmune prostatitis via CXCL1/CXCL2-mediated
neutrophil infiltration. Andrologia. 54(e14455)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zake T, Skuja S, Kalere I, Konrade I and
Groma V: Heterogeneity of tissue IL-17 and tight junction proteins
expression demonstrated in patients with autoimmune thyroid
diseases. Medicine (Baltimore). 97(e11211)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li S, Li S, Lin M, Li Z, He J, Qiu J and
Zhang J: Interleukin-17 and vascular endothelial growth factor: New
biomarkers for the diagnosis of papillary thyroid carcinoma in
patients with Hashimoto's thyroiditis. J Int Med Res.
50(3000605211067121)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu S, Guo P, Wang Z, Zhou Z, Wang R, Zhang
M, Tao J, Tai Y, Zhou W, Wei W and Wang Q: Down-regulation of A3AR
signaling by IL-6-induced GRK2 activation contributes to Th17 cell
differentiation. Exp Cell Res. 399(112482)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Esfahanian F, Ghelich R, Rashidian H and
Jadali Z: Increased levels of serum interleukin-17 in patients with
Hashimoto's thyroiditis. Indian J Endocrinol Metab. 21:551–554.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zake T, Kalere I, Upmale-Engela S,
Svirskis S, Gersone G, Skesters A, Groma V and Konrade I: Plasma
levels of Th17-associated cytokines and selenium status in
autoimmune thyroid diseases. Immun Inflamm Dis. 9:792–803.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Li Q, Wang B, Mu K and Zhang JA: The
pathogenesis of thyroid autoimmune diseases: New T lymphocytes -
Cytokines circuits beyond the Th1-Th2 paradigm. J Cell Physiol.
234:2204–2216. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li J, Zeng M, Yan K, Yang Y, Li H and Xu
X: IL-17 promotes hepatocellular carcinoma through inhibiting
apoptosis induced by IFN-γ. Biochem Biophys Res Commun.
522:525–531. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kristensen B: Regulatory B and T cell
responses in patients with autoimmune thyroid disease and healthy
controls. Dan Med J. 63(B5177)2016.PubMed/NCBI
|
|
20
|
Okda TM, Atwa GMK, Eldehn AF, Dahran N,
Alsharif KF and Elmahallawy EK: A Novel role of galectin-3 and
thyroglobulin in prognosis and differentiation of different stages
of thyroid cancer and elucidation of the potential contribution of
Bcl-2, IL-8 and TNF-α. Biomedicines. 10(352)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang J, Shi C, Chou G, Chen Z, Feng H and
Wang S: Expression of TLR4, NF-κB and Treg/Th17 related cytokines
in patients with Graves' Disease. Chinese Journal of Modern
Medicine, 2017 Feb,. 27:58–61. 2017.
|
|
22
|
Lin SH, Ho JC, Li SC, Cheng YW, Hsu CY,
Chou WY, Hsiao CC and Lee CH: TNF-α activating osteoclasts in
patients with psoriatic arthritis enhances the recruitment of
osteoclast precursors: A Plausible Role of WNT5A-MCP-1 in
osteoclast engagement in psoriatic arthritis. Int J Mol Sci.
23(921)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ma L, Jiang M, Zhao X, Sun J, Pan Q and
Chu S: Cigarette and IL-17A synergistically induce bronchial
epithelial-mesenchymal transition via activating IL-17R/NF-κB
signaling. BMC Pulm Med. 20(26)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wei L, Xiong H, Li W, Li B and Cheng Y:
Upregulation of IL-6 expression in human salivary gland cell line
by IL-17 via activation of p38 MAPK, ERK, PI3K/Akt, and NF-κB
pathways. J Oral Pathol Med. 47:847–855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Salomon BL, Leclerc M, Tosello J, Ronin E,
Piaggio E and Cohen JL: Tumor necrosis factor α and regulatory T
cells in oncoimmunology. Front Immunol. 9(444)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qi G, Zhu Y, Li Y, Li Y, Luo S and Li M:
Role of NF-κB pathway in the pathogenesis of AITD. Journal of
practical medicine, 2018 Oct. 34:3362–3366. 2018.
|
|
27
|
Zhao H, Chen W, Zhu L and Ge H:
1,25(OH)2D3 protects thyroid functions in experimental autoimmune
thyroiditis rats by inhibiting the TLR2 / NF-κB signaling pathway.
Chinese Journal of Comparative Medicine, 2022 Jan,. 32:78–86.
2022.
|
|
28
|
Zake T, Skuja S, Kalere I, Konrade I and
Groma V: Upregulated tissue expression of T helper (Th) 17
pathogenic interleukin (IL)-23 and IL-1β in Hashimoto's thyroiditis
but not in Graves' disease. Endocr J. 66:423–430. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pan Y, Wang M, Chen X, Chen Y, Ai S, Wang
M, Su W and Liang D: Elevated IL-38 inhibits IL-23R expression and
IL-17A production in thyroid-associated ophthalmopathy. Int
Immunopharmacol. 91(107300)2021.PubMed/NCBI View Article : Google Scholar
|