Introduction
Triple-negative breast cancer (TNBC) is a type of
breast cancer in which estrogen receptor (ER), progesterone
receptor (PR) and human epidermal growth factor-2 (HER-2)
expression is absent (1). TNBC
accounts for ~20% of all breast cancer cases, is characterized by a
high recurrence rate and high rate of metastasis, and is difficult
to treat (2). TNBC is the most
common malignant tumor in women, and its incidence and death rates
are increasing annually, and worryingly, the age of onset is
decreasing meaning a larger number of younger individuals are being
diagnosed with TNBC (3).
Non-SMC condensin I complex subunit G (NCAPG) is a
protein related to cell proliferation and division, and it
participates in the occurrence and development of several types of
cancer (4). A previous study found
that NCAPG was associated with a poorer prognosis in breast cancer
patients. Compared with non-TNBC, the expression levels of NCAPG in
TNBC is higher, and knockdown of its expression was found to cause
cell cycle arrest in MCF-7 cells (5). Meanwhile, studies also found that
NCAPG expression is upregulated in TNBC through microarray analysis
where it was found to be associated with a poorer prognosis,
suggesting that NCAPG may serve as a potential biomarker of TNBC
(6,7). Moreover, NCAPG expression has been
shown to be upregulated in trastuzumab-resistant HER2-positive
breast cancer cells, and it enhanced the drug resistance of
HER2-positive breast cancer cells via activation of the SRC/STAT3
signaling pathway (8). However,
there are no studies on the related mechanism of NCAPG action in
TNBC cells.
It was found that NCAPG silencing reduced epidermal
growth factor receptor (EGFR) expression in hepatocellular
carcinoma (9). This suggests that
NCAPG can affect the development of cancer by regulating the
expression of EGFR. EGFR is a glycoprotein receptor on the cell
membrane, which can promote the growth and proliferation of tumor
cells. In TNBC, EGFR was found to promote the development of TNBC
through JAK/STAT3 signaling (10).
Therefore, it was hypothesized that downregulation
of NCAPG affects the development of TNBC cells through
EGFR/JAK/STAT3 signaling. Our study provides a theoretical basis
for the targeted treatment of TNBC.
Materials and methods
Cell culture
Normal mammary epithelial MCF-10A cells, luminal A
human breast cancer MCF-7 cells, luminal B human breast cancer
BT-474 cells, HER2+ breast cancer HCC1954 cells and TNBC
MDA-MB-231 cells were obtained from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences and were cultured in
DMEM supplemented with 10% FBS and 1% penicillin-streptomycin
solution at 37˚C in a humidified incubator supplied with 5%
CO2.
RT-qPCR
Total RNA from the cells was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA from each
sample was reverse transcribed to single-stranded cDNA, which was
next used for qPCR. The mRNA expression levels of NCAPG were
detected using a SYBR Premix Ex Taq kit (Takara Bio, Inc.). The
thermocycling conditions for qPCR were as follows: 95˚C in a 20-µl
reaction volume for 10 min, followed by 40 cycles at 95˚C for 15
sec, 60˚C for 30 sec and 72˚C for 30 sec. The fold changes were
calculated using the 2-ΔΔCq method (11). The sequences were as follows: NCAPG
forward: 5'-TCCACATAGAGAAGAATGATGCTGA-3' and reverse:
5'-GCAAACACGGGGAAGAACAC-3'; GAPDH forward:
5'-AATGGGCAGCCGTTAGGAAA-3' and reverse:
5'-GCGCCCAATACGACCAAATC-3'.
Western blot assay
RIPA lysis buffer was used to extract the total
proteins from the cells, and the protein concentration was
quantified using a BCA kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. A total of 30 µg
protein/lane was loaded on a 10% SDS-gel, resolved using SDS-PAGE
and transferred to a PVDF (Roche Diagnostics GmbH). The membranes
were blocked with 5% nonfat milk for 1 h at room temperature, and
then incubated with the corresponding primary antibodies anti-NCAPG
(1:800, ab70350, Abcam), anti-Bcl-2 (1:1,000, ab32124, Abcam),
anti-Bax (1:1,000, ab182733, Abcam), anti-Bad (1:1,000, ab32445,
Abcam), anti-MMP2 (1:1,000, ab92536, Abcam), anti-MMP9 (1:1,000,
ab76003, Abcam), anti-p-EGFG (1:1,000, ab40815, Abcam), anti-EGFR
(1:1,000, ab52894 Abcam), anti-p-JAK1 (1:1,000, ab138005, Abcam),
anti-JAK1 (1:800, ab133666, Abcam), anti-STAT3 (1:1,000, ab68153,
Abcam), anti-p-STAT3 (1:1,000, ab76315, Abcam), anti-GAPDH
(1:1,000, ab9485, Abcam) overnight at 4˚C. The following day, the
membranes were incubated with goat anti-rabbit IgG H&L
(HRP)-conjugated secondary antibodies (1:5,000, ab7090, Abcam) for
1 h at room temperature. The expression levels of the different
proteins were detected using enhanced chemiluminescence reagent
(Bio-Rad Laboratories, Inc.). The data were analyzed using ImageJ
version 1.46 (National Institutes of Health).
Cell transfection
Short hairpin RNA NCAPG#1 (sh-NCAPG#1), sh-NCAPG#2
and the control adenovirus vector (sh-NC) were obtained from
GeneCopoeia, Inc. For transfections, the cells were seeded into
6-well plates for 24 h, and then transfected according to the
manufacturer's instructions. Two human shRNA-NCAPG sequences were
as follows: sh-NCAPG#1:
CCGGGCTATGCAGAAGCATCTTCTTCTCGAGAAGAAGATGCTTCTGCATAGCTTTTTTG and
sh-NCAPG#2:
CCGGCGGGCAGTGTTATCATGTATTCTCGAGAATACATGATAACACTGCCCGTTTTTTG. sh-NC
is as follows: CAACAAGATGAAGAGCACCAA. After determining the optimal
transfection efficiency, cells were divided into a control group,
sh-NC group and sh-NCAPG group. Then, 50 ng/ml EGF (exogenous EGFR
agonist) and 2 µM colivelin (JAK/STAT3 signaling pathway agonist)
were added, and the transfected cells were divided into a control,
sh-NC and sh-NCAPG, sh-NCAPG+EGF and sh-NCAPG+ colivelin group.
Cell viability assay
The cell viability was measured using a CCK-8 assay
(Dojindo Molecular Technologies, Inc.). Briefly, after treatment of
the cells as above, 10 µl CCK-8 solution was added and cells were
incubated for 2 h. Cell viability was measured at an absorbance
wavelength of 450 nm (optical density) using a microplate reader
(Bio-Rad Laboratories, Inc.).
TUNEL assay
A TUNEL staining kit (Beyotime Institute of
Biotechnology) was used to detect cell apoptosis according to the
manufacturer's instructions. TUNEL solution was then added to stain
the nucleus. Different areas of the sample were randomly selected
and captured under a fluorescence microscope (Olympus Corporation,
magnification, x200) to count the number of TUNEL-positive
cells.
Wound healing assay
Cells were seeded into 6-well plates in serum-free
DMEM. When the cells reached 80-90% confluence, a 10-µl pipette tip
was used to produce a wound in the monolayer at the bottom of the
plate. The wound width was then measured at 0 and 24 h on a light
microscope (magnification, x200). The cell migration rate was
calculated as follows: (Initial width-final width)/Initial
width.
Transwell invasion assay
Cell suspensions (100 µl) were added to the upper
chambers of the Transwell inserts coated with Matrigel™
(50 mg/l; 1:8 diluted solution; BD Biosciences) and 600 µl
supplemented medium was added to the lower chambers. After
incubation for 24 h, the membrane was fixed with 4%
paraformaldehyde for 15 min and sequentially stained with 0.1%
crystal violet solution for 30 min (all at room temperature). The
inside of the membrane was gently wiped with a cotton swab to
remove any cells that had not migrated. Finally, the number of
cells that had migrated were counted under a light microscope
(magnification 200).
Database
The UALCAN database (ualcan.path.uab.edu/) was used to analyze the
expression levels of NCAPG.
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using GraphPad Prism version 6.0
(GraphPad Software, Inc.). A Student's t-test was used for
comparisons between two groups, and a one-way ANOVA followed by
Tukey's post hoc test was used for comparisons between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
NCAPG expression is upregulated in
TNBC MDA-MB-231 cells
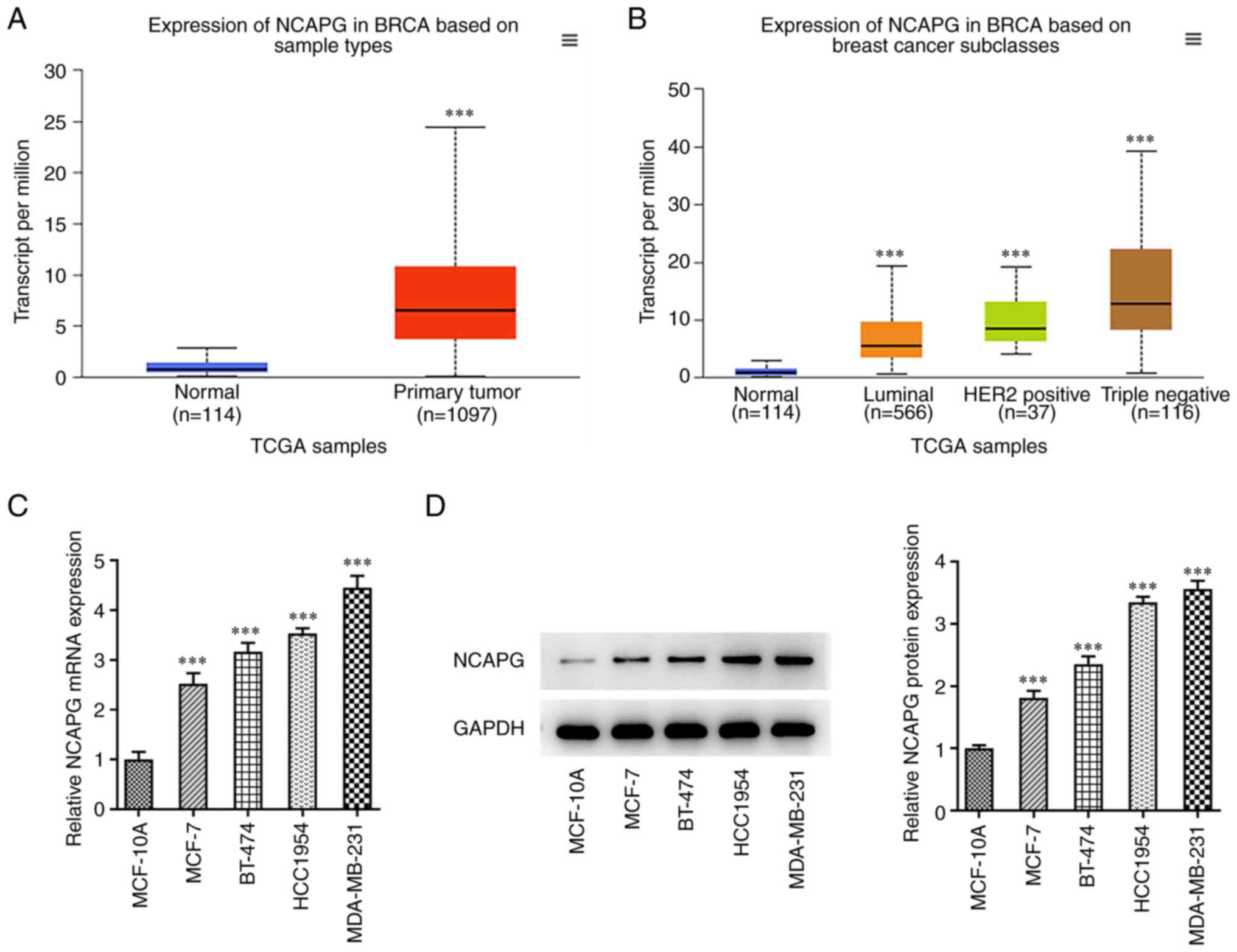
UALCAN database analysis showed that the expression
of NCAPG was significantly increased in TNBC patients compared with
healthy patients (Fig. 1A and
B). RT-qPCR and western blot
analysis were used to detect the expression of NCAPG in various
breast cancer cell lines. The results showed that the expression of
NCAPG was significantly increased in MCF-7, BT-474, HCC1954, and
MDA-MB-231 cells compared with the MCF-10A cells (Fig. 1C and D). The above results indicated that NCAPG
had higher expression in the TNBC cell line. Thus, MDA-MB-231 cells
were selected for the following experiments.
Knockdown of NCAPG promotes apoptosis
of TNBC MDA-MB-231 cells
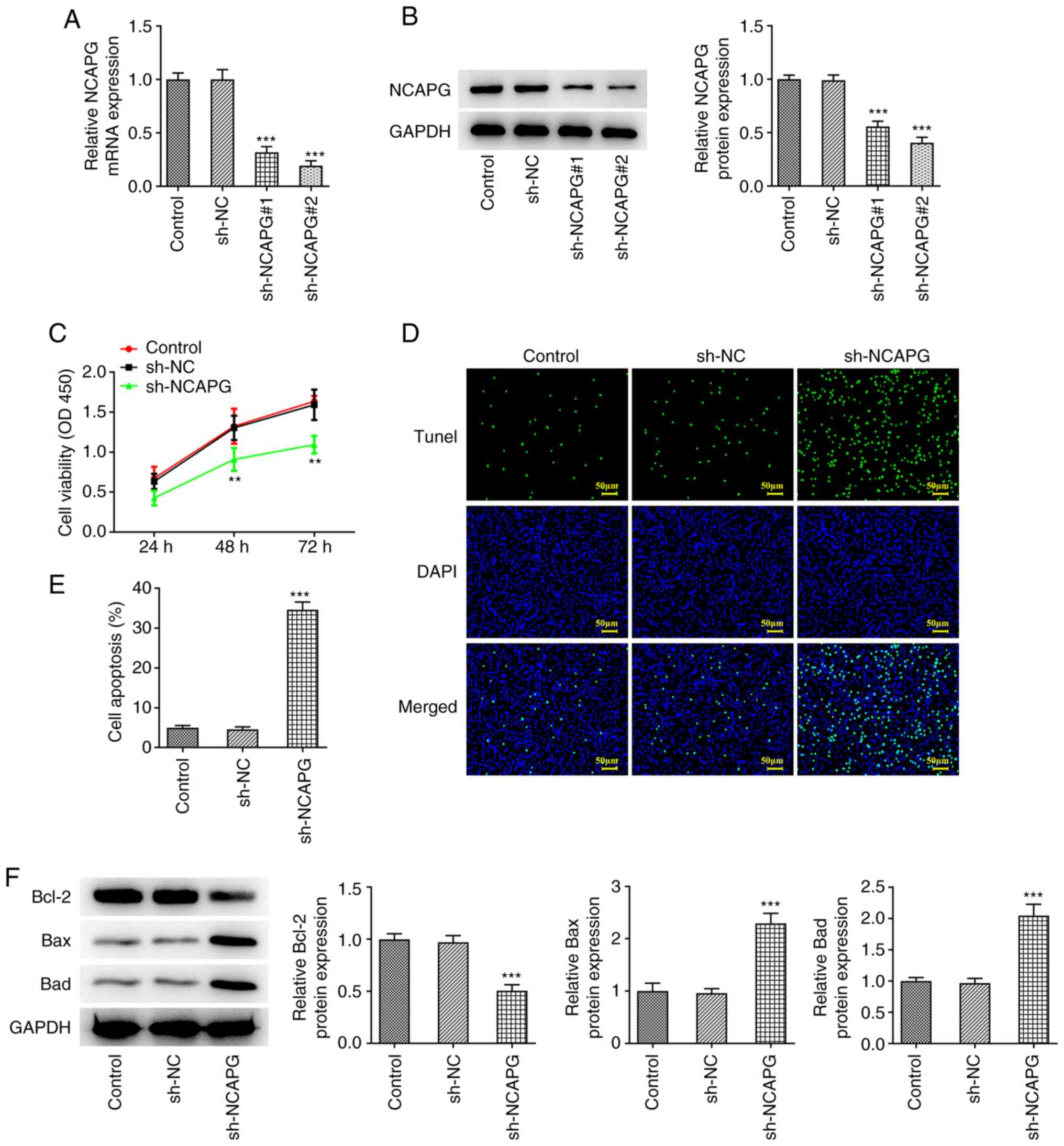
The interference plasmid of NCAPG was constructed,
and the transfection efficiency was detected by RT-qPCR and western
blotting. Compared with the sh-NC group, the expression of NCAPG in
the sh-NCAPG#1 and sh-NCAPG#2 groups was significantly decreased,
and the transfection efficiency of the sh-NCAPG#2 group was the
better of the two, thus it was selected for the subsequent
experiments (Fig. 2A and B). CCK-8 analysis showed that cell
viability in the sh-NCAPG group was significantly decreased
compared with that in the sh-NC group (Fig. 2C). TUNEL staining and western
blotting analysis showed that compared with the sh-NC group,
apoptosis was significantly increased in the sh-NCAPG group, and
this was accompanied by decreased expression of Bcl-2 and increased
expression of Bax and Bad (Fig.
2D-F).
Knockdown of NCAPG inhibits migration
and invasion of TNBC MDA-MB-231 cells
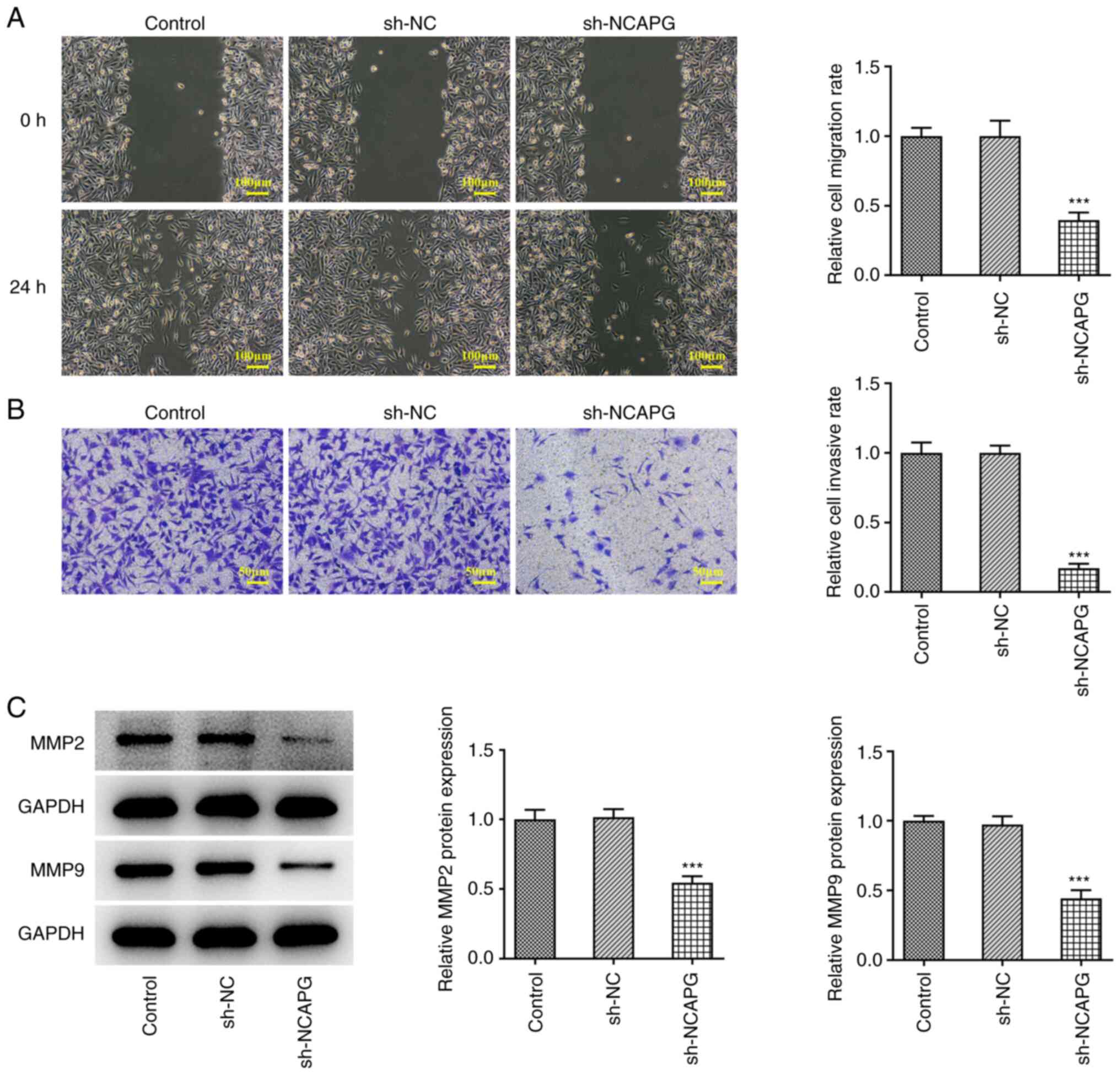
Wound healing and Transwell assays were used to
assess the migration and invasion of TNBC cells, and the results
showed that compared with the sh-NC group, the migration and
invasion of the sh-NCAPG group was significantly decreased
(Fig. 3A and B). Western blotting was used to detect
the expression of matrix metalloproteinase (MMP)2 and MMP9, and the
results showed that the expression of MMP2 and MMP9 in the sh-NCAPG
group were significantly decreased compared with the sh-NC group
(Fig. 3C).
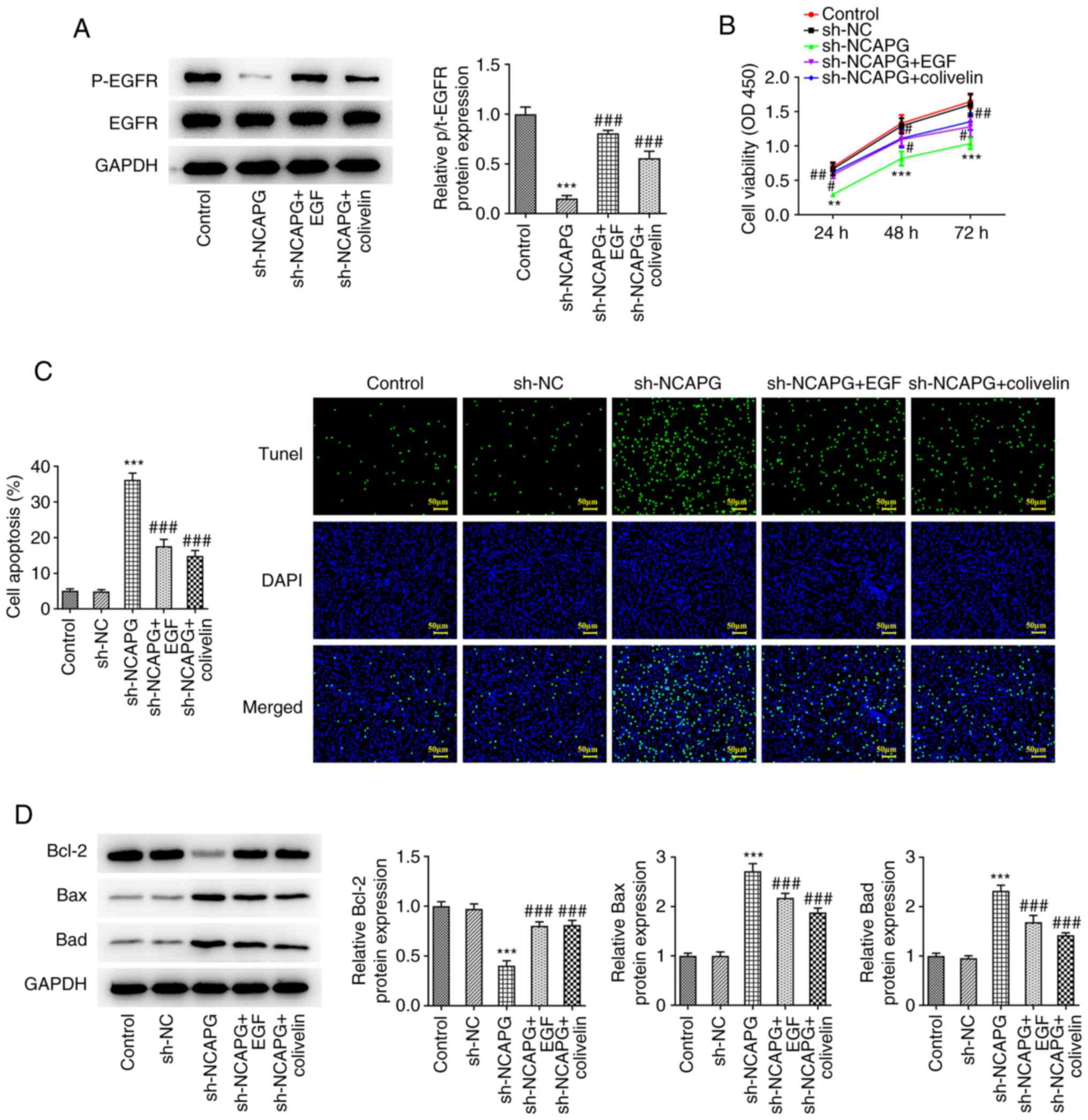
Activation of EGFR/JAK/STAT3 signaling
reduces the effect of the knockdown of NCAPG on the apoptosis of
TNBC cells
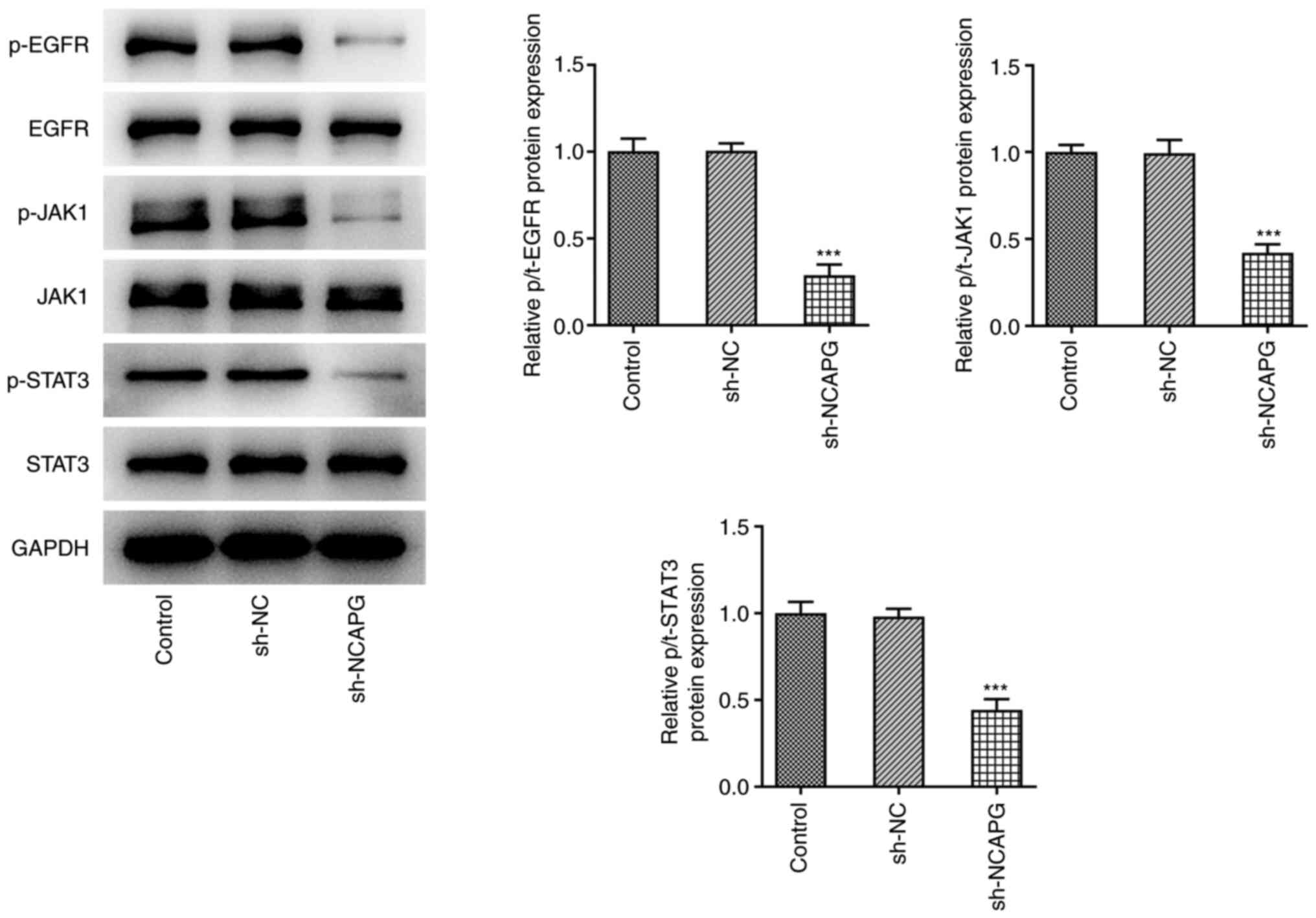
The expression levels of the EGFR/JAK/STAT3
signaling pathway-related proteins were detected by western
blotting, and the results showed that the expression of
phosphorylated (p-)EGFR, phosphorylated Janus kinase 1 (p-JAK1),
and phosphorylated signal transducer and activator of transcription
3 (p-STAT3) were significantly decreased in the sh-NCAPG group
compared with the sh-NC group (Fig.
4). Subsequently, after the addition of EGF and colivelin, the
expression of p-EGFR was increased as detected by western blot
analysis compared with the sh-NCAPG group (Fig. 5A). CCK-8 results showed that
relative to the sh-NC group, cell viability in the sh-NCAPG group
was significantly reduced. Moreover, cell activity was
significantly increased in the sh-NCAPG+ EGF and sh-NCAPG+colivelin
groups compared with the sh-NCAPG group (Fig. 5B). TUNEL and western blotting
results showed that compared with the sh-NCAPG group, apoptosis was
significantly decreased in the sh-NCAPG+ EGF and sh-NCAPG+colivelin
groups, and this was accompanied by increased Bcl-2 expression, and
decreased Bax and Bad expression (Fig.
5C and D).
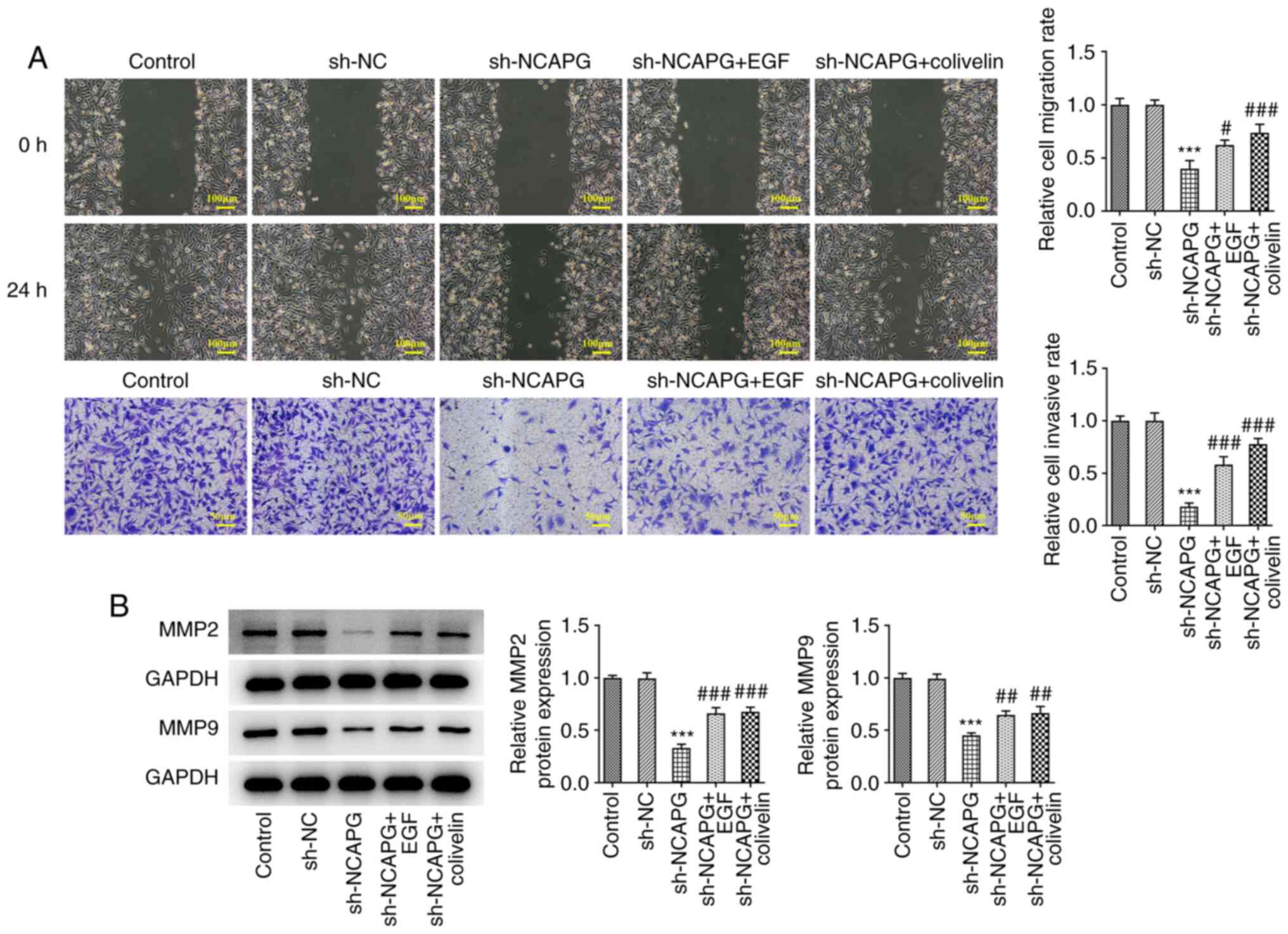
Activation of EGFR/JAK/STAT3 signaling
reduces the inhibitory effect of knockdown of NCAPG on invasion and
migration of TNBC MDA-MB-231 cells
Wound healing and Transwell assays showed that
EGFR/JAK/STAT3 signal activation significantly increased the
invasion and migration of cells (Fig.
6A), and the expression of MMP2 and MMP9 in cells was
significantly increased (Fig. 6B)
compared to the sh-NCAPG group.
Discussion
At present, with the continuous in-depth research on
the pathogenic mechanisms underlying the development and
progression of triple-negative breast cancer (TNBC), several
targeted drugs have been discovered (12). However, due to the heterogeneity of
TNBC and acquisition of drug resistance, the clinical effects of
targeted drugs for TNBC is not always significant (13). Therefore, there is an urgent need
to identify novel effective targets for early screening, prognosis
assessment and treatment of TNBC.
Through UALCAN database analysis, we found that
non-SMC condensin I complex subunit G (NCAPG) expression was
significantly elevated in TNBC. This is consistent with the results
screened out by Zeng et al (14) through microarray analysis. A
previous study showed that the protein expression levels of NCAPG
were significantly increased in 16 different types of tumors, and
upregulated expression of NCAPG was significantly correlated with a
poor survival rate in liver cancer, breast cancer, lung cancer and
ovarian cancer, amongst others (4). NCAPG has also been shown to be
significantly associated with a poor prognosis in TNBC (7). Additionally, it has been shown that
NCAPG promotes hepatocellular carcinoma proliferation and reduces
apoptosis through the PI3K/AKT signaling pathway in hepatocellular
carcinoma cells (15). In lung
cancer, NCAPG promotes lung cancer cell proliferation and migration
through the TGF-β signaling pathway (16). However, the mechanism by which
NCAPG is upregulated in TNBC has not been determined, nor have the
downstream effects of its upregulation. In the present study, it
was shown that the expression of NCAPG in breast cancer cells,
including MCF-7, BT-474, HCC1954 and MDA-MB-231 cells, was
significantly increased. After NCAPG expression was inhibited, the
activity of MDA-MB-231 cells was decreased, apoptosis was
increased, and invasion and migration were significantly decreased.
These results showed that downregulation of NCAPG expression
inhibited the malignant phenotype of TNBC MDA-MB-231 cells.
Subsequently, the downstream regulatory mechanisms
of NCAPG in TNBC MDA-MB-231 cells were explored. It was previously
shown that NCAPG silencing in hepatoma cells inhibited cell
proliferation and promoted cell apoptosis by reducing the
expression of epidermal growth factor receptor (EGFR) (9). In human lung adenocarcinoma,
mutations in the EGFR kinase domain mediate signal transducer and
activator of transcription 3 (STAT3) activation through interleukin
(IL)-6 production (17). EGFR
silencing in vivo can significantly inhibit the growth of
breast cancer cells by regulating the Janus kinase (JAK)/STAT3
signaling pathway (10).
Therefore, whether NCAPG could regulate EGFR and its downstream
JAK/STAT3 signaling pathway was assessed. It was shown that
EGFR/JAK/STAT3 signaling pathway activity was inhibited after NCAPG
inhibition. EGFR/JAK/STAT3 signaling pathway activators can reverse
the effects of NCAPG on proliferation, invasion, migration and
apoptosis of TNBC MDA-MB-231 cells. These results suggest that
downregulation/knockdown of NCAPG promotes apoptosis and inhibits
invasion and migration of TNBC MDA-MB-231 cells through
EGFR/JAK/STAT3 signaling.
There are also certain limitations to the present
study. We only assessed the expression of NCAPG in TNBC MDA-MB-231
cells, which will be verified in more breast cancer cell lines in
future experiments. In addition, we only examined our findings in
cell experiments and did not verify our results in animal
experiments. We will further verify our experimental results in
animal experiments in the future.
In conclusion, the results of the present study
showed that knockdown of NCAPG promoted apoptosis and inhibited
invasion and migration of TNBC MDA-MB-231 cells, and this was
achieved through NCAPG-mediated regulation of EGFR/JAK/STAT3
signaling.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JL, JZ and BL conceived and designed the study. JL,
HS, SL and DW performed experiments. BL and HH wrote the paper. JL,
JZ, HS and HH reviewed and edited the manuscript. Data acquisition
and analysis were performed by JL and HH. JL and SL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sporikova Z, Koudelakova V, Trojanec R and
Hajduch M: Genetic markers in triple-negative breast cancer. Clin
Breast Cancer. 18:e841–e850. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eroles P, Bosch A, Perez-Fidalgo JA and
Lluch A: Molecular biology in breast cancer: Intrinsic subtypes and
signaling pathways. Cancer Treat Rev. 38:698–707. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xiao C, Gong J, Jie Y, Cao J, Chen Z, Li
R, Chong Y, Hu B and Zhang Q: NCAPG is a promising therapeutic
target across different tumor types. Front Pharmacol.
11(387)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong M, Xu T, Cui X, Li H, Li X and Xia W:
NCAPG upregulation mediated by four microRNAs combined with
activation of the p53 signaling pathway is a predictor of poor
prognosis in patients with breast cancer. Oncol Lett.
21(323)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen J, Qian X, He Y, Han X and Pan Y:
Novel key genes in triple-negative breast cancer identified by
weighted gene co-expression network analysis. J Cell Biochem.
120:16900–16912. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiao X, Zhang Z, Luo R, Peng R, Sun Y,
Wang J and Chen X: Identification of potential oncogenes in
triple-negative breast cancer based on bioinformatics analyses.
Oncol Lett. 21(363)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jiang L, Ren L, Chen H, Pan J, Zhang Z,
Kuang X, Chen X, Bao W, Lin C, Zhou Z, et al: NCAPG confers
trastuzumab resistance via activating SRC/STAT3 signaling pathway
in HER2-positive breast cancer. Cell Death Dis.
11(547)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu K, Li Y, Yu B, Wang F, Mi T and Zhao
Y: Silencing non-SMC chromosome-associated polypeptide G inhibits
proliferation and induces apoptosis in hepatocellular carcinoma
cells. Can J Physiol Pharmacol. 96:1246–1254. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Song X, Liu Z and Yu Z: EGFR promotes the
development of triple negative breast cancer through JAK/STAT3
signaling. Cancer Manag Res. 12:703–717. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lyons TG: Targeted therapies for
triple-negative breast cancer. Curr Treat Options Oncol.
20(82)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22(61)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zeng X, Shi G, He Q and Zhu P: Screening
and predicted value of potential biomarkers for breast cancer using
bioinformatics analysis. Sci Rep. 11(20799)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gong C, Ai J, Fan Y, Gao J, Liu W, Feng Q,
Liao W and Wu L: NCAPG promotes the proliferation of hepatocellular
carcinoma through PI3K/AKT signaling. Onco Targets Ther.
12:8537–8552. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu Y, Lin Y, Pan J, Tu X, Xu Y, Li H and
Chen Y: NCAPG promotes the progression of lung adenocarcinoma via
the TGF-β signaling pathway. Cancer Cell Int.
21(443)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B and Bromberg
JF: Mutations in the EGFR kinase domain mediate STAT3 activation
via IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007.PubMed/NCBI View
Article : Google Scholar
|