Introduction
Acute myeloid leukemia (AML) is a group of
aggressive heterogeneous malignancies. The backbone of therapy for
AML is a combination of cytarabine- and anthracycline-based
regimens with hematopoietic stem-cell transplantation (HSCT) for
eligible candidates (1). Acute
promyelocytic leukemia (APL) is a special disease entity of AML,
known as type M3, which is characterized by a specific t(15;17)
chromosome translocation that generates the promyelocytic
leukemia/retinoic acid receptor-α fusion gene (2). Differentiation therapy with all-trans
retinoic acid has been a very successful therapeutic strategy and
has transformed APL into the most curable form of AML (3). However, the increased demand for
nutrients and energy caused by tumor invasion, as well as
chemotherapy-related gastrointestinal mucositis, may affect the
nutritional status of patients (4). An insufficient nutrient supply can
easily lead to malnutrition, which can result in decreased
chemotherapy tolerance and an increased incidence of adverse
complications (5,6). Thus, nutritional status is directly
associated with treatment success.
It has been confirmed that under- and overweight
patients are at an increased risk of complications, non-relapse
mortality and shorter overall survival (OS) after chemotherapy and
HSCT (7). However, as the most
commonly used nutritional indicator in AML, body mass index (BMI)
may not be feasible for the detection of early physical changes,
thereby compromising timely and effective intervention (6). To increase the long-term survival
rate of patients, more sensitive clinical nutritional parameters
should be established, and clinical nutritional interventions
should be implemented for patients with a combined nutritional risk
or those with previous malnutrition.
Phase angle (PhA), which is determined by
bioelectrical impedance analysis (BIA), indicates the amount and
quality of soft tissue. When interpreting PhA in patient
populations, a decrease in PhA is due to loss of soft tissue and
could be seen as an indicator of nutritional status (8). Additionally, PhA has been used in
clinical practice to detect cellular membrane function and fluid
balance that may reveal cellular health and is highly predictive of
impaired clinical outcomes and mortality in renal disease, human
immunodeficiency virus infections, allogeneic HSCT and surgical
patients (9-12).
Previous studies have shown that PhA can be used to predict adverse
clinical outcomes, including survival time, financial cost of
disease and incidence of post-operative complications (11-13).
To the best of our knowledge, few studies have
evaluated the use of PhA in AML. It has been reported by
univariable, but not multivariable analyses, that low baseline PhA
is associated with increased incidence of 60-day mortality
(13). Decreases in morbidity and
mortality of patients warrant improving the understanding of the
nutritional and prognostic value of using PhA in AML. Therefore,
the present study aimed to prospectively evaluate PhA as a
prognostic indicator of the nutritional status and mortality of
patients with AML who were undergoing chemotherapy, excluding APL
for which retinoic acid-based therapeutics have been developed as
aforementioned.
Materials and methods
Study design and population
The present study was conducted in the Department of
Hematology at the First Affiliated Hospital of Chongqing Medical
University (Yuzhong, China) in accordance with the principles of
the Declaration of Helsinki (approval no. 2020-589).
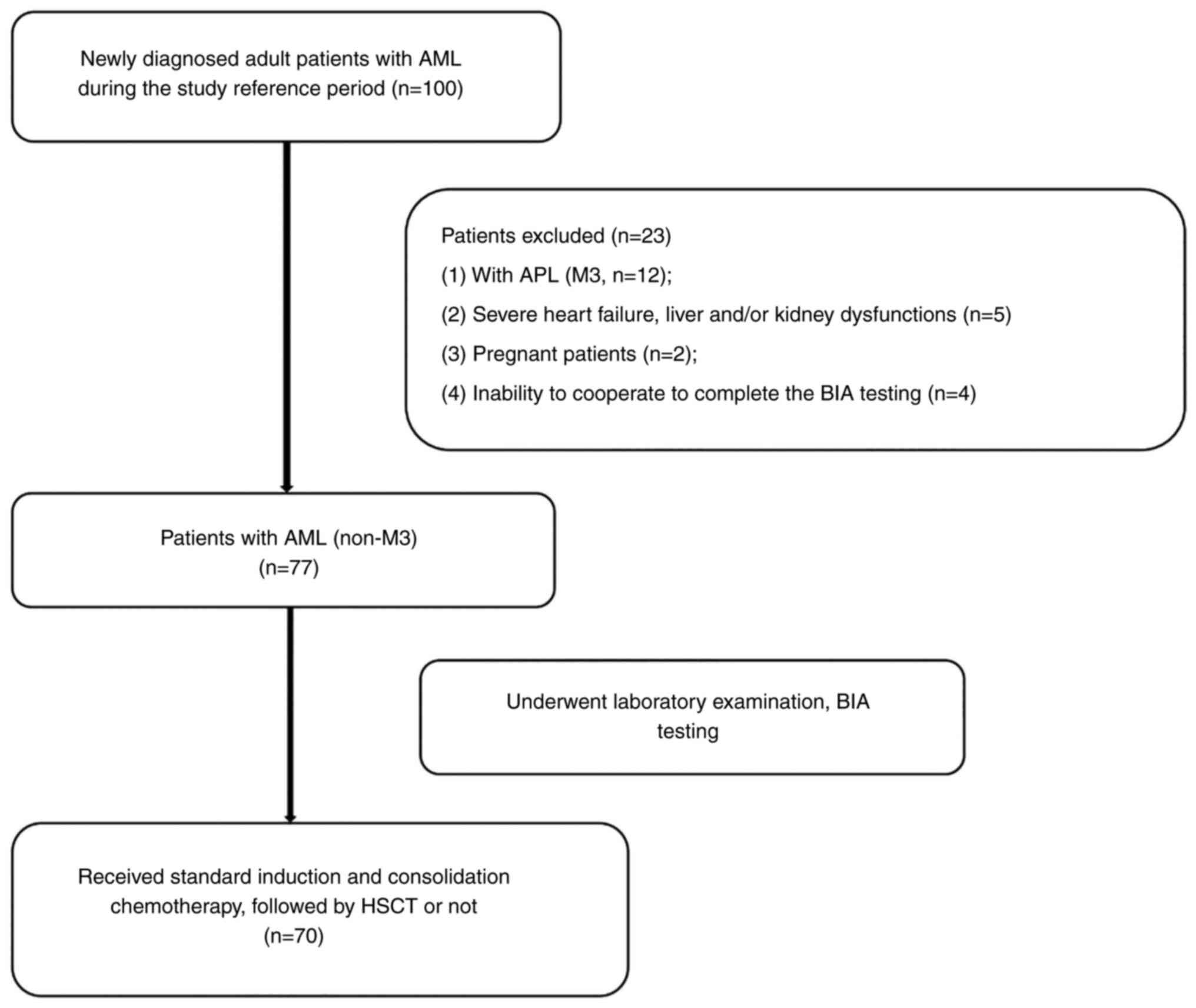
A total of 100 patients were enrolled from July 2020
to February 2021. Baseline clinical data, including age and sex,
are presented in Table I. The
inclusion criteria were as follows: i) Adult patients were aged 19
to 85 years with a diagnosis of AML confirmed by morphology,
immunology, cytogenetics and molecular diagnosis system (14); and ii) indications for chemotherapy
evaluated by a hematologist. The critical exclusion criteria were
as follows: i) Patients diagnosed with APL (type M3); ii) patients
with severe heart failure, liver and kidney dysfunctions or other
concomitant severe diseases, with an absence of tolerability to
chemotherapy after active treatment for the aforementioned
contraindications; iii) pregnant patients; iv) patients who could
not cooperate to complete the BIA testing; and v) chronic drug use
that may affect the body fluid balance. A flowchart of the study
design and patient selection criteria is presented in Fig. 1.
 | Table IComparison of baseline clinical and
body composition indicators according to the baseline PhA. |
Table I
Comparison of baseline clinical and
body composition indicators according to the baseline PhA.
| A, Clinical data |
|---|
| Variable | Total (n=70) | Normal PhA group
(n=51) | Reduced PhA group
(n=19) | P-value |
|---|
| Age, years | 51.9±14.0 | 49.7±12.8 | 58.0±15.6 | 0.025 |
| Sex, male | 35 (50.0%) | 27 (52.9%) | 8 (42.1%) | 0.420 |
| BMI,
kg/m2 | 22.7±2.8 | 23.2±2.9 | 21.4±2.1 | 0.009 |
| SBP, mmHg | 121.0±12.8 | 117.1±11.4 | 113.3±9.0 | 0.647 |
| DBP, mmHg | 70.7±9.8 | 70.5±7.3 | 66.8±10.0 | 0.466 |
| NRS-2002 score | | | | 0.034 |
|
≥3 | 50 (71.4) | 40 (78.4) | 10 (52.6) | |
|
<3 | 20 (28.6) | 11 (21.6) | 9 (47.4) | |
| B, Laboratory
parameters |
| Variable | Total (n=70) | Normal PhA group
(n=51) | Reduced PhA group
(n=19) | P-value |
| WBC,
109/l | 10.3 (2.7-37.8) | 13.5 (2.5-44.0) | 14.0
(8.4-286.0) | 0.219 |
| Hb, g/l | 74.2±24.3 | 83.5±26.6 | 53.5±24.4 | 0.283 |
| Ure, mmol/l | 315 (229-407) | 299 (233-402) | 326 (245-341) | 0.858 |
| Cre, µmol/l | 66.7±16.8 | 63.1±14.4 | 75.7±31.9 | 0.085 |
| Alb, g/l | 41 (38-44) | 41 (38-43) | 40 (37-56) | 0.196 |
| LDH, U/l | 574
(261-1,398) | 404
(182-1,489) | 736
(478-1,646) | 0.093 |
| hs-CRP, mg/l | 10.0
(2.3-20.0) | 1.5 (1.0-7.7) | 15.1
(13.5-20.0) | 0.197 |
| C, Body composition
indicators |
| Variable | Total (n=70) | Normal PhA group
(n=51) | Reduced PhA group
(n=19) | P-value |
| TBW, kg | 33.6±5.7 | 34.0±0.9 | 32.3±4.4 | 0.280 |
| ECW, kg | 13.0±2.2 | 13.0±2.3 | 13.0±1.9 | 0.992 |
| ICW, kg | 19.8
(17.8~22.4) | 21.4
(18.1~23.0) | 19.6
(16.8~21.8) | 0.117 |
| Protein, kg | 8.7 (7.7~9.6) | 9.2 (7.8~10.0) | 8.5 (7.3~9.4) | 0.125 |
| Fat, kg | 13.1
(9.6~16.4) | 14 (9.7~16.6) | 11.5
(7.1~15.6) | 0.088 |
| SMM, kg | 24.3
(21.3~27.1) | 25.9
(21.6~27.8) | 23.5
(20.3~26.3) | 0.121 |
| SLM, kg | 43.0±7.3 | 43.7±7.9 | 41.2±5.5 | 0.220 |
| PBF, kg | 22.5±7.1 | 23.2±7.0 | 20.6±7.2 | 0.169 |
| FFM, kg | 45.6±7.7 | 46.3±8.3 | 43.9±5.8 | 0.246 |
| BCM, kg | 29.5±5.1 | 30.2±5.5 | 27.8±3.6 | 0.122 |
| PhA, ˚ | 5.4±0.9 | 5.9±0.7 | 4.2±0.6 | 0.001 |
| D, Molecular
tests |
| Variable | Total (n=70) | Normal PhA group
(n=51) | Reduced PhA group
(n=19) | P-value |
| FLT3 | 10 (14.3) | 7 (13.7) | 3 (15.8) | 0.548 |
| WT1 | 15 (21.4) | 12 (23.5) | 3 (15.8) | 0.365 |
| E, Cytogenetic
risk |
| Variable | Total (n=70) | Normal PhA group
(n=51) | Reduced PhA group
(n=19) | P-value |
| Risk group | | | | 0.413 |
|
Favorable | 31 (44.3) | 25(49) | 6 (31.6) | |
|
Intermediate | 14(20) | 9 (17.6) | 5 (26.3) | |
|
Adverse | 25 (35.7) | 17 (33.3) | 8 (42.1) | |
| F, HSCT |
| Variable | Total (n=70) | Normal PhA group
(n=51) | Reduced PhA group
(n=19) | P-value |
| Transplant
type | | | | 0.308 |
|
Auto-HSCT | 6 (8.6) | 5 (9.8) | 1 (5.3) | |
|
Allo-HSCT | 17 (24.3) | 10 (19.6) | 7 (36.8) | |
|
Non-HSCT | 47 (67.1) | 36 (70.6) | 11 (57.9) | |
All patients with AML (excluding M3) were given the
standard 3+7 induction chemotherapy comprising idarubicin (10
mg/m2d) or daunorubicin (60 mg/m2d) on days
1-3 plus cytarabine (Ara-c; 100-200 mg/m2d) on days 1-7.
Consolidation chemotherapy consisted of high-dose Ara-c-based
regimens (1-3 g/m2 every 12 h) for a 4-week cycle for
3~6 courses. According to disease remission, risk stratification,
physical status and economic level, patients could choose whether
to undergo HSCT after induction and consolidation chemotherapy.
Measurements
Anthropometric variables including height (cm) and
weight (kg) were measured by doctors. Nutritional Risk Screening
2002 (NRS-2002) system was implemented to screen nutritional risk
(15). Routine laboratory tests
were performed in the Department of Laboratory at the First
Affiliated Hospital of Chongqing Medical University. The data of
tumor-specific variables with prognostic significance were
collected at initial diagnosis of AML, including lactate
dehydrogenase (LDH) level, white blood cell count, creatinine (Cre)
and cytogenetic risk group (based on bone marrow biopsy) (16).
A direct segmental multi-frequency BIA device
(InBody S10; InBody Co., Ltd.) was used to determine the values of
body composition indicators and PhA at 50 kHz before the initiation
of fluid treatment. The participants were instructed not to eat or
drink and to avoid strenuous activity for 2 h before BIA testing.
The results of the parameter tests were obtained using a standard
montage of outer and inner electrodes on the right hand and foot
while patients were laid down with parted legs. All measurements
were undertaken at the time of initial diagnosis of AML and after
completion of therapy. The body composition indicators, including
skeletal muscle mass (SMM), soft lean mass (SLM), percentage of
body fat, fat-free mass (FFM), body cell mass (BCM), intracellular
water (ICW), extracellular water (ECW), total body water (TBW) and
mineral and protein contents, were measured and recorded. The
parameters of resistance and reactance were determined using an
electric alternating current flow of 800 mA and frequencies of 5,
50 and 250 kHz. PhA was calculated using the following equation:
PhA (˚)=(arc tangent reactance/resistance) x (180˚/π). Reduced PhA
was defined as PhA <5˚ for male or PhA <4.6˚ female patients
(17). According to this
established standard, the study population was divided into normal
PhA and reduced PhA groups.
Statistical analysis
The Kolmogorov-Smirnov test was used to analyze data
normality. Normally distributed continuous variables were presented
as the mean ± standard deviation, while non-normally distributed
variables were presented as the median with upper and lower
quartiles. A two-way mixed ANOVA followed by Bonferroni's post hoc
test was used for the comparison of normally distributed body
composition variables between and within groups. For non-normally
distributed body composition variables, a Bonferroni correction
after either Wilcoxon signed-rank test or Mann-Whitney U test was
used. An unpaired Student's t-test or Mann-Whitney U test was used
to analyze the rest of the comparisons. Categorical variables were
presented as frequency (proportions) and compared using Chi-square
test or Fisher's exact test as appropriate. The Kaplan-Meier method
was used to estimate the survival probabilities and the log-rank
test was utilized to compare the differences in progression-free
survival (PFS) and OS between the patient subgroups. A multivariate
analysis was performed by fitting the Cox proportional hazards
model to assess the effects of PhA and other characteristics on PFS
and OS. PFS was defined as the time from diagnosis to relapse,
progression or death from any cause. OS was defined as the time
from diagnosis to the date of the last follow-up examination or the
date of death from any cause. All statistical analyses were
performed using SPSS 21.0 software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients and basic
characteristics
According to the established process of patient
selection, 70 patients consented, underwent baseline laboratory and
BIA measurements and completed the follow-up. The median age of the
patients at diagnosis was 52 years and 50% were males. Of the
enrolled patients, 55.7% had intermediate to adverse cytogenetic
risk. Due to their financial status and treatment tolerance, only
32.9% of the patients underwent HSCT. At initial diagnosis of AML,
28.6% were at nutritional risk according to the NRS-2002 scoring
system and 27.1% had decreased PhA values. The population and basic
characteristics of the normal PhA and reduced PhA groups are
summarized in Table I. There were
no significant statistical differences between the two groups in
laboratory data, body composition indicators and molecular testing
results. Notably, the patients in the normal PhA group were younger
and their basic nutritional risk was lower compared with the
reduced PhA group due to higher BMI and NRS-2002 scores (Table I).
Body composition index after antitumor
therapy
A decrease in body composition parameters from
baseline was observed after chemotherapy or HSCT, although
statistically significant differences existed only in the reduced
PhA group. The post-treatment body composition parameters of the
patients with AML with a reduced baseline PhA were significantly
lower compared with patients with normal PhA, including TBW, ECW,
ICW, protein, fat, SMM, SLM, FFM and BCM (Table II).
 | Table IIComparison of body composition
indicators at the end of therapy according to the baseline PhA. |
Table II
Comparison of body composition
indicators at the end of therapy according to the baseline PhA.
| Variable | Total (n=70) | Normal PhA group
(n=51) | Reduced PhA group
(n=19) | P-value |
|---|
| TBW, kg | 30.7
(27.9-35.0) | 34.8
(28.9-39.7) | 27.9
(25.4-34.6)a | 0.002 |
| ECW, kg | 12.1
(10.8-14.0) | 13.6
(11.2-15.1) | 11.2
(10.1-12.8)a | 0.005 |
| ICW, kg | 19.4±3.8 | 21.1±3.8 |
17.5±2.6a | 0.003 |
| Protein, kg | 8.4±1.7 | 9.1±1.7 |
7.6±1.1a | 0.003 |
| Fat, kg | 14.8±6.3 | 18.4±7.2 |
11.5±5.1a | 0.008 |
| SMM, kg | 23.3±5.0 | 25.6±5.0 |
20.8±3.4a | 0.003 |
| SLM, kg | 39.1
(35.7-44.9) | 44.4
(37.0-51.0) | 35.7
(32.5-41.7)a | 0.002 |
| PBF, kg | 24.9±7.1 | 26.1±7.7 | 22.4±5.1 | 0.062 |
| FFM, kg | 41.7
(38.0-47.5) | 47.1
(39.2-54.1) | 38.0
(34.8-44.1) | 0.002 |
| BCM, kg | 27.8±5.5 | 30.3±5.5 |
25.1±3.7a | 0.007 |
PFS and OS from initial diagnosis of
AML according to baseline PhA
In total, 28 patients experienced progressive
disease (PD), of which 23 deaths occurred at a median observation
time of 9.3 months. Of these deaths, 13 were due to progressive
AML, three deaths were related to treatment complications following
HSCT and 7 patients died of severe infection or acute cerebral
hemorrhage secondary to myelosuppression after chemotherapy. All
the patients were subjected to survival outcome measurements based
on an intention-to-treat analysis.
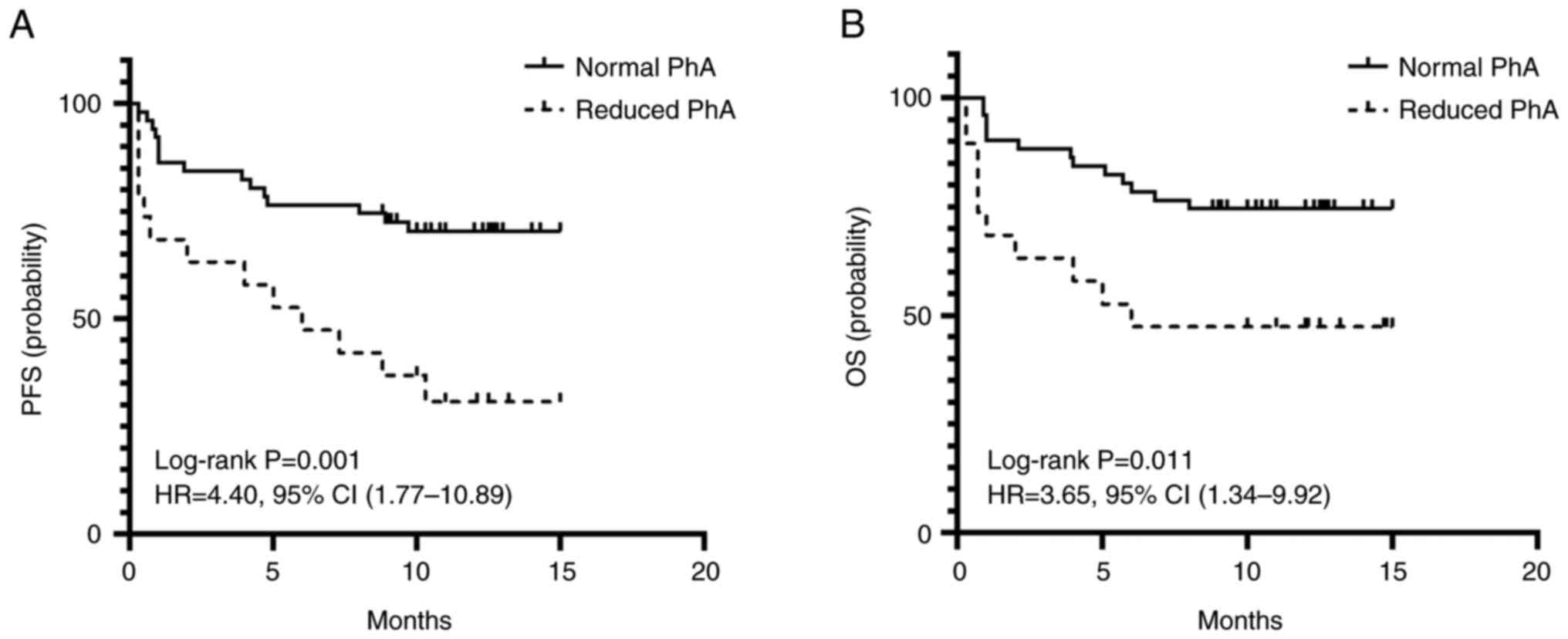
The 1-year PFS and OS rates of the reduced PhA group
were 36.8 and 47.4%, respectively, while those of the normal PhA
group were 76.5 and 76.5%, respectively. The PFS and OS rates of
the reduced PhA group, estimated by Kaplan-Meier analysis, were
shorter compared with those of the patients in the normal PhA group
(median PFS: 7.1 months vs. 11.6 months, P=0.001; median OS: 8.2
months vs. 12.1 months, P=0.011) (Fig.
2).
Cox proportional hazards model for
assessing the association of baseline PhA with PFS and OS
Univariate analysis suggested that both PFS (hazard
ratio [HR], 3.14; 95%CI], 1.49-6.63; P=0.003) and OS (HR, 2.76; 95%
CI, 1.21-6.31, P=0.016) were significantly worse in patients with
reduced baseline PhA. Multivariate adjustments for age, BMI,
NRS-2002 score, LDH and Cre levels confirmed the prognostic value of
PhA in adult patients with AML. A reduced PhA was associated with
decreased PFS (HR, 3.13; 95% CI, 1.21-8.11; P=0.019), but it was
not a significant predictor of OS (HR, 2.19; 95% CI, 0.76-6.30;
P=0.147) (Table III).
 | Table IIICox proportional hazards model for
assessing the association of baseline phase angle with PFS and
OS. |
Table III
Cox proportional hazards model for
assessing the association of baseline phase angle with PFS and
OS.
| Model | PFS [HR (95%
CI)] | OS [HR (95%
CI)] |
|---|
| Univariate | 3.14
(1.49-6.63)a | 2.76
(1.21-6.31)a |
|
Multivariateb | 3.13
(1.21-8.11)a | 2.19
(0.76-6.30) |
Discussion
In the present study, the potential association of
PhA, obtained using BIA, with malnutrition and prognosis was
explored in adults with newly diagnosed AML who were undergoing
chemotherapy. The results demonstrated that chemotherapy caused a
reduction in nutrients, such as water, protein, fat and minerals,
and that these changes were more pronounced in patients with a
reduced baseline PhA. The results also showed that a reduction in
the baseline PhA was significantly associated with an increased
risk of PD and death in AML. When adjusted for age, BMI, NRS-2002
score, Cre and LDH, the present study revealed a reduced baseline
PhA to be a significant predictor of PFS.
Malnutrition is a challenging clinical syndrome in
onco-hematology because of its adverse effects on the patients'
quality of life and survival (18). Furthermore, malnutrition has been
confirmed as a risk factor for infectious complications, treatment
intolerance, prolonged hospitalization and impaired quality of life
(19-23).
A previous study found malnutrition to be highly prevalent,
occurring in 40-80% of patients with cancer (24). In the current study, 28.6% of
patients were at nutritional risk according to the NRS-2002 score
estimated at the time of diagnosis, possibly due to the increased
metabolic demands caused by high tumor load and nutrient
consumption (25). Subsequent
chemotherapy-related mucositis and gastrointestinal side effects,
including nausea and vomiting, may have led to reduced food intake
and nutrient absorption dysfunction, thus further worsening the
nutritional status of patients (26).
At the time of the initial AML diagnosis, ~30% of
the study population had reduced PhA. PhA has been suggested as an
indicator of cellular health, in which higher values reflect higher
cellularity, cell membrane integrity and improved cell function;
therefore, the reduction in baseline PhA can be attributed to the
combination of cell death and the loss of cellular integrity, as
well as changes in membrane selective permeability and fluid
balance (27).
Our previous study revealed that the incidence of
sarcopenia is associated with chemotherapy of patients with AML, as
reflected by body composition changes (28). The present study also observed that
the body composition indicators, including water, muscle mass,
protein and BCM, were significantly decreased after antitumor
therapy in patients with baseline reduced PhA. It is likely that
these patients suffered from malnutrition due to an early shift in
terms of an increased extracellular-to-intracellular fluid ratio
and decreased BCM, both of which lowered PhA (29). In addition, there was no
significant difference between the two groups in baseline
hypersensitive C-reactive protein, thereby excluding the effect of
inflammation-associated excessive hydration on PhA (30). Thus, low PhA could specifically
reflect impaired nutritional status. The negative association
between PhA and malnutrition shows its predictive value for disease
prognosis.
Previous research has confirmed that standardized
PhA (sPhA) is an independent prognostic factor for the 2-year OS
rate in patients with AML that undergo HSCT (11). In another study, the change in sPhA
has been confirmed as a significant predictor of OS, as it
increases the 60-day mortality for patients in the lower 25th
percentile of baseline sPhA values; however, this association is
statistically significant only in univariable analysis (13). A limitation of the aforementioned
studies is the lack of validated cut-offs for PhA, due to previous
studies only focusing on healthy German populations. Thus, PhA was
standardized according to reference values for healthy German
populations as follows: sPhA=observed PhA (˚)-PhA medium for sex,
age and BMI (˚)/standard deviation of PhA for sex, age and BMI. In
this way, the external validity of the results is thereby reduced
(31).
In 2012, Kyle et al (17) evaluated the accuracy of PhA in
identifying the presence of nutritional risk in a large cohort of
patients at the time of hospital admission compared with age-, sex-
and height-matched healthy controls. It was revealed that the
cut-offs for PhA at 5.0˚ in men and 4.6˚ in women are
prognostically relevant and give the highest sensitivity,
specificity and area under the receiver operating characteristic
curve. This established standard was used as the basis for
classifying the patient population in the present study. The
present results add to the growing body of evidence supporting the
prognostic significance of PhA in patients with AML. To the best of
our knowledge, the present study is the first to identify the
association between baseline PhA and disease progression. Reduced
baseline PhA is one of the few predictors of PFS, besides age and
cytogenetic risk, in both univariate and multivariate analyses
(32). These exploratory analyses
suggest that the effect of the baseline PhA on survival may be due
to a combination of the patients' disease response and nutritional
status.
Tumor-specific factors, such as cytogenetics and
gene mutations and other patient-specific factors, including age
and Eastern Cooperative Oncology Group (ECOG) performance status,
have been used to create a prognostic scoring system for OS
(33,34). Thus, the aforementioned prognostic
factors were examined in the present study. In the study
population, ~65% had a favorable or intermediate cytogenic risk. No
significant differences in molecular testing and cytogenetic risk
data have been observed between the two groups. Previous studies
have not confirmed the potential association between PhA and
genetic markers for leukemia (11,13).
These findings considerably reduce the influence of disease
severity on clinical outcomes, improving the interpretation of the
independent effect of PhA on the studied outcomes.
The current study has certain limitations. Firstly,
a follow-up study with an increased sample size is needed to
clarify the impact of PhA on long-term complications and mortality.
Secondly, since there were no data to assess the burden of leukemia
in the bone marrow, it was not possible to determine whether PhA
affects the complete remission status of patients, leading to a
worse prognosis.
In summary, the findings of the present study
demonstrated that PhA is a reproducible and high-precision
indicator that may provide important nutritional and prognostic
information in patients with newly diagnosed AML (excluding type
M3). An increased risk of PD and death exists in patients with a
reduced baseline PhA. Previous studies have suggested that 12 weeks
of progressive resistance training may improve PhA, and nutritional
support can minimize sarcopenia and increase muscle function in
patients with low PhA (35,36).
Future prospective studies are necessary to elucidate the efficacy
of PhA while further exploring the influence of strength training
and personal nutritional support on PhA and clinical outcomes.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by grants provided by
Chongqing Medical Research Project ‘Study on early cardiotoxicity
of antitumor drugs in lymphoma patients’ (grant no. 2021MSXM276)
and Chongqing Natural Science Foundation general project ‘The
mechanism of doxorubicin promoting atherosclerosis in lymphoma
patients through NF-κB/miR-33 signaling pathway’ (grant no.
cstc2019jcyj-msxmX0043).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TJ designed the study, recruited patients, collected
and analyzed data and drafted the manuscript. YW contributed to
data collection and critically reviewed the data analysis and
manuscript preparation. NZ and XT contributed to the data analysis
and interpretation as well as the critical writing and revision of
the manuscript. All authors have read and approved the final
manuscript. TJ and XT confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The protocols involving patients were approved by
the Ethics Committee of the First Affiliated Hospital of Chongqing
Medical University (Yuzhong, China; approval no. 2020-589). The
patients enrolled in this research provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krug U, Röllig C, Koschmieder A, Heinecke
A, Sauerland MC, Schaich M, Thiede C, Kramer M, Braess J,
Spiekermann K, et al: Complete remission and early death after
intensive chemotherapy in patients aged 60 years or older with
acute myeloid leukaemia: A web-based application for prediction of
outcomes. Lancet. 376:2000–2008. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Döhner H, Estey EH, Amadori S, Appelbaum
FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson
RA, et al: Diagnosis and management of acute myeloid leukemia in
adults: Recommendations from an international expert panel, on
behalf of the European LeukemiaNet. Blood. 115:453–474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
De Kouchkovsky I and Abdul-Hay M: ‘Acute
myeloid leukemia: A comprehensive review and 2016 update’. Blood
Cancer J. 6(e441)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Le Blanc K, Ringdén O and Remberger M: A
low body mass index is correlated with poor survival after
allogeneic stem cell transplantation. Haematologica. 88:1044–1052.
2003.PubMed/NCBI
|
|
5
|
Bay JO, Dendoncker C, Angeli M, Biot T,
Chikhi M, Combal C, Jouannic L, Kermeur G, Lopvet L, Marchand T, et
al: Nutritional management for patients hospitalized during
allogeneic stem cell transplantation: Guidelines from the
Francophone society of bone marrow transplantation and cellular
therapy (SFGM-TC). Bull Cancer. 103 (11S):S201–S206.
2016.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
6
|
Sahinoz M, Luther JM, Mashayekhi M, Jung
DK, Ikizler TA and Engelhardt BG: Hematologic malignancies magnify
the effect of body mass index on insulin resistance in cancer
survivors. Blood Adv. 6:1981–1990. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Berro M, Arbelbide JA, Rivas MM, Basquiera
AL, Ferini G, Vitriu A, Foncuberta C, Fernandez Escobar N, Requejo
A, Milovic V, et al: Hematopoietic cell transplantation-specific
comorbidity index predicts morbidity and mortality in autologous
stem cell transplantation. Biol Blood Marrow Transplant.
23:1646–1650. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Norman K, Stobäus N, Pirlich M and
Bosy-Westphal A: Bioelectrical phase angle and impedance vector
analysis-clinical relevance and applicability of impedance
parameters. Clin Nutr. 31:854–861. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oliveira CM, Kubrusly M, Mota RS, Silva
CA, Choukroun G and Oliveira VN: The phase angle and mass body cell
as markers of nutritional status in hemodialysis patients. J Ren
Nutr. 20:314–320. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schwenk A, Beisenherz A, Römer K, Kremer
G, Salzberger B and Elia M: Phase angle from bioelectrical
impedance analysis remains an independent predictive marker in
HIV-infected patients in the era of highly active antiretroviral
treatment. Am J Clin Nutr. 72:496–501. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Urbain P, Birlinger J, Ihorst G, Biesalski
HK, Finke J and Bertz H: Body mass index and bioelectrical
impedance phase angle as potentially modifiable nutritional markers
are independent risk factors for outcome in allogeneic
hematopoietic cell transplantation. Ann Hematol. 92:111–119.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pena NF, Mauricio SF, Rodrigues AMS, Carmo
AS, Coury NC, Correia MITD and Generoso SV: Association between
standardized phase angle, nutrition status, and clinical outcomes
in surgical cancer patients. Nutr Clin Pract. 34:381–386.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yates SJ, Lyerly S, Manuel M, Tooze JA,
Klepin HD, Powell BL, Dralle S, Uprety A and Pardee TS: The
prognostic value of standardized phase angle in adults with acute
leukemia: A prospective study. Cancer Med. 9:2403–2413.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Medinger M, Heim D, Halter JP, Lengerke C
and Passweg JR: Diagnosis and therapy of acute myeloid leukemia.
Ther Umsch. 76:481–486. 2019.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
15
|
Wang F, Dong Q, Yu K, Li RR, Fu J, Guo JY
and Li CW: Nutrition risk screening and related factors analysis of
non-hospitalized cancer survivors: A nationwide online survey in
China. Front Nutr. 9(920714)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Padmakumar D, Chandraprabha VR, Gopinath
P, Vimala Devi ART, Anitha GRJ, Sreelatha MM, Padmakumar A and
Sreedharan H: A concise review on the molecular genetics of acute
myeloid leukemia. Leuk Res. 111(106727)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kyle UG, Soundar EP, Genton L and Pichard
C: Can phase angle determined by bioelectrical impedance analysis
assess nutritional risk? A comparison between healthy and
hospitalized subjects. Clin Nutr. 31:875–881. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qiu M, Zhou YX, Jin Y, Wang ZX, Wei XL,
Han HY, Ye WF, Zhou ZW, Zhang DS, Wang FH, et al: Nutrition support
can bring survival benefit to high nutrition risk gastric cancer
patients who received chemotherapy. Support Care Cancer.
23:1933–1939. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fitzpatrick F, Skally M, O'Hanlon C, Foley
M, Houlihan J, Gaughan L, Smith O, Moore B, Cunneen S, Sweeney E,
et al: Food for thought. Malnutrition risk associated with
increased risk of healthcare-associated infection. J Hosp Infect.
101:300–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barr RD, Gomez-Almaguer D, Jaime-Perez JC
and Ruiz-Argüelles GJ: Importance of nutrition in the treatment of
leukemia in children and adolescents. Arch Med Res. 47:585–592.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alexandre J, Gross-Goupil M, Falissard B,
Nguyen ML, Gornet JM, Misset JL and Goldwasser F: Evaluation of the
nutritional and inflammatory status in cancer patients for the risk
assessment of severe haematological toxicity following
chemotherapy. Ann Oncol. 14:36–41. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Leiva Badosa E, Badia Tahull M, Virgili
Casas N, Elguezabal Sangrador G, Faz Méndez C, Herrero Meseguer I,
Izquierdo González À, López Urdiales R, Oca Burguete FJ, Tubau
Molas M, et al: Hospital malnutrition screening at admission:
Malnutrition increases mortality and length of stay. Nutr Hosp.
34:907–913. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Culine S, Chambrier C, Tadmouri A, Senesse
P, Seys P, Radji A, Rotarski M, Balian A and Dufour P: Home
parenteral nutrition improves quality of life and nutritional
status in patients with cancer: A French observational multicentre
study. Support Care Cancer. 22:1867–1874. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Castillo-Martínez L, Castro-Eguiluz D,
Copca-Mendoza ET, Pérez-Camargo DA, Reyes-Torres CA, Ávila EA,
López-Córdova G, Fuentes-Hernández MR, Cetina-Pérez L and
Milke-García MDP: Nutritional assessment tools for the
identification of malnutrition and nutritional risk associated with
cancer treatment. Rev Invest Clin. 70:121–125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li J, Wang C, Liu X, Liu Q, Lin H, Liu C,
Jin F, Yang Y, Bai O, Tan Y, et al: Severe malnutrition evaluated
by patient-generated subjective global assessment results in poor
outcome among adult patients with acute leukemia: A retrospective
cohort study. Medicine (Baltimore). 97(e9663)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Peric KM and Reeves DJ: Tolerability of
induction chemotherapy dosing practices in acute myeloid leukemia
patients. Leuk Res. 39:173–176. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Player EL, Morris P, Thomas T, Chan WY,
Vyas R, Dutton J, Tang J, Alexandre L and Forbes A: Bioelectrical
impedance analysis (BIA)-derived phase angle (PA) is a practical
aid to nutritional assessment in hospital in-patients. Clin Nutr.
38:1700–1706. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qin Z, Lu K, Jiang T, Wang M, Weng Y, Tang
X and Zhao Y: Evaluating sarcopenia by using the bioelectrical
impedance analysis in patients with acute myeloid leukemia after
chemotherapy. Int J Gen Med. 15:1261–1269. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gonzalez MC, Barbosa-Silva TG, Bielemann
RM, Gallagher D and Heymsfield SB: Phase angle and its determinants
in healthy subjects: Influence of body composition. Am J Clin Nutr.
103:712–716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Demirci MS, Demirci C, Ozdogan O, Kircelli
F, Akcicek F, Basci A, Ok E and Ozkahya M: Relations between
malnutrition-inflammation-atherosclerosis and volume status. The
usefulness of bioimpedance analysis in peritoneal dialysis
patients. Nephrol Dial Transplant. 26:1708–1716. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bosy-Westphal A, Danielzik S, Dörhöfer RP,
Later W, Wiese S and Müller MJ: Phase angle from bioelectrical
impedance analysis: Population reference values by age, sex, and
body mass index. JPEN J Parenter Enteral Nutr. 30:309–316.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sacco AM, Valerio G, Alicante P, Di
Gregorio A, Spera R, Ballarin G and Scalfi L: Raw bioelectrical
impedance analysis variables (phase angle and impedance ratio) are
significant predictors of hand grip strength in adolescents and
young adults. Nutrition. 91-92(111445)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
El-Jawahri A, Abel GA, Traeger L, Waldman
L, Markovitz N, VanDusen H, Fathi A, Steensma DP, LeBlanc TW,
Horick NK, et al: Quality of life and mood of older patients with
acute myeloid leukemia (AML) receiving intensive and non-intensive
chemotherapy. Leukemia. 33:2393–2402. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Röllig C, Bornhäuser M, Thiede C, Taube F,
Kramer M, Mohr B, Aulitzky W, Bodenstein H, Tischler HJ, Stuhlmann
R, et al: Long-term prognosis of acute myeloid leukemia according
to the new genetic risk classification of the European LeukemiaNet
recommendations: Evaluation of the proposed reporting system. J
Clin Oncol. 29:2758–2765. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rondanelli M, Klersy C, Terracol G,
Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M,
Lukaski H and Perna S: Whey protein, amino acids, and vitamin D
supplementation with physical activity increases fat-free mass and
strength, functionality, and quality of life and decreases
inflammation in sarcopenic elderly. Am J Clin Nutr. 103:830–840.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Souza MF, Tomeleri CM, Ribeiro AS,
Schoenfeld BJ, Silva AM, Sardinha LB and Cyrino ES: Effect of
resistance training on phase angle in older women: A randomized
controlled trial. Scand J Med Sci Sports. 27:1308–1316.
2017.PubMed/NCBI View Article : Google Scholar
|