Introduction
Acute promyelocytic leukemia (APL) is a particular
type of acute myeloid leukemia characterized by a balanced
reciprocal translocation, resulting in the formation of the
oncogenic fusion protein, promyelocytic leukemia-retinoic acid
receptor α (PML-RARα) (1,2). It has been reported that PML-RARα, an
oncoprotein, acts as a retinoid receptor involved in suppressing
retinoic acid-induced myeloid differentiation. Currently, all-trans
retinoic acid (ATRA) is considered as an effective drug in treating
APL via promoting the degradation of PML-RARα (3). However, patients with long-term
exposure to ATRA are more likely to experience hyper-inflammation
and differentiation syndrome (DS), thus resulting in poor prognosis
among patients with APL (4,5).
DS, also known as retinoic acid syndrome, is
characterized by the presence of massive inflammatory
differentiating leukemic cells in the bloodstream, which trigger
the release of excessive chemokines and cytokines (6). Previous studies demonstrated that
ATRA can induce the release of early pro-inflammatory cytokines,
such as TNF-α and IL-1β, as well as intercellular adhesion
molecule-1 (ICAM-1) in patients with APL complicated with DS
(7,8). Therefore, it was hypothesized that
the above inflammatory factors could be involved in the
pathogenesis of DS.
Previous studies demonstrated that peptidylarginine
deiminase 4 (PADI4), contributing to ATRA- and 1α,
25-dihydroxyvitamin D3-induced differentiation of human myeloid
leukemia HL-60 cells, is involved in regulating the proliferation
of hematopoietic progenitors (9,10).
As the only member of the PADI family in the nucleus (11), PADI4 is involved in the
pathogenesis of several types of cancer via modulating the
transcriptional network for pluripotency (12,13).
Another study showed that PADI4 is involved in the differentiation
of APL cells (14). However,
little is known regarding the role of PADI4 in APL DS. Therefore,
the current study aimed to investigate the effect of PADI4 in the
pathogenesis of DS. First, the expression levels of PADI4 were
detected in patients with APL DS and patients with APL only.
Subsequently, in vitro experiments were carried out in the
NB4 cell line, an APL cell line mimicking ATRA-induced terminal
neutrophil maturation, transfected with PADI4 overexpression or
silencing plasmids. Furthermore, the secretion levels of the
inflammatory factors TNF-α, IL-1β, IL-8 and ICAM-1, were also
determined to uncover the role of PADI4 in ATRA-induced
differentiation.
Materials and methods
Patients
A total of three patients (1 male and 2 female; age
range 3-8 years) with APL DS admitted to Children's Hospital
Affiliated to Shandong University and Jinan Children's Hospital
between December 2019 and November 2021 were included in the APL +
DS group, while three (1 male and 2 female) age-matched patients
with APL, without DS, served as the control group. Patients with
APL aged <10 years were enrolled in the present study, while
those with other concurrent malignancies or those not willing to
receive ATRA therapy were excluded. Patients were diagnosed with
APL DS according to the guidelines by the Expert Panel of the
European LeukemiaNet, as previously described (15,16).
All six patients received inducing therapy with ATRA (25
mg/m2/day) plus cytarabine and daunorubicin. The parents
of each patient signed the informed consent and the study protocol
was approved by the Ethical Committee of Children's Hospital
Affiliated to Shandong University and Jinan Children's Hospital
(approval no. QLET-IRB/P-2021007).
Extraction of monocytes
Peripheral blood samples were collected from six
patients with APL with or without DS. The peripheral blood
mononuclear cells (PBMCs) were prepared by Ficoll Hypaque gradient
centrifugation at 1,000 x g for 20 min at room temperature and were
then cryopreserved for the subsequent experiments (17).
Cell culture
NB4 cells, purchased from ATCC, were cultured in
1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (Zhejiang Tianhang Biotechnology Co., Ltd.),
penicillin (100 U/ml) and streptomycin (100 µg/ml; both from
Beyotime Institute of Biotechnology) at 37˚C with 5%
CO2. To induce cell differentiation, NB4 cells were
treated with 1 µmol/l ATRA (cat. no. R2625; MilliporeSigma).
Transfection and PADI4 silencing
The PADI4 overexpression plasmid, pPADI4, and the
corresponding control vector were kindly given by Dr Wang Lin from
the Shandong Academy of Medicinal Sciences (Jinan, China). To
silence PADI4 expression, cells were transfected with small
interfering RNA (siRNA) clones targeting PADI4 (siPADI4; sequence,
5'-GCCAACCAGAGCUGUGAAATTUUUCACAGCUCUGGUUGGCTT-3'). The scrambled
negative control sequence (NC-siRNA),
5'-UUCUCCGAACGUGUCACGUUUCUCCGAACGUGUCACGU-3', served as control.
The sequences were synthesized by Shanghai GenePharma Co., Ltd. For
cell transfection, 20 µg pPADI4/empty vector was electroporated
into NB4 cells (1x107) using the Electro square porator
830 (BTX Instrument Division, Harvard Apparatus Inc.) with two 500
V pulses of 10 msec pulse length and pausing for 1 min between
pulses at room temperature. For PADI4 silencing, 5 µl of 20 µmol/l
siRNA was electroporated into NB4 cells (1x107) treated
with ATRA with two 250 V pulses of 8 msec pulse length and pausing
for 30 sec between pulses at room temperature. After incubation on
ice for 10 min, RPMI 1640 medium supplemented with 15% FBS was
added to the mixture, followed by incubation for an additional 36 h
at 37˚C with 5% CO2. The transfection was confirmed by
western blot in the subsequent analysis
Flow cytometry
To investigate the potential effect of PADI4 on cell
differentiation, NB4 cells were divided into the control, ATRA,
ATRA + siPADI4 and ATRA + NC-siRNA groups. Cells at a density of
1x106 were first washed with PBS supplemented with 1%
FCS and 0.01% sodium azide, and were then incubated in FCS at 4˚C
for 30 min. Subsequently, cells were supplemented with
FITC-conjugated anti-human CD11b antibody (cat. no. 562793; BD
Biosciences) followed by incubation at 25˚C for 45 min. The cells
were then fixed with 1% paraformaldehyde and analyzed on FACSVerse
flow cytometer using FACSDiva 6.0 software (BD Biosciences).
ELISA
ELISA was utilized to determine the expression
levels of TNF-α, IL-1β, IL-8, C-C motif chemokine ligand (CCL)2,
CCL4 and C-C motif chemokine receptor (CCR) 1 in each group using
the corresponding commercial kits, according to the manufacturer's
instructions. The commercially available kits for TNF-α (cat. no.
EHC103a), IL-1β (cat. no. EHC002b) and IL-8 (cat. no. EHC008) were
purchased from Neobioscience Technology Co., Ltd., while those for
CCL2 (cat. no. DCP00), CCL4 (cat. no. DMB00) and CCR1 (cat. no.
CT63246) were from Jinan Quantum Trading Co., Ltd.. Finally, the
absorbance at a wavelength of 532 nm was measured using a
microplate reader (Synergy HT; BioTek Instruments Inc.).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from 1x106 NB4
cells and PBMCs using TRIzol® reagent (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Subsequently, total RNA was reverse transcribed into cDNA using the
Reverse Transcription Kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions on the Eppendorf PCR
system. PCR amplification was performed using the SYBR Green
Real-time PCR Master Mix (ToYoBo, Japan) according to
manufacturer's instructions on Stratagene Mx3000P Real-Time PCR
system (Agilent Technologies, Inc.). Each PCR reaction contained 2X
real-time PCR Master Mix, 1 µl of each primer and 1 µl cDNA, in a
total volume of 10 µl. The specific primer sequences for PADI4 were
as follows: 5'-GTTTAGGGTCAGACAGTCCTGG-3',
5'-AGATGTGAGTAGTGGCACATGC'. The primer sequences for GAPDH were:
5'-GTCTCCTCTGACTTCAACAGCG-3', 5'-ACCACCCTGTTGCTGTAGCCAA-3'. The
thermocycling conditions used were as follows: 95˚C for 10 sec; 40
cycles of 60˚C for 5 sec and 72˚C for 10 sec; and 65˚C for 30 sec.
Finally, the amplification results were analyzed using the
2-ΔΔCq method, as previously described (18).
Western blot analysis
NB4 cells were washed twice with ice cold PBS and
were then lysed using RIPA lysis buffer (Applygen Technologies,
Inc.). The protein concentration was determined using the BCA
method. Subsequently, protein samples (20 µg) were separated by 10%
SDS-PAGE and were then transferred onto a PVDF membrane
(MilliporeSigma). The membranes were blocked with 5% skimmed milk
in TBST containing 0.05% Tween-20 for 1 h at room temperature and
incubated with antibodies against PADI4 (dilution, 1:500; cat. no.
p4874; MilliporeSigma), ICAM-1 (dilution, 1:500; cat. no. ER131207)
and GAPDH (dilution, 1:3,000; cat. no. ET1601-4; both from HUABIO,
Inc.) overnight at 4˚C. Subsequently, the membranes were incubated
with HRP-conjugated secondary antibodies (dilution, 1:20,000; cat.
no. HA1001; HUABIO, Inc.) at room temperature for 1 h. The protein
levels were quantified by densitometry and normalized to the
corresponding GAPDH expression levels. Finally, the protein bands
were visualized using the iBright CL750 CL1500 system (Thermo
Fisher Scientific Inc.) and the band intensity was measured using
ImageJ software (Version 1.49, NIH).
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software (SPSS, Inc.). All data are expressed as the mean ±
standard deviation. Significant differences between two groups were
analyzed using unpaired Student's t-test, while those among
multiple groups with one-way ANOVA followed by a Bonferroni's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
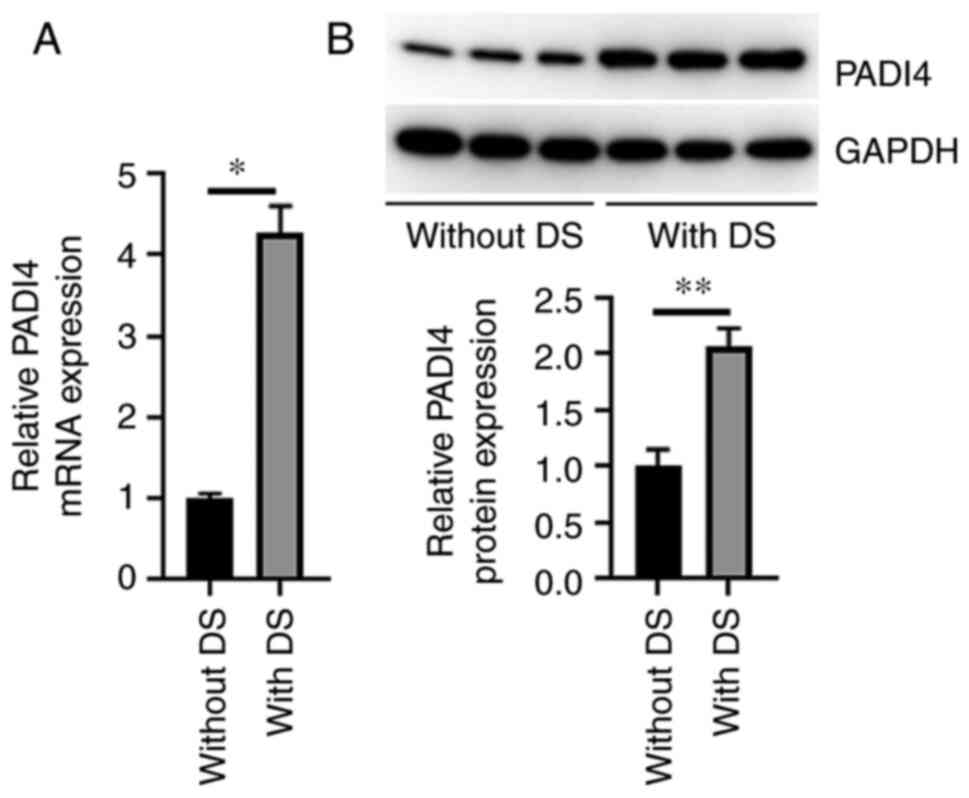
PADI4 is upregulated in APL DS samples
and ATRA-treated NB4 cells
The mRNA and protein expression levels of PADI4 in
PBMCs derived from patients with APL DS were significantly higher
compared with those in patients with APL only (Fig. 1A and B). Compared with the baseline levels, the
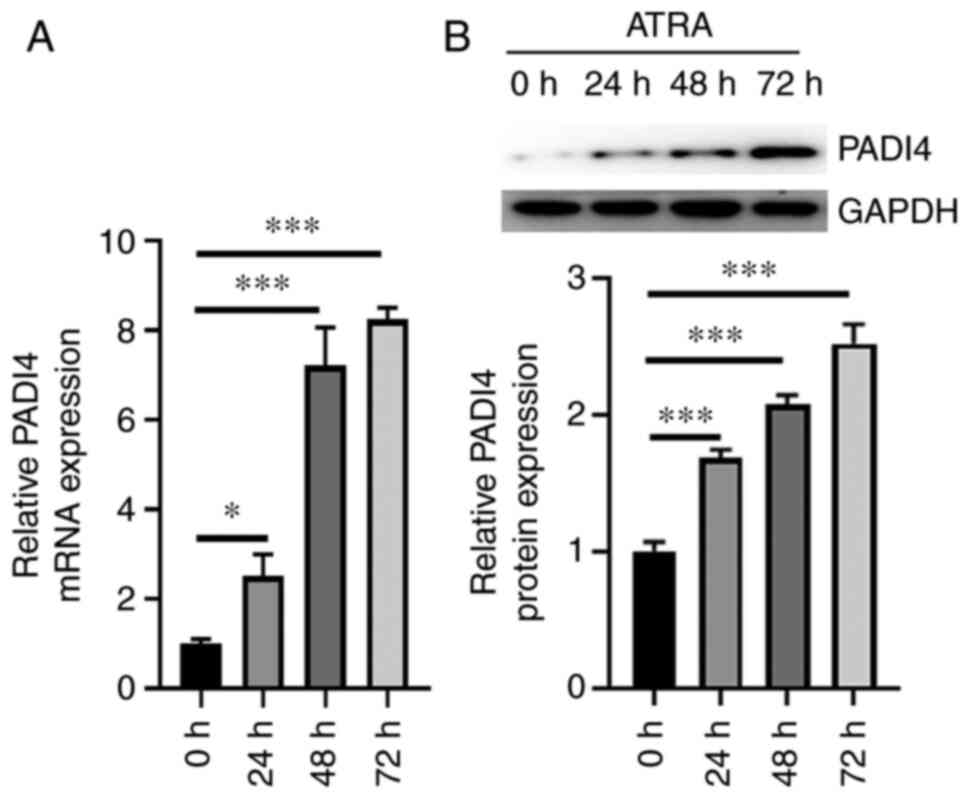
mRNA expression levels of PADI4 were notably increased in NB4 cells
treated with ATRA for 24, 48 and 72 h (P<0.05; Fig. 2A). Similarly, PADI4 was
significantly upregulated at 24, 48 and 72 h in ATRA-treated NB4
cells compared with untreated cells (Fig. 2B). These in vivo and in
vitro experiments indicated that ATRA could upregulate
PADI4.
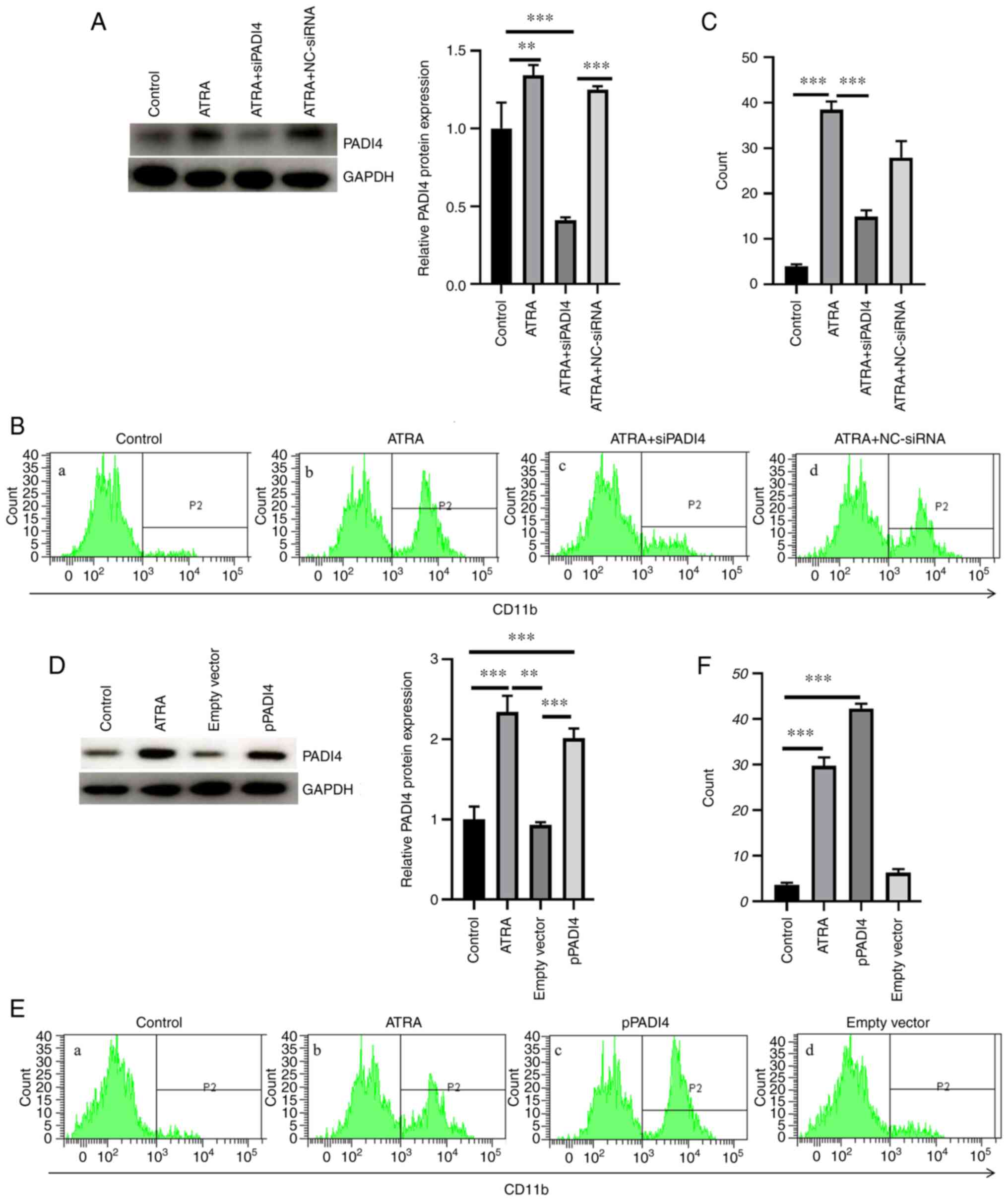
PADI4 serves a crucial role in the
ATRA-induced differentiation of NB4 cells
As shown in Fig.
3A, PADI4 was successfully knocked down in NB4 cells
transfected with the corresponding siRNA clones. No statistically
significant difference was observed in the differentiation capacity
of NB4 cells in the ATRA + NC-siRNA group compared with the ATRA
group (P>0.05; Fig. 3A). The
differentiation ability of NB4 cells was assessed by evaluating the
synthesis of CD11b, a marker of granulocytic differentiation. Flow
cytometric analysis revealed that cell treatment with ATRA induced
NB4 cell differentiation. However, the ATRA-induced NB4 cell
differentiation was reversed following PADI4 silencing (Fig. 3B and C). Electroporation-mediated PADI4
overexpression further promoted the differentiation of NB4 cells
compared with ATRA-treated NB4 cells (P<0.05; Fig. 3D-F). However, the differentiation
ability of ATRA-treated NB4 cells transfected with empty vector was
notably attenuated compared with the ATRA group. Taken together,
the above findings indicated that PADI4 could play a significant
role in the ATRA-induced differentiation of NB4 cells.
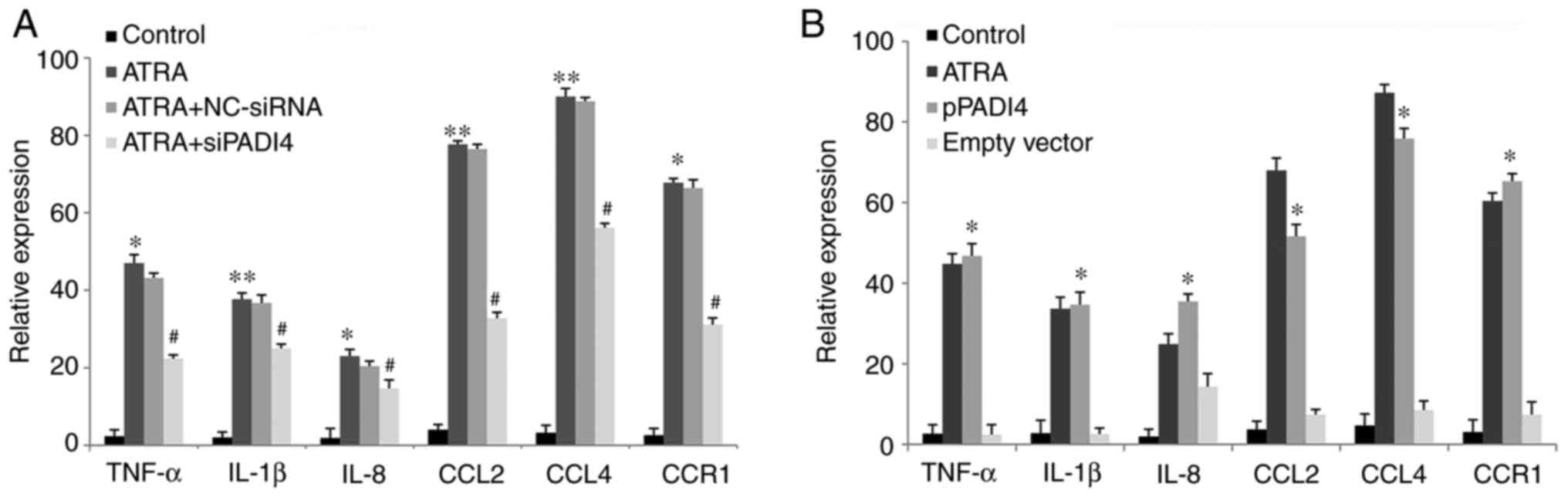
PADI4 promotes the secretion of
inflammatory factors
ELISA was performed to assess the secretion levels
of the inflammatory factors TNF-α, IL-1β, IL-8, CCL2, CCL4 and CCR1
in NB4 cells that were reported to be closely related to the
inflammation process (19,20). The secretion levels of TNF-α,
IL-1β, IL-8, CCL2, CCL4 and CCR1 were significantly increased in
the supernatant of cultured NB4 cells in the ATRA group compared
with those in the control group (all P<0.05; Fig. 4A). However, the secretion levels of
TNF-α, IL-1β, IL-8, CCL2, CCL4 and CCR1 were notably reduced in the
ATRA + siPADI4 group compared with the ATRA group (all P<0.05).
By contrast, no significant differences were observed in the levels
of the above inflammatory factors in the ATRA + NC-siNRA group
compared with the ATRA group. In PADI4 overexpressing cells, the
expression of TNF-α, IL-1β, IL-8, CCL2, CCL4 and CCR1 were
significantly enhanced compared with control cells (P<0.05).
However, there was no significant difference in the secretion
levels of TNF-α, IL-1β, IL-8, CCL2, CCL4 and CCR1 between the ATRA
and PADI4 overexpression group (P>0.05; Fig. 4B). Overall, these results suggested
that PADI4 could be involved in TNF-α, IL-1β, IL-8, CCL2, CCL4 and
CCR1 upregulation in ATRA-treated NB4 cells.
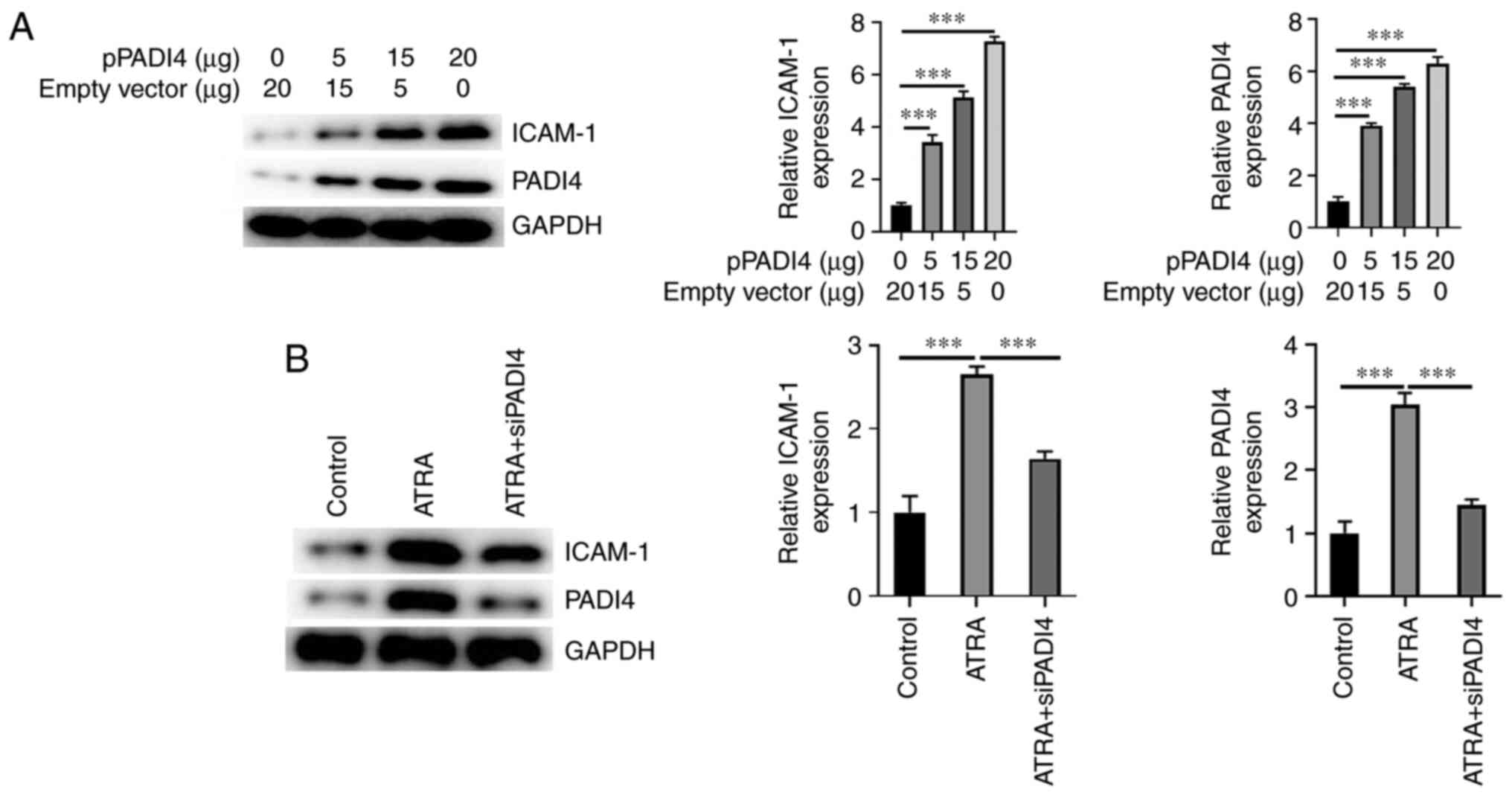
PADI4 upregulates ICAM-1
To determine whether PADI4 could affect the
expression of ICAM-1, the protein expression levels of ICAM-1 were
detected in NB4 cells transfected with 0, 5, 15 and 20 µg pPADI4.
Following transfection for 48 h, the protein expression levels of
ICAM-1 were assessed using western blot analysis. Additionally, to
evaluate whether PADI4 silencing could restore the expression
levels of ICAM-1, NB4 cells were transfected with siRNA clones
targeting PADI4. The results showed that the protein expression
levels of ICAM-1 were increased in PADI4 overexpressing NB4 cells
in a dose-dependent manner (Fig.
5A). As shown in Fig. 5B,
ICAM-1 was markedly upregulated in cells in the ATRA group compared
with the NC group. However, the above effect was reversed in
PADI4-depleted NB4 cells. The aforementioned findings indicated
that PADI4 could be involved in ICAM-1 upregulation in NB4
cells.
Discussion
Differentiation therapy based on ATRA and arsenic
trioxide has been commonly used for treating APL (3). However, several patient may
experience DS (21). Although the
pathogenesis of DS remains to be elucidated, mounting evidence has
suggested that sustained hyperinflammation serves a critical role
in its pathogenesis (16,21,22).
The current study showed that PADI4 could serve an essential role
in regulating the expression of inflammatory cytokines, such as
TNF-α and IL-1β, in ATRA-treated NB4 cells. In addition, PADI4
could also promote the expression of ICAM-1, which was positively
associated with the clinical status of DS.
It has been reported that PADI4 is upregulated in
particular types of cancer (23,24).
Previous studies in solid tumors suggest that PADI4 can bind with
the downstream targets of cytokeratin and p53, including
OKL38 and Elk-1 (13,25,26).
Another study also revealed that PADI4 promoted cellular
differentiation in patients with hematologic neoplasm: Under in
vitro conditions, PADI4 can trigger NB4 cell differentiation by
regulating the PADI4/SOX4/PU.1 signaling pathway (14). In the present study, PADI4 was
upregulated in PBMCs and serum samples of patients with APL DS
compared with patients without DS. However, our understanding of
the effects of PADI4 overexpression during the development of DS in
patients with APL remains limited. Therefore, the present study
aimed to investigate the effect of PADI4 on ATRA-mediated NB4 cell
differentiation in vitro, in PADI4-overexpressing and
-depleted NB4 cells. The results demonstrated that PADI4
overexpression was involved in ATRA-induced NB4 cell
differentiation. By contrast, the ATRA-induced NB4 cell
differentiation was inhibited after PADI4 knockdown. These findings
indicated that PADI4 could serve a critical role in the
ATRA-induced differentiation of NB4 cells.
It has been hypothesized that PADI4 is closely
associated with inflammation. In a previous study, PADI4 activation
was associated with the exacerbation of kidney ischemia-reperfusion
injury by enhancing renal tubular inflammatory responses and
neutrophil infiltration (27). In
addition, PADI4 silencing in a rat model of hemorrhagic shock can
attenuate local inflammatory responses (28). Other studies indicate that PADI4
can be involved in the prevention of bacterial infection by
promoting the formation of neutrophil extracellular traps (29,30).
In ATRA-induced APL cells, the expression levels of cytokines, such
as IL-1β, IL-6, IL-8, TNF-α, L-selectin, lymphocyte
function-associated antigen 1 and ICAM-1 are notably increased
(31,32). For example, Ninomiya et al
(33) show that APL cells can
migrate into lung tissues in the presence of chemotactic factors
secreted by ATRA-induced alveolar epithelial cells, eventually
triggering DS after their metastasis to lung tissues and alveolar
space. These findings suggest that PADI4 could trigger the
pathogenesis and progression of inflammation, while PADI4
inactivation can eliminate or prevent the occurrence of
inflammation. In our previous study, PADI4 was considered to
participate in the expression of TNF-α and IL-1β in ATRA-treated
NB4 cells (8). In the present
study, PADI4 was involved in the regulation of inflammation-related
cytokines, such as TNF-α, IL-1β, IL-8, CCL2, CCL4, CCR1 and ICAM-1.
Following PADI4 silencing, the expression of inflammation-related
cytokines was notably decreased compared with the control
group.
However, the current study has some limitations.
First, the sample size was small. Second, although the current
study revealed the role of PADI4 in the ATRA-induced
differentiation of NB4 cells based on overexpression and silencing
experiments, the particular mechanism underlying the effect of
PADI4 on regulating the expression of IL-1β, TNF-α and ICAM-1 was
not discovered. Therefore, further studies are needed to
investigate the possible mechanism of the above process.
In summary, the present study demonstrated that
PADI4 was upregulated in PBMCs of patients with APL DS and
ATRA-treated NB4 cells. In addition, the results showed that PADI4
was involved in the upregulation of cytokines in ATRA-treated NB4
cells, while PADI4 silencing exhibited the opposite effect. The
aforementioned findings indicated that PADI4 could be involved in
ATRA-induced NB4 differentiation and increased the expression
levels of inflammation-related cytokines. Therefore, PADI4 could
serve as a novel treatment strategy for treating DS.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Jinan Technology
Innovation Plan Project (grant no. 202019072) and Science and
Technology Program of Jinan Municipal Health Commission (grant no.
2021-1-40).
Availability of data and materials
The datasets generated and/or analyzed during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
QG designed the current study. XS, XM and FL
performed the experiments. QG and FL analyzed the data. XY and YW
drafted the manuscript and analyzed data. XM and XY interpreted
data and revised the final manuscript. XS and QG confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was conducted with full adherence
to the international norms of medical ethics, as set out in the
Helsinki Declaration. The patients' parents gave their informed
written consent for enrollment in the study. The study was approved
by the Ethics Committee of the Children's Hospital Affiliated to
Shandong University (approval no. QLET-IRB/P-2021007).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deschler B and Lübbert M: Acute myeloid
leukemia: Epidemiology and etiology. Cancer. 107:2099–2107.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Albanesi J, Noguera NI, Banella C,
Colangelo T, De Marinis E, Leone S, Palumbo O, Voso MT, Ascenzi P,
Nervi C, et al: Transcriptional and metabolic dissection of
ATRA-induced granulocytic differentiation in NB4 acute
promyelocytic leukemia cells. Cells. 9(2423)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Molinaro A, Zanta D, Moleti ML, Giona F,
Conter V, Rizzari C, Biondi A and Testi AM: Challenging management
of severe differentiation syndrome in pediatric acute promyelocytic
leukemia treated with ATRA/ATO. Mediterr J Hematol Infect Dis.
14(e2022027)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Montesinos P, Bergua JM, Vellenga E, Rayón
C, Parody R, de la Serna J, León A, Esteve J, Milone G, Debén G, et
al: Differentiation syndrome in patients with acute promyelocytic
leukemia treated with all-trans retinoic acid and anthracycline
chemotherapy: Characteristics, outcome, and prognostic factors.
Blood. 113:775–783. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jambrovics K, Uray IP, Keresztessy Z,
Keillor JW, Fésüs L and Balajthy Z: Transglutaminase 2 programs
differentiating acute promyelocytic leukemia cells in all-trans
retinoic acid treatment to inflammatory stage through NF-κB
activation. Haematologica. 104:505–515. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dubois C, Schlageter MH, de Gentile A,
Balitrand N, Toubert ME, Krawice I, Fenaux P, Castaigne S, Najean
Y, Degos L, et al: Modulation of IL-8, IL-1 beta, and G-CSF
secretion by all-trans retinoic acid in acute promyelocytic
leukemia. Leukemia. 8:1750–1757. 1994.PubMed/NCBI
|
|
8
|
Guo QW, Li F, Song L, Wang YP and Yang XM:
Effect of PADI4 on the expression of inflammatory cytokines during
NB4 cells differentiation. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
29:1065–1070. 2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
9
|
Nakashima K, Hagiwara T, Ishigami A,
Nagata S, Asaga H, Kuramoto M, Senshu T and Yamada M: Molecular
characterization of peptidylarginine deiminase in HL-60 cells
induced by retinoic acid and 1alpha,25-dihydroxyvitamin D(3). J
Biol Chem. 274:27786–27792. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nakashima K, Arai S, Suzuki A, Nariai Y,
Urano T, Nakayama M, Ohara O, Yamamura K, Yamamoto K and Miyazaki
T: PAD4 regulates proliferation of multipotent haematopoietic cells
by controlling c-myc expression. Nat Commun. 4(1836)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakashima K, Hagiwara T and Yamada M:
Nuclear localization of peptidylarginine deiminase V and histone
deimination in granulocytes. J Biol Chem. 277:49562–49568.
2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Christophorou MA, Castelo-Branco G,
Halley-Stott RP, Oliveira CS, Loos R, Radzisheuskaya A, Mowen KA,
Bertone P, Silva JC, Zernicka-Goetz M, et al: Citrullination
regulates pluripotency and histone H1 binding to chromatin. Nature.
507:104–108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chang X and Han J: Expression of
peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol
Carcinog. 45:183–196. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Song G, Shi L, Guo Y, Yu L, Wang L, Zhang
X, Li L, Han Y, Ren X, Guo Q, et al: A novel PAD4/SOX4/PU.1
signaling pathway is involved in the committed differentiation of
acute promyelocytic leukemia cells into granulocytic cells.
Oncotarget. 7:3144–3157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sanz MA, Fenaux P, Tallman MS, Estey EH,
Löwenberg B, Naoe T, Lengfelder E, Döhner H, Burnett AK, Chen SJ,
et al: Management of acute promyelocytic leukemia: Updated
recommendations from an expert panel of the european LeukemiaNet.
Blood. 133:1630–1643. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang L, Chai W, Ye F, Yu Y, Cao L, Yang M,
Xie M and Yang L: HMGB1 promotes differentiation syndrome by
inducing hyperinflammation via MEK/ERK signaling in acute
promyelocytic leukemia cells. Oncotarget. 8:27314–27327.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fuss IJ, Kanof ME, Smith PD and Zola H:
Isolation of whole mononuclear cells from peripheral blood and cord
blood. Curr Protoc Immunol. 7:7.1.1–7.1.8, (Suppl 85).
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang Y and Dai M: Expression of PADI4 in
patients with ankylosing spondylitis and its role in mediating the
effects of TNF-α on the proliferation and osteogenic
differentiation of human mesenchymal stem cells. Int J Mol Med.
36:565–570. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Harney SM, Meisel C, Sims AM, Woon PY,
Wordsworth BP and Brown MA: Genetic and genomic studies of PADI4 in
rheumatoid arthritis. Rheumatology (Oxford). 44:869–872.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stahl M and Tallman MS: Differentiation
syndrome in acute promyelocytic leukaemia. Br J Haematol.
187:157–162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mohammadzadeh Z, Omidkhoda A, Chahardouli
B, Hoseinzadeh G, Moghaddam KA, Mousavi SA and Rostami S: The
impact of ICAM-1, CCL2 and TGM2 gene polymorphisms on
differentiation syndrome in acute promyelocytic leukemia. BMC
Cancer. 21(46)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chang X, Han J, Pang L, Zhao Y, Yang Y and
Shen Z: Increased PADI4 expression in blood and tissues of patients
with malignant tumors. BMC Cancer. 9(40)2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chang X and Fang K: PADI4 and
tumourigenesis. Cancer Cell Int. 10(7)2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yao H, Li P, Venters BJ, Zheng S, Thompson
PR, Pugh BF and Wang Y: Histone Arg modifications and p53 regulate
the expression of OKL38, a mediator of apoptosis. J Biol Chem.
283:20060–20068. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang X, Gamble MJ, Stadler S, Cherrington
BD, Causey CP, Thompson PR, Roberson MS, Kraus WL and Coonrod SA:
Genome-wide analysis reveals PADI4 cooperates with Elk-1 to
activate c-Fos expression in breast cancer cells. PLoS Genet.
7(e1002112)2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ham A, Rabadi M, Kim M, Brown KM, Ma Z,
D'Agati V and Lee HT: Peptidyl arginine deiminase-4 activation
exacerbates kidney ischemia-reperfusion injury. Am J Physiol Renal
Physiol. 307:F1052–F1062. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He W, Zhou P, Chang Z, Liu B, Liu X, Wang
Y, Li Y and Alam HB: Inhibition of peptidylarginine deiminase
attenuates inflammation and improves survival in a rat model of
hemorrhagic shock. J Surg Res. 200:610–618. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Knight JS, Luo W, O'Dell AA, Yalavarthi S,
Zhao W, Subramanian V, Guo C, Grenn RC, Thompson PR, Eitzman DT, et
al: Peptidylarginine deiminase inhibition reduces vascular damage
and modulates innate immune responses in murine models of
atherosclerosis. Circ Res. 114:947–956. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Knight JS, Subramanian V, O'Dell AA,
Yalavarthi S, Zhao W, Smith CK, Hodgin JB, Thompson PR and Kaplan
MJ: Peptidylarginine deiminase inhibition disrupts NET formation
and protects against kidney, skin and vascular disease in
lupus-prone MRL/lpr mice. Ann Rheum Dis. 74:2199–2206.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Montesinos P and Sanz MA: The
differentiation syndrome in patients with acute promyelocytic
leukemia: Experience of the pethema group and review of the
literature. Mediterr J Hematol Infect Dis.
3(e2011059)2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luesink M, Pennings JL, Wissink WM,
Linssen PC, Muus P, Pfundt R, de Witte TJ, van der Reijden BA and
Jansen JH: Chemokine induction by all-trans retinoic acid and
arsenic trioxide in acute promyelocytic leukemia: Triggering the
differentiation syndrome. Blood. 114:5512–5521. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ninomiya M, Kiyoi H, Ito M, Hirose Y, Ito
M and Naoe T: Retinoic acid syndrome in NOD/scid mice induced by
injecting an acute promyelocytic leukemia cell line. Leukemia.
18:442–448. 2004.PubMed/NCBI View Article : Google Scholar
|