Introduction
Sepsis can be secondary to severe trauma, burn,
infection and other clinical acute and critical diseases,
manifested as systemic inflammatory response disorder and host
autoimmune injury and can result in obvious hemodynamic disorder
and multiple organ dysfunction. In the majority of cases, sepsis is
a syndrome of circulatory, immune and metabolic dysfunction
(1-3).
The gut is the first organ to be affected in sepsis. The normal
intestinal mucosal barrier is composed of mechanical, chemical,
biological and immune barriers (4). Among them, the immune barrier serves
a key role in maintaining the normal operation of the intestinal
tract and preventing bacterial endotoxin translocation (4). The impairment of intestinal mucosal
barrier function, especially the immune barrier damage, leads to
immune inflammatory injury and bacterial translocation, which is an
important cause of sepsis (5).
Therefore, early protection of intestinal immune barrier function
is a crucial consideration to prevent and control the occurrence
and development of sepsis.
Rhodiola is a perennial herb, and exhibits
beneficial effects on cognitive function, cardiovascular system and
fatigue (6). Rhodiola
contains more than 40 chemical constituents including alcohols,
polysaccharides, ketones and phenolic compounds and a variety of
amino acids and trace elements, with extremely complicated
pharmacological activities (6).
Salidroside, one of the most effective and active components
extracted from Rhodiola, is a phenylethanol compound
(7). In recent years, a number of
studies have uncovered that salidroside not only has protective
effects on the cardiovascular, cerebrovascular, immune and central
nervous systems, but also possesses a number of pharmacological
effects such as anti-inflammation and inhibiting cell apoptosis
(8-12).
Salidroside may be a potential candidate for the management of lung
inflammation in cecal ligation and puncture (CLP)-induced
endotoxemia and septic shock (13). However, there are few reports about
the protective effect of salidroside on intestinal barrier
dysfunction during sepsis.
T helper 17 (Th17) cells are a type of CD4
+ T cell which are named for their characteristic
secretion of IL-17(14). The IL-17
family consists of six members: IL-17A (IL-17), IL-17B, IL-17C,
IL-17D, IL-17E (IL-25) and IL-17F (15). IL-17 was discovered in 1993 has
been considered as an important inflammatory factor, which has
profound effects on the anti-infection defenses of the body,
especially against extracellular bacteria and fungi (16). By virtue of its inflammatory
properties, IL-17 is closely related to a number of autoimmune
diseases, such as rheumatoid arthritis, multiple sclerosis,
systemic lupus erythematosus, inflammatory bowel disease and
psoriasis (17,18). Following treatment with IL-17
antibody, the inflammatory reaction and bone destruction in a
collagen-induced arthritis mouse model were significantly reduced
(19,20). In addition, targeting IL-17A can
improve the dysmotility of the small intestine during sepsis
(21) and IL-17-producing γδ T
cells are important for the maintenance and protection of
epithelial barriers in the intestinal mucosa (22). These studies illustrate that IL-17
may serve a vital role in the protection against intestinal barrier
dysfunction during sepsis. In addition, IL-17 could activate p38
MAPK, ERK and NF-κB in various cells and diseases, including human
salivary gland cells, human dental pulp fibroblasts, psoriasis and
ischemic heart failure (23-26).
MAPK and NF-κB pathways are related to sepsis-induced cardiac
inflammation and dysfunction (27)
and inhibiting activation of MAPK/NF-κB can safeguard against
lipopolysaccharide-induced sepsis (28), indicating that MAPK and NF-κB
pathways may be involved in the pathogenesis of sepsis. In
addition, it has been noted that salidroside inhibits MAPK and
NF-κB pathways (9,29).
Based on the above understandings, the present study
constructed a sepsis mouse model to investigate the effects of
salidroside and IL-17 on intestinal barrier dysfunction, so as to
explore an approach for gaining clinical benefit.
Materials and methods
Ethics statement
All animal experiments, were performed in Nanfang
Hospital following the guidelines of the China Council on Animal
Care and Use and were permitted by the Committee of Experimental
Animals of Nanfang Hospital (approval no. S201709024). Pain and
discomfort to the animals were minimized.
Animals
A total of 80 SPF grade C57BL/6 mice (6-8 weeks old,
weighing 18-22 g) were purchased from Vital River Laboratory Animal
Technology Co. Ltd. and raised under the SPF barrier system of the
Laboratory Animal Center of the Nanfang Hospital. The light cycle
was 12-h light/12-h dark, and room temperature was maintained at
21-25˚C, with a humidity of 20-30%. The mice had free access to
food and water and were subjected to the experiment ~3 weeks after
acclimation.
Establishment and administration of
the animal model
Salidroside (CAS: 10338-51-9; cat. no. SMB00072;
purity ≥95%) was purchased from MilliporeSigma. In the sham and
salidroside (320 mg/kg) groups, only the mouse abdominal cavity was
opened and the cecum was turned over and then closed layer by layer
without intestinal puncture and ligation. The sepsis mice models
were established using the CLP method. The main steps were: The
mice were fasted 12 h before operation and drank freely; 2%
pentobarbital sodium (cat. no. P-010; Supelco Inc.) was injected
intraperitoneally into mice at 40 mg/kg for abdominal anesthesia; a
1.5 cm longitudinal incision was made in the middle of the mouse
abdomen; the skin and muscle layers were cut layer by layer; the
abdominal cavity was opened to expose the cecum; the cecum was
ligated and punctured; and the intestine was ligated from ileocecum
to 1/3 of cecum end with sterile silk thread (cat. no. BS0117; 4#;
Changzhou Huawei Medical Supplies Co., Ltd.). A 21G sterile needle
was used to penetrate the intestinal tube and a small amount of
contents were gently squeezed out to ensure smooth perforation.
After the abdomen was sutured, the mice in each group were injected
subcutaneously with 5 ml/100 g normal saline for anti-shock
therapy.
Mice in model, model + salidroside (80 mg/kg), model
+ salidroside (160 mg/kg) and model + salidroside (320 mg/kg)
groups were intraperitoneally injected with salidroside dissolved
in saline according to the dosage of 80, 160 and 320 mg/kg
(30,31) within 30 min after the end of
modeling. The corresponding volume of normal saline was injected
into mice in the sham group and salidroside (320 mg/kg) groups.
Experimental grouping
Mice were randomly divided to eight groups, with 10
mice in each group: Sham group (mice were treated according to the
above conditions); salidroside (320 mg/kg) group (mice treated as
sham group were additionally intraperitoneally injected with 320
mg/kg salidroside); model group (septic model group); model +
salidroside (80 mg/kg) group (mice were intraperitoneally injected
with 80 mg/kg salidroside after modeling); model + salidroside (160
mg/kg) group (mice were intraperitoneally injected with 160 mg/kg
salidroside after modeling); model + salidroside (320 mg/kg) group
(mice were intraperitoneally injected with 320 mg/kg salidroside
after modeling); model + anti-IL-17A group [mice were
intraperitoneally injected with recombinant anti-IL-17A (cat. no.
MAB421; R&D Systems, Inc.) at the dose of 5 mg/kg 6 h before
modeling]; and model + salidroside 160 + anti-IL-17A group [mice
were intraperitoneally injected with recombinant anti-IL-17A (cat.
no. MAB421; R&D Systems, Inc.) at the dose of 5 mg/kg 6 h
before modeling and then intraperitoneally injected with 160 mg/kg
salidroside after modeling].
Histopathological examination
The mice were euthanized by cervical dislocation
under 2% pentobarbital (40 mg/kg) anesthesia 24 h after surgery.
The ileum tissues of the mice were collected, fixed with 4%
paraformaldehyde fixative solution for 24 h at 4˚C, dehydrated with
an increasing alcohol gradient (75, 85, 95 and 100%), cleared with
xylene, routinely embedded in paraffin and sliced into serial cross
sections. The 5 µm ileum tissue sections were stained with
hematoxylin (cat. no. H3136; MilliporeSigma) for 10 min followed by
staining with eosin (cat. no. E4009; MilliporeSigma) for 1 min and
toluidine blue (cat. no. 89640; MilliporeSigma) for 10 min at room
temperature to observe pathological and morphological changes.
Determination of fluorescein
isothiocyanate-dextran (FD4) concentration
At 24 h after modeling, the mouse abdominal cavity
was opened and the ileum (the segment with abundant mesenteric
blood vessels) was taken and ligated at both ends. The ileum was
cut from the loose knot and ligation site and washed with PBS. The
free intestinal segment was tied tightly. Then 0.5 ml of FD4
solution (10 mg/ml) was injected to make FD4 enter the blood
circulation through mesonic vessel. After 30 min, heart blood
samples were harvested and placed into centrifuge tube for 10 min
centrifugation at 10,000 x g at 4˚C, based on which the samples
were transferred to a new centrifuge tube, diluted at 1:8 and
inoculated to a 96-well plate (100 µl/well). FD4 concentration in
blood samples was determined by a fluorospectrophotometer (F-7100;
Hitachi, Ltd.).
Enzyme-linked immune sorbent assay
(ELISA)
Ileum tissues were homogenized for ELISA.
Homogenization was performed in ice-cold homogenate buffer,
containing 10 mM HEPES (pH 7.9), 10 mM KCl, 2 mM MgCl2,
0.1 mM EDTA, 1.0 mM dithiothreitol (DTT) and 0.5 mM
phenylmethanesulfonylfluoride (PMSF). The homogenates were
centrifuged at 3,000 x g for 15 min at 4˚C. The supernatants were
subsequently stored at -70˚C before use. Then TNF-α, IL-6, IL-13
and IL-17 levels in ileum tissues were measured using ELISA kits
(TNF-α; cat. no. RAB0477; MilliporeSigma; IL-6 and IL-13; cat. nos.
KMC0061 and KMC2221; Invitrogen; Thermo Fisher Scientific, Inc.;
IL-17; cat. no. 860.070.048; Diaclone Research, SAS) according to
the instructions of the manufacturers. The protein levels of TNF-α,
IL-6, IL-13 and IL-17 were expressed as pg/ml.
Analysis of NF-κB and p38 MAPK
signaling activation
The activation of NF-κB and p38 MAPK signaling was
measured by DNA-binding activity with TransAM Transcription Factor
ELISA kits (Active Motif, Inc.). Nuclear extracts were collected
using a Nuclear Extract kit (Active Motif, Inc.). Proteins were
quantified in line with the BCA method and subjected to an
ELISA-based TransFactor assay.
Western blotting
Radioimmunoprecipitation assay lysis buffer (cat.
no. P0013K, Beyotime, China) was applied to extract the total
protein from ileum tissues. Protein content was quantified
utilizing the BCA assay reagent kit (cat. no. C503021, Sangon,
China). The SDS-PAGE (with 6-10% gels) was used to separate the
30-µg protein samples and the separated protein was transferred to
the PVDF membrane (cat. no. IPFL00010; MilliporeSigma) which was
then blocked in 5% skimmed milk for 2 h at room temperature. The
membrane was subsequently incubated with primary antibodies at 4˚C
overnight. The primary antibodies were as follows: NF-κB p65
(Abcam; Rabbit; 1:1,000 dilution; cat. no. ab207297; 65 kDa),
phosphorylated (p)-NF-κB p65 (CST; Rabbit; 1:1,000 dilution; cat.
no. 3033; 65 kDa), zonula occludens-1 (ZO-1; Abcam; Rabbit; 1:1,000
dilution; cat. no. ab216880; 245 kDa) and claudin-5 (Abcam; Rabbit;
1:1,000 dilution; cat. no. ab13125; 23 kDa), occluding (CST;
Rabbit; 1:1,000 dilution; cat. no. 91131; 65 kDa), p38 MAPK (CST;
Rabbit; 1:1,000 dilution; cat. no. 8690; 40 kDa), p-p38 MAPK (CST;
Rabbit; 1:1,000 dilution; #4511, 43 kDa), Bcl-2 (Abcam; Rabbit,
1:2,000 dilution; cat. no. ab182858; 26 kDa), Bax (Abcam; Rabbit;
1:1,000; cat. no. ab32503; 21 kDa), cleaved-caspase-3 (CST; Rabbit;
1:1,000 dilution; cat. no. 9664; 17 kDa) and GAPDH (Abcam, Mouse,
1:5,000 dilution; cat. no. ab8245; 36 kDa). The membrane was then
washed by tris-buffered saline with 0.05% Tween-20 (TBST) and
cultivated with HRP-conjugated goat polyclonal anti-rabbit
secondary antibody IgG H&L (Abcam; 1:3,000 dilution; cat. no.
ab97051) and HRP-conjugated goat anti-mouse secondary antibody IgG
H&L (Abcam; 1:3,000 dilution; cat. no. ab205719) at 37˚C for 1
h. The membrane was immersed in ECL Luminous liquid (R30199; 100
ml, Pierce; Thermo Fisher Scientific, Inc.) using a GelDoc XR
Biorad (Bio-Rad Laboratories, Inc.); the gray value of each special
band on the image was analyzed by ImageJ software v1.8 (National
Institute of Health).
Statistical analysis
SPSS 20.0 (IBM Corp.) was used for statistical
analysis. The measurement data are expressed as the mean ± standard
deviation. The comparison among groups was accomplished by one-way
analysis of variance and Tukey's post-hoc test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
Salidroside mitigates the injury of
ileum tissues and intestinal permeability induced by a septic model
and reversely regulated ileum tissue cytokines
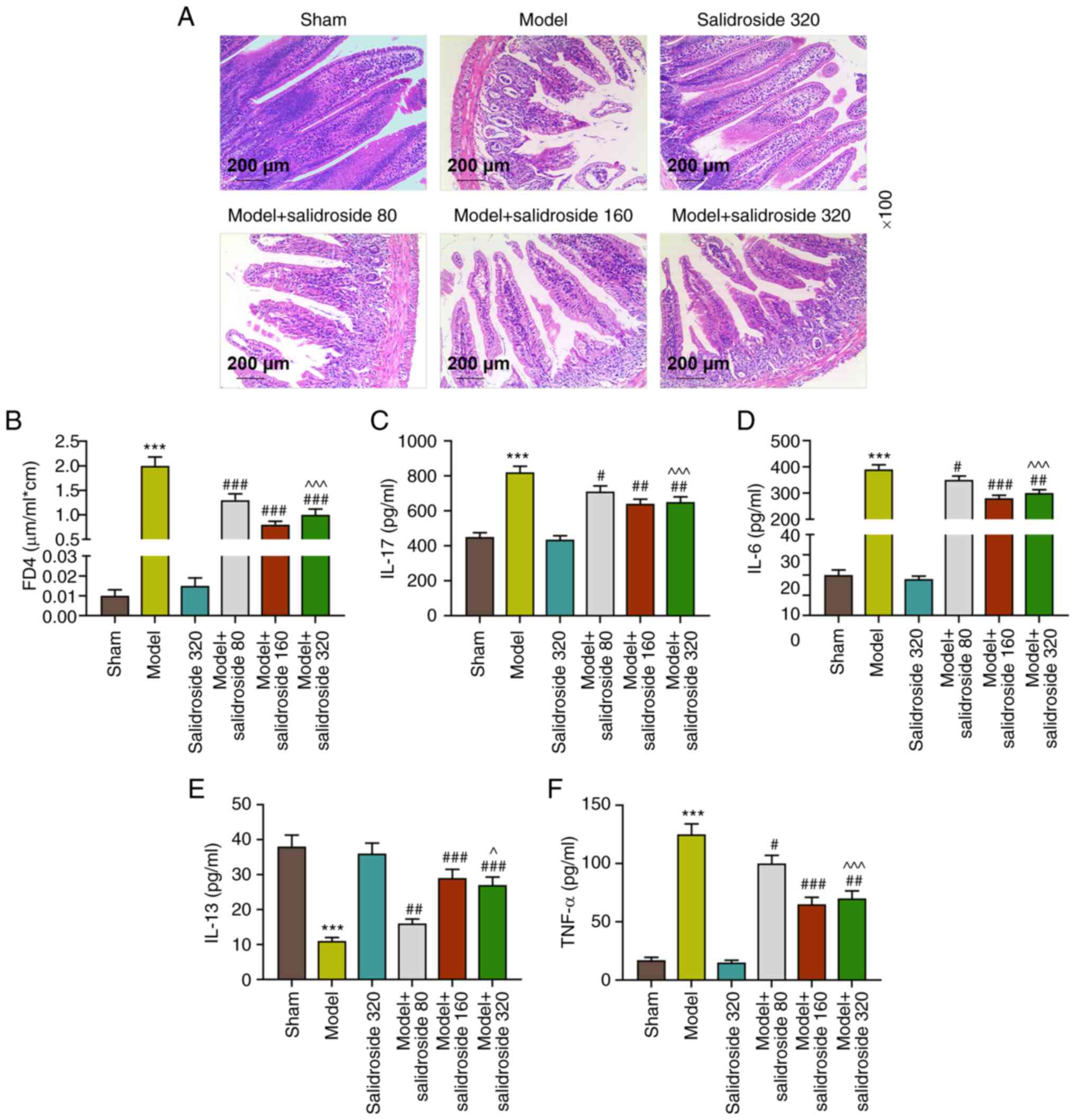
It can be seen from Fig. 1A that in septic model group, the
injury of ileum tissue was the severest, the intestinal villi
became shorter and thicker, the arrangement was disordered, the
epithelial space of intestinal villi was clearly and continuously
broken, the epithelial layer and lamina propria declined and the
capillary congestion and hemorrhage of lamina propria could be
observed. By contrast, the ileum tissue injury was effectively
mitigated in the model + salidroside (80, 160, 320 mg/kg) group,
especially in the model + salidroside (160 mg/kg) group. Fig. 1B shows that FD4 concentration was
significantly higher in septic model group compared with the sham
group (P<0.001; Fig. 1B).
Compared with the model group, FD4 concentration was decreased
evidently in in the model + salidroside (80, 160, 320 mg/kg) group
(P<0.001; Fig. 1B), especially
in the model + salidroside (160 mg/kg) group. The ileum tissue
cytokine levels (Fig. 1C-F)
revealed that the levels of IL-17, IL-6 and TNF-α in septic model
group notably exceeded those in sham group (P<0.001; Fig. 1C, D and F)
and IL-17, IL-6 and TNF-α levels in sepsis group were decreased by
salidroside (80, 160, 320 mg/kg; P<0.01; Fig. 1C, D and F),
especially salidroside (160 mg/kg). The IL-13 level in sham group
was evidently reduced by septic model (P<0.001, 1E). Compared
with the model group, IL-13 level was clearly increased in model +
salidroside (80, 160, 320 mg/kg) group, especially in the model +
salidroside (160 mg/kg) group. These data demonstrated that
salidroside partly reversed the effect of septic model, alleviating
the ileum tissue injury and intestinal permeability and decreasing
the levels of inflammation-related cytokines.
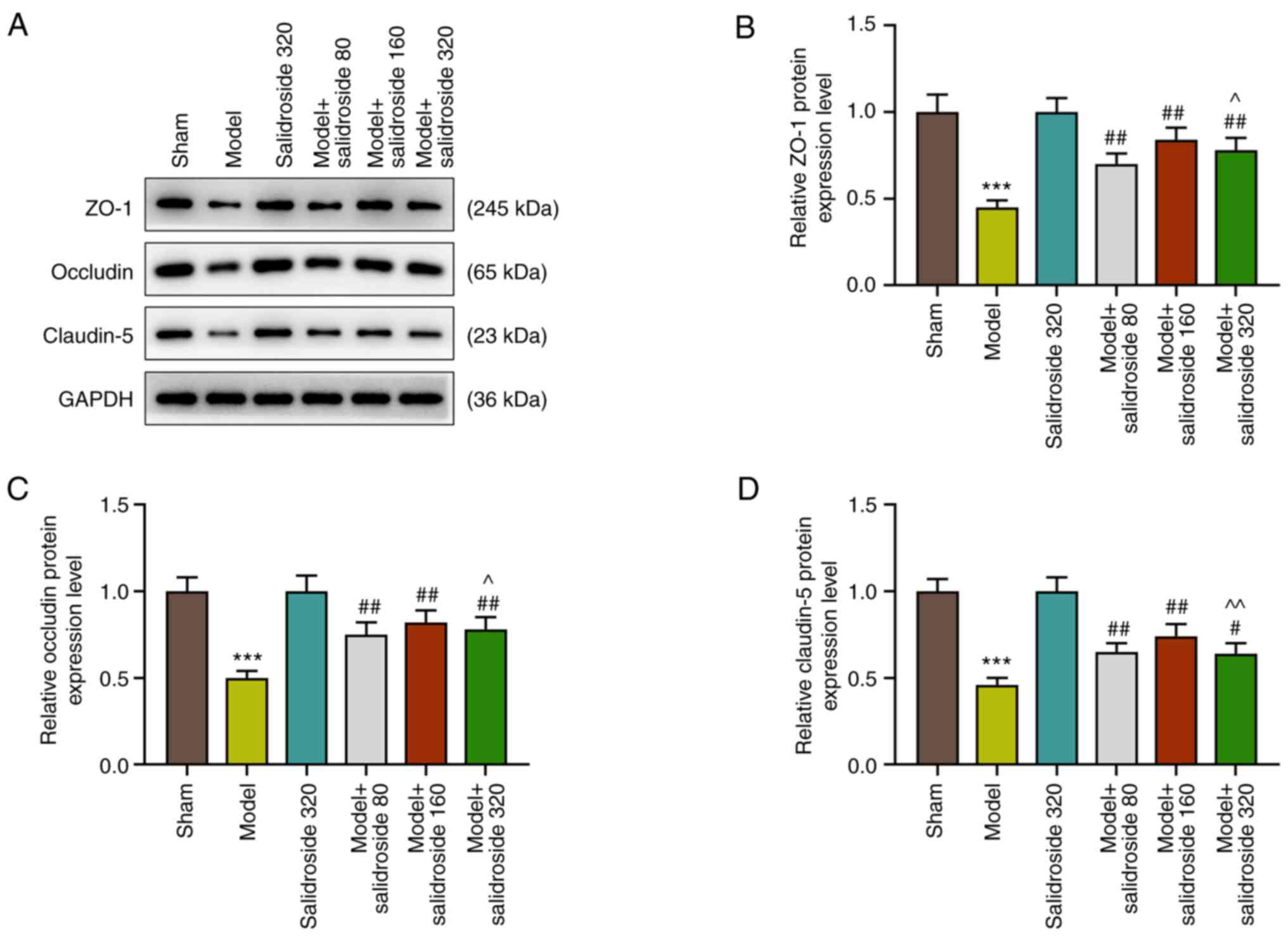
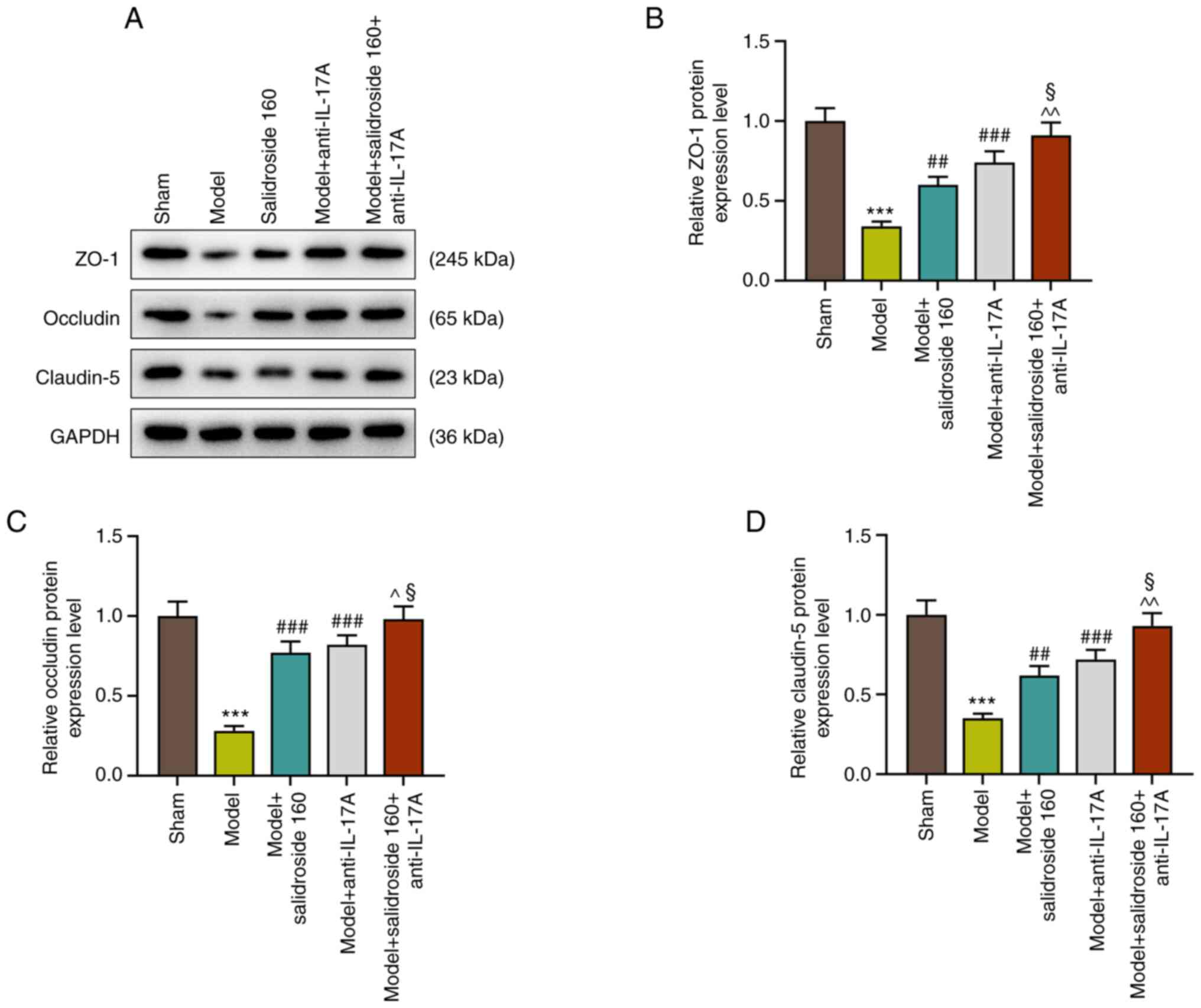
Salidroside attenuates the decreased
in the levels of intestinal tight junction proteins in the septic
model
The intestinal mucosa is a physical and metabolic
barrier, regulated by epithelial junction complexes known as tight
junctions. This barrier function is reflected by the intestinal
permeability. Thus, the levels of intestinal tight junction
proteins: ZO-1, occludin and claudin-5 were further detected in
septic mice. The results showed that the levels of ZO-1, occludin
and claudin-5 were decreased in septic model, which was attenuated
by salidroside (80, 160, 320 mg/kg) (P<0.01; Fig. 2A-D), especially salidroside at 160
mg/kg.
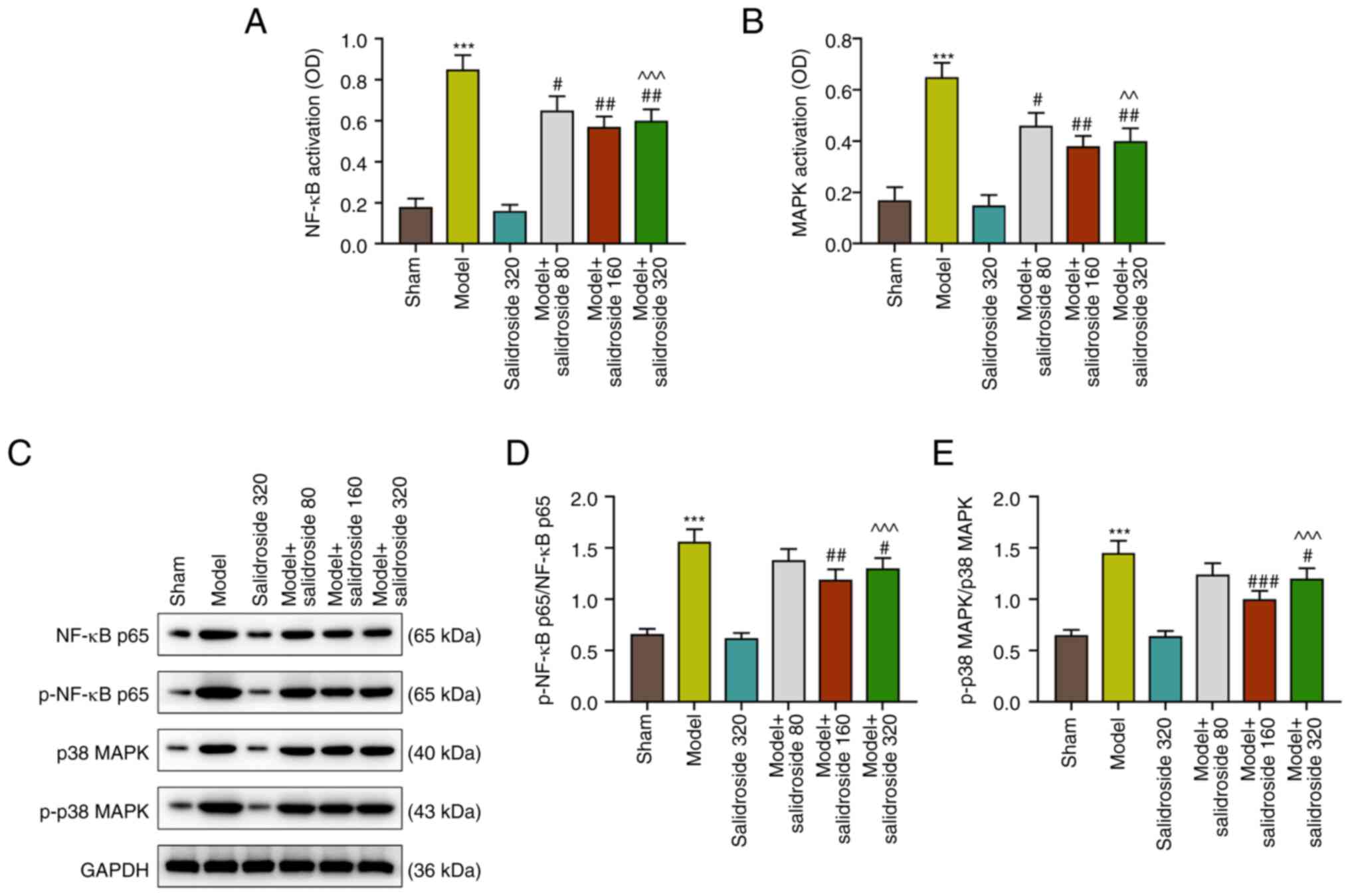
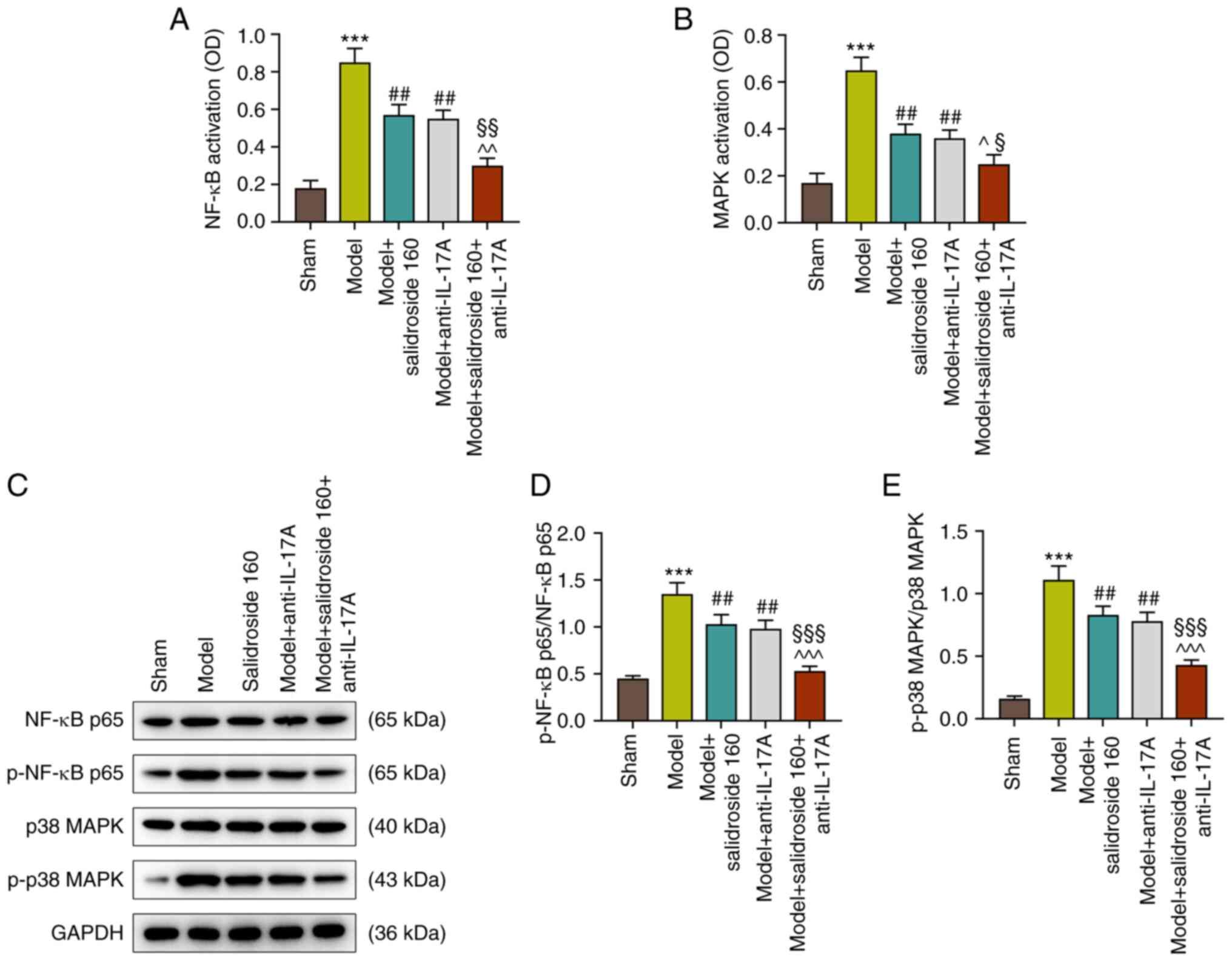
Salidroside suppresses the activation
of NF-κB and p38 MAPK pathways in the septic model
As shown in Fig. 3A
and B, the NF-κB and p38 MAPK
signaling pathways were activated in the septic model (P<0.001;
Fig. 3A and B), which were then inhibited by
salidroside (80, 160, 320 mg/kg) (P<0.01; Fig. 3A and B), especially salidroside at 160 mg/kg.
Fig. 3C to E show that the protein expressions of
p-NF-κB p65/NF-κB p65 and p-p38 MAPK/p38 MAPK were evidently
augmented in septic model mice (P<0.001; Fig. 3C-E). However, the two expressions
were then sharply downregulated by salidroside (80, 160, 320 mg/kg;
P<0.001; Fig. 3C-E), especially
salidroside at 160 mg/kg. The expression levels of Bax and Bcl-2
were also determined, as they are members of the Bcl-2 family
involved in the intrinsic apoptotic pathway. The results showed
that the level of Bcl-2 was decreased, while those of Bax and
cleaved-caspase-3 were elevated in septic model (P<0.001;
Fig. S1A-D); however, salidroside
(80, 160, 320 mg/kg) increased the level of Bcl-2 and decreased the
levels of Bax and cleaved-caspase-3 in septic model (P<0.001;
Fig. S1A-D), especially
salidroside at 160 mg/kg.
Thus, salidroside suppressed activation of NF-κB and
p38 MAPK pathways in the septic model. Considering that salidroside
at 160 mg/kg generated the most effective effect, this dosage was
therefore selected in the following experiments.
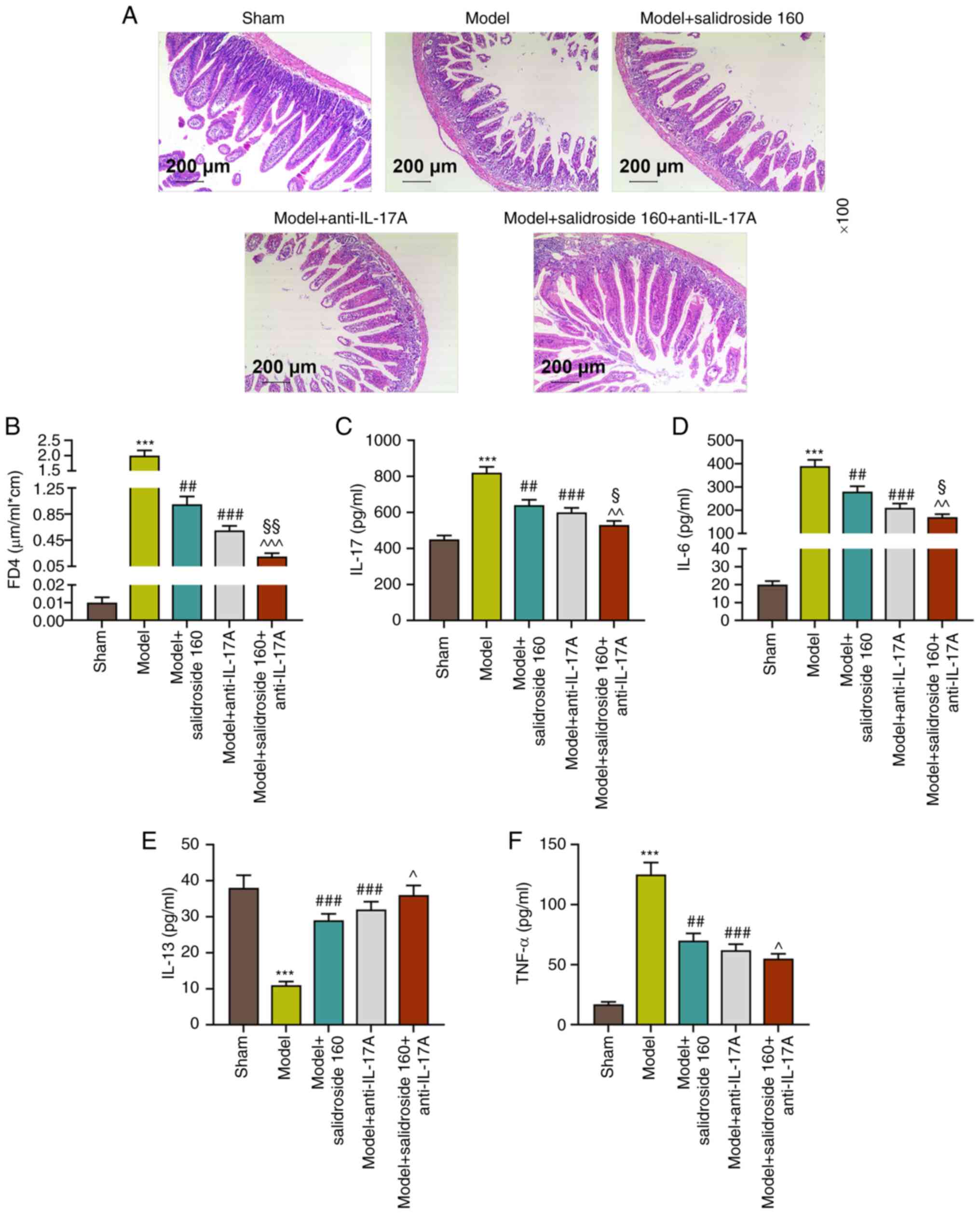
Anti-IL-17A further alleviates the
ileum tissue injury and intestinal permeability and reversely
regulates ileum tissue cytokines in sepsis mice treated with
salidroside
The present study used IL-17A antagonist to verify
the protective mechanism of salidroside in intestinal barrier
dysfunction of sepsis mice. Fig.
4A shows that septic model-induced ileum tissue injury was
mitigated in both the model + anti-IL-17A group and the model +
salidroside (160 mg/kg) group and the mitigative effect was
strengthened in model + salidroside (160 mg/kg) + anti-IL-17A
group, resulting in the ileum tissue in a condition close to that
in the sham group. In addition, FD4 concentration was much lower in
model + anti-IL-17A group compared with the septic model group
(P<0.001; Fig. 4B) and that was
further diminished in model + salidroside (160 mg/kg) + anti-IL-17A
group compared with that in model + anti-IL-17A group (P<0.01;
Fig. 4B). In addition, the ileum
tissue cytokine levels of IL-17, IL-6 and TNF-α that had been
increased in septic model group were decreased in the model +
anti-IL-17A group (P<0.001; Fig.
4C, D and F) and salidroside (160 mg/kg) further
decreased the levels in model + anti-IL-17A group. Anti-IL-17A had
a promoting effect on IL-13 levels. From the above data,
anti-IL-17A could mitigate the ileum tissue injury and intestinal
permeability induced by septic model and reversely modulated ileum
tissue cytokines.
Anti-IL-17A further attenuates the
decreased in the levels of intestinal tight junction proteins in
sepsis mice treated with salidroside
The results showed that anti-IL-17A increased the
levels of ZO-1, occludin and claudin-5 in septic model and further
promoted the effect of salidroside (160 mg/kg) in septic model
(P<0.001; Fig. 5A-D).
Anti-IL-17A further inhibits the
activated NF-κB and p38 MAPK pathways in sepsis mice treated with
salidroside
According to Fig.
4A and B, as with salidroside
(160 mg/kg), anti-IL-17A also suppressed the septic model-induced
NF-κB and p38 MAPK signaling pathways activation (P<0.01;
Fig. 6A and B) and anti-IL-17A combined with
salidroside (160 mg/kg) was more effective than anti-IL-17A alone
(P<0.05; Fig. 6A and B). Similarly, the p-NF-κB p65/NF-κB p65
and p-p38 MAPK/p38 MAPK protein expressions in septic model group
were decreased after the treatment with anti-IL-17A (P<0.01;
Fig. 6C-E) and the effect of
anti-IL-17A was reinforced when combined with salidroside at 160
mg/kg (P<0.001; Fig. 6C-E). As
a consequence, anti-IL-17A could counteract the effect of septic
model, inhibiting the activated NF-κB and p38 MAPK signaling
pathways. Moreover, anti-IL-17A and salidroside (160 mg/kg) in
combination were more effective.
The results showed that anti-IL-17A elevated the
level of Bcl-2 and decreased the levels of Bax and
cleaved-caspase-3 in septic model (P<0.001; Fig. S2A-D). Moreover, anti-IL-17A in
combination with salidroside further elevated the level of Bcl-2
and decreased the levels of Bax and cleaved-caspase-3 in septic
model (P<0.001, Fig. S2A-D).
Finally, the salidroside-associated mechanisms of action against
the sepsis model to mitigate the inflammation is shown in Fig. S3.
Discussion
Sepsis is a systemic inflammatory response syndrome,
mainly caused by infection or suspected infectious factors
(32). The intestinal mucosal
immune barrier is composed of intestinal-associated lymphoid tissue
and diffused immune cells, which serve an important role in
protecting intestinal function and regulating the occurrence and
development of sepsis (33).
Salidroside, a type of phenylethanol compound extracted from
Rhodiola, has been proved to be able to rescue mice from
experimental sepsis induced by CLP through anti-inflammatory and
anti-apoptosis effects (34). In
addition, salidroside alleviates myocarditis induced by sepsis in
rats through regulation of IGF-1/PI3K/Akt/GSK-3β signaling
(35).
The present study established a sepsis mouse model
to identify the effect of salidroside on intestinal mucosal barrier
in mice. The results showed that after the treatment with
salidroside, intestinal villi presented normal basic morphology and
were arranged regularly, the epithelial space was obviously
dilated, capillary congestion was observed and the lamina propria
was still intact. Adherens junctions and intestinal tight junction
proteins, made up of occludin, claudin and ZO-1, serve a crucial
role for protecting the intestinal mucosal barrier (36-38).
This barrier function is reflected by the intestinal permeability.
The intestinal permeability was decreased while the expressions of
intestinal tight junction proteins were increased by the
administration of salidroside. Thus, salidroside could prevent the
increase in mucosal permeability and increase the expressions of
tight junctional proteins, inhibiting intestinal damage and
protecting the intestinal mucosal barrier during sepsis.
The main pathophysiological process of sepsis is
excess activation of inflammatory response caused by pathogen
infection and the secondary immune dysfunction or immunosuppression
(39). Human immune responses to
severe infections are mediated mainly by the primary
proinflammatory cytokine TNF-α and by the secondary proinflammatory
mediators IL-6 and IL-17(40).
TNF-α and IL-6 are both considered as notable elements in the
cytokine network during sepsis (40) and IL-17 is upregulated in both
clinical and experimental sepsis (41). Studies show that IL-6 concentration
in plasma could be a new biomarker for the sepsis diagnosis
(42) and the neutralization of
TNF-α could prolong survival time of patients with moderate/severe
CLP sepsis (43). On the other
hand, the anti-inflammatory cytokine IL-13 neutralizes the
excessive production of proinflammatory cytokines and may induce a
state of immunosuppression in patients with sepsis (44). Previous studies report that
salidroside attenuates the levels of TNF-α and IL-6 in LPS-induced
myocardial injury (45) and the
expression of IL-17 is decreased following salidroside treatment in
heart failure left ventricle (46). In addition, salidroside suppresses
the upregulation of IL-13 in bronchoalveolar lavage fluids and lung
tissues of ovalbumin-induced asthma mice model (47). The present study revealed that the
levels of TNF-α, IL-6 and IL-17 were upregulated and IL-13 level
was downregulated by septic model. Salidroside treatment partially
reversed the effects of septic model, which increased IL-13 level
yet decreased the levels of TNF-α, IL-6 and IL-17, showing
alleviative effects on the septic model-induced inflammation in the
ileum tissues of mice. A previous report indicates that
post-treatment salidroside attenuates CLP-induced increase in the
serum levels of inflammatory factors, including plasma TNF-α and
IL-6, -1β and-10 in septic rats and also significantly attenuate
the activation of NF-κB and increase the release of peroxisome
proliferator-activated receptor γ in the lung tissue (13). Accumulating evidence also reports
the inhibitory effects of natural products on CLP-induced sepsis.
Shenfu decoction may serve a protective role in sepsis by
inhibiting inflammatory response and gut barrier damage in rats
with CLP-induced sepsis (48).
Astragaloside IV protects intestinal epithelium from sepsis-induced
barrier dysfunction through blocking RhoA/NLRP3 inflammasome signal
pathway (49). Sodium tanshinone
IIA sulfonate attenuates cardiac dysfunction and improves survival
of rats with CLP-induced sepsis (50).
It has been reported that glucosamine attenuates
lung injury and inflammation by suppressing the MAPK and NF-κB
activation (51) and maresin 1
alleviates acute kidney injury in septic mice by blocking the
NF-κB/STAT3/MAPK pathways (52).
It is also reported that MAPK/NF-κB serves important roles in the
inflammatory response and apoptosis regulation (53-55).
Increase of Bcl-2 or inhibition of caspases also lead to an
elevated survival in animal models of sepsis (56). Previous studies indicate that
salidroside regulates the MAPK/NF-κB pathway (9,21,57).
IL-17, which has the ability to induce the activation of NF-κB and
MAPK pathways in rheumatoid arthritis synovial fibroblasts and
human colonic myofibroblasts (58,59),
has also been reported to promote the sepsis-induced cardiac
dysfunction via the NF-κB and MAPK pathways (60). Additionally, salidroside protects
mice from CLP-induced sepsis by exerting its anti-inflammatory and
anti-apoptosis effects (34). In
the present study, salidroside also exerted anti-inflammatory and
anti-apoptosis effects on septic mice. The current study
corroborated that septic model-activated MAPK/NF-κB signaling
pathways were suppressed by IL-17 regulated by the treatment of
salidroside.
In brief, the results demonstrated that salidroside
reduced inflammation to protect against intestinal barrier
dysfunction in septic mice by downregulating IL-17, thereby
suppressing the NF-κB and p38 MAPK signaling pathways, which thus
could be a therapeutic option in current therapy of intestinal
barrier dysfunction during sepsis.
Supplementary Material
Salidroside attenuates the regulation
of apoptosis-related proteins in model mice. (A) The levels of
apoptosis-related proteins (B) Bcl-2, (C) Bax and (D)
cleaved-caspase-3 were analyzed by western blotting and GAPDH was
served as the internal reference. The data were presented as the
mean ± standard deviation of three independent experiments;
***P<0.001 vs. sham; ##P<0.01 vs.
model, ###P<0.001; ^^P<0.01;
^^^P<0.001 vs. salidroside (160 mg/kg). p,
phosphorylation.
Anti-IL-17A attenuates apoptosis in
sepsis mice treated with salidroside. (A) The levels of
apoptosis-related proteins (B) Bcl-2, (C) Bax and (D)
cleaved-caspase-3 were analyzed by western blotting and GAPDH was
served as the internal reference. The data were presented as the
mean ± standard deviation of three independent experiments;
***P<0.001 vs. sham; ###P<0.001 vs.
model; ^P<0.05; ^^^P<0.001 vs.
salidroside (160 mg/kg); §P<0.05;
§§P<0.01 vs. model + anti-IL-17A. p,
phosphorylation.
An abstract figure of the salidroside
associated mechanisms of action against the sepsis model to
mitigate the inflammation. CLP, cecal ligation and puncture.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RL made substantial contributions to conception and
design of the present study, drafted the manuscript and critically
revised it for important intellectual content. PZ, JW and KF
performed data acquisition, data analysis and interpretation. RL,
PZ, JW and KF confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments, performed in Nanfang
Hospital following the guidelines of the China Council on Animal
Care and Use, were permitted by the Committee of Experimental
Animals of Nanfang Hospital (approval no. S201709024). The pain and
discomfort to the animals were minimized.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shankar-Hari M, Phillips GS, Levy ML,
Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD and
Singer M: Developing a new definition and assessing new clinical
criteria for septic shock: For the third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:775–787. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Deutschman CS and Tracey KJ: Sepsis:
Current dogma and new perspectives. Immunity. 40:463–475.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hawiger J, Veach RA and Zienkiewicz J: New
paradigms in sepsis: From prevention to protection of failing
microcirculation. J Thromb Haemost. 13:1743–1756. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Adib-Conquy M and Cavaillon JM: Host
inflammatory and anti-inflammatory response during sepsis. Pathol
Biol (Paris). 60:306–313. 2012.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
5
|
de Medina FS, Romero-Calvo I, Mascaraque C
and Martínez-Augustin O: Intestinal inflammation and mucosal
barrier function. Inflamm Bowel Dis. 20:2394–2404. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Panossian A, Wikman G and Sarris J:
Rosenroot (Rhodiola rosea): Traditional use, chemical composition,
pharmacology and clinical efficacy. Phytomedicine. 17:481–493.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xue F, Yang M and Ma L: Microbial
synthesis of salidroside. Sheng Wu Gong Cheng Xue Bao.
35:1184–1192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou F, Ju J, Fang Y, Fan X, Yan S, Wang
Q, Wei P, Duan F, Miao F, Hu Z and Wang M: Salidroside protected
against MPP(+)-induced Parkinson's disease in PC12 cells by
inhibiting inflammation, oxidative stress and cell apoptosis.
Biotechnol Applied Biochem. 66:247–253. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Xu N, Huang F, Jian C, Qin L, Lu F, Wang
Y, Zhang Z and Zhang Q: Neuroprotective effect of salidroside
against central nervous system inflammation-induced cognitive
deficits: A pivotal role of sirtuin 1-dependent Nrf-2/HO-1/NF-κB
pathway. Phytother Res. 33:1438–1447. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Zhong Z, Han J, Zhang J, Xiao Q, Hu J and
Chen L: Pharmacological activities, mechanisms of action, and
safety of salidroside in the central nervous system. Drug Des Devel
Ther. 12:1479–1489. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu R, Wang MQ, Ni SH, Wang M, Liu LY, You
HY, Wu XH, Wang YJ, Lu L and Wei LB: Salidroside ameliorates
endothelial inflammation and oxidative stress by regulating the
AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur J
Pharmacol. 867(172797)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhong ZF, Han J, Zhang JZ, Xiao Q, Chen
JY, Zhang K, Hu J and Chen LD: Neuroprotective effects of
salidroside on cerebral ischemia/reperfusion-induced behavioral
impairment involves the dopaminergic system. Front Pharmacol.
10(1433)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu MW, Su MX, Qin LF, Liu X, Tian ML,
Zhang W and Wang YH: Effect of salidroside on lung injury by
upregulating peroxisome proliferator-activated receptor γ
expression in septic rats. Exp Ther Med. 7:1446–1456.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yasuda K, Takeuchi Y and Hirota K: The
pathogenicity of Th17 cells in autoimmune diseases. Semin
Immunopathol. 41:283–297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gu C, Wu L and Li X: IL-17 family:
Cytokines, receptors and signaling. Cytokine. 64:477–485.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Monin L and Gaffen SL: Interleukin 17
family cytokines: Signaling mechanisms, biological activities, and
therapeutic implications. Cold Spring Harbor perspect Biol.
10(a028522)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuwabara T, Ishikawa F, Kondo M and
Kakiuchi T: The role of IL-17 and related cytokines in inflammatory
autoimmune diseases. Mediators Inflamm.
2017(3908061)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brembilla NC, Senra L and Boehncke WH: The
IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front
Immunol. 9(1682)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lubberts E: IL-17/Th17 targeting: On the
road to prevent chronic destructive arthritis? Cytokine. 41:84–91.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lubberts E, Koenders MI, Oppers-Walgreen
B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA and van den
Berg WB: Treatment with a neutralizing anti-murine interleukin-17
antibody after the onset of collagen-induced arthritis reduces
joint inflammation, cartilage destruction, and bone erosion.
Arthritis Rheum. 50:650–659. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li J, Kong P, Chen C, Tang J, Jin X, Yan J
and Wang Y: Targeting IL-17A improves the dysmotility of the small
intestine and alleviates the injury of the interstitial cells of
cajal during sepsis. Oxid Med Cell Longev.
2019(1475729)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF,
Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan
TK, et al: Interleukin-23-independent IL-17 production regulates
intestinal epithelial permeability. Immunity. 43:727–738.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wei L, Xiong H, Li W, Li B and Cheng Y:
Upregulation of IL-6 expression in human salivary gland cell line
by IL-17 via activation of p38 MAPK, ERK, PI3K/Akt, and NF-κB
pathways. J Oral Pathol Med. 47:847–855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chang SL, Hsiao YW, Tsai YN, Lin SF, Liu
SH, Lin YJ, Lo LW, Chung FP, Chao TF, Hu YF, et al: Interleukin-17
enhances cardiac ventricular remodeling via activating MAPK pathway
in ischemic heart failure. J Mol Cell Cardiol. 122:69–79.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wei L, Liu M, Xiong H and Peng B:
Up-regulation of IL-23 expression in human dental pulp fibroblasts
by IL-17 via activation of the NF-κB and MAPK pathways. Int Endod
J. 51:622–631. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Johansen C, Kragballe K, Westergaard M,
Henningsen J, Kristiansen K and Iversen L: The mitogen-activated
protein kinases p38 and ERK1/2 are increased in lesional psoriatic
skin. Br J Dermatol. 152:37–42. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen H, Wang X, Yan X, Cheng X, He X and
Zheng W: LncRNA MALAT1 regulates sepsis-induced cardiac
inflammation and dysfunction via interaction with miR-125b and p38
MAPK/NFκB. Int Immunopharmacol. 55:69–76. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cai X, Chen Y, Xie X, Yao D, Ding C and
Chen M: Astaxanthin prevents against lipopolysaccharide-induced
acute lung injury and sepsis via inhibiting activation of
MAPK/NF-κB. Am J Transl Res. 11:1884–1894. 2019.PubMed/NCBI
|
|
29
|
Lan KC, Chao SC, Wu HY, Chiang CL, Wang
CC, Liu SH and Weng TI: Salidroside ameliorates sepsis-induced
acute lung injury and mortality via downregulating NF-κB and HMGB1
pathways through the upregulation of SIRT1. Sci Rep.
7(12026)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang XR, Fu XJ, Zhu DS, Zhang CZ, Hou S,
Li M and Yang XH: Salidroside-regulated lipid metabolism with
down-regulation of miR-370 in type 2 diabetic mice. Eur J
Pharmacol. 779:46–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang ZR, Wang HF, Zuo TC, Guan LL and Dai
N: Salidroside alleviates oxidative stress in the liver with
non-alcoholic steatohepatitis in rats. BMC Pharmacol Toxicol.
17(16)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Uhle F, Lichtenstern C, Brenner T and
Weigand MA: Pathophysiology of sepsis. Anasthesiol Intensivmed
Notfallmed Schmerzther. 50:114–122. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hu Q, Ren H, Li G, Wang D, Zhou Q, Wu J,
Zheng J, Huang J, Slade DA, Wu X and Ren J: STING-mediated
intestinal barrier dysfunction contributes to lethal sepsis.
EBioMedicine. 41:497–508. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu S, Yu X, Hu B, Zou Y, Li J, Bo L and
Deng X: Salidroside rescued mice from experimental sepsis through
anti-inflammatory and anti-apoptosis effects. J Surg Res.
195:277–283. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He H, Chang X, Gao J, Zhu L, Miao M and
Yan T: Salidroside mitigates sepsis-induced myocarditis in rats by
regulating IGF-1/PI3K/Akt/GSK-3β signaling. Inflammation.
38:2178–2184. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu F, Liu J, Liu Y, Zhang Y and Ding X:
Shen-Fu Decoction could ameliorate intestinal permeability by
regulating the intestinal expression of tight junction proteins and
p-VASP in septic rats. J Ethnopharmacol. 268(113562)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang L, Cui YL, Zhang Z, Lin ZF and Chen
DC: Rhubarb monomers protect intestinal mucosal barrier in sepsis
via junction proteins. Chin Med J (Engl). 130:1218–1225.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Y, Guo R, Zhang M, Chen P, Li J and Sun
Y: Protective effect of emodin on intestinal epithelial tight
junction barrier integrity in rats with sepsis induced by cecal
ligation and puncture. Exp Ther Med. 19:3521–3530. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kumar V and Sharma A: Innate immunity in
sepsis pathogenesis and its modulation: New immunomodulatory
targets revealed. J Chemother. 20:672–683. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cao YZ, Tu YY, Chen X, Wang BL, Zhong YX
and Liu MH: Protective effect of Ulinastatin against murine models
of sepsis: Inhibition of TNF-α and IL-6 and augmentation of IL-10
and IL-13. Exp Toxicol Pathol. 64:543–547. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lv R, Zhao J, Lei M, Xiao D, Yu Y and Xie
J: IL-33 attenuates sepsis by inhibiting IL-17 receptor signaling
through upregulation of SOCS3. Cell Physiol Biochem. 42:1961–1972.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Franco DM, Arevalo-Rodriguez I, Roqué IFM,
Oleas NG, Nuvials X and Zamora J: Plasma interleukin-6
concentration for the diagnosis of sepsis in critically ill adults.
Cochrane Database Syst Rev. 4(CD011811)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Newham P, Ross D, Ceuppens P, Das S, Yates
JW, Betts C, Reens J, Randall KJ, Knight R and McKay JS:
Determination of the safety and efficacy of therapeutic
neutralization of tumor necrosis factor-α (TNF-α) using AZD9773, an
anti-TNF-α immune Fab, in murine CLP sepsis. Inflamm Res.
63:149–160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Collighan N, Giannoudis PV, Kourgeraki O,
Perry SL, Guillou PJ and Bellamy MC: Interleukin 13 and
inflammatory markers in human sepsis. Br J Surg. 91:762–768.
2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen L, Liu P, Feng X and Ma C:
Salidroside suppressing LPS-induced myocardial injury by inhibiting
ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol
Med. 21:3178–3189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hsiao YW, Tsai YN, Huang YT, Liu SH, Lin
YJ, Lo LW, Hu YF, Chung FP, Lin SF, Chang SL, et al: Rhodiola
crenulata reduces ventricular arrhythmia through mitigating the
activation of IL-17 and inhibiting the MAPK signaling pathway.
Cardiovasc Drugs Ther. 35:889–900. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yan GH and Choi YH: Salidroside attenuates
allergic airway inflammation through negative regulation of nuclear
factor-kappa B and p38 mitogen-activated protein kinase. J
Pharmacol Sci. 126:126–135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu J, Liu F, Liang T, Cao P, Li J, Zhang
Y and Liu Y: Efficacy of Shenfu decoction on sepsis in rats with
condition induced by cecal ligation and puncture. J Tradit Chin
Med. 40:621–628. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xie S, Yang T, Wang Z, Li M, Ding L, Hu X
and Geng L: Astragaloside IV attenuates sepsis-induced intestinal
barrier dysfunction via suppressing RhoA/NLRP3 inflammasome
signaling. Int Immunopharmacol. 78(106066)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Meng ZJ, Wang C, Meng LT, Bao BH, Wu JH
and Hu YQ: Sodium tanshinone IIA sulfonate attenuates cardiac
dysfunction and improves survival of rats with cecal ligation and
puncture-induced sepsis. Chin J Nat Med. 16:846–855.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hwang JS, Kim KH, Park J, Kim SM, Cho H,
Lee Y and Han IO: Glucosamine improves survival in a mouse model of
sepsis and attenuates sepsis-induced lung injury and inflammation.
J Biol Chem. 294:608–622. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sun S, Wang J, Wang J, Wang F, Yao S and
Xia H: Maresin 1 mitigates sepsis-associated acute kidney injury in
mice via inhibition of the NF-κB/STAT3/MAPK pathways. Front
Pharmacol. 10(1323)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chang NC, Yeh CT, Lin YK, Hu P, Kuo KT,
Fong IH, Kounis NG, Hu P and Hung MY: Garcinol attenuates
lipoprotein(a)-induced oxidative stress and inflammatory cytokine
production in ventricular cardiomyocyte through α7-nicotinic
acetylcholine receptor-mediated inhibition of the p38 MAPK and
NF-κB signaling pathways. Antioxidants (Basel).
10(461)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chen Q, Lu H, Duan C, Zhu X, Zhang Y, Li M
and Zhang D: PDCD4 simultaneously promotes microglia activation via
PDCD4-MAPK-NF-κB positive loop and facilitates neuron apoptosis
during neuroinflammation. Inflammation. 45:234–252. 2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jarisarapurin W, Kunchana K,
Chularojmontri L and Wattanapitayakul SK: Unripe carica papaya
protects methylglyoxal-invoked endothelial cell inflammation and
apoptosis via the suppression of oxidative stress and
Akt/MAPK/NF-κB signals. Antioxidants (Basel).
10(1158)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Weber SU, Schewe JC, Putensen C, Stüber F
and Schröder S: Apoptosis as a pathomechanism in sepsis.
Anaesthesist. 53:59–65. 2004.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
57
|
Li R, Guo Y, Zhang Y, Zhang X, Zhu L and
Yan T: Salidroside ameliorates renal interstitial fibrosis by
inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int J Mol
Sci. 20(1103)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hata K, Andoh A, Shimada M, Fujino S,
Bamba S, Araki Y, Okuno T, Fujiyama Y and Bamba T: IL-17 stimulates
inflammatory responses via NF-kappaB and MAP kinase pathways in
human colonic myofibroblasts. Am J Physiol Gastrointestinal Liver
Physiol. 282:G1035–G1044. 2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Hwang SY, Kim JY, Kim KW, Park MK, Moon Y,
Kim WU and Kim HY: IL-17 induces production of IL-6 and IL-8 in
rheumatoid arthritis synovial fibroblasts via NF-kappaB- and
PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 6:R120–R128.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
60
|
He S, Zhao J, Xu X, Cui X, Wang N, Han X,
Guo Y and Liu Q: Uncovering the molecular mechanism of the
Qiang-Xin 1 formula on sepsis-induced cardiac dysfunction based on
systems pharmacology. Oxid Med Cell Longev.
2020(3815185)2020.PubMed/NCBI View Article : Google Scholar
|