Introduction
HER2-positive breast cancer was originally a subtype
with a poor prognosis due to its aggressiveness, but improvements
in anti-HER2 therapy have dramatically reduced the frequency of
recurrence and have prolonged overall survival (1). Perioperative chemotherapy, which
includes trastuzumab (Tmab)- and pertuzumab (Pmab)-containing
regimens for HER2-positive breast cancer, has become the standard
of care when administered sequentially with anthracycline. In the
NeoSphere study, the pathological complete response (pCR) rate was
45.8% after combination of Tmab and Pmab with docetaxel as
neoadjuvant chemotherapy (NAC), which is a significantly higher pCR
rate than that of the other three regimens investigated in this
study; Tmab + Pmab, docetaxel + Tmab, and docetaxel + Pmab
(2). As for the order of taxane
and anthracycline, Wildiers et al reported that taxane-first
regimens were favorable in terms of the relative dose intensity in
an adjuvant setting (3). When
preceded by taxanes, Neo-tAnGo showed a higher pCR rate between
paclitaxel-epirubicin plus cyclophosphamide (EC) and EC-paclitaxel
(20% vs. 15%, P=0.03), and no difference was observed in
disease-free survival (DFS) [HR=0.82, 95% CI (0.63-1.06), P=0.12]
(4). Iwata et al reported a
high pCR rate with taxane-first regimens (docetaxel-FEC) and
confirmed these findings in a Japanese breast cancer series in
which the safety profile was also found to be acceptable (5). These data suggested an advantage of a
taxane-first strategy. As for the combination with anti-HER2
treatment, the FinHER trial showed that the combination of taxane
preceded by anthracycline and T-mab prolonged distant DFS
[docetaxel-FEC plus Tmab vs. no-Tmab, HR=0.32, 95% CI (0.12-0.98),
P=0.029] (6). Preoperative
chemotherapy with sequential Pmab plus Tmab plus docetaxel (PTD)
followed by doxorubicin plus cyclophosphamide (AC) is now covered
by insurance in Japan because of the negative disadvantages
associated with the order of administration. Therefore, the
taxane-first sequence with anti-HER2 targeted therapy in which
PTD-AC might be considered one of the standard regimens for
HER2-positive breast cancer is an option. However, the optimal
clinical positioning of Pmab-containing regimens and anthracyclines
is still debatable, and specifically, the omission of
anthracyclines is still an important issue because of
cardiotoxicity. There is another advantage of investigating the
additional benefit of AC in patients with non-cCR after PTD, if a
PTD-first sequence is planned. Herein to investigate the risk and
benefit of additional AC and taxane-first regimens in HER2-positive
breast cancer, we conducted a prospective observational study of
neoadjuvant PTD-AC.
Patients and methods
Procedures
This prospective observational study was conducted
at a single institute to investigate PTD followed by AC as a
preoperative chemotherapy in patients with HER2-positive breast
cancer and intended to estimate tumor reduction after PTD alone or
after both PTD and AC. The planned study period was from April 2019
to in July 2022, and the registration period was from March 2019 to
July 2021. Key inclusion criteria are: HER2-positive breast cancer
diagnosed pathologically HER2 3+ by immunohistochemistry or
positive results on in situ hybridization if HER2 2+ by
immunohistochemistry; patients indicated to receive neoadjuvant
chemotherapy; an Eastern Cooperative Oncology Group Performance
Status of 0 or 1. Key exclusion criteria were patients with other
active cancers that required treatment, those with heart failure
(EF <60%), and those whose inclusion was determined to be
inappropriate by the investigators. Magnetic resonance imaging
(MRI) and ultrasound (US) were used to assess the maximum tumor
diameter for breast lesions at baseline, after PTD, and after both
PTD and AC; the maximum tumor diameter was measured in accordance
with RECIST ver.1.1. If cancer lesions on images were not solitary
tumors, the maximum diameter of the area including all lesions was
defined as a viable lesion. Contrast-enhanced computed tomography
and ultrasound were used to assess the shape and short axis
diameter of the axillary lymph node (AxLN).
To assess the primary breast tumor, MRI was
evaluated with frequency-selective fat suppression (e-THRIVE) and
maximum intensity projection imaging, and the contrast effect range
was judged as a viable lesion (Ingenia 3.0T Omega HP release 5.41,
Philips Healthcare). Ultrasound was performed by laboratory
technicians, who reported the imaging data in clinical documents
using Aplio 700, Aplio a550 (Canon) and LOGIQ E9 (GE Healthcare
Japan). If a low echoic area with no blood flow was observed with
apparent discrepancies compared with MRI images, MRI findings were
preferred over US findings. Pathological findings included the
diameter of remnant lesions in the surgical pathology report
prepared by pathologists, who were blinded to the patient
information in this study.
The primary endpoint was the pCR rate after PTD
followed by AC, which was defined as ypT0/is and ypN0. Secondary
endpoints were tumor reduction rate and clinical complete response
(cCR) during PTD treatment, AC treatment, and the sequential full
regimen (PTD-AC), and safety, especially cardiotoxicity, at 1 year
after surgery. Left ventricular ejection fraction (LVEF) was
measured by echocardiography, and the same methods were used for
each individual patient throughout the study (7-12).
LVEF assessments were performed at baseline and at 1 year after
initiation of the planned treatment. Significant declines in the
LVEF were defined as decreases over 10% from baseline and to
<50% (7-10).
Left ventricular systolic dysfunction (LVSD) was reported as a
serious adverse event (SAE) (7).
The study protocol was approved by the Clinical
Trial Center of Sapporo Medical University, Japan (approval no.
302-237), was conducted in accordance with The Declaration of
Helsinki and the Ethical Principles for Medical Research Involving
Human Subjects, and is registered with UMIN-CTR (UMIN000046338). We
complied with the latest editions of the ‘Declaration of Helsinki
of the World Medical Association’ and the ‘Ethical Guidelines for
Medical Research Involving Human Subjects’ (Ministry of Health,
Labour and Welfare) with which all medical research involving human
subjects must comply. The consent documents approved by the review
committee were given to the subjects (approval no. 302-237), and
consent was obtained in writing of the subject's own free will
after sufficient explanation.
Treatment protocol
As for the regimen protocol of PTD, patients were
given intravenous Pmab on day 1 for three weeks of every cycle; the
starting loading dose was 840 mg, which decreased to 420 mg in
subsequent cycles. Then, patients were administered Tmab on day 1
of every cycle at a dose of 8 mg/kg, which decreased to 6 mg/kg.
Finally, patients were administered docetaxel on day 1 of every
cycle at a dose of 75 mg/m2 after which they were given
6.6 mg of dexamethasone and 0.75 mg of palonosetron. Those drugs
were given to patients for four cycles.
As for the subsequent four cycles of AC, patients
were given 60 mg/m2 of doxorubicin and 600
mg/m2 of cyclophosphamide on day 1 for three weeks of
every cycle. Aprepitant at 125 mg was also given intravenously on
day 1 and a decreased dose of 80 mg was given orally on days 2 and
3; 1 mg of granisetron was also given intravenously on day 1.
Surgery and perioperative systemic
treatments
If the AxLNs were clinically negative and this was
discovered before the preoperative treatment, sentinel lymph-node
biopsy (SLNB) was performed, while axillary lymph-node dissection
(ALND) was performed in cases of clinically positive AxLNs in
patients who underwent either mastectomy or partial mastectomy.
After surgery, tri-weekly Pmab and Tmab were administered to each
patient. During the study period, 1-year trastuzumab emtansine
(T-DM1) as a postoperative treatment was covered by insurance in
Japan in accordance with the KATHERINE trial (13). If partial mastectomy was performed,
radiation therapy was given to the preserved breast.
Statistical analysis
Descriptive statistics were used to describe the
baseline characteristics. Mann-Whitney U test was performed for the
reduction rate of PTD, AC, and PTD-AC in those with and without ER
positivity. Odds ratios and 95% confidence intervals for pCR for
each clinicopathologic factor were calculated by nominal logistic
analysis. All statistical analyses were performed using JMP 15.1.0
(SAS Institute Inc., Cary NC, USA).
Results
Patients' background
In total, 25 eligible patients were registered, but
one patient declined surgery and was thus ineligible. Therefore,
this analysis included 24 patients who were administered PTD-AC for
NAC. One patient underwent surgery after 4 cycles of PTD with no AC
because of a severe cardiac AE. The baseline patient
characteristics are summarized in Table I. The median age of patients was
54.5 years (range, 39-76 years). All patients were diagnosed with
invasive breast cancer through core needle biopsy or
vacuum-assisted biopsy. As for histological type, patients were
diagnosed with two special types, mucinous and invasive lobular
carcinomas, in addition to invasive carcinoma of no special type.
HER2 overexpression was observed in all patients, and ER and/or PgR
positivity was observed in 15 patients. After neoadjuvant
chemotherapy, 5 patients underwent partial mastectomy and 19
patients underwent mastectomy. Twelve patients with clinically
negative AxLNs underwent SLNB, and of these, one also underwent
ALND because of positive SLNB. Another 12 patients underwent ALND
alone.
 | Table IBaseline patients' characteristics
(n=24). |
Table I
Baseline patients' characteristics
(n=24).
| Characteristic | Values |
|---|
| Median age, years
(range) | 54.5 (39-76) |
| cT, 1/2/≥3 | 4/17/3 |
| cN, 0/≥1 | 12/12 |
| ER,
positive/negative | 15/9 |
| PgR,
positive/negative | 12/12 |
| HER2, 3+/2+ and
ISH-positive | 23/1 |
| NG, 1-2/3 | 18/6 |
| Median Ki67, %
(range) | 32 (14-90) |
| Breast surgery,
mastectomy/partial mastectomy | 19/5 |
| Axillary surgery,
SLNB/ALND after SLNB/ALND | 11/1/12 |
| Adjuvant therapy, 1
year of Pmab + Tmab/1 y of T-DM1 | 22/2 |
| Radiotherapy,
yes/no | 8/16 |
Adjuvant therapy was indicated in all patients; one
year of Pmab + Tmab was given to 22 patients and one year of T-DM1
was given to 2 patients, which was available at the hospital as a
postoperative treatment. Thereafter, irradiation was indicated
after adjuvant therapy in eight patients and was intended for the
preserved breast after partial mastectomy or was intended as
post-mastectomy radiotherapy (PMRT).
Overall, 23 of 24 patients (%) achieved 85% or more
relative dose intensity (RDI) of the planned eight treatment
cycles. Four patients had dose reductions but maintained RDI above
85%, while the other 19 patients achieved 100% RDI without dose
reductions or discontinuation.
Clinical and pathologic complete
response of primary tumors and axillary lymph nodes to NAC
Fig. 1 is sorted by
PTD reduction rate and demonstrates the reduction rate of PTD alone
and subsequent AC as well as the corresponding pathologic findings.
Out of 24 patients, 14 (58.3%) achieved pCR. Of the five patients
who underwent partial mastectomy, no case had positive surgical
margins. In one patient (#20), AC was omitted and surgery was
accelerated because of severe mitral valve disorder after four
cycles of PTD, which also resulted in pCR that was evident in the
surgical pathology findings. Therefore, 4 of 13 patients (30.8%)
achieved pCR after the full regimen (both PTD and AC) and
simultaneously achieved cCR by imaging assessment after PTD, while
4 of 14 patients (28.6%) in the intention-to-treat population
achieved pCR. The remaining 9 of 14 patients (64.3%) achieved pCR
but not cCR at some point after PTD; in other words, they achieved
pCR according to their surgical pathology findings with subsequent
AC. AxLN metastasis behaved differently from the primary breast
tumor (PBT) in some cases, and specifically, there were 5 cases,
all of which were ER-positive in which AxLN metastasis ultimately
remained. As shown in Fig. 1, the
PBTs disappeared in two cases, but AxLN metastases remained (#12
and #19). Table II shows the
details of the lymph nodes corresponding to the shrinkage of the
PBT. In the remaining three cases (#4, #5, and #9) both the PBT and
the AxLN metastases remained. Table
III shows the breakdown of pathologic treatment effects. A
total of 14 cases achieved pCR as defined in this study: 12 cases
of ypT0ypN0 and 2 cases of ypTisypN0.
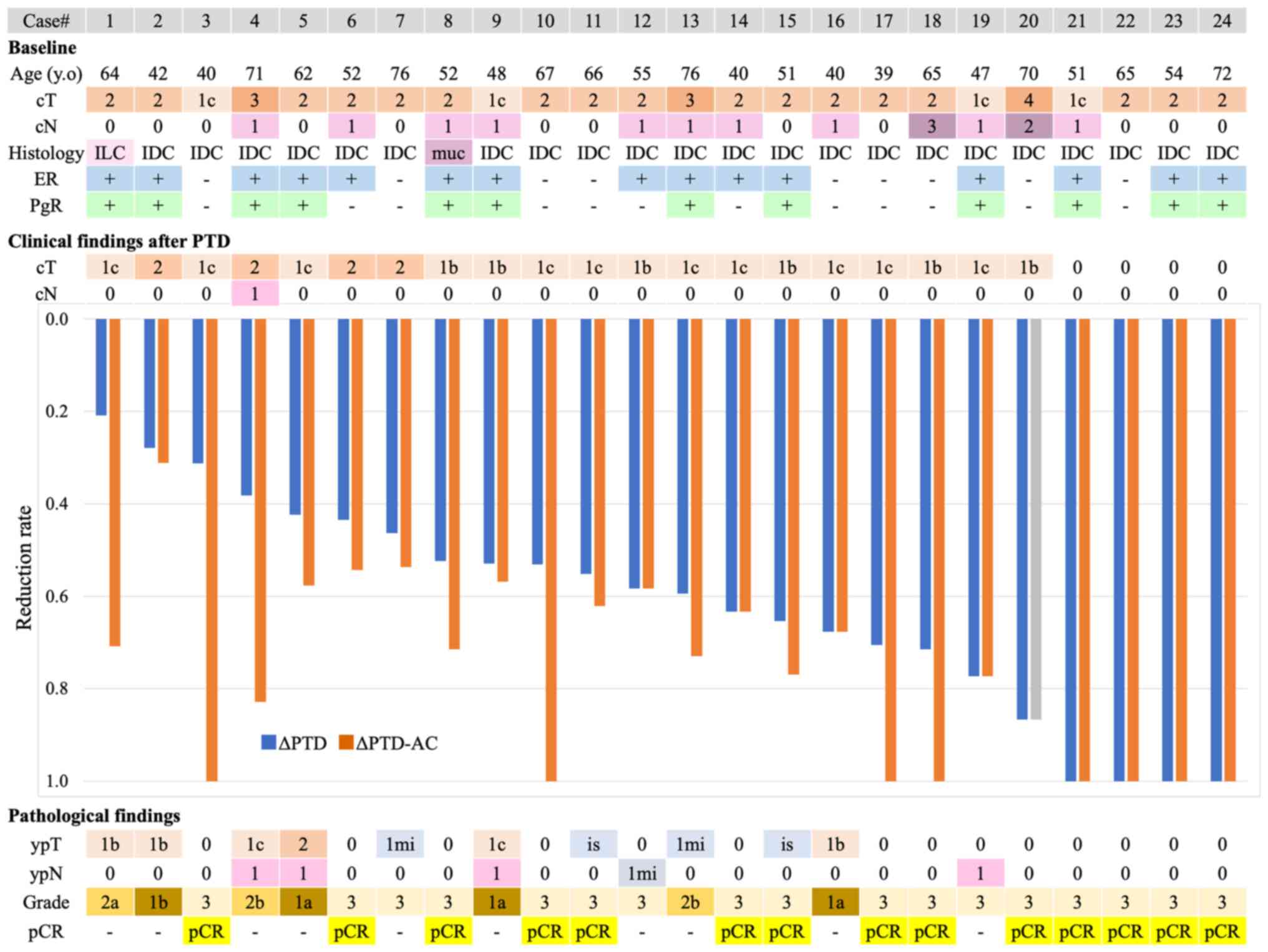 | Figure 1Waterfall plot sorted by PTD reduction
rate and clinical and pathological findings during NAC. cCR was
observed in 8 of 24 patients with primary breast tumors after
sequential PTD-AC, while pCR was observed in 14 of 24 patients.
Notably, four patients achieved apparent cCR, and all four patients
achieved pCR after PTD alone. IDC, invasive ductal carcinoma; ER,
estrogen receptor; PgR, progesterone receptor; NAC, neoadjuvant
chemotherapy; cCR, clinical complete response; pCR, pathological
complete response; AC, adriamycin and cyclophosphamide; PTD,
pertuzumab, trastuzumab and docetaxel; ILC, invasive lobular
carcinoma; muc, mucinous carcinoma. |
 | Table IIClinical and pathological findings on
axillary lymph nodes during NAC. |
Table II
Clinical and pathological findings on
axillary lymph nodes during NAC.
| |
Case no. |
|---|
| Clinical and
pathological findings | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|---|
| Primary breast
tumor | | | | | | | | | | | | | | | | | | | | | | | | |
|
cT of main
tumor baseline | 2 | 2 | 1c | 3 | 2 | 2 | 2 | 2 | 1c | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 1c | 4 | 1c | 2 | 2 | 2 |
|
cT of main
tumor after PTD | 1c | 2 | 1c | 2 | 1c | 2 | 2 | 1b | 1b | 1c | 1c | 1b | 1c | 1c | 1b | 1c | 1c | 1b | 1c | 1b | 0 | 0 | 0 | 0 |
|
ypT | 1b | 1b | 0 | 1c | 2 | 0 | 1mi | 0 | 1c | 0 | is | 0 | 1mi | 0 | is | 1b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Main tumor
reduction rate PTD | 0.21 | 0.28 | 0.31 | 0.38 | 0.42 | 0.44 | 0.46 | 0.52 | 0.53 | 0.53 | 0.55 | 0.58 | 0.6 | 0.63 | 0.65 | 0.68 | 0.71 | 0.71 | 0.77 | 0.87 | 1 | 1 | 1 | 1 |
|
Main tumor
reduction rate PTD-AC | 1 | 0.31 | 1 | 0.83 | 0.58 | 0.54 | 0.54 | 0.71 | 0.57 | 1 | 0.62 | 0.58 | 0.73 | 0.63 | 0.77 | 0.68 | 1 | 1 | 0.77 | Skip | 1 | 1 | 1 | 1 |
| Clinical findings
of AxLN | | | | | | | | | | | | | | | | | | | | | | | | |
|
Swelling
AxLN number | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 4 | 0 | 5 | 0 | 6 | 1 | 1 | 1 | 0 | 0 | 0 |
|
AxLN
FNA | - | - | - | m | - | m | - | m | m | - | - | m | m | ‡ | - | m | - | † | m | m | m | - | - | - |
|
cN
baseline | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 3 | 1 | 2 | 1 | 0 | 0 | 0 |
|
cN after
PTD | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Short diameter of
the largest AxLN | | | | | | | | | | | | | | | | | | | | | | | | |
|
Minor axis
of AxLN before NAC, mm | * | * | * | 37 | * | * | * | 15 | * | * | * | * | * | * | * | 20 | * | 23 | * | * | * | * | * | * |
|
Minor axis
of AxLN after PTD, mm | * | * | * | 32 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
|
Minor axis
of AxLN after PTD-AC, mm | * | * | * | 15 | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Pathological
findings of AxLN | | | | | | | | | | | | | | | | | | | | | | | | |
|
Results of
SLNB for cN0 | | | | | | | | | | | | | | | | | | | | | | | | |
|
SLNB
positive node number | 0 | 0 | 0 | - | 1 | - | 0 | - | - | 0 | 0 | - | - | - | 0 | - | 0 | - | - | - | - | 0 | 0 | 0 |
|
SLNB
total number | 2 | 1 | 1 | - | 1 | - | 1 | - | - | 2 | 1 | - | - | - | 1 | - | 1 | - | - | - | - | 1 | 1 | 2 |
|
Results of
final LN status including ALND | | | | | | | | | | | | | | | | | | | | | | | | |
|
Pathological
positive AxLN number | - | - | - | 1 | 3 | 0 | - | 0 | 3 | - | - | 1 | 0 | 0 | - | 0 | - | 0 | 2 | 0 | 0 | - | - | - |
|
ypN | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1mi | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
 | Table IIIBreakdown of pathologic findings. |
Table III
Breakdown of pathologic findings.
| Pathological
findings | ypT | ypN | Number (n=24) |
|---|
| pCR | ypT0 | ypN0 | 12 |
| | ypTis | ypN0 | 2 |
| non-pCR | ypT1mi | ypN0 | 2 |
| | ypT1 | ypN0 | 3 |
| | ypT2 | ypN0 | 0 |
| | ypT0 | ypN1mi | 1 |
| | ypT0 | ypN1 | 1 |
| | ypTis | ypN1 | 0 |
| | ypT1mi | ypN1 | 0 |
| | ypT1 | ypN1 | 2 |
| | ypT2 | ypN1 | 1 |
Reduction rate of primary tumors by
PTD or AC
The median tumor reduction rate after 4 cycles of
PTD was 58.9% (20.8-100%) in all 24 patients, 58.3% (20.8-100%) in
patients with ER-positive breast cancer, and 67.6% (31.3-100%) in
patients with ER-negative breast cancer (P=0.4733; Fig. 1 and Table II). The median tumor reduction
rate after AC was 7.4% (0-79.2%) in 23 patients, 3.9% (0-79.2%) in
those with ER-positive breast cancer, and 18.0% (0-68.8%) in those
with ER-negative breast cancer (P=0.2915). The overall median tumor
reduction rate of total PTD-AC was 76.9% (31.1-100%) in all
patients; 73.1% (31.3-100%) in patients with ER-positive breast
cancer, and 100% (53.7-100%) in patients with ER-negative breast
cancer (P=0.2579).
Univariate analyses for pCR
As shown in Table
IV, no statistically significant clinicopathologic factor for
pCR was found in the univariate analysis; cT2≤ vs. cT1, OR=2.33,
95% CI [0.24-23.57], P=0.4491; cT1≤ vs. cT0 OR=1.00, 95% CI
[0.18-5.63], P=1.000; ER-negative vs. ER-positive, OR=2.33, 95% CI
[0.39-19.44], P=0.3630; PgR-negative vs. PgR-positive, OR=5.00, 95%
CI [0.84-42.54], P=0.0781; Ki67 ≤20% vs. >20% OR=4.00, 95% CI
[0.27-109.96], P=0.3115. The presence of 0 items indicated that it
was inappropriate to perform a univariate analysis for nuclear
grade.
 | Table IVUnivariate analysis for pCR. |
Table IV
Univariate analysis for pCR.
| Clinicopathological
factors | Odds ratio | 95% CI | P-value |
|---|
| cT (≥cT2 vs.
cT1) | 2.33 | 0.24-23.57 | 0.4491 |
| cN (≥cN1 vs.
cN0) | 1.00 | 0.18-5.63 | 1.0000 |
| ER (negative vs.
positive) | 2.33 | 0.39-19.44 | 0.3630 |
| PgR (negative vs.
positive) | 5.00 | 0.84-42.54 | 0.0781 |
| NG (3 vs. 1-2) | - | - | - |
| Ki67 (≥20% vs.
<20%) | 4.00 | 0.27-109.96 | 0.3115 |
Postoperative relapse events
We conducted an exploratory study in which we
analyzed recurrent events. We found that there were no recurrent
events over a median follow-up of 22.8 (6.8-37.2) months. The
selected adjuvant therapy is described below. Two patients were
planned to receive 14 cycles of adjuvant T-DM1, 21 patients were to
receive 14 cycles of Tmab + Pmab, and 1 patient was to receive 14
cycles of Tmab. However, the planned adjuvant treatment could not
be completed because two patients receiving Tmab + Pmab and one
patient receiving Tmab discontinued the treatment because of
cardiotoxicity.
Safety
The incidence of treatment-related adverse events
(AEs) is summarized in Table V.
Alopecia was the most common grade 1-2 AE (n=24), followed by
nausea (n=13), palmar-plantar erythrodysesthesia syndrome (n=12),
and diarrhea (n=10). Neutropenia or febrile neutropenia was the
most common grade 3/4 severe AEs.
 | Table VSummary of adverse events in
patients. |
Table V
Summary of adverse events in
patients.
| Adverse event | Total | Grade 1-2 | Grade ≥3 |
|---|
| Blood and lymphatic
system disorders | | | |
|
Neutropenia | 4 | 2 | 2 |
|
Anemia | 1 | 1 | 0 |
|
Febrile
neutropenia | 3 | - | 3 |
|
Cardiac
disorders | | | |
|
Left
ventricular systolic disfunction | 2a | - | 2 |
|
Mitral valve
disorder | 1 | 0 | 1 |
| General disorders
and administration site conditions | | | |
|
Fatigue | 8 | 8 | 0 |
|
Peripheral
edema | 6 | 6 | 0 |
|
Mucosal
inflammation | 0 | 0 | 0 |
|
Fever | 2 | 2 | 0 |
| Skin and
subcutaneous tissue disorders | | | |
|
Alopecia | 24 | 24 | 0 |
|
Rash | 9 | 9 | 0 |
|
Nail
disorder | 3 | 3 | 0 |
|
Pruritus | 2 | 2 | 0 |
|
Dry
skin | 1 | 1 | 0 |
|
Skin
hyperpigmentation | 1 | 1 | 0 |
|
Palmer-plantar
erythrodysesthesia syndrome | 12 | 12 | 0 |
|
Pruritus | 2 | 2 | 0 |
| Gastrointestinal
disorders | | | |
|
Diarrhea | 10 | 10 | 0 |
|
Nausea | 13 | 13 | 0 |
|
Vomiting | 1 | 1 | 0 |
|
Constipation | 2 | 2 | 0 |
|
Abdominal
pain | 1 | 1 | 0 |
|
Mucositis
oral | 2 | 2 | 0 |
| Metabolism and
nutrition disorders | | | |
|
Anorexia | 2 | 2 | 0 |
| Nervous system
disorders | | | |
|
Headache | 2 | 2 | 0 |
|
Peripheral
motor neuropathy | 1 | 1 | 0 |
|
Peripheral
sensory neuropathy | 4 | 4 | 0 |
|
Dysgeusia | 3 | 3 | 0 |
| Musculoskeletal and
connective tissue disorders | | | |
|
Myalgia | 2 | 2 | 0 |
|
Arthralgia | 2 | 2 | 0 |
| Respiratory,
thoracic and mediastinal disorders | | | |
|
Cough | 2 | 2 | 0 |
| Other adverse
events | | | |
|
Cystitis
noninfective | 1 | 1 | 0 |
|
Shingles | 2 | 2 | 0 |
|
Middle ear
inflammation | 1 | 1 | 0 |
As for cardiac safety, in this study, the median
observation period for the evaluation of cardiac function (by
echocardiography) was 22.0 (range, 3.0-34.3) months. Severe AEs
were observed in three cases described below (3/24; 12.5%). One
patient had severe mitral regurgitation (grade 3) without
significant LVEF reduction two months after the day neoadjuvant
chemotherapy (NAC) was initiated, and accordingly, she did not
receive anthracycline-containing regimens (#10). Two patients had
significant LVEF reductions of 10% or more but <50% from
baseline, and they also had symptomatic LVSD (grade 3) at 9.9 and
11.5 months after starting NAC. Both patients experienced LVSD
during adjuvant systemic therapy (Tmab + Pmab); the former required
intensive care and had a moderate outcome, and the latter also had
a moderate outcome but received only oral medication. Those three
patients discontinued the planned treatment.
Discussion
This study was conducted as a single-center
prospective observational study with a PTD-first sequence. We
investigated the effect of PTD alone and the additional effects of
anthracycline-containing regimens. As for perioperative systemic
therapy, regimens preceded by anthracyclines have become the
standard of care, and although the order makes no significant
difference in safety and efficacy (4,5) in
patients with HER2-negative breast cancer, it was not ascertained
whether taxane-first sequence would be similarly positioned in
patients with HER2-positive breast cancer. The omission of
anthracycline-containing regimens has been discussed, particularly
to prevent cardiotoxicity during treatment. Simultaneously, the
benefits and conditions of adding anthracycline remain unclear.
Additionally, no conclusion has been reached, about whether a
taxane-first sequence leads to a benefit. pCR in HER2-positive
breast cancer is associated with substantial improvement in
event-free survival and OS, and therefore, pCR was confirmed as a
prognostic surrogate marker in HER2-positive breast cancer
(14). Therefore, it would be
sensible to analyze the effect of PTD alone and the subsequent
additional effect of AC in patients who achieve cCR/non-cCR, if
used as a surrogate marker.
In this study, 14 patients in the intention-to-treat
population achieved pCR at a rate of 58.3%. There were four cases
of cCR with PTD alone, which resulted in pCR at surgery. In
contrast, there were also cases of pCR due to AC after non-cCR
(9/14 patients; 64.3%), which indicated the possibility of an
additional effect of AC administered later. In the NeoSphere study,
pCR was defined as the absence of invasive neoplastic cells on
microscopic examination of the PBT at surgery, and in that study,
the pCR rate of the PTD group was 45.8% (2). Therefore, the result was numerically
favorable and even accounted for anthracycline-containing regimens
that followed. However, it is important to note that pCR and cCR
differ in quality, discussed below. In the GeparQuattro study,
compared the efficacy of the HER2 overexpression status in patients
with breast cancer in the NAC setting, which four cycles of
docetaxel or docetaxel plus capecitabine were administered
preoperatively after 4 cycles of EC (15). In the HER2-positive group, the cCR
rate after four cycles of EC was 22.0% (98/445), and after
subsequent administration of taxane, the pCR rate was 31.7%
(141/445). It is possible to interpret that anthracycline has a
certain effect on HER2-positive breast cancer. Recently,
antibody-drug conjugates, such as trastuzumab deruxtecan (16), for HER2-positive breast cancer have
been introduced sequentially with very impactful results, and
clinical trials focusing on therapies other than anti-HER2 therapy
have become rare except for those testing carboplatin. In this
sense, the data are valuable and show that anthracycline-containing
regimens lead to at least a certain level of response. Since the
results of this study also showed that some cases, which had
non-cCR after PTD and pCR after the entire regimen, responded to
anthracycline, it might be better not to omit anthracycline so
readily but to carefully examine the cases in which the omission
can be considered.
In the NAC setting, trials omitting anthracycline
have been reported, although most protocols use carboplatin instead
of anthracycline. In the TRAIN-2 study, the pCR rate was similar
[the difference was -1.5% between the anthracycline and the
non-anthracycline groups; 95% CI (-11-8), P=0.95(9)], and no substantial difference was
observed in cardiac toxicity with or without anthracyclines
(10). Notably, the results are
similar regardless of whether the cancer was ER-positive or
ER-negative (9). The BCIRG-006
trial also reported similar data on DFS and cardiac toxicity,
albeit in an adjuvant setting (12). Therefore, anti-HER2 therapy using
docetaxel and carboplatin as the chemotherapy components is a
reasonable approach for stage II or III HER2-positive tumors in
either the adjuvant or neoadjuvant setting (17). For anatomic stage I (T1N0),
HER2-positive tumors, paclitaxel plus trastuzumab is a good
de-escalating option according to the APT trial (17-19).
However, the use of carboplatin differs significantly from
taxane-based regimens in that it has high treatment intensity and a
high incidence of side effects (20). Few trials have examined the
omission of anthracycline as a taxane base, but one searchable
retrospective study was found; that study used propensity score
matching and reported a similar 3-year progression-free survival
without anthracycline (20).
Therefore, further analysis of anthracycline omission or
evidence-based use is necessary.
Pivotal studies on the prognostic improvement effect
of adding Pmab or other antiHER2 drugs, in the treatment regimen
have been reported. The combination of Tmab with Pmab improves
invasive disease-free survival (IDFS) in adjuvant chemotherapy for
HER2-positive breast cancer (21).
In the NAC setting, the addition of Pmab to preoperative
chemotherapy improved the pCR rate, but did not directly improved
prognosis (2,11). However, in patients with
HER2-positive breast cancer, the prognostic value of pCR has been
clarified in a study (14). Thus,
further prognostic analysis may reveal an improving effect with the
use of antiHER2 drugs, in the near future. The addition of T-DM1
for 1 year to non-pCR in preoperative chemotherapy for patients
with HER2-positive breast cancer reduced 3yIDFS events from 22.2 to
12.2% compared with the no addition group. However, for OS, the HR
0.70 with 95% CI of 0.47-1.05, which was not significant, but the
absolute value was reduced from 7.5 to 5.7% (22). In the KATHERINE trial, the
combination of Tmab + Pmab as antiHER2 therapy was administered in
18% of patients (22). In the
present study, we analyzed recurrent events after surgery as an
exploratory investigation over a median follow-up of 22.8 months,
and there were no recurrent events observed during this period.
Therefore, the difference between cCR and non-cCR after PTD or that
between pCR and non-pCR after PTD-AC could not be clarified in this
study. However, it is worth noting that there were no recurrences
after these treatments during the postoperative period of
approximately 2 year.
A meta-analysis of the diagnostic performance of MRI
in HER2-positive breast cancer received NAC revealed a positive
predictive value (PPV) of 62.0-94.6% for non-pCR (residual disease)
diagnostic, a negative predictive value (NPV) of 34.9-72%, and
sensitivity of 47-90% (23).
However, in the present study, the PPV for the presence of residual
tumor was 62.5% (10/16), the sensitivity was 100% (10/10), and the
false-negative rate was 0% (0/10). Compared with the meta-analysis,
the PPV was relatively low, which was a limitation of the present
study, although the high sensitivity and low false-negatives rates
appeared favorable.
According to the combination of US, there was no
difference between MRI and US accuracy [P=0.15, (24)]. The accuracy of diagnosing the
presence of residual disease was reported with a PPV of 92.1% and
NPV of 51.0% for MRI alone, whereas for MRI and US, the PPV was
94.0% and the NPV was 57.7%, suggesting that the combination of MRI
and US is superior in detecting residual disease but has problems
in ruling out residual disease (25,26).
Although the limitations in detecting residual
lesions due to PTD, should be noted, diagnoses made by MRI/US are
performed with a certain degree of accuracy in daily practice.
Recently, pCR/NAC diagnosis using core needle biopsy or
vacuum-assisted biopsy improves NPV and resulted in a favorable
false-negative rate (27,28); accordingly, pCR/NAC has become a
considerable option. Unfortunately, we did not perform post-PTD
biopsy in this study. Thus, it is one of the limitations as
described later in this study. However, it is worth noting that in
some cases of non-cCR after PTD, AC can still have an additional
effect and become pCR.
Regarding cardiac disorders, the cardiotoxicity of
anthracycline administration in addition to that of anti-HER2
therapy is a problem that should not be underestimated. In this
study, AEs other than cardiotoxicity did not differ significantly
from those previously reported. However, LVSD was observed in two
patients, and one patient experienced severe mitral regurgitation
but no significant LVEF reduction. The results showed a higher
frequency of LVSD events of any grade (3/24; 12.5%) than TRYPHAENA
(7). Evidence for a taxane-first
sequence is not sufficient (4,5). In
this study, the pCR rate was favorable, and as for safety, the
frequency of cardiac complications was slightly higher.
A limitation of this prospective observational study
is the small number of cases, because of the low frequency of
HER2-positive rates, 10-20% of all breast cancers (29). The second limitation is the lack of
sufficient statistical studies because of the small case number.
However, considering past reports, even with a small number of
cases, issues that were later considered had initially materialized
from observational studies including this study. Third, problems
may arise in MRI/US assessment, where diagnostic imaging is a
different technique than pathology. It is therefore not possible to
make a diagnosis of exactly the same quality, for example, the
diagnosis of residual lesions or complete tumor disappearance
before starting additional AC. In future investigation, it may be
important to perform a minimally invasive biopsy in addition to
diagnostic imaging, which might at least improve the reliability of
the diagnosis of residual disease, and might be supportive to
investigate alternative treatment options based on the diagnosis of
residual disease after prior neoadjuvant systemic therapy; for
example, a study to investigate the benefit of adding AC for
patients with residual lesions after PTD might be more
convincing.
In conclusion, we again found that the pCR rate and
response rate to PTD were high but that some cases experienced an
additional effect of AC, which resulted in pCR. As cardiotoxicity
remains a significant problem, further research on the risk-benefit
treatment strategy is needed to target the omission of AC or for
selection of patients expecting an additional AC effect. For
taxane-first sequences, a high pCR rate was observed, although it
should be noted that the frequency of cardiac AEs could not be
ignored.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS, GK, DK, HK, TO and IT conceived and planned in
detail the present study. YK, FS, NN and TH extracted the entirety
of patient data and performed the data construction and put the
data into a form that could be entered into statistical software.
AW, KS and SU performed analysis and interpretation of the patient
data with HS. DK, HK and TO revised it critically for important
intellectual content. IT provided overall supervision and gave
final approval to the publishable version. All authors read and
approved the final manuscript. HS and GK confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
This study adhered to ethical tenets of The
Declaration of Helsinki and Ethical Principles for Medical Research
Involving Human Subjects, was approved by the Clinical Trial Center
of Sapporo Medical University, Japan, and is registered with
UMIN-CTR (UMIN000046338). The consent documents approved by the
review committee were given to the subjects (302-237), and consent
was obtained in writing of the subject's own free will after
sufficient explanation.
Patient consent for publication
Patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Cronin KA, Kurian AW and
Andridge R: Differences in breast cancer survival by molecular
subtypes in the United States. Cancer Epidemiol Biomarkers Prev.
27:619–626. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomized multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wildiers H, Forceville K, Paridaens R and
Joensuu H: Taxanes and anthracyclines in early breast cancer: Which
first? Lancet Oncol. 11:219–220. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Earl HM, Vallier AL, Hiller L, Fenwick N,
Young J, Iddawela M, Abraham J, Hughes-Davies L, Gounaris I, McAdam
K, et al: Effects of the addition of gemcitabine, and
paclitaxel-first sequencing, in neoadjuvant sequential epirubicin,
cyclophosphamide, and paclitaxel for women with high-risk early
breast cancer (Neo-tAnGo): An open-label, 2x2 factorial randomised
phase 3 trial. Lancet Oncol. 15:201–212. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Iwata H, Sato N, Masuda N, Nakamura S,
Yamamoto N, Kuroi K, Kurosumi M, Tsuda H, Akiyama F, Ohashi Y and
Toi M: Docetaxel followed by
fluorouracil/epirubicin/cyclophosphamide as neoadjuvant
chemotherapy for patients with primary breast cancer. Jpn J Clin
Oncol. 41:867–875. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Joensuu H, Bono P, Kataja V, Alanko T,
Kokko R, Asola R, Utriainen T, Turpeenniemi-Hujanen T, Jyrkkiö S,
Möykkynen K, et al: Fluorouracil, epirubicin, and cyclophosphamide
with either docetaxel or vinorelbine, with or without trastuzumab,
as adjuvant treatments of breast cancer: Final results of the
FinHer trial. J Clin Oncol. 27:5685–5692. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schneeweiss A, Chia S, Hickish T, Harvey
V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, et al:
Pertuzumab plus trastuzumab in combination with standard
neoadjuvant anthracycline-containing and anthracycline-free
chemotherapy regimens in patients with HER2-positive early breast
cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann
Oncol. 24:2278–2284. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Swain SM, Kim SB, Cortés J, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Knott A, et al: Pertuzumab, trastuzumab, and docetaxel for
HER2-positive metastatic breast cancer (CLEOPATRA study): Overall
survival results from a randomised, double-blind,
placebo-controlled, phase 3 study. Lancet Oncol. 14:461–471.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van Ramshorst MS, van der Voort A, van
Werkhoven ED, Mandjes IA, Kemper I, Dezentjé VO, Oving IM, Honkoop
AH, Tick LW, van de Wouw AJ, et al: Neoadjuvant chemotherapy with
or without anthracyclines in the presence of dual HER2 blockade for
HER2-positive breast cancer (TRAIN-2): A multicentre, open-label,
randomized, phase 3 trial. Lancet Oncol. 19:1630–1640.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van der Voort A, van Ramshorst MS, van
Werkhoven ED, Mandjes IA, Kemper I, Vulink AJ, Oving IM, Honkoop
AH, Tick LW, van de Wouw AJ, et al: Three-year follow-up of
neoadjuvant chemotherapy with or without anthracyclines in the
presence of dual ERBB2 blockade in patients with ERBB2-positive
breast cancer: A secondary analysis of the TRAIN-2 randomized,
phase 3 trial. JAMA Oncol. 7:978–984. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gianni L, Pienkowski T, Im YH, Tseng LM,
Liu MC, Lluch A, Starosławska E, de la Haba-Rodriguez J, Im SA,
Pedrini JL, et al: 5-Year analysis of neoadjuvant pertuzumab and
trastuzumab in patients with locally advanced, inflammatory, or
early-stage HER2-positive breast cancer (NeoSphere): A multicentre,
open-label, phase 2 randomised trial. Lancet Oncol. 17:791–800.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et
al: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J
Med. 365:1273–1283. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mamounas EP, Untch M, Mano MS, Huang CS,
Geyer CE Jr, von Minckwitz G, Wolmark N, Pivot X, Kuemmel S,
DiGiovanna MP, et al: Adjuvant T-DM1 versus trastuzumab in patients
with residual invasive disease after neoadjuvant therapy for
HER2-positive breast cancer: Subgroup analyses from KATHERINE. Ann
Oncol. 32:1005–1014. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Broglio KR, Quintana M, Foster M, Olinger
M, McGlothlin A, Berry SM, Boileau JF, Brezden-Masley C, Chia S,
Dent S, et al: Association of pathologic complete response to
neoadjuvant therapy in HER2-positive breast cancer with long-term
outcomes: A meta-analysis. JAMA Oncol. 2:751–760. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Untch M, Rezai M, Loibl S, Fasching PA,
Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B,
et al: Neoadjuvant treatment with trastuzumab in HER2-positive
breast cancer: Results from the GeparQuattro study. J Clin Oncol.
28:2024–2031. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
File D, Curigliano G and Carey LA:
Escalating and de-escalating therapy for early-stage HER2-positive
breast cancer. Am Soc Clin Oncol Educ Book. 40:1–11.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tolaney SM, Guo H, Pernas S, Barry WT,
Dillon DA, Ritterhouse L, Schneider BP, Shen F, Fuhrman K, Baltay
M, et al: Seven-year follow-up analysis of adjuvant paclitaxel and
trastuzumab trial for node-negative, human epidermal growth factor
receptor 2-positive breast cancer. J Clin Oncol. 37:1868–1875.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pernas S and Tolaney SM: Management of
early-stage human epidermal growth factor receptor 2-positive
breast cancer. JCO Oncol Pract. 17:320–330. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fujita N, Fujita K, Kim SJ, Iguchi C,
Nomura T, Aono T, Yanagisawa T, Enomoto Y, Inakami K, Miyagawa Y,
et al: Response-guided omission of anthracycline in patients with
HER2-positive early breast cancer treated with neoadjuvant taxane
and trastuzumab: 5-Year follow-up of prognostic study using
propensity score matching. Oncology. 100:257–266. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
von Minckwitz G, Procter M, de Azambuja E,
Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N,
Clark E, et al: Adjuvant pertuzumab and trastuzumab in early
HER2-positive breast cancer. N Engl J Med. 377:122–131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab emtansine for residual invasive
HER2-positive breast cancer. N Engl J Med. 380:617–628.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu N, Leung VWY and Meterissian S: MRI
performance in detecting pCR after neoadjuvant chemotherapy by
molecular subtype of breast cancer. World J Surg. 43:2254–2261.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marinovich ML, Houssami N, Macaskill P,
Sardanelli F, Irwig L, Mamounas EP, von Minckwitz G, Brennan ME and
Ciatto S: Meta-analysis of magnetic resonance imaging in detecting
residual breast cancer after neoadjuvant therapy. J Natl Cancer
Inst. 105:321–333. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Iwase M, Sawaki M, Hattori M, Yoshimura A,
Ishiguro J, Kotani H, Gondo N, Adachi Y, Kataoka A, Onishi S, et
al: Assessing residual cancer cells using MRI and US after
preoperative chemotherapy in primary breast cancer to omit surgery.
Breast Cancer. 25:583–589. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yuan Y, Chen XS, Liu SY and Shen KW:
Accuracy of MRI in prediction of pathologic complete remission in
breast cancer after preoperative therapy: A meta-analysis. AJR Am J
Roentgenol. 195:260–268. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Heil J, Schaefgen B, Sinn P, Richter H,
Harcos A, Gomez C, Stieber A, Hennigs A, Rauch G, Schuetz F, et al:
Can a pathological complete response of breast cancer after
neoadjuvant chemotherapy be diagnosed by minimal invasive biopsy?
Eur J Cancer. 69:142–150. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Heil J, Kümmel S, Schaefgen B, Paepke S,
Thomssen C, Rauch G, Ataseven B, Große R, Dreesmann V, Kühn T, et
al: Diagnosis of pathological complete response to neoadjuvant
chemotherapy in breast cancer by minimal invasive biopsy
techniques. Br J Cancer. 113:1565–1570. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hayashi N, Kumamaru H, Isozumi U, Aogi K,
Asaga S, Iijima K, Kadoya T, Kojima Y, Kubo M, Miyashita M, et al:
Annual report of the Japanese breast cancer registry for 2017.
Breast Cancer. 27:803–809. 2020.PubMed/NCBI View Article : Google Scholar
|















