Introduction
Head and neck squamous carcinoma (HNSCC), including
oral cavity, lip, laryngeal, nasopharyngeal, oropharyngeal,
hypopharyngeal and salivary glands cancer, is known to be the sixth
most common malignancy in the world (1,2).
More than 931,000 individuals were diagnosed with HNSCC and
>467,000 patients died from HNSCC worldwide in 2020(1). The overall 5-year survival rate was
65.9% in 2002-2006(3) and 67.2% in
a retrospective study (4). Tumour
metastasis is one of the leading causes of death in HNSCC, with
~10% of patients diagnosed with metastatic HNSCC at initial
clinical presentation and 20-30% of patients developing metastasis
during their disease duration (5).
The prognosis of metastatic HNSCC is poor with a median of 10.1
months for overall survival even with improved systemic therapy
(6).
Patients with metastatic HNSCC typically receive
systemic therapy following surgery. The current systemic treatments
include chemotherapy, targeted therapy and immunotherapy.
Platinum-based therapy is the foundation of HNSCC chemotherapy
where the single-agent platinum therapy is superior to non-platinum
chemotherapies or combination platinum therapies in terms of
survival for chemotherapy (7).
Targeted therapy is an emerging treatment for metastatic HNSCC.
High epidermal growth factor receptor (EGFR) expression is
associated with poor overall survival and disease-free survival
(8). Cetuximab, a monoclonal
antibody blocking EGFR signalling, significantly increases patient
survival when combined with radiotherapy (9). Cetuximab in combination with
platinum-fluorouracil chemotherapy has achieved the longest
survival rates in patients with metastatic HNSCC (6,7).
Programmed cell death protein 1 (PD-1) and programmed cell death
ligand 1 (PD-L1) are immune checkpoint proteins that suppress the
anticancer progress of the immune system (10). Antibodies blocking PD-1 or PD-L1
have been approved for recurrent or metastatic HNSCC (11,12).
The benefits of immunotherapy are low toxicity and satisfactory
efficacy. However, only a small portion of patients respond to the
PD-1 or PD-L1 treatment, ranging from 13.3 to 16.0% for HNSCC in
the US (13).
Our previous study investigated the effect of
prodrug-activating suicide gene (PA-SG) therapy on proliferation of
HNSCC and established a method to quantify the effects of PA-SG
therapy (14). The present study
aimed to investigate the effect of PA-SG therapy on metastatic
HNSCC, especially on cancer cell migration. The HSC-3 cell line was
chosen as the metastatic cell model. This cell line is derived from
tongue cancer with lymph node metastasis (15) and is human papillomavirus
16-negative (16). Inoculation of
HSC-3 in nude mice causes lymph node metastasis (15,17).
In the present study, two well-studied PA-SG therapies were
investigated, which are thymidine kinase (TK) with ganciclovir
(GCV) and cytosine deaminase (CD) with 5-fluorocytosine (5-FC)
(18,19). To the best of our knowledge, the
present study is the first to quantify the anti-migratory and
anti-proliferative effects of PA-SG therapies.
Materials and methods
Selection of metastatic HNSCC cell
line
The present study aimed to investigate PA-SG therapy
on metastatic HNSCC cell lines. Information on seven HNSCC cell
lines was searched in the Cellosaurus (20) for a suitable metastatic HNSCC, such
as HSC-1, HSC-2, HSC-3, HSC-4, Ca9-22, KB (15,21-23)
and SAS (24) HSC-3 is the most
suitable cell line for the present study being one of the first
metastatic HNSCC cells established (Table I) (15). Moreover, the metastasis phenotype
of HSC-3 in nude mice has been supported by an independent study
(17). The other cells were not
suitable for our current study, because they were not HNSCC
(HSC-1), lacked the metastatic mice phenotype (HSC-2, HSC-4 and
SAS) or were contaminated by another cell type (Ca9-22 and KB).
 | Table IInformation on cell lines. |
Table I
Information on cell lines.
| First author/s,
year | Cell line | Disease site | Note | (Refs.) |
|---|
| Kondo and Aso,
1981 | HSC-I | Squamous cell
carcinoma of the skin | Not HNSCC | (21) |
| Momose et
al, 1989 | HSC-2 | Squamous cell
carcinoma of the oral cavity | No metastasis
phenotype in nude mice | (15) |
| Momose et
al, 1989 | HSC-3 | Squamous cell
carcinoma of the oral tongue | Metastasis
phenotype in nude mice | (15,17) |
| Matsui et
al, 1998 | | | | |
| Momose et
al, 1989 | HSC-4 | Squamous cell
carcinoma of the oral tongue | No metastasis
phenotype in nude mice | (15) |
| Horikoshi et
al, 1974 | Ca9-22 | Squamous cell
carcinoma of the oral cavity | Partially
contaminated with MSK-922 | (23) |
| | SAS | Squamous cell
carcinoma of the oral tongue | No metastasis
phenotype in nude mice | (24) |
| Eagle, 1955 | KB | Human
papillomavirus-related endocervical adenocarcinoma | Contaminated with a
HeLa derivative | (22) |
Cell culture
The HSC-3 cell line was purchased from Merck KGaA
(cat. no. SCC193). Cells were cultured in DMEM (cat. no. 10569010;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (cat. no.
26140079; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin antibiotics (cat. no. 15140122l; Thermo
Fisher Scientific, Inc.) at 37˚C with 5% CO2. Cells were
subcultured every 2-3 days by digestion with TrypLE™ (Thermo Fisher
Scientific, Inc.). The subculture ratio is 1:6.
Generation of stable cell lines
Stable cell lines were generated as previously
described (14). Briefly, yeast CD
(pCMVtight-UPRT-T2A-RFP-IRES-CD; cat. no. 126677; Addgene, Inc.)
and HSV type 1 TK (pAL119-TK; cat. no. 21911; Addgene, Inc.)
suicide genes were amplified by PCR and ligated to mVenus in the
pROSA26 vector with human ROSA26 1 kb sequence (AC018506.5; bp
114,245 to 115,244) and promoter and polyA signal for mVenus. The
resulting plasmids are mVenus-CD and mVenus-TK, which serve as
templates. The single guide (sg)RNA targeting sequences were
sgRNA1: 5'-TGTCGAGGTTATTGTAATAA-3' and sgRNA4:
5'-CCGTGGGAAGATAAACTAAT-3'. The sgRNA sequences were synthesized by
Integrated DNA Technologies (Integrated DNA Technologies Pte. Ltd.)
and cloned into pX330 vector according to instruction in the
reference (25). The templates,
sgRNA1, and sgRNA4 were co-transfected into HSC-3 cells to
integrate the suicide genes into the ROSA26 locus. One day before
transfection, 1x105 HSC-3 cells were seeded into one
well of a 12-well plate with 1 ml culture medium and cultured at
37˚C with 5% CO2. On the day of transfection, 1 µg
template encoding mVenus-TK, mVenus-CD or mVenus only with 1 µg
sgRNA plasmid targeting the ROSA26 locus were transfected at 37˚C
overnight into HSC-3 cells using FuGENE HD Transfection Reagent kit
(cat. no. E2311; Promega Corporation). One day after transfection,
the transfected HSC-3 cells were trypsinized and subcultured into a
96-well plate at a density of 1 cell/well. After 8 days of culture
at 37˚C fluorescence-positive cell colonies were collected. The
fluorescent images of HSC-3 mVenus-CD or mVenus-TK cell colonies
were taken under Nikon inverted microscope (Eclipse Ti2-U, Nikon
Instruments Inc.) with longpass GFP filter cube (excitation filter
480/30 nm, dichroic mirror 505 nm, barrier filter 515 nm) with the
same exposure time and objective. The fluorescent intensity of
~20-40 individual cells was measure by ImageJ Software (Version
1.53a, National Institutes of Health). The cell colony with the
highest fluorescent intensity was chosen for the experiments.
Verification of insertion of the
suicide gene in HSC-3 cells
To extract genomic DNA, 1x106 HSC-3 cells
were incubated in 300 µl 0.5 M NaOH for 30 min at 37˚C. After
centrifuging at 10,000 x g at room temperature for 10 min, HSC-3
supernatant was diluted to 1:1,000 in 0.1 M Tris-HCl (pH 8.0) as a
template. The suicide gene inserts at the ROSA36 locus were
amplified by Taq DNA Polymerase (cat. No. 11304011; Thermo Fisher
Scientific, Inc.) using the following primer pair: Forward,
5'-CGGCCGAGACTTCTGGATGG-3' and reverse,
5'-CCCAGCTAAGGAAAAAGGATAAAATGAAAATCAAG-3', which target to ROSA26
locus, The thermocycling conditions used for PCR were as follows:
Initial denaturation at 95˚C for 30 sec followed by 30 cycles at
95˚C for 30 sec, annealing at 60˚C for 15 sec and elongation at
72˚C for 90 sec. The PCR products were resolved on 1% agarose gel
with ethidium bromide.
Prodrug treatment
GCV and 5-FC were purchased from Sigma-Aldrich (cat.
nos. PHR1593 and F7129, respectively). GCV was dissolved in DMSO at
a concentration of 30 mM as a stock solution. 5-FC was dissolved in
water at a concentration of 100 mM. HSC-3 mVenus-TK cells were
treated with GCV and HSC-3 mVenus-CD cells were treated with 5-FC
at 37˚C at indicated concentration for indicated duration. For the
dose-response experiment, HSC-3 cells were treated with two-fold
dilution of GCV or 5-FC ranging from ~0.1 µM to 100 µM for 3 days
at 37˚C. For the time-course response experiment, HSC-3 mVenus-TK
cells were treated with 25 µM GCV and HSC-3 mVenus-CD cells were
treated with 100 µM 5-FC for 0, 24, 48, 72, and 96 h respectively
at 37˚C. For the bystander effect experiment, the cells were
treated with 25 µM GCV or 100 µM 5-FC for 72 h at 37˚C. Before
treating cells, drugs were diluted to the desired concentration
using the culture medium. For the prodrug treatments, the old
culture medium was replaced with medium containing the prodrug.
Cell viability analysis via MTT
assay
MTT was dissolved in PBS at a concentration of 5
mg/ml (cat. no. M2003-1G; Signa-Aldrich; Merck KGaA). A total of
1x104 cells was seeded into each well of a 96-well
plate. Following drug treatment, 10 µl MTT reagent was added to
each well. Following 1 h incubation at 37˚C, 100 µl solubilization
solution (10% SDS in 0.01 M HCl) was added to each well. The plate
was kept at 37˚C overnight. The soluble formazan was measured by
using FlexStation 3 Multi-Mode Microplate Reader (Molecular
Devices, LLC) at 570 nm wavelength. For the dose-response
experiment, MTT assay was performed 3 days after drug treatment.
For the time-course response experiment, MTT assays were performed
every 24 h from 0 to 96 h. For the bystander effect experiment, MTT
assay was carried out after prodrug treatment for 72 h.
Quantitation of bystander effect
Bystander effect is a phenomenon of suicide gene
therapy, in which adjacent cancer cells that do not express suicide
gene are killed by prodrug treatment. Bystander effects were
quantitively measured using a method described previously (14). HSC-3 mVenus-TK or mVenus-CD cells
were mixed with HSC-3 mVenus cells at the ratios of 100, 75, 50, 25
and 0% of suicide gene-positive cells. Then, the cell mixtures were
treated with GCV at 25 µM or with 5-FC at 100 µM for three days.
Next, cell viabilities were measured by MTT assay. The cell
viability was plotted against the percentage of suicide gene
positive cells ratio. The data were fitted to exponential equation:
y=a*e-bx + c,
where y is cell viability and x is percentage of suicide gene
positive cells. In this exponential equation, b is the decay
constant, which represents how fast the cell viability decrease
with increase of percentage of suicide gene positive cell. The
bigger b value means prodrug will kill more cells with the same
ratio of suicide gene positive cells. Thus, a bigger b value
represents a better bystander effect.
Wound healing assay and prodrug
treatment
A total of 6x105 cells was seeded into a
6-well plate with 2 ml culture medium with 10% FBS. Serum
starvation was not performed during wound healing assay because
serum starvation may complex HSC-3 cell migration (26) and would healing assay protocol
without serum starvation is feasible (27). The wound was generated by using 1
ml pipette tip. Detached cells and cell debris were removed by
washing with medium. Wound images were captured immediately with a
Nikon inverted light microscope equipped with a 10x objective
(Nikon Corporation). The healing images were captured after 24 h
culture at 37˚C. Inhibition of cell migration was calculated as the
ratio of the wound area of the healing image and the mean area of
the wound image at three time intervals: 0-24, 24-48, and 48-72 h.
Wound areas were measured using ImageJ Software (Version 1.53a,
National Institutes of Health) with the Wound Healing Tool
(28).
Wound healing was measured at three time periods:
0-24, 24-48 and 48-72 h. For the 0-24 h period experiment, a wound
was generated immediately before prodrug treatment and the healing
images were captured after 24 h prodrug treatment. For the 24-48 h
period experiment, cells were treated with prodrug for 24 h before
wound generation while the healing image was captured after a
further 24 h for a total of 48 h prodrug treatment. For the 48-72 h
experiment, cells were treated with prodrug for 48 h before the
wound strip generation while the healing images were captured after
a further 24 h for a total of 72 h prodrug treatment.
Statistical analysis
GraphPad Prism 9 software (GraphPad Software, Inc.)
was used to perform statistical analysis. One-way analysis of
variance with Tukey's post hoc test was performed for multiple
comparisons. Data are expressed as the mean ± standard deviation or
standard error of the mean from three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Properties of HSC-3 cell lines stably
expressing CD or TK
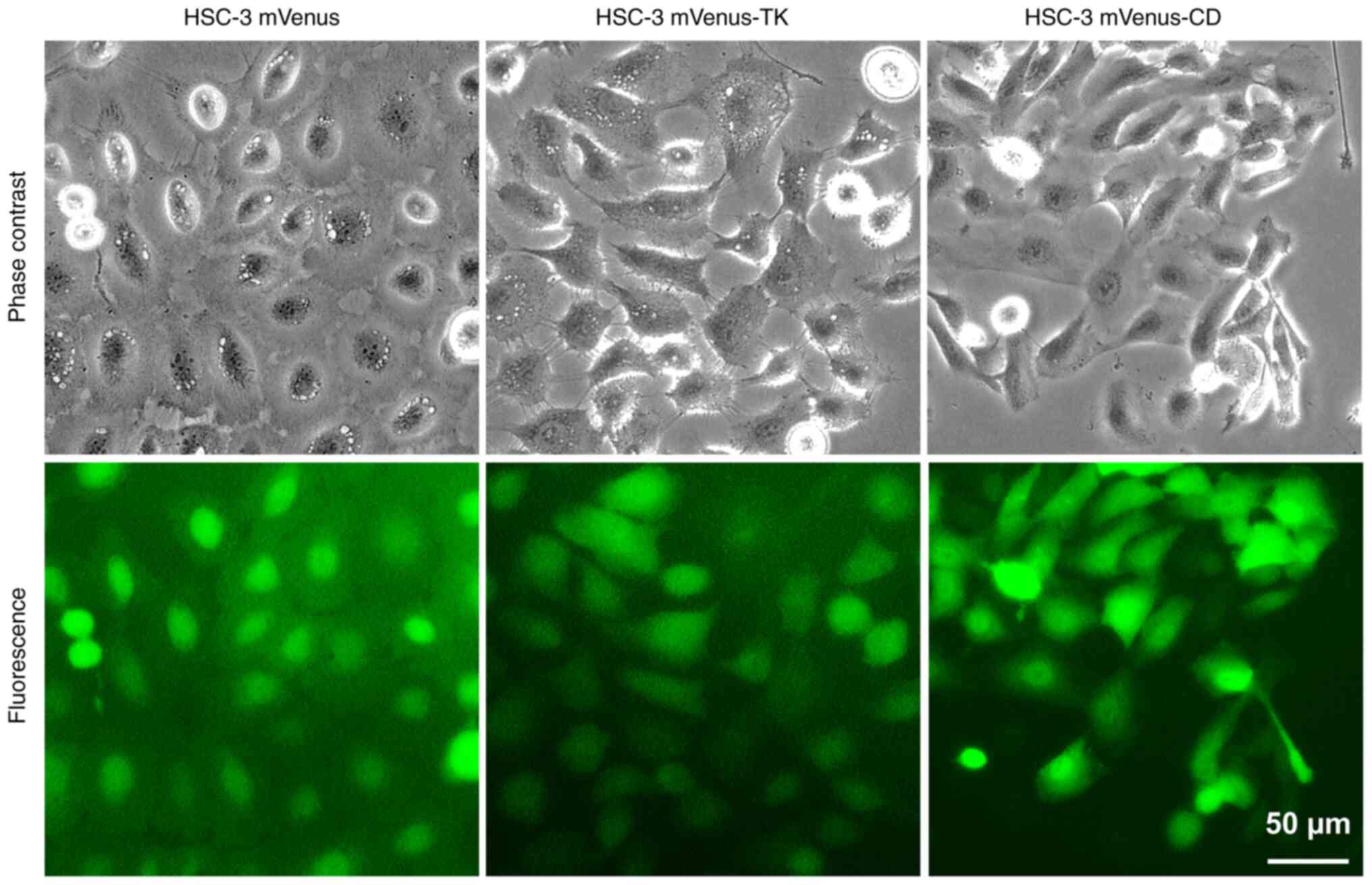
HSC-3 cell lines stably expressing mVenus tagged TK
(mVenus-TK) or CD (mVenus-CD) were generated. The mVenus tag serves
as a fluorescent marker to facilitate the screening of stable cell
lines. A control cell line, which expresses the mVenus tag only,
was generated as a negative control. Most HSC-3 cells were
fluorescence-positive, indicating the three stable cell lines were
homogenous (Fig. 1). To
demonstrate the incorporation of genes into the ROSA26 locus, PCR
was performed to amplify the DNA sequence around the insert site of
the ROSA26 locus from HSC-3 mVenus, mVenus-CD or mVenus-TK cells.
mVenus and suicide genes were successfully inserted into the ROSA26
locus of HSC-3 (Fig. S1A). The
proliferation curve of HSC-3 mVenus and HSC3 mVenus-CD cells were
the same as the wild-type HSC-3 cells. However, HSC-3 mVenus-TK
cells grew more slowly than wild-type HSC-3 cells (Fig. S1). The proliferation rate of other
independent HSC-3 mVenus-TK cells was also measured, which showed a
slower proliferation rate than wild-type HSC-3 cells (data not
shown). This indicated that the expression of mVenus-TK reduces the
HSC-3 cell proliferation rate.
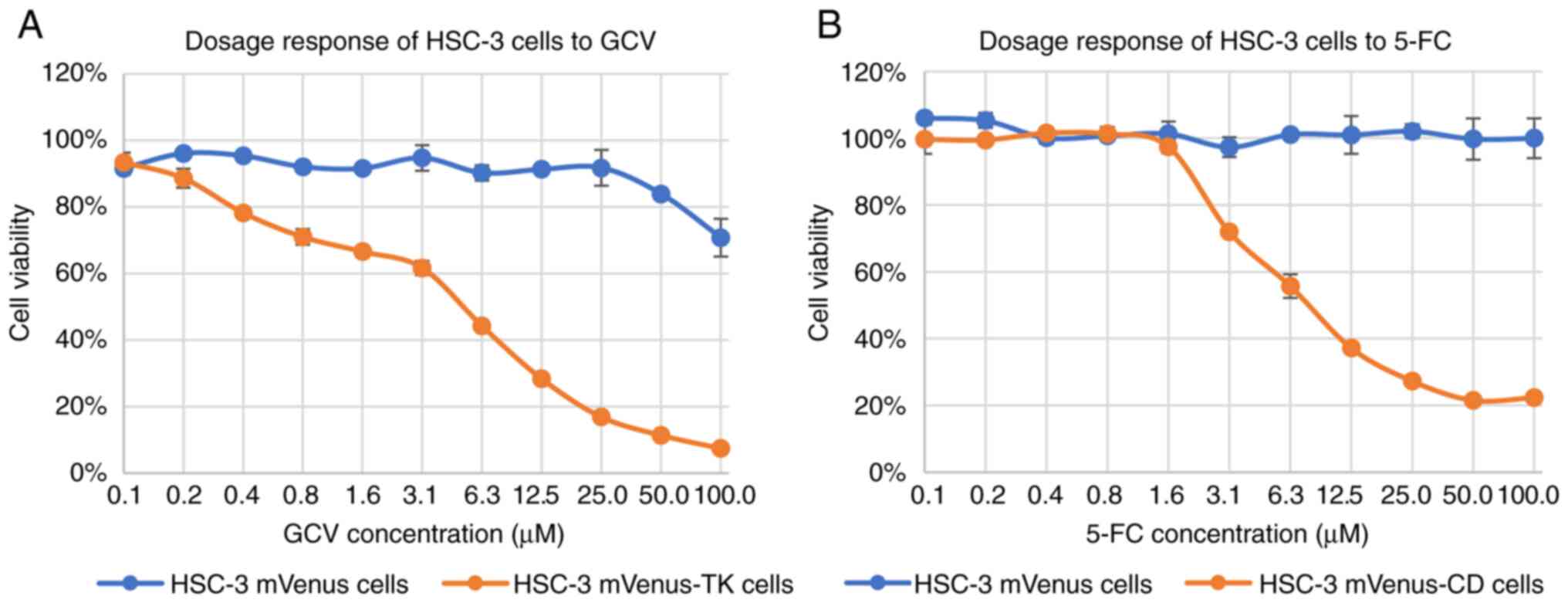
Dose-response of HSC-3 cells to GCV or
5-FC
To evaluate the response of HSC-3 cells to GCV and
5-FC, HSC-3 cells expressing TK or CD were treated with a series of
2-fold dilutions of GCV or 5-FC for three days. The highest
concentration of prodrug was 100 µM.
For HSC-3 mVenus-TK cells, GCV concentrations from
0.2 µM started to inhibit HSC-3 cell viability increase (Fig. 2A). However, high concentrations of
GCV, including 50 and 100 µM GCV, inhibited HSC-3 mVenus cells
viability increase, which did not express TK (Fig. 2A). The highest concentration of GCV
that did not inhibit HSC-3 mVenus viability increase was 25 µM. At
this concentration, GCV inhibited 83% of HSC-3 mVenus-TK cell
viability increase. Cell viability reduction rate increased at GCV
concentration >3.1 µM (Fig.
2A). Consequently, the dose-response curve was divided into two
phases based on the viability reduction rate: i) A slow phase at
GCV <3.1 µM and ii) a quick phase at GCV ≥3.1 µM. The shape of
each phase was sigmoidal, whereas the dose-response curve of HSC-3
mVenus-TK to GCV was double sigmoidal (Fig. 2A).
5-FC started to inhibit the viability increase of
HSC-3 mVenus-CD cells at a concentration of 3.1 µM (Fig. 2B). The inhibitory effect plateaued
at 50 µM 5-FC. At 100 µM, 5-FC caused 78% inhibition of HSC-3
mVenus-CD cells viability increase, whereas 5-FC at this
concentration did not inhibit the viability increase of HSC-3
mVenus cells (Fig. 2B).
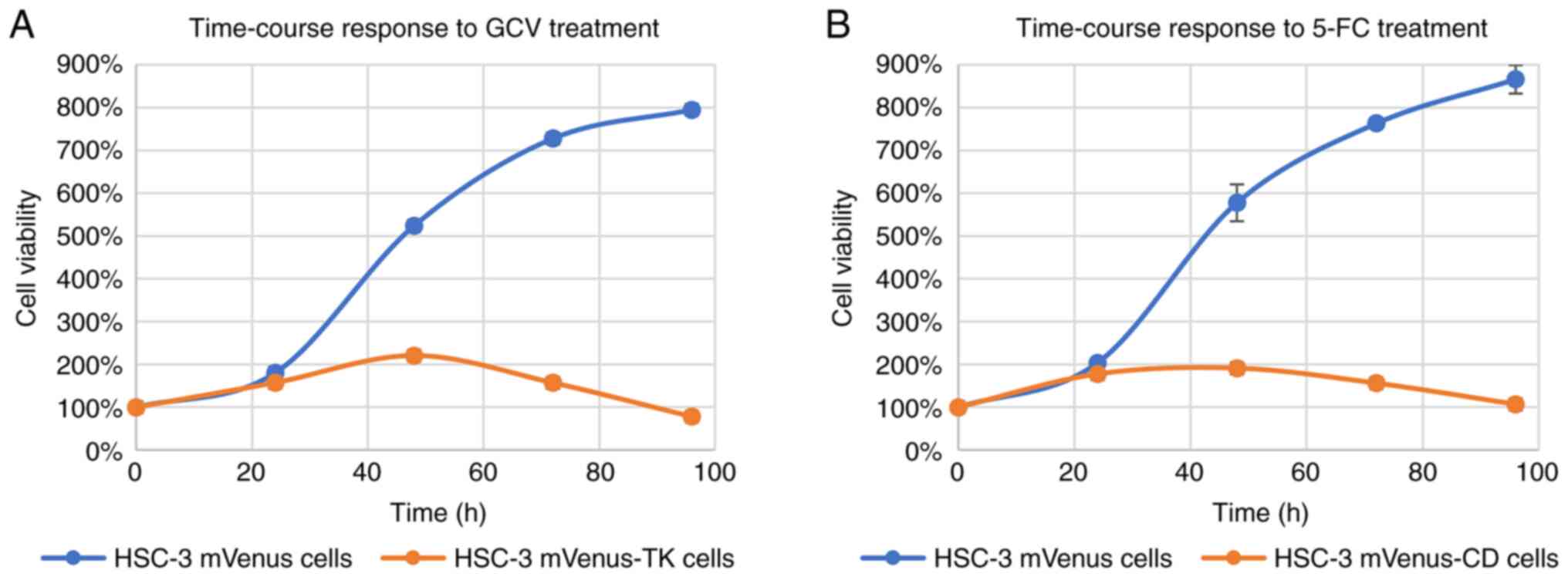
Time-course response of HSC-3 cells to
prodrug treatment
To evaluate time-course response, HSC-3 mVenus-TK
and HSC-3 mVenus-CD cells were treated with GCV at 25 µM and 5-FC
at 100 µM. The cell viability was measured via MTT assay every 24
h.
GCV and 5-FC treatments started to inhibit the
viability increase of HSC-3 mVenus-TK and HSC-3 mVenus-CD cells
after 24 h treatment (Fig. 3A and
B). By contrast, the two prodrugs
did not inhibit HSC-3 mVenus cells viability increase. Compared
with HSC-3 mVenus cells, cell viability of HSC-3 mVenus-TK and
HSC-3 mVenus-CD cells was 78 and 107% after 96 h treatment with GCV
and 5-FC, respectively. By contrast, viability of HSC-3 cells
reached to 795 and 866% respectively. The time-course response
profiles of HSC-3 cells to GCV or 5-FC treatment exhibited a
similar profile (Fig. S2).
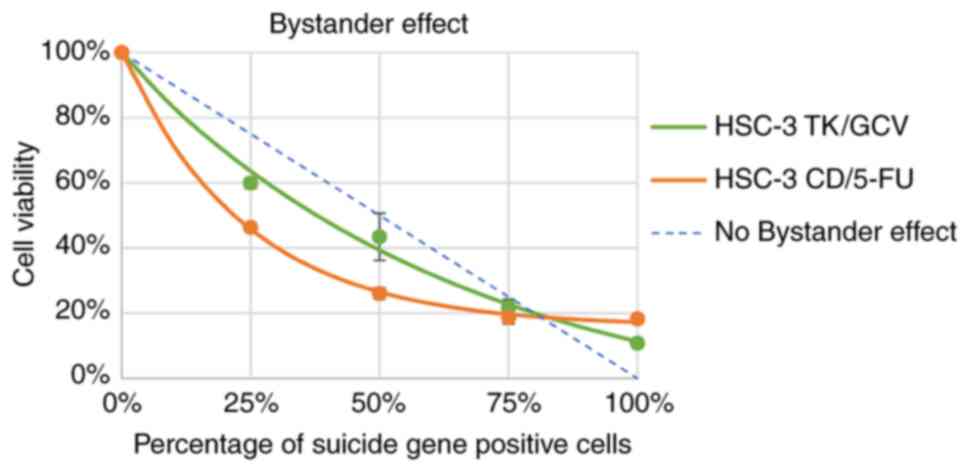
Bystander effect of TK/GCV and CD/5-FC
on HSC-3 cells
Bystander effects of TK/GCV and CD/5-FC on HSC-3
cells were investigated. The cell viability and the percentage of
suicide gene positive cells were fitted to the exponential
equation: y=a*e-bx + c. The
fitting results is satisfactory with R2 equal to 0.9995
and 0.9934 for CD/5-FC and TK/GCV respectively (Table II). It was found that CD/5-FC pair
had a bigger b value than the TK/GCV pair (Fig. 4 and Table II), indicating that CD/5-FC had a
better bystander effect than TK/GCV on HSC-3 cells.
 | Table IIQuantitative evaluation of bystander
effects of prodrug-activating-suicide gene therapies on HSC-3
cells. |
Table II
Quantitative evaluation of bystander
effects of prodrug-activating-suicide gene therapies on HSC-3
cells.
| Cell-prodrug
pair | Prodrug
concentration | R2 | b-value | % of area |
|---|
| Cytosine
deaminase/5-fluorocytosine | 100 µM | 0.9995 | 4.14 | 31.4 |
| Thymidine
kinase/ganciclovir | 25 µM | 0.9934 | 1.51 | 13.2 |
To measure the overall bystander effect, the % of
area was calculated based on curves in Fig. 4. First, the area between fitted
exponential curve and theoretical no bystander effect curve was
calculated. This area is directly related to by-stander effect of
suicide gene. Then the percentage of this area to the area of
triangle surrounded by x-axis, y-axis, and the theoretical no
bystander effect curve was calculated. As shown in Table II, CD/5-FC still has a larger % of
area than TK/GCV (31.4 vs 13.2%), confirming that CD/5-FC has a
better bystander effect than TK/GCV on HSC-3 cells.
Inhibition of HSC-3 cell migration by
PA-SG therapy
To investigate the effect of prodrugs on HSC-3 cell
migration, an improved wound-healing assay was performed. In the
improved wound-healing assay, instead of evaluating prodrug effects
within the first 24 h treatment, the effect of prodrugs on cell
migration was evaluated during the last 24 h of prodrug
treatment.
For HSC-3 mVenus-TK cells, 25 µM GCV inhibited cell
migration at all three different prodrug treatment durations. By
contrast, migration of HSC-3 mVenus cells was not affected by GCV
treatment (Fig. 5A-C). GCV
inhibited 57-95% of HSC-3 mVenus-TK cell migration in all prodrug
treatment durations and the inhibitory effect increased by
increasing the duration of treatments (Fig. 5D).
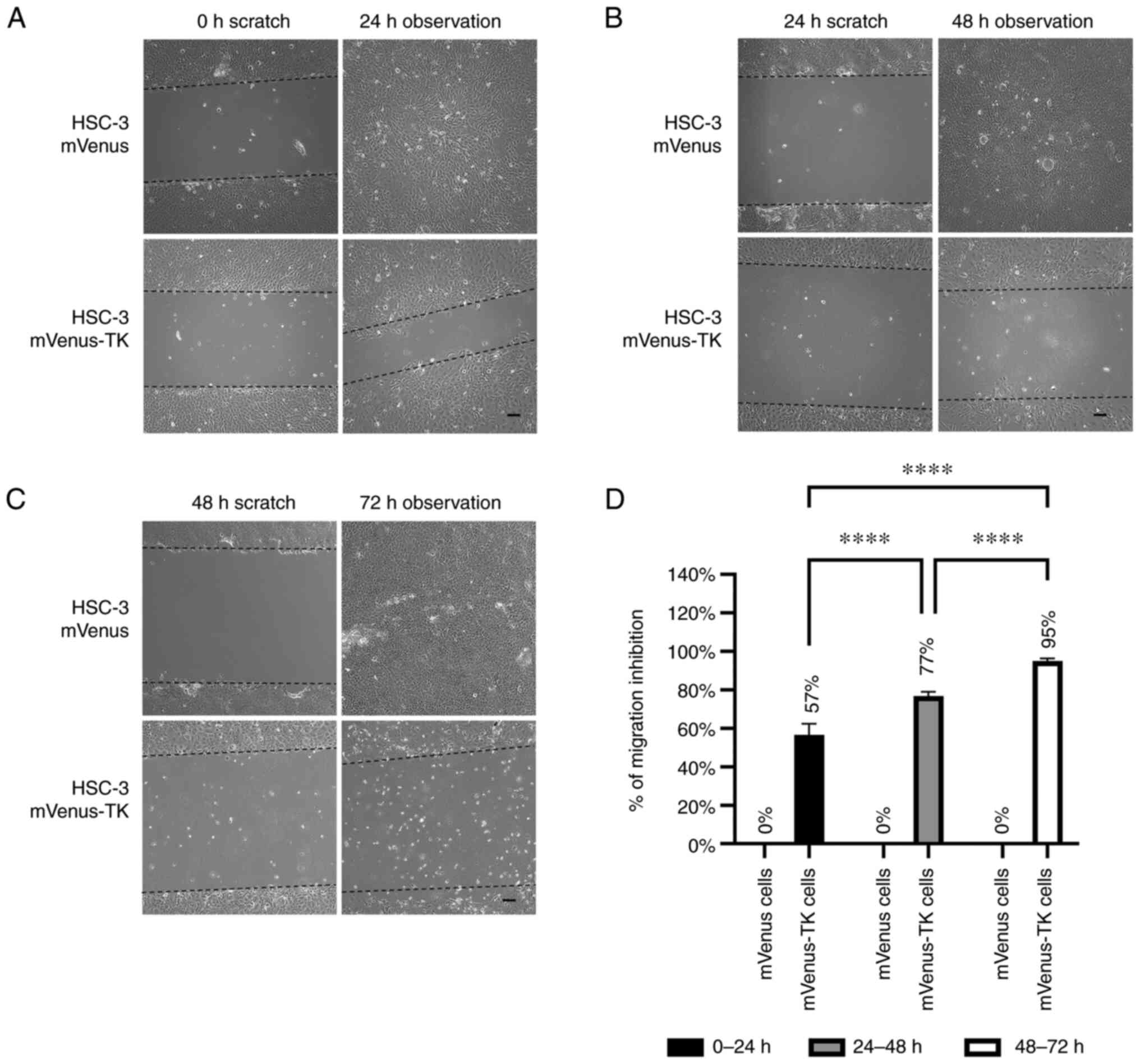 | Figure 5Inhibition of HSC-3 mVenus-TK cell
migration following treatment with GCV. (A) GCV treatment inhibited
HSC-3 mVenus-TK but not HSC-3 mVenus cell migration during the 0-24
h period. Following generation of the wound, scratch images were
captured and cells were treated with 25 µM GCV. The healing images
were captured after a further 24 h GCV treatment (scale bar, 100
µm). (B) GCV treatment inhibited HSC-3 mVenus-TK cell migration
during the 24-48 h period. HSC-3 mVenus and HSC-3 mVenus-TK cells
were treated with 25 µM GCV for 24 h and wounds were generated.
After taking the scratch images, cells were treated with 25 µM GCV
for a further 24 h, then the healing images were captured (scale
bar, 100 µm). (C) GCV treatment inhibited HSC-3 mVenus-TK cell
migration during the 48-72 h period. The wounds were generated
after cells were treated with 25 µM GCV for 48 h. The cells were
treated for a further 24 h, then healing images were captured.
Scale bar, 100 µm. (D) Quantification of inhibition of cell
migration for treatment durations. The percentage of migration
inhibition is the ratio of the area of healing images to the
average strip area of the scratch images. Data are presented as the
mean ± SD of >3 healing images. Data were analysed using one-way
ANOVA followed by Tukey's post hoc test for multiple comparisons.
****P<0.0001. CD, cytosine deaminase; TK, thymidine
kinase; GCV, ganciclovir; SD, standard deviation. |
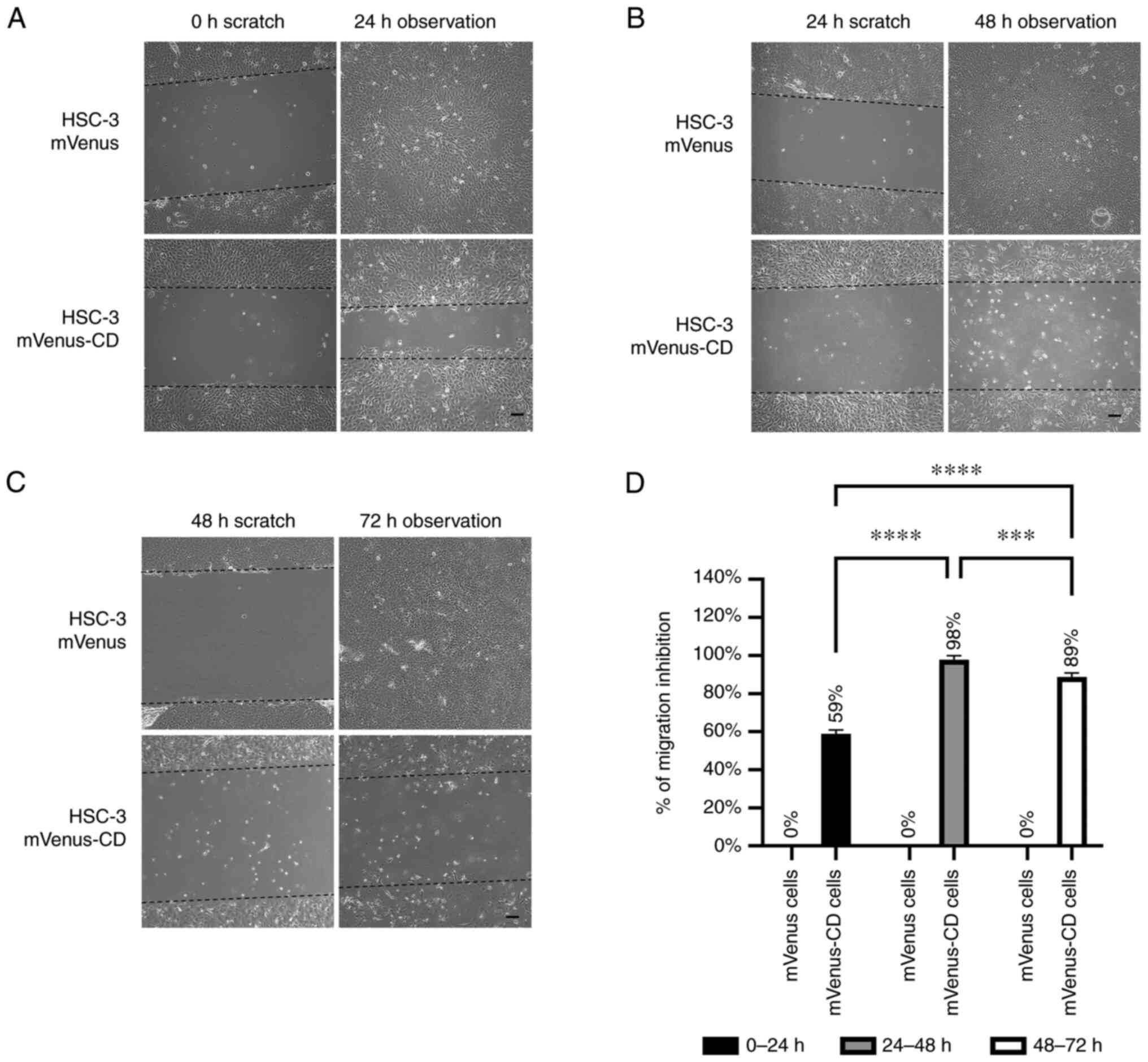
For HSC-3 mVenus-CD cells, 100 µM 5-FC inhibited
cell migration in all treatment durations but had no effects on the
HSC-3 mVenus cells (Fig. 6A-C).
However, the inhibitory effect decreased from 98 to 89% during the
last 24 h of the longest prodrug treatment (Fig. 6C and D).
Discussion
In the present study, the anti-proliferative and
anti-migratory effects of two well-studied PA-SG therapies on
metastatic HSC-3 cells were quantitively evaluated (18,19).
To the best of our knowledge, the present study is the first
quantitative study of anti-migratory effects of PA-SG therapy on a
metastatic HNSCC cell model. In addition, the methods used in the
present study are common and can be used to evaluate other PA-SG
therapies for various types of cancer.
The anti-migration effect of TK/GCV was different
from CD/5-FC. TK/GCV continuously inhibited HSC-3 migration during
72 h treatment, whereas the anti-migration effect of CD/5-FC
decreased during the 48-72 h treatment period. This difference
implied that the anti-migratory mechanisms were different between
TK/GCV and CD/5-FC. In a previous study, a mechanistic difference
was observed between CD/5-FC and TK/GCV in terms of cell-killing
effect (29). TK/GCV rapidly
induces phosphorylation of Bcl-xL and consequently apoptotic cell
death. By contrast, CD/5-FC treatment decreases expression of
Bcl-xL. This implies that TK/GCV and CD/5-FC activate different
intracellular signalling pathways. These differences between TK/GCV
and CD/5-FC may be associated with differences in the
anti-migratory effect found in the present study. The
anti-migratory mechanisms of TK/GCV and CD/5-FC are clear. TK/GCV
and CD/5-FC block DNA synthesis and cause cell cycle arrest.
However, their effects on cell cycle arrest are different.
5-fluorouracil, which is an effective product of CD/5-FC, blocks
two thirds of cells in G0/G1 phase and
one-third of cells at S phase (30). By contrast, TK/GCV arrests more
cells at the S phase than CD/5-FC (31). A previous study reported that, when
arrested in S phase, cells are not sensitive to movement
stimulation (32). Thus TK/GCV has
an improved anti-migratory effect compared with that of CD/5-FC,
potentially due to TK/GCV arresting more cells at the S phase than
CD/5-FC and S phase cells having weaker mobility.
The dose-response curve of HSC-3 cells was compared
with the previous CAL-27 cell results (14). The IC50, which is the
concentration when prodrug inhibits 50% of cell viability increase,
of GCV to HSC-3 and CAL-27 were 3.96 and 0.024 µM, respectively.
These results showed that HSC-3 cells were less sensitive to GCV
treatment than CAL-27 cells. It was previously demonstrated that
HSC-3 cells are more malignant and tend to metastasize following
implantation into immunodeficient mice (17). In the present study, the
IC50 of GCV for HSC-3 cells was 165-fold higher than
that for CAL-27 cells. At 0.39 µM, GCV effectively kills most of
the CAL-27 cells (14). By
contrast, in the present study, 25 µM GCV killed most HSC-3 cells
(Fig. 2A), which is consistent
with the malignancy of HSC-3 cells (17). Dose-response curve of HSC-3
mVenus-TK-GCV was double sigmoid. The underlying mechanism remains
unclear. HSC-3 cells may express endogenous protein that processes
TK to decrease its activity. This protein may not be potent and
only be activated at a high concentration of GCV. IC50
of 5-FC for HSC-3 cells was 2-fold higher than that for CAL-27
cells, which also showed the malignancy of HSC-3 cells.
The time-course response of HSC-3 cells to prodrugs'
treatment was compared with CAL-27 cells' response . It was found
that HSC-3 mVenus cells proliferated more quickly than the CAL-27
mVenus cells in the presents of prodrugs. Consistent with the
dose-response result, HSC-3 cells expressing suicide genes were
less sensitive to prodrug treatment than CAL-27 cells in the
time-course response. After 96 h treatment, HSC-3 mVenus-TK and
mVenus-CD cell viabilities were 78 and 107% for GCV and 5-FC
treatment respectively. By contrast, prodrugs can kill >85% of
CAL-27 cells (14), indicating
that HSC-3 cells are more resistant to PA-SC therapies than CAL-27
cells.
In the present study, CD/5-FC has a stronger
bystander effect than TK/GCV on HSC-3 cells (Fig. 4 and Table II). This phenomenon was also
observed on CAL-27 cells, in which bystander effect of CD/5-FC was
stronger than that of TK/GCV (Table
SI) (14). We also found that
the bystander effects of CD/5-FC and TK/GCV on HSC-3 cells were
weaker than that on CAL-27 cells The b-value of 5-FC on HSC-3 was
4.14, whereas the b-value of 5-FC on CAL-27 is 14.0. The percentage
of area (% of area) was 31.4 and 63.2% for CD/5-FC on HSC-3 and
CAL-27 cells respectively. The TK/GCV also has a weaker bystander
effect on HSC-3 than on CAL-27 (HSC-3 vs CAL-27 b-value: 1.51 vs
5.85; % of are: 13.2 vs 52.3%). These results indicated that HSC-3
cells were more malignant than CAL-27 cells.
Despite advances in cancer treatment, cancer
metastasis is a major concern for solid tumour treatment (33). TK/GCV may be more beneficial on
patients with HNSCC than CD/5-FC because the present study found
that TK/GCV had an improved anti-migratory effect compared with
that of CD/5-FC. Prevention of metastasis of cancer may lead to
improved morbidity and mortality (34).
In the present study, the human ROSA26 locus was
selected for the knock-in site of suicide genes, which is located
on human chromosome 3(35). HSC-3
cell line is a near-triploid cell line with at least three copies
of chromosome 3(36). Compared
with normal diploid cells, chromosome 3 is not evenly distributed
into daughter cells after mitosis (36). As a result, one copy of suicide
gene integration would not be sufficient for stable cell screening.
In the process of stable cell screening, a portion of cells lost
fluorescence following cell division into one single colony (data
not shown). Consequently, in the present study, the number of HSC-3
cell colonies screened to obtain a stable cell line was higher than
that for CAL-27 cells. Therefore, it is recommended to use a novel
knock-in locus instead of ROSA26 for HSC-3 cell line.
The method in the present study could be extended to
evaluate PA-SG therapies on metastatic NHSCC models in vivo.
HSC-3 cells develop lymph nodes and pulmonary metastasis following
injection into the tongue of a immunodeficient mouse model
(17). A similar animal model
could be used to inoculate HSC-3 mVenus-TK and mVenus-CD cells in
the tongues of immunodeficient mice. Finally, lymph node and
pulmonary metastases could be evaluated with or without
prodrugs.
Supplementary Material
HSC-3 stable cell lines expressing
mVenus, mVenus-CD or mVenus-TK. (A) Amplification of sequence
around the ROSA26 locus insertion site from the HSC-3 mVenus,
mVenus-CD, or mVenus-TK cells. (B) Growth curve of HSC-3 stable
cell lines expressing mVenus, mVenus-CD or mVenus-TK. A total of
5,000 cells was seeded into one well of 96-well plate. Cell
viability was measured every 24 h via MTT assay. Data are presented
as the mean ± standard deviation of three samples. CD, cytosine
deaminase; TK, thymidine kinase; OD, optical density; WT,
wild-type.
Comparison of time-course response of
HSC-3 cells to prodrug treatments. Time-course response curves of
HSC-3 cells to GCV and 5-FC treatment in this study were plotted
together.
Quantitative comparison of bystander
effects of prodrug-activating suicide gene therapies on HSC-3 cells
in the present study and CAL-27 cells from a previous report .
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Science
Foundation of China (grant no. 31670728), Hong Kong Special
Administrative Region (grant nos. 16103719 and 16101120), Research
Program of Shenzhen Innovation Council (grant nos.
JCYJ20200109140208058 and JCYJ20190809104803572), Guangdong Basic
and Applied Basic Research Foundation (grant no. 2021A1515220104),
Shenzhen Fund for Guangdong Provincial High-level Clinical Key
Specialties (grant no. SZGSP008) and Sanming Project of Medicine in
Shenzhen (grant no. SZSM 202111012; Oral and Maxillofacial Surgery
Team, Peking University Hospital of Stomatology).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NX, HT and HY participated in the research design.
NX, HT, CF, YL and YC performed the experiments, analysed data and
wrote the manuscript. YS, GZ, CG and HY provided technical guidance
and participated in data acquisition and analysis. YS and HY
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cramer JD, Burtness B, Le QT and Ferris
RL: The changing therapeutic landscape of head and neck cancer. Nat
Rev Clin Oncol. 16:669–683. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pulte D and Brenner H: Changes in survival
in head and neck cancers in the late 20th and early 21st century: A
period analysis. Oncologist. 15:994–1001. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gonzalez-Garcia R, Naval-Gias L,
Roman-Romero L, Sastre-Perez J and Rodriguez-Campo FJ: Local
recurrences and second primary tumors from squamous cell carcinoma
of the oral cavity: A retrospective analytic study of 500 patients.
Head Neck. 31:1168–1180. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pisani P, Airoldi M, Allais A, Valletti
PA, Battista M, Benazzo M, Briatore R, Cacciola S, Cocuzza S,
Colombo A, et al: Metastatic disease in head & neck oncology.
Acta Otorhinolaryngol Ital. 40:S1–S86. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Price KA and Cohen EE: Current treatment
options for metastatic head and neck cancer. Curr Treat Options
Oncol. 13:35–46. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R,
Hammond EH, Fu KK and Milas L: Impact of epidermal growth factor
receptor expression on survival and pattern of relapse in patients
with advanced head and neck carcinoma. Cancer Res. 62:7350–7356.
2002.PubMed/NCBI
|
|
9
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
11
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cohen EEW, Soulieres D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haslam A and Prasad V: Estimation of the
percentage of us patients with cancer who are eligible for and
respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw
Open. 2(e192535)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu N, Tian H, Fung CP, Lin Y, Zhu G, Shen
Y and Yang H: Quantitative evaluation and comparison of two
prodrug-activating suicide gene therapies on oral squamous cell
carcinoma. Am J Cancer Res. 11:1672–1682. 2021.PubMed/NCBI
|
|
15
|
Momose F, Araida T, Negishi A, Ichijo H,
Shioda S and Sasaki S: Variant sublines with different metastatic
potentials selected in nude mice from human oral squamous cell
carcinomas. J Oral Pathol Med. 18:391–395. 1989.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sugiyama M, Bhawal UK, Dohmen T, Ono S,
Miyauchi M and Ishikawa T: Detection of human papillomavirus-16 and
HPV-18 DNA in normal, dysplastic, and malignant oral epithelium.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 95:594–600.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsui T, Ota T, Ueda Y, Tanino M and
Odashima S: Isolation of a highly metastatic cell line to lymph
node in human oral squamous cell carcinoma by orthotopic
implantation in nude mice. Oral Oncol. 34:253–256. 1998.PubMed/NCBI
|
|
18
|
Karjoo Z, Chen X and Hatefi A: Progress
and problems with the use of suicide genes for targeted cancer
therapy. Adv Drug Deliv Rev. 99:113–128. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bali A, Bali D and Sharma A: An overview
of gene therapy in head and neck cancer. Indian J Hum Genet.
19:282–290. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bairoch A: The cellosaurus, a cell-line
knowledge resource. J Biomol Tech. 29:25–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kondo S and Aso K: Establishment of a cell
line of human skin squamous cell carcinoma in vitro. Br J Dermatol.
105:125–132. 1981.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Eagle H: Propagation in a fluid medium of
a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med.
89:362–364. 1955.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Horikoshi M, Kimura Y, Nagura H, Ono T and
Ito H: A new human cell line derived from human carcinoma of the
gingiva. I. Its establishment and morphological studies. Nihon Koku
Geka Gakkai Zasshi. 20:100–106. 1974.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
24
|
Takahashi K, Kanazawa H, Akiyama Y, Tazaki
S, Takahara M, Muto T, Tanzawa H and Sato KI: Establishment and
characterization of a cell line (SAS) from poorly differentiated
human squamous cell carcinoma of the tongue. J Jpn Stomatol Soc.
38:20–28. 1989.
|
|
25
|
Cong L, Ran FA, Cox D, Lin S, Barretto R,
Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA and Zhang F:
Multiplex genome engineering using CRISPR/Cas systems. Science.
339:819–823. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pirkmajer S and Chibalin AV: Serum
starvation: Caveat emptor. Am J Physiol Cell Physiol.
301:C272–C279. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yarrow JC, Perlman ZE, Westwood NJ and
Mitchison TJ: A high-throughput cell migration assay using scratch
wound healing, a comparison of image-based readout methods. BMC
Biotechnol. 4(21)2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jonkman JE, Cathcart JA, Xu F, Bartolini
ME, Amon JE, Stevens KM and Colarusso P: An introduction to the
wound healing assay using live-cell microscopy. Cell Adh Migr.
8:440–451. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fischer U, Steffens S, Frank S, Rainov NG,
Schulze-Osthoff K and Kramm CM: Mechanisms of thymidine
kinase/ganciclovir and cytosine deaminase/ 5-fluorocytosine suicide
gene therapy-induced cell death in glioma cells. Oncogene.
24:1231–1243. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gao J, Yan Q, Liu S and Yang X: Knockdown
of EpCAM enhances the chemosensitivity of breast cancer cells to
5-fluorouracil by downregulating the antiapoptotic factor Bcl-2.
PLoS One. 9(e102590)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liang L, Bi W, Chen W, Lin Y and Tian Y:
Combination of MPPa-PDT and HSV1-TK/GCV gene therapy on prostate
cancer. Lasers Med Sci. 33:227–232. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bonneton C, Sibarita JB and Thiery JP:
Relationship between cell migration and cell cycle during the
initiation of epithelial to fibroblastoid transition. Cell Motil
Cytoskeleton. 43:288–295. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pulley JM, Jerome RN, Ogletree ML, Bernard
GR, Lavieri RR, Zaleski NM, Hong CC, Shirey-Rice JK, Arteaga CL,
Mayer IA, et al: Motivation for launching a cancer metastasis
inhibition (CMI) program. Target Oncol. 13:61–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Eccles SA and Welch DR: Metastasis: Recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Irion S, Luche H, Gadue P, Fehling HJ,
Kennedy M and Keller G: Identification and targeting of the ROSA26
locus in human embryonic stem cells. Nat Biotechnol. 25:1477–1482.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ribeiro IP, Rodrigues JM, Mascarenhas A,
Kosyakova N, Caramelo F, Liehr T, Melo JB and Carreira IM:
Cytogenetic, genomic, and epigenetic characterization of the HSC-3
tongue cell line with lymph node metastasis. J Oral Sci. 60:70–81.
2018.PubMed/NCBI View Article : Google Scholar
|