Introduction
The pathology of acute lung injury (ALI) or acute
respiratory distress syndrome (ARDS) is characterized by elevations
in alveolar-capillary permeability caused by inflammation, trauma
and burns (1). This in turn
triggers atelectasis and excessive inflammatory responses,
resulting in progressively worsening respiratory failure (2). In particular, sepsis is one of the
main causes of ALI, which is frequently mediated indirectly by
lipopolysaccharides (LPSs) released from the outer layer of the
cell wall of gram-negative bacteria (3). LPS can activate inflammation by
interacting with cell surface receptors including LBP, CD14, MD2
and TLR4 to promote structural damage and dysfunction in the
physiological epithelial and endothelial barriers (4,5).
This is facilitated following the activation of second messengers
including cAMP and cGMP and related signaling pathways including
NFκB signaling and the PI3K/Akt pathway (6). Although numerous studies have
previously revealed that inflammatory factors including TNF-α and
IL-6 can become activated during ALI (7-9),
the use of anti-inflammatory drugs (such as sivelestat and
simvastatin) in clinical practice was not able to effectively
reduce the mortality rate which is estimated as 30-40% globally
(10). To date, no effective
prevention or treatment measures exist for septic ALI (11). Clinically, mechanical ventilation
is the main supportive treatment strategy (12); however, the mechanical tension
generated by the ventilation process itself can also cause lung
injury (13). Therefore, novel
treatment methods for ALI remain in demand.
Remifentanil is a synthetic piperidine derivative
and a selective opioid receptor agonist (14). Remifentanil has high affinity for
µ-opioid receptors but weaker affinity for δ and κ receptors
(15). Its analgesic effect has
been reported to be 250 times that of morphine (16). The pharmacokinetics of remifentanil
are characterized by a rapid onset of action followed by rapid
clearance (17). Since its
clearance is mainly dependent on non-specific esterase degradation
in the plasma or tissue, it is not affected by liver and kidney
function, sex, age and body weight; Remifentanil has a short
half-life and high plasma clearance rate, and is the first choice
for special populations (over 65 years old, liver and kidney
insufficiency, children or obesity) (18). In addition, there were no
alterations in its metabolic rate or accumulation in the body after
long-term infusion or repeated injections (19). Owing to these aforementioned
advantages, remifentanil is used extensively as a general
anesthesia (20). Previous studies
have reported that remifentanil pre-treatment can alleviate
myocardial ischemia/reperfusion injury, the mechanism of which is
associated with the inhibition of oxidative stress and apoptosis
(21,22). Remifentanil can also inhibit the
LPS-induced inflammatory response in human aortic endothelial cells
through the poly (ADP-ribose) polymerase 1 (PARP-1)/NF-κB signaling
pathway (23). Furthermore,
remifentanil has been found to inhibit the expression and release
of high mobility group box protein 1 in the liver, lungs, and
kidney tissues of septic rats (24). TLR4 is a member of TLRs family,
regulating inflammatory response (25). Increased expression of MMP9 and
increased ratio of MMP-9/tissue inhibitors of metalloproteinase 1
(TIMP1) expression are considered to be primary indicators of
chronic airway injury and emphysema (26). Notably, TLR4 has been reported to
regulate hippocampal MMP/TIMP imbalance in perioperative
neurocognitive disorder in diabetes (27). Therefore, remifentanil treatment
may exert regulatory effects on TLR4-mediated MMP-9/TIMP1 balance
and inhibit inflammatory injury in lung epithelial cells.
A549 lung adenocarcinoma cells have been extensively
used as a cell model for type II pulmonary epithelial cells
(28,29). Therefore, in the present study,
A459 cells were selected and LPS was used as the treatment to
establish inflammatory damage models. The aim of the present study
was to investigate the effects of remifentanil on inflammatory
injury in the cells, the MMP-9/TIMP1 balance and possible
regulatory pathways.
Material and methods
Cell culture
A549 lung adenocarcinoma cells (Procell Life Science
& Technology Co., Ltd.) were cultured in the DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) in a humidified chamber at 37˚C
with 5% CO2. Remifentanil was obtained from Jiangsu Nhwa
Pharmaceutical Co., Ltd. (Xuzhou, China), with the concentrations
(0.625, 1.25 and 2.5 µM) set at 37˚C according to previous studies
(23,30). LPS (10 µg/ml; Beyotime Institute of
Biotechnology) was used to treat the cells at 37˚C for 24 h
(31). The TLR4 inhibitor CLI-095
(also known as resatorvid; 3 µM; Selleck Chemicals) was added at
37˚C for 6 h to suppress TLR4 signaling (32). Cells in the treatment group were
treated with remifentanil for 30 min prior to LPS stimulation.
Untreated cells were regarded to be the control group.
Cell Counting Kit-8 (CCK-8) assay
A549 cells were seeded into 96-well plates at a
density of 5x103 cells/well (100 µl) and treated with
remifentanil or LPS, as aforementioned. Following 24 h of
incubation, 10 µl CCK-8 reagent (AmyJet Scientific, Inc.) was added
to each well before the cells were incubated at 37˚C for a 1 h. The
optical density in each well was measured using a microplate reader
(450 nm; Nanjing Detie Laboratory Equipment Co., Ltd.).
Lactate dehydrogenase (LDH) release
assay
A549 cells (5x103 cells/well; 150 µl)
were treated with remifentanil and LPS at 37˚C, as aforementioned.
At 23 h incubation, the LDH release reagent (15 µl) provided by the
LDH Cytotoxicity Assay Kit (cat. no. C0016; Beyotime Institute of
Biotechnology) was added to the wells, and the cells were incubated
for 1 h (24 h total incubation). The cells were then centrifuged at
400 x g for 5 min at room temperature before 120 µl supernatant was
taken from each well for measurement. LDH release was measured by
plotting a standard curve after OD values were obtained using a
microplate reader (490 nm).
Flow cytometry
Apoptosis was analyzed by flow cytometry using an
Annexin V-FITC Apoptosis Detection Kit (cat. no. C1062; Beyotime
Institute of Biotechnology). Briefly, treated or untreated A549
cells (1x105), aforementioned, were washed twice with
pre-cooled PBS and suspended in 195 µl binding buffer. The cell
suspension (5x105 cells/ml) was then transferred into a
tube and incubated with Annexin V-FITC (5 µl) and propidium iodide
(10 µl) at room temperature in the dark for 15 min. Results were
obtained using a BD FACSCanto II flow cytometer (BD Biosciences)
and FlowJo version 10 software (FlowJo LLC).
Western blotting
Protein extracts of A549 cells were obtained using
the RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.), cytoplasmic and nuclear protein were extracted using
NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol
and protein concentrations were determined using the BCA assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Protein samples (30
µg/lane) were subjected to SDS-PAGE (10%) and transferred onto PVDF
membranes (MilliporeSigma). Following blocking in 5% non-fat milk
for 1 h at room temperature, the membranes were incubated with the
indicated primary antibodies at 4˚C overnight and then with the
respective HRP-conjugated secondary antibodies at room temperature
for 2 h; all antibodies used in the present study are listed in
Table I. Protein bands were
visualized using an DBI TOP-ECL chemiluminescence reagent (Shanghai
Xinghan Biotechnology Co., Ltd.) and quantified using ImageJ
software (version 1.52v; National Institutes of Health).
 | Table IAntibodies used for western blot
analysis. |
Table I
Antibodies used for western blot
analysis.
| Antibody | Catalog number | Host | Dilution ratio | Company |
|---|
| Bcl-2 | AB112 | Rabbit | 1:1,000 | Beyotime Institute
of Biotechnology |
| Bax | AF1270 | Rabbit | 1:2,000 | Beyotime Institute
of Biotechnology |
| Cleaved-PARP | ab32064 | Rabbit | 1:5,000 | Abcam |
| PARP | orb88930 | Mouse | 1:1,000 | Biorbyt, Ltd. |
|
Cleaved-caspase3 | ab32042 | Rabbit | 1:500 | Abcam |
| Caspase3 | GTX110543 | Rabbit | 1:5,000 | GeneTex, Inc. |
| IL-6 | ab233706 | Rabbit | 1:1,000 | Abcam |
| IL-1β | ab283818 | Rabbit | 1:1,000 | Abcam |
| TNF-α | ab183218 | Rabbit | 1:1,000 | Abcam |
| Toll-like receptor
4 | ab13556 | Rabbit | 1:500 | Abcam |
| MMP9 | ab283575 | Rabbit | 1:1,000 | Abcam |
| Tissue inhibitor of
metalloproteinase 1 | MA1-773 | Mouse | 1:500 | Invitrogen; Thermo
Fisher Scientific, Inc. |
| p-NF-κB p65 | MA5-15160 | Rabbit | 1:1,000 | Invitrogen; Thermo
Fisher Scientific, Inc. |
| NF-κB p65 | 14-6731-81 | Rabbit | 1:1,000 | Invitrogen; Thermo
Fisher Scientific, Inc. |
| p-STAT3 | 44-384G | Rabbit | 1:1,000 | Invitrogen; Thermo
Fisher Scientific, Inc. |
| STAT3 | MA1-13042 | Mouse | 1:5,000 | Invitrogen; Thermo
Fisher Scientific, Inc. |
| H3 | ab1791 | Rabbit | 1:1,000 | Abcam |
| β-actin | orb181785 | Rabbit | 1:500 | Biorbyt, Ltd. |
| HRP-conjugated
anti-rabbit IgG | A0208 | Goat | 1:1,000 | Beyotime Institute
of Biotechnology |
| HRP-conjugated
anti-mouse IgG | A0216 | Goat | 1:1,000 | Beyotime Institute
of Biotechnology |
ELISA
ELISA kits were used to measure the levels of
inflammatory factors, including IL-6 (cat. no. ZN2272), IL-1β (cat.
no. ZN2236) and TNF-α (cat. no. ZN2460; Beijing Biolab Technology
Co., Ltd.), as well as the secretion levels MMP9 (cat. no.
BMS2016-2; Invitrogen; Thermo Fisher Scientific, Inc.) and TIMP1
(cat. no. PT888; Beyotime Institute of Biotechnology). The
operating steps were performed according to the respective
protocols. Briefly, cell culture media (500 µl) from A549 cells
treated with remifentanil and LPS following 18 h of incubation at
room temperature was centrifuged at 2,000 x g for 10 min at room
temperature to remove debris before the supernatant was collected
for assaying. The samples (100 µl) were then incubated at room
temperature with (in order): Biotin-labeled antibodies (100 µl) for
1 h, avidin-peroxidase complex (100 µl) for 25 min, TMB chromogenic
solution (100 µl) for 20 min and stop solution (50 µl). Protein
levels were measured by plotting a standard curve after the OD
values were obtained using a microplate reader (450 nm).
Oxidative stress assessment
Superoxide dismutase (SOD; cat. no. A001-1),
glutathione peroxidase (GSH; cat. no. A005-1) and malondialdehyde
(MDA; cat. no. A003-4-1; Nanjing Jiancheng Bioengineering
Institute) assay kits were used to assess their levels. Briefly,
following indicated treatment, supernatant of A549 cells
(1x106) were collected using centrifugation at 1,000 x g
for 5 min at 4˚C and 10-50 µl of supernatant was used to analyze
the levels of oxidative stress according to the manufacturer's
protocol of corresponding kits. The OD values at 450 nm were
measured using a microplate reader.
Immunofluorescence (IF)
IF was performed to verify the localization of NF-κB
p65. Briefly, treated or untreated A549 cells (5x104
cells/well) plated in a 24-well plate for 24 h were fixed with 4%
paraformaldehyde at room temperature for 15 min and permeabilized
with 0.5% Triton X-100 at room temperature for 15 min. The cells
were then blocked with 10% goat serum (Beijing Solarbio Science
& Technology Co., Ltd.) for 1 h at room temperature, incubated
with the NF-κB p65 primary antibody (1:100) overnight at 4˚C and
FITC-conjugated goat anti-rabbit secondary antibodies (1:1,000;
cat. no. orb688925; Biorbyt, Ltd.) for 1 h at room temperature in
the dark. The nuclei were stained with DAPI (5 µg/ml) for 5 min at
room temperature, and the immunostaining was examined under a
fluorescence microscope (Leica Microsystems GmbH) at x200
magnification.
Statistical analysis
All experiments were performed at least three times,
and normally distributed data were expressed as the mean ± standard
deviation. The data were analyzed by one-way ANOVA followed by
Tukey's post hoc test using the SPSS version 13 software (SPSS,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Remifentanil improves the viability in
LPS-treated cells
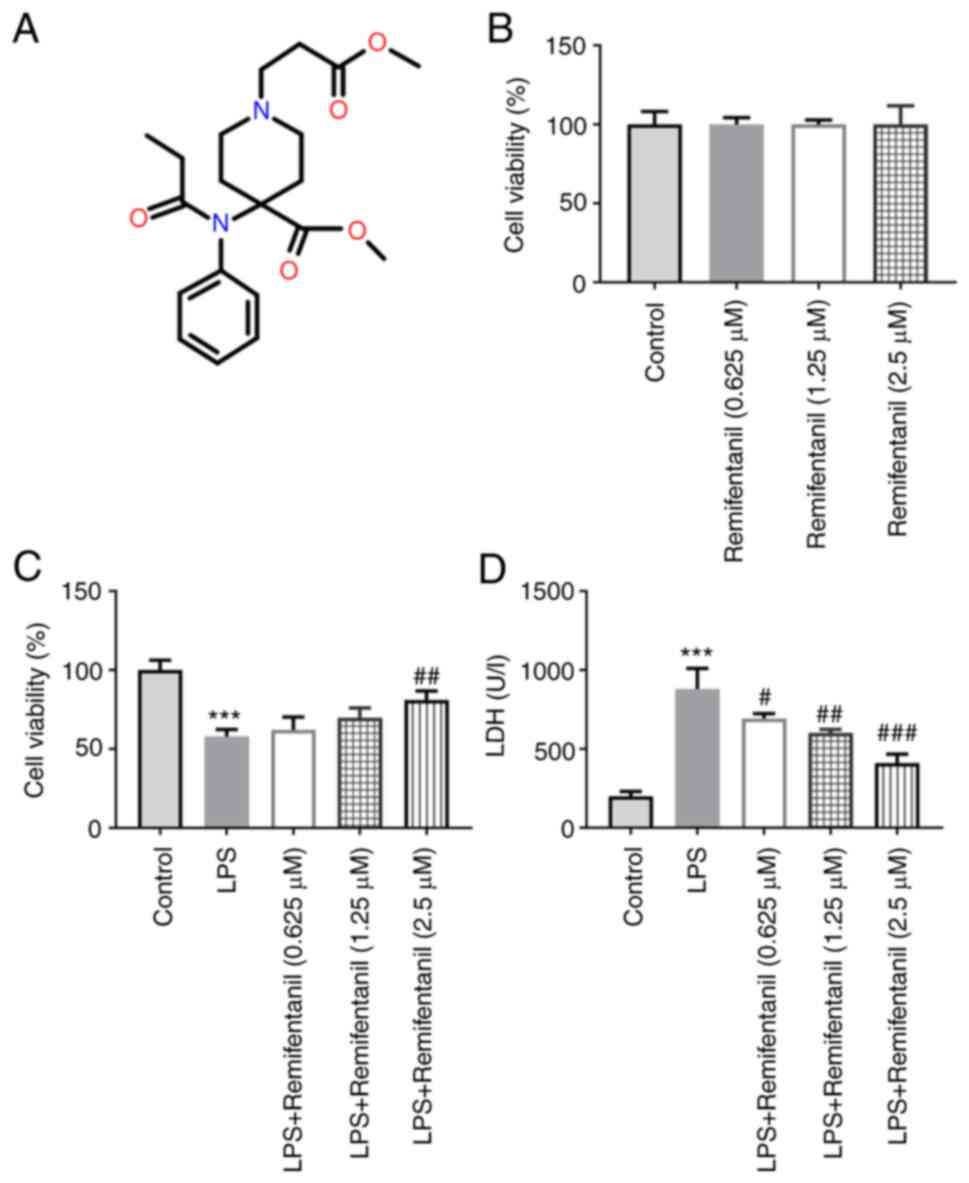
The chemical structure of remifentanil is displayed
in Fig. 1A. The effects of
different concentrations of remifentanil on A549 cell viability was
determined using CCK-8 assay (Fig.
1B); the highest remifentanil concentration tested (2.5 µM) did
not affect cell viability, suggesting it is a safe cell treatment
concentration. The viability of cells treated with LPS was
decreased significantly compared with untreated cells (Fig. 1C), whereas the viability of cells
co-treated with remifentanil at a concentration of 2.5 µM was
markedly increased compared with that in the LPS-only group. In
addition, the level of LDH release in each treatment group of cells
was measured. The results revealed that the degree of LDH release
by cells in the LPS-only group was significantly higher compared
with that in the control group (Fig.
1D). The LDH levels in cells co-treated with remifentanil were
significantly reduced compared with that in the LPS group.
Remifentanil suppresses LPS-induced
apoptosis
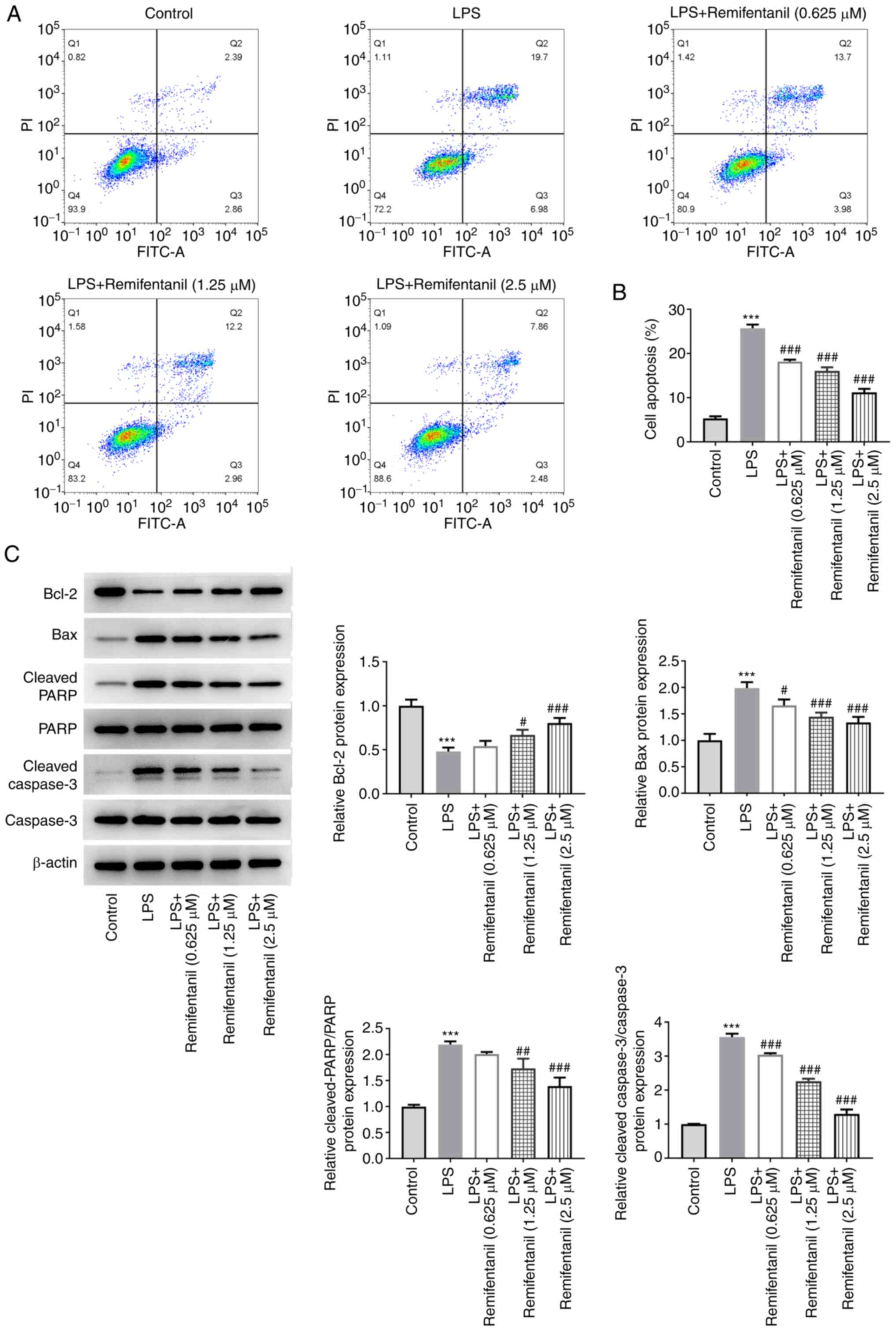
To evaluate the effect of remifentanil on apoptosis,
the apoptotic rates of cells in each treatment group were detected
by flow cytometry. The proportion of early-(Q3) and late-stage (Q2)
apoptotic cells in the LPS group was significantly increased
compared with that in the control group (Fig. 2A and B). Compared with that in the LPS-only
group, the proportion of apoptotic cells in the remifentanil
co-treated groups was all significantly decreased. In addition, the
expression levels of apoptosis-related proteins were measured using
western blotting (Fig. 2C).
Compared with the control group, the protein expression levels of
Bax, cleaved PARP and cleaved caspase 3 in the LPS group were
significantly increased, whereas those of the Bcl-2 protein were
significantly decreased. By contrast, remifentanil co-treatment
reversed the alterations in the protein expression levels, with the
differences reaching significance at higher concentrations tested.
These data suggested that remifentanil may alleviate LPS-induced
apoptosis.
Remifentanil reduces inflammation and
oxidative stress caused by LPS
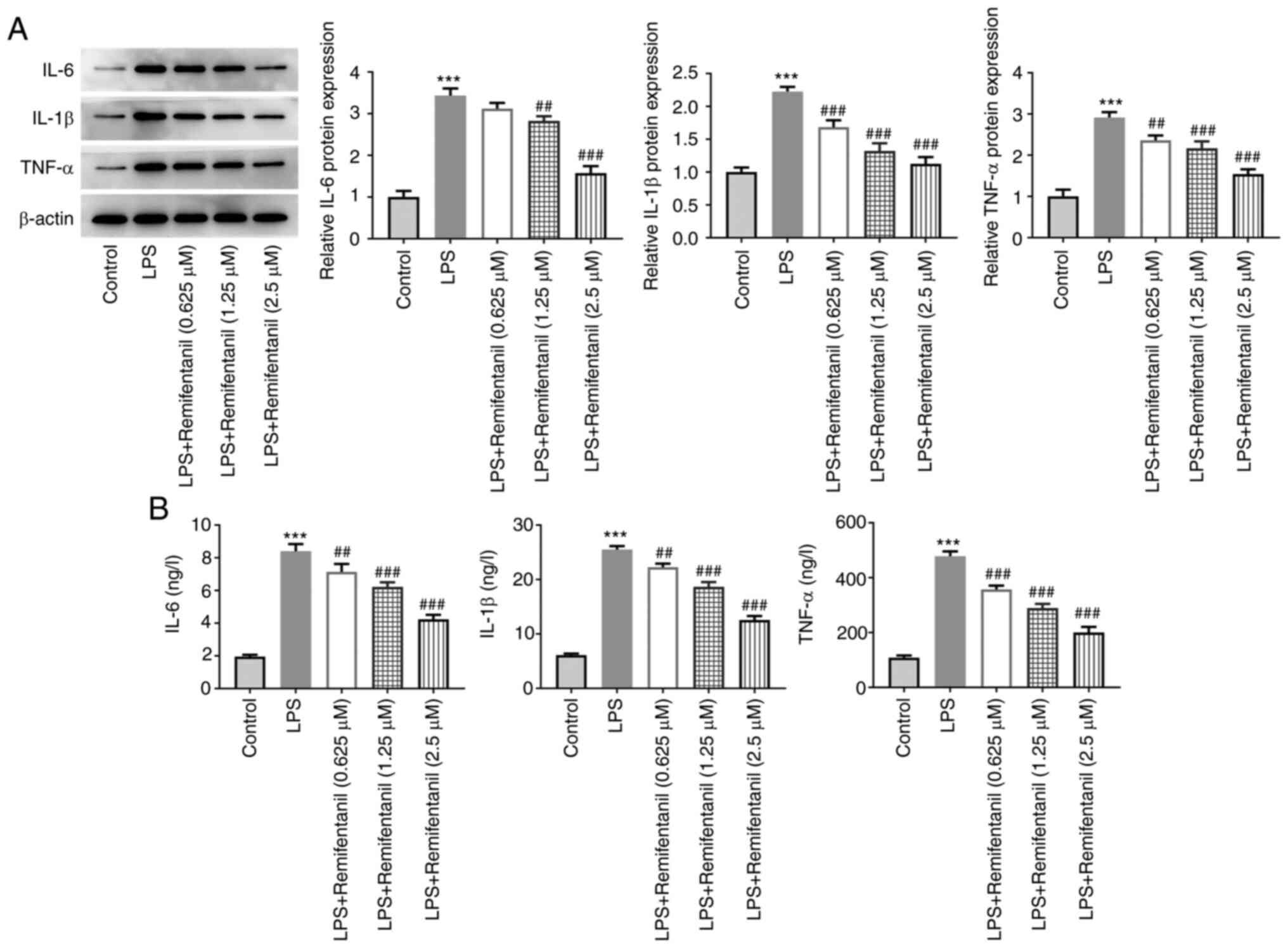
Inflammation-related cytokine levels were determined
using western blotting and ELISA (Fig.
3A and B, respectively). The
levels of IL-6, IL-1β and TNF-α expression and secretion were
significantly elevated in the LPS-only group compared with the
untreated control group, and these were reversed by remifentanil
co-treatment in a concentration-dependent manner.
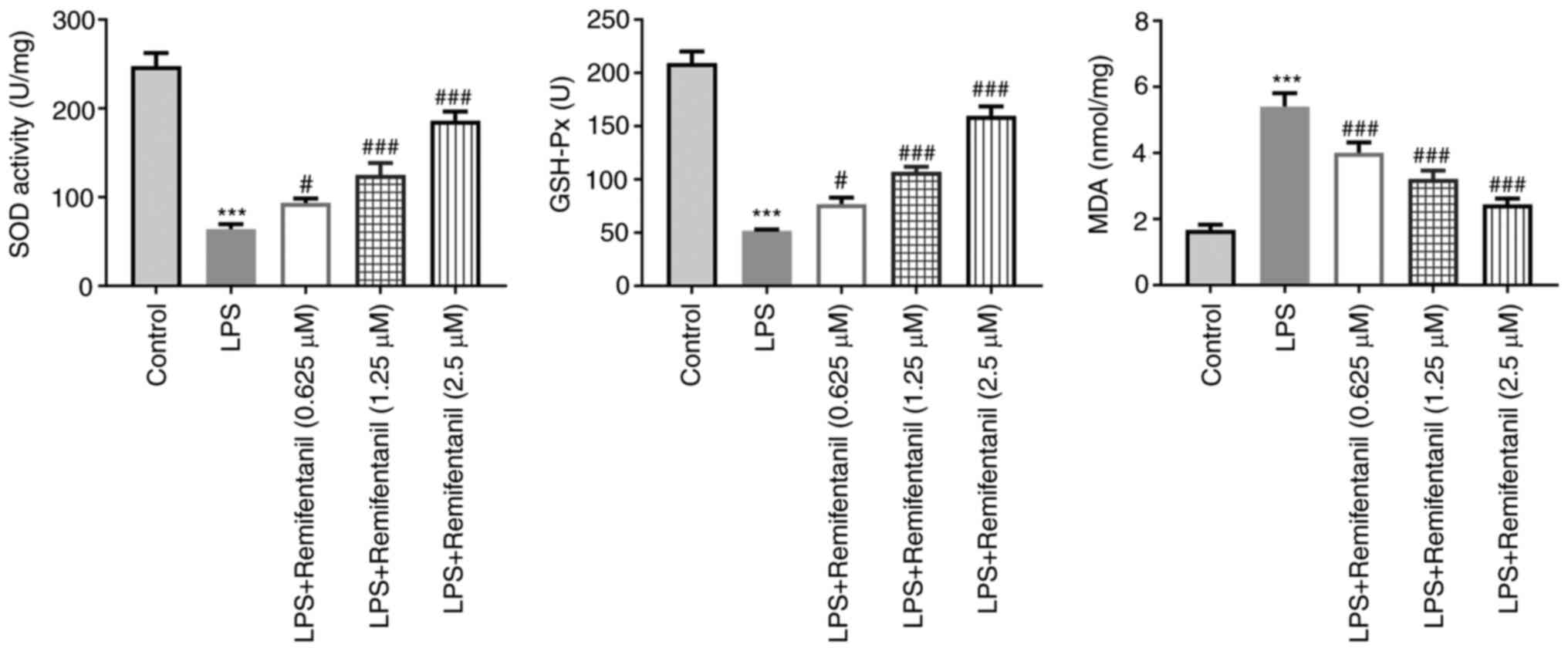
The levels of oxidative stress markers were also
measured using their corresponding assay kits. The levels of SOD
and GSH-Px were significantly reduced in the LPS group compared
with the control group whereas those of MDA were increased
(Fig. 4). Co-treatment with
remifentanil significantly reversed the aforementioned LPS-induced
effects (Fig. 4). These
observations suggested that remifentanil treatment may reduce
oxidative stress in the cells caused by LPS.
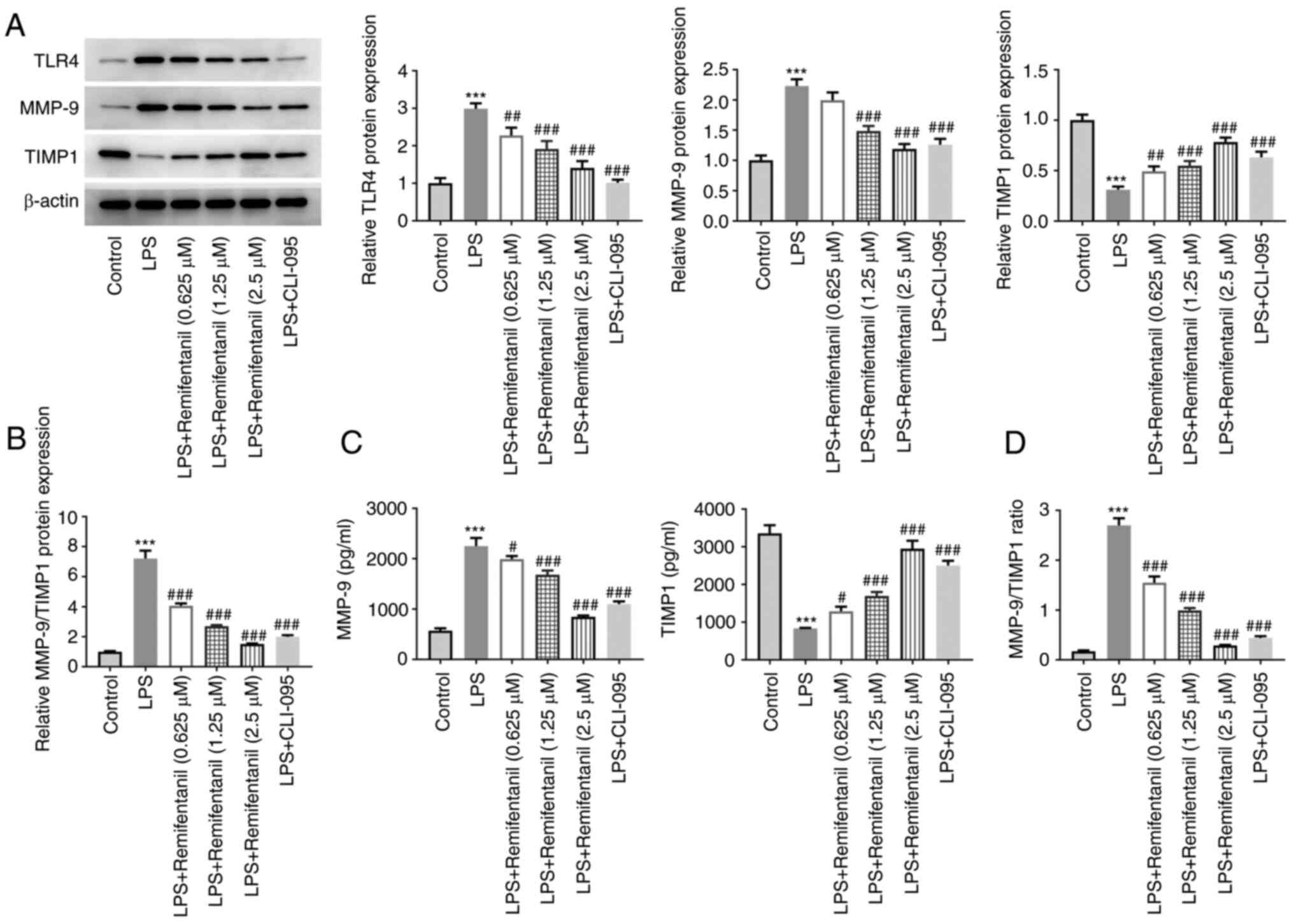
Remifentanil modulates TLR4 to mediate
MMP-9/TIMP1 imbalance in LPS-treated cells
The expression levels of TLR4, MMP-9 and TIMP1 were
evaluated using western blotting (Fig.
5A and B). The expression
levels of TLR4 and MMP-9 were significantly elevated in the
LPS-treated group, whilst those of TIMP1 were decreased.
MMP-9/TIMP1 ratio significantly elevated upon LPS treatment. By
contrast, remifentanil co-treatment significantly reversed the
LPS-induced increase in TLR4 and MMP-9 expressions and the
decreased TIMP1 expression. To further assess the role of TLR4 in
the MMP-9/TIMP1 expression balance, the cells were treated with the
TLR4 inhibitor, CLI-095. Compared with those in the LPS group,
CLI-095 co-treatment significantly reduced the expression of TLR4.
Moreover, relative to LPS group, additional CLI-095 treatment
weakened MMP-9 expression and content, whilst increasing those of
TIMP1, resulting in a decrease in MMP-9/TIMP1 ratio. The effect of
CLI-095 on the secretion of MMP-9 and TIMP1 was similar to that
induced by LPS + 2.5 µM remifentanil group (Fig. 5C and D). These data suggested that remifentanil
may restore MMP-9/TIMP1 imbalance to the normal level in
LPS-treated cells via mediatingTLR4 signaling.
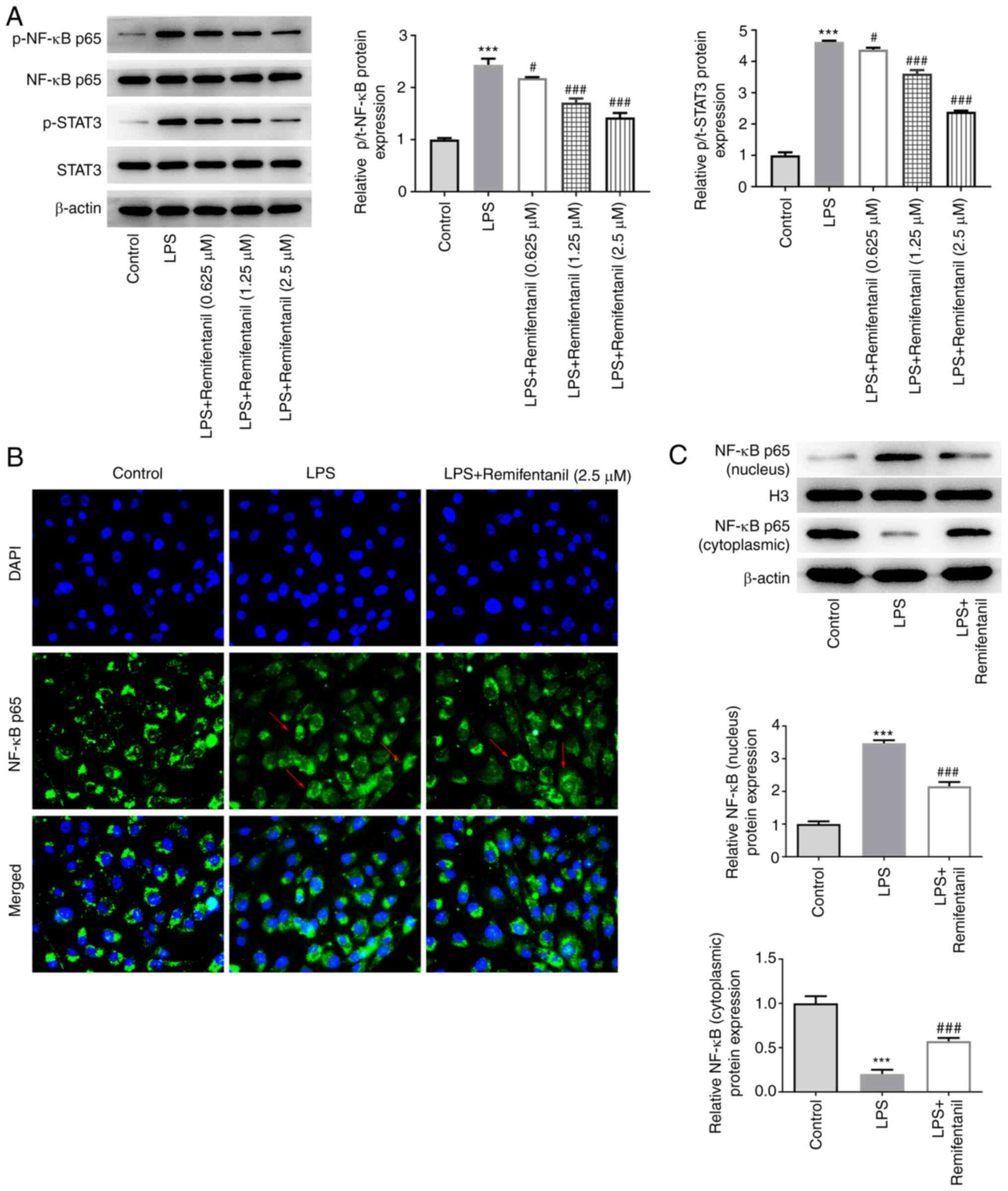
Remifentanil regulates the NF-κB/STAT3
signaling pathway
The expression levels of proteins in the NF-κB/STAT3
signaling pathway were assessed using western blotting. The
phosphorylation levels of NF-κB and STAT3 proteins were found to be
significantly elevated in the LPS group compared with the control,
and these were significantly reversed by remifentanil co-treatment
(Fig. 6A). To verify whether NF-κB
signaling was activated or blocked, nuclear translocation of NF-κB
p65 was examined using IF assay (Fig.
6B). The fluorescence of NF-κB p65 in the nucleus appeared to
be enhanced in the LPS-treated group compared with the control
group, and remifentanil co-treatment appeared to be lower compared
with the LPS-only group, suggesting that remifentanil suppressed
the nuclear translocation of NF-κB p65. However, the difference in
the IF images was not obvious, so western blotting was performed.
According to the western blotting data, the protein expression
level of NF-κB p65 in the nucleus was significantly increased
following LPS stimulation, which was accompanied by its reduction
in the cytoplasm (Fig. 6C). After
remifentanil co-treatment, the protein expression level of NF-κB
p65 decreased in the nucleus and increased in the cytoplasm
compared with that in the LPS-only group (Fig. 6C).
Discussion
Previous studies reported that aberrant coagulation,
fibrinolysis and extravascular fibrosis form the key features of
ALI (33-35).
Among them, coagulation and fibrinolysis are closely associated
with the inflammatory response and together promote ALI progression
(36). When ALI occurs, endogenous
endotoxemia is induced through a multitude of mechanisms, such as
stress and the immune response, which stimulates the release of
inflammatory mediators IL-1β and IL-6 to enhance the inflammatory
response and aggravate lung dysfunction (37,38).
By contrast, fibrous deposits activate endothelial cells to also
produce proinflammatory cytokines including TNF-α, IL-1 and IL-6 to
induce activated neutrophil aggregation (39). Persistent protein deposition can
also cause vascular wall thickening, pulmonary artery thrombosis
and increased risk of mortality (34). These previous findings suggest that
the inflammatory response is an important cause of ALI, where the
imbalance between the systemic inflammatory response and
compensatory anti-inflammatory response serves a key role in its
pathogenesis, during which LDH will be released into the
bloodstream (40). Therefore, from
the perspective of inflammation, seeking a novel therapeutic
strategy for ALI prevention and treatment would be of significance
for reducing the risk of morbidity and mortality. The results of
the present study suggest that remifentanil may reduce apoptosis
whilst alleviating inflammation and oxidative stress in LPS-treated
A549 cells. Therefore, remifentanil may serve an anti-inflammatory
role in ALI.
In the present study, it was found that remifentanil
reduced the expression level of TLR4 and ratio of MMP-9/TIMP1 in
LPS-treated cells. TLRs belong to a class of pattern recognition
receptors that can mediate the inflammatory response to pathogens
by inducing proinflammatory effects (25). It has been previously revealed that
TLR4 is involved in the regulation of immune defense responses and
inflammatory mediators in the respiratory tract during ALI
progression in an animal model (4). In addition, another previous study
demonstrated that remifentanil can ameliorate hepatic
ischemia-reperfusion injury by upregulating the expression of
β-arrestin 2, a well-known µ-opioid receptor desensitizer (41). Since β-arrestin 2 is also a
negative regulator of the TLR4-mediated inflammatory response,
β-arrestin 2 may serve as a key molecule connecting the µ-opioid
receptor and TLR4 pathways (41).
These findings imply that remifentanil might elevate β-arrestin 2
expression to suppress TLR4-mediated inflammatory response, which
was consistent with the results in the present study. Reactive
oxidants and free radicals can directly obstruct cell function,
causing proteases, such as MMP-9, to be released. MMP-9 serves a
role in various physiological processes, including extracellular
matrix degradation and lung tissue remodeling (42-44),
both of which have been reported to be associated with the
occurrence of various lung diseases including chronic obstructive
pulmonary disease and idiopathic pulmonary fibrosis (42-44).
MMP9 activity is regulated by the specific endogenous inhibitor
TIMP1(45). Under the action of
external stimuli, the proportion of their expression and the
interaction between the two can be regulated, the dysregulation of
which can result in lung pathology (45). Moreover, increased expression of
MMP9 and increased ratio of MMP-9/TIMP1 expression are considered
to be primary indicators of chronic airway injury and emphysema
(26). Notably, TLR4 has been
reported to regulate hippocampal MMP/TIMP imbalance in
perioperative neurocognitive disorder in diabetes (27). Therefore, identifying the upstream
pathway(s) that may alleviate the imbalance and, in turn, designing
putative intervention methods is currently garnering research
interest. In the present study, remifentanil was found to restore
their imbalance to the normal level, indicating it has the
potential to treat ALI.
As a proinflammatory factor, LPS induces the
inflammatory response, mainly by activating the NF-κB pathway
(6). Previous studies have shown
that remifentanil can alleviate endothelial cell inflammation
(23) and myocardial injury
(46) through inhibiting NF-κB
signaling, where activating TLR4 can lead to the activation of
NF-κB signaling (47). NF-κB is
one of the most important nuclear transcription factors in cells
and serves a central role in the transcriptional regulation of
cellular messages mediated by stimuli including inflammation and
infection (48). NF-κB activation
can promote the expression and release of ILs and TNF-α, which can
be applied for diagnosis and severity assessment of ALI (49). Furthermore, activation of the STAT3
signaling pathway can also promote the production of inflammatory
cytokines and chemokines to aggravate the process of ALI (50). Following the action of Janus
kinase, STAT3 in the cytoplasm dimerizes due to following Y705 and
S727 phosphorylation in its protein structure, where STAT3 can also
be activated by reversible acetylation (51). Activated STAT3 will then
translocate into the nucleus and bind to genomic DNA to regulate
transcription (52). Previous
studies have revealed that in innate immunity, adaptive immunity,
acute and chronic inflammation, and tumor occurrence, STAT3
activity is aberrantly activated with high frequencies (43,53-55).
STAT3 cooperates with NF-κB to drive inflammatory response
(56). Therefore, the apparent
suppressive effects of remifentanil on NF-κB/STAT3 signaling may be
one of the mechanism by which it alleviates ALI. However, the
present study is limited to in vivo experiments using A549
cells. Other cell types, such as primary cells, in addition to
in vivo studies, are required.
In conclusion, the present study found that
remifentanil restored MMP-9/TIMP1 imbalance to the normal level via
mediating TLR4 and inhibited inflammatory injury in LPS-treated
cells by regulating NF-κB/STAT3 signaling. It is hoped that these
findings may facilitate the development of novel ALI treatment
methods.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and WZ contributed to the experimental design,
conducting experiments and data analysis. JC contributed to the
writing of the manuscript. JC and WZ approved the final version of
the manuscript and confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swenson KE and Swenson ER: Pathophysiology
of acute respiratory distress syndrome and COVID-19 lung injury.
Crit Care Clin. 37:749–776. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhou Y, Li P, Goodwin AJ, Cook JA,
Halushka PV, Chang E, Zingarelli B and Fan H: Exosomes from
endothelial progenitor cells improve outcomes of the
lipopolysaccharide-induced acute lung injury. Crit Care.
23(44)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li J, Lu K, Sun F, Tan S, Zhang X, Sheng
W, Hao W, Liu M, Lv W and Han W: Panaxydol attenuates ferroptosis
against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1
pathway. J Transl Med. 19(96)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang YM, Ji R, Chen WW, Huang SW, Zheng
YJ, Yang ZT, Qu HP, Chen H, Mao EQ, Chen Y and Chen EZ: Paclitaxel
alleviated sepsis-induced acute lung injury by activating MUC1 and
suppressing TLR-4/NF-κB pathway. Drug Des Devel Ther. 13:3391–3404.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cheng KT, Xiong S, Ye Z, Hong Z, Di A,
Tsang KM, Gao X, An S, Mittal M, Vogel SM, et al:
Caspase-11-mediated endothelial pyroptosis underlies
endotoxemia-induced lung injury. J Clin Invest. 127:4124–4135.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Tang J, Xu L, Zeng Y and Gong F: Effect of
gut microbiota on LPS-induced acute lung injury by regulating the
TLR4/NF-kB signaling pathway. Int Immunopharmacol.
91(107272)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McVey MJ, Steinberg BE and Goldenberg NM:
Inflammasome activation in acute lung injury. Am J Physiol Lung
Cell Mol Physiol. 320:L165–L178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gouda MM and Bhandary YP: Acute lung
injury: IL-17A-mediated inflammatory pathway and its regulation by
curcumin. Inflammation. 42:1160–1169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen C, He Y, Feng Y, Hong W, Luo G and Ye
Z: . Long non-coding RNA review and implications in acute lung
inflammation. Life Sci. 269(119044)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen T, Wei Y, Zhu G, Zhao H and Zhang X:
Design, synthesis and structure-activity relationship studies of
4-indole-2-arylaminopyrimidine derivatives as anti-inflammatory
agents for acute lung injury. Eur J Med Chem.
225(113766)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Song W, Yang X, Wang W, Wang Z, Wu J and
Huang F: Sinomenine ameliorates septic acute lung injury in mice by
modulating gut homeostasis via aryl hydrocarbon receptor/Nrf2
pathway. Eur J Pharmacol. 912(174581)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brochard L, Slutsky A and Pesenti A:
Mechanical ventilation to minimize progression of lung injury in
acute respiratory failure. Am J Respir Crit Care Med. 195:438–442.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Beitler JR, Malhotra A and Thompson BT:
Ventilator-induced lung injury. Clin Chest Med. 37:633–646.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cappoli N, Aceto P, Tabolacci E, Mezzogori
D, Sollazzi L, Navarra P and Dello Russo C: Effects of remifentanil
on human C20 microglial pro-inflammatory activation. Eur Rev Med
Pharmacol Sci. 25:5268–5274. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nowoczyn M, Marie N, Coulbault L, Hervault
M, Davis A, Hanouz JL and Allouche S: Remifentanil produces
cross-desensitization and tolerance with morphine on the mu-opioid
receptor. Neuropharmacology. 73:368–379. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Azzam AAH, McDonald J and Lambert DG: Hot
topics in opioid pharmacology: Mixed and biased opioids. Br J
Anaesth. 122:e136–e145. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grillot N, Garot M, Lasocki S, Huet O,
Bouzat P, Le Moal C, Oudot M, Chatel-Josse N, El Amine Y, Danguy
des Déserts M, et al: Assessment of remifentanil for rapid sequence
induction and intubation in patients at risk of pulmonary
aspiration of gastric contents compared to rapid-onset paralytic
agents: Study protocol for a non-inferiority simple blind
randomized controlled trial (the REMICRUSH study). Trials.
22(237)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kato M, Satoh D, Okada Y, Sugiyama K, Toda
N and Kurosawa S: Pharmacodynamics and pharmacokinetics of
remifentanil: Overview and comparison with other opioids. Masui.
56:1281–1286. 2007.PubMed/NCBI(In Japanese).
|
|
19
|
Bevans T, Deering-Rice C, Stockmann C,
Light A, Reilly C and Sakata DJ: Inhaled remifentanil in rodents.
Anesth Analg. 122:1831–1838. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Grape S, Kirkham KR, Frauenknecht J and
Albrecht E: Intra-operative analgesia with remifentanil vs
dexmedetomidine: A systematic review and meta-analysis with trial
sequential analysis. Anaesthesia. 74:793–800. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ni XQ and Hu ZY: Remifentanil improves
myocardial ischemia-reperfusion injury in rats through inhibiting
IL-18 signaling pathway. Eur Rev Med Pharmacol Sci. 24:3915–3922.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cao D, Liu S, Yang M, Xie K, Zheng Z, Wen
H and Xie X: Remifentanil preconditioning alleviates myocardial
ischemia/reperfusion injury in rats via activating Jagged-1/Notch
signaling pathway. Biosci Rep: BSR20210534, 2021 (Epub ahead of
print).
|
|
23
|
Zhang JN, Ma Y, Wei XY, Liu KY, Wang H,
Han H, Cui Y, Zhang MX and Qin WD: Remifentanil protects against
lipopolysaccharide-induced inflammation through PARP-1/NF-κB
signaling pathway. Mediators Inflamm. 2019(3013716)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Seo KH, Choi JW, Jung HS, Yoo H and Joo
JD: The effects of remifentanil on expression of high mobility
group box 1 in septic rats. J Korean Med Sci. 32:542–551.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tall AR and Yvan-Charvet L: Cholesterol,
inflammation and innate immunity. Nat Rev Immunol. 15:104–116.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Chung FT, Huang HY, Lo CY, Huang YC, Lin
CW, He CC, He JR, Sheng TF and Wang CH: Increased ratio of matrix
metalloproteinase-9 (MMP-9)/tissue inhibitor metalloproteinase-1
from alveolar macrophages in chronic asthma with a fast decline in
FEV(1) at 5-year follow-up. J Clin Med. 8(1451)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y, Liu H, Chen Z, Yu M, Li J, Dong
H, Li N, Ding X, Ge Y, Liu C, et al: TLR4-mediated hippocampal
MMP/TIMP imbalance contributes to the aggravation of perioperative
neurocognitive disorder in db/db mice. Neurochem Int.
140(104818)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang C, Zhu X, Hua Y, Zhao Q, Wang K,
Zhen L, Wang G, Lü J, Luo A, Cho WC, et al: YY1 mediates
TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial
cells. Respir Res. 20(249)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Foster KA, Oster CG, Mayer MM, Avery ML
and Audus KL: Characterization of the A549 cell line as a type II
pulmonary epithelial cell model for drug metabolism. Exp Cell Res.
243:359–366. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lei S, Zhang Y, Su W, Zhou L, Xu J and Xia
ZY: Remifentanil attenuates lipopolysaccharide-induced oxidative
injury by downregulating PKCβ2 activation and inhibiting autophagy
in H9C2 cardiomyocytes. Life Sci. 213:109–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu B, Wang H and Chen Z: Puerarin inhibits
ferroptosis and inflammation of lung injury caused by sepsis in LPS
induced lung epithelial cells. Front Pediatr.
9(706327)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang M, Xue Y, Chen H, Meng L, Chen B,
Gong H, Zhao Y and Qi R: Resveratrol inhibits MMP3 and MMP9
expression and secretion by suppressing TLR4/NF-κB/STAT3 activation
in Ox-LDL-treated HUVECs. Oxid Med Cell Longev.
2019(9013169)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abedi F, Hayes AW, Reiter R and Karimi G:
Acute lung injury: The therapeutic role of Rho kinase inhibitors.
Pharmacol Res. 155(104736)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
White CW, Rancourt RC and Veress LA:
Sulfur mustard inhalation: Mechanisms of injury, alteration of
coagulation, and fibrinolytic therapy. Ann N Y Acad Sci.
1378:87–95. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yu Y, Jiang P, Sun P, Su N and Lin F:
Pulmonary coagulation and fibrinolysis abnormalities that favor
fibrin deposition in the lungs of mouse antibody-mediated
transfusion-related acute lung injury. Mol Med Rep.
24(601)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gouda MM, Shaikh SB and Bhandary YP:
Inflammatory and fibrinolytic system in acute respiratory distress
syndrome. Lung. 196:609–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang HX, Liu SJ, Tang XL, Duan GL, Ni X,
Zhu XY, Liu YJ and Wang CN: H2S attenuates LPS-induced acute lung
injury by reducing oxidative/nitrative stress and inflammation.
Cell Physiol Biochem. 40:1603–1612. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gao M, Yu T, Liu D, Shi Y, Yang P, Zhang
J, Wang J, Liu Y and Zhang X: Sepsis plasma-derived exosomal
miR-1-3p induces endothelial cell dysfunction by targeting SERP1.
Clin Sci (Lond). 135:347–365. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ojima M, Yamamoto N, Hirose T, Hamaguchi
S, Tasaki O, Kojima T, Tomono K, Ogura H and Shimazu T: Serial
change of neutrophil extracellular traps in tracheal aspirate of
patients with acute respiratory distress syndrome: Report of three
cases. J Intensive Care. 8(25)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu Y, Yang Y, Zhang C, Huang F, Wang F,
Yuan J, Wang Z, Li J, Li J, Feng C, et al: Clinical and biochemical
indexes from 2019-nCoV infected patients linked to viral loads and
lung injury. Sci China Life Sci. 63:364–374. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang Y, Chen C, Cui C, Jiao Y, Li P, Zhu
L, Yu W, Xia Q, Wen D and Yang L: Indispensable role of β-arrestin2
in the protection of remifentanil preconditioning against hepatic
ischemic reperfusion injury. Sci Rep. 9(2087)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tao Z, Jie Y, Mingru Z, Changping G, Fan
Y, Haifeng W and Yuelan W: The Elk1/MMP-9 axis regulates E-cadherin
and occludin in ventilator-induced lung injury. Respir Res.
22(233)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liang Y, Yang N, Pan G, Jin B, Wang S and
Ji W: Elevated IL-33 promotes expression of MMP2 and MMP9 via
activating STAT3 in alveolar macrophages during LPS-induced acute
lung injury. Cell Mol Biol Lett. 23(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Corbel M, Boichot E and Lagente V: Role of
gelatinases MMP-2 and MMP-9 in tissue remodeling following acute
lung injury. Braz J Med Biol Res. 33:749–754. 2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen G, Ge D, Zhu B, Shi H and Ma Q:
Upregulation of matrix metalloproteinase 9 (MMP9)/tissue inhibitor
of metalloproteinase 1 (TIMP1) and MMP2/TIMP2 ratios may be
involved in lipopolysaccharide-induced acute lung injury. J Int Med
Res. 48(300060520919592)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhou Q, Song J, Wang Y and Lin T:
Remifentanil attenuates cardiac dysfunction, lipid peroxidation and
immune disorder in rats with isoproterenol-induced myocardial
injury via JNK/NF-KB p65 inhibition. Ann Transl Med.
8(551)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Min Y, Kim MJ, Lee S, Chun E and Lee KY:
Inhibition of TRAF6 ubiquitin-ligase activity by PRDX1 leads to
inhibition of NFKB activation and autophagy activation. Autophagy.
14:1347–1358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bianco C, Thompson L and Mohr I:
Repression of eEF2K transcription by NF-κB tunes translation
elongation to inflammation and dsDNA-sensing. Proc Natl Acad Sci
USA. 116:22583–22590. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yang H, Lv H, Li H, Ci X and Peng L:
Oridonin protects LPS-induced acute lung injury by modulating
Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB
pathways. Cell Commun Signal. 17(62)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao J, Yu H, Liu Y, Gibson SA, Yan Z, Xu
X, Gaggar A, Li PK, Li C, Wei S, et al: Protective effect of
suppressing STAT3 activity in LPS-induced acute lung injury. Am J
Physiol Lung Cell Mol Physiol. 311:L868–L880. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dubový P, Hradilová-Svíženská I, Klusáková
I, Kokošová V, Brázda V and Joukal M: Bilateral activation of STAT3
by phosphorylation at the tyrosine-705 (Y705) and serine-727 (S727)
positions and its nuclear translocation in primary sensory neurons
following unilateral sciatic nerve injury. Histochem Cell Biol.
150:37–47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Peron M, Dinarello A, Meneghetti G,
Martorano L, Betto RM, Facchinello N, Tesoriere A, Tiso N, Martello
G and Argenton F: Y705 and S727 are required for the mitochondrial
import and transcriptional activities of STAT3, and for regulation
of stem cell proliferation. Development.
148(dev199477)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hirano T: IL-6 in inflammation,
autoimmunity and cancer. Int Immunol. 33:127–148. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang Y, Shen Y, Wang S, Shen Q and Zhou X:
The role of STAT3 in leading the crosstalk between human cancers
and the immune system. Cancer Lett. 415:117–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zou S, Tong Q, Liu B, Huang W, Tian Y and
Fu X: Targeting STAT3 in cancer immunotherapy. Mol Cancer.
19(145)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Fan Y, Mao R and Yang J: NF-κB and STAT3
signaling pathways collaboratively link inflammation to cancer.
Protein Cell. 4:176–185. 2013.PubMed/NCBI View Article : Google Scholar
|